Abstract
Objective
To investigate the impact of CYP2C19 gene polymorphism on clopidogrel reactivity and its association with long-term clinical outcome in patients with coronary heart disease (CHD) undergoing percutaneous coronary intervention (PCI).
Methods
In total, 675 patients were enrolled. Based on the platelet inhibition rate, patients were categorized into two groups: clopidogrel low responsiveness (CLR) and normal clopidogrel responsiveness (NCR). The CLR group was divided into ticagrelor and clopidogrel group based on the antiplatelet drugs used in the follow-up treatment. Patients were classified into three groups (normal metabolizer, intermediate metabolizer, and poor metabolizer) based on the CYP2C19 genotype. We aimed to evaluate the impact of CYP2C19 gene polymorphism on clopidogrel reactivity. The cumulative rates of 12-month all-cause deaths, major adverse cardiovascular events (MACCEs), and bleeding events were calculated.
Results
CLR was observed in 44.4% of the overall population. Significant differences were observed in the platelet inhibition rate of clopidogrel among the three metabolic genotypes (P < 0.05). At the 12-month follow-up, 13 patients (1.9%) died and 96 patients (14.2%) experienced MACCEs. Patients with CLR (9.6% vs. 11.7% vs. 22.1%, P < 0.05) or poor metabolizer (10.7% vs. 16.4% vs. 22.6%, P = 0.026) experienced a higher rate of MACCEs. A MACCEs risk score between zero and two was calculated. The highest incidence of MACCEs significantly increased with the 2-positive results, and the area under the curve (AUC) was 0.712 (95% CI: 0.650–0.774, P < 0.05). There was no significant difference between the group with a score of one and the occurrence of MACCEs (P > 0.05).
Conclusions
Low response to clopidogrel in CHD patients is correlated with CYP2C19 gene polymorphism. CYP2C19 genotyping combined with platelet reactivity is an independent predictor of 12-months MACCEs in patients with clopidogrel treatment after PCI, which is better than either test alone.
Dual antiplatelet therapy with aspirin and clopidogrel is the standard treatment for the prevention of stent thrombosis and improving clinical prognosis after percutaneous coronary intervention (PCI).[1] However, platelet responses to clopidogrel show a marked interindividual variability, relevant studies have found that 4%-30% of patients still fail to achieve an adequate antiplatelet effect after standard dose treatment, which still lead to cardiovascular and cerebrovascular adverse events such as stent restenosis, stent thrombosis and clinical death, which is clinically known as Clopidogrel Resistance (CPGR).[2,3] Several independent clinical researches have demonstrated that patients with CPGR were at increased risk of stent thrombosis and other cardiac complications.[4,5] Consequently, there is a clinical need to measure the efficacy of antiplatelet therapy and apply more effective ADP-receptor blocking agents.
At present, there is still no unified diagnostic standard for CPGR worldwide. According to Muller, et al.,[6] CPGR is defined as a decrease in ADP-induced platelet aggregation rate of less than 10% from baseline 4 h after a 600 mg clopidogrel loading dose. A decrease of 10%–29% is considered partial resistance, while an inhibition exceeding 30% is classified as a normal response. Therefore, thrombelastography (TEG) is commonly employed for clinical evaluation of platelet response to clopidogrel. TEG can provide real-time monitoring of blood coagulation, platelet aggregation, fibrinolysis, and other dynamic changes. Moreover, the platelet inhibition rate can precisely and comprehensively reflect the responsiveness of clopidogrel in patients, thereby guiding clinical medication to reduce the occurrence of cardiovascular events and mortality.[7]
Clopidogrel is a prodrug with no activity in itself, approximately 15% of the drug is absorbed in the gastrointestinal tract and undergoes hepatic metabolism by cytochrome P450 enzymes to form its active metabolite. The active metabolite of clopidogrel irreversibly inhibits the P2Y12 receptor on platelets, leading to the inhibition of platelet aggregation. The CYP2C19 gene encodes the protease that serves as the key enzyme influencing clopidogrel metabolism.[8]
Hence, the CYP2C19 gene is widely believed to have a crucial role in the activation of clopidogrel and serves as a significant intrinsic factor influencing its antiplatelet effect.[9–11] As we known, the 2022 updated CPIC guideline[12] has clearly stated that “CYP2C19 genotype impacts clopidogrel active metabolite formation”. However, compared with that in White patients, the frequency of CYP2C19 LoF carriage and the level of on-clopidogrel platelet reactivity were higher in East Asian patients (65% vs. 30%, respectively). But in fact, the risk of ischemic events following PCI was similar or even lower in East Asians than that in Whites, which is often described as the “East Asian Paradox[13–14]”. Therefore, the prognostic implications of a comprehensive assessment of clopidogrel low responsiveness and the CYP2C19 genotype remain poorly characterized in East Asians, and the prognostic effects of platelet reactivity and CYP2C19 genotype have not been established, particularly in East Asians. So, this study aimed to investigate the impact of CYP2C19 gene polymorphism on clopidogrel reactivity and their association with long-term clinical outcome in patients with coronary heart disease (CHD) undergoing PCI.
METHODS
Study Population
In this retrospective study, we investigated the impact of CYP2C19 gene polymorphism on clopidogrel reactivity and long-term clinical outcomes in patients with coronary heart disease who underwent PCI. We enrolled patients with coronary heart disease who underwent PCI at Xuanwu Hospital, Capital Medical University, between March 2015 and March 2018.
To be eligible for the study, patients had to meet the following criteria: they were undergoing elective coronary stent placement after receiving a pretreatment of 100 mg aspirin and 75 mg clopidogrel per day for 2–3 days, or they received loading doses of 300 mg of both medications at least 6 h before the procedure.[15] Additionally, all enrolled patients underwent simultaneous detection of the CYP2C19 gene and TEG. Patients with severe liver disease or abnormal coagulation function, those taking chronic oral anticoagulation or thienopyridine treatment within two weeks prior to admission, those with contraindications to aspirin, clopidogrel, ticagrelor, or heparin, patients with cancer or undergoing hemodialysis, and those with acute or chronic inflammatory disease were excluded from the study. The follow-up information of patients was collected from medical records or telephone contact with the patient at 3rd and 6th months, and then annually. The minimum follow-up duration was 12 months. The cumulative rates of 12-month all-cause death and major adverse cardiac, cerebrovascular events (MACCEs) and bleeding events were assessed as adverse clinical outcomes. The MACCEs[16] encompassed cardiac deaths, stent thrombosis, repeat revascularization, myocardial infarction (MI), and stroke. Bleeding events[17] comprised massive bleeding (BARC type 3–5), which referred to fatal or clinically obvious bleeding that necessitated blood transfusion or hospitalization, such as intracerebral hemorrhage or massive gastrointestinal bleeding. Moderate bleeding (BARC type 2) referred to bleeding that was apparent but did not require blood transfusion or hospitalization. Small bleeding (BARC type 0-1) indicated gingival bleeding or subcutaneous bleeding less than 2 mm in diameter, such as bleeding in the mucosa, skin, nose, and other minor bleeding sites.
The study was approved by the Ethics Committee of the Xuanwu Hospital Capital Medical University. Written informed consent was obtained from all participants.
Study Protocol
All patients underwent PCI and intracoronary stenting. The choice of stent type (bare-metal or drug-eluting stent) was left to the operator’s discretion, considering the clinical situation and anatomy of the coronary artery. Before the operation, patients received 100 mg aspirin and 75 mg clopidogrel per day for 2–3 days and loading doses of 300 mg both, respectively, at least 6 h prior to the procedure. During the procedure, the patients received an intra-arterial dose of 100 U/kg heparin; the use of glycoprotein IIb/IIIa inhibitors was only permitted in bailout situations. Following PCI, all patients were prescribed lifelong aspirin (100 mg/day) and clopidogrel (75 mg/day) for a duration of 12 months. Among them, some patients in the clopidogrel low responsiveness (CLR) group were advised by doctors to switch from clopidogrel to ticagrelor 90 mg twice daily for antiplatelet therapy, while the rest of the patients declined the replacement of clopidogrel. In addition to antiplatelet therapy, all patients received statins to modify their blood lipid levels. Administration of antihypertensive agents and hypoglycemic drugs followed the guidelines, based on individual patient needs.
Platelet Function Assay
Blood samples for platelet function testing were drawn after loading with clopidogrel (with 300 mg once or 75 mg/day for at least 2 days), 2–3 mL fasting venous blood was collected in the morning, and the thrombologram coagulation analyzer and supporting reagents were used. The antiplatelet inhibition rate of clopidogrel was determined using TEG software with ADP (2 μmol/L) as the activator, which included kaolin, activator F (a mixture of Agkistrodon hemagglutinin and platelet XI-IIa factor). According to relevant studies[18,19] and product description, platelet ADP receptor inhibition rate < 30% is defined as CLR, platelet inhibition rate ≥ 30% is normal reactivity. Based on the platelet inhibition rate whether less than 30%, the patients were categorized into two groups: the clopidogrel CLR group and the normal response to clopidogrel group (NRC). In this study, some patients in the CLR group were recommended by their doctors to switch to ticagrelor for antiplatelet therapy, while the remaining patients continued to receive antiplatelet therapy with clopidogrel. Therefore, within the CLR group, patients were further divided into two subgroups based on the actual antiplatelet drugs used: the ticagrelor group and the clopidogrel group. The specific process of the above items was completed by Xuanwu Hospital laboratory.
Detection of CYP2C19 Polymorphisms
A fasting venous blood sample of 2–3 mL was collected from the patient’s median cubital vein in the early morning, using EDTA-Na2 as the anticoagulant. Genomic DNA was then extracted. The CYP2C19 gene detection kit (utilizing DNA microarray chip method) was employed for PCR amplification, hybridization, and color visualization. Following the reaction, the biochip was read, and BaiO gene chip image analysis software was utilized to scan the images, perform data analysis, and obtain the detection results. Based on the results of gene detection, they were divided into three groups: normal metabolizer (wild type homozygous CYP2C19 *1*1); intermediate metabolizer (wild type and mutant heterozygous CYP2C19*1*2, CYP2C19*1* 3); poor metabolizer (mutation gene homozygous and heterozygous CYP2C19 *2*2, CYP2C19 *2*3, CYP2C19 *3*3) groups. The specific procedures for the aforementioned steps were carried out in the gene amplification room of Xuanwu Hospital.
Statistical Analysis
Continuous variables were presented as either mean ± SD or median (interquartile range), depending on the data distribution. Differences among the groups were evaluated using appropriate statistical tests, such as the independent t-test, one-way analysis of variance (ANOVA) test, or the Wilcoxon rank-sum test. Categorical variables were presented as counts (n) with corresponding percentages (%). Differences among the groups were assessed using appropriate statistical tests, such as the chi-square test or Fisher exact test. Following the identification of significant differences among variables using the ANOVA test, post hoc comparisons between the groups were conducted using Tukey’s multiple comparison test. The factors associated with clopidogrel hypo-responsiveness were identified using multivariate binary logistic regression analysis. Kaplan-Meier methods were employed to estimate the rates of all-cause deaths and major adverse cardiac and cerebrovascular events (MACCEs) over a 12-month period, and to visualize the cumulative occurrence of MACCEs in the three groups. The significance of differences among the groups was assessed using the log-rank test. Statistical analysis was conducted using SPSS version 22.0 (IBM Corp., Chicago, IL), and a P-value < 0.05 was considered statistically significant.
RESULTS
Patients Demography
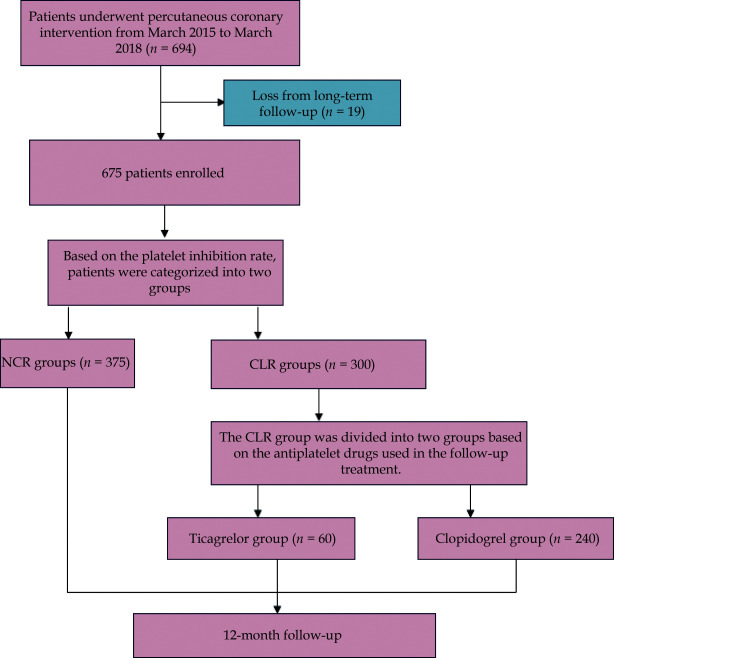
From March 2015 to March 2018, a total of 694 patients met the inclusion criteria. Out of the total 675 patients, 19 people lost contact during the follow-up period, leaving 675 patients who were followed up for at least one time. The mean follow-up period was 14.8 months. There were 60 patients in the CLR group who switched from clopidogrel to ticagrelor (Figure 1). The baseline characteristics of the different patient groups are listed in Table 1. The nationality, the sex, smoking history, drinking history, platelet count, aspartate amino transferase, creatine kinase isoenzyme, creatine kinase and CYP2C19 gene was significantly higher in the NCR group, while hypercholesterolemia,[20] Killip class 3–4 and triglyceride was higher in the CLR group.
Figure 1.
Flow chart of the study.
CHD: coronary heart disease; CLR: clopidogrel low responsiveness; NCR: normal clopidogrel responsiveness.
Table 1. Baseline characteristics.
| Characteristic | CLR group (n = 300) |
NCR group (n = 375) |
P-value |
| Data are presented as n (%), mean ± SD or median (interquartile range). ALT: alanine aminotransferase; Apo AI: apolipoprotein AI; Apo B: apolipoprotein B; AST: aspartate amino transferase; APTT: activated partial thromboplastin time; BMI: body mass index; CK: creatine kinase; CKMB: creatine kinase isoenzyme; DBIL: direct bilirubin; FIB: fibrinogen; GGT: gamma-glutamyl transpeptidase; HbA1c: glycated hemoglobin; HCY: homocysteine; HDL: high-density lipoprotein; LDL: Low-density lipoprotein; PLT: platelet count; PT: prothrombin time; SIB: indirect bilirubin; TG: triglyceride; TC: total cholesterol; TT: thrombin time; UA: uric acid. | |||
| Baseline characteristics | |||
| Age, yrs | 63.65 ± 11.72 | 62.47 ± 12.04 | 0.203 |
| Nationality (Han) | 280 (93.3%) | 366 (97.9%) | 0.003 |
| Sex (male) | 194 (64.7%) | 292 (77.9%) | 0.000 |
| BMI, kg/m2 | 25.06 (23.03, 27.34) | 25.34 (22.93, 27.68) | 0.430 |
| Past Medical History | |||
| Diabetes mellitus | 126 (42.0%) | 143 (38.1%) | 0.308 |
| Hypercholesterolemia | 75 (25.0%) | 68 (18.1%) | 0.030 |
| Hypertension | 198 (66.0%) | 247 (65.9%) | 0.971 |
| Smoking history | 150 (50.0%) | 225 (60.0%) | 0.009 |
| Drinking history | 97 (32.3%) | 154 (41.1%) | 0.020 |
| The number of vascular lesions ≥ 2 | 85 (28.3%) | 101 (26.9%) | 0.686 |
| Killip class 3–4 | 80 (26.7%) | 137 (36.5%) | 0.006 |
| Laboratory results | |||
| PLT, *109/L | 213.33 ± 61.76 | 228.92 ± 76.97 | 0.005 |
| APTT, s | 38.32 ± 7.66 | 38.47 ± 10.86 | 0.840 |
| PT(s) | 13.41 ± 0.98 | 13.48 ± 1.38 | 0.392 |
| FIB, g/L | 3.61 (3.14, 4.33) | 3.46 (3.02, 4.26) | 0.395 |
| TT, s | 16.82 ± 3.34 | 16.63 ± 2.24 | 0.385 |
| DBIL, μmol/L | 4.10 (3.16, 5.61) | 4.51 (3.23, 5.95) | 0.050 |
| SIB, μmol/L | 9.20 ± 5.26 | 9.91 ± 4.85 | 0.075 |
| UA, μmol/L | 343 ± 89.25 | 347 ± 87.30 | 0.591 |
| Urea, μmol/L | 5.91 ± 2.66 | 6.28 ± 2.98 | 0.102 |
| HCY, μmol/L | 17.03 ± 9.70 | 17.50 ± 10.29 | 0.564 |
| HDL-C, mmol/L | 1.05 ± 0.30 | 1.07 ± 0.31 | 0.321 |
| LDL-C, mmol/L | 2.53 ± 0.88 | 2.56 ± 0.91 | 0.756 |
| ApoAI, g/L | 1.13 ± 0.22 | 1.14 ± 0.24 | 0.810 |
| ApoB, g/L | 0.88 ± 0.39 | 0.91 ± 0.48 | 0.436 |
| TG, mmol/L | 1.71 (1.20, 2.27) | 1.51 (1.11, 2.17) | 0.046 |
| TC, mmol/L | 4.09 ± 1.03 | 4.15 ± 2.35 | 0.709 |
| HbA1c | 6.70% ± 1.58% | 6.78% ± 2.57% | 0.675 |
| Creatinine, μmol/L | 77.94 ± 44.60 | 79.95 ± 38.38 | 0.536 |
| GGT, IU/L | 36.75 ± 33.28 | 34.78 ± 29.14 | 0.421 |
| AST, IU/L | 32.00 (23.00, 69.00) | 46.50 (25.00, 110.50) | 0.000 |
| ALT, IU/L | 30.57 ± 31.89 | 34.64 ± 36.49 | 0.135 |
| CKMB, IU/L | 17.00 (11.00, 44.00) | 28.00 (17.75, 89.25) | 0.000 |
| CK, IU/L | 143.00 (71.00, 461.00) | 285.00 (103.75, 883.25) | 0.000 |
| CYP2C19 gene | 300 (44.4%) | 375 (55.6%) | 0.001 |
| Normal metabolizer | 109 (36.3%) | 186 (49.6%) | < 0.05 |
| Intermediate metabolizer | 148 (49.3%) | 160 (42.7%) | > 0.05 |
| Poor metabolizer | 43 (14.3%) | 29 (7.7%) | < 0.05 |
Correlation Analysis Between CYP2C19 Gene Polymorphism and Low Reactivity of Clopidogrel
Platelet inhibition rate in patients with different CYP2C19 genotypes
One-way analysis of variance (ANOVA) was performed to compare the ADP-induced platelet inhibition rate among different CYP2C19 genotypes, and a statistically significant difference was observed (P < 0.05) (Table 2).
Table 2. Platelet inhibition rate in patients with different CYP2C19 genotypes.
| Groups | CYP2C19 gene | ADP-induced platelet inhibition rate | P-value | |
| Data are presented as n (%), mean ± SD. | ||||
| Normal metabolizer | *1*1 | 295 (43.7%) | 43.65 ± 28.18 | 0.001 |
| Intermediate metabolizer | *1*2 | 265 (39.3%) | 38.53 ± 29.60 | |
| *1*3 | 43 (6.4%) | |||
| Poor metabolizer | *2*2 | 56 (8.3%) | 29.61 ± 27.52 | |
| *2*3 | 14 (2.1%) | |||
| *3*3 | 2 (0.3%) | |||
Clopidogrel Reactivity Among Patients with Different CYP2C19 Genotypes
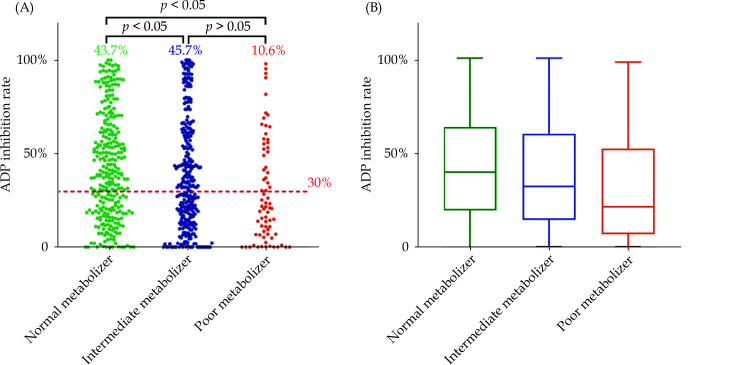
The chi-square test revealed significant differences in clopidogrel reactivity among patients with different genotypes (X2=7.425, P = 0.001). Statistically significant differences were observed between patients with normal metabolizer and Intermediate metabolizer (P < 0.05), as well as between patients with normal metabolizer and poor metabolizer (P < 0.05). No significant difference was found between patients with intermediate metabolizer and poor metabolizer (P > 0.05) (Figure 2).
Figure 2.
Clopidogrel reactivity and CYP2C19 gene.
(A): A scatter plot of ADP inhibition rate in the 3 strata defined by CYP2C19 genotype. The percentage indicates proportions for genotype and red dashed line displays threshold for NCR (30%). The p value by chi-square test between strata. (B): Box and whisker plots of ADP inhibition rate by genotype. The boxes show medians and upper and lower quartiles of the data, whereas the whiskers indicate the minimum and maximum values. ADP: adenosine diphosphate.
Multivariate Binary Logistic Regression Analysis for Low Response to Clopidogrel
These variables were included in a multivariate binary logistic regression model to predict high ADP inhibition rate. As shown in Table 3, this model confirmed that CYP2C19 gene was the strongest predictor of high ADP inhibition rate. Additionally, sex, nationality, and PLT prevailed as significant predictors in the multivariable analysis.
Table 3. Multivariate logistic regression analyses.
| Variables | B | S.E. | Wald | P | OR | 95% CI |
| AST: aspartate amino transferase; CKMB: creatine kinase isoenzyme; CK: creatine kinase; PLT: platelet count; TG: triglyceride | ||||||
| Sex (Male) | 0.751 | 0.278 | 7.295 | 0.007 | 2.120 | 1.229–3.657 |
| Nationality (Han) | 1.378 | 0.489 | 7.940 | 0.005 | 3.966 | 1.521–10.341 |
| Smoking history | 0.117 | 0.245 | 0.227 | 0.634 | 1.124 | 0.695–1.817 |
| Drinking history | -0.038 | 0.233 | 0.027 | 0.870 | 0.962 | 0.609–1.520 |
| Hypercholesterolemia | -0.379 | 0.234 | 2.630 | 0.105 | 0.684 | 0.433–1.082 |
| Killip class 3–4 | 0.321 | 0.211 | 2.311 | 0.128 | 1.379 | 0.911–2.085 |
| PLT (109/L) | 0.004 | 0.002 | 6.920 | 0.009 | 1.004 | 1.001–1.007 |
| AST (IU/L) | 0.000 | 0.003 | 0.020 | 0.887 | 1.000 | 0.993–1.006 |
| CKMB | 0.004 | 0.004 | 1.028 | 0.311 | 1.004 | 0.996–1.013 |
| CK | 0.000 | 0.000 | 0.240 | 0.624 | 1.000 | 0.999–1.001 |
| TG | -0.108 | 0.062 | 2.999 | 0.083 | 0.897 | 0.794–1.014 |
| CYP2C19 gene (Normal metabolizer) | 1.076 | 0.329 | 10.711 | 0.001 | 2.932 | 1.540-5.583 |
Clinical Outcomes
At the 12-month follow-up, 13 (1.9%) patients died, and 96 (14.2%) patients experienced MACCEs. The Kaplan-Meier survival curves demonstrated that the incidence of the cumulative rates of MACCEs, stent thrombosis and myocardial infarction (MI) was the lowest in normal clopidogrel responsiveness (NCR) group, and CLR group patients had the highest rates of MACCEs, stent thrombosis and MI (P < 0.05) (Table 4) (Figure 3).
Table 4. Clinical outcomes for NCR group vs. CLR group at one year follow-up.
| Adverse Events | NCR group (n = 375) | CLR group | P-value | |
| Ticagrelor group (n = 60) | Clopidogrel group (n = 240) | |||
| Data are presented as n (%). MACCEs: major adverse cardiac and cerebrovascular event, includes cardiac death, stent thrombosis, repeat revascularization, MI and stroke; MI: myocardial infarction. aP < 0.05 vs. NCLR group, bP < 0.05 vs. Ticagrelor group, cP < 0.05 vs. clopidogrel group. | ||||
| All-cause death | 5 (1.3%) | 1 (1.7%) | 7 (2.9%) | 0.390 |
| MACCEs | 36b,c (9.6%) | 7a,c (11.7%) | 53a,b (22.1%) | 0.000 |
| Cardiac death | 5(1.3%) | 1(1.7%) | 4(1.7%) | 0.939 |
| Stent thrombosis | 10b,c (2.7%) | 3a,c (5.0%) | 19a,b (7.9%) | 0.013 |
| MI | 9b,c (2.4%) | 2a,c (3.3%) | 16a,b (6.7%) | 0.035 |
| Repeat revascularization | 11 (2.9%) | 1 (1.7%) | 12 (5.0%) | 0.283 |
| Stroke | 1 (0.3%) | 0 (-) | 2 (0.8%) | 0.473 |
| Massive bleeding | 5 (1.3%) | 1 (1.7%) | 3 (1.3%) | 0.970 |
| Moderate bleeding | 11 (2.9%) | 3 (5.0%) | 9 (3.8%) | 0.685 |
| Small bleeding | 28 (7.5%) | 7 (11.7%) | 19 (7.9%) | 0.571 |
Figure 3.
Comparison of clinical outcomes in the overall population at 12-month follow-up.
(A): Comparison of MACCE rates in the overall population; (B): comparison of stent thrombosis rates in the overall population; (C): comparison of myocardial infarction rates in the overall population. MACCEs: major adverse cardiac and cerebrovascular events including cardiac death, stent thrombosis, repeat revascularization, myocardial infarction, and stroke.
At the 12-month clinical follow-up, excluding patients treated with oral ticagrelor antiplatelet therapy (n = 60), 12 (1.9%) patients died, and 89 (14.4%) patients experienced MACCEs. Based on the Kaplan-Meier survival curves, it was observed that the normal metabolizer group had the lowest cumulative rates of MACCEs (10.7%), and the poor metabolizer group exhibited the highest rates of MACCEs (22.6%) (P < 0.05). However, there was no significant difference in rates between the intermediate metabolizer and poor metabolizer groups (P > 0.05) (Table 5) (Figure 4).
Table 5. Clinical outcomes with different CYP2C19 genotypes at one year follow-up.
| Adverse Events | Normal metabolizer (n = 272) | Intermediate metabolizer (n = 281) | Poor metabolizer (n = 62) | P-value |
| Values expressed are in n (%). MACCEs major adverse cardiac and cerebrovascular event, includes cardiac death, stent thrombosis, repeat revascularization, MI and stroke, MI myocardial infarction. MI: myocardial infarction. aP < 0.05 vs. NCLR group, bP < 0.05 vs. Ticagrelor group, cP < 0.05 vs. Clopidogrel group.. | ||||
| All-cause death | 5 (1.8%) | 6 (2.1%) | 1 (1.6%) | 0.948 |
| MACCEs | 29 (10.7%) | 46a (16.4%) | 14a (22.6%) | 0.026 |
| Cardiac death | 4 (1.5%) | 4 (1.4%) | 1 (1.6%) | 0.994 |
| Stent thrombosis | 10 (3.7%) | 15 (5.3%) | 4 (6.5%) | 0.519 |
| MI | 6b,c (2.2%) | 13a,c (4.6%) | 6ab (9.7%) | 0.034 |
| Repeat revascularization | 9 (3.3%) | 12 (4.3%) | 2 (3.2%) | 0.817 |
| Stroke | 0 (-) | 2 (0.7%) | 1 (1.6%) | 0.141 |
| Massive bleeding | 3 (1.1%) | 4 (1.4%) | 1 (1.6%) | 0.922 |
| Moderate bleeding | 13 (4.8%) | 4 (1.4%) | 3 (4.8%) | 0.051 |
| Small bleeding | 19 (7.0%) | 24 (8.5%) | 4 (6.5%) | 0.736 |
Figure 4.
Comparison of Clinical outcomes with different CYP2C19 genotypes at 12-month follow-up.
(A): Comparison of MACCE rates in the overall population except for patients treated with oral ticagrelor antiplatelet therapy (n = 60); (B): Comparison of MI rates in the overall population except for patients treated with oral ticagrelor antiplatelet therapy (n = 60). MACCEs: major adverse cardiac and cerebrovascular events including cardiac death, stent thrombosis, repeat revascularization, myocardial infarction, and stroke; MI: myocardial infarction.
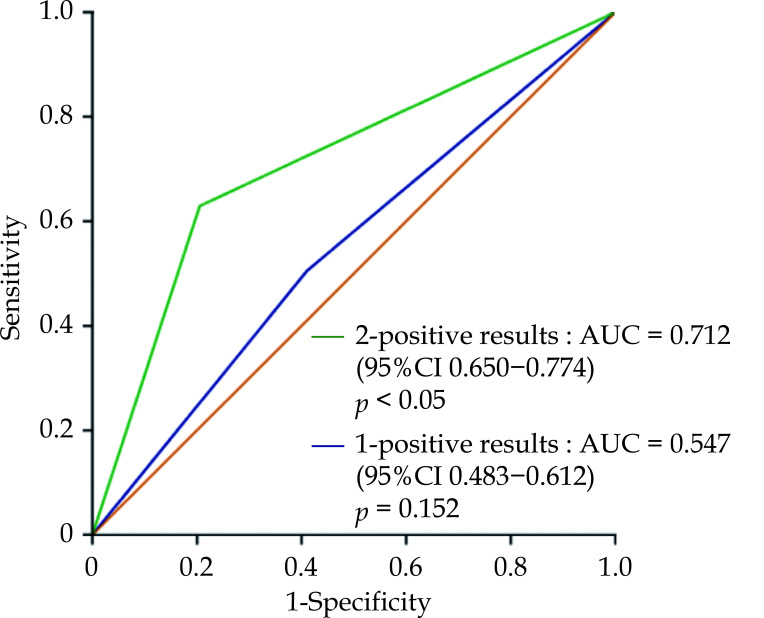
Based on the number of positive results of the two tests, patients were classified into three groups: 0-positive results (the patients with normal responses to clopidogrel and normal metabolizer; n = 189); 1-positive result (the patients with clopidogrel low responsiveness or intermediate metabolizer or poor metabolizer; n = 261); 2-positive results (the patients with clopidogrel low responsiveness and Intermediate metabolizer or poor metabolizer; n = 165). We evaluated the prognostic value of the aggregate positive results of the two detection methods for 12-month MACCEs. The highest incidence of MACCEs significantly increased with the 2-positive results, with an area under the curve (AUC) was 0.712 (95% CI: 0.650–0.774, P < 0.05). However, there was no significant difference between the group with the score of one and MACCEs (P > 0.05) (Figure 5).
Figure 5.
Relationships between MACCEs and the aggregate positive results of CYP2C19 genotyping and on-clopidogrel platelet reactivity.
Combined with platelet function to detect the additional risk predictive power of severe adverse cardiovascular events during one year follow-up of patients with coronary artery disease. AUC: area under the curve; MACCEs: major adverse cardiac and cerebrovascular events including cardiac death, stent thrombosis, repeat revascularization, myocardial infarction, and stroke.
DISCUSSION
CYP2C19 Gene Impact on Platelet Function
In the present study, we demonstrated that the interaction of genomic and clinical factors with on-clopidogrel platelet function in a large cohort of patients undergoing PCI is that the CYP2C19 gene polymorphisms and several clinical variables show a statistically highly significant association with CLR. Nevertheless, these variables do not suffice to predict CLR with the precision needed for clinical decision-making.
Previous studies have shown that the protease encoded by the CYP2C19 gene is the key enzyme responsible for the metabolism of clopidogrel during its activation in the liver.[8] Based on the ability of CYP2C19 to oxidative metabolism of drugs, it can be divided into two phenotypes: extensive metabolizer (EM) and poor metabolizer (PM). Among them,[21–23] CYP2C19 * 1 (wild type), which is the most common allele in the population, determines the drug normal metabolizers (extensive metabolizers); CYP2C19*2 and CYP2C19*3 are loss-of-function alleles that impede the synthesis of active clopidogrel metabolites, leading to reduced antiplatelet effects and an increased risk of adverse cardiovascular events.[24] Therefore, as a result, CYP2C19 gene polymorphism is considered an independent factor contributing to individual variations in clopidogrel treatment outcomes.[25] However, prior studies of genetic polymorphisms in CYP2C19 and their relationship with clinical efficacy have not included East Asian populations.[26] The frequency of CYP2C19 LoF alleles and the level of platelet reactivity differ between East Asians and Whites.[27] Nevertheless, the prognostic implications of combined platelet reactivity and the CYP2C19 genotype are undetermined, specifically in East Asians.
Our study revealed that the initial prevalence of the CLR in the overall patient population (44%) was similar to that reported in previous studies conducted in different populations (4%-44%).[28,29] We observed significant differences in the platelet inhibition rate among the three metabolic genotypes (P < 0.05). Specifically, significant differences were found between normal metabolizer and Intermediate metabolizer (P < 0.05), as well as between normal metabolizer and poor metabolizer (P < 0.05). However, no significant difference was observed between Intermediate metabolizer and poor metabolizer (P > 0.05), as shown in Figure 1. The presence of CYP2C19*2 and/or CYP2C19*3 mutation genes in patients with intermediate metabolizer and poor metabolizer suggested an impact on clopidogrel responsiveness, resulting in clopidogrel resistance.[30,31] Previous studies have also identified CYP2C19 gene polymorphism as predictors of clopidogrel resistance.[32–34]
Application of Antiplatelet Drugs in Patients with Coronary Heart Disease in the Real World
Currently, the prognostic benefit of dual antiplatelet therapy (aspirin and clopidogrel) following acute coronary syndromes (ACS) has been well established.[35,36] However, there are interindividual differences in clopidogrel metabolism that can significantly increase the risk of ischemic events.[37,38] Therefore, finding ways to achieve safe and effective antiplatelet therapy for patients with poor response to clopidogrel has become a prominent topic in the field of antithrombotic therapy. Ticagrelor is a novel oral antiplatelet drug that binds reversibly to P2Y12 receptors without the need for metabolic activation, thereby facilitating a rapid antiplatelet effect.[39] Ticagrelor exhibits more prompt and potent inhibition of platelet aggregation when compared to clopidogrel.[40] The RESPOND studies[41] demonstrated that ticagrelor exhibited comparable antiplatelet effects in patients with both CLR and NCR conditions. Moreover, the ticagrelor was superior to clopidogrel in preventing cardiovascular events in patients diagnosed with ACS, acute myocardial infarction (AMI), ST-segment elevation myocardial infarction (STEMI), and non-ST-segment elevation myocardial infarction (NSTEMI), while maintaining a similar risk of bleeding.[42,43] Additionally, studies of ONSET/OFFSET and RESPOND genotypes[44] revealed that ticagrelor possessed greater pharmacodynamic efficacy than clopidogrel, regardless of the individual’s CYP2C19 genotype. Consequently, ticagrelor is frequently employed as an antiplatelet therapy for CHD patients with CLR.
In our study, 675 patients were enrolled, 375 in the NCR group and 300 in the CLR group. Some patients in the CLR group were replaced by ticagrelor for antiplatelet therapy, so the CLR group was divided into ticagrelor group and clopidogrel group according to the actual antiplatelet drugs used by the patients, and the incidence of clinical adverse events within 12 months in the three groups was observed. At the 12-month follow-up, 13(1.9%) patients died, and 96(14.2%) patients experienced MACCEs. Notably, we found significant differences in the incidence of MACCEs among the three groups, patients with NCR had a better prognosis after PCI than patients with CLR undergoing PCI in terms of MACCEs, and ticagrelor demonstrated superiority to clopidogrel in reducing rates of the primary endpoint of death from MACCEs at 12 months. Remarkably, there was also a significant reduction in the rate of individual endpoints with ticagrelor, including stent thrombosis (5.0% vs. 7.9%, P = 0.009), MI (3.3% vs. 6.7%, P = 0.017). In terms of bleeding events, there was no statistical difference among the three groups, but the Ticagrelor group had a higher risk of bleeding. According to the above, for patients with CLR, ticagrelor was proven to be superior to clopidogrel in reduction of the composite endpoint of MACCEs, MI, or stent thrombosis, and resulted in enhanced survival without an increase in bleeding events.
However, there is controversy regarding the safety of ticagrelor and its impact on long-term prognosis. In clinical practice, the use of ticagrelor is frequently restricted by various factors, including drug-related side effects (such as dyspnea, ventricular pauses ≥ 3 s, and elevated levels of serum creatinine and uric acid), advanced age, poor liver and kidney function, and patient non-compliance.[45] The majority of patients favored a combination of clopidogrel and aspirin for antiplatelet therapy. In this study, certain patients with CLR withdrew from the study group due to a modification in their antiplatelet medication during the administration of ticagrelor antiplatelet therapy.
Effect of CYP2C19 Gene Polymorphism and Clopidogrel Hyporesponsiveness on Long-term Prognosis of Patients with Coronary Heart Disease After PCI
Current studies conducted domestically and internationally have confirmed that CYP2C19 gene polymorphism contributes to variable platelet response to clopidogrel. However, the relationship between CYP2C19 genotype and long-term prognosis of patients with CHD after PCI remains controversial.[46] In this study, the CYP2C19 genotype was categorized into three groups based on metabolic rate: normal, intermediate, and poor. Subsequently, the incidence of clinical adverse events within a 12-month period was observed in each group. At the 12-month clinical follow-up, except for patients treated with oral ticagrelor antiplatelet therapy (n = 60), 12(1.9%) patients died, and 89(14.4%) patients experienced MACCEs. Normal metabolizer demonstrated superiority to intermediate metabolizer and poor metabolizer in reducing rates of the primary endpoint of death from MACCEs at 12 months. Additionally, a notable decrease was observed in the rate of individual endpoints, such as myocardial infarction (2.2% vs. 4.6% vs. 9.7%, P = 0.034), among patients with fast metabolism. In terms of bleeding events, there was no statistical difference among the three groups. These findings align with previous studies, such as meta-analyses,[10,47,48] which highlight the increased risk of severe adverse cardiovascular events in patients who carry even one variant of the diminished CYP2C19 allele and undergo percutaneous coronary intervention with clopidogrel. Guided by the findings of CYP2C19 genotype testing, patients harboring CYP2C19*2 and/or CYP2C19*3 mutations (e.g., ticagrelor) are provided with alternative antiplatelet therapy. Notably, there was no discernible discrepancy in major adverse cardiovascular events (MACE) between individuals with normal metabolic genotypes who received standard therapies and those carrying the mutant gene who were administered alternative treatments.[49,50]
In light of the above study, we found that both CYP2C19 genotyping and platelet function testing are able to predict MACCEs in Chinese patients treated with clopidogrel and undergoing stent implantation, but the accurate prognostic technique is still disputed. Therefore, a combination of both techniques was employed to ascertain the potential for a more precise prognosis. Our findings revealed a substantial increase in the highest MACCEs incidence among individuals with 2-positive results, suggesting that combining the positive results from both testing methods may serve as an independent and favorable predictor for MACCEs.
CYP2C19 polymorphisms are more prevalent, occurring in approximately 30% of whites, 40% of blacks, and over 55% of East Asians.[32,51] Therefore, CYP2C19 gene polymorphism resulting in a reduced clopidogrel responsiveness leads to cardiovascular events in roughly half of Chinese patients, highlighting the need for greater attention. However, current guidelines[52] do not endorse genetic testing or platelet response testing (Class IIb) as standard prior to prescribing clopidogrel. Hence, additional research is necessary, particularly in the form of large-scale randomized controlled trials, to evaluate the efficacy of this approach.
Conclusions
In conclusion, low response to clopidogrel in patients with coronary heart disease is correlated with CYP2C19 gene polymorphism, and CYP2C19 genotyping combined with platelet reactivity is an independent and favorable predictor of 12-months MACCEs in patients undergoing stenting with clopidogrel treatment, which is better than either test alone. Detection of CYP2C19 genotype and clopidogrel reactivity has guiding significance in the clinical application of antiplatelet drugs, improves clinical prognosis.
Study Limitations
The limitations of this study include limited detection technology for CYP2C19 polymorphisms, such as the inability to detect CYP2C19 *17, CYP2C19 *9,[12] etc. Additionally, the small sample size of this study prevented an accurate reflection of the clinical effectiveness of CYP2C19 gene polymorphism and platelet function detection in personalized antiplatelet therapy. Therefore, further studies are needed, particularly in large randomized controlled trials, to evaluate the efficacy of this strategy.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No.62172288). All authors had no conflicts of interest to disclose. The authors would like to thank all participants and all staff members for data collection, management, and monitoring of this study.
References
- 1.Levine GN, Bates ER, Bittl J A, et al 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082–1115. doi: 10.1016/j.jacc.2016.03.513. [DOI] [PubMed] [Google Scholar]
- 2.Mejin M, Tiong WN, Lai LY, et al CYP2C19 genotypes and their impact on clopidogrel responsiveness in percutaneous coronary intervention. Int J Clin Pharm. 2013;35:621–628. doi: 10.1007/s11096-013-9783-y. [DOI] [PubMed] [Google Scholar]
- 3.Yi X, Lin J, Zhou Q, et al Clopidogrel resistance increases rate of recurrent stroke and other vascular events in Chinese population. J Stroke Cerebrovasc Dis. 2016;25:1222–1228. doi: 10.1016/j.jstrokecerebrovasdis.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Buonamici P, Marcucci R, Migliorini A, et al Impact of platelet reactivity after clopidogrel administration on drug-eluting stent thrombosis. J Am Coll Cardiol. 2007;49:2312–2317. doi: 10.1016/j.jacc.2007.01.094. [DOI] [PubMed] [Google Scholar]
- 5.Sibbing D, Braun S, Morath T, et al Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J Am Coll Cardiol. 2009;53:849–856. doi: 10.1016/j.jacc.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Müller I, Besta F, Schulz C, et al. Prevalence of clopidogrel non-responders among patient. Thromb Haemost 2003: 783–787.
- 7.Price MJ Bedside evaluation of thienopyridine antiplatelet therapy. Circulation. 2009;119:2625–2632. doi: 10.1161/CIRCULATIONAHA.107.696732. [DOI] [PubMed] [Google Scholar]
- 8.Kazui M, Nishiya Y, Ishizuka T, et al Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92–99. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]
- 9.Xie HG, Zou JJ, Hu ZY, et al Individual variability in the disposition of and response to clopidogrel: pharmacogenomics and beyond. Pharmacol Ther. 2011;129:267–289. doi: 10.1016/j.pharmthera.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Mega JL, Simon T, Collet JP, et al Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott SA, Sangkuhl K, Stein CM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther 2013, 94: 317–323.
- 12.Lee C R, Luzum JA, Sangkuhl K, et al Clinical pharmacogenetics implementation consortium guideline for CYP2C19 genotype and clopidogrel therapy: 2022 update. Clin Pharmacol Ther. 2022;112:959–967. doi: 10.1002/cpt.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SE, Jeon HS, Go TH, et al High platelet reactivity combined with CYP2C19 genotype in predicting outcomes in east Asian patients undergoing percutaneous coronary intervention. Clin Pharmacol Ther. 2023;114:1104–1115. doi: 10.1002/cpt.3026. [DOI] [PubMed] [Google Scholar]
- 14.Kim HK, Tantry US, Smith SC, Jr. , et al. The East Asian Paradox: an updated position statement on the challenges to the current antithrombotic strategy in patients with cardiovascular disease. Thromb Haemost 2021; 121: 422–432.
- 15.Tan K, Lian Z, Shi Y, et al The effect of CYP2C19 genotype-guided antiplatelet therapy on outcomes of selective percutaneous coronary intervention patients: an observational study. Per Med. 2019;16:301–312. doi: 10.2217/pme-2018-0087. [DOI] [PubMed] [Google Scholar]
- 16.Li KHC, Wong KHG, Gong M, et al Percutaneous coronary intervention versus medical therapy for chronic total occlusion of coronary arteries: a systematic review and meta-analysis. Curr Atheroscler Rep. 2019;21:42. doi: 10.1007/s11883-019-0804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehran R, Rao SV, Bhatt DL, et al Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 18.Bliden KP, Dichiara J, Tantry US, et al. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: is the current antiplatelet therapy adequate? J Am Coll Cardiol 2007; 49: 657–666.
- 19.Shanker JGA, Kitas GD, Kakkar VV. Platelet function and antiplatelet therapy in cardiovascular disease implications of genetic polymorphisms. Curr Vasc Pharmacol 2011: 479–489.
- 20.Civeira F, Arca M, Cenarro A, et al. A mechanism-based operational definition and classification of hypercholesterolemia. J Clin Lipidol 2022, 16: 813–821.
- 21.Anderson CD, Biffi A, Greenberg SM, et al. Personalized approaches to clopidogrel therapy: are we there yet? Stroke 2010; 41: 2997–3002.
- 22.Terpening C Clopidogrel: a pharmacogenomic perspective on its use in coronary artery disease. Clin Med Insights Cardiol. 2010;4:117–128. doi: 10.4137/CMC.S4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erlinge D, James S, Duvvuru S, et al Clopidogrel metaboliser status based on point-of-care CYP2C19 genetic testing in patients with coronary artery disease. Thromb Haemost. 2014;111:943–950. doi: 10.1160/TH13-09-0767. [DOI] [PubMed] [Google Scholar]
- 24.Ali ZO, Bader L, Mohammed S, et al Effect of CYP2C19 genetic variants on bleeding and major adverse cardiovascular events in a cohort of Arab patients undergoing percutaneous coronary intervention and stent implantation. Pharmacogenet Genomics. 2022;32:183–191. doi: 10.1097/FPC.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 25.Brown SA, Pereira N. Pharmacogenomic impact of CYP2C19 variation on clopidogrel therapy in precision cardiovascular medicine. J Pers Med 2018, 8: 8.
- 26.Magavern EF, Jacobs B, Warren H, et al. CYP2C19 Genotype Prevalence and Association With Recurrent Myocardial Infarction in British-South Asians Treated With Clopidogrel. JACC Adv 2023. Doi: 10.1016/j.jacadv.2023.100573.
- 27.Kang J, Park KW, Palmerini T, et al Racial differences in ischaemia/bleeding risk trade-off during anti-platelet therapy: individual patient level landmark meta-analysis from seven RCTs. Thromb Haemost. 2019;119:149–162. doi: 10.1055/s-0038-1676545. [DOI] [PubMed] [Google Scholar]
- 28.Vlachojannis GJ, Dimitropoulos G, Alexopoulos D. Clopidogrel resistance: current aspects and future directions. Hellenic J Cardiol 2011: 235–245.
- 29.Gurbel PA, Bliden KP, Hiatt BL, et al Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908–2913. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 30.Collet JP, Hulot JS, Anzaha G, et al High doses of clopidogrel to overcome genetic resistance: the randomized crossover CLOVIS-2 (Clopidogrel and Response Variability Investigation Study 2) JACC Cardiovasc Interv. 2011;4:392–402. doi: 10.1016/j.jcin.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Thomas CD, Williams AK, Lee CR, et al. Pharmacogenetics of P2Y12 receptor inhibitors. Pharmacotherapy 2023, 43: 158–175.
- 32.Simon T, Verstuyft C, Mary-Krause M, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med 2009: 363–375.
- 33.Mao L, Jian C, Changzhi L, et al Cytochrome CYP2C19 polymorphism and risk of adverse clinical events in clopidogrel-treated patients: a meta-analysis based on 23, 035 subjects. Arch Cardiovasc Dis. 2013;106:517–527. doi: 10.1016/j.acvd.2013.06.055. [DOI] [PubMed] [Google Scholar]
- 34.Xi Z, Fang F, Wang J, et al CYP2C19 genotype and adverse cardiovascular outcomes after stent implantation in clopidogrel-treated Asian populations: A systematic review and meta-analysis. Platelets. 2019;30:229–240. doi: 10.1080/09537104.2017.1413178. [DOI] [PubMed] [Google Scholar]
- 35.Yusuf S, Zhao F, Mehta SR, et al Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 36.Sabatine MS, Cannon CP, Gibson CM, et al Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352:1179–1189. doi: 10.1056/NEJMoa050522. [DOI] [PubMed] [Google Scholar]
- 37.Bernlochner I, Byrne RA, Kastrati A, et al. The future of platelet function testing to guide therapy in clopidogrel low and enhanced responders. Expert Rev Cardiovasc Ther 2011: 999–1014.
- 38.Paré G, Mehta S R, Yusuf S, et al Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Engl J Med. 2010;363:1704–1714. doi: 10.1056/NEJMoa1008410. [DOI] [PubMed] [Google Scholar]
- 39.Tunströmer K, Faxälv L, Larsson P, et al Thrombus remodelling by reversible and irreversible P2Y(12) inhibitors. Platelets. 2023;34:2157805. doi: 10.1080/09537104.2022.2157805. [DOI] [PubMed] [Google Scholar]
- 40.Tapp L, Shantsila E, Lip G Y. Role of ticagrelor in clopidogrel nonresponders: resistance is futile? Circulation 2010; 121: 1169–1171.
- 41.Gurbel PA, Bliden KP, Butler K, et al Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation. 2010;121:1188–1199. doi: 10.1161/CIRCULATIONAHA.109.919456. [DOI] [PubMed] [Google Scholar]
- 42.Qian W, Chen L, Zhang L, et al Comparison of ticagrelor and clopidogrel in the treatment of patients with coronary heart disease carrying CYP2C19 loss of function allele. J Thorac Dis. 2022;14:2591–2601. doi: 10.21037/jtd-22-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollack CV, Davoudi F, Diercks DB, et al Relative efficacy and safety of ticagelor vs clopidogrel as a function of time to invasive management in non-ST-segment elevation acute coronary syndrome in the PLATO trial. Clin Cardiol. 2017;40:390–398. doi: 10.1002/clc.22733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tantry US, Bliden KP, Wei C, et al First analysis of the relation between CYP2C19 genotype and pharmacodynamics in patients treated with ticagrelor versus clopidogrel: the ONSET/OFFSET and RESPOND genotype studies. Circ Cardiovasc Genet. 2010;3:556–666. doi: 10.1161/CIRCGENETICS.110.958561. [DOI] [PubMed] [Google Scholar]
- 45.Davis EM, Knezevich JT, Teply RM Advances in antiplatelet technologies to improve cardiovascular disease morbidity and mortality: a review of ticagrelor. Clin Pharmacol. 2013;5:67–83. doi: 10.2147/CPAA.S41859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Empey PE, Stevenson JM, Tuteja S, et al Multisite investigation of strategies for the implementation of CYP2C19 genotype-guided antiplatelet therapy. Clin Pharmacol Ther. 2018;104:664–674. doi: 10.1002/cpt.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramirez AH, Delaney JT, Shuldiner AR, et al Carrying one or two reduced-function CYP2C19 alleles is associated with an increased risk of major adverse cardiovascular events in people undergoing percutaneous coronary intervention and treated with clopidogrel. Evid Based Med. 2011;16:124–125. doi: 10.1136/ebm1203. [DOI] [PubMed] [Google Scholar]
- 48.Jin B, Ni HC, Shen W, et al Cytochrome P450 2C19 polymorphism is associated with poor clinical outcomes in coronary artery disease patients treated with clopidogrel. Mol Biol Rep. 2011;38:1697–1702. doi: 10.1007/s11033-010-0282-0. [DOI] [PubMed] [Google Scholar]
- 49.Cavallari LH, Lee CR, Beitelshees AL, et al Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. JACC Cardiovasc Interv. 2018;11:181–191. doi: 10.1016/j.jcin.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallentin L, James S, Storey RF, et al Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–1328. doi: 10.1016/S0140-6736(10)61274-3. [DOI] [PubMed] [Google Scholar]
- 51.Caudle KE, Dunnenberger HM, Freimuth RR, et al Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC) Genet Med. 2017;19:215–223. doi: 10.1038/gim.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levine GN, Bates ER, Bittl JA, et al 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg. 2016;152:1243–1275. doi: 10.1016/j.jtcvs.2016.07.044. [DOI] [PubMed] [Google Scholar]