Abstract
Background
The sensitivity of amyloid to pre-analytic factors complicates cerebrospinal fluid (CSF) diagnostics for Alzheimer disease. We report reliability and validity evidence for automated immunoassays from frozen and fresh CSF samples in an ongoing, single-site research program.
Methods
CSF samples were obtained from 2 Wisconsin cohorts (1256 measurements; 727 participants). Levels of amyloid beta 1–42 (), phosphorylated tau 181 (), and total tau (tTau) were obtained using an Elecsys cobas e 601 platform. Repeatability and fixed effects of storage tube type, extraction method, and freezing were assessed via mixed models. Concordance with amyloid positron emission tomography (PET) was investigated with 238 participants having a temporally proximal PET scan.
Results
Repeatability was high with intraclass correlation (ICC) ≥0.9, but tube type strongly affected measurements. Discriminative accuracy for PET amyloid positivity was strong across tube types (area under the curve [AUC]: , 0.87; , 0.96), although optimal thresholds differed.
Conclusions
Under real-world conditions, the Elecsys platform had high repeatability. However, strong effects of pre-analytic factors suggest caution in drawing longitudinal inferences.
Introduction
Alzheimer disease (AD) is a proteinopathy which can be diagnosed and staged via amyloid and tau biomarkers (1). Consequently, laboratory tests to verify these biomarkers in cerebrospinal fluid (CSF) are important for diagnosing AD (2, 3). However, the sensitivity of amyloid to pre-analytic factors (4–7) poses a challenge for accurate measurement that looms larger as interest in fluid biomarkers grows.
The emergence of fully automated immunoassays such as the Roche Elecsys platform for amyloid beta 1–42 (), phosphorylated tau 181 (), and total tau (tTau) has improved measurement of AD-relevant analytes, recently receiving U.S. Food and Drug Administration clearance for diagnostic use (8). The Elecsys platform has high precision, with repeatability coefficient of variation (CV) for amyloid <2% and both intermediate and interlaboratory precision CV < 5% (9). In a community clinical population, Elecsys results correlated well with those of Innotest, an earlier method (10). However, even with full automation, certain pre-analytic factors may impact positivity thresholds (4). To our knowledge, repeatability has not been examined with Elecsys in the context of longitudinal cohorts in which such factors change over time.
In this study, we report reliability and validity evidence for the Elecsys 601 platform obtained from an historical archive of frozen and fresh CSF samples in an ongoing, single-site research program (11). We report between-subjects estimates of the effects of several pre-analytic factors, including tube type, extraction method, and exposure to a freeze–thaw cycle, along with reliability in a small subset with repeated measurements. Using brain amyloid scans as a reference standard, we report criterion validity and estimate local cutpoints, which we compare to published values (12–14).
Materials and Methods
Ethics approval and consent to participate
The research protocols were approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board (IRB00000366), and all participants provided written informed consent.
Participants
Samples were obtained from participants in the Wisconsin Registry for Alzheimer’s Prevention (WRAP) (15) and the Wisconsin Alzheimer’s Disease Research Center (ADRC) (16) cohorts. Participants were classified by cognitive status at the nearest assessment (mean temporal difference (SD): 0.44 (0.78) years). The present analyses include data from 1256 measurements ( = 1101 unique person-visits; = 727 individual people), comprising 40 measurements on people with dementia ( = 40), 100 with mild cognitive impairment (MCI; = 72), 20 with non-MCI impairment ( = 15), and 1085 cognitively unimpaired ( = 612), with 17 participants having CSF samples at multiple stages of progression. A total of 683 observations ( = 430) were from ADRC participants and 573 ( = 297) were from WRAP. This comprises all samples run from January 2010 to July 2021, including some reruns of the same subject-visit. Full sample characteristics are shown in Table 1.
Table 1.
Participant characteristics by lumbar puncture protocol. Samples were categorized by cognitive diagnosis at the nearest visit. Some participants provided samples more than once, some in more than one tube type, and some at more than one stage of cognitive impairment.
| Variable | pull/frozen/Sarstedt 0.5 mL screw top | pull/frozen/Fisher 1.5 mL microcentrifuge | pull/frozen/Fisher 2.0 mL cryovial | pull/frozen/Matrix 1.0 mL cryotube | drip/fresh/Sarstedt 2.5 mL non-stick | drip/frozen/Sarstedt 2.5 mL non-stick |
|---|---|---|---|---|---|---|
| Number of participants | 249 | 393 | 36 | 201 | 43 | 95 |
| Number of observations | 252 | 501 | 36 | 223 | 43 | 95 |
| Number of measurements | 290 | 543 | 60 | 225 | 43 | 95 |
| Diagnosis, unimpaired, n (%) | 222 (89%) | 322 (82%) | 32 (89%) | 168 (84%) | 37 (86%) | 82 (86%) |
| Diagnosis, MCI, n (%) | 17 (7%) | 37 (9%) | 4 (11%) | 18 (9%) | 3 (7%) | 7 (7%) |
| Diagnosis, dementia, n (%) | 3 (1%) | 27 (7%) | — | 10 (5%) | — | — |
| Diagnosis, missing, n (%) | 7 (3%) | 7 (2%) | — | 5 (2%) | 3 (7%) | 6 (6%) |
| Age at lumbar puncture, mean (SD) | 65.33 (7.58) | 64.86 (8.58) | 63.65 (8.98) | 64.20 (8.48) | 67.97 (7.29) | 65.33 (8.30) |
| Number of cognitive visits, mean (SD) | 4.95 (2.27) | 5.59 (2.14) | 5.53 (1.92) | 5.28 (2.71) | 5.47 (2.33) | 4.79 (2.66) |
Concordance with amyloid imaging was investigated in 238 participants with an amyloid scan within 2 years of their CSF. For consistency, concordance was investigated separately by tube type. The first tube type with sufficient samples (“Sarstedt 0.5 mL screw top”; see details in CSF methods below) includes data from 96 cognitively unimpaired individuals, 10 with MCI, 0 with dementia, and 1 with other cognitive impairment; 27 were amyloid positive. The second (“Fisher 1.5 mL microcentrifuge”) includes data from 119 cognitively unimpaired individuals, 11 with MCI, 1 with dementia, and 0 with other cognitive impairment (see CSF methods below); 30 were amyloid positive.
CSF Sample collection and preparation methods
Participants underwent lumbar puncture (LP), most after an 8 to 12 h fast (89% compliance). Participants provided 22 mL of CSF. After collection, samples were transferred into a single 30 mL polypropylene tube, gently mixed, and centrifuged for 10 min at 2000g (3000 rpm) at 4°C within 30 min of collection, before aliquoting for freezing at −80°C. Other pre-analytic protocol features varied as described below. Detailed counts of participants, observations, and assay results for each combination of tube type, extraction method, and freeze–thaw cycle are presented in Table 1.
Collection tube type
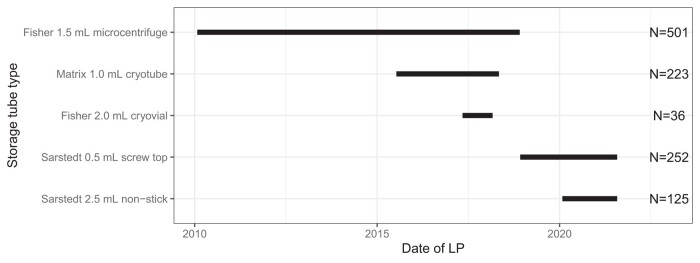
Samples were obtained between January 27, 2010, and July 21, 2021, during which time tube types (see Supplemental Table 1) changed in response to evolving best practices for LP collection (5). For a schematic time line, see Fig. 1. Briefly, prior to July 2015, most samples were stored in Fisher 1.5 mL polypropylene tubes (Fisher 1.5 mL microcentrifuge; 0.5 mL aliquots; = 501). After that date, some were stored in Thermo Matrix tubes (“Matrix 1.0 mL cryotube”; ThermoFisher Scientific; 0.5 mL aliquots; = 223), and some in Fisher 2.0 mL cryovial tubes (“Fisher 2.0 mL cryovial”; 0.5 mL aliquots; = 36). Starting in December 2018, most samples were stored in Sarstedt 0.5 mL screw-top tubes (Sarstedt 0.5 mL screw top; 0.5 mL aliquots; = 252). Beginning in January 2020, one sample per participant was also collected in Sarstedt 2.5 mL tubes (“Sarstedt 2.5 mL non-stick”; 2.0 mL aliquots; = 125). For some LPs, more than one tube type was used to freeze samples for planned within-subject analyses (see Precision analysis). Concordance with brain amyloid positivity was examined in the Fisher 1.5 mL and Sarstedt 0.5 mL subsets; other subsets had too few proximal amyloid measurements available.
Fig. 1.
Schematic illustrating start and end dates of tube types used for CSF storage.
Extraction method and freeze–thaw cycle
Prior to January 29, 2020, participants provided CSF via gentle extraction (“pull”) with a Sprotte 24- or 25-gauge atraumatic spinal needle into a series of 6 polypropylene syringes. On that date, the center implemented a standardized fresh drip protocol (5), allowing CSF to drip at a slower rate for initial collection (“drip”), and then proceeding with gentle extraction for subsequent vials. Some samples obtained via fresh drip were analyzed immediately (“Sarstedt 2.5 mL from fresh”), whereas others were frozen first (“Sarstedt 2.5 mL from frozen”). Although the standardized protocol specifies immediate analysis, limited reagent availability forced freezing in some cases. The selection of samples into these categories was largely administrative, with a small set of observations selected for analysis in both conditions as part of planned within-subjects comparisons (see Precision analysis). For analysis, frozen samples were allowed to thaw for 20 min, and then rolled at room temperature for 20 min on a Stuart roller mixer.
Elecsys
Concentrations of , , and tTau were obtained using an Elecsys cobas e 601 instrument (9). The first-generation assay used for has a measuring range of 200 to 1700 pg/mL; observations outside this range were imputed at the boundary ( = 209; 17%).
PET Collection methods
Pittsburgh compound B positron emission tomography (PiB-PET) was used to quantify brain amyloidosis and create parametric distribution volume ratio (DVR) images. Participants were classified as amyloid positive based on a visual rating, and amyloid negative otherwise. Detailed PET acquisition methods are described elsewhere (17, 18).
Statistical methods
Statistical analysis was performed with R (19). Between-subjects effects of pre-analytic factors were analyzed with mixed effects models, with random effects including subject and observation nested within subject, and fixed effects including pre-analytic protocol (see CSF sample collection and preparation methods), reagent lot, cognitive diagnosis, and age at LP. Exploratory locally-smoothed ("loess") regression curves investigated storage time as a potential factor. Test–retest associations were examined using mixed effects models. For models examining (a) repeated assays of the same aliquot on the same day and (b) different aliquots from the same pre-analytic protocol on different days, random intercepts were fit for subject–observation clusters and assay date. In models examining (c) tube and freeze–thaw cycle differences and (d) tube and run date, random intercepts were fit for subject clusters only. Repeatability was assessed via intraclass correlation (ICC). Mean differences and limits of agreement (LOA) were examined with Bland–Altman plots. Agreement with PiB visual rating was examined using receiver operating characteristic (ROC) curves, and thresholds for and were chosen by maximizing the Youden index. Thresholds for and tTau were established by determining a nonparametric upper confidence limit equivalent to z = 2 on a set of younger (45 to 60 years of age), cognitively unimpaired participants with a negative PiB scan within 2 years of their LP.
Availability of data and materials
Coded data and R scripts underlying all analyses may be shared at the request of any qualified investigator for purposes of replication. Requests for data should be submitted separately to the WRAP and ADRC executive committees via https://wrap.wisc.edu/data-requests-2/ (accessed November 17, 2023).
Results
Effects of pre-analytic factors on measured values
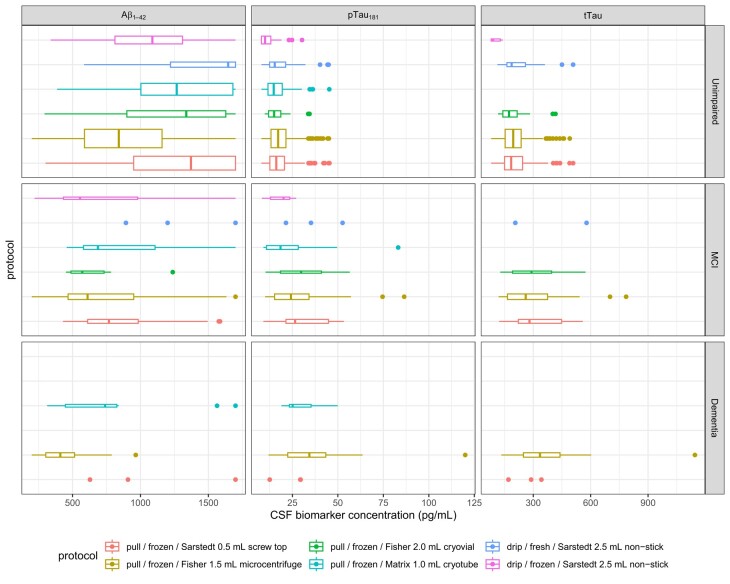
Fig. 2 shows observed values of the 3 analytes, stratified by cognitive diagnosis and pre-analytic protocol. Repeatability was strong (all ICC ≥ 0.9). is strongly affected by protocol, with lower concentrations for samples stored in the Fisher 1.5 mL tube than the reference condition, the Sarstedt 0.5 mL tube (37% of the mean value, P < 0.0001). Smaller differences were observed for the fresh-drip samples stored in Sarstedt 2.5 mL tubes, both when analyzed from fresh, which led to higher estimates of (14% of the mean, P < 0.0001), and from frozen, which resulted in lower estimates (16% of the mean, P < 0.0001). The small number of samples stored in other tube types also differed somewhat, except for the Matrix 1.0 mL cryotubes (Fisher 2.0 mL cryovial: 13% of the mean, P = 0.0071; Matrix 1.0 mL cryotube: 7% of the mean, P = 0.15). No effect of reagent lot was observed. Statistically significant differences were also observed for other analytes with substantially lower and tTau concentrations linked to the Sarstedt 2.5 mL tubes when analyzed from frozen (: 25% of the mean, P < 0.0001; tTau: 36% of the mean, P < 0.0001), and slightly higher measured and tTau concentrations for the Sarstedt 2.5 mL tubes when analyzed from fresh (: 3% of the mean, P = 0.032; tTau: 6% of the mean, P = 0.00053). , but not tTau, values were also affected by reagent lot. As expected, cognitive impairment and higher age at LP were also associated with lower concentrations of and higher levels of and tTau. No clear relationship with storage time was observed (online Supplemental Fig. 1). Full model results are shown in Table 2.
Fig. 2.
Between-subjects estimates of effects of protocol on measured values of , , and tTau, stratified by concurrent cognitive diagnosis.
Each boxplot reflects the median (central line) and two hinges (upper and lower lines, reflecting 25th and 75th percentile), along with whiskers extending from the hinge to the largest value within 1.5 times the interquartile range (IQR) of each hinge. Boxplot widths are proportional to sample size.
Table 2.
Coefficient estimates from linear mixed effects models on full (between-subjects) dataset. Bold entries indicate coefficient estimates significantly different from 0 (nominal α = 0.05). , amyloid beta 1–42; CI, 95% confidence interval; MCI, mild cognitive impairment; , phosphorylated tau 181; tTau, total tau.
| Aβ42 | pTau181 | tTau | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | Estimates | CI | P | Estimates | CI | P | Estimates | CI | P |
| (Intercept) | 1307.40 | 1262.09 to 1352.72 | <0.001 | 17.11 | 16.25 to 17.96 | <0.001 | 194.36 | 184.81 to 203.91 | <0.001 |
| Sarstedt 0.5 mL screw top, gentle extraction, frozen | Reference | Reference | Reference | ||||||
| Fisher 1.5 mL microcentrifuge, gentle extraction, frozen | −389.59 | −434.33 to −344.85 | <0.001 | −0.05 | −0.62 to 0.51 | 0.858 | −5.93 | −12.79 to 0.93 | 0.090 |
| Fisher 2.0 mL cryovial, gentle extraction, frozen | −132.80 | −229.27 to −36.34 | 0.007 | −0.37 | −1.49 to 0.75 | 0.515 | 0.49 | −12.08 to 13.06 | 0.939 |
| Matrix 1.0 mL cryotube, gentle extraction, frozen | −70.98 | −168.64 to 26.68 | 0.154 | 0.91 | −0.13 to 1.94 | 0.086 | |||
| Sarstedt 2.5 mL non-stick, drip method, never frozen | 153.82 | 99.25 to 208.39 | <0.001 | 0.56 | 0.05 to 1.07 | 0.031 | 12.10 | 5.40 to 18.79 | <0.001 |
| Sarstedt 2.5 mL non-stick, drip method, frozen | −174.00 | −257.73 to −90.27 | <0.001 | −4.60 | −5.41 to −3.79 | <0.001 | −77.76 | −111.72 to −43.80 | <0.001 |
| Age, centered at 60, years | −5.10 | −8.42 to −1.77 | 0.003 | 0.32 | 0.25 to 0.38 | <0.001 | 3.43 | 2.70 to 4.16 | <0.001 |
| Unimpaired | Reference | Reference | Reference | ||||||
| Impaired—other | 107.83 | −45.17 to 260.84 | 0.167 | −0.08 | −1.97 to 1.82 | 0.936 | 11.63 | −15.43 to 38.69 | 0.399 |
| MCI | −196.54 | −282.89 to −110.20 | <0.001 | 4.52 | 3.24 to 5.80 | <0.001 | 43.14 | 28.62 to 57.66 | <0.001 |
| Dementia | −392.05 | −517.20 to −266.89 | <0.001 | 9.65 | 7.32 to 11.99 | <0.001 | 125.05 | 91.75 to 158.35 | <0.001 |
| Aβ42 reagent lot 470571 | 50.51 | −29.05 to 130.07 | 0.213 | ||||||
| Aβ42 reagent lot 518749 | 6.06 | −86.39 to 98.52 | 0.898 | ||||||
| pTau181 reagent lot 460998 | 0.60 | −0.32 to 1.52 | 0.202 | ||||||
| pTau181 reagent lot 527946 | −2.17 | −3.13 to −1.21 | <0.001 | ||||||
| tTau reagent lot 456531 | 9.57 | −3.28 to 22.43 | 0.144 | ||||||
| Random effects | |||||||||
| σ2 | 15541.60 | 1.29 | 140.69 | ||||||
| τ00 | 23560.22 Reggieid:sample_date | 2.62 Reggieid:sample_date | 298.06 Reggieid:sample_date | ||||||
| 115943.10 Reggieid | 77.19 Reggieid | 7334.23 Reggieid | |||||||
| ICC | 0.90 | 0.98 | 0.98 | ||||||
| N | 721 Reggieid | 721 Reggieid | 588 Reggieid | ||||||
| 902 sample_date | 902 sample_date | 654 sample_date | |||||||
| Observations | 1245 | 1244 | 903 | ||||||
| Marginal R2/conditional R2 | 0.223/0.922 | 0.176/0.987 | 0.192/0.985 | ||||||
To explore the effects of censored observations on model estimates, we fitted the same model to a dataset for which log normally distributed values above the boundary had been multiply imputed (see online Supplemental Table 2). Briefly, although confidence intervals were wider, the estimated beta coefficients for the protocol were of larger magnitude. Further, the average ICC was lower at 0.89.
Precision analysis
Same aliquot
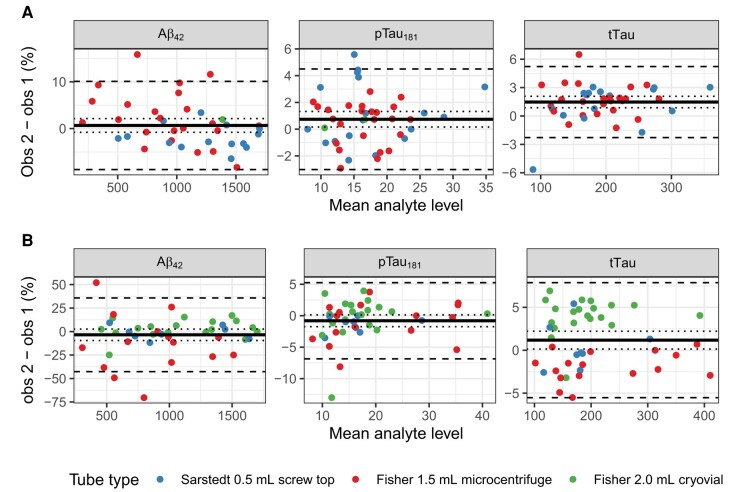
Online Supplemental Table 3A shows the results of linear mixed effects models of analyte levels obtained via repeated analyses of 44 single aliquots within a 24 h period. Six observations were missing tTau values; available analyte levels for these observations were included. Agreement was very strong for each analyte (all ICC ≥ 0.99). The repeatability of , , and tTau assays from the same aliquot are illustrated in Fig. 3A. In these plots, the percent difference between 2 runs (obs1, first observation; obs2, second observation) of the same aliquot on the same day [] is plotted against their mean value. The mean percent difference, LOA (mean percent difference 2 SD), and error CV were as follows: , 0.7% (LOA: −8.8%, 10.1%), = 3.02%; , 0.7% (LOA: −3%, 4.5%), = 1.49%; tTau, 1.5% (LOA: −2.3%, 5.2%), = 1.69%.
Fig. 3.
Bland–Altman (mean-difference) plots showing repeatability of assays.
Dashed lines represent upper and lower 95% limits of agreement (); dotted lines reflect the 95% confidence interval around the mean difference (). Scatter represents the percentage difference between the first and second measurement plotted against their mean value. (A), Same aliquot, same analysis date; (B), different aliquot from the same lumber puncture, different analysis date.
Different aliquot, same LP and protocol, same tube type
Online Supplemental Table 3B summarizes mixed models of analyte levels in 44 pairs of aliquots drawn from the same LP under the same pre-analytic protocol. Agreement was again strong (all ICC > 0.9; for and tTau, ICC ≥ 0.99). The intermediate precision of , , and tTau assays is illustrated in Fig. 3B. The mean percent difference, LOA, and CV corresponding to run date were as follows: , −3.5% (LOA: −42.7%, 35.8%), = 5.61%; , −0.8% (LOA: −6.9%, 5.3%), = 1.61%; tTau, 1.2% (LOA: −5.5%, 7.9%), = 1.69%.
Different aliquot, same LP, analyzed fresh versus frozen
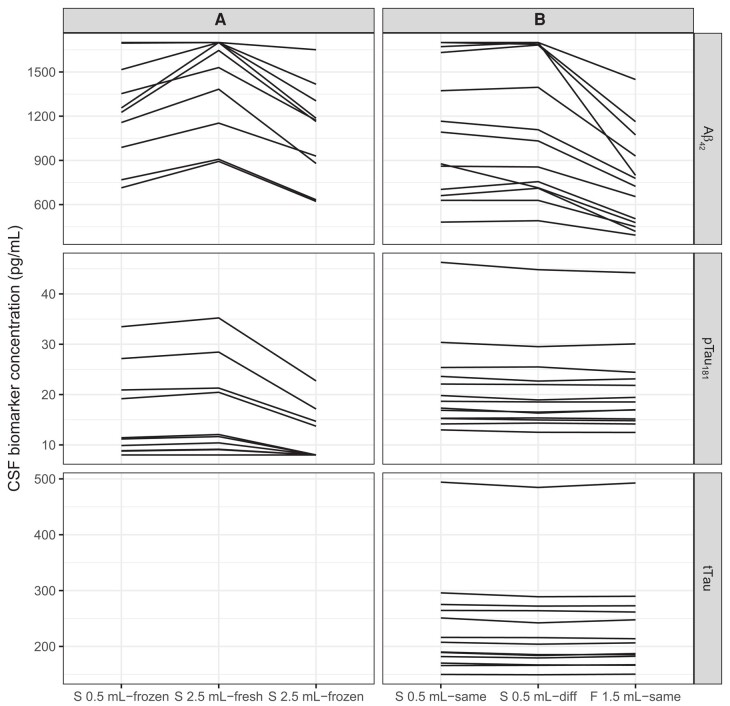
Online Supplemental Table 3C summarizes mixed models of analyte levels in 10 sets of fresh and frozen samples from the same LP. For this analysis, samples obtained via gentle extraction and frozen in Sarstedt 0.5 mL tubes were compared vs those obtained via drip, placed in Sarstedt 2.5 mL tubes, and analyzed either fresh or frozen. Because this study spanned a short interval with constant reagents, run date was not included. Repeatability was again strong (ICC > 0.9). However, compared to samples frozen in Sarstedt 0.5 mL tubes, samples in Sarstedt 2.5 mL fresh tubes had substantially higher measured values of by almost 200 pg/mL (15%, P = 0.00017), and those in the same tubes analyzed from frozen had substantially lower values by close to 150 pg/mL (11%, P = 0.0029). For , Sarstedt 0.5 mL frozen values and Sarstedt 2.5 mL fresh values were similar; however, Sarstedt 2.5 frozen values were lower by more than 4 pg/mL (29%, P = 0.00078). Results are depicted in Fig. 4A.
Fig. 4.
Spaghetti plots showing 3-level within-subjects comparisons.
(A), Tube type and freeze–thaw. First column: Sarstedt 0.5 mL tube, frozen; second column: Sarstedt 2.5 mL tube, fresh; third column: Sarstedt 2.5 mL tube, frozen. (B), Tube type and run date. First column: Sarstedt 0.5 mL tube, run date A; second column: Sarstedt 0.5 mL tube, run date B; third column: Fisher 1.5 mL tube, run date A. Note: tTau analyte values not available for study A.
Different aliquot, same LP, different tube type
Online Supplemental Table 3D summarizes mixed models of analyte levels from 13 sets of aliquots from the same LP, placed into Sarstedt 0.5 mL vs Fisher 1.5 mL storage tubes, and analyzed on the same or different dates. Repeatability was again high (ICC > 0.9; for and tTau, ICC ≥ 0.99). However, as expected based on results of our between-subjects analysis, a large effect of tube type was seen for , with measurements approximately 360 pg/mL lower (37%, P < 0.0001) in the Fisher 1.5 mL polypropylene tubes than in the Sarstedt 0.5 mL screw-top tubes. Small negative effects of both run conditions were also observed for both (Sarstedt 0.5 mL tube, different run date: 2% of mean analyte level, P = 0.0033; Fisher 1.5 mL tube, same run date: 2%, P = 0.0042) and tTau (Sarstedt 0.5 mL, different run date: 2% of mean level, P < 0.0001; Fisher 1.5 mL, same run date: 1%, P = 0.010). Results are depicted in Fig. 4B.
Concordance with PiB-PET
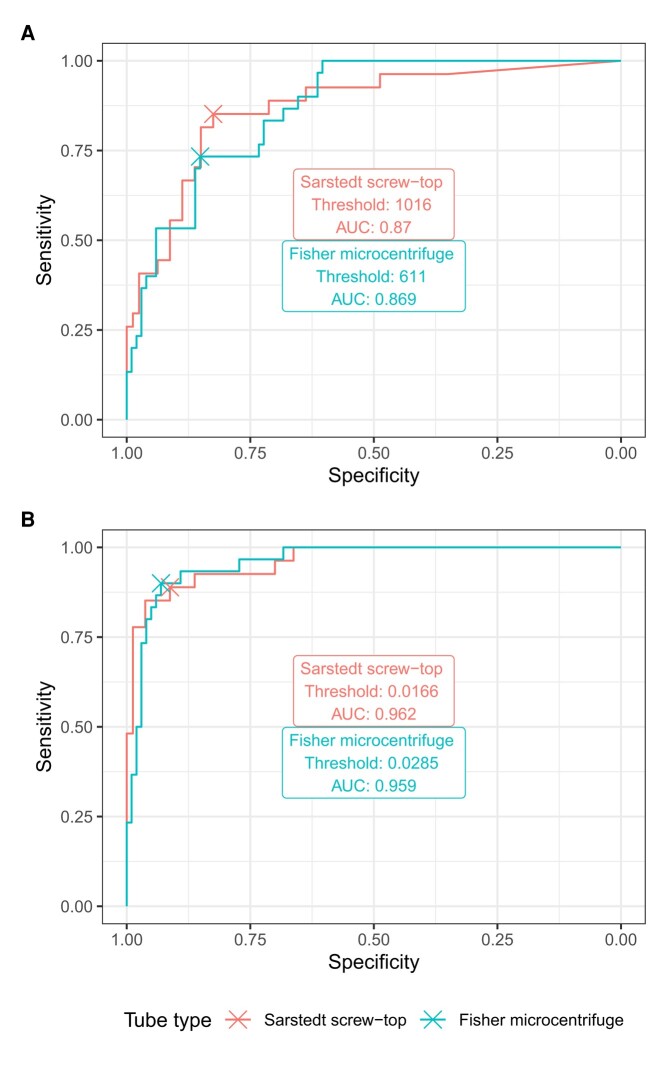
In the subset of participants with a concurrent PiB visual rating, we observed good agreement between CSF and PET measures of amyloid positivity. Because of the tube dependence, we calculated fit measures and thresholds separately. For samples in Sarstedt 0.5 mL tubes, AUCs for predicting PiB visual rating from CSF and were 0.87 and 0.96, respectively. For , a threshold of 1016 produced sensitivity and specificity of 0.85 and 0.82, respectively. This threshold is quite similar to the prototype package insert value of 1000 (12) and reasonably close to the value of 1100 established in the BioFINDER cohort (4). For , a threshold of 0.017 produced sensitivity and specificity of 0.89 and 0.91, respectively. This threshold is slightly lower than the published value of 0.024 suggested in the prototype package insert (12) and a bit closer to the value of 0.022 found in BioFINDER (4). Applying these thresholds, 34% of participants overall, and 28% of unimpaired participants, were categorized as CSF ; for , the figures were 29% and 22%, respectively.
In the subset of participants in which fresh 2.5 mL Sarstedt samples were compared to frozen 0.5 mL Sarstedt samples, we classified each result using these thresholds for frozen samples and published thresholds for fresh samples (20), and observed substantial agreement between the two (: percent agreement = 86.67, Cohen’s κ = 0.56; : percent agreement = 86.36, Cohen κ = 0.62). For each binary indicator, no samples were flagged as positive only using the fresh samples, but a small number were flagged as positive only using the frozen 0.5 mL samples (: n = 6; : n = 6).
Turning to the Fisher 1.5 mL tubes, the AUCs for predicting amyloid positivity from CSF and were 0.87 and 0.96, respectively—equivalent within 2 decimal points to the AUC for the Sarstedt 0.5 mL tubes. For , a threshold of 611 produced sensitivity and specificity of 0.73 and 0.85, respectively. This low threshold is unsurprising given the strong effect of tube type on levels of this analyte in our data (Table 2; Supplemental Table 3C). For , a threshold of 0.028 produced sensitivity and specificity of 0.9 and 0.93, respectively. ROC plots and optimality points are shown in Fig. 5. Applying these tube-specific thresholds, 35% of participants overall, and 24% of unimpaired participants, were categorized as CSF ; for , the figures were 32% and 20%, respectively. If we instead applied the Sarstedt 0.5 mL screw-top thresholds, the percentages CSF for all participants and unimpaired participants, respectively, would have been 74% and 59%; the percentages would have been 69% and 54%. In the PiB subset, the Sarstedt thresholds would yield sensitivity values for PiB positivity of 1 for and 1 for , but the corresponding specificity values would be only 0.46 and 0.5.
Fig. 5.
Receiver operating characteristic (ROC) curves for classification of PiB-PET positivity by visual rating using CSF analyte values from Sarstedt subset (PiB+, N = 27; PiB−, 80). (A), ; (B), .
Finding local pTau and tTau cutpoints
We have no gold standard for pTau181 and tTau. Instead, we selected a subset likely to be : 59 younger (45 to 60 years of age), cognitively unimpaired individuals having a negative PiB scan within 2 years of their LP. We then constructed a bootstrapped (R = 10 000) nonparametric upper confidence limit at a percentile equivalent to z = 2 (approximately 97.7%). For , this procedure produced a threshold of 23.6; this is consistent with several other published values, but is lower than the threshold of 27 suggested on the package insert (13). For tTau, the selected threshold was 273.7, also lower than the package insert value of 300 (14).
Discussion
The importance of pre-analytic protocol choices in harmonizing Alzheimer fluid biomarker data is widely acknowledged (5). Encouragingly, data collected in our center after implementing a lower-binding Sarstedt 0.5 mL tube supports a locally computed positivity threshold for similar to other centers, facilitating harmonization, collaboration, and interpretation. Further, within-aliquot repeatability, between-aliquot precision, and LOA were reasonable across analytes, with ICCs all exceeding 0.9. Our research setting, prioritizing individuals who were unimpaired at baseline, may differ from other settings, especially in who contributes both LP and PET (21).
In the full between-subjects dataset, including LP procedures performed over more than a decade, we observed strong effects of both extraction method and tube type. In particular, compared to the Sarstedt 0.5 mL tubes, markedly lower amyloid concentrations were measured in the Fisher 1.5 mL tubes, alongside somewhat lower concentrations in the Fisher 2.0 mL tubes, echoing earlier results on other platforms (7). Because our recruitment criteria have varied in tandem with protocol changes, these between-subjects results should be interpreted cautiously, but a small, within-subjects analysis provides additional evidence that results are not comparable without adjustment for tube type, even on a fully automated platform. Happily, the similarly high AUC we observed for in both tube types against PiB suggests that results retain similar fidelity to PET amyloid positivity when cutpoints are allowed to vary. However, forcing uniformity across tubes substantially inflates the proportion of participants in the higher-binding tubes identified as CSF , potentially doubling the number of unimpaired participants so identified.
Within-subjects data comparing fresh-drip samples collected in Sarstedt 2.5 mL tubes to those frozen in Sarstedt 0.5 mL tubes are scant, but suggest substantial agreement. However, although we did not assess PiB correspondence directly, we saw no samples that could be classified as amyloid positive by fresh drip only, indirectly suggesting that this more resource-intensive method may not improve sensitivity. The logistics of applying the fresh-drip protocol in our lower-throughput research setting, compared to a high-throughput central clinical laboratory, have been prohibitive, necessitating freezing most samples before analysis. In between-subjects examinations of the fresh-drip samples, we observed a strong effect of the freeze–thaw cycle on our results for and , indicating that for credible use, separate fresh and frozen cutpoints are required. The median time in a freezer for these samples was 28 days, reflecting the contemporary state of the supply chain for reagents, but little published data on stability at this interval is available (5, 22). Taken together, our experience with the fresh-drip protocol suggests limited practical benefit in pure research settings.
Limitations
This study has several limitations. Our largest between-subjects analysis is a convenience sample, and coefficient estimates for pre-analytic protocol effects must be interpreted cautiously, as unidentified confounders may be present. Although our within-subjects analyses partially address this, those subsets were kept small to preserve scarce banked fluid and may not be broadly representative. A second difficulty results from the censoring imposed by the measuring ranges of these assays, which likely biases our coefficient estimates downward, and perhaps our reliability as well. Relatedly, the predominance of cognitively unimpaired participants in our sample may limit generalizability to broader cohorts. A third limitation is the lack of amyloid beta 1–40 ), which is often used in ratio with to improve discriminative performance. As argued by the manufacturer, can substitute, but lacks the biological interpretability of . Finally, CSF may be more sensitive to pathologic than PET, and a better assessment of performance vs criterion requires pathology data.
Conclusion
The present work provides general evidence for the reliability of CSF AD biomarker analyses using a commercially available platform. In highlighting the sensitivity of measured analyte levels to pre-analytic protocol differences, our analyses also suggest caution in drawing longitudinal inferences from datasets in which these factors have not remained constant. Similar challenges will be presented by improvements in assays over time, such as the advent of second-generation Elecsys assays, which we do not report here. In such situations, when binary categorization is enough to support inferences, cutpoints should be established separately by tube type or other relevant factors where possible. Future research should include participants from a broader section of the AD continuum, as well as measurements of novel CSF biomarkers (11).
Supplemental Material
Supplemental material is available at Clinical Chemistry online.
Supplementary Material
Acknowledgments
We extend our deepest thanks to the WRAP participants and staff for their invaluable contributions to the study.
Contributor Information
Erin M Jonaitis, Wisconsin Alzheimer’s Institute, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States; Wisconsin Alzheimer’s Disease Research Center, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States; Department of Medicine, Division of Geriatrics and Gerontology, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Beckie Jeffers, Wisconsin Alzheimer’s Institute, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States; Wisconsin Alzheimer’s Disease Research Center, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States; Department of Medicine, Division of Geriatrics and Gerontology, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Monica VandenLangenberg, Wisconsin Alzheimer’s Disease Research Center, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States; Department of Medicine, Division of Geriatrics and Gerontology, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Yue Ma, Wisconsin Alzheimer’s Disease Research Center, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Carol Van Hulle, Wisconsin Alzheimer’s Disease Research Center, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Rebecca Langhough, Wisconsin Alzheimer’s Institute, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States; Wisconsin Alzheimer’s Disease Research Center, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States; Department of Medicine, Division of Geriatrics and Gerontology, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Lianlian Du, Wisconsin Alzheimer’s Disease Research Center, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States; Department of Biostatistics and Medical Informatics, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Nathaniel A Chin, Department of Medicine, Division of Geriatrics and Gerontology, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Robert J Przybelski, Department of Medicine, Division of Geriatrics and Gerontology, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Kirk J Hogan, Department of Anesthesiology, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Bradley T Christian, Wisconsin Alzheimer’s Disease Research Center, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States; Department of Medical Physics, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States; Department of Psychiatry, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Tobey J Betthauser, Wisconsin Alzheimer’s Disease Research Center, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States; Department of Medicine, Division of Geriatrics and Gerontology, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Ozioma C Okonkwo, Wisconsin Alzheimer’s Disease Research Center, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States; Department of Medicine, Division of Geriatrics and Gerontology, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Barbara B Bendlin, Wisconsin Alzheimer’s Disease Research Center, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States; Department of Medicine, Division of Geriatrics and Gerontology, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Sanjay Asthana, Geriatric Research Education and Clinical Center of the Wm. S. Middleton Memorial Veterans Hospital, Madison, WI, United States; Wisconsin Alzheimer’s Disease Research Center, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Cynthia M Carlsson, Geriatric Research Education and Clinical Center of the Wm. S. Middleton Memorial Veterans Hospital, Madison, WI, United States; Wisconsin Alzheimer’s Institute, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States; Wisconsin Alzheimer’s Disease Research Center, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Sterling C Johnson, Geriatric Research Education and Clinical Center of the Wm. S. Middleton Memorial Veterans Hospital, Madison, WI, United States; Wisconsin Alzheimer’s Institute, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States; Wisconsin Alzheimer’s Disease Research Center, School of Medicine and Public Health, University of Wisconsin —Madison, Madison, WI, United States.
Nonstandard Abbreviations
CSF, cerebrospinal fluid; , amyloid beta 1–42; , phosphorylated tau 181; tTau, total tau; PET, positron emission tomography; ICC, intraclass correlation; AUC, area under the curve; AD, Alzheimer disease; MCI, mild cognitive impairment; LP, lumbar puncture; PiB, Pittsburgh compound B; LOA, limits of agreement.
Author Contributions
The corresponding author takes full responsibility that all authors on this publication have met the following required criteria of eligibility for authorship: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved. Nobody who qualifies for authorship has been omitted from the list.
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form.
Research Funding
This work was supported by the National Institutes of Health (grant numbers P30AG062715, RO1AG021155, R01AG027161, R01AG037639, R01AG062167, S10 OD025245-01, UL1TR000427), the Alzheimer’s Association, and Roche Diagnostics.
Disclosures
S.C. Johnson has served as a consultant to Prothena, Eisai, Merck, ALZpath, and Roche Diagnostics, has received an equipment grant from Roche Diagnostics, and has received support (sponsoring of an observational study and provision of precursor for tau imaging) from Cerveau Technologies. B.B. Bendlin and B.T. Christian have received imaging agents and precursor from Avid Radiopharmaceuticals. T.J. Betthauser has received speaking honoraria from Intermountain Healthcare. B.B. Bendlin, advisory board, New Amsterdam Pharma. C.M. Carlsson, Chair, HHS Advisory Council on Alzheimer’s Research, Care, and Services (NAPA Council) and has received travel support for NAPA meetings; honorarium received as Co-Chair NIH/NIA ADRC Program Clinical Task Force Clinical Measures and Diagnosis Subcommittee; received grants to institution: VA Merit 1 I01 CX001261; Armarin Corp (study drugs for VA Merit); NIH/NIA U19 AG010483/Lilly (A4 Trial); NIH/NIA R01 AG049872; Alz Assn/FNIH “LEARN Study”; NIH/NIA R01 AG021155; NIA/NIA R01 AG054059; NIH/NIA U2CAG057441; NIH/NIA RF1AG052324; NIH/NIA R01 AG060737; BAN2401-G000-303 (NIH/Eisai); NIH/NIA 1U19AG065188-01; NIH/NIA 1RF1AG066837; UW Department of Medicine Pilot Funding; NIH/NIA P30 AG062715; NIH/NIA 2RF1 AG027161; honoraria given for talks at Indiana Neurological Society and Northwestern University Medical Grand Rounds; travel funding from Alzheimer’s Association (Chair of US POINTER DSMB) and CTAD (Plenary Speaker at CTAD 2021); honorarium for chairing DSMB for 2 NIH clinical trials (TDAD [U Kansas], D-CARE [UCLA]); Amarin Corp (Vascepa/placebo study drugs for VA-funded trial) study drugs for trial given to institution. R.J. Przybelski, Genentech advisory panel for one meeting. S. Asthana, received NIH/NIA T32 Training Grant; royalty as an editor of a textbook entitled Hazzard's Geriatrics and Gerontology (McGraw-Hill).
Role of Sponsor
The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
References
- 1. Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement 2018;14:535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mattsson N, Lönneborg A, Boccardi M, Blennow K, Hansson O; Geneva Task Force for the Roadmap of Alzheimer’s Biomarkers . Clinical validity of cerebrospinal fluid Aβ42, tau, and phospho-tau as biomarkers for Alzheimer’s disease in the context of a structured 5-phase development framework. Neurobiol Aging 2017;52:196–213. [DOI] [PubMed] [Google Scholar]
- 3. Ashton NJ, Leuzy A, Karikari TK, Mattsson-Carlgren N, Dodich A, Boccardi M, et al. The validation status of blood biomarkers of amyloid and phospho-tau assessed with the 5-phase development framework for AD biomarkers. Eur J Nucl Med Mol Imaging 2021;48:2140–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hansson O, Seibyl J, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T, et al. CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimer’s Dement 2018;14:1470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hansson O, Batrla R, Brix B, Carrillo MC, Corradini V, Edelmayer RM, et al. The Alzheimer’s Association international guidelines for handling of cerebrospinal fluid for routine clinical measurements of amyloid β and tau. Alzheimer’s Dement 2021;17:1575–82. [DOI] [PubMed] [Google Scholar]
- 6. Hansson O, Mikulskis A, Fagan AM, Teunissen C, Zetterberg H, Vanderstichele H, et al. The impact of preanalytical variables on measuring cerebrospinal fluid biomarkers for Alzheimer’s disease diagnosis: a review. Alzheimer’s Dement 2018;14:1313–33. [DOI] [PubMed] [Google Scholar]
- 7. Vanderstichele HMJ, Janelidze S, Demeyer L, Coart E, Stoops E, Herbst V, et al. Optimized standard operating procedures for the analysis of cerebrospinal fluid Aβ42 and the ratios of Aβ isoforms using low protein binding tubes. J Alzheimer’s Dis 2016;53:1121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roche Diagnostics. Roche Alzheimer's disease Cerebrospinal Fluid (CSF) assays receive FDA clearance, supporting more accurate and timely diagnosis. 2022. https://diagnostics.roche.com/us/en/news-listing/2022/roche-alzheimers-disease-cerebrospinal-fluidassays-receive-fda-clearance.html (Accessed January 2024).
- 9. Bittner T, Zetterberg H, Teunissen CE, Ostlund RE, Militello M, Andreasson U, et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1-42) in human cerebrospinal fluid. Alzheimer’s Dement 2016;12:517–26. [DOI] [PubMed] [Google Scholar]
- 10. Willemse EAJ, van Maurik IS, Tijms BM, Bouwman FH, Franke A, Hubeek I, et al. Diagnostic performance of Elecsys immunoassays for cerebrospinal fluid Alzheimer’s disease biomarkers in a nonacademic, multicenter memory clinic cohort: the ABIDE project. Alzheimer’s Dement (Amst) 2018;10:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Hulle C, Jonaitis EM, Betthauser TJ, Batrla R, Wild N, Kollmorgen G, et al. An examination of a novel multipanel of CSF biomarkers in the Alzheimer’s disease clinical and pathological continuum. Alzheimer’s Dement 2021;17:431–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roche Diagnostics International Ltd. Electrochemiluminescence immunoassay (ECLIA) for the in vitro quantitative determination of ß-Amyloid (1-42) in human Cerebrospinal fluid (CSF). 2017. http://web.archive.org/web/20190713224323/https://diagnostics.roche.com/global/en/products/params/elecsys-abeta42.html (Accessed November 2021).
- 13.Roche Diagnostics International Ltd. Electrochemiluminescence immunoassay (ECLIA) for the in vitro quantitative determination of phosphorylated Tau in human Cerebrospinal fluid (CSF). 2017. https://diagnostics.roche.com/global/en/products/params/elecsysptau.html (Accessed November 2021).
- 14.Roche Diagnostics International Ltd. Electrochemiluminescence immunoassay (ECLIA) for the in vitro quantitative determination of total Tau in human Cerebrospinal fluid (CSF). 2017. https://diagnostics.roche.com/global/en/products/params/elecsys-ttau.html (Accessed November 2021).
- 15. Johnson SC, Koscik RL, Jonaitis EM, Clark LR, Mueller KD, Berman SE, et al. The Wisconsin Registry for Alzheimer’s prevention: a review of findings and current directions. Alzheimer’s Dement (Amst) 2018;10:130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Besser L, Kukull W, Knopman DS, Chui H, Galasko D, Weintraub S, et al. Version 3 of the National Alzheimer’s Coordinating Center’s Uniform Data Set. Alzheimer Dis Assoc Disord. 2018;32:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson SC, Christian BT, Okonkwo OC, Oh JM, Harding S, Xu G, et al. Amyloid burden and neural function in people at risk for Alzheimer’s disease. Neurobiol Aging 2014;35:576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koscik RL, Betthauser TJ, Jonaitis EM, Allison SL, Clark LR, Hermann BP, et al. Amyloid duration is associated with preclinical cognitive decline and tau PET. Alzheimer’s Dement (Amst) 2020;12:e12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. R Core Team . R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2020. [Google Scholar]
- 20.Mayo Clinic Laboratories. Test definition: ADEVL (Alzheimer's disease evaluation, CSF). 2021. https://www.mayocliniclabs.com/test-catalog/download-setup.php?format=pdf&unit_code=607273 (Accessed August 2021).
- 21. Blazel MM, Lazar KK, Van Hulle CA, Ma Y, Cole A, Spalitta A, et al. Factors associated with lumbar puncture participation in Alzheimer’s disease research. J Alzheimer’s Dis 2020;77:1559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Janelidze S, Stomrud E, Brix B, Hansson O. Towards a unified protocol for handling of CSF before β-amyloid measurements. Alzheimer’s Res Ther 2019;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Coded data and R scripts underlying all analyses may be shared at the request of any qualified investigator for purposes of replication. Requests for data should be submitted separately to the WRAP and ADRC executive committees via https://wrap.wisc.edu/data-requests-2/ (accessed November 17, 2023).