Abstract
Background.
Plague is a rare and severe zoonotic illness with limited empiric evidence to support treatment recommendations. We summarize treatment information for all patients with plague in the United States (US) as collected under the auspices of public health surveillance.
Methods.
We reviewed use of specific antimicrobials and illness outcome among cases of plague reported from 1942–2018. Antimicrobials were a priori classified into high-efficacy (aminoglycosides, tetracyclines, fluoroquinolones, sulfonamides, and chloramphenicol) and limited-efficacy classes (all others). Logistic regression models were created to describe associations between use of specific antimicrobial classes and illness outcome while controlling for potential confounding factors.
Results.
Among 533 total reported plague cases during 1942–2018, 426 (80%) received high-efficacy antimicrobial therapy. Mortality differed significantly among those receiving high-efficacy therapy (9%) and only limited-efficacy therapy (51%). Aminoglycosides and tetracyclines were used more commonly than other classes, and their use was associated with increased odds of survival of plague. Gentamicin use was associated with higher mortality than streptomycin, and aminoglycoside use was linked to higher mortality than for tetracyclines. Fluoroquinolones have been used in treatment of >30% of patients in recent years and limited data suggest clinical effectiveness.
Conclusions.
Most US patients with plague have received effective antimicrobials. Aminoglycosides and tetracyclines substantially improve survival of plague, and fluoroquinolones may be equally as effective, yet lack sufficient data. Early recognition and early treatment with any of these antimicrobial classes remain the most important steps to improving survival of plague.
Keywords: plague, treatment, surveillance, Yersinia pestis
Plague is a rare but life-threatening zoonosis that occurs in many areas of the world including Africa, Asia, and the Americas [1-3]. The etiologic agent, the gram-negative bacterium Yersinia pestis, survives in a complex transmission cycle involving rodents and their fleas. Humans are incidentally exposed through flea bites, direct handling or ingestion of infected animal tissues, or inhalation of infectious droplets. Specific clinical presentations of plague vary and are linked to the route through which the bacterium enters the human body [4]. The bubonic form of plague, characterized by a swollen and painful lymph node, is the most common manifestation of plague worldwide. Septicemic plague occurs absent localizing signs. Pneumonic plague is the most severe form and can occur as a result of direct inhalation of infectious droplets coughed by a human or animal with pneumonic plague or dissemination from other parts of the body. One can survive bubonic plague absent treatment, but septicemic plague and pneumonic plague are typically fatal [4]. Overall mortality due to plague in the United States (US) was 66% before antimicrobials, and 16% since their advent [5].
Currently, streptomycin, several tetracyclines, and 3 fluoroquinolones are approved by the US Food and Drug Administration (FDA) for treatment of human plague. Fluoroquinolones were approved in recent years on the basis of animal studies; evidence of clinical effectiveness in humans is limited [6]. Conversely, gentamicin has been used successfully in clinical practice but is not FDA-approved for plague treatment [7-9]. Well-powered clinical trials to define effectiveness of various plague treatment regimens have not occurred due to both feasibility and ethical concerns. Accordingly, comprehensive data on relative effectiveness of various antimicrobials in treating human plague are lacking. To supplement available information on treatment of human plague, we describe patterns of antimicrobials received by all documented plague patients in the US and their associated illness outcomes.
METHODS
Information on human plague cases occurring in the US has been maintained by the US Public Health Service, and more recently by the Centers for Disease Control and Prevention, since Y. pestis was first imported in 1900. As described elsewhere [5], these data include not only basic demographic and exposure information for each reported case, but often information on clinical course and antimicrobial treatments used. For this summary, cases of plague were defined as those with a clinically compatible illness and supportive or confirmatory laboratory evidence of Y. pestis infection. As 1942 was the first year with record of an antimicrobial used in treatment of a US patient with plague, this summary reflects treatment patterns from 1942 through 2018.
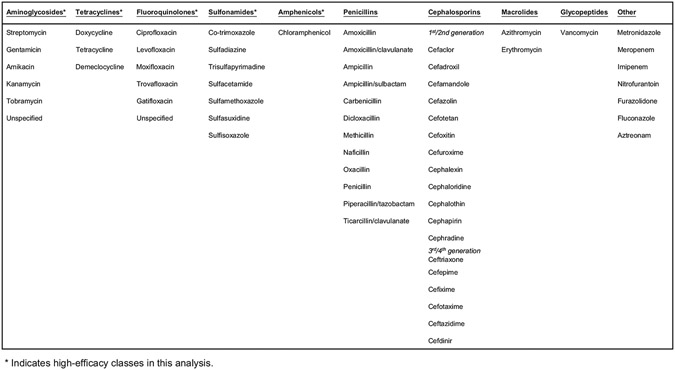
Use of any given antimicrobial was defined as a record of having received at least 1 dose. All antimicrobials indicated in patient records were categorized according to antimicrobial class (Figure 1). Aminoglycosides, tetracyclines, fluoroquinolones, sulfonamides, and amphenicols were considered to be of “high efficacy” for certain analyses; all other antimicrobials were considered to be of limited efficacy for treatment of plague. Use of various antimicrobial classes are described according to time period, patient age, and primary clinical form of plague (ie, bubonic, septicemic, or pneumonic). The overall period under review was divided into 5 time periods with similar case counts to account for improvements in general medical practice over time: before 1970, 1970s, 1980s, 1990s, and 2000–2018. Dosage, timing of each specific antimicrobial administration, and duration of therapy are incompletely captured in these data and not explicitly considered in this review. However, for a subset of data, number of days from onset until administration and associated mortality are described.
Figure 1.
Antimicrobials and associated classes used in treatment of patients with plague—United States, 1942–2018.
Nonparametric and categorical descriptive statistics are presented where appropriate. Univariate and multivariable logistic regression models were created using data from treated patients to identify factors that potentially confound the association between antimicrobial usage and survival of plague, consequently control for these effects, and tease apart relative associations between high-efficacy treatments and illness outcome. Binary variables for use of each specific antimicrobial class were included together in a multivariable model to better clarify the association of use of each antimicrobial class with odds of survival, thereby removing effects of coadministration of multiple antimicrobial classes. The outcome variable for all models was survival of plague. Covariates assessed for their potential role as confounders were time period (as described above), primary clinical form of plague, patient sex, number of days between reported illness onset and first healthcare visit, number of days between reported illness onset and first receipt of any antimicrobial, secondary plague manifestations such as septicemia secondary to bubonic plague, and illness complications such as non-plague pneumonia or amputation. Purposeful variable selection for the final set of covariates incorporated strength of association and statistical significance, as well as minimization of overall variable count given available sample size. All data analyses were conducted using SAS version 9.4 software (SAS Institute, Cary, North Carolina). Statistical significance was considered as P < .05.
RESULTS
Overall Patient Characteristics
A total of 533 human plague cases were reported in the US during 1942–2018; 408 (77%) were bubonic, 93 (17%) were septicemic, and 18 (3%) were pneumonic; 14 were of unknown or other rare primary clinical form. Among these records, 469 (88%) contained information indicating the patient received at least 1 dose of any named antimicrobial or class (Table 1; Figure 1). There were no differences between patients who received any antimicrobials and those who received none in any of the following: sex, age, race/ethnicity, or primary clinical form (data not shown). The median age of treated patients increased over time (P < .0001; Table 1). Overall, 426 patients received at least 1 dose of an antimicrobial considered to be of high efficacy (80% overall, 91% among those who received any treatment), with no appreciable difference in proportion over time (Table 1). Among those receiving any antimicrobial treatment, overall mortality due to plague decreased over time from 28% before 1970 to 8% during 2000–2018 and was lower among the subset of patients receiving high-efficacy antimicrobials (Table 1). Male and female patients with plague equally received high-efficacy antimicrobials (89% vs 93%, respectively). However, males were more likely to have a fatal outcome than females despite receiving high-efficacy antimicrobials (P = .026). Median age and age range among treated individuals who died was not different than among those who survived their illness (P = .76). Similarly, the age distribution among those with fatal outcomes did not differ between males and females (P = .92).
Table 1.
Frequency of Any Antimicrobial Use and Characteristics of Patients With Plague Who Received Antimicrobials—United States, 1942–2018
| Time Period | ||||||
|---|---|---|---|---|---|---|
| Antimicrobial Use | 1942–1969 | 1970–1979 | 1980–1989 | 1990–1999 | 2000–2018 | Total |
| ≥1 dose of any antimicrobial | 43/49 (88) | 105/107 (98) | 138/180 (77) | 81/91 (89) | 102/106 (96) | 469/533 (88) |
| Mortality among patients receiving any antimicrobials | 12/43 (28) | 13/105 (12) | 20/135 (15) | 8/81 (10) | 8/99 (8) | 61/463 (13) |
| Age at onset among those receiving antimicrobials, y, median (range) | 13 (2–74) | 16 (0–79) | 25 (1–82) | 39 (0–94) | 46 (1–83) | 28 (0–94) |
| ≥1 dose of any high-efficacy antimicrobial | 39/43 (91) | 100/105 (95) | 120/138 (87) | 72/81 (89) | 95/102 (93) | 426/469 (91) |
| Mortality among patients receiving high-efficacy antimicrobials | 8/39 (21) | 9/100 (9) | 13/117 (11) | 5/72 (7) | 4/92 (4) | 39/420 (9) |
| Age at onset among those receiving high-efficacy antimicrobials, y, median (range) | 14 (2–74) | 16 (0–79) | 28 (1–82) | 39 (0–94) | 46 (1–83) | 30 (0–94) |
Data are presented as No./Total (%) unless otherwise indicated.
Overall and Temporal Patterns in Antimicrobial Use
Coadministration of multiple antimicrobial classes was common; 139 of 469 patients (30%) received only a single recorded drug class during their illness, referred to as monotherapy. The most commonly used high-efficacy antimicrobials were aminoglycosides (320 [68% of all treated patients]), and tetracyclines (267 [57%]) (Figure 2). Streptomycin was used in treatment of 180 patients (38%), with decreasing frequency over time (only 5 patients since 2000) (P < .0001). Gentamicin was used in 149 (31%), with increasing frequency over time (P < .0001). Doxycycline accounted for 39% of recorded uses of tetracyclines. Although tetracycline use was common throughout the time period under review, use of doxycycline specifically increased over time (P < .0001).
Figure 2.
Percentage of patients with plague who received specific high-efficacy antimicrobial classes over time—United States, 1942–2018.
Use of fluoroquinolones for treatment of human plague increased over time (Figure 2; P <.0001). Ciprofloxacin and levofloxacin each accounted for 19 of 48 US patients with recorded use of fluoroquinolones for plague (40%). Among these, 2 patients received both ciprofloxacin and levofloxacin during the course of therapy. Although relatively rare overall, use of chloramphenicol or sulfonamides in treatment of plague in the US decreased over time (P < .0001) (Figure 2). Only 17 patients in the US specifically received cotrimoxazole for treatment of plague.
Penicillin use was recorded for 42% of all patients. Although use of penicillins decreased over time (P < .0001), their use remained substantial in the 2000s and 2010s (28% of patients).
Time From Illness Onset to First Healthcare Visit and Antimicrobial Treatment
Among 393 records with information allowing calculation of time from onset to first healthcare visit, 77% of patients sought care within the first 2 days of illness (median, 1 day [range, 0–17]). Time between onset of symptoms to receipt of any antimicrobials was a median of 2 days (interquartile range [IQR], 1–3 days) and time from onset to receipt of highly effective antimicrobials was a median of 3 days (IQR, 2–5 days). Among those who received monotherapy with a high-efficacy antimicrobial, time to treatment was a median of 3 days for those who survived and 5 days for those who died (P = .03). There were similar times to treatment between those receiving aminoglycosides vs tetracyclines (Table 2). Overall, fluoroquinolones and chloramphenicol appear to be initiated slightly later in illness and sulfonamides earlier in illness compared to the aminoglycosides and tetracyclines (Table 2).
Table 2.
Clinical Course of Patients With Plague According to Overall Use and Use in the Absence of Other High-efficacy Antimicrobial Classes—United States, 1942–2018
| Antimicrobial Class | No. | Days From Onset to Treatment With Specified Class, Median (IQR) |
No. (%) Bubonic | Secondary Disease Manifestations, No. (%) |
Died, No. (%) |
|---|---|---|---|---|---|
| Aminoglycosides | |||||
| Overall | 300 | 3 (2–5) | 237 (80) | 93 (31) | 31 (10) |
| No other high efficacy | 73 | 3 (1–5) | 55 (75) | 24 (32) | 12 (17) |
| Tetracyclines | |||||
| Overall | 253 | 3 (2–6) | 211 (84) | 72 (29) | 12 (5) |
| No other high efficacy | 51 | 3 (2–4) | 45 (88) | 8 (16) | 1 (2) |
| Fluoroquinolones | |||||
| Overall | 44 | 4.5 (2.5–6) | 23 (53) | 24 (55) | 3 (7) |
| No other high efficacy | 12 | 4 (3–6) | 4 (33) | 6 (50) | 2 (18) |
| Chloramphenicol | |||||
| Overall | 82 | 5 (3–7) | 67 (81) | 39 (48) | 16 (20) |
| No other high efficacy | 9 | 5 (3–6) | 7 (78) | 2 (22) | 2 (22) |
| Sulfonamides | |||||
| Overall | 27 | 1 (1–6) | 21 (81) | 6 (23) | 2 (7) |
| No other high efficacy | 11 | 1 (0–12) | 10 (91) | 1 (10) | 2 (18) |
Abbreviation: IQR, interquartile range.
Antimicrobial Usage According to Primary Clinical Manifestation
Overall, receipt of high-efficacy treatment was not associated with primary clinical form of plague; however, there was a tendency for patients with primary septicemic plague to receive effective therapy less frequently than persons with bubonic or pneumonic plague (85%, 92%, and 94%, respectively; P = .13). Aminoglycosides and tetracyclines were the most common high-efficacy therapy, regardless of primary clinical form (Table 3). Fluoroquinolones have been used for 7% of patients with bubonic plague but 24% of patients with pneumonic and septicemic plague (Table 3).
Table 3.
Illness Outcome According to Receipt of Any High-efficacy Antimicrobial, Primary Clinical Form of Plaguea, and Patient Age—United States, 1942–2018b
| Bubonic (Total No. Treated = 364) |
Pneumonic and Septicemic (Total No. Treated = 97) |
||||
|---|---|---|---|---|---|
| Antimicrobial | Age ≤15 y | Age >15 y | Age ≤15 y | Age >15 y | All (N = 463) |
| Aminoglycosides | 86 (6) | 163 (8) | 11 (18) | 50 (20) | 315 (10) |
| Streptomycin | 63 (5) | 93 (9) | 6 (17) | 15 (20) | 179 (8) |
| Gentamicin | 27 (7) | 76 (9) | 7 (29) | 36 (22) | 148 (13) |
| Tetracyclines | 65 (5) | 156 (4) | 4 (0) | 38 (8) | 265 (5) |
| Fluoroquinolones | 0 (0) | 24 (4) | 1 (0) | 22 (9) | 47 (6) |
| Chloramphenicol | 35 (6) | 34 (24) | 4 (0) | 11 (55) | 84 (19) |
| Sulfonamides | 19 (11) | 11 (9) | 1 (0) | 4 (0) | 36 (6) |
Data are presented as No. (%) fatal.
Rarer primary clinical forms included in total figures.
Due to treatment with multiple antimicrobial classes, totals are >100%.
Patients with septicemic plague received streptomycin during the course of therapy less often than for other clinical forms, even after controlling for decreasing use of streptomycin over time and increasing frequency of septicemic plague over time (P = .0003). In contrast, gentamicin treatment was more common among patients with septicemic plague than among those with bubonic and pneumonic plague, after controlling for changes in use and presentation frequency over time (P = .036). Use of any tetracycline was more common among patients with bubonic plague than among those with pneumonic or septicemic plague (P = .0003).
Among 17 patients with primary pneumonic plague who received any antimicrobial, 13 received aminoglycosides, 9 received tetracyclines, 6 received chloramphenicol, 3 received fluoroquinolones, and 1 received a sulfonamide. Three patients received 1 high-efficacy drug while the remainder received 2–3 classes of high-efficacy therapy.
Mortality Patterns
Overall mortality was markedly lower for patients who received any high-efficacy antimicrobial (9%) as compared to those who received only limited-efficacy antimicrobials (51%) (P < .0001). Those with primary pneumonic and septicemic plague were more likely to have a fatal outcome despite receiving high-efficacy antimicrobials than those who presented with bubonic plague (25% and 16% vs 7%, respectively) after controlling for time period (P = .0004). There were no appreciable differences across antimicrobial classes in mortality between children ≤15 years of age and adults, with the possible exception of chloramphenicol, which appeared to have higher mortality among adults than among children (Table 3).
Forty-three (29%) patients with noted secondary plague manifestations died as compared to 18 (6%) of those without (P < .0001). Among the 113 patients with other illness complications, 41% died vs 3% of those without noted complications. In both instances, these differences remained even after stratifying according to receipt of high-efficacy antimicrobials (P < .0001).
The most common high-efficacy combination therapy, whether concurrent or sequential, was use of an aminoglycoside and a tetracycline (n = 196). Mortality associated with use of aminoglycosides only (10%) was higher than for tetracyclines only (2%) or for aminoglycoside and tetracycline coadministration (3%). Independent and combined use of aminoglycosides and tetracyclines were associated with similar time from symptom onset until treatment initiation.
Adjusted Odds of Survival Associated With Use of High-efficacy Antimicrobials
Final covariates in multivariable models to assess association between receipt of high-efficacy antimicrobials and survival were primary clinical form (bubonic vs non-bubonic), time period, secondary plague manifestations, and illness complications. Importantly, those with secondary manifestations were more likely to have noted illness complications, however these variables were not correlated with one another to the point of introducing multicollinearity.
Among all patients receiving any antimicrobial treatment, use of aminoglycosides and tetracyclines were independently associated with significantly increased odds of survival (aminoglycosides: adjusted odds ratio [aOR], 4.8 [95% confidence interval {CI}, 1.9–12.2]; tetracyclines: aOR, 6.2 [95% CI, 2.6–15.1]) compared to those receiving only limited-efficacy antimicrobials (Figure 3). The high odds of survival associated with aminoglycoside use may have been driven more by use of streptomycin (aOR, 4.3 [95% CI, 1.7–10.8]) than of gentamicin (aOR, 2.1 [95% CI, .9–4.9]). Use of fluoroquinolones, sulfonamides, and chloramphenicol each displayed point estimates for odds of survival >1, however CIs included 1 (Figure 3).
Figure 3.
Adjusted odds ratios and 95% confidence intervals associated with use of high-efficacy antimicrobial classes and survival of plague, controlling for effects of antimicrobial coadministration, primary clinical form, secondary manifestations, and illness complications—United States, 1942–2018.
Use of penicillins, first- and second-generation cephalosporins, and third- and fourth-generation cephalosporins independent of a high-efficacy antimicrobial were not significantly associated with increased odds of survival of plague compared to other antimicrobials (penicillins: aOR, 0.9 [95% CI, .4–2.0]; first-/second-generation cephalosporins: aOR, 1.5 [95% CI, .5–3.9]; third-/fourth-generation cephalosporins: aOR, 1.4 [95% CI, .5–4.2]).
DISCUSSION
Plague is a rare condition, and robust clinical data for assessing the effectiveness of specific antimicrobial treatments are limited. We found that 80% of US patients with plague received at least 1 dose of an antimicrobial considered a priori to be highly effective for plague. Treatment with aminoglycosides or tetracyclines was associated with at least 4 times higher odds of survival than treatment with limited-efficacy antimicrobials. Although data for fluoroquinolones are limited, a high point estimate for odds of survival associated with fluoroquinolone use provides support for clinical effectiveness of these agents.
Aminoglycosides and tetracyclines were the most commonly used antimicrobial classes in treatment of US patients with plague. Patterns in use of specific drugs or classes to treat plague in the US have evolved over time. Sulfonamides and streptomycin were among the first antimicrobials available [10-12], and their declining use over time for treatment of plague reflects changes in availability and release of new antimicrobials with more appealing safety profiles and ease of administration.
Some results from this analysis bear further discussion. Overall mortality associated with gentamicin use was 60% higher than for streptomycin use. Although there are some in vitro data suggesting greater efficacy for streptomycin [13], this result may also reflect early suspicion of plague by the treating providers. Streptomycin is in limited supply in the US, and its use suggests that providers may be specifically treating the patient for possible plague. Conversely, gentamicin may be used empirically for patients with severe sepsis of unknown etiology, as occurred for several fatal cases included in this summary.
The lower mortality rate among those receiving tetracycline as compared to aminoglycosides is similarly notable. This may result from selective administration of tetracyclines to less severely ill patients or a survival bias in that it is given on discharge to those patients who survive their initial hospitalization. Nevertheless, there are pharmacologic reasons to suspect that tetracyclines may be more effective at controlling some aspects of plague. Generally bactericidal, aminoglycosides are characterized by poor abscess penetration and efficacy in acidic environments, which likely limits their activity within the bubo [14]. Based on case-fatality rates of independent aminoglycoside and tetracycline use vs their combined usage, there does not appear to be a clear benefit to their coadministration in terms of improving likelihood of survival.
In these data, use of chloramphenicol and sulfonamides was not independently associated with increased odds of survival. This finding is in line with evidence from in vitro and animal studies and suggests that not all antimicrobials considered “high-efficacy” in this analysis are equally effective in treating plague and thus should not all be considered first-line therapy [9, 13]. Treatment recommendations necessarily must consider potential for antimicrobial resistance; however, resistance to commonly effective antimicrobials is exceedingly rare in naturally occurring plague [9, 15, 16]. Additionally, our findings reinforce previous in vivo and in vitro assessments that penicillins and cephalosporins are of limited efficacy in treating human plague [7, 9, 17].
Comparative analysis of the effectiveness of various antimicrobials in this retrospective observational analysis is limited by several factors. Survival of plague is influenced by many factors in addition to use of specific antimicrobials. Available data could conceivably be biased toward more thorough treatment and clinical course information for more severe illnesses. Time from disease onset to receipt of effective therapy is an important predictor of survival of plague [18] but was hard to discern in many of these records. Furthermore, lack of detailed data on dosage, duration, and coadministration vs sequential administration of multiple effective antimicrobial classes also limits ability to make robust, generalizable conclusions regarding comparative effectiveness of various antimicrobials. These data include proportionally more primary septicemic plague cases than are reflected in reviews that summarize data published in the worldwide scientific literature; this difference is likely due to the increasing commonality of blood cultures in hospitals in the US. Some of the cases reviewed here were also published in the scientific literature as case reports and aggregate summaries; thus, these data do not represent a wholly independent source of data on treatment of plague. Despite these challenges, these retrospective data do not suffer from potential publication bias that may reflect more severe or unusual cases of plague, or those from specific time periods or geographic areas.
Our findings support recommendations that aminoglycosides and tetracyclines are independently effective in substantially improving survival of plague, and fluoroquinolones may be equally as effective yet presently lack the robust data associated with the other 2 classes. Results from this analysis suggest that further investigation is necessary to determine differences in effectiveness of streptomycin vs gentamicin and aminoglycosides vs tetracyclines. Early recognition and early treatment with any of these antimicrobial classes remain the most important steps to improving chances of survival of plague infection.
Acknowledgments.
The authors acknowledge the public health professionals who have investigated human cases of plague in the United States and shared information with the Centers for Disease Control and Prevention (CDC).
Footnotes
Supplement sponsorship. This article appears as part of the supplement “Plague and Bioterrorism Preparedness,” sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Pollitzer R. Plague. Geneva, Switzerland: World Health Organization, 1954. [Google Scholar]
- 2.Perry RD, Fetherston JD. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev 1997; 10:35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dennis DT, Gage KL, Gratz ND, Poland JD, Tikhomirov E. Plague manual: epidemiology, distribution, surveillance and control. Geneva, Switzerland: World Health Organization, 1999. [Google Scholar]
- 4.Mead PS. Yersinia species (including plague). In: Bennett JE, Dolin R, Blaser MJ. Principles and practice of infectious diseases. 8th ed, vol. 2. Philadelphia: Elsevier, 2015:2607–18. [Google Scholar]
- 5.Kugeler KJ, Staples JE, Hinckley AF, Gage KL, Mead PS. Epidemiology of human plague in the United States, 1900–2012. Emerg Infect Dis 2015; 21:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apangu T, Griffith K, Abaru J, et al. Successful treatment of human plague with oral ciprofloxacin. Emerg Infect Dis 2017; 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulanger LL, Ettestad P, Fogarty JD, Dennis DT, Romig D, Mertz G. Gentamicin and tetracyclines for the treatment of human plague: review of 75 cases in new Mexico, 1985–1999. Clin Infect Dis 2004; 38:663–9. [DOI] [PubMed] [Google Scholar]
- 8.Mwengee W, Butler T, Mgema S, et al. Treatment of plague with gentamicin or doxycycline in a randomized clinical trial in Tanzania. Clin Infect Dis 2006; 42:614–21. [DOI] [PubMed] [Google Scholar]
- 9.Inglesby TV, Dennis DT, Henderson DA, et al. Plague as a biological weapon: medical and public health management. JAMA 2000; 283:2281–90. [DOI] [PubMed] [Google Scholar]
- 10.Wagle P. Recent advances in the treatment of bubonic plague. Indian J Med Sci 1948; 2:489–94. [Google Scholar]
- 11.Meyer KF. Modern therapy of plague. J Am Med Assoc 1950; 144:982–5. [DOI] [PubMed] [Google Scholar]
- 12.Sokhey SS, Wagle PM, Habbu MK. Treatment of bubonic plague with sulfonamides and antibiotics. Bull World Health Organ 1953; 9:637–43. [PMC free article] [PubMed] [Google Scholar]
- 13.Wendte JM, Ponnusamy D, Reiber D, Blair JL, Clinkenbeard KD. In vitro efficacy of antibiotics commonly used to treat human plague against intracellular Yersinia pestis. Antimicrob Agents Chemother 2011; 55:3752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leggett JE. Aminoglycosides. In: Bennet JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 9th ed, vol. 1. Philadelphia: Elsevier, 2020:305–17. [Google Scholar]
- 15.Urich SK, Chalcraft L, Schriefer ME, Yockey BM, Petersen JM. Lack of antimicrobial resistance in Yersinia pestis isolates from 17 countries in the Americas, Africa, and Asia. Antimicrob Agents Chemother 2012; 56:555–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guiyoule A, Gerbaud G, Buchrieser C, et al. Transferable plasmid-mediated resistance to streptomycin in a clinical isolate of Yersinia pestis. Emerg Infect Dis 2001; 7:43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byrne WR, Welkos SL, Pitt ML, et al. Antibiotic treatment of experimental pneumonic plague in mice. Antimicrob Agents Chemother 1998; 42:675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crook LD, Tempest B. Plague. A clinical review of 27 cases. Arch Intern Med 1992; 152:1253–6. [DOI] [PubMed] [Google Scholar]