Abstract
Objectives
Gastric cancer can be diagnosed even in patients long after Helicobacter pylori eradication. Most cases involve intramucosal lesions; however, some are invasive and require surgery. To clarify appropriate long‐term surveillance methods, this study compared invasive gastric cancer diagnosed ≥10 and <10 years after eradication.
Methods
This retrospective multicenter study included 14 institutions. We included 377 patients with gastric cancer with submucosal or deep invasion after surgical or endoscopic resection. Ordered logistic regression analysis was used to explore the factors contributing to the pathological stage and histological type.
Results
Invasive gastric cancer was detected in 84 patients (Group L) and 293 patients (Group S) ≥10 and <10 years after H. pylori eradication, respectively. Endoscopic mucosal atrophy at the time of cancer detection was similar in both groups; 50% of the patients had severe atrophy. Annual endoscopy correlated with early pathological stage (odds ratio [OR] 0.28, 95% confidence interval [CI] 0.14–0.54, p < 0.001). Group L exhibited an independent correlation with the advanced pathological stage (OR 2.27, 95% CI 1.06–4.88, p = 0.035) and the undifferentiated type (OR 2.12, 95% CI 1.16–3.90, p = 0.015). The pure differentiated type and early pathological stage significantly (p = 0.001) correlated with severe mucosal atrophy in Group S but not in Group L.
Conclusions
Invasive cancers diagnosed ≥10 years after H. pylori eradication were likely to be more malignant in histological type and pathological stage. Gastric cancer surveillance should continue regardless of endoscopic atrophy, particularly ≥10 years after eradication.
Keywords: endoscopic resection, gastric cancer surveillance, mucosal atrophy, pathological stage, undifferentiated type
INTRODUCTION
In 2013, the Japanese National Health Insurance approved Helicobacter pylori eradication therapy for H. pylori gastritis. 1 Gastric cancer has been diagnosed in patients followed up after H. pylori eradication, although H. pylori eradication reduces the risk. 2 , 3 The incidence of gastric cancers diagnosed ≥10 years after H. pylori eradication has increased along with the rise in long‐term post‐eradication cases. 4 , 5
Small differentiated early‐stage carcinomas are more common in severe mucosal atrophy, 3 , 6 whereas undifferentiated carcinomas are more common in mild or moderate atrophy cases. 5 , 7 The risk of diffuse‐type gastric cancer is reportedly greater in the second decade of follow‐up than in the first decade following H. pylori eradication. 5 Even though gastric cancer diagnosis after H. pylori eradication takes years, many are differentiated intramucosal carcinomas that can be treated endoscopically. 8 , 9 However, some lesions are invasive cancer and require surgery, making them a growing concern. 9 , 10 , 11 No comprehensive studies have examined invasive gastric cancer after H. pylori eradication 12 , 13 ; therefore, its characteristics are unknown. An appropriate surveillance method for long‐term post‐H. pylori eradication is required.
This multicenter study aimed to clarify the clinical, endoscopic, and histopathologic characteristics of invasive gastric cancer diagnosed ≥10 years after H. pylori eradication. We collected cases from participating institutions and compared them with those of invasive gastric cancer diagnosed <10 years after H. pylori eradication.
METHODS
We retrospectively reviewed 377 patients with primary gastric cancer from 14 Japanese institutions who met the inclusion criteria between January 2004 and September 2022. We compared 84 patients with gastric cancers diagnosed ≥10 years after H. pylori eradication (Long‐term group; Group L) with 293 patients with gastric cancers diagnosed <10 years after eradication (Short‐term group; Group S).
Inclusion criteria
Surgically or endoscopically resected gastric cancer with submucosal or deeper histological invasion.
Patients with histories of H. pylori eradication.
Confirmed negative results for H. pylori infection after eradication.
Successful H. pylori eradication therapy was assessed using either the 13C‐urea breath test, stool antigen test, culture, or quantification of anti–H. pylori Immunoglobulin G levels. Patients with one or more negative results in any of these assays were classified as H. pylori–negative. Each institution was confirmed to be negative for H. pylori according to the criteria of the respective test method.
Exclusion criteria
Patients with an unknown period of eradication of H. pylori.
Patients who have undergone gastrectomy in the past.
Patients with gastroesophageal junction cancer.
Patients with gastric adenocarcinoma of fundic gland type or fundic gland mucosa type.
Patients who could not provide informed consent to participate in this study.
Nineteen patients with an unknown period of H. pylori eradication (n = 10), gastric adenocarcinoma of fundic gland type (n = 5), or non‐resection, biopsy‐based diagnosis (n = 4) were excluded.
Study variables
Patient data
Age, sex, smoking history, history of gastric or other cancers, family history of first‐degree gastric cancer, disease eligible for H. pylori eradication therapy, post–H. pylori eradication period and timing of the last endoscopy before gastric cancer detection were retrospectively recorded. We defined annual endoscopy as an endoscopic examination performed within 2 years before the lesion was detected.
Histological assessment
According to the Japanese Classification of Gastric Carcinoma, gastric cancer size, macroscopic type, histological type, location, invasive depth, lymphovascular invasion, lymph node metastasis, and distant metastasis were recorded. 14 Pathological stage was evaluated in surgically resected lesions. Each institution performed pathological diagnosis without a central review. For comparison, the histological type was divided into (1) pure differentiated, (2) differentiated‐predominant mixed, and (3) undifferentiated (pure undifferentiated or undifferentiated‐predominant mixed).
Endoscopic assessment of mucosal atrophy
The endoscopic grade of gastric mucosal atrophy was evaluated based on the endoscopic classification system described by Kimura and Takemoto 15 to classify cases into three grades: mild (C‐1 and C‐2), moderate (C‐3 and O‐1), and severe (O‐2 and O‐3). The endoscopic assessment was performed at gastric cancer diagnosis.
Ethics
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethical committee of Cancer Institute Hospital, Japanese Foundation for Cancer Research (2021GA1053), and those of each participating institution. The participants provided informed consent using the opt‐out method. Junko Fujisaki was the chief investigator, and Ken Namikawa was in charge of the study secretariat office. The responsible co‐investigator at each participating institution completed the case report forms anonymously and sent them to the study secretariat office, which then compiled and analyzed the study data.
Statistical analysis
Continuous and categorical variables were expressed as medians (ranges) and numbers (percentages), respectively. Differences in clinicopathological data between the two groups post‐H. pylori eradication period and the three groups of mucosal atrophy were evaluated using the Mann–Whitney U test for continuous data and the chi‐squared test for categorical variables. Ordered logistic regression analysis was used to explore the factors contributing to pathological stage and histological type. Odds ratios and 95% confidence intervals were determined. All statistical analyses were performed using SPSS software (v. 27.0; IBM). All reported p‐values are two‐sided, with p < 0.05 indicating significant differences.
RESULTS
Patients’ characteristics
We treated 377 patients with invasive gastric cancer either endoscopically (n = 38) or surgically (n = 339), including additional surgery after endoscopic resection. We compared 84 patients with gastric cancers diagnosed ≥10 years after H. pylori eradication (Group L) with 293 patients with gastric cancers diagnosed <10 years after eradication (Group S; Table 1).
TABLE 1.
Patients’ characteristics.
| Overall | Group L | Group S | p‐value | |
|---|---|---|---|---|
| Age, years, median (range) | 70 (38–94) | 72 (49–94) | 70 (38–91) | 0.017 |
| Sex | 0.309 | |||
| Male | 288/377 (76.4%) | 68/84 (81.0%) | 220/293 (75.0%) | |
| Female | 89/377 (23.6%) | 16/84 (19.0%) | 73/293 (25.0%) | |
| History of smoking | 237/377 (62.9%) | 54/84 (64.3%) | 183/293 (62.5%) | 0.799 |
| Past history of GC | 74/368 (20.1%) | 13/80 (16.3%) | 61/288 (21.2%) | 0.430 |
| Past history of other cancer | 90/367 (24.5%) | 23/81 (28.4%) | 67/286 (23.4%) | 0.381 |
| Family history of GC | 98/317 (30.9%) | 22/73 (30.1%) | 76/244 (31.1%) | 1.000 |
| Diseases eligible for eradication | <0.001 | |||
| Gastritis | 228/377 (60.5%) | 38/84 (45.2%) | 190/293 (64.8%) | |
| Gastric ulcer | 40/377 (10.6%) | 15/84 (17.9%) | 25/293 (8.53%) | |
| Duodenal ulcer | 6/377 (1.59%) | 1/84 (1.19%) | 5/293 (1.70%) | |
| EMR/ESD of GC | 57/377 (15.1%) | 9/84 (10.7%) | 48/293 (16.4%) | |
| Others or unknown | 46/377 (12.2%) | 21/84 (25.0%) | 25/293 (8.53%) | |
| Post‐eradication period, months | Not tested | |||
| Median (range) | 60 (1–558) | 143 (120–528) | 49 (1–119) | |
| <12 | 22/377 (5.83%) | 22/293 (7.51%) | ||
| 12 ≤ X < 60 | 141/377 (37.4%) | 141/293 (48.1%) | ||
| 60 ≤ X < 120 | 130/377 (34.5%) | 130/293 (44.3%) | ||
| 120 ≤ X < 180 | 67/377 (17.8%) | 67/84 (79.8%) | ||
| 180 ≤ X < 240 | 13/377 (3.45%) | 13/84 (15.5%) | ||
| 240≤ | 4/377 (1.06%) | 4/84 (4.76%) | ||
| Last endoscopy before detection | 0.060 | |||
| ≤24 months | 186/258 (72.1%) | 31/51 (60.8%) | 155/207 (74.9%) | |
| 24 < X ≤ 48 months | 40/258 (15.5%) | 9/51 (17.6%) | 31/207 (15.0%) | |
| 48 months< | 32/258 (12.4%) | 11/51 (21.6%) | 21/207 (10.1%) | |
| Unknown | 119/377 (31.6%) | 33/84 (39.3%) | 86/293 (29.4%) | 0.057 |
Abbreviations: EMR/ESD, endoscopic mucosal resection/endoscopic submucosal dissection; GC, gastric cancer.
Group L, group of invasive gastric cancers diagnosed ≥10 years after H. pylori eradication; Group S, group of invasive gastric cancers diagnosed <10 years after H. pylori eradication.
The median ages of these patients were Group L: 72 years (49–94 years) and Group S: 70 years (38–91 years). Patients in Group L were significantly older than those in Group S (p = 0.017). No significant differences were observed between Groups L and S in terms of sex, history of smoking, history of gastric or other cancers, or family history of gastric cancer within first‐degree relatives. The diseases eligible for H. pylori eradication therapy differed significantly (p < 0.001). In Group S, 65% of the patients were treated for H. pylori gastritis. The median post‐H. pylori eradication was Group L: 143 months (120–528 months), Group S: 49 months (1–119 months). Annual endoscopy was more common in Group S (75%) than in Group L (61%), although the difference was not statistically significant (p = 0.060).
Histopathological findings and endoscopic mucosal atrophy
Table 2 shows the histopathological findings and endoscopic mucosal atrophy. No significant differences were observed between Groups L and S in terms of size, macroscopic type, location, and invasion depth. Regarding the histological type, the undifferentiated type was significantly (p = 0.023) more common in Group L (38%) than in Group S (23%). No significant differences were observed between Groups L and S in terms of lymphovascular invasion or lymph node metastasis. However, the incidence of distant metastasis was significantly (p = 0.031) higher in Group L (6.7%) than in Group S (1.5%). The pathological stage was more advanced in Group L than in Group S; however, the difference was not statistically significant (p = 0.060).
TABLE 2.
Histopathological findings and endoscopic mucosal atrophy.
| Overall | Group L | Group S | p‐value | |
|---|---|---|---|---|
| Size, mm (median, range) | 24 (3–170) | 23 (3–160) | 25 (4–170) | 0.310 |
| Macroscopic type | 0.760 | |||
| Type 0‐I, 0‐IIa, 0‐IIb | 29/377 (7.69%) | 8/84 (9.52%) | 21/293 (7.17%) | |
| Type 0‐IIc, 0‐IIa+IIc | 282/377 (74.8%) | 61/84 (72.6%) | 221/293 (75.4%) | |
| Type 1, 2, 3, 4, 5 | 66/377 (17.5%) | 15/84 (17.9%) | 51/293 (17.4%) | |
| Location | 0.713 | |||
| Upper | 120/377 (31.8%) | 28/84 (33.3%) | 92/293 (31.4%) | |
| Middle | 140/377 (37.1%) | 33/84 (39.3%) | 107/293 (36.5%) | |
| Lower | 117/377 (31.0%) | 23/84 (27.4%) | 94/293 (32.1%) | |
| Invasive depth | 0.256 | |||
| SM1 | 137/377 (36.3%) | 25/84 (29.8%) | 112/293 (38.2%) | |
| SM2 | 149/377 (39.5%) | 34/84 (40.5%) | 115/293 (39.2%) | |
| MP or deeper | 91/377 (24.1%) | 25/84 (29.8%) | 66/293 (22.5%) | |
| Histological type | 0.023 | |||
| Pure differentiated | 169/377 (44.8%) | 29/84 (34.5%) | 140/293 (47.8%) | |
| Differentiated‐predominant mixed | 110/377 (29.2%) | 24/84 (28.6%) | 86/293 (29.4%) | |
| Undifferentiated | 98/377 (26.0%) | 31/84 (36.9%) | 67/293 (22.9%) | |
| Lymphovascular invasion | 186/366 (50.8%) | 42/78 (53.8%) | 144/288 (50.0%) | 0.700 |
| Lymph node metastasis | 68/339 (20.1%) | 17/75 (22.7%) | 51/264 (19.3%) | 0.628 |
| Distant metastasis | 9/339 (2.65%) | 5/75 (6.67%) | 4/264 (1.51%) | 0.031 |
| Pathological stage | 0.060 | |||
| IA | 227/339 (67.0%) | 42/75 (56.0%) | 185/264 (70.1%) | |
| IB | 44/339 (13.0%) | 14/75 (18.7%) | 30/264 (11.4%) | |
| IIA | 17/339 (5.01%) | 4/75 (5.33%) | 13/264 (4.92%) | |
| IIB | 13/339 (3.83%) | 4/75 (5.33%) | 9/264 (3.41%) | |
| IIIA or IIB or IIIC | 29/339 (8.55%) | 6/75 (8.00%) | 23/264 (8.71%) | |
| IV | 9/339 (2.65%) | 5/75 (6.67%) | 4/264 (1.52%) | |
| Endoscopic mucosal atrophy† | 0.608 | |||
| Mild, C‐1, 2 | 32/377 (8.49%) | 9/84 (10.7%) | 23/293 (7.84%) | |
| Moderate, C‐3, O‐1 | 157/377 (41.6%) | 32/84 (38.1%) | 125/293 (42.7%) | |
| Severe, O‐2, 3 | 188/377 (49.9%) | 43/84 (51.2%) | 145/293 (49.5%) |
†Kimura‐Takemoto classification.
Abbreviations: C, closed; O, open.
Group L, Group of invasive gastric cancers diagnosed ≥10 years after H. pylori eradication; Group S, Group of invasive gastric cancers diagnosed <10 years after H. pylori eradication; SM1, submucosal invasion <0.5mm; SM2, submucosal invasion ≥0.5mm; MP, muscularis propria.
The endoscopic grade of the gastric mucosal atrophy was similar at gastric cancer detection. Approximately half of the patients in both groups had severe gastric atrophy.
Clinical and histological factors contributing to pathological stage
We analyzed the factors contributing to the pathological stage of all the reviewed patients (Table 3). Size and Group L contributed significantly and independently to a more advanced pathological stage. Annual endoscopy and the pure differentiated type significantly correlated with a less advanced pathological stage.
TABLE 3.
Ordered logistic regression analysis of pathological stage with clinical and histological factors
| OR | 95% CI | p‐Value | |
|---|---|---|---|
| Size | 1.06 | 1.04–1.07 | <0.001 |
| Pure differentiated type | 0.31 | 0.14–0.69 | 0.004 |
| Annual endoscopy | 0.28 | 0.14–0.54 | <0.001 |
| Endoscopic severe mucosal atrophy | 0.61 | 0.31–1.20 | 0.160 |
| Diagnosis ≥10 years after H. pylori eradication | 2.27 | 1.06–4.88 | 0.035 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Clinical and endoscopic factors contributing to histological type
We analyzed the factors contributing to histological type in all the reviewed patients (Table 4). Older age, male sex, and severe endoscopic mucosal atrophy contributed significantly to the pure differentiated type. Group L exhibited a significant and independent correlation with the undifferentiated type.
TABLE 4.
Ordered logistic regression analysis of histological types with clinical and endoscopic factors.
| OR | 95% CI | p‐Value | |
|---|---|---|---|
| Age | 0.96 | 0.93–0.98 | 0.003 |
| Male | 0.38 | 0.21–0.69 | 0.002 |
| Annual endoscopy | 0.62 | 0.36–1.05 | 0.077 |
| Endoscopic severe mucosal atrophy | 0.53 | 0.32–0.87 | 0.013 |
| Diagnosis ≥10 years after H. pylori eradication | 2.12 | 1.16–3.90 | 0.015 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Association of histopathological findings with endoscopic mucosal atrophy
We compared the endoscopic grade of gastric mucosal atrophy with histological types and pathological stages in Groups L and S (Figures 1 and 2). In Group S, the endoscopic grade of mucosal atrophy significantly correlated with the histological type and pathological stage (p < 0.001 and p = 0.001, respectively). However, these correlations were not observed in Group L. The pure differentiated type was lower in Group L than in Group S, even with severe mucosal atrophy (Figure 1). Similarly, there was no evidence of a higher proportion of stage IA cases with severe or moderate mucosal atrophy in Group L (Figure 2).
FIGURE 1.

Number of patients with invasive gastric cancer classified as mild, moderate, or severe according to the Kimura and Takemoto classification of endoscopic mucosal atrophy. In Group S, the histological type significantly correlated with the endoscopic grade of mucosal atrophy (p < 0.001, Chi‐squared test). However, these correlations were not observed in Group L. Group L included patients with invasive gastric cancers diagnosed ≥10 years after Helicobacter pylori (H. pylori) eradication, whereas Group S included patients with invasive gastric cancers diagnosed <10 years after H. pylori eradication. Pure D, pure differentiated type; D‐mix, differentiated‐predominant mixed type; Undiff, undifferentiated type.
FIGURE 2.
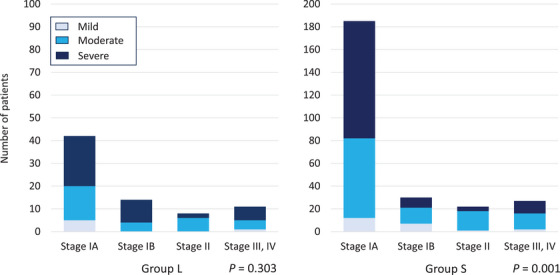
Number of patients with invasive gastric cancer classified as mild, moderate, or severe according to the Kimura and Takemoto classification of endoscopic mucosal atrophy. In Group S, the pathological stage significantly correlated with the endoscopic grade of mucosal atrophy (p = 0.001, Chi‐squared test). However, these correlations were not observed in Group L. Group L: Patients with invasive gastric cancers diagnosed ≥10 years after Helicobacter pylori (H. pylori) eradication; Group S: Patients with invasive gastric cancers diagnosed <10 years after H. pylori eradication.
DISCUSSION
This multicenter database analysis revealed the characteristics of patients with invasive gastric cancer after H. pylori eradication. Endoscopic or histological findings of early‐stage gastric cancer after H. pylori eradication are becoming clearer with more previous reports. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 After H. pylori eradication, few reports of invasive gastric cancer exist, 12 , 13 and its characteristics are unknown. To our knowledge, this is the first study to compare the clinicopathological features of invasive gastric cancer diagnosed ≥10 years after H. pylori eradication with those detected <10 years after eradication.
Herein, most patients were older men aged ≥70 years and had moderate to severe degrees of endoscopic mucosal atrophy, which is consistent with the findings of previous reports on early‐stage gastric cancer after H. pylori eradication. Mucosal atrophy improves with long‐term follow‐up after H. pylori eradication. 16 , 17 However, at ≥10 years of H. pylori eradication, the degree of endoscopic background mucosal atrophy was similar to that observed <10 years of eradication, showing moderate to severe atrophy. These prolonged risks, older men, and endoscopic mucosal atrophy were not reduced by H. pylori eradication.
A population‐based prospective cohort study reported the highest risk of gastric cancer in patients with both smoking habits and H. pylori infection. 18 However, few studies have reported an association between smoking and gastric cancer post‐H. pylori eradication cases. 19 In this study, smoking was also strongly associated with gastric cancer in cases that occurred after H. pylori eradication cases; however, no significant differences were observed between the status observed ≥10 and that observed <10 years after eradication. Similarly, no differences were found in the history of gastric cancer, other cancers, or family history of gastric cancer. We identified no promising risk factors predicting cancer development 10 years later.
In this study, the pure differentiated type was more common in invasive gastric cancer cases diagnosed <10 years after H. pylori eradication than it was in those diagnosed ≥10 years after eradication. The pure differentiated type was also more common in patients with severe mucosal atrophy in invasive gastric cancer diagnosed <10 years after H. pylori eradication than it was in those diagnosed ≥10 years after eradication (Figure 1). The pathological stage was also associated with endoscopic mucosal atrophy. More patients with severe mucosal atrophy had pathological stage IA in invasive gastric cancers diagnosed <10 years after H. pylori eradication than they did in those diagnosed ≥10 years after eradication (Figure 2).
Invasive gastric cancers diagnosed ≥10 years after H. pylori eradication were more often of the undifferentiated or differentiated‐predominant mixed type and had little association with endoscopic mucosal atrophy (Figure 1). The mucosal atrophy did not affect the pathological stage in invasive gastric cancers diagnosed after ≥10 years (Figure 2). Long‐term post‐eradication increases the risk of undifferentiated or differentiated mixed‐type gastric cancer, which is pathologically staged as high‐grade. No studies have reported a higher frequency of undifferentiated intramucosal carcinomas in long‐term post‐eradication patients. Speculations suggest that over the long‐term post‐eradication period, improvement in histological mucosal atrophy may facilitate the development of undifferentiated or differentiated mixed‐type cancers. This study did not histologically examine mucosal atrophy. However, long‐term follow‐up after H. pylori eradication may restore histological atrophic changes, even if the endoscopic mucosal atrophy is severe or moderate. Endoscopic atrophy improved less than histological atrophy by 36–153 months (mean, 78 months) after H. pylori eradication. 20
Similar to this study, another study reported that patients with mild to moderate gastric atrophy were at an increased risk of developing diffuse‐type gastric cancer 10–20 years after H. pylori eradication. 5 Although the study design (prospective cohort vs. retrospective case‐control), histological type (diffuse type vs. undifferentiated type), the timing of endoscopic background mucosal assessment (at time of eradication vs. at time of lesion discovery), and main diseases deemed eligible for H. pylori eradication (peptic ulcer vs. gastritis or after endoscopic mucosal resection/endoscopic submucosal dissection) were different between the two studies, their results were congruent.
Endoscopic images and histological findings of invasive gastric cancer after H. pylori eradication are highly variable, and accurately detecting all lesions is difficult. In the macroscopic type, lesions showing depression were the most common, accounting for 75% of the cases, including IIc‐like advanced cancers that invaded below the proper muscle. The median diameter of the lesion was 24 mm. Regardless of the time of detection, small advanced cancers <30 mm were present (n = 17), while relatively large submucosal cancers >50 mm were observed (n = 15). In this study, annual endoscopy significantly correlated with the early pathological stage. As 44% (164/377) of invasive cancers were detected within 5 years of H. pylori eradication, appropriate examinations must be performed for 5 years or longer following eradication. Endoscopic examination before H. pylori eradication cannot be neglected, and the accuracy of the endoscopic examination before and after eradication is also a factor in determining whether gastric cancer can be detected before invasion.
After H. pylori eradication, the disease is generally discovered at an invasive stage owing to irregular follow‐up; however, such lesions also develop in patients undergoing annual endoscopy. After H. pylori eradication, the endoscopist must detect gastric cancer sufficiently early for endoscopic or curative surgery. Although numerous gastric cancers can occur after H. pylori eradication, and each lesion develops differently, we believe that many lesions can be diagnosed at a curable stage with diligent periodic examinations.
This study had some limitations. First, although this study included many patients from 14 institutions, it had a retrospective design. Second, as time passes after H. pylori eradication, it becomes increasingly difficult for patients to recall the reason for eradication and to continue periodic examinations. Third, as the participating institutions were specialized oncology hospitals, the frequency of referred patients was high. The assessment of H. pylori infection is very important after eradication; however, it was sometimes performed by referring physicians using methods other than the recommended 13C‐urea breath test or stool antigen test. Finally, histological types may change before and after cancer invasion. Therefore, we have planned a separate study to determine the frequency of undifferentiated types, including intramucosal cancers.
In conclusion, invasive gastric cancers diagnosed ≥10 years after H. pylori eradication are often undifferentiated types. Irrespective of endoscopic mucosal atrophy, these tumors were more biologically malignant in terms of their histological types and pathological stages. Thus, whether this was caused by a delayed diagnosis or histological restoration of mucosal atrophy requires further investigation. In clinical practice, gastric cancer surveillance should be continued regardless of endoscopic atrophy, particularly after 10 years of H. pylori eradication.
CONFLICT OF INTEREST STATEMENT
Seiichiro Abe is an associate editor of DEN Open. The other authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the Affiliated Research Group for the development of endoscopic diagnosis for the detection of gastric cancer in the H. pylori uninfected and post‐eradication era (2020–2022) in JGES. We thank Kaoru Nakano and Hiroshi Kawachi (Department of Pathology, Cancer Institute Hospital, Japanese Foundation for Cancer Research) for their collaboration during the registration.
All authors (1) made substantial contributions to the study concept, data analysis, or interpretation; (2) drafted the manuscript or revised it critically for important intellectual content; (3) approved the final version of the manuscript to be published; and (4) agreed to be accountable for all aspects of the work.
The authors formed the Japan Gastrointestinal Endoscopy Society, Affiliated Research Group for the development of endoscopic diagnosis for the detection of gastric cancer in the H. pylori uninfected and post‐eradication era.
REFERENCES
- 1. Tsuda M, Asaka M, Kato M. Effect on Helicobacter pylori eradication therapy against gastric cancer in Japan. Helicobacter 2017; 22: e12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kamada T, Hata J, Sugiu K et al. Clinical features of gastric cancer discovered after successful eradication of Helicobacter pylori: Results from a 9‐year prospective follow‐up study in Japan. Aliment Pharmacol Ther 2005; 21: 1121–1126. [DOI] [PubMed] [Google Scholar]
- 3. Fukase K, Kato M, Kikuchi S et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open‐label, randomised controlled trial. Lancet 2008; 372: 392–397. [DOI] [PubMed] [Google Scholar]
- 4. Take S, Mizuno M, Ishiki K et al. Seventeen‐year effects of eradicating Helicobacter pylori on the prevention of gastric cancer in patients with peptic ulcer; A prospective cohort study. J Gastroenterol 2015; 50: 638–644. [DOI] [PubMed] [Google Scholar]
- 5. Take S, Mizuno M, Ishiki K et al. Risk of gastric cancer in the second decade of follow‐up after Helicobacter pylori eradication. J Gastroenterol 2020; 55: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamamoto K, Kato M, Takahashi M et al. Clinicopathological analysis of early‐stage gastric cancers detected after successful eradication of Helicobacter pylori . Helicobacter 2011; 16: 210–216. [DOI] [PubMed] [Google Scholar]
- 7. Tanaka M, Hoteya S, Kikuchi D et al. Effect of Helicobacter pylori infection on malignancy of undifferentiated‐type gastric cancer. BMC Gastroenterol 2022; 22: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mori G, Nakajima T, Asada K et al. Incidence of and risk factors for metachronous gastric cancer after endoscopic resection and successful Helicobacter pylori eradication: Results of a large‐scale, multicenter cohort study in Japan. Gastric Cancer 2016; 19: 911–918. [DOI] [PubMed] [Google Scholar]
- 9. Hata K, Ito M, Boda T et al. Gastric cancer with submucosal invasion after successful Helicobacter pylori eradication: A propensity score‐matched analysis of patients with annual patient endoscopic survey. Digestion 2019; 99: 59–65. [DOI] [PubMed] [Google Scholar]
- 10. Kodama M, Mizukami K, Hirashita Y et al. Differences in clinical features and morphology between differentiated and undifferentiated gastric cancer after Helicobacter pylori eradication. PLoS One 2023; 18: e0282341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suzuki H, Nonaka S, Maetani I et al. Clinical and endoscopic features of metachronous gastric cancer with possible lymph node metastasis after endoscopic submucosal dissection and Helicobacter pylori eradication. Gastric Cancer 2023; 26: 743–754. [DOI] [PubMed] [Google Scholar]
- 12. Tanaka M, Kikuchi D, Odagiri H et al. Advanced gastric cancer detected during regular follow‐up after eradication of Helicobacter pylori . Clin J Gastroenterol 2022; 15: 358–362. [DOI] [PubMed] [Google Scholar]
- 13. Tokura J, Namikawa K, Nakano K et al. Clinicopathological characteristics of advanced gastric cancer after Helicobacter pylori eradication. JGH Open 2022; 6: 833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Japanese Gastric Cancer Association . Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14: 101–112. [DOI] [PubMed] [Google Scholar]
- 15. Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969; 3: 87–97. [Google Scholar]
- 16. Kodama M, Murakami K, Okimoto T et al. Ten‐year prospective follow‐up of histological changes at 5 points on the gastric mucosa as recommended by the updated Sydney system after Helicobacter pylori eradication. J Gastroenterol 2012; 47: 394–403. [DOI] [PubMed] [Google Scholar]
- 17. Kodama M, Okimoto T, Mizukami K et al. Gastric mucosal changes, and sex differences therein, after Helicobacter pylori eradication: A long‐term prospective follow‐up study. J Gastroenterol Hepatol 2021; 36: 2210–2216. [DOI] [PubMed] [Google Scholar]
- 18. Shikata K, Doi Y, Yonemoto K et al. Population‐based prospective study of the combined influence of cigarette smoking and Helicobacter pylori infection on gastric cancer incidence: The Hisayama study. Am J Epidemiol 2008; 168: 1409–1415. [DOI] [PubMed] [Google Scholar]
- 19. Hatta W, Koike T, Asonuma S et al. Smoking history and severe atrophic gastritis assessed by pepsinogen are risk factors for the prevalence of synchronous gastric cancers in patients with gastric endoscopic submucosal dissection: A multicenter prospective cohort study. J Gastroenterol 2023; 58: 433–443. [DOI] [PubMed] [Google Scholar]
- 20. Kodama M, Okimoto T, Ogawa R et al. Endoscopic atrophic classification before and after H. pylori eradication is closely associated with histological atrophy and intestinal metaplasia. Endosc Int Open 2015; 03: E311–E317. [DOI] [PMC free article] [PubMed] [Google Scholar]


