Abstract
The mechanosensory lateral line system is the flow sensing system present in all 34 000+ species of fishes. Its neuromast receptor organs, located on the skin or in bony canals on the head and tubed scales on the trunk, respond to the near field component of acoustic stimuli as well as short range, low frequency (0–200 Hz) water flows of biotic and abiotic origin. Here, I discuss the genesis of my research career and its focus on the structural and functional evolution of the lateral line system among a wide taxonomic range of fishes including those from different aquatic habitats (tropical lakes to coral reefs and the deep sea). I discuss the importance of investigating structure before function, using investigations in my laboratory that had unexpected outcomes, as well as the role of serendipity in the evolution of a career and in the nature of scientific discovery.
I. INTRODUCTION
Two mechanosensory systems (auditory/vestibular, lateral line) were present in the first fishes and have evolved over 400 million years in diverse aquatic environments all characterized by complex acoustics (Fay et al., 2008; Webb et al., 2008). The mechanosensory lateral line (LL) system was first described in the 17th century but was not recognized as a sensory system until almost 200 years later (reviewed by Coombs and Bleckmann, 2014). In the late 19th century, the anatomy and development of the LL system in different taxa were described and illustrated in expeditionary reports (e.g., Garman, 1899) and anatomical monographs (e.g., Allis, 1889; Collinge, 1895; Clapp, 1898). Anatomical studies of the LL system of fishes (among the 34 000+ extant species) continue to this day (reviewed in Webb, 1989a, 2014a,b; Sato, 2022; also see Webb and Ramsay, 2017; Marranzino and Webb, 2018; Nickles et al., 2020).
The LL system mediates flow sensing by responding to low frequency (0–200 Hz) near field acoustic stimuli and short range hydrodynamic stimuli of abiotic and biotic origin; these play critical roles in the behavior and ecology of fishes (reviewed in Kasumyan, 2003; Montgomery et al., 2014; Coombs and Montgomery, 2014; Mogdans, 2019). Historically, the auditory/vestibular, mechanosensory LL, and electrosensory systems were considered to comprise a single octavolateralis system (Platt et al., 1989) based on some common developmental, structural, and functional attributes (see Coombs and Bleckmann, 2014). The sensory organs of the LL system (neuromasts; see Webb et al., 2019) are similar to the sensory maculae of the inner ear, in that they are comprised of sensory hair cells, which along with the afferent sensory neurons that innervate them and arise from cranial ectodermal placodes in the embryo (the otic placode and a series of LL placodes, respectively). Each hair cell has a single kinocilium and multiple stereocilia (stereovilli) to one side of it on the cell surface, which imparts both structural and functional asymmetry (an axis of best physiological sensitivity). The ciliary bundles of all of the hair cells in a sensory epithelium (macula or neuromast, respectively) are embedded in a gelatinous matrix (an otolithic membrane or dome-shaped cupula, respectively) that covers the entire hair cell epithelium. Sensory transduction occurs when fluid flows cause the deflection of the ciliary bundles. Despite these similarities, the two systems are distinct with respect to their gross morphology and functional roles (Platt et al., 1989). The auditory system is comprised of a bilateral pair of complex sense organs (the inner ears) that include several sensory epithelia (maculae), while the LL system is comprised of dozens to thousands of individual sense organs (neuromasts; Fig. 1) distributed on the skin (superficial neuromasts, SNs) and in hollow, fluid-filled canals (canal neuromasts, CNs) on the head, trunk, and tail. The sensory hair cells are innervated by neurons of different cranial nerves—the auditory nerve (VIII, = auditory-vestibular nerve) or the anterior, middle, and posterior LL nerves, which form distinct ganglia and central projections in the hindbrain (Wullimann and Grothe, 2014).
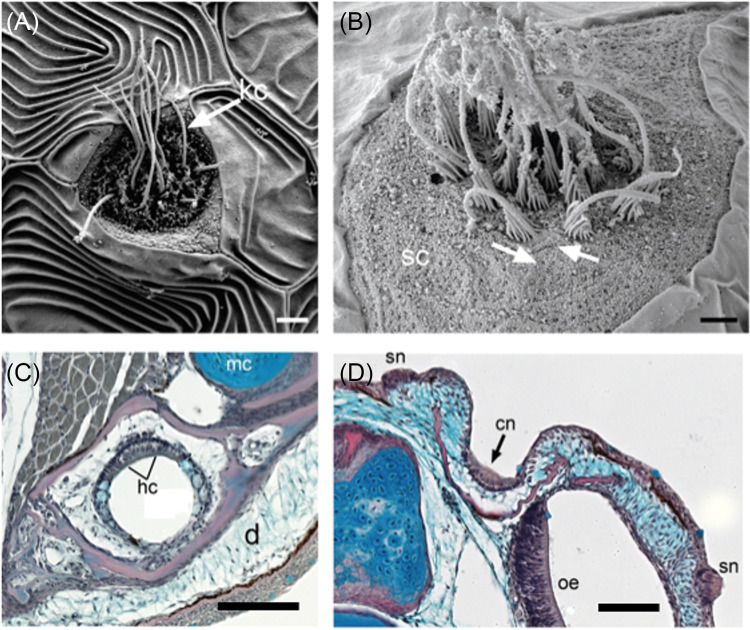
FIG. 1.
(Color online) Neuromasts in teleost fishes. (A) Scanning electron micrograph (SEM) of a SN on the head of a larval cichlid (Aulonocara stuartgranti). Long kinocilium (kc, arrow) and multiple shorter stereocilia (= stereovilli; not visible) extend from the surface of the hair cells. Scale bar = 2 μm. (B) SEM of SN on the trunk of a newly settled juvenile goby (Elacatinus lori) with ciliary bundles (each has one long kinocilium, and several stereovilli, which are graded in length). Hair cells have opposing polarities (arrows) and are surrounded by non-sensory support cells (sc). Scale bar =1 μm. (C) Histological section through the mandibular canal (in lower jaw) in a cichlid (Labeotropheus fuelleborni). The canal is located deep in the dermis (d). Hair cells (hc) in the canal neuromast have prominent nuclei; non-sensory cells and mucus-secreting cells (blue). Meckel's cartilage (mc) and the LL canal are incorporated into the dentary bone (pink), Scale bar = 50 μm. (D) Histological section through a canal neuromast in the supraorbital canal of a juvenile cichlid (Tramitichromis sp.), located within the trough-like nasal bone (pink), which has not yet enclosed the neuromast within the canal. Two superficial neuromasts (sn) and the ciliated olfactory epithelium (oe) are visible, Scale bar = 50 μm. Reprinted with permission from Webb, Collin, Kuciel, Schulz-Mirbach, Zuwala, Denizot, and Kirschbaum, “Sensory organs,” in The Histology of Fishes, 1st ed. Copyright 2019 Taylor and Francis Group, LLC, a division of Informa plc.
The auditory and LL systems both respond to the near-field component of acoustic stimuli (particle displacement, close to a source), which deflects the hair cells. However, the LL system responds to lower frequencies (0–200 Hz) in the near-field and is thus complementary to the ear's ability to respond to a wider range of frequencies in the near-field and as well as in the far-field (sound pressure waves) if a gas-filled swim bladder is present (Platt et al., 1989; Webb et al., 2008; Braun and Grande, 2008; Schulz-Mirbach et al., 2013b; Schulz-Mirbach and Ladich, 2021; Tricas and Webb, 2016). An acoustic stimulus may simultaneously provide inputs to the auditory and LL systems via the recessus lateralis in clupeomorphs (herrings and relatives, Denton and Gray, 1983, 1993), and perhaps the laterophysic connection (LC) present in some chaetodontids (coral reef butterflyfishes; see Sec. III C). Central integration of auditory and LL inputs (e.g., Bleckmann and Mogdans, 2014; Higgs and Radford, 2016; Braun and Sand, 2014; Mensinger, 2016) provides fishes with information about acoustic stimuli that cannot be derived from input to just one of the two systems.
The neuromast organs of the LL system (Fig. 1), found in stereotyped locations on the skin (SNs) and within pored canals (canal neuromasts, CNs; Fig. 1, 2; see Webb, 2014a,b) define two LL sub-modalities. SNs function as velocimeters, responding directly to fluid flows, but CNs function as accelerometers (responding indirectly to water flows as the result of the pressure differentials at adjacent canal pores). On the head of bony fishes, the cranial LL canals are typically associated with an evolutionarily conserved subset of the flat, dermal bones that cover the skull (the “LL bones”; Fig. 2), despite considerable variation in bone size and shape among species. Nevertheless, five cranial canal phenotypes (defined by canal width and degree of development) are present among bony fishes [Webb, 2014b; Figs. 3(A)–3(E)].
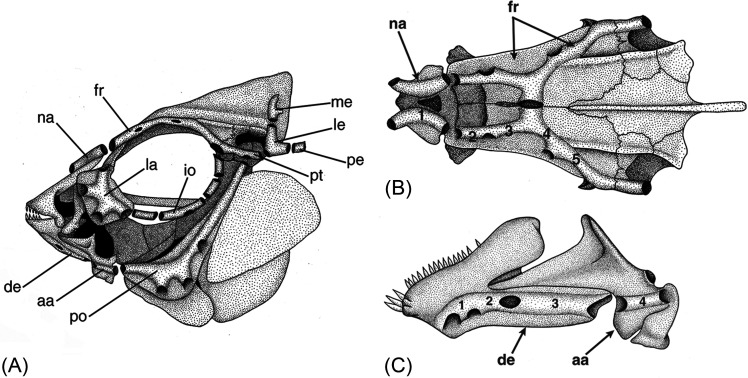
FIG. 2.
Skull of a bony fish (a teleost), the convict cichlid (Amatitlania nigrofasciata = Archocentrus nigrofasciatus), illustrating pored LL canals contained within dermatocranial bones. (A) Lateral view showing the supraorbital canal in the nasal (na) and frontal (fr) bones, infraorbital canals in the lacrimal (la) and infraorbital series (e.g., io), preopercular canal in the preoperculum (po), mandibular canal in the dentary (de) and angulo-articular (aa), otic canal in the pterotic (pt), supratemporal commissure in the lateral and medial extrascapular bones (le, me), and post-otic canal in the posttemporal bone (pe). (B) Dorsal view showing the supraorbital canal in the nasal (na) and frontal (fr) bones. (C) Ventrolateral view of mandibular canal in dentary (de) and angulo-articular (aa) bones. Numbers (in B and C) indicate the location of neuromasts between adjacent canal pores within the supraorbital and mandibular canals. (A) Reprinted with permission from Webb, “Mechanosensory lateral line: functional morphology and neuroanatomy,” in Handbook of Experimental Animals—The Laboratory Fish, Copyright 2000 Elsevier. (B) and (C) Reprinted with permission from Tarby and Webb, “Development of the supraorbital and mandibular lateral line canals in the cichlid, Archocentrus nigrofasciatus,” J. Morphol. 254, 44–57 (2003). Copyright 2003 Wiley-Liss, Inc.
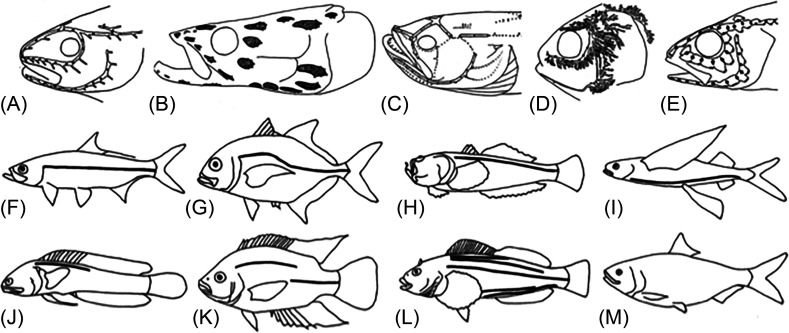
FIG. 3.
Diversity in cranial LL canals (A)–(E), five canal patterns and trunk canals [(F)–(M), eight patterns] found among teleost fishes. (A) Narrow-simple canals, in saithe, Pollachius virens. (B) narrow canals with widened tubules (gray), in Arapaima gigas, (C) reduced canals with lines of SNs (dots) in the plainfin midshipman (Porichthys notatus), (D) narrow canals with branched tubules in Atlantic menhaden (Brevoortia tyrannus), (E) widened canals in common percarina (Percarina demidoffi). (F) complete straight canal in Atlantic tarpon (Megalops atlanticus), (G) complete arched canal in a carangid, (H) dorsally placed canal in a stonefish, (I) ventrally placed canal in a flying fish, (J) incomplete canal in a blenny, (K) disjunct canal in a cichlid, (L) multiple canals in a greenling (see Sec. III A), (M) absence of trunk canal in a menhaden). Sources: (A) Redrawn with permission from Marshall, “Systematic and biological studies of the macrourid fishes (Anacanthini Teleostii),” Deep Sea Res. 12, 299–322 (1965). Copyright 1965 Elsevier. (B) Redrawn with permission from Nelson, “Infraorbital bones and their bearing on the phylogeny and geography of osteoglossomorphs fishes,” Am. Mus. Novit. No. 2394 (1969). Copyright 1969 the American Museum of Natural History. (C) Redrawn with permission from Greene, “The phosphorescent organs in the toadfish, Porichthys notatus Girard,” J. Morphol. 15, 667–696 (1899). Copyright 1899 Wiley. (D) Redrawn with permission from Hoss and Blaxter, “Development and function of the swimbladder-inner ear system in the Atlantic menhaden, Brevoortia tyrannus (Latrobe),” J. Fish Biol. 20, 131–142 (1982). Copyright 1982 Wiley & Sons, Inc. (E) Redrawn with permission from Jakubowski, “Cutaneous sense organs of fishes. Part VII. The structure of the system of lateral-line canal organs in the Percidae,” Acta Biol. Cracov. Ser. Zool. 10, 69–81 (1967); licensed under an Attribution-NonCommercial-NoDerivs 3.0 Unported (CC BY-NC-ND 3.0; https://creativecommons.org/licenses/by-nc-nd/3.0/license. (F)–(M) Modified with permission from Nelson, Grande, and Wilson, Fishes of the World, 5th ed. Copyright 2016 Wiley-Liss, Inc.
The trunk canal (“the LL” of the classic ichthyology literature), is comprised of a series of small canal segments, typically embedded within overlapping LL scales in a horizontal series extending along the length of the trunk (Fig. 4). Eight patterns [Figs. 3(F)–3(M); see Sec. III A] describe the diversity of the trunk canals among bony fishes. In addition to the CNs in the cranial and trunk canals, SNs (Fig. 1) are found on the skin of the head, trunk and tail (e.g., Nickles et al., 2020; Sato et al., 2017; Sato et al., 2021; see Sec. III C).
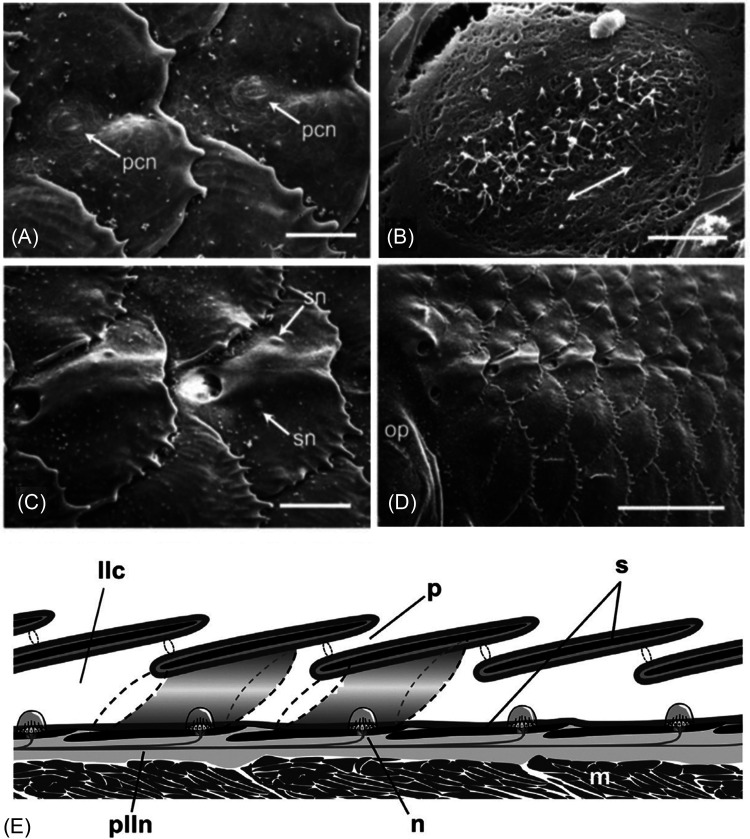
FIG. 4.
LL scales on the trunk of teleost fishes. (A) SEM of the developing trunk canal in the convict cichlid, Amatitlania nigrofasciata (=Archocentrus nigrofasciatus). Two LL scales prior to enclosure of tubular canal segment (pcn, presumptive canal neuromasts), scale bar = 100 μm; (B) Close-up of a presumptive canal neuromast as in (A); double-headed arrow indicates the axis of best physiological sensitivity of hair cells, scale bar = 10 μm; (C) SEM of two LL scales after canal segments have formed, with additional superficial neuromasts (sn) on the skin, scale bar = 1 mm; and (D) anterior end of the trunk canal (just caudal to operculum, op) showing several overlapping scales, scale bar = 1 mm; (E) Schematic of overlapping LL scales (based on ten species of pomacentrids, embiotocids, and pleuronectids). LL scales (black) sit beneath the epidermis (gray) at a shallow angle in the dermis (light gray). Tubular canal segments (see shaded tubes ending in dotted lines) form a continuous, epithelium-lined canal that runs parallel to the skin surface. The infrascalar and suprascalar pores [right and left, respectively, in two scales depicted three-dimensionally (3-D)] are represented by dashed lines. Rostral to left. Abbreviations: llc, LL canal; m, trunk muscle; n, canal neuromast; p, pore (small circles); plln, posterior LL nerve; s, scale. (A)–(D) Modified from Webb, J. F., Maruska, K. P., Butler, J. M., and Schwalbe, M. A. B. (2021). “The mechanosensory lateral line system of cichlid fishes: From anatomy to behavior,” in The Behavior, Ecology and Evolution of Cichlid Fishes, edited by M. E. Abate and D. L. G. Noakes (Springer, Dordrecht), pp. 401–442 (https://link.springer.com/book/10.1007/978-94-024-2080-7) with permission of Springer Nature. (E) Reprinted from Fig. 8 of Webb, J. F. and Ramsay, J. B. (2017). “New interpretation of the 3-D configuration of lateral line scales and the lateral line canal contained within them,” Copeia 105, 339–347, with permission from the American Society of Ichthyologists and Herpetologists.
II. THE GENESIS OF MY WORK ON THE LL SYSTEM
I always knew that I wanted to study biology, but I could not have predicted that I would study the LL system, or even fishes. Yet hard work and persistence, as well as naivete and serendipity, all played key roles in setting me on my career path (students, take note!). In the 1970s, Jacques Cousteau was thrilling us with his explorations of the “Undersea World” on television and the New York Aquarium (on the iconic Coney Island boardwalk) was down the street from our apartment building in Brooklyn, New York. I attended John Dewey High School, an experimental New York City public high school that was a unique alternative to the traditional neighborhood high schools. Founded in 1969, it was filled with young, energetic, idealistic, and creative teachers who made it seem like anything was possible. It was also the only high school that offered courses in marine biology. These included beach surveys, trips to Woods Hole (a bit prophetic) and to local marshes to collect fishes and invertebrates that filled our large student-run aquarium room, and annual lobster feasts (thinly disguised as “dissections”). We were riding the wave of the environmental movement after the first Earth Day and were encouraged to become docents (volunteers) at the NY Aquarium where we shared our excitement about the marine organisms on exhibit with aquarium visitors. This was not your typical New York City public high school experience, and it is something for which I will be ever grateful.
As a major in Biological Sciences at Cornell University, I followed a rather traditional curriculum, but I also took an immersive summer course at Cornell's Shoals Marine Laboratory (shoalsmarinelaboratory.org). That was the transformative experience that solidified my desire to pursue a career in marine biology. However, I applied to graduate school still not knowing what I really wanted to study. I attended Boston University whose Marine Program was in residence at the world-renowned Marine Biological Laboratory (MBL) in Woods Hole at the time (to which I now return for part of each summer). Jelle Atema became my Ph.D. advisor and introduced me to the field of sensory biology. When I met Harvard ichthyologist Karel Liem at a seminar he gave at Cornell during my senior year, I asked if I could sit in on his Biology of Fishes course when I arrived in Boston (Boston University did not offer such a course at the time). He said yes, which was the first of many acts of generosity he bestowed upon me throughout my career. The stimulating and entertaining lectures in the course introduced me to the fields of comparative and functional morphology as well as systematics—this was the game changer. Had Boston University offered a course in fish biology in at the time, I would be telling a very different story. Serendipity! Throughout graduate school, I continued to visit Karel's lab, volunteer in the fish collection (in Harvard's Museum of Comparative Zoology, MCZ) with invaluable mentorship from then fish collection manager, Karsten Hartel, and mine the literature in the MCZ's incredible Ernst Mayr Library. The MCZ provided me with an intellectual home, something that I did not realize would be so important for my career trajectory. It gave me access to resources that were critical to my success as a Ph.D. student, and to this day, given my appointment as an Associate in Ichthyology (2009–present).
The 1980s were an exciting time at Harvard—the giants of evolutionary biology (Gould, Lewontin, Mayr, Wilson, etc.) engaged in lively discussions at seminars given by prominent speakers from Harvard and elsewhere, and the key ideas being debated at that time would form the conceptual basis of my research program. Cladistic systematics (evolutionary relationships based on nested shared derived characteristics instead of overall similarity) was transforming the field of ichthyology, in particular, and provided the framework in which I would interpret the evolution of the LL system. The iconic paper, “The Spandrels of San Marco and the Panglossian Paradigm: A Critique of the Adaptationist Programme” (Gould and Lewontin, 1979) rid us of the idea that every feature of a fish skeleton, for instance, was the result of adaptation via natural selection. The emerging field of evolutionary developmental biology (“evo-devo”) revealed how variation in developmental patterns and processes could be used to predict and interpret evolutionary trends in morphology (e.g., heterochrony). The importance of “developmental constraints” was first made clear to me in a conversation with Per Alberch (herpetologist and evolutionary biologist). When I told him that I was interested in evolutionary variation in the LL system, he told me to pay attention to morphological variants that are not present instead of just those that are present. All of these ideas are reflected in many of my papers (see Webb, 1989a,b, 1999; Webb, et al., 2014; Bird and Webb, 2014).
Yet, how did I start working on the LL system in the first place? At Harvard, I was surrounded by people studying the functional morphology of feeding in fishes and I became interested in filter feeding (in herrings, anchovies, menhadens, etc.). One summer day in Woods Hole I pulled a preserved menhaden (Brevoortia tyrannus) out of a jar with the intention of measuring and counting its gill rakers (which enable filter feeding). I left the lab for about 15 min (OK, bad form) and when I came back, the alcohol had evaporated from the surface of the fish revealing what I then learned were the highly branched tubules of the cranial LL canals. I ran to the MBL Library and read everything I could about the LL system, especially in menhaden and its relatives by John Blaxter and his colleagues (e.g., Hoss and Blaxter, 1982; Blaxter, 1987). That is how it all started. Serendipity!
Soon after, Jelle Atema, along with Richard Fay, Arthur Popper, and William Tavolga started organizing the Sensory Biology of Aquatic Animals conference in Sarasota, FL, 1985 (see Atema et al., 1988). I was certainly intrigued by the LL system, but I was still searching for the Ph.D. project. When I asked if I could attend the conference, Jelle told me that I could do so, but that I would need to write a book chapter on the evolution of the LL system with Sheryl Coombs and John Janssen (then at Loyola University in Chicago) neither of whom I knew. I never figured out how an unknown graduate student, who had not yet published, was offered up to two faculty members as a co-author (for an invited book chapter, no less), but I ran with it and my naivete worked to my benefit. I called Sheryl Coombs and introduced myself (as if this was normal), then I travelled to Chicago to make plans for the work that would comprise this book chapter (Coombs et al., 1988).
As this was long before Google, I started to search for data on the LL system by scouring the MCZ Ichthyology Department's reprint collection and it proved to be a gold mine. Buried within species descriptions and data matrices used to construct new phylogenies were descriptions of the LL canals and neuromasts in all sorts of fishes. I also started attending meetings of the American Society of Ichthyologists and Herpetologists (ASIH) and absorbed all of the information on the LL system that I could, which was scattered among talks and posters on fish morphology, taxonomy, and systematics. I asked questions of experts on different groups of fishes, and I was the recipient of a great deal of good will. Ichthyologists from all over the world sent me their papers, copies of file cards with pertinent paper citations, and names of species they said I must look at. At the time, few in the ichthyology community were studying the LL system, per se, but everyone wanted to learn more about “their fish,” so it was a win-win.
At this time, research on the LL physiology and LL-mediated behavior was largely focused on a few small, robust, freshwater or nearshore marine fishes (see Coombs and Bleckmann, 2014; Coombs, 2023). This work had not yet been integrated with what was known about phylogenetic variation in LL morphology, and this was the niche that I set out to fill. At the Sarasota conference, I met all the luminaries in sensory biology of aquatic animals and was truly inspired by all of them. Sheryl Coombs gave a talk on the diversity of the LL system based on our extensive literature review (my first publication; Coombs et al., 1988) and I presented a poster on variation in LL morphology in a phylogenetic framework (my first dissertation chapter; Webb, 1989b). The degree of structural diversity and the evolutionary patterns demonstrated by the LL system turned out to be a revelation for many. When John Blaxter asked me to explain my poster to Eric Denton, John Gray, and himself, one of them (I cannot recall whom) commented that he had no idea that there was so much diversity in the system. This admission was shocking to me (after all, they were the world's experts on the system), but it convinced me that there was something important and unique in the approach that I was taking. The validation I received at this conference was unprecedented and invaluable. Art Popper told me to forget about gill rakers and filter feeding and stick to the LL system (almost a direct quote) and I followed his sage advice. During the conference, I was also offered post-docs in two labs, but this was a bit premature.
The balance of my dissertation focused on the LL system of cichlids and their relatives (wrasses, parrotfishes, damselfishes, surfperches—the suborder “Labroidei”; Webb, 1989c, 1990a,b). This decision was intentional as Karel Liem, his students, and new Harvard faculty member, Melanie Stiassny (now curator at the American Museum of Natural History in New York) were all working on these fishes, which provided interest in and a broader context for my work. At Boston University, I started rearing two species of cichlids, Nile tilapia (Oreochromis niloticus), which were being maintained by another student, and the convict cichlid (Amatitlania nigrofasciata = Cichlasoma nigrofasciatum), which was common in pet stores, in order to study the development of the LL system. Art Popper invited me to do scanning electron microscopy in his department at Georgetown Medical School, and the images that I acquired were the basis for my second dissertation chapter (Webb, 1989c; see Fig. 4). This set the scene for my subsequent work on the multiple trunk canals of hexagrammid fishes (see Sec. III A) and on the cranial LL canals in the convict cichlid (Tarby and Webb, 2003), other cichlids (see Sec. III C), and in the zebrafish (Webb and Shirey, 2003). Soon after I finished my Ph.D., I gave an invited talk at the 1987 Lateral Line Conference in Bielefeld, Germany (see Coombs et al., 1988) in which I talked about how heterochrony [alteration in the timing (rate, onset, offset) of the process of canal formation] could be used to explain the diversification of cranial LL canal and trunk canal phenotypes among species (Webb, 1989a).
To broaden my training, I did NIH/NRSA post-docs with Glenn Northcutt at the Scripps Institution of Oceanography on neuroanatomy of the LL system in non-teleost bony fishes (Webb and Northcutt, 1991, 1997) and with Drew Noden at the New York State College of Veterinary Medicine at Cornell, where I studied the development the neuronal placodes using the chick-quail chimera system. My intention was to transfer these skills to the study of the placodal origins of the LL system in fishes; however, this project never came to fruition (but see Webb and Noden, 1993). I then taught Vertebrate Developmental Biology at Illinois Wesleyan University for a semester and did a summer Grass Fellowship at Friday Harbor Laboratories (University of Washington), before accepting my first faculty job in 1993 at Villanova University. Thirteen years later I moved my research lab to the University of Rhode Island where I run its Marine Biology Program and hold the George and Barbara Young Chair in Biology (2016–present).
III. STRUCTURE AND FUNCTION OF THE LL SYSTEM
Throughout my career, my research has drawn inspiration from the work that I did as a graduate student. I have continued to focus on the structural evolution of the LL system using morphological and developmental approaches (with an expanding toolbox of visualization methods, e.g., μCT, vital fluorescent staining) as well as experimental approaches in order to determine the role of the LL system in prey detection. Over the years, I have chosen study species depending on the questions I wanted to ask. Some projects investigated the morphological attributes of a particular taxon (butterflyfishes, flatfishes, minnows, cichlids), and others used the attributes of model species (convict cichlid, Tarby and Webb, 2003; zebrafish, Webb and Shirey, 2003; little skate, Webb and Gillis, 2013; line snout goby, Nickles et al., 2020; brook trout, Jones, 2023) to learn about fundamental features of the LL system in the larger taxonomic groups to which they belong. Here, I will focus on just a few projects involving detailed anatomical analyses that have provided critical contexts for the consideration of the functional evolution of the LL system.
A. Multiple trunk canals—Are five canals better than one?
My dissertation work showed that eight types of trunk canals are found among bony fishes [Coombs et al., 1988; Webb, 1989b; Fig. 3(F)–3(M)]. These are defined by canal position [mid-lateral (and either straight, or arched over the pectoral fin), or dorsally or ventrally displaced], degree of development (complete, incomplete, disjunct), and number (multiple canals; up to five canals; Fig. 3). The multiple canal phenotype [Fig. 3(L)] is the rarest of the eight patterns, occurring only in a handful of fish families (Webb, 2014b). A simple hypothesis to explain the adaptive significance of multiple canals was that the larger number of neuromasts within these canals enhances the overall sensitivity of the system or increases spatial resolution for the interpretation of water flows. This hypothesis was based on an a priori assumption that a neuromast is present in each LL scale in each of the five canals, and there was no reason to think otherwise (see Klein et al., 2013).
To explore this further, my first MS student Angela (Wonsettler) Ridgel (now on the faculty at Kent State University) determined the distribution of neuromasts within the five trunk canals in two species of greenlings (Family Hexagrammidae, Hexagrammos decagrammus and Hexagrammos stelleri). She found that in H. stelleri, the tubular LL scales comprising the five canals were almost identical in morphology. However, she showed that neuromasts are found in only one of the five canals—the one that runs along the mid-flank (and is likely the homologue of the single canal in other fishes)—and that none of the canals are connected to the canals on the head (Wonsettler and Webb, 1997). So, what could their function possibly be? How do the canals develop in the absence of neuromasts given the long-standing idea that neuromasts induce the formation of canal segments around them (discussed in Webb, 2014a,b)?
Our histological analysis of a lab-raised growth series of H. stelleri (provided by Jeffrey Marliave, Vancouver Aquarium), showed that the canal that contains neuromasts develops like the single canal found in other species [Figs. 4(A)–4(D)]; however, neuromasts are not present in the other four canal series either before or after the tubed LL scales that comprise those canals have formed (in larvae and juveniles; Wonsettler and Webb, 1997). This was a surprising result (and contrary to work on other taxa with multiple canals; Klein et al., 2013). In order to explain this, we hypothesized that placode-derived primordia (like those described in detail in zebrafish; López-Shier et al., 2004; Chitnis et al., 2011; Ghysen et al., 2014) migrate along five discrete paths on the trunk, but cells differentiate into neuromasts along only one of these paths, and that undifferentiated placode-derived cells are sufficient to guide or induce the development of the tubed LL scales that comprise the other four canals. This work taught us an important lesson—that the construction of meaningful functional hypotheses requires an understanding of structure. Our developmental hypothesis remains untested (but see Wada et al., 2014) and the functional significance of multiple canals in these fishes remains unknown.
B. How a bit of obscure skeletal anatomy revealed the importance of bioacoustics in butterflyfishes on coral reefs
Our work on coral reef butterflyfishes (Family Chaetodontidae) is a prime example of the importance of serendipity in scientific discovery. In his Ph.D. dissertation at the University of Hawaii, Stanley Blum (now working for Biodiversity Information Standards, TDWG) determined that the butterflyfishes in the genus Chaetodon have a small hole (fossa) in the medial (inside) surface of the supracleithrum, a small bone located at the posterior margin of the skull [Fig. 5(F); Blum, 1988]. It was suggested to him that this medial fossa might be the site of a connection to the swim bladder, so he asked me if I could do histology to determine if this was indeed the case. I purchased an eightband butterflyfish (Chaetodon octofasciatus), which was the least expensive butterflyfish at a local pet store, based on the assumption that since the medial fossa was a synapomorphy (shared derived characteristic) of the genus Chaetodon, all species would be similar. My initial histological analysis revealed a bilateral pair of anterior extensions of the swim bladder (horns) that adhere to the medial fossa in the supracleithrum forming a multi-layered soft tissue “tympanum” between the air-filled swim bladder horn and the fluid-filled LL canal within the supracleithrum, which is contiguous with the other cranial LL canals [Figs. 5(A) and 5(B)]. I coined the term “laterophysic connection” (LC) to describe this association (Webb, 1998), as an analog to the otophysic connections between the swim bladder and ear found in other fishes; Braun and Sand, 2014). I hypothesized that sound pressure waves originating in the swim bladder would be propagated along the swimbladder horns, causing deflections of the tympanum and fluid movements in the LL canal thus stimulating nearby CNs (Webb, 1998). If this were the case, this would expand the functional repertoire of the LL system to include pressure reception (normally the realm of the ear) in these fishes.
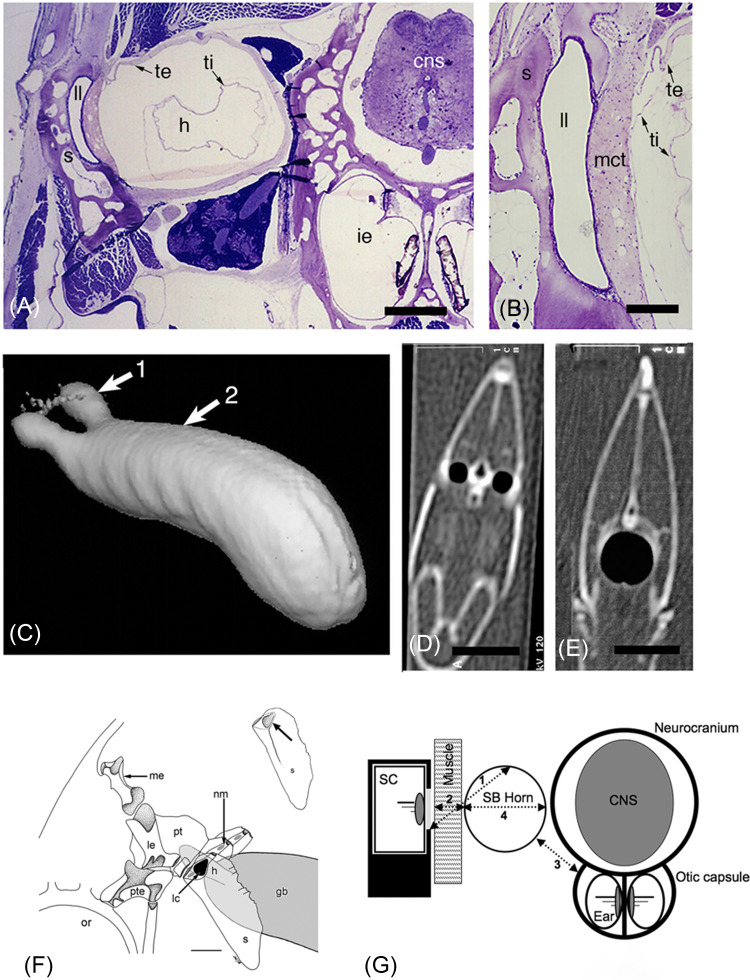
FIG. 5.
(Color online) LC (laterophysic connection) in Chaetodon spp. (A) Transverse histological section through the LC in C. octofasciatus. Scale bar = 500 μm. (B) Close-up of tympanum between the air-filled swim bladder horn and fluid-filled LL canal in C. octofasciatus. Scale bar = 200 μm. (C) Computed tomographic (CT) 3-D reconstruction of the volume of air in the anterior swim bladder horns and swim bladder in C. ephippium (lateral view, rostral to left) derived from two-dimensional (2-D) slices. (D) CT slice at level of arrow 1 (swim bladder horns) in C. (E) CT slice at level of arrow 2 (body of swim bladder) in (C). Scale bars in (D) and (E) =10 mm. Abbreviations: cns, central nervous system; h, horn; i.e., inner ear; ll, LL canal; mct, mucoid connective tissue; s, supracleithrum; te, tunica externa; ti, tunica interna. (F) The bones just caudal to the left eye (orbit) in C. octofasciatus (lateral view, rostral to left). The anterior swim bladder horn (shaded) sits deep to the medial opening in the supracliethrum (black teardrop, = site of laterophysic connection), and in the vicinity of several CNs within the LL canals (gray ovals). Abbreviations: gb, gas bladder (swim bladder); h, horn; lc, laterophysic connection; le, lateral extrascapular; me, medial extrascapular; nm, neuromast in LL scale; or, orbit (eye); pt, post-temporal; pte, pterotic; s, supracleithrum. (G) Schematic representation (in transverse view) of the relationship between the left LL canal in the supracleithrum (sc), medial fossa in the suoracleithrum (light gray), swim bladder horn (sb horn), neuromast in canal, and muscle tissue that sits deep to the fossa (defining an indirect LC), neurocranium containing the central nervous system (brain, cns) and the bilateral sacculae (otolithic organs of the two ears) that are vertical and sit at the midline ventral to the brain in C. ocellatus. (Numbers represent distances measured for description of the anatomy in Webb et al., 2012). (A)–(E) Reprinted with permission from Webb, Smith, and Ketten, “The laterophysic connection and swim bladder in butterflyfishes in the genus Chaetodon (Perciformes: Chaetodontidae),” J. Morphol. 267, 1338–1355 (2006). Copyright 2006 Wiley and Sons, Wiley-Liss, Inc. (F) and (G) Reprinted with permission from Webb, Walsh, Casper, Mann, Kelly, and Cicchino, “Development of the ear, hearing capabilities, and laterophysic connection in the spotfin butterflyfish (Chaetodon ocellatus),” Environ. Biol. Fishes 95, 275–290 (2012). Copyright 2012 Springer Nature.
Given this exciting discovery, my MS student Leo Smith (now on the faculty at the University of Kansas) and a group of talented and energetic undergraduates did a histological analysis of 22 Chaetodon species distributed among the 11 Chaetodon subgenera (despite a suggestion that we just focus one or two species). This work revealed unexpected and extensive variation in the soft tissues in the vicinity of the medial opening in the supracleithrum [Fig. 5(F)]. We identified two LC types among the first few species Chaetodon species that we examined—one defined by close opposition of the wall of the swim bladder horn and the medial fossa in the supracleithrum forming a “tympanum” [e.g., as in C. octofasciatus, a direct LC; Figs. 5(A) and 5(B)], and the other in which muscle tissue lies between the swim bladder horn and the media fossa in the supracleithrum [an indirect LC; Fig. 5(G)]. Ultimately, we found six LC variants, defined by the presence of either a direct or indirect LC, as well as by variation in the diameter (narrow, wide) and length (long, short) of the swim bladder horns (Smith et al., 2003). We also found that LC type (direct, indirect) is correlated with the thickness of the outer wall of the swim bladder (suggesting differences in the ability of the swim bladder to transmit pressure stimuli), and that the LC variant present in a species is predictive of its placement in a Chaetodon subgenus (a taxonomic arrangement established prior to this study; discussed in Smith et al., 2003). These observations strongly suggested that the LC is a part of a larger functional complex, involving not only the swim bladder horns, but the entire swim bladder (Woods et al., 2006), and perhaps the ear (Webb and Smith, 2000; Webb et al., 2002; Webb et al., 2006).
If the LC does play a role in hearing, then it could just be another type of otophysic connection, so we looked at the ear in Chaetodon to determine if it has the same sorts of modifications present in other fishes with otophysic connections. We found that in several species of Chaetodon (and in a member of another genus that lacks an LC, Forcipiger flavissimus) the size of the otoliths, the shape of the sensory maculae (sensory hair cell epithelia in the three otolithic organs—utriculus, sacculus, lagena), and the orientation pattern of the hair cells in those maculae were “unremarkable” and similar to other perciform (perch-like) fishes that lack an otophysic connection. Experimental work would be needed to determine if the LC (defined by the presence of the anterior swim bladder extensions) enhances the sensitivity of the ear to sound pressure. Nevertheless, we showed that the evolution of the LC and its diversification among Chaetodon species was not accompanied by the sorts of structural modifications of the ear found in species with otophysic connections (Webb et al., 2010).
All of this work generated a good deal of excitement and inspired several important collaborations. I had never met Darlene Ketten (Woods Hole Oceanographic Institution and Harvard Medical School), but after I gave my talk on some of our early work at the 1999 conference on Sensory Processing in the Aquatic Environment (Heron Island, GBR, Australia; see Collin and Marshall, 2003; Webb and Smith, 2000) she approached me and said, “Nice X-rays, but I can do better.” I was not sure exactly what she meant, but a few months later I found myself in the radiology department at the Massachusetts Eye and Ear Infirmary in Boston with a bucket containing a live butterflyfish (C. uliatensis), a species chosen deliberately based on our understanding of its anatomy. Using high resolution CT (computed tomographic) imaging, which was then in its infancy for non-medical applications, Darlene generated 2-D and 3-D images of the volume of air within the swim bladder and within its anterior horns [Figs. 5(C)–5(E)], thus confirming our observations in histological material at a comparable level of resolution. CT imaging of about a dozen species of butterflyfishes and two species of squirrelfishes (Family Holocentridae, one of which has swim bladder horns and an otophysic connection) taught us how to interpret swim bladder anatomy using a combination of CT, histology, and dissection (Webb, et al., 2006). With this project, we pioneered CT imaging of the swim bladders of live, anaesthetized fishes [Webb et al., 1999; Figs. 5(C)–5(E)]. CT imaging is now a standard method for the visualization of the relationship between the swim bladder and the inner ear (e.g., Schulz-Mirbach and Ladich, 2021; Schulz-Mirbach et al., 2013a; Schulz-Mirbach et al., 2013b; Schulz-Mirbach et al., 2014), of the skeletal and soft tissue anatomy of fishes and other vertebrates (Gignac et al., 2016; Hilton et al., 2019), and of the cranial LL canals within the dermatocranial bones of bony fishes (e.g., Webb et al., 2010; Webb, 2014b; Marranzino and Webb, 2018).
Based on my initial description of the LC in Chaetodon octofasciatus (Webb, 1998), Tim Tricas (University of Hawaii) suggested that sound should be important in the lives of butterflyfishes on coral reefs. On his way to the Heron Island conference in 1999 he put hydrophones on the reef off the Island of Hawaii. When he provoked social interactions among monogamous pairs of C. multicinctus (a monogamous and territorial species, with an indirect LC) he discovered that they do indeed make sounds. He and his Ph.D. student, Kelly Boyle (now on the faculty at the University of New Orleans) followed up on this observation by describing the sound production repertoire among several Chaetodon species with different LC variants and in a non-Chaetodon butterflyfish (Forcipiger flavissimus), which lacks a LC and anterior swim bladder extensions. Using auditory evoked potentials (AEP), they showed that deflation of the swim bladder extensions or deflation of the entire swim bladder raised the hearing threshold in species with an LC (Chaetodon spp.), but not in a species that lacks an LC (F. flavissimus). They concluded that the evolution of anterior extensions of the swim bladder horns, which define the LC in Chaetodon species increase sensitivity to sound pressure at frequencies used for acoustic communication (Boyle and Tricas, 2010, 2011; Tricas and Boyle, 2015a,b; Tricas et al., 2006).
A unique opportunity also arose to study the development of the LC in the larvae and juveniles of the spotfin butterflyfish, Chaetodon ocellatus, which stray northward from the Caribbean to the coast of New Jersey. Our histological analysis of a growth series [larvae, tholichthys (specialized late-stage larva), and juveniles] that was provided by Kenneth Able (director of the Rutgers University Marine Field Station) revealed that the medial fossa in the supracleithrum, the three otolithic organs of the ear, and an inflated swim bladder are already present in tholichthys, but that the anterior swim bladder horn form at transformation to the juvenile stage (Webb et al., 2012). David Mann and Brandon Casper (both from University of South Florida) and I then collaborated on the analysis of AEPs in live juvenile C. ocellatus collected at the Rutgers Field Station. This work showed that small juveniles have lower auditory thresholds (∼30–40 dB lower) than comparably sized damselfishes (Family Pomacentridae) that lack swim bladder extensions (see Webb et al., 2012). Thus we concluded that sound is likely not critical for the ability of late-stage larvae to navigate to their coral reef settlement sites (see general discussion of this process in Majoris et al., 2021) but is likely to be important at some point after transformation to the juvenile stage, perhaps in the context of their territorial and reproductive behavior as adults, as shown by Tricas and Boyle in other species (reviewed in Tricas and Webb, 2016).
Before I started this work in 1990, little was known about the acoustic sensory biology of coral reef fishes and of butterflyfishes, in particular. Had I not purchased a butterflyfish based solely on its low price (C. octofasciatus), which happened to have a direct LC (as opposed to an indirect LC), the idea that the medial opening in the supracleithrum is the site of a connection between the LL system and anterior extensions of the swim bladder (Webb, 1998) would not have been borne out, and our work would likely have stopped right there. Furthermore, had we followed the suggestion that we focus on the study of the LC in only one or two species, we would have never found the striking variation in the LC found among Chaetodon species, which has functional consequences as well as taxonomic correlates (Smith et al., 2003; Webb et al., 2006). We had no idea that the initial question posed to me by Stan Blum concerning an admittedly esoteric bit of anatomy would lead us to the description of the LC, CT imaging of the swim bladder, and ultimately to the discovery of sound production and its importance in the social behavior of butterflyfishes. Serendipity! This work also raised important questions about the potential impacts of increasing levels of anthropogenic sound on coral reefs, which may compromise the ability of these highly social fishes to communicate (discussed in Tricas and Webb, 2016). Nevertheless, my original hypothesis, that the LC is the site of transduction of sound pressure from the swim bladder into the LL canals, which would expand the functional repertoire of the LL system to include reception and interpretation of sound pressure stimuli in these fishes (Webb, 1998) remains untested, so there is still much to learn.
C. Widened LL canals—Convergent evolution for enhanced prey detection?
The occurrence of five different cranial LL canal phenotypes among bony fishes [Figs. 3(A)–3(E); Webb, 2014b) raises the obvious question about their adaptive significance; however, few studies have directly addressed this (but see Herzog et al., 2017; Kaldenbach et al., 2019; Mogdans, 2019). Widened canals are characterized by wide canal diameter, large CNs, and large bony pores covered by skin that is pierced by small pores that connect the fluid in the canal lumen to the external environment (Fig. 6). These canals are quite obvious when examining live or preserved specimens, so much so that the widened canals of the Eurasian Ruffe (Gymnocephalus cernuus) were the first to be illustrated in the literature (Leydig, 1850) followed soon after by the widened canals on the blind side of the head in the witch flounder (Glyptocephalus zachirus; McDonnell, 1871). The convergent evolution of widened canals in ∼20 taxonomically diverse families of bony fishes (Webb, 2014b) has always intrigued me as an important example of adaptive evolution in the LL system and ecological correlates provide hints about their adaptive value.
FIG. 6.
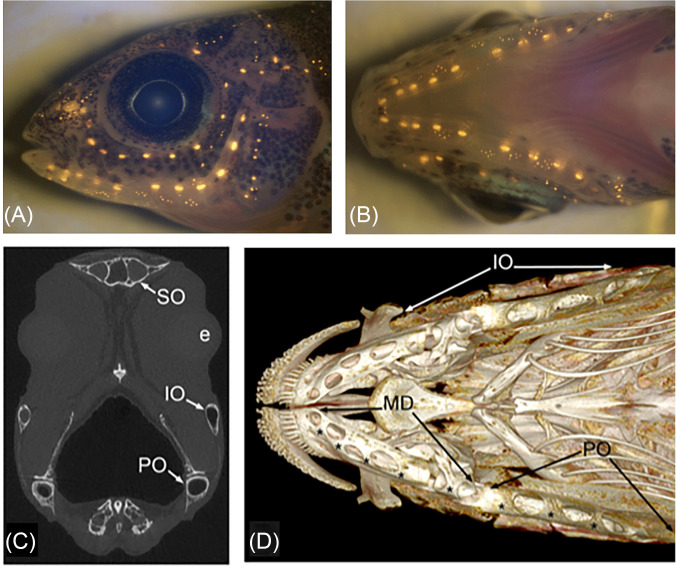
(Color online) Neuromasts in the peacock cichlid, Aulonocara stuartgranti in (A) lateral and (B) ventral views as revealed by vital fluorescent staining of hair cells in neuromasts (with 4-Di-2-ASP). CNs are larger than SNs. (C) Transverse μCT (micro-computed tomographic) slice through the head of adult Aulonocara baenschi at the level of the lens of the eye (e), indicating the lumen of the preopercular (PO), infraorbital (IO), and supraorbital (SO) canals. (D) 3-D reconstructions (μCT) of cranial skeleton, in ventral view, showing the mandibular (MD), preopercular (PO), and infraorbital (IO) LL canals in adult Aulonocara baenschi. Asterisks (*) indicate the location of CNs within the MD canal, found within the dentary and anguloarticular bones of the mandible, and in the PO canal in the ventral portion of the L-shaped preopercular bone. (A) and (B) Reprinted from Figs. 2(E) and 2(F) from Becker, Bird, and Webb, “Post-embryonic development of canal and superficial neuromasts and the generation of two cranial lateral line phenotypes,” J. Morphol. 277(10), 1273–1291 (2016). Copyright 2016 Wiley Periodicals, Inc., John Wiley and Sons. (C) and (D) Reprinted from Figs. 3(D) and 3(C) from Webb, Bird, Carter, and Dickson, “Comparative development and evolution of two lateral line phenotypes in Lake Malawi cichlids,” J. Morphol. 275(6), 678–692 (2014). Copyright 2014 Wiley Periodicals, Inc., John Wiley and Sons.
Fishes with widened canals feed on prey in the water column or sandy or muddy sediments. They are active under light-limited conditions (are crepuscular or nocturnal) or live at great depths in both marine and freshwater habitats (Webb, 2014b; Edgley and Genner, 2019; Webb et al., 2021). Theoretical, biomechanical, and behavioral studies have demonstrated that neuromasts in widened canals are more sensitive to flows (especially at low frequencies) but respond more slowly than those in narrow canals. Further, fishes with widened canals react to flow stimuli at shorter distances than those with narrow canals (Denton and Gray, 1988, 1989; Gray and Best, 1989; Janssen, 1997). Experimental work on the feeding behavior of two members of the family Percidae, the Eurasian ruffe, Gymnocephalus cernuus (which has widened canals) and European perch, Perca fluviatilis (which has narrow canals), showed that they feed on similar prey in the water column but that ruffe are also able to feed at night thus giving them an ecological advantage (Bergman, 1991; Janssen, 1997; Schleuter and Eckmann, 2006).
When I moved to the University of Rhode Island in 2006 my goal was to develop a system in which we could study the developmental basis for the structural evolution of LL phenotypes and the functional significance of variation in canal phenotype (e.g., widened vs narrow canals). Based on my prior work on cichlid fishes I chose two species from the iconic adaptive radiation of cichlids in Lake Malawi (Africa): the flavescent peacock cichlid, Aulonocara stuartgranti (widened canals) and Tramitichromis sp. (narrow canals), both of which feed on benthic invertebrates in sandy substrates. Peacock cichlids in the genus Aulonocara swim closely over sandy bottoms and strike at prey (hence their nickname “sonar fishes”; Fryer and Iles, 1972; Konings, 2007). This suggested to us that their widened LL canals (Fig. 6), and the ventrally facing mandibular, lower preopercular, and infraorbital LL canals, in particular [Figs. 6(C) and 6(D)], could detect fluid flows generated by invertebrate prey in sandy substrates. My M.S. student, Emily Becker, showed that despite differences in canal morphology and neuromast size, the number and distribution of neuromasts (spatial patterning) on the head of A. stuartgranti and Tramitichromis sp. were identical [Becker et al., 2016; Figs. 6(A) and 6(B)]. A post-doc, Nathan Bird (now on the faculty at the University of Northern Iowa), demonstrated that mosaic heterochrony, a combination of acceleration and deceleration of the development of neuromasts and canals, respectively, as well as truncation of the process of canal ossification, could explain the evolution of widened canals from narrow canals (Bird and Webb, 2014).
My Ph.D. student, Margot Schwalbe (now on the faculty at Lake Forest College), designed a series of behavioral experiments to test the hypothesis that the detection of flows generated by benthic prey is mediated by the LL system in A. stuartgranti (widened canals; Fig. 6) but not in Tramitichromis sp. (narrow canals). First, she showed that A. stuartgranti could locate live prey (adult brine shrimp) tethered to platforms nestled in sandy substrates, which generate measurable hydrodynamic flows. Then she quantified prey detection behavior under light and dark conditions in fish with an intact LL system and those in fish in which the LL system was ablated with cobalt chloride. Results showed that A. stuartgranti detects and strikes at prey at low light intensities as well as in the dark, and that they prefer live (mobile, stimulus generating) over dead (immobile) prey (Schwalbe et al., 2012; Schwalbe and Webb, 2014, 2015). These results also showed that A. stuartgranti uses a combination of visual and LL cues to detect prey when light is present. In the dark, they also detect live prey, but this ability is lost when the LL system is ablated, thus demonstrating the role of the LL system in flow sensing. Further, this work showed that in the dark, when neither visual nor LL cues are available, chemical cues (olfaction and/or gustation) were insufficient to mediate the detection and localization of prey (Schwalbe et al., 2012). However, it was subsequently shown that cobalt chloride also inactivates the olfactory system in another cichlid (Astatotilapia burtoni; Butler et al., 2016), which might explain our results. In stark contrast to A. stuartgranti, Tramitichromis sp., which has narrow canals, detects prey even when the LL system is ablated, but will not feed at all in the dark. Thus, it appears that this species depends on vision (and perhaps chemoreception) for detection of its benthic invertebrate prey in the lab and likely in the field.
The contribution of flow sensing by the LL system in prey detection behavior was further confirmed by successfully training A. stuartgranti to respond to artificial water flows emerging from tubes in a sandy substrate and characterized using digital particle image velocimetry (DPIV). This experimental design thus controlled for the effect of any visual and/or chemical cues, which may have been present with the use of live and dead prey (Schwalbe et al., 2016). The results of this novel work showed that A. stuartgranti could successfully detect artificial flows but could not accomplish this task when the LL system was ablated. It also showed that flow sensing behavior (strikes on tubes from which flows emerged) returned within a week, the timeframe required for hair cell regeneration (e.g., in zebrafish), thus providing a link between behavior and cell-level processes.
In total, this work provided the first experimental evidence for the role of the LL system (and widened canals, in particular) in prey detection behavior. It also established the sensory basis for prey detection in two cichlids that share a food resource in Lake Malawi (invertebrates in sandy substrates) but use different prey strike strategies, provided the first demonstration of LL-mediated feeding behavior in a cichlid, and revealed the potential for nocturnal activity and feeding in cichlids, which has important implications for our understanding of their ecology and evolution.
Widened canals have evolved in other taxa in which all the canals are similarly widened. However, two taxa, a North American freshwater minnow, and a small genus of coastal marine flounders are characterized by regional specialization of the cranial LL canals. The silverjaw minnow Notropis buccatus (=Ericymba buccata), which feeds nocturnally on benthic invertebrates in sandy-bottomed streams, has narrow dorsal canals (supraorbital canal) and ventral or ventrally-directed widened canals (mandibular, preopercular, infraorbital canals; Jones, 2023). Flatfishes in the small genus Glyptocephalus (three species; rex sole, witch flounder) live on sandy and muddy bottoms, at relatively deep depths, and feed on small benthic, tube-dwelling invertebrates. The cranial canals on the eyed (right, functionally dorsal) side of the head are narrow, but those on the blind (left, functionally ventral) side are widened with large diamond-shaped CNs (Webb and New, 1994). Thus, in both cases, widened canals are found only on the functionally ventral side of the head, facing the substrate, suggesting that they mediate the detection of water flows generated by benthic prey. The evolution of widened canals in these fishes would require an alteration in the genetic program that maintains phenotypic uniformity among canals and CNs, either across the axis of bilateral symmetry (in Glyptocephalus) or along the dorsal-ventral body axis (in Notropis buccatus). In both cases, this is likely a result of a change in developmental timing among canals (and CNs) during the larval stage (heterochrony; see Bird and Webb, 2014; Webb et al., 2014; Jones, 2023). Given the different functional attributes of narrow and widened canals, the presence of regional modification of canals as described here provides strong evidence for functional adaptation in the LL system.
D. The LL system of deep-sea fishes—It is not just about the eyes
Two significant gaps in our knowledge of the LL system in fishes became apparent when I was compiling a list of key papers on LL morphology for every order of bony fishes (see Table I in Webb, 2014b). The literature indicated that virtually nothing was known about the mechanosensory LL system of the weakly electric fishes (Mormyriformes, Gymnotiformes) or the deep-sea fishes (Stomiiformes and Myctophiformes, in particular, amongst a larger assemblage of taxonomically diverse deep-sea fishes). This was not surprising given the great deal of attention that has been given to the electrosensory system of weaky electric fishes and the visual system (and bioluminescence) of deep-sea fishes.
Deep-sea fish assemblages live in hydrodynamically quiet environments, to depths of thousands of meters (in the twilight of the mesopelagic and the darkness of the bathypelagic), often at low population densities, conditions that should promote adaptations in the LL system (and the other non-visual senses) for detection of prey, avoidance of predators, and identification of potential mates. Deep-sea fishes that lack photophores (light-producing organs) tend to have widened canals (e.g., morids, macrourids, melamphaeids, cetomimids), which were illustrated starting more than 100 years ago (e.g., Garman, 1899). Some deep-sea anglerfishes (Ceratioidei) have photophores (including on their esca, or feeding lure), and others have an elaboration of SNs on their head (Marshall, 1965, 1996; Pietsch, 2009). However, the LL system in the two largest orders of exclusively deep-sea fishes, the Stomiiformes and Myctophiformes, remained unexplored.
My M.S. student, Ashley Marranzino (now working for the NOAA Office of Exploration) had expressed an interest in deep-sea fishes, and I suggested that she look at the LL system of the stomiiform fishes (hatchetfishes, bristlemouths, dragonfishes). She started by examining specimens that I had collected at sea many years earlier and she found the only paper on the LL system of stomiiforms, which reported that one specimen of the Pacific hatchetfish (Argyropelecus affinis) had 27 neuromasts (Handrick, 1901). Given little to go on, we did not know what she would find, although published images of the cranial skeleton of these fishes suggested that the LL canals and dermatocranial bones with which they are associated are highly reduced. After studying a beautifully preserved specimen of the Pacific hatchetfish (the same species that Handrick had studied) in some detail Ashley informed me that she was “seeing white dots.” After some detailed observations, she counted 350–550 of them arranged in distinct lines on the head and body in three species of hatchetfishes [Marranzino and Webb, 2018; see Figs. 7(A) and 7(B)]. Their arrangement in lines suggested that they were SNs (not external taste buds, which tend not to occur in lines) and SEM imaging confirmed this [see Figs. 7(F)–7(H)]. Ashley continued to search for SNs among species in three families of stomiiforms — hatchetfishes (Family Sternoptychidae), dragonfishes [Family Stomiidae, Figs. 7(D) and 7(E)], and bristlemouths [Family Gonostomatidae, Fig. 7(C)] — and found proliferations of hundreds of SNs on the head and body of all 17 of the species she examined (Marranzino and Webb, 2018). Based on this, we hypothesized that SN proliferations are likely a unifying feature (synapomorphy) of all 400+ species in the order Stomiiformes, which is represented in all of the world's oceans and is of global ecological significance. This work now provides a strong rationale for the future study of the LL system in these and other deep-sea fish groups (especially the Myctophiformes). Most importantly, it demands that any consideration of the ecology of deep-sea fishes must include the potential contribution of flow sensing by the LL system in their behavior and ecology.
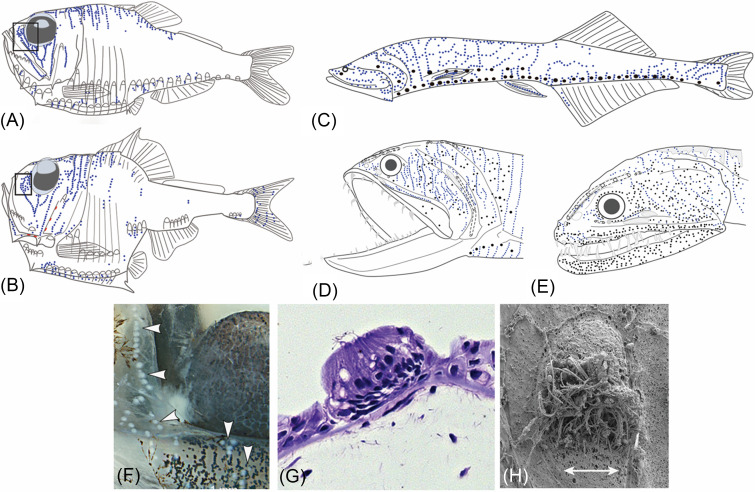
FIG. 7.
(Color online) Proliferation of SNs in deep-sea stomiiform fishes: Hatchetfishes, Family Sternoptychidae, (A) Argyropelecus affinis, (B) A. hemigymnus. Bristlemouths, Family Gonostomatidae; (C) Cyclothone spp. (based on whole preserved specimens of C. acclinidens, C. braueri, C. microdon, C. signata). Dragonfishes, Family Stomiidae: (D) Gonostoma elongatum, (E) Idiacanthus antrostomus. (A)–(E) CNs (red), SNs (blue), photophores (black), pores (open circles), LL canals (dotted lines) are illustrated; neuromasts are slightly enlarged to enhance visibility. (F) SNs (“white dots,” indicated by arrowheads) rostral to eye in Argyropelecus affinis. (G) A histological section revealing the structure of a SN with central hair cells on head of A. aculeatus. (H) SEM of a SN [the same tissue as in (F)] revealing the oval shape and long kinocilia of the sensory hair cells in a central area (sensory strip); double-headed arrow = axis of best physiological sensitivity. (A)–(F) Modified from Marranzino and Webb, “Flow sensing in the deep sea: The lateral line system of stomiiform fishes,” Zool. J. Linn. Soc. 183(4), 945–965 (2018), by permission of The Linnean Society of London. (G) and (H) Licensed under a Creative Commons Attribution-Noncommercial-ShareAlike license, Museum of Comparative Zoology, Harvard University; Copyright President and Fellows of Harvard College.
IV. REFLECTIONS ON THE PAST AND LOOKING FORWARD
My work on the LL system started quite serendipitously in graduate school and my research continues to be propelled by the desire to understand the structural diversity and evolution of the system. I remain fascinated by the interplay of the development and phenotypic evolution and its link to functional evolution (which has required an increasing number of exciting collaborations). Furthermore, I am intrigued by the dual identity of the cranial LL canals as components of the vertebrate skull (and scales) and as a sensory system, and how this idea can be used to understand how different (competing?) selection pressures and both developmental opportunities and constraints can generate the structural and functional diversity in the LL system that we see among fishes.
The work in my lab has focused on a relatively small number of taxa (<100 of the 34 000+ extant fish species; Nelson et al., 2016). However, most of these (including some not discussed here) belong to the largest families of freshwater fishes (Cichlidae, Cyprinidae, Characidae) and marine fishes (Gobiidae, Stomiiformes), which collectively represent one-third of all bony fishes. Furthermore, the fishes that we have studied occupy critical habitats—coral reefs (Percomorpha: Chaetodontidae, Gobiidae), the deep-sea (Stomiiformes), and both temperate (Cypriniformes, Salmoniformes) and tropical (Cichlidae, Characidae) freshwaters. Thus, this work provides valuable insights into the diversity and evolution of the LL system more broadly. Nevertheless, there is still a great deal of work to be done.
Future studies of both functional (physiological, behavioral, neuroethological) correlates of variation in cranial canal and trunk canal morphology and work on the interaction of LL and auditory inputs are needed, especially in species that exhibit behaviors in which both acoustic stimuli and flow sensing appear to be important (e.g., sound production and complex locomotory behaviors; discussed by Tricas and Webb, 2016), and in species that are active nocturnally and/or live in deep waters (e.g., Marranzino and Webb, 2018). Further, the functional distinctions and relative contributions to flow sensing by the two LL submodalities—CNs (which act as accelerometers) and SNs (which act as velocimeters)—need additional investigation (see Engelmann et al., 2002; Montgomery et al., 2014; van Netten and McHenry, 2014). Finally, structure-function relationships at the level of the neuromast (with respect to their size, shape, and orientation), and SN proliferations (e.g., Nickles et al., 2020; Sato et al., 2021; Sato, 2022), whose distributions are underestimated and functional roles are not understood, require additional study.
The LL system of chondrichthyan fishes [elasmobranchs (sharks, skates, rays), chimaeras], provides a fascinating contrast to the system in bony fishes, given anatomical distinctions that arose more than 400 million years ago with the origin of these two evolutionary lineages. The LL canals of elasmobranchs are reported to contain CNs that are either continuous (Johnson, 1917) or discrete (Gillis et al., 2012; as in bony fishes), and sparsely distributed SNs have been reported to occur on the skin between specialized dermal denticles in a limited number of species (“pit organs,” Peach and Rouse, 2000; Maruska, 2001; Peach and Marshall, 2009). Some preliminary work (Webb and Gillis, 2013) has shown that the cranial LL canals and the trunk canal in the little skate, Leucoraja erinacea (a new and important model species), develop in a completely different way than they do in bony fishes (also see Johnson, 1917). In the absence of dermatocranial bones (with which the LL canals of bony fishes are typically associated), the narrow LL canals of elasmobranchs are found in the soft tissue overlying the cranial cartilages that comprise the neurocranium. They demonstrate a great deal of variation in their distribution and course, which is correlated with head shape in sharks (e.g., Chu and Wen, 1979; Maruska and Tricas, 1998; Maruska, 2001; Jordan et al., 2009). In skates and rays, they are found on the dorsal and ventral surfaces of the head and on the greatly enlarged pectoral fins. (Chu and Wen, 1979; Jordan et al., 2009). Some species also have non-pored canals or vesicles of Savi (Chu and Wen, 1979; Maruska and Tricas, 2004; Wueringer et al., 2011), which are considered to be part of the mechanosensory LL system but whose development, morphology, and functional roles deserve more attention. All of these fascinating fishes provide fertile ground for future studies of the structural and functional evolution of the LL system.
Finally, all aquatic habitats on Earth are being affected by global change with important implications for sensory ecology and behavior of the fishes that inhabit them (Kelley et al., 2018; Draper and Weissburg, 2019; Rivest et al., 2019; Tigert and Porteus, 2023). Changes are already being seen in the horizontal and vertical distributions of fishes (due to changes in temperature profiles), as well as in light environments (due to increased turbidity and changes in color profiles), soundscapes (due to noise pollution and habitat degradation), and flow regimes (due to damming, etc.), in both marine and freshwater habitats. Thus, it is essential that we understand structure-function relationships in the LL system, as well as in the other sensory systems of fishes, so that we may understand, and perhaps mitigate, the challenges that fishes face in their natural (and not so natural) habitats that may ultimately threaten their survival.
V. CONCLUSION
My work has contributed to our knowledge of the anatomy, development, and evolution of the LL and has included discoveries that have enhanced our understanding of the importance of the non-visual sensory biology of fishes more generally. The study of anatomy has always been and will continue to be the science of discovery that is fundamental to our understanding of organismal biology and biodiversity at all levels of organization. To make appropriate predictions and test hypotheses about function in a single species, structure must be understood first, as we have shown in several instances. Anatomy must be studied unapologetically! New technologies are driving a renaissance in the field (Gignac et al., 2016; Hilton et al., 2019; Danos et al., 2022) and the integration of data from classic and cutting-edge methods for visualization and analysis of structure must frame the analysis of function. The study of comparative morphology, which is informed by taxonomy and systematics, is essential for the analysis of both structural and functional evolution (Danos et al., 2022; Ford et al., 2023). In addition, the considerable knowledge acquired through the intense study of model species (e.g., zebrafish, medaka, stickleback, cichlids, little skate) needs to be placed into comparative and evolutionary contexts so that it may contribute to our understanding of the larger taxonomic groups to which these model species belong (see Webb and Shilling, 2006; Schilling and Webb, 2007). Finally, all of these efforts will be meaningless unless the teaching of anatomy at all levels and thus the training of anatomists (in both comparative evolutionary and biomedical contexts), which has been in decline for decades, is valued, re-invigorated, and sustained into the future (Collins et al., 1994; Singh et al., 2015; Danos et al., 2022; Ford, 2023). We still have much to discover and it is impossible to predict the impacts of serendipitous discoveries.
ACKNOWLEDGEMENTS
I would like to thank the organizers for the invitation to contribute to this special issue on Fish Bioacoustics: Hearing and Sound Communication. I thank my students and post-docs, mentors, and collaborators who have all enriched the work that I do. The work in my lab has been funded by the National Institutes of Health (NRSA), National Science Foundation, American Philosophical Society, Marine Biological Laboratory, and the George and Barbara Young Chair in Biology at the University of Rhode Island.
This paper is part of a special issue on Fish Bioacoustics: Hearing and Sound Communication.
AUTHOR DECLARATIONS
Conflict of Interest
The author has no conflicts of interest.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Allis, E. P. (1889). “ The anatomy and development of the lateral line system in Amia calva,” J. Morphol. 2, 463–542. 10.1002/jmor.1050020303 [DOI] [Google Scholar]
- 2.Atema, J. , Fay, R. R. , Popper, A. N. , and Tavolga, W. N. (1988). Sensory Biology of Aquatic Animals ( Springer, New York: ). [Google Scholar]
- 3. Becker, E. A. , Bird, N. C. , and Webb, J. F. (2016). “ Post-embryonic development of canal and superficial neuromasts and the generation of two cranial lateral line phenotypes,” J. Morphol. 277, 1273–1291. 10.1002/jmor.20574 [DOI] [PubMed] [Google Scholar]
- 4. Bergman, E. (1991). “ Changes in abundance of two percids, Perca fluviatilis and Gymnocephalus cernuus, along a productivity gradient: Relations to feeding strategies and competitive abilities,” Can. J. Fish. Aquat. Sci. 48, 536–545. 10.1139/f91-068 [DOI] [Google Scholar]
- 5. Bird, N. C. , and Webb, J. F. (2014). “ Heterochrony and modularity in the functional evolution of the lateral line system,” EvoDevo 5, 21. 10.1186/2041-9139-5-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blaxter, J. H. S. (1987). “ Structure and development of the lateral line,” Biol. Rev. 62, 471–514. 10.1111/j.1469-185X.1987.tb01638.x [DOI] [Google Scholar]
- 7. Bleckmann, H. , and Mogdans, J. (2014). “ Central processing of lateral line information,” in The Lateral Line System, edited by Coombs S., Bleckmann H., Fay R. R., and Popper A. N. ( Springer, New York: ), pp. 253–279. [Google Scholar]
- 8. Blum, S. D. (1988). “ The osteology and phylogeny of the Chaetodontidae (Teleostei: Perciformes),” Ph.D. thesis, University of Hawaii at Manoa, Manoa, HI. [Google Scholar]
- 9. Boyle, K. S. , and Tricas, T. C. (2010). “ Pulse sound generation, anterior swim bladder buckling, and associated muscle activity in the pyramid butterflyfish, Hemitaurichthys polylepis,” J. Exper. Biol. 213, 3881–3893. 10.1242/jeb.048710 [DOI] [PubMed] [Google Scholar]
- 10. Boyle, K. S. , and Tricas, T. C. (2011). “ Sound production in the longnose butterflyfishes (genus Forcipiger): Cranial kinematics, muscle activity and honest signals,” J. Exper. Biol. 214, 3829–3842. 10.1242/jeb.062554 [DOI] [PubMed] [Google Scholar]
- 11. Braun, C. B. , and Grande, T. (2008). “ Evolution of peripheral mechanisms for the enhancement of sound reception,” in Fish Bioacoustics, edited by Webb J. F., Fay R. R., and Popper A. N. ( Springer-Verlag, New York: ), pp. 99–144. [Google Scholar]
- 12. Braun, C. B. , and Sand, O. (2014). “ Functional overlap and nonoverlap between lateral line and auditory systems,” in The Lateral Line System, edited by Coombs S., Bleckmann H., Fay R. R., and Popper A. N. ( Springer, New York: ), pp. 281–312. [Google Scholar]
- 13. Butler, J. M. , Field, K. E. , and Maruska, K. P. (2016). “ Cobalt chloride treatment used to ablate the lateral line system also impairs the olfactory system in three freshwater fishes,” PLoS One 11, 7. 10.1371/journal.pone.0159521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chitnis, A. J. , Nogare, D. D. , and Matsuda, M. (2011). “ Building the posterior lateral line system in zebrafish,” Devel. Neurol. 72, 234–255. 10.1002/dneu.20962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chu, Y. T. , and Wen, M. C. (1979). Monograph of Fishes of China (No. 2): A Study of the Lateral-Line Canals System and That of Lorenzini Ampulla and Tubules of Elasmobranchiate Fishes of China ( Science and Technology Press, Shanghai, China: ). [Google Scholar]
- 16. Clapp, C. M. (1898). “ The lateral line system of Batrachus tau,” J. Morphol. 15, 223–264. 10.1002/jmor.1050150203 [DOI] [Google Scholar]
- 17. Collin, S. P. , and Marshall, N. J. (2003). Sensory Processing in Aquatic Environments ( Springer, New York: ). [Google Scholar]
- 18. Collinge, W. E. (1895). “ On the sensory canal system of fishes. Teleostei—Suborder A. Physostomi,” Proc. Zool. Soc. London 63, 274–298. 10.1111/j.1469-7998.1895.tb00011.x [DOI] [Google Scholar]
- 19. Collins, T. J. , Given, R. L. , Hulsebosch, C. E. , and Miller, B. T. (1994). “ Status of gross anatomy in the U.S. and Canada: Dilemma for the 21st century,” Clin. Anat. 7, 275–296. 10.1002/ca.980070509 [DOI] [Google Scholar]
- 20. Coombs, S. (2023). “ A multisensory perspective on near-field detection and localization of hydroacoustic sources,” J. Acoust. Soc. Am. 153, 2545–2561. 10.1121/10.0017926 [DOI] [PubMed] [Google Scholar]
- 21. Coombs, S. , and Bleckmann, H. (2014). “ The gems of the past: A brief history of lateral line research in the context of the hearing sciences,” in The Lateral Line System, edited by Coombs S., Bleckmann H., Fay R. R., and Popper A. N. ( Springer, New York: ), pp. 1–16. [Google Scholar]
- 22. Coombs, S. , Janssen, J. , and Webb, J. F. (1988). “ Diversity of lateral line systems: Phylogenetic and functional considerations,” in Sensory Biology of Aquatic Animals, edited by Atema J., Fay R. R., Popper A. N., and Tavolga W. N. ( Springer, New York), pp. 553-593. [Google Scholar]
- 23. Coombs, C. , and Montgomery, J. C. (2014). “ The role of flow and the lateral line in the multisensory guidance of orienting behaviors,” in Flow Sensing in Air and Water—Behavioural, Neural and Engineering Principles of Operation, edited by Bleckmann H., Mogdans J., and Coombs S. ( Springer-Verlag, Berlin: ), pp. 65–102. [Google Scholar]
- 24. Danos, N. , Staab, K. L. , and Whitenack, L. B. (2022). “ The core concepts, competencies, and grand challenges of comparative vertebrate anatomy and morphology,” Integr. Comp. Biol. 4(1), obac019. 10.1093/iob/obac019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Denton, E. J. , and Gray, J. A. B. (1983). “ Mechanical factors in the excitation of clupeid lateral lines,” Proc. R. Soc. London B 218, 1–26. 10.1098/rspb.1983.0023 [DOI] [PubMed] [Google Scholar]
- 26. Denton, E. J. , and Gray, J. A. B. (1988). “ Mechanical factors in the excitation of the lateral lines of fish,” in Sensory Biology of Aquatic Animals, edited by Atema J., Fay R. R., Popper A. N., and Tavolga W. N. ( Springer-Verlag, New York: ), pp. 595–617. [Google Scholar]
- 27. Denton, E. J. , and Gray, J. A. B. (1989). “ Some observations on the forces acting on neuromasts in fish lateral line canals,” in The Mechanosensory Lateral Line: Neurobiology and Evolution, edited by Coombs S., Görner P., and Münz H. ( Springer-Verlag, New York: ), pp. 229–246. [Google Scholar]
- 28. Denton, E. J. , and Gray, J. A. B. (1993). “ Stimulation of the acoustico-lateralis system of clupeid fish by external sources and their own movements,” Philos. Trans. R. Soc. London B 341, 113–127. 10.1098/rstb.1993.0096 [DOI] [Google Scholar]
- 29. Draper, A. M. , and Weissburg, M. J. (2019). “ Impacts of global warming and elevated CO2 on sensory behavior in predator-prey interactions: A review and synthesis,” Front. Ecol. Evol. 7, 72. 10.3389/fevo.2019.00072 [DOI] [Google Scholar]
- 30. Edgley, D. E. , and Genner, M. J. (2019). “ Adaptive diversification of the lateral line system during cichlid fish radiation,” iScience 16, 1–11. 10.1016/j.isci.2019.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Engelmann, J. , Hanke, W. , and Bleckmann, H. (2002). “ Lateral line reception in still- and running water,” J. Compar. Physiol. A 188, 513–526. 10.1007/s00359-002-0326-6 [DOI] [PubMed] [Google Scholar]
- 32. Fay, R. R. , Popper, A. N. , and Webb, J. F. (2008). “ Introduction,” in Fish Bioacoustics, edited by Webb J. F., Fay R. R., and Popper A. N. ( Springer-Verlag, New York: ), pp. 1–15. [Google Scholar]
- 33. Ford, K. L. , Albert, J. S. , Summers, A. P. , Hedrick, B. P. , Schachner, E. R. , Jones, A. S. , Evans, K. , and Chakrabarty, P. (2023). “ A new era of morphological investigations: Reviewing methods for comparative anatomical studies,” Integ. Org. Biol 5(1), obad008. 10.1093/iob/obad008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fryer, G. , and Iles, T. D. (1972). The Cichlid Fishes of the Great Lakes of Africa: Their Biology and Evolution ( Oliver and Boyd, Edinburgh, UK: ). [Google Scholar]
- 35. Garman, S. (1899). “ Reports on an exploration off the west coasts of Mexico, Central and South America, and off the Galapogos Islands, in charge of Alexander Agassiz, by the US fish commission steamer ‘Albatross’ during 1891, Lieut. Commander Z.L. Tanner, USN, Commanding, XXVI—The fishes,” Mem. Mus. Comp. Zool. 24, 1–431. [Google Scholar]
- 36. Ghysen, A. , Wada, H. , and Dambly-Chaudière, C. (2014). “ Patterning the posterior lateral line in teleosts: Evolution of development,” in Flow Sensing in Air and Water—Behavioural, Neural and Engineering Principles of Operation, edited by Bleckmann H., Mogdans J., and Coombs S. ( Springer, Berlin: ), pp. 295–318. [Google Scholar]
- 37. Gignac, P. , Kley, N. , Clarke, J. A. , Colbert, M. W. , Morhardt, A. , Cerio, D. , Cost, I. N. , Cox, P. G. , Daza, J. D. , Early, C. M. , Echolis, M. S. , Henkelman, R. M. , Herdina, A. N. , Holliday, C. M. , Li, Z. , Mahlow, K. , Merchant, S. , Müller, J. , Orsbon, C. P. , Paluh, D. J. , Thies, M. L. , Tsai, H. P. , and Witmer, L. M. (2016). “ Diffusible iodine-based contrast-enhanced computed tomography (diceCT): An emerging tool for rapid, high-resolution, 3-D imaging of metazoan soft tissues,” J. Anat. 228, 889–909. 10.1111/joa.12449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gillis, J. A. , Modrell, M. S. , Northcutt, R. G. , Catania, K. C. , Luer, C. A. , and Baker, C. V. (2012). “ Electrosensory ampullary organs are derived from lateral line placodes in cartilaginous fishes,” Development 139, 3142–3146. 10.1242/dev.084046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gould, S. J. , and Lewontin, R. C. (1979). “ The spandrels of San Marco and the Panglossian paradigm: A critique of the adaptationist programme,” Proc. R. Soc. London 205, 581–598. 10.1098/rspb.1979.0086 [DOI] [PubMed] [Google Scholar]
- 40. Gray, J. A. B. , and Best, A. C. G. (1989). “ Patterns of excitation of the lateral line of the ruffe,” J. Mar. Biol. Ass. 69, 289–306. 10.1017/S0025315400029416 [DOI] [Google Scholar]
- 41. Greene, C. W. (1899). “ The phosphorescent organs in the toadfish, Porichthys notatus Girard,” J. Morphol. 15, 667–696. 10.1002/jmor.1050150304 [DOI] [Google Scholar]
- 42. Handrick, K. (1901). “ Nervensystems und der leuchtorgane von Argyropelecus hemigymnus” (“Nervous system and the light organs of Argyropelecus hemigymnus”), Zoologica 32, 1–69. [Google Scholar]
- 43. Herzog, H. , Klein, B. , and Ziegler, A. (2017). “ Form and function of the teleost lateral line revealed using three-dimensional imaging and computational fluid dynamics,” J. R. Soc. Interf. 14, 20160898. 10.1098/rsif.2016.0898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Higgs, D. M. , and Radford, C. A. (2016). “ The potential overlapping roles of the ear and lateral line in driving ‘acoustic’ responses,” in Fish Hearing and Bioacoustics: An Anthology in Honor of Arthur N. Popper and Richard R. Fay, edited by Sisneros J. A. ( Springer Verlag, New York: ), pp. 255–270. [DOI] [PubMed] [Google Scholar]
- 45. Hilton, E. J. , Warth, P. , and Konstantinidis, P. (2019). “ The morphology, development, and evolution of the head of fishes: Foundational studies for a renaissance of comparative morphology,” Acta Zool. 100, 221–231. 10.1111/azo.12299 [DOI] [Google Scholar]
- 46. Hoss, D. E. , and Blaxter, J. H. S. (1982). “ Development and function of the swimbladder-inner ear system in the Atlantic menhaden, Brevoortia tyrannus (Latrobe),” J. Fish Biol. 20, 131–142. 10.1111/j.1095-8649.1982.tb03914.x [DOI] [Google Scholar]
- 47. Jakubowski, M. (1967). “ Cutaneous sense organs of fishes. Part VII. The structure of the system of lateral-line canal organs in the Percidae,” Acta Biol. Cracov. Ser. Zool. 10, 69–81. [Google Scholar]
- 48. Janssen, J. (1997). “ Comparison of response distance to prey via the lateral line in the ruffe and yellow perch,” J. Fish Biol. 51, 921–930. 10.1111/j.1095-8649.1997.tb01531.x [DOI] [Google Scholar]
- 49. Johnson, S. E. (1917). “ Structure and development of the sense organs of the lateral canal system of selachians (Mustelus canis and Squalus acanthias),” J. Compar. Neurol. 28, 1–74. 10.1002/cne.900280102 [DOI] [Google Scholar]
- 50. Jones, A. E. (2023). “ Form and function of the lateral line system in three North American fishes: Development, evolutionary insights and impacts of climate change,” Ph.D. thesis, University of Rhode Island, Kingston, RI. [Google Scholar]
- 51. Jordan, L. K. , Kajiura, S. M. , and Gordon, M. S. (2009). “ Functional consequences of structural differences in stingray sensory systems. Part I: Mechanosensory lateral line canals,” J. Exper. Biol. 212, 3037–3043. 10.1242/jeb.028712 [DOI] [PubMed] [Google Scholar]
- 52. Kaldenbach, F. , Klein, A. , and Bleckmann, H. (2019). “ Form-function relationship in artificial lateral lines,” Bioinspir. Biomim. 14, 026001. 10.1088/1748-3190/aaf488 [DOI] [PubMed] [Google Scholar]
- 53. Kasumyan, A. O. (2003). “ The lateral line in fish: Structure, function and role in behavior,” J. Ichthyol. 43, S175–S213. [Google Scholar]
- 54. Kelley, J. L. , Chapuis, L. , Davies, W. I. , and Collin, S. P. (2018). “ Sensory system responses to human-induced environmental change,” Front. Ecol. Evol. 6, 95. 10.3389/fevo.2018.00095 [DOI] [Google Scholar]
- 55. Klein, A. , Münz, H. , and Bleckmann, H. (2013). “ The functional significance of lateral line canal morphology on the trunk of the marine teleost Xiphister atropurpureus (Stichaeidae),” J. Comput. Physiol. A 199, 735–750. 10.1007/s00359-013-0834-6 [DOI] [PubMed] [Google Scholar]
- 56. Konings, A. (2007). Malaŵi Cichlids in Their Natural Habitat, 4th ed. ( Cichlid Press, El Paso, TX: ). [Google Scholar]
- 57. Leydig, F. (1850). “ Ueber die Schleimkanäle der Knochenfische” (“About the mucous canals of bony fish”), Müller's Archiv fur Anatomie, Physiologie und Wissenschaftliche Medicin, Jahrgang 1850, 170–181. [Google Scholar]
- 58. López-Shier, H. , Starr, C. J. , Kappler, F. A. , Kollmar, R. , and Hudspeth, A. J. (2004). “ Directional cell migration establishes the axes of planar polarity in the posterior lateral-line organ of the zebrafish,” Develop. Cell 7, 401–412. 10.1016/j.devcel.2004.07.018 [DOI] [PubMed] [Google Scholar]
- 59. Majoris, J. E. , Foretich, M. A. , Hu, Y. , Nickles, K. R. , Di Persia, C. L. , Chaput, R. , Webb, J. F. , Paris, C. B. , and Buston, P. M. (2021). “ An integrative investigation of sensory organ development and orientation behavior throughout the larval phase of a coral reef fish,” Sci. Rep. 11, 12377. 10.1038/s41598-021-91640-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Marranzino, A. N. , and Webb, J. F. (2018). “ Flow sensing in the deep sea: The mechanosensory lateral line system of stomiiform fishes,” Zool. J. Linn. Soc. 183, 945– 956. 10.1093/zoolinnean/zlx090 [DOI] [Google Scholar]
- 61. Marshall, N. B. (1965). “ Systematic and biological studies of the macrourid fishes (Anacanthini-Teleostii),” Deep Sea Res. 12, 299–322. 10.1016/0011-7471(65)90004-5 [DOI] [Google Scholar]
- 62. Marshall, N. J. (1996). “ The lateral line systems of three deep-sea fish,” J. Fish Biol. 49, 239–258. 10.1111/j.1095-8649.1996.tb06079.x [DOI] [Google Scholar]
- 63. Maruska, D. P. (2001). “ Morphology of the mechanosensory lateral line system in elasmobranch fishes: Ecological and behavioral considerations,” Env. Biol. Fish. 60, 47–75. 10.1023/A:1007647924559 [DOI] [Google Scholar]
- 64. Maruska, K. P. , and Tricas, T. C. (1998). “ Morphology of the mechanosensory lateral line system in the Atlantic stingray, Dasyatis sabina: The mechanotactile hypothesis,” J. Morphol. 238, 1–22. [DOI] [PubMed] [Google Scholar]
- 65. Maruska, K. P. , and Tricas, T. C. (2004). “ Test of the mechanotactile hypothesis: Neuromast morphology and response dynamics of mechanosensory lateral line primary afferents in the stingray,” J. Exper. Biol. 207, 3463–3476. 10.1242/jeb.01140 [DOI] [PubMed] [Google Scholar]
- 66. McDonnell, R. (1871). “ On the system of the ‘lateral line’ in fishes,” Trans. R. Irish Acad. Sci. 24, 161–188. [Google Scholar]
- 67. Mensinger, A. F. (2016). “ Multimodal sensory input in the utricle and lateral line of the toadfish, Opsanus tau,” in Fish Hearing and Bioacoustics: An Anthology in Honor of Arthur N. Popper and Richard R. Fay, edited by Sisneros J. A. ( Springer Verlag, New York: ), pp. 271–289. [DOI] [PubMed] [Google Scholar]
- 68. Mogdans, J. (2019). “ Sensory ecology of the fish lateral-line system: Morphological and physiological adaptations for the perception of hydrodynamic stimuli,” J. Fish Biol. 95, 53–72. 10.1111/jfb.13966 [DOI] [PubMed] [Google Scholar]
- 69. Montgomery, J. C. , Bleckmann, H. , and Coombs, S. (2014). “ Sensory ecology and neuroethology of the lateral line,” in The Lateral Line System, edited by Coombs S., Bleckmann H., Fay R. R., and Popper A. N. ( Springer, New York: ), pp. 121–150. [Google Scholar]
- 70. Nelson, G. J. (1969). “ Infraorbital bones and their bearing on the phylogeny and geography of osteoglossomorphs fishes,” Am. Mus. Novit. 2394, 1–37. [Google Scholar]
- 71. Nelson, J. S. , Grande, T. C. , and Wilson, M. V. H. (2016). Fishes of the World, 5th ed. ( John Wiley and Sons, Inc., Hoboken, NJ: ). [Google Scholar]
- 72. Nickles, K. R. , Hu, Y. , Majoris, J. E. , Buston, P. M. , and Webb, J. F. (2020). “ Organization and ontogeny of a complex lateral line system in a goby (Elacatinus lori), with a consideration of function and ecology,” Copeia 108, 863–885. 10.1643/CG-19-341 [DOI] [Google Scholar]
- 73. Peach, M. B. , and Marshall, N. J. (2009). “ The comparative morphology of pit organs in elasmobranchs,” J. Morphol. 270, 688–701. 10.1002/jmor.10715 [DOI] [PubMed] [Google Scholar]
- 74. Peach, M. B. , and Rouse, G. W. (2000). “ The morphology of the pit organs and lateral line canal neuromasts of Mustelus antarcticus (Chondrichthyes: Triakidae),” J. Mar. Biol. Assoc. 80, 155–162. 10.1017/S0025315499001678 [DOI] [Google Scholar]
- 75. Pietsch, T. W. (2009). Oceanic Anglerfishes—Extraordinary Diversity in the Deep Sea ( University of California Press, Berkeley, CA: ), p. 557. [Google Scholar]
- 76. Platt, C. , Popper, A. N. , and Fay, R. R. (1989). “ The ear as part of the octavolateralis system,” in The Mechanosensory Lateral Line: Neurobiology and Evolution, edited by Coombs S., Görner P., and Münz H. ( Springer, New York: ). [Google Scholar]
- 77. Rivest, E. B. , Jellison, B. , Ng, G. , Satterthwaite, E. V. , Bradley, H. L. , Williams, S. L. , and Gaylord, B. (2019). “ Mechanisms involving sensory pathway steps inform impacts of global climate change on ecological processes,” Front. Mar. Sci. 6, 346. 10.3389/fmars.2019.00346 [DOI] [Google Scholar]
- 78. Sato, M. (2022). “ Morphological diversity of the lateral line system in teleostei,” in Fish Diversity of Japan, edited by Kai Y., Motomura H., and Matsuura K. ( Springer, Singapore: ), pp. 283–310. [Google Scholar]
- 79. Sato, M. , Asaoka, R. , Nakae, M. , and Sasaki, K. (2017). “ The lateral line system and its innervation in Lateolabrax japonicus (Percoidei incertae sedis) and two apogonids (Apogonidae), with special reference to superficial neuromasts (Teleostei: Percomorpha),” Ichthyol. Res. 64, 308–330. 10.1007/s10228-016-0568-x [DOI] [Google Scholar]
- 80. Sato, M. , Nakae, M. , and Sasaki, K. (2021). “ The lateral line system in the nurseryfish Kurtus gulliveri (Percomorpha: Kurtidae): A distribution and innervation of superficial neuromasts unique within percomorphs,” Ichthyol. Herpetol. 109, 31–42. 10.1643/i2020017 [DOI] [Google Scholar]
- 81. Schilling, T. F. , and Webb, J. F. (2007). “ Considering the zebrafish in a comparative context,” J. Exp. Zool. B 308B, 515–522. 10.1002/jez.b.21191 [DOI] [PubMed] [Google Scholar]
- 82. Schleuter, D. , and Eckmann, R. (2006). “ Competition between perch (Perca fluviatilis) and ruffe (Gymnocephalus cernuus): The advantage of turning night into day,” Freshw. Biol. 51, 287–297. 10.1111/j.1365-2427.2005.01495.x [DOI] [Google Scholar]
- 83. Schulz-Mirbach, T. , Heß, M. , and Metscher, B. D. (2013a). “ Sensory epithelia of the fish inner ear in 3D: Studied with high resolution contrast enhanced microCT,” Front. Zool. 10, 63. 10.1186/1742-9994-10-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schulz-Mirbach, T. , Heß, M. , Metscher, B. D. , and Ladich, F. (2013b). “ A unique swim bladder-inner ear connection in a teleost fish revealed by a combined high-resolution microtomographic and three-dimensional histological study,” MBC Biol. 11, 75. 10.1186/1741-7007-11-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schulz-Mirbach, T. , and Ladich, F. (2021). “ The evolution of enhanced cichlid hearing: Functional morphology and the role of ecoacoustical factors,” in The Behavior, Ecology and Evolution of Cichlid Fishes, edited by Abate M. E. and Noakes D. L. ( Springer, Dordrecht, the Netherlands: ), pp. 503–539. [Google Scholar]
- 86. Schulz-Mirbach, T. , Ladich, F. , Plath, M. , Metscher, B. D. , and Heß, M. (2014). “ Are accessory hearing structures linked to inner ear morphology? Insights from 3D orientation patterns of ciliary bundles in three cichlid species,” Front. Zool. 11, 25. 10.1186/1742-9994-11-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Schwalbe, M. A. B. , Bassett, D. K. , and Webb, J. F. (2012). “ Feeding in the dark: Lateral line mediated feeding behavior in the peacock cichlid, Aulonocara stuartgranti,” J. Exper. Biol. 215, 2060–2071. 10.1242/jeb.065920 [DOI] [PubMed] [Google Scholar]
- 88. Schwalbe, M. A. B. , Sevey, B. , and Webb, J. F. (2016). “ Detection of artificial water flows by the lateral line system of a benthic feeding cichlid fish,” J. Exp. Biol. 219, 1050–1059. 10.1242/jeb.136150 [DOI] [PubMed] [Google Scholar]
- 89. Schwalbe, M. A. B. , and Webb, J. F. (2014). “ Sensory basis for detection of benthic prey in two Lake Malawi cichlids,” Zoology 117, 112–121. 10.1016/j.zool.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 90. Schwalbe, M. A. B. , and Webb, J. F. (2015). “ Effect of light intensity on prey detection behavior in two Lake Malawi cichlids, Aulonocara stuartgranti and Tramitichromis sp,” J. Comp. Physiol. A. 201, 341–356. 10.1007/s00359-015-0982-y [DOI] [PubMed] [Google Scholar]
- 91. Singh, R. , Shane Tubbs, R. , Gupta, K. , Singh, M. , Jones, D. G. , and Kumar, R. (2015). “ Is the decline of human anatomy hazardous to medical education/profession?—A review,” Surg. Radiol. Anat. 37, 1257–1265. 10.1007/s00276-015-1507-7 [DOI] [PubMed] [Google Scholar]
- 92. Smith, W. L. , Webb, J. F. , and Blum, S. D. (2003). “ The evolution of the laterophysic connection with a revised phylogeny and taxonomy of butterflyfishes (Teleostei: Chaetodontidae),” Cladistics 19, 287–306. 10.1111/j.1096-0031.2003.tb00374.x [DOI] [Google Scholar]
- 93. Tarby, M. L. , and Webb, J. F. (2003). “ Development of the supraorbital and mandibular lateral line canals in the cichlid, Archocentrus nigrofasciatus,” J. Morphol. 255, 44–57. 10.1002/jmor.10045 [DOI] [PubMed] [Google Scholar]
- 94. Tigert, L. R. , and Porteus, C. S. (2023). “ Invited review—The effects of anthropogenic abiotic stressors on the sensory systems of fishes,” Comp. Biochem. Physiol. A 277, 111366. 10.1016/j.cbpa.2022.111366 [DOI] [PubMed] [Google Scholar]
- 95. Tricas, T. C. , and Boyle, K. S. (2015a). “ Diversity and evolution of sound production in the social behavior of Chaetodon butterflyfishes,” J. Exp. Biol. 218, 1572–1584. 10.1242/jeb.114256 [DOI] [PubMed] [Google Scholar]
- 96. Tricas, T. C. , and Boyle, K. S. (2015b). “ Sound pressure enhances the hearing sensitivity of Chaetodon butterflyfishes on noisy coral reefs,” J. Exp. Biol. 218, 1585–1595. 10.1242/jeb.114264 [DOI] [PubMed] [Google Scholar]
- 97. Tricas, T. C. , Kajiura, S. M. , and Kosaki, R. K. (2006). “ Acoustic communication in territorial butterflyfish: Test of the sound production hypothesis,” J. Exp. Biol. 209, 4994–5004. 10.1242/jeb.02609 [DOI] [PubMed] [Google Scholar]
- 98. Tricas, T. C. , and Webb, J. F. (2016). “ Acoustic communication in butterflyfishes: Anatomical novelties, physiology, evolution, and behavioral ecology,” in Fish Hearing and Bioacoustics: An Anthology in Honor of Arthur N. Popper and Richard R. Fay, edited by Sisneros J. A. ( Springer Verlag, New York: ), pp. 57–92. [DOI] [PubMed] [Google Scholar]
- 99. Van Netten, S. M. , and McHenry, M. J. (2014). “ The biophysics of neuromasts,” in The Lateral Line System, edited by Coombs S., Bleckmann H., Fay R. R., and Popper A. N. ( Springer, New York: ), pp. 99–120. [Google Scholar]
- 100. Wada, H. , Iwasaki, M. , and Kawakami, K. (2014). “ Development of the lateral line canal system through a bone remodeling process in zebrafish,” Dev. Biol. 392, 1–14. 10.1016/j.ydbio.2014.05.004 [DOI] [PubMed] [Google Scholar]
- 101. Webb, J. F. (1989a). “ Developmental constraints and evolution of the lateral line system in teleost fishes,” in The Mechanosensory Lateral Line: Neurobiology and Evolution, edited by Coombs S., Görner P., and Münz H. ( Springer, New York: ), pp. 79–97. [Google Scholar]
- 102. Webb, J. F. (1989b). “ Gross morphology and evolution of the mechanoreceptive lateral line system in teleost fishes,” Brain. Behav. Evol. 33, 34–53. 10.1159/000115896 [DOI] [PubMed] [Google Scholar]
- 103. Webb, J. F. (1989c). “ Neuromast morphology and lateral line trunk canal ontogeny in two species of cichlids: An SEM Study,” J. Morphol. 202, 53–68. 10.1002/jmor.1052020105 [DOI] [PubMed] [Google Scholar]
- 104. Webb, J. F. (1990a). “ Comparative morphology and evolution of the lateral line system in the Labridae (Perciformes: Labroidei),” Copeia 1990, 137–146. 10.2307/1445830 [DOI] [Google Scholar]
- 105. Webb, J. F. (1990b). “ Ontogeny and phylogeny of the trunk lateral line system in cichlid fishes,” J. Zool. (London) 221, 405–418. 10.1111/j.1469-7998.1990.tb04010.x [DOI] [Google Scholar]
- 106. Webb, J. F. (1998). “ Laterophysic connection: A unique link between the swim bladder and the lateral-line system in Chaetodon (Perciformes: Chaetodontidae),” Copeia 1998, 1032–1036. 10.2307/1447353 [DOI] [Google Scholar]
- 107. Webb, J. F. (1999). “ Fish larvae in development and evolution,” in Origin and Evolution of Larval Forms, edited by Hall B. K. and Wake M. H. ( Academic Press, San Diego, CA: ), pp. 109–158. [Google Scholar]
- 108. Webb, J. F. (2000). “ Mechanosensory lateral line: Functional morphology and neuroanatomy,” in Handbook of Experimental Animals—The Laboratory Fish, edited by Ostrander G. ( Academic Press, London: ), pp. 236–244. [Google Scholar]
- 109. Webb, J. F. (2014a). “ Lateral line morphology and development and implications for the functional ontogeny of flow sensing of fishes,” in Flow Sensing in Air and Water - Behavioural, Neural and Engineering Principles of Operation, edited by Bleckmann H., Mogdans J., and Coombs S. ( Springer-Verlag, Berlin), pp. 247–270. [Google Scholar]
- 110. Webb, J. F. (2014b). “ Morphological diversity, development, and evolution of the mechanosensory lateral line system,” in The Lateral Line System, edited by Coombs S., Bleckmann H., Fay R. R., and Popper A. N. ( Springer, New York: ), pp. 17–72. [Google Scholar]
- 111. Webb, J. F. (2021). “ Morphology of the mechanosensory lateral line system of fishes,” in The Senses: A Comprehensive Reference, 2nd ed., edited by Fritzsch B. and Bleckmann H. ( Elsevier, New York: ), pp. 29–46. [Google Scholar]
- 112. Webb, J. F. , Bird, N. C. , Carter, L. , and Dickson, J. (2014). “ Comparative development and evolution of two lateral line phenotypes in Lake Malawi cichlids,” J. Morphol. 275, 678–692. 10.1002/jmor.20285 [DOI] [PubMed] [Google Scholar]
- 113. Webb, J. F. , Collin, S. P. , Kuciel, M. , Schulz-Mirbach, T. , Zuwala, K. , Denizot, J.-P. , and Kirschbaum, F. (2019). “ Sensory organs,” in The Histology of Fishes, edited by Kirschbaum F. and Formicki K. ( CRC Press/Francis and Taylor, Boca Raton, FL: ), pp. 267–338. [Google Scholar]
- 114. Webb, J. F. , and Gillis, J. A. (2013). “ Lateral line morphogenesis in chondrichthyan vs. osteichthyan fishes: New perspectives on an old problem,” Integ. Comp. Biol. 53, E223. [Google Scholar]
- 115. Webb, J. F. , Herman, J. L. , Woods, C. F. , and Ketten, D. R. (2010). “ The ears of butterflyfishes: ‘Hearing generalists’ on noisy coral reefs?,” J. Fish Biol. 77, 1434–1451. 10.1111/j.1095-8649.2010.02765.x [DOI] [PubMed] [Google Scholar]
- 116. Webb, J. F. , Maruska, K. P. , Butler, J. M. , and Schwalbe, M. A. B. (2021). “ The mechanosensory lateral line system of cichlid fishes: From anatomy to behavior,” in The Behavior, Ecology and Evolution of Cichlid Fishes, edited by Abate M. E. and Noakes D. L. ( Springer, Dordrecht, the Netherlands: ), pp. 401–442. [Google Scholar]
- 117. Webb, J. F. , Montgomery, J. C. , and Mogdans, J. (2008). “ Bioacoustics and the lateral line system of fishes,” in Fish Bioacoustics, edited by Webb J. F., Fay R. R., and Popper A. N. ( Springer-Verlag, New York: ), pp. 145–182. [Google Scholar]
- 118. Webb, J. F. , and New, J. G. (1994). “ Hindbrain morphology reflects asymmetry in the lateral line canal system of the flatfish, Glyptocephalus zachirus,” J. Morphol. 220, 409. [Google Scholar]
- 119. Webb, J. F. , and Noden, D. M. (1993). “ Ectodermal placodes: Contributions to the development of the vertebrate head,” Am. Zool. 33, 434–447. 10.1093/icb/33.4.434 [DOI] [Google Scholar]
- 120. Webb, J. F. , and Northcutt, R. G. (1991). “ Ciliated epithelium in non-teleost actinopterygian fishes,” Acta Zoologica (Stockh). 72, 107–111. 10.1111/j.1463-6395.1991.tb00323.x [DOI] [Google Scholar]
- 121. Webb, J. F. , and Northcutt, R. G. (1997). “ Morphology and distribution of pit organs and canal neuromasts in non-teleost bony fishes,” Brain, Behav. Evol. 50, 139–151. 10.1159/000113328 [DOI] [PubMed] [Google Scholar]
- 122. Webb, J. F. , and Ramsay, J. (2017). “ New interpretation of the 3-D configuration of lateral line scales and the lateral line canal contained within them,” Copeia 105, 339–347. 10.1643/CG-17-601 [DOI] [Google Scholar]
- 123. Webb, J. F. , and Schilling, T. F. (2006). “ Zebrafish in comparative context: A symposium,” Integ. Comp. Biol. 46, 569–576. 10.1093/icb/icl017 [DOI] [PubMed] [Google Scholar]
- 124. Webb, J. F. , and Shirey, J. E. (2003). “ Post-embryonic development of the lateral line canals and neuromasts in zebrafish,” Devel. Dyn. 228, 370–385. 10.1002/dvdy.10385 [DOI] [PubMed] [Google Scholar]
- 125. Webb, J. F. , and Smith, W. L. (2000). “ The laterophysic connection in chaetodontid butterflyfish: Morphological variation and speculations on sensory function,” Philos. Trans. R. Soc. London B 355, 1125–1129. 10.1098/rstb.2000.0652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Webb, J. F. , Smith, W. L. , and Ketten, D. R. (1999). “ CT (Computerized X-Ray Tomography) provides new views of the swim bladder of butterflyfishes (Perciformes: Chaetodon) with a novel specialization of the lateral line system,” Am. Zool. 39, 16A. [Google Scholar]
- 127. Webb, J. F. , Smith, W. L. , and Ketten, D. R. (2006). “ The laterophysic connection and swim bladder in butterflyfishes in the genus Chaetodon (Perciformes: Chaetodontidae),” J. Morphol. 267, 1338–1355. 10.1002/jmor.10480 [DOI] [PubMed] [Google Scholar]
- 128. Webb, J. F. , Smith, W. L. , Tricas, T. C. , and Ketten, D. R. (2002). “ The laterophysic connection: A novel specialization thought to enhance sound pressure sensitivity in butterflyfishes (Chaetodontidae, Chaetodon),” J. Acoust. Soc. Am. 112, 2203. 10.1121/1.1526542 [DOI] [Google Scholar]
- 129. Webb, J. F. , Walsh, R. M. , Casper, B. , Mann, D. A. , Kelly, N. , and Cicchino, N. (2012). “ Ontogeny of the ear, hearing capabilities, and laterophysic connection in the spotfin butterflyfish (Chaetodon ocellatus),” Environ. Biol. Fish. 95, 275–290. 10.1007/s10641-012-9991-7 [DOI] [Google Scholar]
- 130. Wonsettler, A. L. , and Webb, J. F. (1997). “ Morphology and development of the multiple lateral line canals on the trunk in two species of Hexagrammos (Scorpaeniformes: Hexagrammidae),” J. Morphol. 233, 195–214. [DOI] [PubMed] [Google Scholar]
- 131. Woods, C. , Webb, J. F. , and Ketten, D. R. (2006). “ The physoclistous swim bladder of chaetodontid butterflyfishes: Implications for acoustic function,” J. Acoust. Soc. Am. 120, 3326. 10.1121/1.4781233 [DOI] [Google Scholar]
- 132. Wueringer, B. E. , Peverell, S. C. , Seymour, J. , Squire, L., Jr. , and Collin, S. P. (2011). “ Sensory systems in sawfishes. 2. The lateral line,” Brain, Behav. Evol. 78, 150–161. 10.1159/000329518 [DOI] [PubMed] [Google Scholar]
- 133. Wullimann, M. F. , and Grothe, B. (2014). “ The central nervous organization of the lateral line system,” in The Lateral Line System, edited by Coombs S., Bleckmann H., Fay R. R., and Popper A. N. ( Springer, New York: ), pp. 195–251. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.