Abstract
Individuals aged ≥65 yr will comprise ∼20% of the global population by 2030. Cardiovascular disease remains the leading cause of death in the world with age-related endothelial “dysfunction” as a key risk factor. As an organ in and of itself, vascular endothelium courses throughout the mammalian body to coordinate blood flow to all other organs and tissues (e.g., brain, heart, lung, skeletal muscle, gut, kidney, skin) in accord with metabolic demand. In turn, emerging evidence demonstrates that vascular aging and its comorbidities (e.g., neurodegeneration, diabetes, hypertension, kidney disease, heart failure, and cancer) are “channelopathies” in large part. With an emphasis on distinct functional traits and common arrangements across major organs systems, the present literature review encompasses regulation of vascular ion channels that underlie blood flow control throughout the body. The regulation of myoendothelial coupling and local versus conducted signaling are discussed with new perspectives for aging and the development of chronic diseases. Although equipped with an awareness of knowledge gaps in the vascular aging field, a section has been included to encompass general feasibility, role of biological sex, and additional conceptual and experimental considerations (e.g., cell regression and proliferation, gene profile analyses). The ultimate goal is for the reader to see and understand major points of deterioration in vascular function while gaining the ability to think of potential mechanistic and therapeutic strategies to sustain organ perfusion and whole body health with aging.
Keywords: endothelial function, K+ channels, myoendothelial coupling, TRP channels, vascular aging
INTRODUCTION
With a level of intensity once elevated to that of published ad hominem attacks in the early 19th century (1), the desire to understand how the cardiopulmonary system delivers life throughout the body via an organized circulatory system is as old as the field of physiology itself. In hindsight as well as the present, this passion is understandable as cardiovascular disease remains the number one killer throughout the world (2) despite an approximate doubling in average life expectancy since then from ∼40 to ∼80 yr old. As a result, modern biomedical science and medicine primarily revolve around the process of aging and the development of comorbidities that accompany it such as sarcopenia, chronic kidney disease, coronary artery disease, heart failure, hypertension, diabetes, stroke, and dementia [(3); Fig. 1]. At a fundamental level, aging has been an inexorable process defined primarily by nuclear telomere shortening, mitochondrial DNA mutations, lysosomal dysfunction, and deficiencies in nutrient/caloric sensing (4, 5). Such molecular and cellular alterations likely manifest most overtly as an initial impairment in cardiopulmonary function and then development of stiffness of the aorta and large, conduit arteries (6, 7). As a result, a lack of optimal attenuation in pulse pressure as bulk blood flow travels from the heart to other organs will ultimately damage the parenchymal microcirculation throughout the body over time (7, 8). Although not covered here in detail, recent studies indeed demonstrate biological sex-based differences for the development of arterial stiffness during advancing age with a key role for G protein-coupled estrogen receptors (9, 10). Almost all basic science investigation and data surrounding vascular aging have been focused on pathological impacts on isolated cell and organ systems (e.g., brain, skeletal muscle, gut) using reductionist in vitro and ex vivo methods. In addition, there are other groups who are continuously working to adapt the signaling pathways and experimental interventions that have emerged in such studies to living human subjects (e.g., Refs. 11–16).
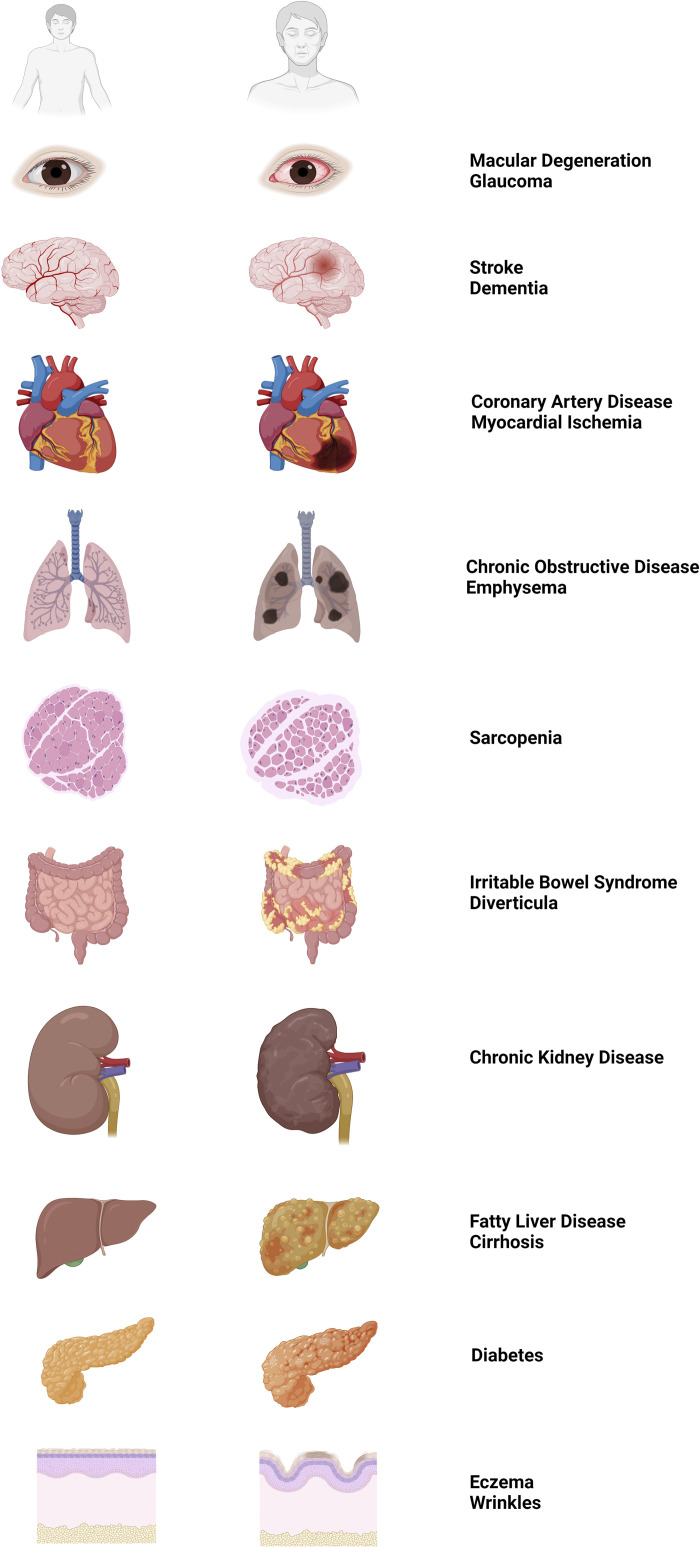
Figure 1.
Aging and the development of chronic diseases among organs throughout the body. Left: cartoon illustrations of healthy organs during young adult age (≈25 yr) as eye, brain, heart, lungs, skeletal muscle, gut, kidney, liver, pancreas, and skin (top to bottom). Middle: corresponding illustrations of diseased organs during old adult age (≥65 yr). Right: list of common diseases that become more prevalent aging as associated with the disease states of each organ illustrated in middle. Images were created with a licensed version of Biorender.com.
In the context of molecular, cellular, and integrative physiology, the current literature review is a concise effort to assemble the most recent data accompanied by refined perspective for the interplay of vascular receptors and ion channels during aging. Prior reviews have examined the biophysical interactions of key vascular ion channels [e.g., calcium-activated K+ (KCa) and transient receptor potential (TRP) channels] and handling of Ca2+ and electrical signals during aging (17–21). Currently, we seek to address distinct functional traits and common arrangements across major organs systems and the role of biological sex where possible as key knowledge gaps. Endothelial cells (ECs) are notably vulnerable with the aging process (22, 23) and thus, the narrative of this review is biased toward endothelial function as a well-coupled cellular network with regard to coordination of both second messengers and electrical signals (17). An overview of classic myoendothelial coupling mechanisms of resistance arteries is presented with updates across major organ systems regarding aging. Furthermore, local and conducted vascular signaling arrangements are discussed in the context of aging and/or chronic disease. Although equipped with awareness of knowledge gaps in the vascular aging field, a section has been included to encompass general feasibility, role of biological sex, additional considerations for cell regression and proliferation, informative value of gene profile analyses, and data interpretation. This manuscript concludes with a sample of numerous outstanding questions that remain for cellular mechanisms underlying optimal balance of discrete and conducted vascular signaling modes to meet metabolic demands throughout the body. The ultimate goal is for the reader to see and understand major points of deterioration in vascular function while gaining the ability to think of potential mechanistic and therapeutic strategies to sustain organ perfusion and whole body health with aging. References to “young” and “old” age throughout the manuscript correspond to rodent ages of ∼3–6 mo (humans: 20–30 yr old) and 22–26 mo (humans: 65–75 yr), respectively (24). Furthermore, unless noted otherwise, discussed data and findings were collected from male animals or where biological sex was not specified for a given study.
CLASSIC SIGNALING ANATOMY OF RESISTANCE ARTERIES
Vascular supply throughout the body is crucial to sustain life through the delivery of oxygen to meet metabolic demand, whereby smooth muscle cells (SMCs) and ECs of the vascular wall regulate resistance to blood flow as vascular dilation or constriction in response to hormones, transmitters, intravascular pressure, and/or shear forces (25). As fully recognized since the 1980s (see Refs. 26–28 for historical perspective), the endothelium lining the intima of the resistance vasculature plays a central role as an intermediary organ that “feeds” all other organs via ≥100 km of blood circulation throughout the body (29, 30). Beginning with an overview, the EC layer communicates with surrounding SMCs to relax and thereby increase blood flow to regions of increased metabolic demand (17). The major endothelium-dependent signaling pathways underlying vasodilation entail 1) production and diffusion of soluble compounds, such as nitric oxide (NO), carbon monoxide (CO), hydrogen sulfide (H2S), prostaglandins, and epoxyeicosatrienoic acids (EETs); and 2) an electrical signal in the form of an intracellular negative charge (hyperpolarization) that conducts through gap junctions between ECs, ECs to SMCs, and vice versa (17) (Fig. 2). Regulation of blood flow to meet tissue metabolic demand involves coordination of vasodilation and constriction over millimeters along the vascular wall within and across different precapillary networks (19, 27). As an electrical property unique to blood vessels (vs. “excitable” cardiac myocytes or neurons), ECs generate a hyperpolarizing signal that is transmitted to surrounding SMCs through myoendothelial gap junctions in an electrotonic manner, a process called endothelium-derived hyperpolarization (EDH) (17).
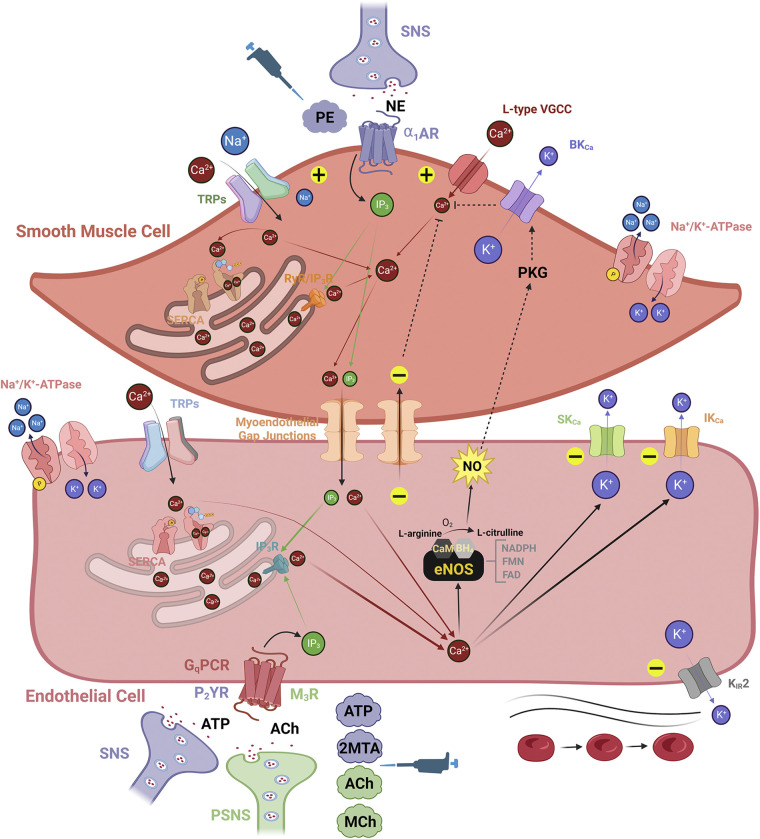
Figure 2.
Classic fundamental anatomy of vascular signaling within resistance arteries: an endothelial perspective. Smooth muscle cells (SMCs) serve the mechanical compliance function of the vascular wall for controlling resistance to blood flow, whereas endothelial cells (ECs) transduce stimuli evoked by signaling molecules carried in the blood within the vascular lumen. The level of [Ca2+]i among cell types in the vascular wall plays a dichotomous role, whereby its global increase in SMCs and ECs generates vasodilation and vasoconstriction, respectively. Either as a cause or effect of increased [Ca2+]i in the vascular wall, vasoconstriction and vasodilation are associated with membrane potential (Vm), indicated as depolarization (“+” sign) and hyperpolarization (“−” sign), respectively. SMC: the primary contributors to elevations in SMC [Ca2+]i are norepinephrine (NE)-stimulated α1-adrenergic receptors (α1ARs) sourced from the sympathetic nervous system (SNS), L-type voltage-gated Ca2+ channels (VGCCs), and Ca2+-permeant transient receptor potential (TRP) channels (e.g., TRPC3, TRPV3, TRPV4). Also, Na+-permeant TRP channels (e.g., TRPM4) can depolarize SMC Vm and activate L-type VGCCs. A distinct event known as Ca2+ “sparks” in SMCs released from endoplasmic reticulum ryanodine receptors (RyRs) will selectively activate high-conductance Ca2+-activated K+ (BKCa) channels to hyperpolarize SMC Vm while deactivating L-type VGCCs. Note that α1ARs can also be activated by the experimental agonist phenylephrine (PE, see pipettor icon). EC: the contributors to increased EC [Ca2+]i are Gq protein-coupled receptors (GqPCRs; purinergic, P2YR and muscarinic, M3R) activated by adenosine triphosphate (ATP) or acetylcholine (ACh) and Ca2+-permeant TRP channels (e.g., TRPV4). Increased EC [Ca2+]i primarily generates nitric oxide (NO) via endothelial NO synthase (eNOS, see main text for more information) and activates small- and intermediate-conductance Ca2+-activated K+ (SKCa and IKCa) channels. In addition to activated SKCa and IKCa channels, hyperpolarization of EC Vm can occur through direct activation of inward-rectifying K+ (KIR) channels via the shear of blood flow or elevated extracellular K+ (6–15 mM). Experimental agonists for P2YRs (2-methylthioadenosine diphosphate or 2MTA) and M3Rs (methacholine or MCh) are also used to mimic actions of the SNS and parasympathetic nervous system (PSNS), respectively (see pipettor icon). Myoendothelial feedback: activation of SMC α1ARs by NE generates inositol-trisphosphate (IP3) to elicit Ca2+ release through IP3 receptors (IP3Rs) in the sarcoplasmic reticulum and thereby produce constriction. When elevated in SMCs for a prolonged period, IP3 and Ca2+ diffuse through myoendothelial gap junctions into the endothelium to activate SKCa and IKCa channels and/or NO production, providing negative feedback to smooth muscle contraction (see broken lines indicating signaling from EC back to SMC). Images were created with a licensed version of Biorender.com.
SMCs and ECs share second messenger signaling molecules such as Ca2+, inositol-1,4,5-trisphosphate (IP3), cyclic adenosine monophosphate (cAMP), and cyclic guanosine monophosphate (cGMP) via myoendothelial gap junctions. Electrogenic ions (e.g., Na+, K+, or Cl−) are also distributed from ECs to SMCs in response to stimulation of EC GqPCRs (e.g., muscarinic, M3R; purinergic, P2YR) (31, 32), or vice versa subsequent to SMC α1-adrenergic receptor (α1AR) stimulation (25). Following EC GqPCR activation, IP3 is produced, which in turn activates IP3 receptors (IP3Rs) to release Ca2+ from the endoplasmic reticulum (ER) into the cytosol. ER Ca2+ release is observed as the initial “peak” response in the increase in [Ca2+]i (33, 34). The ER Ca2+ stores are filled or refilled through the uptake of Ca2+ from the cytoplasm into the ER through smooth sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) pumps to support repetitive physiological signals. The influx of Ca2+ occurs through TRP channels [e.g., canonical family, TRPC1/3/4/5/6; vanilloid, TRPV1/3/4; ankyrin, TRPA1; melastatin, TRPM3/8; and polycystin, TRPP2 (35–37)] to help refill ER Ca2+ stores and/or sustain elevated [Ca2+]i signals while integral to the secondary “plateau” phase of the Δ[Ca2+]i response following GqPCR stimulation (33, 34). Similar to a Ca2+-induced Ca2+ release mechanism in neurons governed by voltage-gated Ca2+ channels (VGCCs) and ryanodine receptors (RyRs) (38), direct pharmacological activation of TRPV4 channels can trigger a cellular influx of Ca2+ that is then amplified by intracellular Ca2+ release via ER IP3Rs in ECs (39) or RyRs in SMCs (40).
As the central mediator of vascular reactivity, Ca2+ plays opposing roles in SMCs versus ECs for vasoconstriction and vasodilation. Depolarization of SMC membrane potential (Vm) sequentially activates VGCCs [L-type, CaV1.2 and/or T-type, CaV3.1–3.2 (41, 42)] and increases cytoplasmic Ca2+ ([Ca2+]i) via Ca2+ influx into the cell interior to activate myosin light-chain kinase for myosin light-chain phosphorylation and vasoconstriction. In contrast, following G protein-coupled receptor (GPCR) stimulation, EC [Ca2+]i increases activate EC NO synthase (eNOS) and the production of NO concomitant with stimulation of small- and intermediate-conductance Ca2+-activated K+ (SKCa and IKCa) channels for EDH, resulting in overall myosin light-chain dephosphorylation and vasodilation. In a process known as myoendothelial “feedback” (43–45), activation of SMC α1ARs increases SMC [Ca2+]i and IP3, whereby excess Ca2+ ions and/or IP3 molecules will pass from SMCs to ECs to activate EC IP3Rs, increase EC [Ca2+]i, and cease further constriction (46). The differential vasoactive responses to increased [Ca2+]i among respective cell layers in combination with open cross talk via myoendothelial gap junctions prevent extreme variations in blood flow up to potential hemorrhage or ischemia.
The production of NO is dependent on the conversion of l-arginine to l-citrulline in the presence of O2 via eNOS activity. The eNOS enzyme contains oxygenase [binds l-arginine, heme, Zn2+, and tetrahydrobiopterin (BH4)], calmodulin (CaM; binds Ca2+), and reductase [binds with reducing agents nicotinamide adenine dinucleotide phosphate (NADPH), flavin mononucleotide (FMN), and flavin adenine dinucleotide (FAD)] domains (47). SMC relaxation to NO occurs via soluble guanylate cyclase (sGC) to produce cyclic guanosine monophosphate (cGMP) and thereby activate cGMP-dependent protein kinase (PKGI) for phosphorylation of several ion pumps and channels including T-type VGCCs (48), sarcoplasmic reticulum Ca2+-ATPases (SERCAs) (49), and high-conductance Ca2+-activated K+ (BKCa) channels (50). In such a manner, the relaxant effects of NO can occur as a result of reduced [Ca2+]i (49, 51), hyperpolarized Vm (52, 53), and/or direct interaction with myosin light-chain phosphatase (54, 55) to ultimately decrease myosin light-chain phosphorylation in SMCs. Note that such signaling events at the plasma membrane and throughout the cytoplasm for vascular mechanical movement are rapidly occurring while interdigitated on a timeframe of milliseconds. Thus, their magnitude and sequence of occurrence are extremely difficult to delineate and thus, findings and interpretations among studies may differ in accord with vascular size (e.g., aorta vs. parenchymal arteriole) and/or approach for measuring vascular reactivity (e.g., preconstriction via α1AR activation vs. spontaneous tone due to intravascular pressure alone).
In addition to NO, there are novel gasotransmitters to influence vascular ion channel activity as CO and H2S. CO is generated from heme oxygenases [HO-1, inducible and HO-2, constitutive; expressed in both ECs and SMCs (56)] as a by-product of heme degradation and, like NO, CO stimulates the sGC/cGMP/PKGI pathway (57). Endogenous production of CO favors endothelium-dependent dilation (58), whereby activators include NO (59), cAMP (60), platelet-derived growth factor (61), and glutamate (62). CO can also cause inhibition of SMC T-type VGCCs (CaV3.2) (63) while enhancing the coupling of RyR-generated Ca2+ “sparks” with activation of BKCa channels (64, 65). Note that SMC T-type VGCCs, RyRs, and BKCa channels operate in “microdomains” to influence Vm hyperpolarization while acting as a brake to arterial constriction (66, 67). In contrast to oxidizing hydrogen peroxide (H2O2), H2S is a biological reducing agent sourced enzymatically from thiosulfate (cystathionine-γ-lyase), homocysteine (cystathionine-β-synthase), and cysteine (3-mercaptopyruvate sulfurtransferase) (68) and nonenzymatically via sulfate-reducing bacteria in the gut (69). H2S has been shown to regulate cardiovascular function at the level of the central nervous system (CNS; hypothalamic paraventricular nucleus, rostral and caudal ventrolateral medulla, nucleus tractus solitarius, nucleus ambiguus, intermediolateral medulla; decreased mean arterial pressure and heart rate, increased baroreflex sensitivity), peripheral nerve bodies (sympathetic ganglia; reduced catecholamine release), heart (decreased rate and contractility), and isolated blood vessels (sustained EC function) (reviewed in Ref. 70). Similar to CO, H2S also influences the coupling of cytoplasmic Ca2+ signals with Vm hyperpolarization via sulfhydration of TRPV4 channels (Ca2+ influx) and subsequent activation of BKCa channels (71).
SKCa and IKCa channels are abundantly expressed in arteries and arterioles for blood flow control (72). As demonstrated thus far for the brain in particular, SKCa and IKCa channels are virtually absent in capillary ECs (72, 73) where direct exchange of nutrients and metabolites with metabolically active neurons takes place. Cytoplasmic Ca2+ binds to free CaM which is then tethered to CaM-binding domains of respective carboxyl termini of SKCa (74) and IKCa (75) channels to open their pores and thereby allow for K+ efflux from the cellular interior. Through this mechanism, hyperpolarization of EC Vm occurs in response to sequential ER Ca2+ release and Ca2+ influx, notably during treatment with a GqPCR agonist [e.g., acetylcholine (ACh)]. The resulting hyperpolarization of EC Vm transmits to the SMCs via myoendothelial gap junctions (76, 77), whereby L-type voltage-gated Ca2+ channels are deactivated, and similar to the outcome of the NO/cGMP/PKGI pathway, SMC [Ca2+]i is reduced to promote vasodilation (78). Note that when KCa channel function is absent as in ECs of capillaries in the brain (73) or collecting lymphatics of the popliteal fossa (79), an otherwise rapid Vm hyperpolarization response converts into slow Vm depolarization because of accumulation of intracellular positive charges in the form of Na+ and Ca2+ having entered through activated nonselective cation channels containing TRPV4. Consequently, there may be an impairment in the electrical coordination of capillary to precapillary vasodilation and/or production of Ca2+-dependent NO that will relax pericytes for a local increase in capillary blood flow only (80). A similar set of mechanisms for shaping discrete versus conducted vascular signals may guide contraction waves among collecting lymphatics to propel lymph flow as well (79).
IMPACT OF AGING ON RESISTANCE ARTERY FUNCTION: RECEPTOR AND ION CHANNEL FUNCTION ACROSS MAJOR ORGAN SYSTEMS
Old age has been associated with decreased vascular NO bioavailability since the early 2000s across vascular types of several different organs such as the brain, heart, skeletal muscle, and gut (reviewed in Ref. 81). A key mechanism is described by deficiencies in l-arginine and/or BH4 that result in eNOS “uncoupling,” whereby superoxide (O2•−) is produced instead of NO (82). O2•− is directly converted to H2O2 via superoxide dismutase or peroxynitrite (ONOO−) upon reacting with NO (83). In turn, ONOO− will oxidize BH4 to trihydrobiopterin radical (BH3•) and decay to dihydrobiopterin (BH2) to sustain uncoupled eNOS as well (84). The production of ONOO− in tandem with formation of H2O2 and hydroxyl radicals (HO•) also upregulates SKCa and IKCa channel function through direct and indirect mechanisms (17). A multitude of known effects on ion channel function during aging is primarily due to reactive oxygen species (ROS) signaling as illustrated in Fig. 3. Increased SKCa and IKCa channel function during old age sustains local vasodilatory signals. However, at the same time, the consequence of enhanced ion channel activity is the “leak” of electrical charge through the plasma membrane of reduced input resistance and a restricted spatial domain of conducted signaling (reviewed in Ref. 17). As illustrated in Fig. 4, this working paradigm effectively explains dyscoordination of blood flow control along entire vascular resistance networks during aging and development of chronic disease.
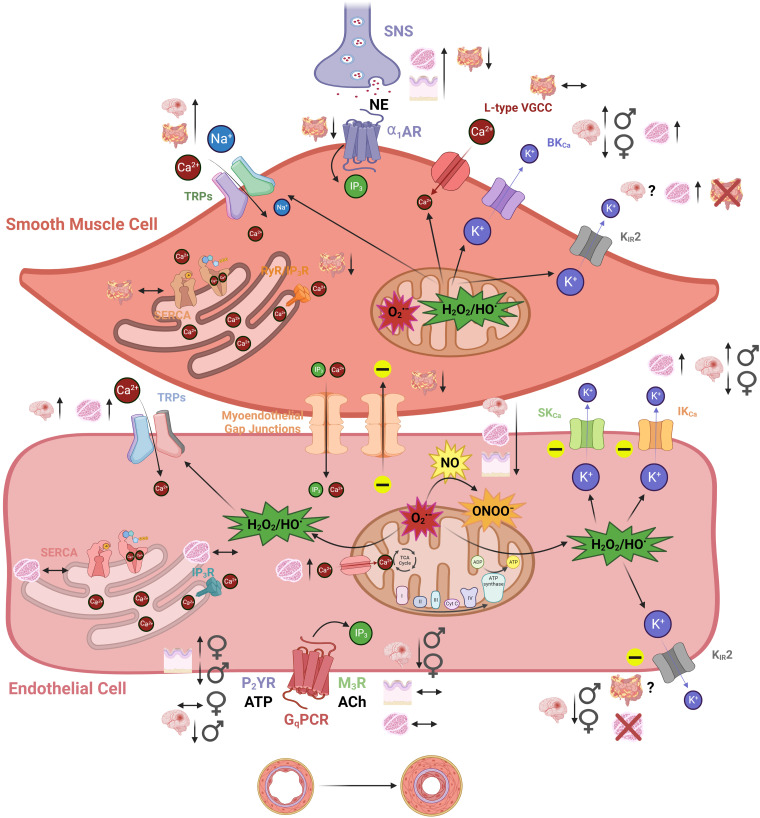
Figure 3.
The impact of aging on classical signaling components within the vascular wall of resistance arteries. There are increases (↑), decreases (↓), or stability (↔) among various smooth muscle cell (SMC) and endothelial cell (EC) receptors and ion channels that govern vascular reactivity during old vs. young age. With brain, gut, skeletal muscle, and skin blood vessels most prominently used for aging studies, various vascular types (and biological sex where applicable/known) are illustrated for both SMCs and ECs. Note that KIR2 channels in particular are expressed in both cell types in brain arteries but not skeletal muscle (SMCs only) or gut (ECs only). Mitochondrial reactive oxygen species signaling via superoxide (O2•−), and hydrogen peroxide/peroxyl radicals (H2O2/HO•) play more of a role during and old age and impact various Ca2+-permeant pathways (e.g., TRP channels) and K+ channels (e.g., SKCa and IKCa). Although not illustrated, other major sources of O2•− include the nicotinamide adenine dinucleotide phosphate (NADPH) oxidases and uncoupled eNOS (see main text). Note that nitric oxide (NO) bioavailability is decreased during old age because of a greater propensity for uncoupled endothelial NO synthase (eNOS) and the reaction of O2•− with NO form peroxynitrite (ONOO−). A primary consequence of altered signaling during aging could be elevated basal constriction and higher vascular resistance as the basis for hypertension. Another outcome may be sustained discrete reactivity to metabolites and neurotransmitters with a decrease in the spatial domain along the vascular wall for conducted signals through endothelial and myoendothelial gap junctions among capillaries, precapillary arterioles, and arteries (see Fig. 4). Images were created with a licensed version of Biorender.com.
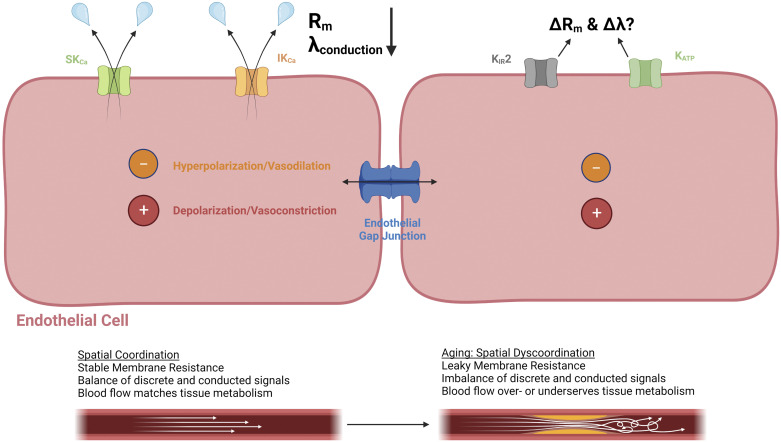
Figure 4.
Conducted vascular signaling and the endothelium as an electrical conduit: leaky ion channels and a restricted spatial domain with aging. In addition to modes of discrete/local changes in vasoreactivity, conducted signaling along the endothelium through gap junctions is crucial for coordinating vascular resistance among arteries, arterioles, and capillaries to match tissue metabolism. With aging, ion channels such as small- and intermediate-conductance Ca2+-activated K+ (SKCa and IKCa) channels become “leaky” because of reactive oxygen species and enhanced Ca2+ entry through transient receptor potential channels (TRPs). Where applicable, changes in modulators of KIR2 (phosphatidylinositol 4,5-bisphosphate or PIP2, cholesterol) and ATP-sensitive K+ channels (KATP) channels (ATP, H2S) with aging may influence optimal distribution of charge (hyperpolarization, depolarization) and corresponding vasoreactivity (vasodilation, vasoconstriction) as well. The length constant of conduction (λ) is favored by a high membrane resistance (Rm) and low-axial resistance as defined by the open-state probability of ion channels and gap junctions, respectively. The consequences of leaky ion channels with aging are a decrease in Rm and thereby a restricted spatial domain for optimally coordinating changes in vascular resistance to blood flow to meet metabolic demand. Images were created with a licensed version of Biorender.com.
Our view of EC respiration has seen a substantial shift from a glycolysis-centered one (85) to our current understanding, whereby mitochondrial production of ATP is indispensable for the control of vascular tone (86). Especially during old age, EC mitochondria also engage in cytoplasmic Ca2+ buffering and production of ROS (85, 87). It is worth noting that high concentrations of H2O2 (≥100 µM) can depolarize mitochondria and inhibit IP3Rs to altogether decrease mitochondrial Ca2+ uptake, resulting in less production of O2•−/H2O2 as well as ATP (88). During aging, the structure for vascular mitochondria becomes relatively larger, immobile, and elongated (89) with increased Ca2+ buffering capacity (33) relative to young adult animals. With recent remarkable breakthroughs in high-throughput cell respiration assays (90, 91) and imaging (92), the research area of vascular mitochondrial signaling and mitophagy (93) requires additional investigation in the context of aging and the development of cardiovascular disease.
With aging, there are also differences in modes of vascular signaling across organs to consider such as how skeletal muscle ECs display robust, sustained [Ca2+]i signals following GqPCR stimulation, whereas [Ca2+]i signals in cerebral artery ECs are relatively low in amplitude and transient (94). Furthermore, there are ion channel expression differences to be aware of such as inward-rectifying K+ (KIR2.1) channels differentially expressed on ECs and/or SMCs depending on vascular type. In the brain, both cell layers express KIR2.1 (95), whereas they are located primarily in only SMCs of skeletal muscle (96) and only ECs in mesentery (97). Note that the KIR2.2 isoform is primarily expressed in arterial SMCs of mouse skeletal muscle (96) and brain (98). However, in young rats, SMC KIR2.1 remains dominant with an absence of SMC KIR2.2 mRNA transcripts in basilar, coronary, and mesenteric arteries (99). In addition, there may be varying degrees of myoendothelial coupling among vascular types and disease states. Although not covered in this review in detail, there are also structural changes to consider such as arterial stiffening, smooth muscle hypertrophy, and an increased wall-to-lumen ratio (100–104) that may manifest differently across organs and further challenge known receptor and ion channel function deficiencies during aging. Indeed, as metabolic demands are different among various organs, the mode of EC function is tailored accordingly, especially when comparing across the microcirculation among the CNS and periphery. For example, blood flow to skeletal muscle is highly dynamic when shifting from rest to exercise (≥50-fold increase) as has been shown throughout numerous demonstrations of robust EC function in cellular, vascular, and integrated study models (17), whereas cerebral blood flow remains relatively stable upon transition to physical activity (105). Thus, it is reasonable to expect an array of differences and similarities in ion channel function among major organ systems during aging as discussed next. A summary of representative studies for altered function of vascular TRP and K+ channels during aging and/or associated diseases across brain, eye, skeletal muscle, mesentery, heart, lung, kidney, and skin is illustrated in Table 1. Accordingly, Figs. 3, 4, and 5 provide visual complementarity as to how vascular receptors and ion channels are impacted by aging.
Table 1.
A summary of representative studies illustrating how aging and associated diseases impact vascular TRP and K+ channels that govern moment-to-moment blood flow across major organ systems
| Animal Model/Species/Age/Sex(es) | Vascular Type | Physiological Approach and Ion Channel Pharmacological Intervention(s) |
Outcome: Ion Channel Function, Old (or diseased) vs. Young (or Healthy Control) |
Reference No. and PMID |
|
|---|---|---|---|---|---|
| Brain | |||||
| Aging | Mouse C57BL/6N mCAT mouse 4 to 6, 12 to 16, 24 to 28 mo Male ♂ and Female♀ |
Posterior cerebral artery | *Endothelial tubes; sharp electrode Vm recordings and [Ca2+]i photometry *NS309, elevated [K+]o, H2O2 |
Endothelium SKCa/IKCa channels: ♂↑; ♀↓ TRP channels: ♂↑; ♀↓ KIR2 channels: ♂↓; ♀↓ |
(94) 31760422 |
| Aging | Rat Sprague-Dawley 3 to 5 and 22 to 26 mo Male ♂ and Female♀ |
Middle cerebral artery | *Intravascular pressure myography, patch clamp *NS1619, IbTX, paxilline, 4-AP |
Smooth muscle BKCa channels: ♂↑; ♀↓ |
(106) 31738403 |
| Aging, hypertension | Mouse C57BL/6N 3 and 24 mo ± ANG II Male ♂ |
Middle cerebral artery | *Intravascular pressure myography *SKF96365, IbTX |
Smooth muscle TRP channels: ♂↑ |
(107) 24097425 |
| Hypertension | Mouse C57BL/6 20 to 23 wk ± ANG II Male ♂ and Female♀ |
Cerebral parenchymal arteriole | *Behavior, cerebral blood flow, intravascular pressure myography *GSK1016790A, NS309, TRAM-34, apamin |
Endothelium SKCa/IKCa channels: ♂↔; ♀↔ TRPV4 channels: ♂↓; ♀↔ |
(108) 36897751 |
| Alzheimer’s disease | Mouse 3xTg-AD 1 to 2, 4 to 5, 6 to 8, 12 to 15 mo Male ♂ and Female♀ |
Posterior cerebral artery | *Endothelial tubes; sharp electrode Vm recordings and [Ca2+]i photometry *NS309, elevated [K+]o |
Endothelium SKCa/IKCa channels: ♂↑; ♀↔ TRP channels: ♂↑; ♀↔ KIR2 channels: ♂↓; ♀↓ |
(109) 32651315 |
| Alzheimer’s disease | Mouse 5xFAD and B6SJLF1/J 4 to 5 mo Male ♂ and Female♀ Mouse |
Posterior communicating artery, parenchymal arteriole | *Intravascular pressure myography, en face [Ca2+]i imaging *NS309, apamin, TRAM-34 |
Endothelium SKCa/IKCa channels: ♂+♀↔ |
(110) 34465229 |
| Alzheimer’s disease |
5xFAD and B6SJLF1/J 12 to 13 mo Male ♂ and Female♀ |
Capillary | *Cerebral blood flow (whisker stimulation) and patch clamp *diC16-PIP2, elevated [K+]o, BaCl2 |
Endothelium | (111) 33763649 |
| KIR2 channels: ♂+♀↓ | |||||
| Alzheimer’s disease,cerebrovascular inflammation | Mouse hAPP and C57BL/6J TGF-β1 and C57BL/6J hAPP/TGF-β1 and C57BL/6J 3 to 6 mo Male ♂ and Female♀ |
Posterior cerebral artery | *Intravascular pressure myography *GSK1016790A, HC-067047, NS309, apamin, ChTX, SOD, CAT |
Endothelium SKCa/IKCa channels: ♂+♀↔ TRPV4 channels: ♂+♀↓ |
(112) 23889563 |
| Alzheimer’s disease,cerebrovascular inflammation | Mouse hAPP and C57BL/6J TGF-β1 and C57BL/6J 3 to 4 and 7 to 10 mo Male ♂ and Female♀ |
Posterior and middle cerebral arteries | *Neuronal activity, cerebral blood flow and intravascular pressure myography *Elevated [K+]o, BaCl2, ML133, CAT, indomethacin |
Smooth muscle and endothelium KIR2 channels: ♂+♀↓ |
(113) 34820829 |
| Eye | |||||
| Diabetic retinopathy | Rat Sprague-Dawley ± STZ Trpv2+/− 3 wk, 3 mo, and 1 yr Male ♂ |
Retinal arterioles | *Electroretinography, intravascular pressure myography, patch clamp *TRPV2 pore-blocking antibodies |
Smooth muscle TRPV2 channels: ♂↓ |
(114) 36134661 |
| Skeletal muscle | |||||
| Aging | Mouse C57BL/6N 4 to 6, 12 to 14, 24 to 26 mo Male ♂ |
Superior epigastric arteries | *Sharp electrode Vm measurements of endothelial tubes *NS309, apamin, ChTX, H2O2, CAT |
Endothelium SKCa/IKCa channels: ♂↑ local Vm hyperpolarization to ACh or NS309; conducted hyperpolarization/ depolarization↓ |
(115) 23723370 |
| Aging | Mouse C57BL/6N 3 to 4 and 24 to 26 mo Male ♂ |
Superior epigastric arteries | *Intravascular pressure myography, patch clamp *Paxilline, IbTX, TEA |
Smooth muscle BKCa channels: ♂↑ |
(102) 24705555 |
| Aging | Mouse C57BL/6N 3 to 4 and 24 to 26 mo Male ♂ |
Superior epigastric arteries | *Intravascular pressure myography, patch clamp *Elevated [K+]o, BaCl2 |
Smooth muscle KIR2 channels: ♂↑ |
(96) 28432059 |
| Aging | Rat Fischer 344 4 and 24 mo Male ♂ |
Soleus arterioles (oxidative, slow twitch) Gastrocnemius arterioles (glycolytic, fast twitch) |
*Intravascular pressure myography *4-AP, IbTX |
Smooth muscle BKCa channels: ♂↑ (soleus only) Kv1 channels: ♂↑ (soleus and gastrocnemius) |
(116) 19407249 |
| Obesity | Rat Sprague-Dawley ± high-fat diet 24 to 28 wk Male ♂ |
Saphenous arteries | *Intravascular pressure myography *1-EBIO, CyPPA, apamin, TRAM-34 |
Endothelium IKCa channels: ♂↑ |
(117) 20671071 |
| Heart | |||||
| Aging | Human < 64 and ≥64 yr Male ♂ and Female♀; 83% ♂ (25/30 subjects) |
Coronary arteriole of right atrium | *Intravascular pressure myography *Apamin, TRAM-34 |
Endothelium SKCa/IKCa channels: ♂+♀↑ as local vasodilation to bradykinin; conducted vasodilation↓ |
(118) 24778172 |
| Aging, oxidative stress | Mouse C57BL/6 129S4/SvJae × C57BL/6 CerS2 null GPX1/CAT double KO 15 to 25 and 75 to 100 wk Male ♂ and/or Female♀; not specified |
Thoracic aorta | *Wire myography, ROS fluorescence of ECs *1-EBIO, CAT |
Endothelium IKCa channels↑ |
(119) 27363720 |
| Obesity | Rat Obese Zucker and Lean Zucker 17 to 18 wk Male ♂ |
Second-order branches of the left anterior descending coronary artery | *Wire myography, EC [Ca2+]i photometry *GSK1016790A, NS309, apamin, TRAM-34 |
Endothelium SKCa/IKCa channels: ♂↑ |
(120) 25302606 |
| Diabetes | Canine Mongrel dogs Age not specified Male ♂ and/or Female♀; not specified |
Subepicardial collateral coronary arteries and arterioles | *Intravital vascular diameter measurements *Apamin, ChTX |
Endothelium SKCa/IKCa channels: ↑ as compensation to decrease in NO signaling |
(121) 29665152 |
| Lung | |||||
| Chronic hypoxia | Sheep High altitude (3,801 m) and normal altitude (720 m); >100 days Adult; ∼2 yr Female♀ |
Pulmonary artery (4th to 5th branch order) |
*wire myography, [Ca2+]i imaging *TEA |
Smooth muscle BKCa channels: ♀↓ |
(122) 29513562 |
| Chronic hypoxia and pulmonary arterial hypertension | Rat Wistar; hypoxic and normoxic Age not specified Male ♂ |
Pulmonary artery | *wire myography, patch clamp of SMCs *IbTX |
Smooth muscle BKCa channels: ♂↓ |
(123) 14613862 |
| Idiopathic pulmonary arterial hypertension | Human patients with IPAH ∼40 to 50 yr Male ♂ and Female ♀ |
Pulmonary artery | *Pulmonary hemodynamic measurements, patch-clamp of SMCs (single and whole cell) *correolide, bepridil |
Smooth muscle Kv1.5 channels: ♂+♀↓ via KCNA5 gene sequence mutations/single nucleotide polymorphisms |
(124) 17267549 |
| Inherited and idiopathic pulmonary arterial hypertension | Human patients with PAH <1 to 79 yr Male ♂ and Female ♀ |
Pulmonary artery | *Gene profiling of patients with PAH and whole-cell patch clamp of COS-7 cells with SUR1 variants *Diazoxide, glibenclamide |
Smooth muscle and endothelium KIR6.2 + SUR1 (KATP) channels: ♂+♀↓ via ABCC8 gene sequence mutations/single nucleotide polymorphisms |
(125) 30354297 |
| Inherited and idiopathic pulmonary arterial hypertension | Human patients with PAH 8 to 49 yr Male ♂ and Female♀ |
Pulmonary artery | *Gene profiling of patients with PAH and whole-cell patch clamp of COS-7 cells with KCNK3 variants *pH < 7.4>, ONO-RS-082 |
Smooth muscle and endothelium K2P channels: ♂+♀↓ via KCNK3 gene sequence mutations/single nucleotide polymorphisms |
(126) 23883380 |
| Mesentery | |||||
| Aging | Mouse C57BL/6 5 to 6 and 29 to 30 mo Male ♂ |
Mesenteric arteries | *[Ca2+]i photometry *Zero to 2 mM [Ca2+]o, CPA, caffeine |
Smooth muscle TRP channels: ♂↑ |
(127) 19017540 |
| Hypertension | Rat Spontaneously hypertensive Wistar–Kyoto 5 mo Male ♂ |
Mesenteric arteries (2nd order) | *Isometric tension myography *NS309, UCL-1684, TRAM-34, ChTX, NS1619, IbTX |
Endothelium and smooth muscle SKCa channels: ♂↓ IKCa channels: ♂↑ BKCa channels: ♂↔ |
(128) 19766962 |
| Hypertension | Rat Spontaneously hypertensive and Wistar-Kyoto 12, 36, and 64 wk Male ♂ |
Mesenteric arteries | *Isometric tension myography, patch clamp *IbTX |
Smooth muscle BKCa channels: ♂↑ |
(129) 29093563 |
| Diabetes | Rat Zucker diabetic fatty and Zucker lean 18 wk Male ♂ |
Mesenteric arteries (3rd order) | *Intravascular pressure myography *1-EBIO, apamin, ChTX, TRAM-34, NS1619, IbTX |
Endothelium SKCa/IKCa channels: ♂↑ BKCa channels: ♂↔ |
(130) 25128173 |
| Diabetes and hyperglycemia | Mouse db/db (leptin KO) and C57BLKS/J 11 to 18 wk Male ♂ and/or Female♀; not specified |
Mesenteric artery (feed artery + two side branches) |
*Intravascular pressure myography *Apamin, TRAM-34 |
Endothelium SKCa/IKCa channels: ↔ as locally stimulated by SLIGRL; conducted vasodilation↓ |
(131) 29339138 |
| Kidney | |||||
| Hypertension | Rat Spontaneous hypertensive Normotensive Sprague–Dawley ∼13 to 16 wk Male ♂ and/or Female♀; not specified |
Renal blood circulation, renal interlobular artery |
*Ultrasonic flow probe, wire myography *(l-NAME and indomethacin; remaining dilation/flow |
Endothelium SKCa/IKCa channels: ↔ |
(132) 31368238 |
| Diabetes | Rat Wistar ± STZ 16 to 17 wk Male ♂ |
Renal blood circulation | *Ultrasonic flow probe *Apamin, ChTX |
Endothelium SKCa/IKCa channels: ♂↔ |
(133) 18635451 |
| Diabetes | Rat Sprague–Dawley ± STZ 10 to 12 wk Male ♂ |
Renal afferent arteriole |
*In vitro blood-perfused juxtamedullary nephron *BaCl2, tertiapin-Q, glibenclamide |
Smooth muscle and endothelium KIR and KATP channels: ♂↑ |
(134) 22252401 |
| Skin | |||||
| Aging | Human 18 to 30 and 60 to 80 yr Male ♂ and Female♀ |
Microcirculation of the ventral forearm | *Microdialysis, Laser Doppler *TEA |
Endothelium KCa channels: ♂+♀↑ |
(14) 32401629 |
| Diabetes | Human 18 to 75 yr; diabetic and nondiabetic Male ♂ and Female♀ |
Subcutaneous artery | *Wire myography *UCL1684, TRAM-34 |
Endothelium SKCa/IKCa channels: ♂ +♀↑ |
(135) 26768833 |
Each row illustrates a select assortment of individual studies that used a specific aging/disease study model; biological sexes (male and/or female); vascular type and segment (arteries, arterioles, or capillaries); physiological approach and use of pharmacological interventions; primary outcome as to whether the function of an endothelial or smooth muscle TRP or K+ channel goes up, down, or remains the same with aging/disease; and the manuscript reference number with accompanying PMID. Note that this table is not necessarily exhaustive of all studies involved in vascular aging and associated pathology. As most abundant and consistent throughout the literature for aging and disease, only salient information for vascular TRP and K+ channel function has been included. Furthermore, this table does not necessarily include all approaches/experiments performed for each study, whereby some studies may have also included use of novel pharmacological agents (s-equol, Δ9-tetrahydrocannabinol), gene transfection, molecular expression assays, histology, and examination of other types of receptors (e.g., N-methyl-d-aspartate receptors) and ion channels (e.g., epithelial Na+ channels). Also, due their general stability with aging/disease relative to TRP and K+ channels while controversial for interrogation via various pharmacological and genetic tools (136, 137), information for potential alterations in vascular gap junction function has not been included. See Figs. 3, 4, and 5 and corresponding text for additional complementary information regarding altered functions of select GPCRs and organellar channels/pumps with aging. ACh, acetylcholine; ANG II, angiotensin II; 4-AP, 4-aminopyridine; BKCa channels, high-conductance Ca2+-activated K+ channels; CAT, catalase; CerS2, ceramide synthase 2; ChTX, charybdotoxin; GSK1016790A, N-[(1S)-1-[[4-[(2S)-2-[[(2,4-dichlorophenyl)sulfonyl]amino]-3-hydroxy-1-oxopropyl]-1-piperazinyl]carbonyl]-3-methylbutyl]benzo[b]thiophene-2-carboxamide; [Ca2+]i, intracellular Ca2+ concentration; [Ca2+]o, extracellular Ca2+ concentration; CPA, cyclopiazonic acid, CyPPA, N-Cyclohexyl-N-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-4-pyrimidinamine; diC16-PIP2, phosphatidylinositol 4,5-bisphosphate diC16; 1-EBIO, 1-ethyl-2-benzimidazolinone; GPX1, glutathione peroxidase 1; hAPP, human amyloid precursor protein; HC-067047, 2-methyl-1-[3-(4-morpholinyl)propyl]-5-phenyl-N-[3-(trifluoromethyl)phenyl]-1H-pyrrole-3-carboxamide; H2O2, hydrogen peroxide; IbTX, iberiotoxin; IKCa channels, intermediate-conductance Ca2+-activated K+ channels; IPAH, idiopathic pulmonary arterial hypertension; KO, knockout; l-NAME, NG-nitro-l-arginine methyl ester hydrochloride; [K+]o, extracellular K+ concentration; KATP channels, ATP-sensitive K+ channels; KIR channels, inward-rectifying K+ channels; KATP channels, ATP-sensitive K+ channels; K2P channels, two-pore domain K+ channels; Kv channels, voltage-gated K+ channels; mCAT mouse, mitochondrial catalase overexpressing mouse; ML133, N-[(4-methoxyphenyl)methyl]-1-naphthalenemethanamine hydrochloride; NS1619, 1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one; SKF96365, 1-[2-(4-methoxyphenyl)-2-[3-(4-methoxyphenyl)propoxy]ethyl-1H-imidazole hydrochloride; NS309, 6,7-dichloro-1H-indole-2,3-dione 3-oxime; ONO-RS-082, 4-chloro-2-[[(2E)-1-oxo-3-(4-pentylphenyl)-2-propen-1-yl]amino]-benzoic acid; SKF96365, 1-[2-(4-methoxyphenyl)-2-[3-(4-methoxyphenyl)propoxy]ethyl-1H-imidazole hydrochloride; SKCa channels, small-conductance Ca2+-activated K+ channels; SLIGRL, Ser-Leu-Ile-Gly-Arg-Leu-NH2; SOD, superoxide dismutase; STZ, streptozotocin; TEA, tetraethylammonium; TGF-β1, transforming growth factor-β1; TRAM-34, 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole; TRP channels, transient receptor potential channels; TRPV channels, TRP vanilloid channels; UCL-1684, 6,12,19,20,25,26-hexahydro-5,27:13,18:21,24-trietheno-11,7-metheno-7H-dibenzo [b,n] [1,5,12,16]tetraazacyclotricosine-5,13-diium dibromide; ΔVm, change in membrane potential.
Figure 5.
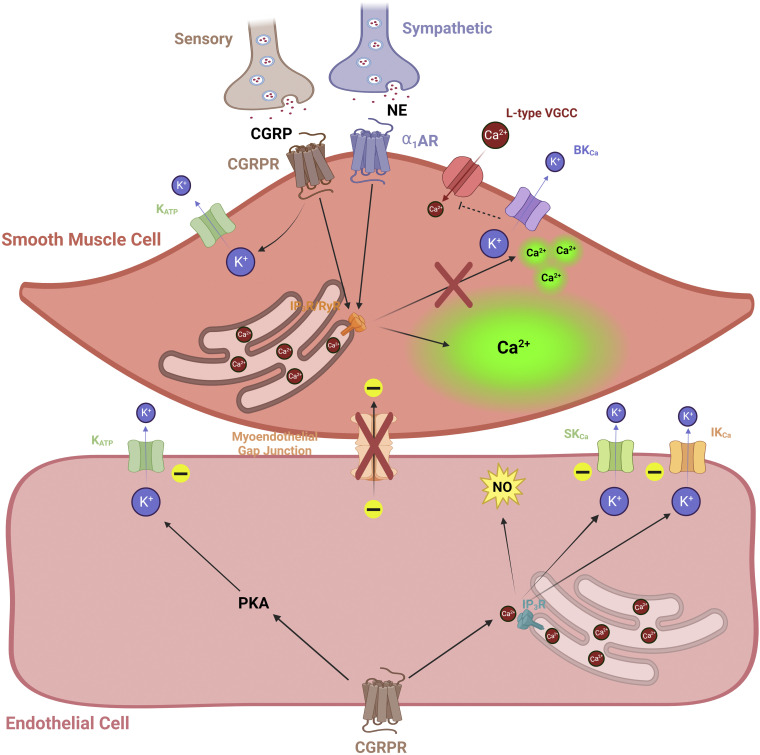
Novel perivascular nerve interactions to influence Ca2+ and K+ channel activity during aging. A role for afferent (sensory) nerve-signaling mechanisms has recently emerged during aging as a vasodilatory counterbalance to sympathetic (vasoconstrictive) input (209, 211) (see main text). A diminishment in sensory nerve density and signaling [via calcitonin gene-related peptide (CGRP) and its receptor] occurs in tandem with enhanced sympathetic activity [via α1-adrenergic receptor (α1AR)]. Consequently, smooth muscle cell (SMC), Ca2+ “waves” dominate versus “spark” events and thereby enhance cross-bridge interaction of actin and myosin fibers for constriction while inhibiting SMC hyperpolarization via high-conductance Ca2+-activated K+ channels (BKCa). As shown in mesenteric arteries, myoendothelial coupling is reduced during old age as well, potentially precluding the vasodilatory influence of calcitonin gene-related peptide receptors (CGRPRs) on endothelial cells (ECs) via activated K+ channels (KATP, SKCa, and IKCa). Although not as well known in the context of aging and competition with sympathetic nerves, note that substance P and its receptor are also important for mediating perivascular sensory nerve signals particularly via KCa channels and nitric oxide (NO) production. Images were created with a licensed version of Biorender.com.
Brain
Aside from the heart itself, the brain is a highly demanding organ as it commands a constant ∼20% of the body’s cardiac output and oxygen delivery, albeit only ∼2% of the total body weight (140). As young adults (∼20–40 yr), 21% of the cardiac output is allocated to the brain in female subjects versus 15% for males (141). With advancing age, this cerebral blood flow-to-cardiac output ratio decreases linearly by ∼1.7% in females versus ∼0.9% in males per decade of life throughout middle (mid-40s to early 60s) and old (mid-60s to 80 yr) age and is associated with decreases in cerebral blood flow despite a stable cardiac output (141). Remarkably, the pattern of decrease in EC KIR2 channel function in posterior cerebral arteries of aging C57BL/6N mice follows this general trend (94). As expressed on both SMC and EC layers (95, 142), KIR2 channels are indeed integral to cerebral blood flow control at rest and during functional hyperemia (73, 143). Note that EC KIR2 channels are highly sensitive to membrane levels of lipids, including phosphatidylinositol 4,5-bisphosphate (PIP2) (111) and cholesterol (144) as demonstrated in cerebral vessels of animal models of Alzheimer’s disease (AD). PIP2 degrades into IP3 and diacylglycerol following activation of Gq protein-coupled receptors and is known to facilitate KIR2 activity (145, 146), whereas cholesterol mediates KIR2 inactivation (146, 147). Loss of cholesterol homeostasis, in particular, is known to underlie atherosclerosis during aging (148). A consequence of reduced EC KIR2 channel function is impaired vasodilation, either initiated at capillaries (conducted to arterioles) or directly at the arteriolar level (73). Consequently, impaired blood flow distribution throughout cerebrovascular resistance networks during metabolic demand leaves active cell types of the parenchyma (e.g., neurons, astrocytes) at risk of aberrant excitability or death. Evidence shows that exogenous PIP2 (111) or methyl-β-cyclodextrin (144) restores EC KIR2 channel function of brain capillaries and arteries, respectively, in mouse models of AD. Furthermore, oxidative stress and inflammation associated with AD pathology also impair cerebrovascular KIR2 channels and can be targeted using catalase and indomethacin, respectively (113). How combination interventions for dyslipidemia, oxidative stress, and inflammation can be modulated to stabilize KIR2 channel activity and cerebral blood flow control with aging and dementia is a topic of future investigation.
Prior literature reviews have highlighted the role of vascular TRP channels during health (149) and age-related vascular cognitive impairment and dementia (150). TRP channels are not cation-selective, whereby Ca2+ and/or Na+ can enter cells to influence vasoreactivity via activation of K+ channels and direct Vm depolarization, respectively. In general, SMC TRP currents are higher in the middle cerebral arteries of old versus young male mice (107). In response to H2O2 as a primary signaling molecular of vascular aging, the vulnerability of SMCs in posterior cerebral arteries of male mice may center on TRPC3-containing channels in particular, an effect that is mitigated via myoendothelial coupling with ECs (151). In posterior cerebral ECs, TRP-induced Ca2+ influx in response to Vm hyperpolarization progressively increases and decreases with aging in males and females, respectively (94). This sensitization of Ca2+ entry to Vm hyperpolarization is prominent in male AD mice as well, whereas it is relatively stable among both Pre-AD and AD conditions in females (109). Additional studies have underscored the importance of TRPV4 channels during the dilation of cerebral parenchymal arterioles and maintenance of cognitive function (152). Angiotensin II-induced hypertension (systolic blood pressure ≥140 mmHg) diminishes TRPV4 channel function in cerebral parenchymal arterioles of young male C57BL/6 mice, whereas females maintain TRPV4 channel function regardless of hypertension (108). Also, during conditions of cerebrovascular amyloidosis and inflammation, impaired EC TRPV4 channel has been observed in posterior cerebral arteries among male and female mice combined (112). Note that although PIP2 supports KIR2 channel function (145), it can also inhibit TRPV4 channel function in capillary ECs and thereby support capillary-to-arteriole signaling to coordinate cerebral perfusion (80). Thus, biological sex, vascular segment (capillaries, arterioles, arteries), local versus conducted vascular signaling, and severity of pathology associated with aging are important factors to bear in mind for experimental design and development of treatment strategies. Altogether, at least in males, Ca2+ overload through heightened vascular TRP channel activity in the brain may impair neurovascular coupling and blood-brain barrier integrity (150).
In the brain, KCa channels appear to be minimally impacted relative to KIR2 and TRP channels. In male cerebral arteries (94), the underlying components of EDH signaling (i.e., increased [Ca2+]i and SKCa and IKCa channel function) may be enhanced in old age (≥24 mo) because of increased levels and basal activity of H2O2. Indeed, Vm hyperpolarization of posterior cerebral ECs to a half-maximal effective concentration (EC50, ≈1 µM) of the potent SKCa and IKCa channel opener 6,7-dichloro-1H-indole-2,3-dione-3-oxime (NS309) in old mitochondrial catalase-overexpressing (mCAT) mice was reduced by ∼30% relative to old wild-type mice (94). Also, note that EDH is decreased in posterior cerebral arteries of old females relative to young females and old males (94). Note that for conditions of AD versus healthy controls, EDH is enhanced in males, whereas relatively stable in females (109, 110). As consistent with EC SKCa and IKCa channels, SMC BKCa channel function is decreased in females but increased in males in middle cerebral arteries of old rats as well (106). Although, BKCa channel function may be less sensitive in the middle cerebral arteries of old versus young male rats following pharmacological modulation of certain GPCRs, such as in the case of isoproterenol activation of β-adrenergic receptors and phosphorylation of BKCa channels via protein kinase A (PKA) (153). For overall EDH signaling in the brain, females appear to favor GqPCR (purinergic) signaling versus K+ channel function for males during aging (94) and conditions of AD (109). As a widespread appreciation for neurovascular support of healthy cognition continues to emerge (154, 155), additional work will further illuminate the myoendothelial interactions of cerebral blood vessels in the aging brain.
Eye
Age-related diseases associated with the impaired vascular health of the eye include glaucoma (156) and diabetic retinopathy (157). In addition, retinal imaging has also been developed as a noninvasive method for monitoring the development of a major cognitive disorder such as Alzheimer’s disease (158, 159). A multitude of studies have focused on enhanced proliferation and migration of ECs via vascular endothelial growth factor (160, 161) and inflammatory signaling itself regarding the arrangement of vascular receptors and ion channels and how they alter with aging. It is known that retinal arterioles contain the standard voltage-gated K+ (e.g., BKCa) and Cl− [e.g., Ca2+-activated Cl− (ClCa)] channels (162) and VGCCs (T-type, CaV3.1) (163) for myogenic autoregulation. With respect to differences among vascular segments, capillaries and arterioles of the eye are enriched in ATP-sensitive K+ (KATP) channels and VGCCs, respectively (164, 165). With going in the direction from retinal arterioles (containing ECs and SMCs) to distal capillaries (ECs and pericytes) of pressurized whole retinas ex vivo, elegant electrophysiology measurements demonstrate progressively declining participation of VGCCs and slowing constriction speed to increasing pressure (20–100 mmHg) at the ophthalmic artery inlet (165). Other studies have highlighted how various TRP family members (canonical, melanostatin, mucolipin, and vanilloid) are expressed among the corneal epithelium, lens, ciliary body, trabecular meshwork, and retina of the eye (reviewed in Ref. 166). In particular, Ca2+-permeant TRPV channels (e.g., TRPV2, TRPV4) govern vascular autoregulation of retinal arterioles (166, 167), whereby loss in TRP channel function generally underlies diabetic neuropathy (114, 166). A deficiency in caveolin-1 as a key regulator of intravascular pressure-induced myogenic tone (168) accelerates age-related loss of contractile SMCs of retinal arterioles (169). Furthermore, per conditions of central serous chorioretinopathy, aldosterone will activate mineralocorticoid receptors to upregulate choroid EC SKCa (KCa2.3) channel expression and thereby cause excessive vascular dilation and leak underneath the retina (170).
Electrotonic mapping of retinal arterioles and capillaries demonstrated that decay in axially spreading changes in Vm (depolarization) occur at ∼5% per 100 μm of vascular distance along capillaries, tertiary arterioles and secondary arterioles (171). This calculation matches well with the decay rate observed for the endothelium of skeletal muscle (superior epigastric) arteries as ∼4.4% per 100 μm (172). In the presence of angiotensin II as a key indicator of vascular aging and hypertension (173), this decay along the retinal microcirculation increases 10-fold from ∼5% to ∼50% decay per 100 μm (171), likely because of an impairment in axial EC-to-EC transmission of charge and not local EC-to-SMC coupling or SMC-to-SMC conduction (164). Note that these studies were performed using acute applications of angiotensin II (500 nM; ∼5-min exposure) in microvasculature isolated from young rats, whereby detectable changes in membrane resistance (Rm) were not observed using hyperpolarization pulses (20 mV) via the perforated-patch configuration of intact vessels (171). Instead of a profound alteration in plasma membrane ion channel activity to influence Rm, a potential difference in connexin (Cx) composition among endothelial and myoendothelial junctions and their respective sensitivity to angiotensin II treatment has been speculated for these findings (164). Exploring this possibility has been complicated by the controversial nature of pharmacological and genetic tools intended to interrogate gap junctions (136, 137). The vascular biophysics underlying coordination of blood flow throughout the eye during youth, healthy aging, and pathogenesis require further investigation. In addition, the eye as an extension of the brain and CNS may have prognosticative value for development of neurodegenerative disease (174), further reinforcing its importance for diagnosing and treating dementia in addition to impairment in sight.
Skeletal Muscle
The impact of aging on skeletal muscle arteries has been described in detail throughout prior reviews (17–19). In brief, skeletal muscle arteries contain robust EDH signaling whereby classic M3R stimulation with ACh evokes a “peak” Δ[Ca2+]i response (IP3 binds to IP3R and release of Ca2+ from the endoplasmic reticulum) that is followed by a secondary “plateau” Δ[Ca2+]i phase (Ca2+ influx through TRPs) (17). In tandem, SKCa and IKCa channels are activated and hyperpolarization is present and proportional to the elevated [Ca2+]i signal during exposure to ACh (17). In males, the “peak” Δ[Ca2+]i response is maintained during old age but the TRP channel contribution during the “plateau” phase is elevated (33). Because of heightened H2O2 signaling, basal activity of SKCa and IKCa channels is elevated during old age whereby EC conduction of both hyperpolarization and depolarization through gap junctions is impaired [(115); Fig. 4]. In turn, robust Vm hyperpolarization to H2O2 (200 µM, 20 min) has been demonstrated in an SKCa and IKCa channel-dependent manner, whereby electrical conduction was abolished (115) similar to what is observed during a maximal concentration (10 µM) of the SKCa and IKCa channel opener NS309 (172). Remarkably, prolonged treatment of superior epigastric arteries with H2O2 (200 µM, 50 min) evokes less cell death in old versus young animals whereby ECs are more resilient than SMCs (175). Follow-up studies have revealed that vascular cells of females are significantly more protected against H2O2 than vascular cells of males, whereby interleukin-10 (IL-10) and a caspase-dependent apoptotic pathway mediate cell death (176).
General increases in SMC K+ channel function occur in male skeletal muscle arteries (96, 102) and arterioles (116) during advanced age as well. Enhanced BKCa (102) and KIR2 channel (96) function has been observed in superior epigastric arteries of old versus young animals. With regard to muscle fiber during old age (177), both BKCa and voltage-gated K+ (Kv) channel activities oppose myogenic tone in arterioles of soleus as an oxidative, slow-twitch muscle type with a high mitochondrial content (116). With a negligible contribution of BKCa channels in both young and old animals, arteriolar myogenic tone within the gastrocnemius (glycolytic, fast twitch) is predominantly regulated by KV channels during old age (116).
Note that enhanced KCa function is also present in skeletal muscle arteries during obesity (117) and that impaired conducted hyperpolarization and vasodilator signals occur during conditions of hypertension (178) and hyperhomocysteinemia (179). Also, in response to chronic hypobaric hypoxia (380 Torr; 48 h), the expression of BKCa emerges in ECs with microdomains formed among caveolin-1, TRPV4, and BKCa channels in male rat gracilis arteries (180). With relevance to sarcopenia and osteoporosis with advancing age, uncoupling of eNOS and higher levels of ONOO− have also been found in old versus young male rats in tandem with elevated superoxide dismutase 2 (SOD2), glutathione peroxidase 1 (GPX1), and NADPH oxidase 2 (NOX2) (181).
Heart
The myocardium of the heart extracts ∼70–80% of the oxygen contained within coronary blood flow (182). Similar to skeletal muscle, many of the same trends are observed with coronary arteries or aorta during aging (119) or chronic disease such as obesity (120) and diabetes (121) as generally elevated KCa channel function in the face of decreased NO signaling coupled with an impairment in conducted vasodilation via gap junctions (118). In addition, fundamental work and insight has emerged for the interaction among KIR and KATP channels in capillaries among pericytes and ECs as primary “electrometabolic” sensors of blood flow regulation (see Ref. 183 for a comparative analysis among the heart and brain). In addition, other groups are actively researching mineralocorticoid receptor signaling and EC Na+ channels per their contribution to cardiovascular fibrosis and arterial stiffness with aging and insulin resistance (184).
Lung
As recently reviewed in detail (185), numerous receptor and ion channel components govern the tone of pulmonary arteries as they receive the entire cardiac output of deoxygenated blood from the heart’s right ventricle in a high-flow, low-resistance system with a mean pressure of ∼14 mmHg (reference: ∼100 mmHg for systemic arteries). This arrangement is appropriate for the purpose of the lungs to extract oxygen from the environment via alveoli and replenish oxygen within the pulmonary circulation to be returned to the left atrium of the heart via the pulmonary veins. As a factor to consider for potential dysregulation of blood pH (e.g., acidosis), the lungs normally remove excess carbon dioxide carried within the entryway of the pulmonary circulation back out into the environment. In such manner, the pulmonary vasculature is especially sensitive to mechanical stretch and shear stress of EC membranes that then transmit intracellular signals to command movement of SMCs to influence vasoreactivity of the entire vascular wall.
Pulmonary arteries contain primary receptor and ion channel components such as TRPV4 (186), SKCa and IKCa channels (187), and Cx40 (188) for EDH. However, in contrast, to mesenteric arteries, for example, TRPV4 channel signaling in pulmonary arteries favors coupling with eNOS and NO production in a negative feedback loop manner versus SKCa and IKCa channel activation (186). Pulmonary EC TRPV4 channels are generally colocalized with eNOS while containing negligible levels of hemoglobin α (Hbα; NO scavenger) at myoendothelial gap junctions (189). However, with challenges of decreased EC NO levels and NO-mediated dilation, pharmacological activators of SKCa and IKCa channels may still be used to treat pulmonary arterial hypertension (PAH; mean pressure > 20 mmHg) (187). Not surprisingly, mechanosensitive TRPC6 and PIEZO1 channels in arterial ECs have also emerged as primary contributors to Ca2+ influx and associated increases in [Ca2+]i (190). Chronically elevated EC (191) and SMC (192) PIEZO1 channel function in particular contributes to Ca2+-induced cell proliferation that is representative of the pathogenesis of pulmonary hypertension. As PIEZO1 channels are also permeant to Na+ (193), prolonged depolarization and vascular constriction will accompany such structural remodeling as well.
Impaired function of various SMC K+ channels susceptible to hypoxia such as KV1.5 (124, 194), BKCa (122, 123, 195), KATP (196, 197), and two-pore domain K+ (K2P) (198, 199) accompanies and/or exacerbates PAH. One molecular mechanism entails a high expression of microRNAs that negatively regulate KCNA5 (KV1.5 mRNA) and KCNMB1 (BKCa β1 subunit mRNA) as miR-29b, miR-138, and miR-222 (200). Loss-of-function mutations of ABCC8 [KATP sulfonylurea receptor 1 (SUR1) subunit] (125) or enhanced endothelin signaling (201) can directly counteract normal KATP channel expression and function, respectively. Outwardly rectifying K2P channels are pH sensitive and set K+ background currents in pulmonary arteries (reviewed in Ref. 138), whereby their loss of function leads to pulmonary vasoconstriction and PAH (126), because of a depolarized resting Vm of SMCs and activation of L-type VGCCs (202). Another novel therapeutic target of PAH responsible for SMC depolarization includes a ClCa channel as TMEM16A as a plasma membrane gateway for the efflux of Cl− ions (139). Downregulation in an EC gap junction component as Cx40 in response to chronic hypoxia (10% O2; 4 wk) relative to normoxia (21% O2) also contributes to PAH via a decline in the spread of ACh-induced EDH (188). Note that gap junctions containing Cx40 are instrumental or conducted depolarization and vasoconstriction in response to acute hypoxia (1% O2; 10 min) as well (203), underscoring the dichotomous role of EC-to-EC coupling for modulating pulmonary vascular resistance throughout health and disease. Note that in the case of endothelin exposure and Ca2+ activation of rho kinase [i.e., Ca2+ “sensitization” for myosin light-chain phosphatase inhibition (204)], Vm depolarization is not necessarily required for vasoconstriction in response to chronic hypoxia (205).
Finally, general features of perivascular innervation of the pulmonary vasculature as sympathetic, parasympathetic, and sensory are known across several species including rodents and humans (reviewed in Ref. 206). However, as is the case with other organs, there is a paucity of knowledge as to how various nerves and their receptors interact to sculpt exchange of electrical signals and second messengers among pulmonary artery ECs and SMCs (Fig. 5). A foundational study has carefully separated examination of SMCs and ECs to reveal that sensory nerve signaling via CGRP receptors and KATP channels are present on both cell layers of male and female mouse pulmonary arteries (207). Progressive concentrations of CGRP (100 pM to 1 µM) evoke up to ∼20-mV hyperpolarization in a like manner for both SMCs and ECs that is dependent on PKA and activation of KATP channels (207). Referring back to the central importance of KATP channels for pulmonary blood flow during health and PAH (125, 201), such studies will expand considerations for the depth of integrative vascular signaling and the therapeutic toolbox accordingly. Unfortunately, how biological sex and aging influence receptor and ion channel function in the pulmonary vessels is altogether unknown, and thus, studies are desperately needed in this area of research.
Mesentery
In the mesentery, an accumulated body of novel research has revealed competing contributions of sympathetic versus sensory perivascular nerves during aging in the context of SMC and EC ion channels, Ca2+ signaling, and myoendothelial coupling (208–212) (Fig. 5). Overall, note that the density and function of perivascular nerves are diminished with advancing age in mesenteric arteries (211, 212). Sympathetic nerves release norepinephrine and activate αARs, and with treatment of phenylephrine as a selective α1AR agonist, the potency of αAR activation has been shown to diminish with old age (211). Sensory nerves release respective transmitters as substance P and calcitonin gene-related peptide (CGRP) (44). There are differential effects of respective agents among ECs and SMCs. Substance P hyperpolarizes both ECs and SMCs via KCa channels while also involving endothelium-derived NO signaling for the relaxation of smooth muscle (208). In response to CGRP, SMC and EC hyperpolarization require KATP and KCa channels, respectively (208). Note that vasodilation to CGRP is diminished with old age in male rodents (212), whereby myoendothelial coupling among SMCs and ECs is substantially reduced (210). Furthermore, sympathetic induction of SMC Ca2+ events is also unbalanced for local Ca2+ “sparks” that underlie vasodilation versus global intracellular Ca2+ “waves” producing vasoconstriction (209). Altogether, these data highlight an example of the interaction of vasodilatory (sensory) and vasoconstrictor (sympathetic) perivascular nerve pathways to regulate blood flow and how this balance is impacted with aging.
In contrast to dominant NO signaling in pulmonary arteries, TRPV4 channels of mesenteric ECs favor activation of SKCa and IKCa channels (186) with the additional presence of NO-scavenging Hbα at myoendothelial gap junctions (189). As consistent with other organs during aging, KCa channel function is generally increased during chronic disease conditions such as hypertension (128, 129) and diabetes (130). Furthermore, with local EDH unaffected, conducted hyperpolarization/vasodilation to the peptide SLIGRL (selective protease-activated receptor agonist) along mesenteric arteries is impaired during chronic hyperglycemia [(131); 40 mM glucose, nonfasting blood glucose level in young diabetic db/db mice lacking functional leptin receptors) as likely influenced by SMC BKCa and KV channel activity (213). The function of L-type VGCCs is preserved (214), whereby SMC TRP currents are generally higher (127) during old versus young age. The coupling of SMC T-type VGCCs and RyRs may also alter with advancing age to influence EC function (215, 216). In contrast to the microcirculation of the eye (171), angiotensin II infusion (4 ng/min; raised mean arterial blood pressure by 6 ± 2 mmHg) enhances conducted, but not local, vasoconstriction to local norepinephrine or electrical stimulation treatments in rat mesenteric arterioles in vivo (217). This finding is most consistent with a broad downregulation of the expression and/or function of various SMC K+ channels (e.g., KV, KIR, and BKCa) in response to angiotensin II and an increased SMC Rm (reviewed in Ref. 218). Note that local vasoreactivity is sustained during angiotensin II in mesenteric arterioles (217) as similar to the eye (171). The differential effects of angiotensin II on Rm and/or axial resistance (Ra) among vascular types to shape an overall decline or enhancement in conducted electrical signals along and between respective SMC and EC layers requires further examination. With a large, informative body of work constructed for the gut microcirculation nonetheless (77), additional studies are also needed in the context of aging, biological sex, and the emerging gut-brain axis research field (219) for investigation.
Kidney
Similar to the brain, the kidney occupies only ∼1% of the total body weight and receives a constant ∼20% of the cardiac output to maintain renal perfusion and the blood-urine filtration interface for elimination of excess metabolites (e.g., NH4+, H+; via glomerular filtration and tubular excretion) while retaining nutritive glucose, amino acids, and ions (e.g., HCO3−; via tubular reabsorption and venous return) for maintaining overall fluid and electrolyte homeostasis (220). The anatomy, physiology, and pathophysiology of the renal microcirculation have been covered in depth elsewhere (reviewed in Refs. 221, 222) but, in brief, afferent arterioles branching off of the interlobular artery feed into glomeruli and regulate glomerular hydrostatic pressure and filtration. During healthy conditions, there is autoregulatory cross talk among the inherent myogenic reactivity of the afferent arterioles and changes in Na+, K+, and Cl− sensed by Na+:2Cl−:K+ cotransporters on macula densa cells of the thick ascending limb and distal convoluted tubule junction (i.e., “tubuloglomerular feedback”) to ultimately determine an optimal glomerular filtration rate (GFR) (223). For example, an increased NaCl concentration detected by the macula densa will evoke acute constriction of the afferent arteriole and a brief decrease in GFR. Chronic disruptions in the autoregulation of afferent arteriolar tone as sustained vasodilation or vasoconstriction underly the progression of nephropathy and the development of systemic hypertension and diabetes (224). Furthermore, the convergence of systemic and intrarenal renin-angiotensin-aldosterone signaling, sympathetic and sensory nerves, and intravascular fluid-shear forces detected by primary cilia also shape vascular tone among afferent and efferent arterioles of the pre- and postglomerular apparatus, respectively (222).
As a result of limited accessibility to the blood microcirculation contained within the renal parenchyma (221), information for mechanisms of vascular cell-specific receptor and ion channel function of the kidney is relatively scarce among conditions of both health and disease (virtually nothing for aging). Starting with the interlobar arteries of male and female mice, it is known that a functional EDH mechanism is present with the inclusion of SKCa/IKCa and KIR channels, Na+/K+-ATPases, and patent myoendothelial gap junctions that contain Cx40 (225). The presence of Cx40 is crucial for transmission of the tubuloglomerular response in afferent arterioles (226). Furthermore, EDH signaling via SKCa/IKCa function also appears to be present in afferent but not in efferent arterioles (227), pointing to distinct differences in EC ion channel function in the pre- versus postglomerular apparatus. Remarkably, both arterioles contain functional KIR2.1 channels, whereby stimulating KIR2 currents enough to evoke ∼10 mV of hyperpolarization has a negligible effect on vascular diameter in efferent versus >5 µm vasodilation in afferent arterioles preconstricted with angiotensin II (228). Thus, note that, even with a known similarity in K+ channel expression/function, the close coupling of electrical responses (changes in Vm) with mechanical vasodilation and vasoconstriction is indeed a consistent feature of afferent but not efferent arterioles (229). As determined by renal blood flow measurements in vivo and/or isolated interlobar arteries ex vivo, SKCa/IKCa function is maintained throughout conditions of both hypertension (132) and diabetes (133), whereas NO-dependent dilation is impaired. Additional studies using an in vitro blood-perfused juxtamedullary nephron technique have demonstrated a threefold higher contribution of KIR channels [sensitive to BaCl2 and/or tertiapin-Q (KIR1.1, KIR2.1, KIR3.x)] to vasodilation of afferent arterioles in male diabetic versus nondiabetic rats (134, 230). With current priorities centered on renin-angiotensin, oxidative, and sympathetic nerve signaling (221, 222, 224), a focus on diseases of chronic aging and biological sex will pave an exciting future for new mechanisms and therapy surrounding renal pathophysiology.
Skin
Due to their noninvasive nature, skin blood flow studies are common for assessing the vascular function of human subjects. For cutaneous vasodilation, old age augments and attenuates purinergic receptor function in human females (231) and males (232), respectively, with no apparent effect on muscarinic receptors. As consistent with the observation of increased EDH throughout organ systems in rodents, general K+ channel-dependent EC function is enhanced in older (∼65 yr) versus younger (∼25 yr) mixed male and female human subject cohorts (14). Furthermore, EDH also compensates for reduced NO activity during diabetic conditions in the subcutaneous arteries of humans (135). Note that novel vascular agents such as menthol (putative opener of Ca2+-permeant TRPM8 channels; theoretically stimulates both EDH and NO signaling) are being explored as therapeutic agents in randomized controlled trials of human subjects (233–235). The capacity for cutaneous vasodilation by menthol in particular is preserved across conditions of normotension and hypertension in both men and women (15).
VASCULAR AGING STUDIES: CONSIDERATIONS FOR GENERAL FEASIBILITY, ROLE OF BIOLOGICAL SEX, CELL REGRESSION VERSUS PROLIFERATION, GENE PROFILE ANALYSES, AND DATA INTERPRETATION
Biological aging has been recognized as the most prominent risk factor for the development of major chronic disorders as cardiovascular disease, neurodegeneration, and cancer (236). However, there are significant logistical limitations to consider for a research environment that is preparing for, or conducting, aging studies. There is a time and husbandry issue concerning the experimental animals themselves, by which investigators may or may not be able to procure animals at approximate ages throughout the animal’s lifespan upon demand. Typically (and with permission of agencies such as the National Institute on Aging) only wild-type animals of the C57BL6 and Fischer 344 lineages have been available for purchase in an “as needed” basis. Most to all other animal types suitable for studying cardiovascular disease will require breeding and local housing at the investigator’s institution for up to 2 years or longer (24). Consequently, because of availability of animal study models at any one time, aging research may encounter problems of diminished scientific rigor while limited to “observational” or “phenomenology” studies that lack critical mechanistic insight. Thus, at least with respect to mammalian models, biology of aging investigators must be more encompassing with their efforts for fundamental studies using young, healthy animals and dedicated models of disease as well. In the aggregate, perhaps the strongest experimental design for understanding cardiovascular disease is indeed one that examines models of poor, normal, and optimal aging models in parallel. In turn, the investigator increases their chances of capturing subtle mechanistic transitions from physiology to pathology. Accordingly, the scope and timing of preparing individual manuscripts for peer review must be decided carefully and in a highly organized manner as well.
Studies focused on the role of biological sex have been a persistent problem with what was once determined in 2009 as ∼70% of studies focused on a single sex or were unspecified (237). As of the most recent meta-analysis in 2019, this overarching number has decreased to ∼50% (238) but progress or improvements in this regard depend on interpretation concerning discipline while addressing the greatest need for data collection among females in particular. For example, there has been a specified increase in the inclusion of both sexes among studies in physiology (from 13 to 36%) but the use of males only remained the same (51% throughout), whereas the percentage of female-only studies actually decreased (14 to 4%) within that time frame (238). As a much more dire outcome, the field of pharmacology has shown an overall decrease in studies specifying inclusion of both sexes (from 33 to 29%), with an increase in male only (from 50 to 58%) and stability of a low participation (10% throughout) in female-only research (238). Thus, sex as a biological variable and inclusion of females remains a primary priority for improvement among biomedical research. However, with referring to a key limitation of aging research (availability across multiple time points throughout the animal’s lifespan), this consideration effectively doubles the number of animals required relative to the majority of prior efforts and can thereby constrain financial and logistical resources further.
The estrous cycle and the potential confounding effects thereof have been a convenient excuse for excluding females from biomedical studies (237, 238). However, it is the experience and bias of the author that, for the most part, only long-term depletion of estrogen on the order of months (e.g., menopause) will significantly alter its genomic and corresponding physiological imprint for female vascular function (239). In support of this notion, recent studies have demonstrated that ex vivo vascular EC function [(240); mesenteric and cerebral arteries] and in vivo cerebral autoregulation (241) are unaffected by individual phases of the estrous cycle in young adult mice and female humans, respectively. Note that in vivo measurements of pulse wave velocity (PWV; putative index of aortic stiffness) are lower during estrus relative to diestrus (240) but, the PWV also reflects changes in mean arterial blood pressure throughout the estrous cycle (242) and not necessarily vascular structure or mechanics per se (243). Also, in past studies of the EC function of rodent mesenteric and cerebral arteries, an extreme surgical intervention such as ovariectomy and a ∼1 mo waiting period is required to effectively deplete estrogen to the extent that significant alterations in NO and/or EDH signaling are observed (244, 245). Furthermore, another chemical method to induce menopause as application of 4-vinylcyclohexene diepoxide requires at least 20 consecutive days of treatment followed by an additional 3-mo period to evoke marked differences in the structure and function of cerebral arterioles relative to control females (246). During old age and postmenopause, when estrogen levels are at their lowest in the female lifespan, of course, there are considerations for estrogen-sensitive receptors and ion channels such as EC purinergic (P2Y) receptors (247) coupled through KCa channels for producing EDH (94). For models of poor or accelerated aging, note that altered hormone signaling per menopause may not be relevant coincident with the peak of pathology during middle age (e.g., 3xTg-AD mice; 12–15 mo) (109) or perhaps younger (5x-FAD mice; 4–5 mo) (110). Regardless, changes in estrogen and testosterone levels (and/or corresponding receptor expression) occur chronically throughout the lifespan of both males and females, particularly a stable drop upon and throughout advanced age [(248, 249); human subjects, 50–97 yr]. Thus, especially within investigational settings on the order of a week or less within the animal’s lifespan, hormone cycling and signaling should not be used as a sole justification to exclude either biological sex among studies of physiology and pharmacology.
As vascular physiology and pathology during aging is a rapidly growing field of research, this review could not cover all relevant literature in detail including chronic vascular remodeling. The current scope entailed how vascular receptor and ion channel function is altered for moment-to-moment regulation of blood flow during aging. Note that there is a broader literature that focuses on EC death (regression) and replicative senescence in response to varying degrees and exposure times of disturbed blood flow patterns and inflammation (reviewed in Ref. 5). Typically, such remodeling carries consequences of altered cellular structure and proliferation for impaired vascular permeability and aberrant angiogenesis, respectively (5, 250). Furthermore, the structural maladies of the vascular wall tend to be rooted in genomic disturbances (5) and posttranscriptional regulation by noncoding miRNAs (251, 252). In contrast, vascular receptor and ion channel expression is relatively stable with advancing age (253) and are likely best served by posttranslational modifications because of their high metabolic expense for altered expression/turnover. In addition, recent studies demonstrate mechanisms of insufficient lysosomal function and autophagy to degrade intracellular molecules and dysfunctional organelles that thereby accelerate vascular aging and establish hypertension (254, 255). Furthermore, during conditions of repressed autophagy in old mice and human subjects, activating EC purinergic (P2Y1) receptors ameliorates age-related restrictions in vasodilator capacity (256). More information is required for crossover interactions for acute and chronic events among vascular structure and vasoreactivity and their corresponding investigational layers as preprotein coding and regulation of tetrameric proteins assembled within the plasma membrane.
Finally, we as academic scientists (and human beings) tend to look for meaningful patterns and overarching narratives/stories in life events that occur inside and outside of the laboratory (257, 258). In turn, there is always a risk of “homogenizing” and effectively overinterpreting experimental findings in the context of the broader literature accordingly. The perspectives conveyed in this manuscript are not necessarily an exception. As always, the reader is encouraged to read and consider all cited studies independently while locating ways to challenge and/or complement current working paradigms stated in the text, tables, and figures. Next, although seldom studied, there are indeed notable species differences even among rodents themselves as mice, rats, and hamsters of the same sex and similar age. For example, third-order mesenteric arteries of the standard C57BL/6J mouse are less responsive to agonist (phenylephrine) activation of α1AARs and vasoconstriction relative to the Wistar rat (259). In response to stimulation of perivascular sympathetic nerves, C57BL/6J mice engage both ARs and purinergic receptors for vasoconstriction, whereas arteries from the Wistar rat do not involve purinergic receptors (259). Furthermore, functional presence of the α1DAR subtype during phenylephrine-mediated constriction is evident in cremasteric arterioles of male golden hamsters (260) but not in mesenteric arteries of male mice and rats (259). With gleaning from this very small, directed comparison showing significant differences in adrenergic activity among resistance arteries of one sex (male) and age group (young) of rodent species alone, it is not a surprise as to why there are “failed” human clinical trials for new drugs to treat age-related cardiovascular diseases (e.g., hypertension) and dementia. In fact, one could argue that any success to be had for extracting meaning from biomedical research toward the application of therapy is at least partially fortuitous. Hopefully, where reasonable, the current literature review also adds awareness and resolution to the details of various studies of vascular aging so that luck, coincidence, and disingenuous accounts are minimized in studies moving forward for progress.
SUMMARY, CONCLUSIONS, AND FUTURE DIRECTIONS
Gerontology experts have projected that individuals of old age (≥65 yr) will compose ∼20% of the US population by 2030 (261). Aging is the key risk factor for chronic conditions throughout the world such as cardiovascular disease (2). In such manner, aging is also the source of numerous morbidities such as sarcopenia, chronic kidney disease, hypertension, heart failure, diabetes, stroke, and dementia (3). Effective therapeutic targets of vascular aging entail ion channels among the smooth muscle and endothelial layers of resistance arteries throughout the body. With some distinct differences in vascular receptor and ion channel arrangements, a common theme of aging includes altered function of TRP and K+ channels and imbalanced local and conducted signal coordination, leaving the health of major organs at risk. In addition, there is a disturbance among the optimal signaling counterbalances of smooth muscle cells and endothelial cells via myoendothelial coupling needed to stabilize a healthy vascular resistance throughout conditions of rest and physical activity.
Numerous knowledge gaps remain concerning vascular aging throughout the body and role of various ion channels governing the electrical and metabolic signals for blood flow control (Table 2). Sex as a biological variable, and particularly data for female animals, has still not been sufficiently met for a comprehensive understanding of the cardiovascular system during aging and the development of disease. As a big picture consideration, how should we distribute strategies for treatment among cardiopulmonary impairment, aorta and large artery stiffening, microcirculatory dysfunction, and end-organ damage that occur with aging? Can novel therapeutic applications such as novel histone deacetylase inhibitors (262), pharmacological ion channel openers (263), novel vasoactive gases (70), mitochondria-targeted antioxidants (264), or BH4 and vitamin C supplements (265) be used to restore NO bioavailability and/or activate endothelial SKCa and IKCa channels without impacting cell-to-cell coupling? Other more fundamental questions include: what direct (receptor/ion channel expression) and indirect (e.g., posttranslational modifications) roles do vascular miRNAs play for vascular health during aging? How does the function of myoendothelial gap junctions alter across individual organs for aging and various chronic diseases? Relative to other ion channels (e.g., KCa), how do endothelial KIR2 (266) and KATP (267) channels shape electrical conduction of hyperpolarization (vasodilation) and depolarization (vasoconstriction) through gap junctions? How does the function of novel cation nonselective ion channels [e.g., PIEZO1 (268)] alter with aging? With aging and chronic disease pathology such as hypertension and development of dementia, what mechanistic changes are inherent to vascular ion channels themselves versus a spatial localization/separation from otherwise intact cell signaling events for ion channel activation/inhibition (269)? What relative contributions to energy production are made via glycolysis versus oxidative phosphorylation in ECs and SMCs across organs during aging? Finally, outside of the blood circulation, there is consideration for another type of contractile vessel in the body as lymphatics that control tissue fluid homeostasis and immunity. How do their cellular functions change with the aging process while equipped with a fundamentally different structure and signaling arrangement relative to blood vessels (79, 270)? These are just a few research questions to consider as we continue to resolve mechanisms of healthy aging throughout a standard lifespan.
Table 2.
A select list of outstanding research questions remaining in the field of vascular aging for individual major organ systems and the body as a whole
| Remaining Questions/Knowledge Gaps for Vascular Aging |
|---|
| Brain |
|
|
|
|
|
|
|
| Eye |
|
|
|
| Skeletal muscle |
|
| Heart |
|
| Lung |
|
| Mesentery |
|
|
|
| Kidney |
|
|
|
| Skin |
|
|
| Whole body |
|
|
|
|
|
|
|
|
|
|
|
|
BKCa, high-conductance Ca2+-activated K+ channels; CO, carbon monoxide; H2S, hydrogen sulfide; IKCa channels, intermediate-conductance Ca2+-activated K+ channels; KATP channels, ATP-sensitive K+ channels; KIR2 channels, inward-rectifying K+ channel; ROS, reactive oxygen species; SKCa channels, small-conductance Ca2+-activated K+ channels; TRP channels, transient receptor potential channels; TRPA, TRP ankyrin class TRPC, TRP canonical class; TRPM, TRP melastatin class; TRPV, TRP vanilloid class.
GRANTS
This work was supported by National Institute on Aging Grant R01AG073230 (to E.J.B.).
DISCLAIMERS
The content of this original article is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
E.J.B. prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
REFERENCES
- 1.Observations on the Harveian doctrine of the circulation of the blood. Edinb Med Surg J 13: 501–507, 1817. [PMC free article] [PubMed] [Google Scholar]
- 2. Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol 80: 2361–2371, 2022. doi: 10.1016/j.jacc.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 3. Divo MJ, Martinez CH, Mannino DM. Ageing and the epidemiology of multimorbidity. Eur Respir J 44: 1055–1068, 2014. doi: 10.1183/09031936.00059814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Camici GG, Savarese G, Akhmedov A, Lüscher TF. Molecular mechanism of endothelial and vascular aging: implications for cardiovascular disease. Eur Heart J 36: 3392–3403, 2015. doi: 10.1093/eurheartj/ehv587. [DOI] [PubMed] [Google Scholar]
- 5. Bloom SI, Islam MT, Lesniewski LA, Donato AJ. Mechanisms and consequences of endothelial cell senescence. Nat Rev Cardiol 20: 38–51, 2023. doi: 10.1038/s41569-022-00739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paneni F, Diaz Canestro C, Libby P, Lüscher TF, Camici GG. The aging cardiovascular system: understanding it at the cellular and clinical levels. J Am Coll Cardiol 69: 1952–1967, 2017. doi: 10.1016/j.jacc.2017.01.064. [DOI] [PubMed] [Google Scholar]
- 7. Fleenor BS, Carlini NA, Martens CR. Nutraceuticals in the prevention and therapeutic treatment of cardiovascular and cerebrovascular disease. J Cardiopulm Rehabil Prev 43: 162–169, 2023. doi: 10.1097/HCR.0000000000000773. [DOI] [PubMed] [Google Scholar]
- 8. Winder NR, Reeve EH, Walker AE. Large artery stiffness and brain health: insights from animal models. Am J Physiol Heart Circ Physiol 320: H424–H431, 2021. doi: 10.1152/ajpheart.00696.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ogola BO, Abshire CM, Visniauskas B, Kiley JX, Horton AC, Clark-Patterson GL, Kilanowski-Doroh I, Diaz Z, Bicego AN, McNally AB, Zimmerman MA, Groban L, Trask AJ, Miller KS, Lindsey SH. Sex differences in vascular aging and impact of GPER deletion. Am J Physiol Heart Circ Physiol 323: H336–H349, 2022. doi: 10.1152/ajpheart.00238.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kehmeier MN, Walker AE. Sex differences in large artery stiffness: implications for cerebrovascular dysfunction and Alzheimer’s disease. Front Aging 2: 791208, 2021. doi: 10.3389/fragi.2021.791208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hearon CM Jr, Richards JC, Racine ML, Luckasen GJ, Larson DG, Dinenno FA. Amplification of endothelium-dependent vasodilatation in contracting human skeletal muscle: role of KIR channels. J Physiol 597: 1321–1335, 2019. doi: 10.1113/JP276998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Vasodilatory responsiveness to adenosine triphosphate in ageing humans. J Physiol 588: 4017–4027, 2010. doi: 10.1113/jphysiol.2010.197814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. ATP-mediated vasodilatation occurs via activation of inwardly rectifying potassium channels in humans. J Physiol 590: 5349–5359, 2012. doi: 10.1113/jphysiol.2012.234245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Serviente C, Berry CW, Kenney WL, Alexander LM. Healthy active older adults have enhanced K+ channel-dependent endothelial vasodilatory mechanisms. Am J Physiol Regul Integr Comp Physiol 319: R19–R25, 2020. doi: 10.1152/ajpregu.00049.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Craighead DH, Alexander LM. Menthol-induced cutaneous vasodilation is preserved in essential hypertensive men and women. Am J Hypertens 30: 1156–1162, 2017. doi: 10.1093/ajh/hpx127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, Chonchol M, Seals DR. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun 9: 1286, 2018. doi: 10.1038/s41467-018-03421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Behringer EJ. Calcium and electrical signaling in arterial endothelial tubes: new insights into cellular physiology and cardiovascular function. Microcirculation 24: 10.1111/micc.12328, 2017. doi: 10.1111/micc.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Behringer EJ, Hakim MA. Functional interaction among KCa and TRP channels for cardiovascular physiology: modern perspectives on aging and chronic disease. Int J Mol Sci 20: 1380, 2019. doi: 10.3390/ijms20061380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Behringer EJ, Segal SS. Spreading the signal for vasodilatation: implications for skeletal muscle blood flow control and the effects of ageing. J Physiol 590: 6277–6284, 2012. doi: 10.1113/jphysiol.2012.239673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harraz OF, Jensen LJ. Aging, calcium channel signaling and vascular tone. Mech Ageing Dev 191: 111336, 2020. doi: 10.1016/j.mad.2020.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harraz OF, Jensen LJ. Vascular calcium signalling and ageing. J Physiol 599: 5361–5377, 2021. doi: 10.1113/JP280950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Tabula Muris Consortium. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 583: 590–595, 2020. doi: 10.1038/s41586-020-2496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parab S, Setten E, Astanina E, Bussolino F, Doronzo G. The tissue-specific transcriptional landscape underlines the involvement of endothelial cells in health and disease. Pharmacol Ther 246: 108418, 2023. doi: 10.1016/j.pharmthera.2023.108418. [DOI] [PubMed] [Google Scholar]
- 24. Fox JG, Barthold S, Davisson M, Newcomer CE, Quimby FW, Smith A. The Mouse in Biomedical Research (2nd ed.). Amsterdam; Boston: Elsevier, 2007. [Google Scholar]
- 25. King DR, Sedovy MW, Eaton X, Dunaway LS, Good ME, Isakson BE, Johnstone SR. Cell-to-cell communication in the resistance vasculature. Compr Physiol 12: 3833–3867, 2022. doi: 10.1002/cphy.c210040. [DOI] [PubMed] [Google Scholar]
- 26. Bagher P, Segal SS. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol (Oxf) 202: 271–284, 2011. doi: 10.1111/j.1748-1716.2010.02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Segal SS. Integration and modulation of intercellular signaling underlying blood flow control. J Vasc Res 52: 136–157, 2015. doi: 10.1159/000439112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garland CJ, Dora KA. EDH: endothelium-dependent hyperpolarization and microvascular signalling. Acta Physiol (Oxf) 219: 152–161, 2017. doi: 10.1111/apha.12649. [DOI] [PubMed] [Google Scholar]
- 29. Aird WC. Spatial and temporal dynamics of the endothelium. J Thromb Haemost 3: 1392–1406, 2005. doi: 10.1111/j.1538-7836.2005.01328.x. [DOI] [PubMed] [Google Scholar]
- 30. Fishman AP. Endothelium: a distributed organ of diverse capabilities. Ann N Y Acad Sci 401: 1–8, 1982. doi: 10.1111/j.1749-6632.1982.tb25702.x. [DOI] [PubMed] [Google Scholar]
- 31. Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res 87: 474–479, 2000. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 32. Garland CJ, Bagher P, Powell C, Ye X, Lemmey HAL, Borysova L, Dora KA. Voltage-dependent Ca2+ entry into smooth muscle during contraction promotes endothelium-mediated feedback vasodilation in arterioles. Sci Signal 10: eaal3806, 2017. doi: 10.1126/scisignal.aal3806. [DOI] [PubMed] [Google Scholar]
- 33. Behringer EJ, Segal SS. Impact of aging on calcium signaling and membrane potential in endothelium of resistance arteries: a role for mitochondria. J Gerontol A Biol Sci Med Sci 72: 1627–1637, 2017. doi: 10.1093/gerona/glx079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Behringer EJ, Segal SS. Membrane potential governs calcium influx into microvascular endothelium: integral role for muscarinic receptor activation. J Physiol 593: 4531–4548, 2015. doi: 10.1113/JP271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dietrich A, Gudermann T. TRP channels in the cardiopulmonary vasculature. Adv Exp Med Biol 704: 781–810, 2011. doi: 10.1007/978-94-007-0265-3_41. [DOI] [PubMed] [Google Scholar]
- 36. Yue Z, Xie J, Yu AS, Stock J, Du J, Yue L. Role of TRP channels in the cardiovascular system. Am J Physiol Heart Circ Physiol 308: H157–H182, 2015. doi: 10.1152/ajpheart.00457.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gees M, Colsoul B, Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol 2: a003962, 2010. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Behringer EJ, Vanterpool CK, Pearce WJ, Wilson SM, Buchholz JN. Advancing age alters the contribution of calcium release from smooth endoplasmic reticulum stores in superior cervical ganglion cells. J Gerontol A Biol Sci Med Sci 64: 34–44, 2009. doi: 10.1093/gerona/gln053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heathcote HR, Lee MD, Zhang X, Saunter CD, Wilson C, McCarron JG. Endothelial TRPV4 channels modulate vascular tone by Ca2+-induced Ca2+ release at inositol 1,4,5-trisphosphate receptors. Br J Pharmacol 176: 3297–3317, 2019. doi: 10.1111/bph.14762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97: 1270–1279, 2005. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 41. Abd El-Rahman RR, Harraz OF, Brett SE, Anfinogenova Y, Mufti RE, Goldman D, Welsh DG. Identification of L- and T-type Ca2+ channels in rat cerebral arteries: role in myogenic tone development. Am J Physiol Heart Circ Physiol 304: H58–H71, 2013. doi: 10.1152/ajpheart.00476.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jensen LJ, Salomonsson M, Jensen BL, Holstein-Rathlou N-H. Depolarization-induced calcium influx in rat mesenteric small arterioles is mediated exclusively via mibefradil-sensitive calcium channels. Br J Pharmacol 142: 709–718, 2004. doi: 10.1038/sj.bjp.0705841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tran CHT, Taylor MS, Plane F, Nagaraja S, Tsoukias NM, Solodushko V, Vigmond EJ, Furstenhaupt T, Brigdan M, Welsh DG. Endothelial Ca2+ wavelets and the induction of myoendothelial feedback. Am J Physiol Cell Physiol 302: C1226–C1242, 2012. doi: 10.1152/ajpcell.00418.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Westcott EB, Segal SS. Perivascular innervation: a multiplicity of roles in vasomotor control and myoendothelial signaling. Microcirculation 20: 217–238, 2013. doi: 10.1111/micc.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mishra RC, Rahman MM, Davis MJ, Wulff H, Hill MA, Braun AP. Alpha(1)-adrenergic stimulation selectively enhances endothelium-mediated vasodilation in rat cremaster arteries. Physiol Rep 6: e13703, 2018. doi: 10.14814/phy2.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci USA 94: 6529–6534, 1997. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garcia V, Sessa WC. Endothelial NOS: perspective and recent developments. Br J Pharmacol 176: 189–196, 2019. doi: 10.1111/bph.14522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harraz OF, Brett SE, Welsh DG. Nitric oxide suppresses vascular voltage-gated T-type Ca2+ channels through cGMP/PKG signaling. Am J Physiol Heart Circ Physiol 306: H279–H285, 2014. doi: 10.1152/ajpheart.00743.2013. [DOI] [PubMed] [Google Scholar]
- 49. Lalli MJ, Shimizu S, Sutliff RL, Kranias EG, Paul RJ. [Ca2+]i homeostasis and cyclic nucleotide relaxation in aorta of phospholamban-deficient mice. Am J Physiol Heart Circ Physiol 277: H963–H970, 1999. doi: 10.1152/ajpheart.1999.277.3.H963. [DOI] [PubMed] [Google Scholar]
- 50. Sausbier M, Schubert R, Voigt V, Hirneiss C, Pfeifer A, Korth M, Kleppisch T, Ruth P, Hofmann F. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ Res 87: 825–830, 2000. doi: 10.1161/01.res.87.9.825. [DOI] [PubMed] [Google Scholar]
- 51. Jackson WF. Endothelial ion channels and cell-cell communication in the microcirculation. Front Physiol 13: 805149, 2022. doi: 10.3389/fphys.2022.805149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 265: C299–C303, 1993. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- 53. Tare M, Parkington HC, Coleman HA, Neild TO, Dusting GJ. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature 346: 69–71, 1990. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- 54. Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha. Science 286: 1583–1587, 1999. doi: 10.1126/science.286.5444.1583. [DOI] [PubMed] [Google Scholar]
- 55. Cole WC, Welsh DG. Role of myosin light chain kinase and myosin light chain phosphatase in the resistance arterial myogenic response to intravascular pressure. Arch Biochem Biophys 510: 160–173, 2011. doi: 10.1016/j.abb.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 56. Leffler CW, Parfenova H, Jaggar JH. Carbon monoxide as an endogenous vascular modulator. Am J Physiol Heart Circ Physiol 301: H1–H11, 2011. doi: 10.1152/ajpheart.00230.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Christodoulides N, Durante W, Kroll MH, Schafer AI. Vascular smooth muscle cell heme oxygenases generate guanylyl cyclase-stimulatory carbon monoxide. Circulation 91: 2306–2309, 1995. doi: 10.1161/01.cir.91.9.2306. [DOI] [PubMed] [Google Scholar]
- 58. Samora JB, Goodwill AG, Frisbee JC, Boegehold MA. Growth-dependent changes in the contribution of carbon monoxide to arteriolar function. J Vasc Res 47: 23–34, 2010. doi: 10.1159/000231718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Durante W, Kroll MH, Christodoulides N, Peyton KJ, Schafer AI. Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ Res 80: 557–564, 1997. doi: 10.1161/01.res.80.4.557. [DOI] [PubMed] [Google Scholar]
- 60. Durante W, Christodoulides N, Cheng K, Peyton KJ, Sunahara RK, Schafer AI. cAMP induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle. Am J Physiol Heart Circ Physiol 273: H317–H323, 1997. doi: 10.1152/ajpheart.1997.273.1.H317. [DOI] [PubMed] [Google Scholar]
- 61. Durante W, Peyton KJ, Schafer AI. Platelet-derived growth factor stimulates heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 19: 2666–2672, 1999. doi: 10.1161/01.atv.19.11.2666. [DOI] [PubMed] [Google Scholar]
- 62. Parfenova H, Neff RA 3rd, Alonso JS, Shlopov BV, Jamal CN, Sarkisova SA, Leffler CW. Cerebral vascular endothelial heme oxygenase: expression, localization, and activation by glutamate. Am J Physiol Cell Physiol 281: C1954–C1963, 2001. doi: 10.1152/ajpcell.2001.281.6.C1954. [DOI] [PubMed] [Google Scholar]
- 63. Boycott HE, Dallas ML, Elies J, Pettinger L, Boyle JP, Scragg JL, Gamper N, Peers C. Carbon monoxide inhibition of Cav3.2 T-type Ca2+ channels reveals tonic modulation by thioredoxin. FASEB J 27: 3395–3407, 2013. doi: 10.1096/fj.13-227249. [DOI] [PubMed] [Google Scholar]
- 64. Xi Q, Tcheranova D, Parfenova H, Horowitz B, Leffler CW, Jaggar JH. Carbon monoxide activates KCa channels in newborn arteriole smooth muscle cells by increasing apparent Ca2+ sensitivity of alpha-subunits. Am J Physiol Heart Circ Physiol 286: H610–C618, 2004. doi: 10.1152/ajpheart.00782.2003. [DOI] [PubMed] [Google Scholar]
- 65. Jaggar JH, Leffler CW, Cheranov SY, Tcheranova D, E S, Cheng X. Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ Res 91: 610–617, 2002. [Erratum in Circ Res 91: e34, 2002]. doi: 10.1161/01.res.0000036900.76780.95. [DOI] [PubMed] [Google Scholar]
- 66. Harraz OF, Abd El-Rahman RR, Bigdely-Shamloo K, Wilson SM, Brett SE, Romero M, Gonzales AL, Earley S, Vigmond EJ, Nygren A, Menon BK, Mufti RE, Watson T, Starreveld Y, Furstenhaupt T, Muellerleile PR, Kurjiaka DT, Kyle BD, Braun AP, Welsh DG. CaV3.2 channels and the induction of negative feedback in cerebral arteries. Circ Res 115: 650–661, 2014. doi: 10.1161/CIRCRESAHA.114.304056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Harraz OF, Brett SE, Zechariah A, Romero M, Puglisi JL, Wilson SM, Welsh DG. Genetic ablation of CaV3.2 channels enhances the arterial myogenic response by modulating the RyR-BKCa axis. Arterioscler Thromb Vasc Biol 35: 1843–1851, 2015. doi: 10.1161/ATVBAHA.115.305736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kanagy NL, Kevil CG. The pleiotropic effects of hydrogen sulfide. Am J Physiol Heart Circ Physiol 314: H1–H2, 2018. doi: 10.1152/ajpheart.00613.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shen X, Carlstrom M, Borniquel S, Jadert C, Kevil CG, Lundberg JO. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med 60: 195–200, 2013. doi: 10.1016/j.freeradbiomed.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Huerta de la Cruz S, Medina-Terol GJ, Tapia-Martinez JA, Silva-Velasco DL, Beltran-Ornelas JH, Sanchez-Lopez A, Sancho M, Centurion D. Hydrogen sulfide as a neuromodulator of the vascular tone. Eur J Pharmacol 940: 175455, 2023. doi: 10.1016/j.ejphar.2022.175455. [DOI] [PubMed] [Google Scholar]
- 71. Naik JS, Osmond JM, Walker BR, Kanagy NL. Hydrogen sulfide-induced vasodilation mediated by endothelial TRPV4 channels. Am J Physiol Heart Circ Physiol 311: H1437–H1444, 2016. doi: 10.1152/ajpheart.00465.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Garcia DCG, Longden TA. Ion channels in capillary endothelium. Curr Top Membr 85: 261–300, 2020. doi: 10.1016/bs.ctm.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 73. Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, Hill-Eubanks D, Nelson MT. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci 20: 717–726, 2017. doi: 10.1038/nn.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schumacher MA, Rivard AF, Bachinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature 410: 1120–1124, 2001. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- 75. Fanger CM, Ghanshani S, Logsdon NJ, Rauer H, Kalman K, Zhou J, Beckingham K, Chandy KG, Cahalan MD, Aiyar J. Calmodulin mediates calcium-dependent activation of the intermediate conductance KCa channel, IKCa1. J Biol Chem 274: 5746–5754, 1999. doi: 10.1074/jbc.274.9.5746. [DOI] [PubMed] [Google Scholar]
- 76. Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res 86: 94–100, 2000. doi: 10.1161/01.res.86.1.94. [DOI] [PubMed] [Google Scholar]
- 77. Garland CJ, Dora KA. Endothelium-dependent hyperpolarization: the evolution of myoendothelial microdomains. J Cardiovasc Pharmacol 78: S3–S12, 2021. doi: 10.1097/FJC.0000000000001087. [DOI] [PubMed] [Google Scholar]
- 78. Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol 268: C799–C822, 1995. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 79. Behringer EJ, Scallan JP, Jafarnejad M, Castorena-Gonzalez JA, Zawieja SD, Moore JE Jr, Davis MJ, Segal SS. Calcium and electrical dynamics in lymphatic endothelium. J Physiol 595: 7347–7368, 2017. doi: 10.1113/JP274842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Harraz OF, Longden TA, Hill-Eubanks D, Nelson MT. PIP2 depletion promotes TRPV4 channel activity in mouse brain capillary endothelial cells. eLife 7: e38689, 2018. doi: 10.7554/eLife.38689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Muller-Delp JM, Gurovich AN, Christou DD, Leeuwenburgh C. Redox balance in the aging microcirculation: new friends, new foes, and new clinical directions. Microcirculation 19: 19–28, 2012. doi: 10.1111/j.1549-8719.2011.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 33: 829–837, 2012. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278: 22546–22554, 2003. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 85. Groschner LN, Waldeck-Weiermair M, Malli R, Graier WF. Endothelial mitochondria–less respiration, more integration. Pflugers Arch 464: 63–76, 2012. doi: 10.1007/s00424-012-1085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wilson C, Lee MD, Buckley C, Zhang X, McCarron JG. Mitochondrial ATP production is required for endothelial cell control of vascular tone. Function (Oxf) 4: zqac063, 2023. doi: 10.1093/function/zqac063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wilson C, Lee MD, Heathcote HR, Zhang X, Buckley C, Girkin JM, Saunter CD, McCarron JG. Mitochondrial ATP production provides long-range control of endothelial inositol trisphosphate-evoked calcium signaling. J Biol Chem 294: 737–758, 2019. doi: 10.1074/jbc.RA118.005913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhang X, Lee MD, Wilson C, McCarron JG. Hydrogen peroxide depolarizes mitochondria and inhibits IP3-evoked Ca2+ release in the endothelium of intact arteries. Cell Calcium 84: 102108, 2019. doi: 10.1016/j.ceca.2019.102108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chalmers S, Saunter CD, Girkin JM, McCarron JG. Age decreases mitochondrial motility and increases mitochondrial size in vascular smooth muscle. J Physiol 594: 4283–4295, 2016. doi: 10.1113/JP271942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sakamuri SSVP, Sure VN, Kolli L, Liu N, Evans WR, Sperling JA, Busija DW, Wang X, Lindsey SH, Murfee WL, Mostany R, Katakam PVG. Glycolytic and oxidative phosphorylation defects precede the development of senescence in primary human brain microvascular endothelial cells. Geroscience 44: 1975–1994, 2022. doi: 10.1007/s11357-022-00550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sure VN, Sakamuri SSVP, Sperling JA, Evans WR, Merdzo I, Mostany R, Murfee WL, Busija DW, Katakam PVG. A novel high-throughput assay for respiration in isolated brain microvessels reveals impaired mitochondrial function in the aged mice. Geroscience 40: 365–375, 2018. doi: 10.1007/s11357-018-0037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rutkai I, Evans WR, Bess N, Salter-Cid T, Cikic S, Chandra PK, Katakam PVG, Mostany R, Busija DW. Chronic imaging of mitochondria in the murine cerebral vasculature using in vivo two-photon microscopy. Am J Physiol Heart Circ Physiol 318: H1379–H1386, 2020. doi: 10.1152/ajpheart.00751.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Schreckenberger ZJ, Wenceslau CF, Joe B, McCarthy CG. Mitophagy in hypertension-associated premature vascular aging. Am J Hypertens 33: 804–812, 2020. doi: 10.1093/ajh/hpaa058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hakim MA, Chum PP, Buchholz JN, Behringer EJ. Aging alters cerebrovascular endothelial GPCR and K+ channel function: divergent role of biological sex. J Gerontol A Biol Sci Med Sci 75: 2064–2073, 2020. doi: 10.1093/gerona/glz275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sancho M, Gao Y, Hald BO, Yin H, Boulton M, Steven DA, MacDougall KW, Parrent AG, Pickering JG, Welsh DG. An assessment of KIR channel function in human cerebral arteries. Am J Physiol Heart Circ Physiol 316: H794–H800, 2019. doi: 10.1152/ajpheart.00022.2019. [DOI] [PubMed] [Google Scholar]
- 96. Hayoz S, Pettis J, Bradley V, Segal SS, Jackson WF. Increased amplitude of inward rectifier K+ currents with advanced age in smooth muscle cells of murine superior epigastric arteries. Am J Physiol Heart Circ Physiol 312: H1203–H1214, 2017. doi: 10.1152/ajpheart.00679.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Crane GJ, Walker SD, Dora KA, Garland CJ. Evidence for a differential cellular distribution of inward rectifier K channels in the rat isolated mesenteric artery. J Vasc Res 40: 159–168, 2003. doi: 10.1159/000070713. [DOI] [PubMed] [Google Scholar]
- 98. Kowalewska PM, Fletcher J, Jackson WF, Brett SE, Kim MS, Mironova GY, Haghbin N, Richter DM, Tykocki NR, Nelson MT, Welsh DG. Genetic ablation of smooth muscle KIR2.1 is inconsequential to the function of mouse cerebral arteries. J Cereb Blood Flow Metab 42: 1693–1706, 2022. doi: 10.1177/0271678X221093432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bradley KK, Jaggar JH, Bonev AD, Heppner TJ, Flynn ER, Nelson MT, Horowitz B. Kir2.1 encodes the inward rectifier potassium channel in rat arterial smooth muscle cells. J Physiol 515: 639–651, 1999. doi: 10.1111/j.1469-7793.1999.639ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chaston DJ, Baillie BK, Grayson TH, Courjaret RJ, Heisler JM, Lau KA, Machaca K, Nicholson BJ, Ashton A, Matthaei KI, Hill CE. Polymorphism in endothelial connexin40 enhances sensitivity to intraluminal pressure and increases arterial stiffness. Arterioscler Thromb Vasc Biol 33: 962–970, 2013. doi: 10.1161/ATVBAHA.112.300957. [DOI] [PubMed] [Google Scholar]
- 101. Diaz-Otero JM, Garver H, Fink GD, Jackson WF, Dorrance AM. Aging is associated with changes to the biomechanical properties of the posterior cerebral artery and parenchymal arterioles. Am J Physiol Heart Circ Physiol 310: H365–H375, 2016. doi: 10.1152/ajpheart.00562.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hayoz S, Bradley V, Boerman EM, Nourian Z, Segal SS, Jackson WF. Aging increases capacitance and spontaneous transient outward current amplitude of smooth muscle cells from murine superior epigastric arteries. Am J Physiol Heart Circ Physiol 306: H1512–H1524, 2014. doi: 10.1152/ajpheart.00492.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tumer N, Toklu HZ, Muller-Delp JM, Oktay S, Ghosh P, Strang K, Delp MD, Scarpace PJ. The effects of aging on the functional and structural properties of the rat basilar artery. Physiol Rep 2: e12031, 2014. doi: 10.14814/phy2.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Aguilar V, Paul A, Lazarko D, Levitan I. Paradigms of endothelial stiffening in cardiovascular disease and vascular aging. Front Physiol 13: 1081119, 2023. doi: 10.3389/fphys.2022.1081119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Musch TI, Friedman DB, Pitetti KH, Haidet GC, Stray-Gundersen J, Mitchell JH, Ordway GA. Regional distribution of blood flow of dogs during graded dynamic exercise. J Appl Physiol (1985) 63: 2269–2277, 1987. doi: 10.1152/jappl.1987.63.6.2269. [DOI] [PubMed] [Google Scholar]
- 106. Reed JT, Pareek T, Sriramula S, Pabbidi MR. Aging influences cerebrovascular myogenic reactivity and BK channel function in a sex-specific manner. Cardiovasc Res 116: 1372–1385, 2020. doi: 10.1093/cvr/cvz314. [DOI] [PubMed] [Google Scholar]
- 107. Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Schwartzman ML, Sonntag WE, Ungvari Z. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol 305: H1698–H1708, 2013. doi: 10.1152/ajpheart.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chambers LC, Yen M, Jackson WF, Dorrance AM. Female mice are protected from impaired parenchymal arteriolar TRPV4 function and impaired cognition in hypertension. Am J Physiol Heart Circ Physiol 324: H581–H597, 2023. doi: 10.1152/ajpheart.00481.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hakim MA, Behringer EJ. Development of Alzheimer’s disease progressively alters sex-dependent KCa and Sex-independent KIR channel function in cerebrovascular endothelium. J Alzheimers Dis 76: 1423–1442, 2020. doi: 10.3233/JAD-200085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Peters EC, Gee MT, Pawlowski LN, Kath AM, Polk FD, Vance CJ, Sacoman JL, Pires PW. Amyloid-beta disrupts unitary calcium entry through endothelial NMDA receptors in mouse cerebral arteries. J Cereb Blood Flow Metab 42: 145–161, 2022. doi: 10.1177/0271678X211039592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mughal A, Harraz OF, Gonzales AL, Hill-Eubanks D, Nelson MT. PIP2 improves cerebral blood flow in a mouse model of Alzheimer’s disease. Function (Oxf) 2: zqab010, 2021. doi: 10.1093/function/zqab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang L, Papadopoulos P, Hamel E. Endothelial TRPV4 channels mediate dilation of cerebral arteries: impairment and recovery in cerebrovascular pathologies related to Alzheimer’s disease. Br J Pharmacol 170: 661–670, 2013. doi: 10.1111/bph.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lacalle-Aurioles M, Trigiani LJ, Bourourou M, Lecrux C, Hamel E. Alzheimer's disease and cerebrovascular pathology alter inward rectifier potassium (KIR2.1) channels in endothelium of mouse cerebral arteries. Br J Pharmacol 179: 2259–2274, 2022. [Erratum in Br J Pharmacol 179: 4458, 2022]. doi: 10.1111/bph.15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. O'Hare M, Esquiva G, McGahon MK, Hombrebueno JMR, Augustine J, Canning P, Edgar KS, Barabas P, Friedel T, Cincola P, Henry J, Mayne K, Ferrin H, Stitt AW, Lyons TJ, Brazil DP, Grieve DJ, McGeown JG, Curtis TM. Loss of TRPV2-mediated blood flow autoregulation recapitulates diabetic retinopathy in rats. JCI Insight 7: e155128, 2022. doi: 10.1172/jci.insight.155128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Behringer EJ, Shaw RL, Westcott EB, Socha MJ, Segal SS. Aging impairs electrical conduction along endothelium of resistance arteries through enhanced Ca2+-activated K+ channel activation. Arterioscler Thromb Vasc Biol 33: 1892–1901, 2013. doi: 10.1161/ATVBAHA.113.301514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kang LS, Kim S, Dominguez JM 2nd, Sindler AL, Dick GM, Muller-Delp JM. Aging and muscle fiber type alter K+ channel contributions to the myogenic response in skeletal muscle arterioles. J Appl Physiol (1985) 107: 389–398, 2009. doi: 10.1152/japplphysiol.91245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chadha PS, Haddock RE, Howitt L, Morris MJ, Murphy TV, Grayson TH, Sandow SL. Obesity up-regulates intermediate conductance calcium-activated potassium channels and myoendothelial gap junctions to maintain endothelial vasodilator function. J Pharmacol Exp Ther 335: 284–293, 2010. doi: 10.1124/jpet.110.167593. [DOI] [PubMed] [Google Scholar]
- 118. Feher A, Broskova Z, Bagi Z. Age-related impairment of conducted dilation in human coronary arterioles. Am J Physiol Heart Circ Physiol 306: H1595–H1601, 2014. doi: 10.1152/ajpheart.00179.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Choi S, Kim JA, Li H-Y, Shin K-O, Oh GT, Lee Y-M, Oh S, Pewzner-Jung Y, Futerman AH, Suh SH. KCa3.1 upregulation preserves endothelium-dependent vasorelaxation during aging and oxidative stress. Aging Cell 15: 801–810, 2016. doi: 10.1111/acel.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Climent B, Moreno L, Martinez P, Contreras C, Sanchez A, Perez-Vizcaino F, Garcia-Sacristan A, Rivera L, Prieto D. Upregulation of SK3 and IK1 channels contributes to the enhanced endothelial calcium signaling and the preserved coronary relaxation in obese Zucker rats. PLoS One 9: e109432, 2014. doi: 10.1371/journal.pone.0109432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yada T, Shimokawa H, Tachibana H. Endothelium-dependent hyperpolarization-mediated vasodilatation compensates nitric oxide-mediated endothelial dysfunction during ischemia in diabetes-induced canine coronary collateral microcirculation in vivo. Microcirculation 25: e12456, 2018. doi: 10.1111/micc.12456. [DOI] [PubMed] [Google Scholar]
- 122. Blum-Johnston C, Thorpe RB, Wee C, Opsahl R, Romero M, Murray S, Brunelle A, Blood Q, Wilson R, Blood AB, Zhang L, Longo LD, Pearce WJ, Wilson SM. Long-term hypoxia uncouples Ca2+ and eNOS in bradykinin-mediated pulmonary arterial relaxation. Am J Physiol Regul Integr Comp Physiol 314: R870–R882, 2018. doi: 10.1152/ajpregu.00311.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bonnet S, Savineau J-P, Barillot W, Dubuis E, Vandier C, Bonnet P. Role of Ca2+-sensitive K+ channels in the remission phase of pulmonary hypertension in chronic obstructive pulmonary diseases. Cardiovasc Res 60: 326–336, 2003. doi: 10.1016/s0008-6363(03)00527-3. [DOI] [PubMed] [Google Scholar]
- 124. Remillard CV, Tigno DD, Platoshyn O, Burg ED, Brevnova EE, Conger D, Nicholson A, Rana BK, Channick RN, Rubin LJ, O'Connor DT, Yuan JX-J. Function of Kv1.5 channels and genetic variations of KCNA5 in patients with idiopathic pulmonary arterial hypertension. Am J Physiol Cell Physiol 292: C1837–C1853, 2007. doi: 10.1152/ajpcell.00405.2006. [DOI] [PubMed] [Google Scholar]
- 125. Bohnen MS, Ma L, Zhu N, Qi H, McClenaghan C, Gonzaga-Jauregui C, et al. Loss-of-function ABCC8 mutations in pulmonary arterial hypertension. Circ Genom Precis Med 11: e002087, 2018. doi: 10.1161/CIRCGEN.118.002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ma L, Roman-Campos D, Austin ED, Eyries M, Sampson KS, Soubrier F, Germain M, Tregouet DA, Borczuk A, Rosenzweig EB, Girerd B, Montani D, Humbert M, Loyd JE, Kass RS, Chung WK. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med 369: 351–361, 2013. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Goyal R, Angermann JE, Ostrovskaya O, Buchholz JN, Smith GD, Wilson SM. Enhanced capacitative calcium entry and sarcoplasmic-reticulum calcium storage capacity with advanced age in murine mesenteric arterial smooth muscle cells. Exp Gerontol 44: 201–207, 2009. doi: 10.1016/j.exger.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Giachini FR, Carneiro FS, Lima VV, Carneiro ZN, Dorrance A, Webb RC, Tostes RC. Upregulation of intermediate calcium-activated potassium channels counterbalance the impaired endothelium-dependent vasodilation in stroke-prone spontaneously hypertensive rats. Transl Res 154: 183–193, 2009. doi: 10.1016/j.trsl.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhang Y, Liao J, Zhang L, Li S, Wu Y, Shi L. BKCa channel activity and vascular contractility alterations with hypertension and aging via beta1 subunit promoter methylation in mesenteric arteries. Hypertens Res 41: 96–103, 2018. doi: 10.1038/hr.2017.96. [DOI] [PubMed] [Google Scholar]
- 130. Schach C, Resch M, Schmid PM, Riegger GA, Endemann DH. Type 2 diabetes: increased expression and contribution of IKCa channels to vasodilation in small mesenteric arteries of ZDF rats. Am J Physiol Heart Circ Physiol 307: H1093–H1102, 2014. doi: 10.1152/ajpheart.00240.2013. [DOI] [PubMed] [Google Scholar]
- 131. Lemmey HAL, Ye X, Ding HC, Triggle CR, Garland CJ, Dora KA. Hyperglycaemia disrupts conducted vasodilation in the resistance vasculature of db/db mice. Vascul Pharmacol 103–105: 29–35, 2018. doi: 10.1016/j.vph.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Stannov SU, Brasen JC, Salomonsson M, Holstein-Rathlou NH, Sorensen CM. Interactions between renal vascular resistance and endothelium-derived hyperpolarization in hypertensive rats in vivo. Physiol Rep 7: e14168, 2019. doi: 10.14814/phy2.14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Edgley AJ, Tare M, Evans RG, Skordilis C, Parkington HC. In vivo regulation of endothelium-dependent vasodilation in the rat renal circulation and the effect of streptozotocin-induced diabetes. Am J Physiol Regul Integr Comp Physiol 295: R829–R839, 2008. doi: 10.1152/ajpregu.00861.2007. [DOI] [PubMed] [Google Scholar]
- 134. Troncoso Brindeiro CM, Lane PH, Carmines PK. Tempol prevents altered K+ channel regulation of afferent arteriolar tone in diabetic rat kidney. Hypertension 59: 657–664, 2012. doi: 10.1161/HYPERTENSIONAHA.111.184218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Mokhtar SS, Vanhoutte PM, Leung SW, Yusof MI, Wan Sulaiman WA, Mat Saad AZ, Suppian R, Rasool AH. Endothelium dependent hyperpolarization-type relaxation compensates for attenuated nitric oxide-mediated responses in subcutaneous arteries of diabetic patients. Nitric Oxide 53: 35–44, 2016. doi: 10.1016/j.niox.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 136. Behringer EJ, Socha MJ, Polo-Parada L, Segal SS. Electrical conduction along endothelial cell tubes from mouse feed arteries: confounding actions of glycyrrhetinic acid derivatives. Br J Pharmacol 166: 774–787, 2012. doi: 10.1111/j.1476-5381.2011.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Billaud M, Lohman AW, Johnstone SR, Biwer LA, Mutchler S, Isakson BE. Regulation of cellular communication by signaling microdomains in the blood vessel wall. Pharmacol Rev 66: 513–569, 2014. doi: 10.1124/pr.112.007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 90: 559–605, 2010. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 139. Forrest AS, Joyce TC, Huebner ML, Ayon RJ, Wiwchar M, Joyce J, Freitas N, Davis AJ, Ye L, Duan DD, Singer CA, Valencik ML, Greenwood IA, Leblanc N. Increased TMEM16A-encoded calcium-activated chloride channel activity is associated with pulmonary hypertension. Am J Physiol Cell Physiol 303: C1229–C1243, 2012. doi: 10.1152/ajpcell.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Raichle ME, Gusnard DA. Appraising the brain’s energy budget. Proc Natl Acad Sci USA 99: 10237–10239, 2002. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Xing C-Y, Tarumi T, Liu J, Zhang Y, Turner M, Riley J, Tinajero CD, Yuan LJ, Zhang R. Distribution of cardiac output to the brain across the adult lifespan. J Cereb Blood Flow Metab 37: 2848–2856, 2017. doi: 10.1177/0271678X16676826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sancho M, Samson NC, Hald BO, Hashad AM, Marrelli SP, Brett SE, Welsh DG. KIR channels tune electrical communication in cerebral arteries. J Cereb Blood Flow Metab 37: 2171–2184, 2017. doi: 10.1177/0271678X16662041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Longden TA, Nelson MT. Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation 22: 183–196, 2015. doi: 10.1111/micc.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Hakim MA, Behringer EJ. Methyl-beta-cyclodextrin restores KIR channel function in brain endothelium of female Alzheimer’s disease mice. J Alzheimers Dis Rep 5: 693–703, 2021. doi: 10.3233/ADR-210016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Harraz OF, Longden TA, Dabertrand F, Hill-Eubanks D, Nelson MT. Endothelial GqPCR activity controls capillary electrical signaling and brain blood flow through PIP2 depletion. Proc Natl Acad Sci USA 115: E3569–E3577, 2018. doi: 10.1073/pnas.1800201115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Sancho M, Fabris S, Hald BO, Brett SE, Sandow SL, Poepping TL, Welsh DG. Membrane lipid-KIR2.x channel interactions enable hemodynamic sensing in cerebral arteries. Arterioscler Thromb Vasc Biol 39: 1072–1087, 2019. doi: 10.1161/ATVBAHA.119.312493. [DOI] [PubMed] [Google Scholar]
- 147. Fancher IS, Ahn SJ, Adamos C, Osborn C, Oh M-J, Fang Y, Reardon CA, Getz GS, Phillips SA, Levitan I. Hypercholesterolemia-induced loss of flow-induced vasodilation and lesion formation in Apolipoprotein E-deficient mice critically depend on inwardly rectifying K+ channels. J Am Heart Assoc 7: e007430, 2018. doi: 10.1161/JAHA.117.007430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Felix-Redondo FJ, Grau M, Fernandez-Berges D. Cholesterol and cardiovascular disease in the elderly. Facts and gaps. Aging Dis 4: 154–169, 2013. [PMC free article] [PubMed] [Google Scholar]
- 149. Kuppusamy M, Ottolini M, Sonkusare SK. Role of TRP ion channels in cerebral circulation and neurovascular communication. Neurosci Lett 765: 136258, 2021. doi: 10.1016/j.neulet.2021.136258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Negri S, Sanford M, Shi H, Tarantini S. The role of endothelial TRP channels in age-related vascular cognitive impairment and dementia. Front Aging Neurosci 15: 1149820, 2023. doi: 10.3389/fnagi.2023.1149820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Norton CE, Shaw RL, Mittler R, Segal SS. Endothelial cells promote smooth muscle cell resilience to H2O2-induced cell death in mouse cerebral arteries. Acta Physiol (Oxf) 235: e13819, 2022. doi: 10.1111/apha.13819. [DOI] [PubMed] [Google Scholar]
- 152. Diaz-Otero JM, Yen TC, Ahmad A, Laimon-Thomson E, Abolibdeh B, Kelly K, Lewis MT, Wiseman RW, Jackson WF, Dorrance AM. Transient receptor potential vanilloid 4 channels are important regulators of parenchymal arteriole dilation and cognitive function. Microcirculation 26: e12535, 2019. doi: 10.1111/micc.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Li N, Shi R, Ye Y, Zhang Y, Zhang Y, Wang Z, Gu Y, Yin Y, Chen D, Tang J. Aging-induced down-regulation of PKA/BKCa pathway in rat cerebral arteries. Physiol Res 71: 811–823, 2022. doi: 10.33549/physiolres.934944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Santisteban MM, Iadecola C, Carnevale D. Hypertension, neurovascular dysfunction, and cognitive impairment. Hypertension 80: 22–34, 2023. doi: 10.1161/HYPERTENSIONAHA.122.18085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zhu WM, Neuhaus A, Beard DJ, Sutherland BA, DeLuca GC. Neurovascular coupling mechanisms in health and neurovascular uncoupling in Alzheimer’s disease. Brain 145: 2276–2292, 2022. doi: 10.1093/brain/awac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Seki M, Lipton SA. Targeting excitotoxic/free radical signaling pathways for therapeutic intervention in glaucoma. Prog Brain Res 173: 495–510, 2008. doi: 10.1016/S0079-6123(08)01134-5. [DOI] [PubMed] [Google Scholar]
- 157. Leley SP, Ciulla TA, Bhatwadekar AD. Diabetic retinopathy in the aging population: a perspective of pathogenesis and treatment. Clin Interv Aging 16: 1367–1378, 2021. doi: 10.2147/CIA.S297494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Elahi FM, Ashimatey SB, Bennett DJ, Walters SM, La Joie R, Jiang X, Wolf A, Cobigo Y, Staffaroni AM, Rosen HJ, Miller BL, Rabinovici GD, Kramer JH, Green AJ, Kashani AH. Retinal imaging demonstrates reduced capillary density in clinically unimpaired APOE ε4 gene carriers. Alzheimers Dement (Amst) 13: e12181, 2021. [Erratum in Alzheimers Dement (Amst) 13: e12210, 2021]. doi: 10.1002/dad2.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Williams MA, McGowan AJ, Cardwell CR, Cheung CY, Craig D, Passmore P, Silvestri G, Maxwell AP, McKay GJ. Retinal microvascular network attenuation in Alzheimer’s disease. Alzheimers Dement (Amst) 1: 229–235, 2015. doi: 10.1016/j.dadm.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Wang H, Geisen P, Wittchen ES, King B, Burridge K, D'Amore PA, Hartnett ME. The role of RPE cell-associated VEGF189 in choroidal endothelial cell transmigration across the RPE. Invest Ophthalmol Vis Sci 52: 570–578, 2011. doi: 10.1167/iovs.10-5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Dervenis P, Dervenis N, Smith JM, Steel DH. Anti-vascular endothelial growth factors in combination with vitrectomy for complications of proliferative diabetic retinopathy. Cochrane Database Syst Rev 5: CD008214, 2023. doi: 10.1002/14651858.CD008214.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Needham M, McGahon MK, Bankhead P, Gardiner TA, Scholfield CN, Curtis TM, McGeown JG. The role of K+ and Cl- channels in the regulation of retinal arteriolar tone and blood flow. Invest Ophthalmol Vis Sci 55: 2157–2165, 2014. doi: 10.1167/iovs.13-12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Fernandez JA, McGahon MK, McGeown JG, Curtis TM. CaV3.1 T-type Ca2+ channels contribute to myogenic signaling in rat retinal arterioles. Invest Ophthalmol Vis Sci 56: 5125–5132, 2015. doi: 10.1167/iovs.15-17299. [DOI] [PubMed] [Google Scholar]
- 164. Puro DG. Retinovascular physiology and pathophysiology: new experimental approach/new insights. Prog Retin Eye Res 31: 258–270, 2012. doi: 10.1016/j.preteyeres.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Klug NR, Sancho M, Gonzales AL, Heppner TJ, O'Brien RIC, Hill-Eubanks D, Nelson MT. Intraluminal pressure elevates intracellular calcium and contracts CNS pericytes: Rrole of voltage-dependent calcium channels. Proc Natl Acad Sci USA 120: e2216421120, 2023. doi: 10.1073/pnas.2216421120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Guarino BD, Paruchuri S, Thodeti CK. The role of TRPV4 channels in ocular function and pathologies. Exp Eye Res 201: 108257, 2020. doi: 10.1016/j.exer.2020.108257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. McGahon MK, Fernandez JA, Dash DP, McKee J, Simpson DA, Zholos AV, McGeown JG, Curtis TM. TRPV2 channels contribute to stretch-activated cation currents and myogenic constriction in retinal arterioles. Invest Ophthalmol Vis Sci 57: 5637–5647, 2016. doi: 10.1167/iovs.16-20279. [DOI] [PubMed] [Google Scholar]
- 168. Adebiyi A, Zhao G, Cheranov SY, Ahmed A, Jaggar JH. Caveolin-1 abolishment attenuates the myogenic response in murine cerebral arteries. Am J Physiol Heart Circ Physiol 292: H1584–H1592, 2007. [Erratum in Am J Physiol Heart Circ Physiol 292: H2556–H2557, 2007]. doi: 10.1152/ajpheart.00584.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Reagan AM, Gu X, Paudel S, Ashpole NM, Zalles M, Sonntag WE, Ungvari Z, Csiszar A, Otalora L, Freeman WM, Stout MB, Elliott MH. Age-related focal loss of contractile vascular smooth muscle cells in retinal arterioles is accelerated by caveolin-1 deficiency. Neurobiol Aging 71: 1–12, 2018. doi: 10.1016/j.neurobiolaging.2018.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Zhao M, Celerier I, Bousquet E, Jeanny J-C, Jonet L, Savoldelli M, Offret O, Curan A, Farman N, Jaisser F, Behar-Cohen F. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest 122: 2672–2679, 2012. doi: 10.1172/JCI61427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Zhang T, Wu DM, Xu G-Z, Puro DG. The electrotonic architecture of the retinal microvasculature: modulation by angiotensin II. J Physiol 589: 2383–2399, 2011. doi: 10.1113/jphysiol.2010.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Behringer EJ, Segal SS. Tuning electrical conduction along endothelial tubes of resistance arteries through Ca2+-activated K+ channels. Circ Res 110: 1311–1321, 2012. doi: 10.1161/CIRCRESAHA.111.262592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Arnold AC, Gallagher PE, Diz DI. Brain renin-angiotensin system in the nexus of hypertension and aging. Hypertens Res 36: 5–13, 2013. doi: 10.1038/hr.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol 9: 44–53, 2013. doi: 10.1038/nrneurol.2012.227. [DOI] [PubMed] [Google Scholar]
- 175. Socha MJ, Boerman EM, Behringer EJ, Shaw RL, Domeier TL, Segal SS. Advanced age protects microvascular endothelium from aberrant Ca2+ influx and cell death induced by hydrogen peroxide. J Physiol 593: 2155–2169, 2015. doi: 10.1113/JP270169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Norton CE, Sinkler SY, Jacobsen NL, Segal SS. Advanced age protects resistance arteries of mouse skeletal muscle from oxidative stress through attenuating apoptosis induced by hydrogen peroxide. J Physiol 597: 3801–3816, 2019. doi: 10.1113/JP278255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 282: H1843–H1854, 2002. doi: 10.1152/ajpheart.00666.2001. [DOI] [PubMed] [Google Scholar]
- 178. Jobs A, Schmidt K, Schmidt VJ, Lubkemeier I, van Veen TA, Kurtz A, Willecke K, de Wit C. Defective Cx40 maintains Cx37 expression but intact Cx40 is crucial for conducted dilations irrespective of hypertension. Hypertension 60: 1422–1429, 2012. doi: 10.1161/HYPERTENSIONAHA.112.201194. [DOI] [PubMed] [Google Scholar]
- 179. Givvimani S, Narayanan N, Armaghan F, Pushpakumar S, Tyagi SC. Attenuation of conducted vasodilation in skeletal muscle arterioles during hyperhomocysteinemia. Pharmacology 91: 287–296, 2013. doi: 10.1159/000350394. [DOI] [PubMed] [Google Scholar]
- 180. Naik JS, Walker BR. Endothelial-dependent dilation following chronic hypoxia involves TRPV4-mediated activation of endothelial BK channels. Pflugers Arch 470: 633–648, 2018. doi: 10.1007/s00424-018-2112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Pandya CD, Lee B, Toque HA, Mendhe B, Bragg RT, Pandya B, Atawia RT, Isales C, Hamrick M, Caldwell RW, Fulzele S. Age-dependent oxidative stress elevates arginase 1 and uncoupled nitric oxide synthesis in skeletal muscle of aged mice. Oxid Med Cell Longev 2019: 1704650, 2019. doi: 10.1155/2019/1704650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- 183. Longden TA, Zhao G, Hariharan A, Lederer WJ. Pericytes and the control of blood flow in brain and heart. Annu Rev Physiol 85: 137–164, 2023. doi: 10.1146/annurev-physiol-031522-034807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Hill MA, Jaisser F, Sowers JR. Role of the vascular endothelial sodium channel activation in the genesis of pathologically increased cardiovascular stiffness. Cardiovasc Res 118: 130–140, 2022. doi: 10.1093/cvr/cvaa326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Balistrieri A, Makino A, Yuan JX-J. Pathophysiology and pathogenic mechanisms of pulmonary hypertension: role of membrane receptors, ion channels, and Ca2+ signaling. Physiol Rev 103: 1827–1897, 2023. doi: 10.1152/physrev.00030.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Marziano C, Hong K, Cope EL, Kotlikoff MI, Isakson BE, Sonkusare SK. Nitric oxide-dependent feedback loop regulates transient receptor potential vanilloid 4 (TRPV4) channel cooperativity and endothelial function in small pulmonary arteries. J Am Heart Assoc 6: e007157, 2017. doi: 10.1161/JAHA.117.007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Daneva Z, Chen Y-L, Ta HQ, Manchikalapudi V, Bazaz A, Laubach VE, Sonkusare SK. Endothelial IK and SK channel activation decreases pulmonary arterial pressure and vascular remodeling in pulmonary hypertension. Pulm Circ 13: e12186, 2023. doi: 10.1002/pul2.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Si R, Zhang Q, Cabrera JTO, Zheng Q, Tsuji-Hosokawa A, Watanabe M, Hosokawa S, Xiong M, Jain PP, Ashton AW, Yuan JX-J, Wang J, Makino A. Chronic hypoxia decreases endothelial connexin 40, attenuates endothelium-dependent hyperpolarization-mediated relaxation in small distal pulmonary arteries, and leads to pulmonary hypertension. J Am Heart Assoc 9: e018327, 2020. doi: 10.1161/JAHA.120.018327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Ottolini M, Daneva Z, Chen Y-L, Cope EL, Kasetti RB, Zode GS, Sonkusare SK. Mechanisms underlying selective coupling of endothelial Ca2+ signals with eNOS vs. IK/SK channels in systemic and pulmonary arteries. J Physiol 598: 3577–3596, 2020. doi: 10.1113/JP279570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Zhao T, Parmisano S, Soroureddin Z, Zhao M, Yung L, Thistlethwaite PA, Makino A, Yuan JX-J. Mechanosensitive cation currents through TRPC6 and Piezo1 channels in human pulmonary arterial endothelial cells. Am J Physiol Cell Physiol 323: C959–C973, 2022. doi: 10.1152/ajpcell.00313.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Wang Z, Chen J, Babicheva A, Jain PP, Rodriguez M, Ayon RJ, Ravellette KS, Wu L, Balistrieri F, Tang H, Wu X, Zhao T, Black SM, Desai AA, Garcia JGN, Sun X, Shyy JY, Valdez-Jasso D, Thistlethwaite PA, Makino A, Wang J, Yuan JX-J. Endothelial upregulation of mechanosensitive channel Piezo1 in pulmonary hypertension. Am J Physiol Cell Physiol 321: C1010–C1027, 2021. doi: 10.1152/ajpcell.00147.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Chen J, Rodriguez M, Miao J, Liao J, Jain PP, Zhao M, Zhao T, Babicheva A, Wang Z, Parmisano S, Powers R, Matti M, Paquin C, Soroureddin Z, Shyy JY, Thistlethwaite PA, Makino A, Wang J, Yuan JX-J. Mechanosensitive channel Piezo1 is required for pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 322: L737–L760, 2022. doi: 10.1152/ajplung.00447.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Gnanasambandam R, Bae C, Gottlieb PA, Sachs F. Ionic selectivity and permeation properties of human PIEZO1 channels. PLoS One 10: e0125503, 2015. doi: 10.1371/journal.pone.0125503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Firth AL, Platoshyn O, Brevnova EE, Burg ED, Powell F, Haddad GH, Yuan JX. Hypoxia selectively inhibits KCNA5 channels in pulmonary artery smooth muscle cells. Ann N Y Acad Sci 1177: 101–111, 2009. doi: 10.1111/j.1749-6632.2009.05040.x. [DOI] [PubMed] [Google Scholar]
- 195. Barnes EA, Lee L, Barnes SL, Brenner R, Alvira CM, Cornfield DN. beta1-Subunit of the calcium-sensitive potassium channel modulates the pulmonary vascular smooth muscle cell response to hypoxia. Am J Physiol Lung Cell Mol Physiol 315: L265–L275, 2018. doi: 10.1152/ajplung.00060.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Le Ribeuz H, Masson B, Capuano V, Dutheil M, Gooroochurn H, Boet A, Ghigna M-R, De Montpreville V, Girerd B, Lambert M, Mercier O, Chung WK, Humbert M, Montani D, Antigny F. SUR1 as a new therapeutic target for pulmonary arterial hypertension. Am J Respir Cell Mol Biol 66: 539–554, 2022. doi: 10.1165/rcmb.2021-0180OC. [DOI] [PubMed] [Google Scholar]
- 197. Breen E, Yuan JX-J. Targeting ATP-sensitive K+ channels to treat pulmonary hypertension. Am J Respir Cell Mol Biol 66: 476–478, 2022. doi: 10.1165/rcmb.2021-0549ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Lambert M, Mendes-Ferreira P, Ghigna MR, LeRibeuz H, Adao R, Boet A, Capuano V, Rucker-Martin C, Bras-Silva C, Quarck R, Domergue V, Vachiery JL, Humbert M, Perros F, Montani D, Antigny F. Kcnk3 dysfunction exaggerates the development of pulmonary hypertension induced by left ventricular pressure overload. Cardiovasc Res 117: 2474–2488, 2021. doi: 10.1093/cvr/cvab016. [DOI] [PubMed] [Google Scholar]
- 199. Nielsen G, Wandall-Frostholm C, Sadda V, Olivan-Viguera A, Lloyd EE, Bryan RM Jr, Simonsen U, Kohler R. Alterations of N-3 polyunsaturated fatty acid-activated K2P channels in hypoxia-induced pulmonary hypertension. Basic Clin Pharmacol Toxicol 113: 250–258, 2013. doi: 10.1111/bcpt.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Babicheva A, Ayon RJ, Zhao T, Ek Vitorin JF, Pohl NM, Yamamura A, Yamamura H, Quinton BA, Ba M, Wu L, Ravellette KS, Rahimi S, Balistrieri F, Harrington A, Vanderpool RR, Thistlethwaite PA, Makino A, Yuan JX-J. MicroRNA-mediated downregulation of K+ channels in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 318: L10–L26, 2020. doi: 10.1152/ajplung.00010.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Lopez-Valverde V, Andersen CU, Laursen BE, Mulvany MJ, Simonsen U. Glibenclamide reveals role for endothelin in hypoxia-induced vasoconstriction in rat intrapulmonary arteries. J Cardiovasc Pharmacol 46: 422–429, 2005. doi: 10.1097/01.fjc.0000175877.25296.bd. [DOI] [PubMed] [Google Scholar]
- 202. Moudgil R, Michelakis ED, Archer SL. The role of K+ channels in determining pulmonary vascular tone, oxygen sensing, cell proliferation, and apoptosis: implications in hypoxic pulmonary vasoconstriction and pulmonary arterial hypertension. Microcirculation 13: 615–632, 2006. doi: 10.1080/10739680600930222. [DOI] [PubMed] [Google Scholar]
- 203. Wang L, Yin J, Nickles HT, Ranke H, Tabuchi A, Hoffmann J, Tabeling C, Barbosa-Sicard E, Chanson M, Kwak BR, Shin H-S, Wu S, Isakson BE, Witzenrath M, de Wit C, Fleming I, Kuppe H, Kuebler WM. Hypoxic pulmonary vasoconstriction requires connexin 40-mediated endothelial signal conduction. J Clin Invest 122: 4218–4230, 2012. doi: 10.1172/JCI59176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389: 990–994, 1997. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 205. Norton CE, Jernigan NL, Walker BR, Resta TC. Membrane depolarization is required for pressure-dependent pulmonary arterial tone but not enhanced vasoconstriction to endothelin-1 following chronic hypoxia. Pulm Circ 10: 2045894020973559, 2020. doi: 10.1177/2045894020973559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Kummer W. Pulmonary vascular innervation and its role in responses to hypoxia: size matters! Proc Am Thorac Soc 8: 471–476, 2011. doi: 10.1513/pats.201101-013MW. [DOI] [PubMed] [Google Scholar]
- 207. Norton CE, Segal SS. Calcitonin gene-related peptide hyperpolarizes mouse pulmonary artery endothelial tubes through KATP channel activation. Am J Physiol Lung Cell Mol Physiol 315: L212–L226, 2018. doi: 10.1152/ajplung.00044.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Norton CE, Boerman EM, Segal SS. Differential hyperpolarization to substance P and calcitonin gene-related peptide in smooth muscle versus endothelium of mouse mesenteric artery. Microcirculation 28: e12733, 2021. doi: 10.1111/micc.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Boerman EM, Segal SS. Aging alters spontaneous and neurotransmitter-mediated Ca2+ signaling in smooth muscle cells of mouse mesenteric arteries. Microcirculation 27: e12607, 2020. doi: 10.1111/micc.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Boerman EM, Everhart JE, Segal SS. Advanced age decreases local calcium signaling in endothelium of mouse mesenteric arteries in vivo. Am J Physiol Heart Circ Physiol 310: H1091–H1096, 2016. doi: 10.1152/ajpheart.00038.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Westcott EB, Segal SS. Ageing alters perivascular nerve function of mouse mesenteric arteries in vivo. J Physiol 591: 1251–1263, 2013. doi: 10.1113/jphysiol.2012.244483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Boerman EM, Segal SS. Depressed perivascular sensory innervation of mouse mesenteric arteries with advanced age. J Physiol 594: 2323–2338, 2016. doi: 10.1113/JP270710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Beleznai TZ, Yarova PL, Yuill KH, Dora KA. Smooth muscle Ca2+-activated and voltage-gated K+ channels modulate conducted dilation in rat isolated small mesenteric arteries. Microcirculation 18: 487–500, 2011. doi: 10.1111/j.1549-8719.2011.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. del Corsso C, Ostrovskaya O, McAllister CE, Murray K, Hatton WJ, Gurney AM, Spencer NJ, Wilson SM. Effects of aging on Ca2+ signaling in murine mesenteric arterial smooth muscle cells. Mech Ageing Dev 127: 315–323, 2006. doi: 10.1016/j.mad.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 215. Fan G, Kaßmann M, Cui Y, Matthaeus C, Kunz S, Zhong C, Zhu S, Xie Y, Tsvetkov D, Daumke O, Huang Y, Gollasch M. Age attenuates the T-type CaV3.2-RyR axis in vascular smooth muscle. Aging Cell 19: e13134, 2020. doi: 10.1111/acel.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Thuesen AD, Andersen K, Lyngso KS, Burton M, Brasch-Andersen C, Vanhoutte PM, Hansen PBL. Deletion of T-type calcium channels Cav3.1 or Cav3.2 attenuates endothelial dysfunction in aging mice. Pflugers Arch 470: 355–365, 2018. doi: 10.1007/s00424-017-2068-x. [DOI] [PubMed] [Google Scholar]
- 217. Gustafsson F, Holstein-Rathlou NH. Angiotensin II modulates conducted vasoconstriction to norepinephrine and local electrical stimulation in rat mesenteric arterioles. Cardiovasc Res 44: 176–184, 1999. doi: 10.1016/s0008-6363(99)00174-1. [DOI] [PubMed] [Google Scholar]
- 218. Tykocki NR, Boerman EM, Jackson WF. Smooth muscle ion channels and regulation of vascular tone in resistance arteries and arterioles. Compr Physiol 7: 485–581, 2017. doi: 10.1002/cphy.c160011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Ticinesi A, Tana C, Nouvenne A, Prati B, Lauretani F, Meschi T. Gut microbiota, cognitive frailty and dementia in older individuals: a systematic review. Clin Interv Aging 13: 1497–1511, 2018. doi: 10.2147/CIA.S139163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Blantz RC, Deng A, Miracle CM, Thomson SC. Regulation of kidney function and metabolism: a question of supply and demand. Trans Am Clin Climatol Assoc 118: 23–43, 2007. [PMC free article] [PubMed] [Google Scholar]
- 221. Krishnan S, Suarez-Martinez AD, Bagher P, Gonzalez A, Liu R, Murfee WL, Mohandas R. Microvascular dysfunction and kidney disease: challenges and opportunities? Microcirculation 28: e12661, 2021. doi: 10.1111/micc.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Dennis MR, Pires PW, Banek CT. Vascular dysfunction in polycystic kidney disease: a mini-review. J Vasc Res. 2023 Aug. 3: 1–12. doi: 10.1159/000531647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Vallon V. Tubuloglomerular feedback and the control of glomerular filtration rate. News Physiol Sci 18: 169–174, 2003. doi: 10.1152/nips.01442.2003. [DOI] [PubMed] [Google Scholar]
- 224. Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev 95: 405–511, 2015. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Brasen JC, de Wit C, Sorensen CM. Myoendothelial coupling through Cx40 contributes to EDH-induced vasodilation in murine renal arteries: evidence from experiments and modelling. Acta Physiol (Oxf) 222: e12906, 2018. doi: 10.1111/apha.12906. [DOI] [PubMed] [Google Scholar]
- 226. Sorensen CM, Giese I, Braunstein TH, Brasen JC, Salomonsson M, Holstein-Rathlou N-H. Role of connexin40 in the autoregulatory response of the afferent arteriole. Am J Physiol Renal Physiol 303: F855–F863, 2012. doi: 10.1152/ajprenal.00026.2012. [DOI] [PubMed] [Google Scholar]
- 227. Wang X, Loutzenhiser R. Determinants of renal microvascular response to ACh: afferent and efferent arteriolar actions of EDHF. Am J Physiol Renal Physiol 282: F124–F132, 2002. doi: 10.1152/ajprenal.0157.2001. [DOI] [PubMed] [Google Scholar]
- 228. Chilton L, Loutzenhiser K, Morales E, Breaks J, Kargacin GJ, Loutzenhiser R. Inward rectifier K+ currents and Kir2.1 expression in renal afferent and efferent arterioles. J Am Soc Nephrol 19: 69–76, 2008. doi: 10.1681/ASN.2007010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Loutzenhiser R, Chilton L, Trottier G. Membrane potential measurements in renal afferent and efferent arterioles: actions of angiotensin II. Am J Physiol Renal Physiol 273: F307–F314, 1997. doi: 10.1152/ajprenal.1997.273.2.F307. [DOI] [PubMed] [Google Scholar]
- 230. Troncoso Brindeiro CM, Fallet RW, Lane PH, Carmines PK. Potassium channel contributions to afferent arteriolar tone in normal and diabetic rat kidney. Am J Physiol Renal Physiol 295: F171–F178, 2008. doi: 10.1152/ajprenal.00563.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Fujii N, McGarr GW, Sigal RJ, Boulay P, Nishiyasu T, Kenny GP. Ageing augments nicotinic and adenosine triphosphate-induced, but not muscarinic, cutaneous vasodilatation in women. Exp Physiol 104: 1801–1807, 2019. doi: 10.1113/EP088144. [DOI] [PubMed] [Google Scholar]
- 232. Fujii N, Nishiyasu T, Sigal RJ, Boulay P, McGarr GW, Kenny GP. Aging attenuates adenosine triphosphate-induced, but not muscarinic and nicotinic, cutaneous vasodilation in men. Microcirculation 25: e12462, 2018. doi: 10.1111/micc.12462. [DOI] [PubMed] [Google Scholar]
- 233. Craighead DH, Alexander LM. Topical menthol increases cutaneous blood flow. Microvasc Res 107: 39–45, 2016. doi: 10.1016/j.mvr.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Craighead DH, McCartney NB, Tumlinson JH, Alexander LM. Mechanisms and time course of menthol-induced cutaneous vasodilation. Microvasc Res 110: 43–47, 2017. doi: 10.1016/j.mvr.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Dillon GA, Lichter ZS, Alexander LM. Menthol-induced activation of TRPM8 receptors increases cutaneous blood flow across the dermatome. Microvasc Res 139: 104271, 2022. doi: 10.1016/j.mvr.2021.104271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol 22: R741–R752, 2012. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 237. Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35: 565–572, 2011. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Woitowich NC, Beery A, Woodruff T. A 10-year follow-up study of sex inclusion in the biological sciences. eLife 9: e56344, 2020. doi: 10.7554/eLife.56344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Novella S, Perez-Cremades D, Mompeon A, Hermenegildo C. Mechanisms underlying the influence of oestrogen on cardiovascular physiology in women. J Physiol 597: 4873–4886, 2019. doi: 10.1113/JP278063. [DOI] [PubMed] [Google Scholar]
- 240. Kehmeier MN, Bedell BR, Cullen AE, Khurana A, D'Amico HJ, Henson GD, Walker AE. In vivo arterial stiffness, but not isolated artery endothelial function, varies with the mouse estrous cycle. Am J Physiol Heart Circ Physiol 323: H1057–H1067, 2022. doi: 10.1152/ajpheart.00369.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Favre ME, Serrador JM. Sex differences in cerebral autoregulation are unaffected by menstrual cycle phase in young, healthy women. Am J Physiol Heart Circ Physiol 316: H920–H933, 2019. doi: 10.1152/ajpheart.00474.2018. [DOI] [PubMed] [Google Scholar]
- 242. Takezawa H, Hayashi H, Sano H, Saito H, Ebihara S. Circadian and estrous cycle-dependent variations in blood pressure and heart rate in female rats. Am J Physiol Regul Integr Comp Physiol 267: R1250–R1256, 1994. doi: 10.1152/ajpregu.1994.267.5.R1250. [DOI] [PubMed] [Google Scholar]
- 243. McCallinhart PE, Lee YU, Lee A, Anghelescu M, Tonniges JR, Calomeni E, Agarwal G, Lincoln J, Trask AJ. Dissociation of pulse wave velocity and aortic wall stiffness in diabetic db/db mice: the influence of blood pressure. Front Physiol 14: 1154454, 2023. doi: 10.3389/fphys.2023.1154454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Geary GG, Krause DN, Duckles SP. Estrogen reduces mouse cerebral artery tone through endothelial NOS- and cyclooxygenase-dependent mechanisms. Am J Physiol Heart Circ Physiol 279: H511–H519, 2000. doi: 10.1152/ajpheart.2000.279.2.H511. [DOI] [PubMed] [Google Scholar]
- 245. Golding EM, Kepler TE. Role of estrogen in modulating EDHF-mediated dilations in the female rat middle cerebral artery. Am J Physiol Heart Circ Physiol 280: H2417–H2423, 2001. doi: 10.1152/ajpheart.2001.280.6.H2417. [DOI] [PubMed] [Google Scholar]
- 246. Blackwell JA, Silva JF, Louis EM, Savu A, Largent-Milnes TM, Brooks HL, Pires PW. Cerebral arteriolar and neurovascular dysfunction after chemically induced menopause in mice. Am J Physiol Heart Circ Physiol 323: H845–H860, 2022. doi: 10.1152/ajpheart.00276.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Duckles SP, Krause DN. Cerebrovascular effects of oestrogen: multiplicity of action. Clin Exp Pharmacol Physiol 34: 801–808, 2007. doi: 10.1111/j.1440-1681.2007.04683.x. [DOI] [PubMed] [Google Scholar]
- 248. Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer's disease. Neurobiol Aging 32: 604–613, 2011. doi: 10.1016/j.neurobiolaging.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. JAMA 292: 1431–1432, 2004. doi: 10.1001/jama.292.12.1431-b. [DOI] [PubMed] [Google Scholar]
- 250. Jullienne A, Quan R, Szu JI, Trinh MV, Behringer EJ, Obenaus A. Progressive vascular abnormalities in the aging 3xTg-AD mouse model of Alzheimer’s disease. Biomedicines 10: 1967, 2022. doi: 10.3390/biomedicines10081967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Chum PP, Hakim MA, Behringer EJ. Cerebrovascular microRNA expression profile during early development of Alzheimer’s disease in a mouse model. J Alzheimers Dis 85: 91–113, 2022. doi: 10.3233/JAD-215223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Chum PP, Bishara MA, Solis SR, Behringer EJ. Cerebrovascular miRNAs track early development of Alzheimer’s disease and target molecular markers of angiogenesis and blood flow regulation. J Alzheimers Dis. 2023 Jul 14. doi: 10.3233/JAD-230300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Boerman EM, Sen S, Shaw RL, Joshi T, Segal SS. Gene expression profiles of ion channels and receptors in mouse resistance arteries: effects of cell type, vascular bed, and age. Microcirculation 25: e12452, 2018. doi: 10.1111/micc.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. McCarthy CG, Wenceslau CF, Calmasini FB, Klee NS, Brands MW, Joe B, Webb RC. Reconstitution of autophagy ameliorates vascular function and arterial stiffening in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 317: H1013–H1027, 2019. doi: 10.1152/ajpheart.00227.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Nussenzweig SC, Verma S, Finkel T. The role of autophagy in vascular biology. Circ Res 116: 480–488, 2015. doi: 10.1161/CIRCRESAHA.116.303805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Cho JM, Park SK, Kwon OS, Taylor La Salle D, Cerbie J, Fermoyle CC, Morgan D, Nelson A, Bledsoe A, Bharath LP, Tandar M, Kunapuli SP, Richardson RS, Anandh Babu PV, Mookherjee S, Kishore BK, Wang F, Yang T, Boudina S, Trinity JD, Symons JD. Activating P2Y1 receptors improves function in arteries with repressed autophagy. Cardiovasc Res 119: 252–267, 2023. doi: 10.1093/cvr/cvac061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Mattson MP. Superior pattern processing is the essence of the evolved human brain. Front Neurosci 8: 265, 2014. doi: 10.3389/fnins.2014.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Masis-Obando R, Norman KA, Baldassano C. Schema representations in distinct brain networks support narrative memory during encoding and retrieval. eLife 11: e70445, 2022. doi: 10.7554/eLife.70445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Mittal A, Park PD, Mitchell R, Fang H, Bagher P. Comparison of adrenergic and purinergic receptor contributions to vasomotor responses in mesenteric arteries of C57BL/6J mice and Wistar rats. J Vasc Res 58: 1–15, 2020. doi: 10.1159/000511462. [DOI] [PubMed] [Google Scholar]
- 260. Jackson WF, Boerman EM, Lange EJ, Lundback SS, Cohen KD. Smooth muscle alpha1D-adrenoceptors mediate phenylephrine-induced vasoconstriction and increases in endothelial cell Ca2+ in hamster cremaster arterioles. Br J Pharmacol 155: 514–524, 2008. doi: 10.1038/bjp.2008.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.United States. Administration on Aging., and American Association of Retired Persons. A profile of older Americans. Washington, DC: U.S. Dept. of Health and Human Services, Administration on Aging. https://www.loc.gov/item/2002230325/. Library of Congress Control Number 2002230325. [Google Scholar]
- 262. Kolski-Andreaco A, Balut CM, Bertuccio CA, Wilson AS, Rivers WM, Liu X, Gandley RE, Straub AC, Butterworth MB, Binion D, Devor DC. Histone deacetylase inhibitors (HDACi) increase expression of KCa2.3 (SK3) in primary microvascular endothelial cells. Am J Physiol Cell Physiol 322: C338–C353, 2022. doi: 10.1152/ajpcell.00409.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. John CM, Khaddaj Mallat R, Mishra RC, George G, Singh V, Turnbull JD, Umeshappa CS, Kendrick DJ, Kim T, Fauzi FM, Visser F, Fedak PWM, Wulff H, Braun AP. SKA-31, an activator of Ca2+-activated K+ channels, improves cardiovascular function in aging. Pharmacol Res 151: 104539, 2020. doi: 10.1016/j.phrs.2019.104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Murray KO, Ludwig KR, Darvish S, Coppock ME, Seals DR, Rossman MJ. Chronic mitochondria antioxidant treatment in older adults alters the circulating milieu to improve endothelial cell function and mitochondrial oxidative stress. Am J Physiol Heart Circ Physiol 325: H187–H194, 2023. doi: 10.1152/ajpheart.00270.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. DuBose LE, Ozemek C, Wick T, Richardson V, Hildreth KL, Moreau KL. Role of BH4 deficiency as a mediator of oxidative stress-related endothelial dysfunction in menopausal women. Am J Physiol Heart Circ Physiol 323: H975–H982, 2022. doi: 10.1152/ajpheart.00435.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Hakim MA, Behringer EJ. KIR channel regulation of electrical conduction along cerebrovascular endothelium: enhanced modulation during Alzheimer’s disease. Microcirculation 30: e12797, 2023. doi: 10.1111/micc.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Sancho M, Klug NR, Mughal A, Koide M, Huerta de la Cruz SH, Heppner TJ, Bonev AD, Hill-Eubanks D, Nelson MT. Adenosine signaling activates ATP-sensitive K+ channels in endothelial cells and pericytes in CNS capillaries. Sci Signal 15: eabl5405, 2022. doi: 10.1126/scisignal.abl5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Harraz OF, Klug NR, Senatore AJ, Hill-Eubanks DC, Nelson MT. Piezo1 is a mechanosensor channel in central nervous system capillaries. Circ Res 130: 1531–1546, 2022. doi: 10.1161/CIRCRESAHA.122.320827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Taylor JL, Walsh KR, Mosneag IE, Danby TGE, Luka N, Chanda B, Schiessl I, Dunne RA, Hill-Eubanks D, Hennig GW, Allan SM, Nelson MT, Greenstein AS, Pritchard HAT. Uncoupling of Ca2+ sparks from BK channels in cerebral arteries underlies hypoperfusion in hypertension-induced vascular dementia. Proc Natl Acad Sci USA 120: e2307513120, 2023. doi: 10.1073/pnas.2307513120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Davis MJ, Kim HJ, Nichols CG. KATP channels in lymphatic function. Am J Physiol Cell Physiol 323: C1018–C1035, 2022. doi: 10.1152/ajpcell.00137.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]