Abstract
Objective
To assess the efficacy of dalfampridine in patients with neuromyelitis optica spectrum disorder.
Methods
We included 15 consecutive patients, who were started on a treatment of dalfampridine 10 mg twice daily for 2 weeks. Efficacy assessment was based on walking ability improvement using Timed-25-Foot Walk and 12-item Multiple Sclerosis Walking Scale tests.
Results
The mean Timed-25-Foot Walk score was reduced from 14.8 (±2.4) to 11.3 (±1.9) seconds (p = 0.01). The mean score on the 12-item Multiple Sclerosis Walking Scale was reduced from 41.2 (±3.5) to 31.4 (±3.2) (p = 0.004).
Conclusion
Dalfampridine seems to be useful for symptomatic treatment of walking impairment in neuromyelitis optica spectrum disorder.
Keywords: Fampridine, neuromyelitis optica spectrum disorder, neuromyelitis optica
Introduction
Dalfampridine improves walking capacities in multiple sclerosis (MS). 1 Neuromyelitis optica spectrum disorder (NMOSD), like MS, is an inflammatory disease of the central nervous system, but particularly affects the spinal cord and optic nerve. In patients with NMOSD, walking impairment is common and frequently severe. However, to date, there are no data on the value of dalfampridine in NMOSD but the drug is used off-label. In the present study, we assessed the effect of dalfampridine tablets in clinical practice in patients with NMOSD for walking disability.
Patients and methods
Fifteen patients with NMOSD (diagnosed according to the 2015 NMOSD criteria) 2 with Expanded Disability Status Scale (EDSS) scores between 4 and 7 were prospectively included in this pilot study. All patients gave their written informed consent to participate in the study. All patients were stabilized with their symptomatic and disease-modifying drugs and had not had any relapses for at least 6 months. All patients received dalfampridine tablets 10 mg twice daily and were evaluated at day 0 and after 2 weeks of treatment.
The primary end point was the Timed 25-Foot Walk (T25FW) reduction after two weeks of treatment. The secondary end points were three self-questionnaires usually used in MS. These questionnaires were administered to each patient to evaluate walking ability (12-item Multiple Sclerosis Walking Scale (MSWS-12)), health-related-quality of life (SEP-59) and fatigue (EMIF-SEP).
In summary:
T25FW assesses how much time in seconds it takes a patient to walk, with or without aid, as quickly as possible along a well-marked 25-foot (7.62 m) linear course. 3 We compared T25FW scores before and after 2 weeks of treatment. We considered, as for MS studies, that responders were patients with at least a 20% reduction.
MSWS-12 is a 12-item questionnaire that assesses patient-perceived walking ability and evaluates the impact of MS on walking speed, distance, and various other parameters related to walking quality. Item scores are summed and transformed to a 0–100 scale, with higher scores indicating greater limitation in walking ability. 4 In MS, a significant reduction is considered to be a reduction of 5 points or more.
SEP-59 is the French-validated questionnaire of health-related quality of life (HRQOL), which includes 59 questions grouped into 15 subscales. Scores are obtained by averaging items within subscales and transforming average scores linearly to 0–100 possible scores, with higher values indicating better HRQOL. 5
-
EMIF-SEP is the French-validated version of the fatigue impact scale in MS. 6 It comprises 40 items grouped into four subscales (cognitive, physical, psychological and social aspects of fatigue). Scores are obtained by averaging items within subscales and transforming average scores linearly to 0–100 possible scores, with higher values indicating higher fatigue. 6
Statistical analysis: Differences in data at baseline (T0) and after two weeks (T2w) were assessed using the two-tailed Student's-test (Paired t test).
Results
Twelve of the 15 patients were women (80%). Median age was 46.4 years (28–62). Eleven of the 15 patients (73.3%) were positive for aquaporin 4 (AQP4) antibodies. None of the patients were positive for MOG antibodies. The median EDSS score was 5.5 (range: 4.0–7.0) and the median disease duration was 10.6 years (range: 1–36).
One patient experienced adverse events (nausea, gait ataxia, and tremor) that resulted in early discontinuation of treatment without control clinical assessments. No other side effects were reported by the other 14 patients during the study.
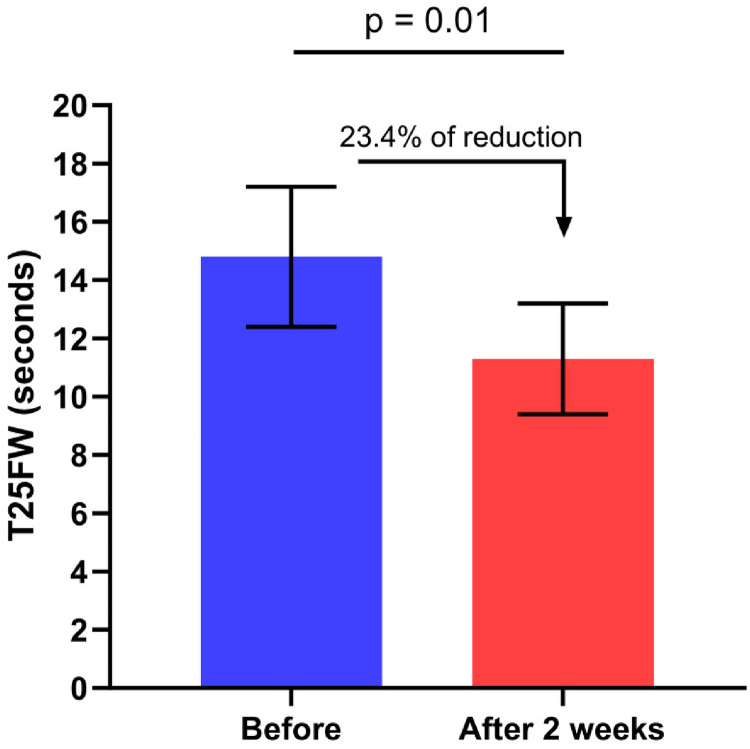
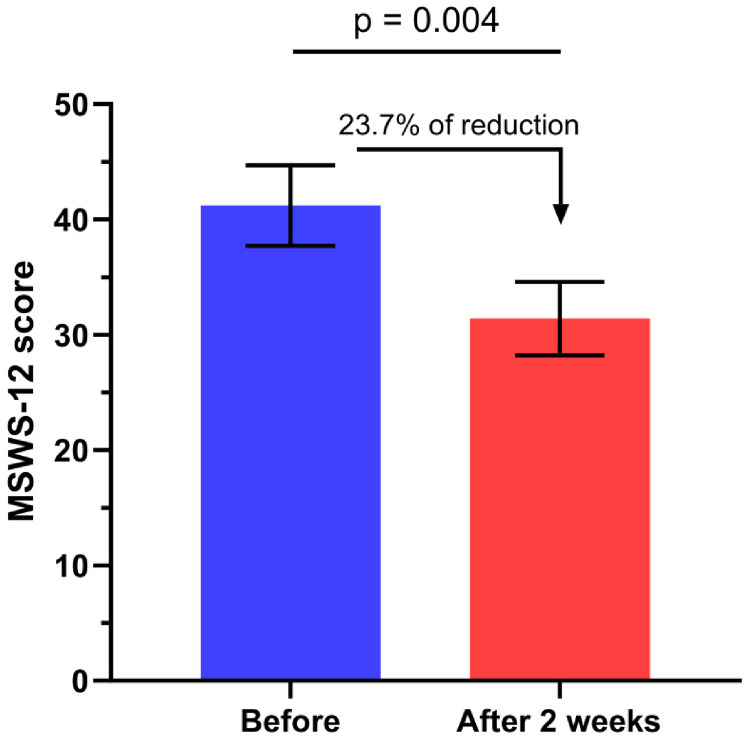
In the remaining 14 patients, the mean T25FW score was reduced from 14.8 (±2.4) to 11.3 (±1.9) seconds corresponding to a walking speed reduction of 23.4% (p = 0.01, Figure 1). The reduction was ≥20% for 8 patients (59.1% of patients). The mean score on the MSWS-12 was reduced from 41.2 (±3.5) to 31.4 (±3.2), corresponding to a reduction of 23.7% (p = 0.004, Figure 2). When we compared AQP4 positive and negative patients we did not observed any differences regarding both T25FW and MSWS. The percentage of responders (reduction of 5 points or more) was 64.2% (9 patients). The SEP-59 questionnaire scores improved in all the subscales (except for the physical limitation dimension) but the difference did not reach the level of statistical significance. The EMIF-SEP scores improved in all the subscales, the improvement being statistically significant for the psychological dimension only (p = 0.04) and showed a tendency towards significance for the fatigue global score (p = 0.055).
Figure 1.
Change in Timed 25-Foot Walk (T25FW) duration before and after 2 weeks of dalfampridine treatment.
Figure 2.
Change in 12-item Multiple Sclerosis Walking Scale (MSWS-12) score before and after 2 weeks of dalfampridine treatment.
Discussion
Our results show that dalfampridine improved walking disorder in NMOSD, with a possibly greater level of improvement than that previously demonstrated in MS. In MS, the proportion of patients with a demonstrated clinical benefit in terms of walking speed and self-reported walking ability varies according to the case series.1,3 An improvement of 20% or greater on the T25FW or a reduction of the MSWS-12 score superior or equal to 5 points is usually considered clinically meaningful in MS. An improvement was observed in 27% and 36% of MS patients, respectively, after dalfampridine treatment. 3 In our series, 59.1% of the patients who completed the study had a reduction of at least 20% for T25FW and 64.2% of the patients had a reduction of at least 5 points for MSWS-12. These results suggest that NMOSD patients could respond to dalfampridine as well as or even slightly better than MS patients better compared with MS patients.
The classical mode of action of fampridine discussed in MS is a restoration of nerve conduction in demyelinated axons. NMOSD is primarily not a demyelinating disease, as it is an astrocytopathy but there is a secondary demyelinisation (demonstrated for example by increase latency of P100 wave on VEP) that could explain the positive effect observed in a majority of our patients.
As in MS, NMOSD has a strong impact on patients’ quality of life, including fatigue. 7 Dalfampridine seems to have a positive effect on fatigue and HRQL in NMOSD and this might not be solely due to improved ambulation. However, we should highlight some limitations of our study: small population, open-label trial design, and short-term evaluation.
Conclusion
As could be expected, in view of previous results in the MS population and the mechanism of action of the drug, dalfampridine seems to be useful for symptomatic treatment of walking impairment in NMOSD. Randomized studies with larger cohorts of patients will be needed to confirm these preliminary results and to determine the real impact of dalfampridine on fatigue and quality of life in NMOSD.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Jérôme de Seze https://orcid.org/0000-0002-7197-7578
Nicolas Collongues https://orcid.org/0000-0002-3683-5582
Kevin Bigaut https://orcid.org/0000-0002-5176-580X
Contributor Information
Jérôme de Seze, Clinical Investigation Center (CIC), CHU Strasbourg, France; Department de Neurology, CHU Strasbourg, France.
Christine Clerc, Department de Neurology, CHU Strasbourg, France.
Matthieu Béreau, Departement of Neurology, CHU Besançon, France.
Bertrand Bourre, Department of Neurology, CHU Rouen, France.
Hélène Zephir, University of Lille, CHU Lille, France.
Nicolas Collongues, Clinical Investigation Center (CIC), CHU Strasbourg, France; Department de Neurology, CHU Strasbourg, France.
Laurent Kremer, Department de Neurology, CHU Strasbourg, France.
Patrick Vermersch, University of Lille, LilNCog, CHU Lille, FHU PRECISE, Lille, France.
Kevin Bigaut, Department de Neurology, CHU Strasbourg, France.
References
- 1.Blight AR, Henney HR, 3rd, Cohen R. Development of dalfampridine, a novel pharmacologic approach for treating walking impairment in multiple sclerosis. Ann Y Acad Sci 2014. Nov; 1329: 33–44. [DOI] [PubMed] [Google Scholar]
- 2.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hobart J, Blight AR, Goodman A, et al. Timed 25-Foot Walk. Direct evidence that improving 20% or greater is clinically meaningful in MS. Neurology 2013; 80: 1–9. [DOI] [PubMed] [Google Scholar]
- 4.Hobart JC, Riazi A, Lamping DL, et al. Measuring the impact of MS on walking ability: The 12-item MS Walking Scale (MSWS-12). Neurology 2003; 60: 31–36. [DOI] [PubMed] [Google Scholar]
- 5.Vernay D, Gerbaud L, Biolay S, et al. Quality of life and multiple sclerosis: Validation of the French version of the self-questionnaire (SEP-59). Rev Neurol (Paris) 2000; 156: 247–243. [PubMed] [Google Scholar]
- 6.Debouverie M, Pittion-Vouyovitch S, Louis S, et al. Validity of a French version of the fatigue impact scale in multiple sclerosis. Mult Scler 2007; 13: 1026–1032. [DOI] [PubMed] [Google Scholar]
- 7.Chanson J-B, Zéphir H, Collongues N, et al. Evaluation of health-related quality of life, fatigue and depression in neuromyelitis optica. Eur J Neurol 2011; 18: 836–841. [DOI] [PubMed] [Google Scholar]