Abstract
The Sendai virus P/C mRNA expresses eight primary translation products by using a combination of ribosomal choice and cotranscriptional mRNA editing. The longest open reading frame (ORF) of the mRNA starts at AUG104 (the second initiation site) and encodes the 568-amino-acid P protein, an essential subunit of the viral polymerase. The first (ACG81), third (ATG114), fourth (ATG183), and fifth (ATG201) initiation sites are used to express a C-terminal nested set of polypeptides (collectively named the C proteins) in the +1 ORF relative to P, namely, C′, C, Y1, and Y2, respectively. Leaky scanning accounts for translational initiation at the first three start sites (a non-ATG followed by ATGs in progressively stronger contexts). Consistent with this, changing ACG81/C′ to ATG (GCCATG81G) abrogates expression from the downstream ATG104/P and ATG114/C initiation codons. However, expression of the Y1 and Y2 proteins remains normal in this background. We now have evidence that initiation from ATG183/Y1 and ATG201/Y2 takes place via a ribosomal shunt or discontinuous scanning. Scanning complexes appear to assemble at the 5′ cap and then scan ca. 50 nucleotides (nt) of the 5′ untranslated region before being translocated to an acceptor site at or close to the Y initiation codons. No specific donor site sequences are required, and translation of the Y proteins continues even when their start codons are changed to ACG. Curiously, ATG codons (in good contexts) in the P ORF, placed either 16 nt upstream of Y1, 29 nt downstream of Y2, or between the Y1 and Y2 codons, are not expressed even in the ACGY1/ACGY2 background. This indicates that ATG183/Y1 and ATG201/Y2 are privileged start sites within the acceptor site. Our observations suggest that the shunt delivers the scanning complex directly to the Y start codons.
The vast majority of eukaryotic mRNAs are monocistronic and express a single open reading frame (ORF) which initiates from the ATG codon nearest the capped 5′ end. This initiation site is chosen by a scanning mechanism in which initiation factors, 40S ribosomal subunits, and initiator Met-tRNA bind to the capped 5′ end of the mRNA and linearly scan the nucleotide sequence for the first start codon (the 5′ scanning model; for a review, see reference 29). Some eukaryotic viral mRNAs, like the Sendai virus (SeV) P/C mRNA, however, express two ORFs which overlap (Fig. 1). Such mRNAs therefore also initiate proteins at start codons which are not 5′ proximal. To account for initiation on these bicistronic mRNAs, a modified scanning model which allows for leaky scanning, i.e., scanning in which the scanning complex can bypass the first start codon to some extent when it is not in an optimal context for initiation and thereby can initiate at a downstream start codon, was proposed (23). This model followed from the realization that the nucleotide sequence surrounding the start codon (the context) was important for the efficiency with which ribosomes initiate protein synthesis, with the strongest determinant for efficiency being a purine at position −3, followed by a G at position +4.
FIG. 1.
Schematic representation of the SeV P/C mRNA. The 1,943-nt-long SeV P/C mRNA is shown as a horizontal line, with the various ORFs as boxes. The P ORF is shown as a shaded box, the C ORF (position +1 relative to P, encoding the C′, C, Y1, and Y2 proteins) is shown as an open box, and the internal V ORF (position −1 relative to P) is shown as a black box. The V and W proteins are generated by cotranscriptional editing of the P/C mRNA, during which a +1 G (for V) and +2 Gs (for W) are inserted at nucleotide position 1053. This fuses these ORFs to the N-terminal half of P. The 81-nt 5′ UTR is also indicated. Numbers refer to nucleotide positions relative to the 5′ end of the P/C mRNA.
Eukaryotic mRNAs known to initiate proteins at two start codons are nevertheless uncommon, and those that initiate at more than two start codons are rare. The most extreme example of an mRNA known to initiate proteins at multiple start sites remains the SeV P/C mRNA, which uses five start codons located 81 to 201 nucleotides (nt) from the 5′ end and a start codon located more than 1500 nt from the 5′ end to generate eight primary translation products (C′, P, C, Y1, Y2, V, W, and X) (Fig. 1). The second start site (and 5′-proximal ATG codon) generates three proteins (P, V, and W) containing the same N-terminal 317 amino acids (aa) but different C-terminal regions, as this mRNA is cotranscriptionally edited by the SeV polymerase via the programmed insertion of zero, one, or two G residues (45). The X protein, which represents approximately the C-terminal 95 aa of the 568-aa-long P protein, is thought to be initiated in a cap-dependent but scanning-independent manner (5). The first ribosomal start site (an unusual ACG start codon) and the third, fourth, and fifth start sites (all ATG codons) generate a nested set of four C proteins (termed C′, C, Y1, and Y2, of 215, 204, 183, and 175 aa, respectively) with a common C terminus, irrespective of whether the mRNA is edited (6, 18, 33). The Y proteins do not appear to be generated by proteolytic cleavage of the C or C′ protein, because a recombinant SeV which does not express either C or C′ (due to mutation of their start codons) overexpresses the Y proteins (25a). The available evidence suggests that whereas the first three start sites (for the C′, P, and C proteins) are accessed by leaky scanning, it is unlikely that the last two start sites (for the Y proteins) are accessed by a mechanism involving only linear movement of the scanning complex. For example, the leaky-scanning model posits that when successive initiation codons are used, they will be increasingly efficient as start sites. However, the Y1 and Y2 start sites are in the weakest context of the five, with neither a purine at position −3 nor a G at position +4. Only the first, inherently weak, ACG81 start codon is in an otherwise optimal context (GCCACGGAT) for initiation (2, 16). More importantly, mutation of the 5′-proximal ACG initiation site to ATG (GCCA81TGG; hereafter referred to as ATG81/C′) increased C′ expression by as much as fivefold and eliminated initiation from the downstream ATG104/P and ATG114/C start codons, presumably because the very favorable context of ATG81/C′ precludes any leaky scanning. Expression from ATG183/Y1 and ATG201/Y2, in contrast, was either normal or, in some instances, increased (6). The continued expression of the Y proteins under these conditions suggested that initiation from these distal start sites was scanning independent, or discontinuous.
Some mRNAs (e.g., picornavirus and hepatitis C virus [HCV] RNAs, mammalian Bip RNA, and Drosophila antennapedia mRNA; for reviews, see references 20, 21, and 29) are thought to initiate protein synthesis by an alternate mechanism, in which preinitiation complexes bind directly to an internal ribosome entry site (IRES) without scanning from the 5′ end of the mRNA. IRES-directed initiation is distinguished from that of 5′ scanning by its independence of both a 5′ cap group on the mRNA and the cap-binding initiation factor eIF-4E, although the other canonical initiation factors of the eIF-4F complex (namely, eIF-4A and eIF-4G) are all generally involved (32, 35, 36). Recent work, however, has found that the HCV and classical swine fever virus IRESs, which are structurally distinct from those of picornaviruses, can assemble 43S preinitiation complexes independently of these canonical eIFs as well as eIF-4E (36a).
In earlier work, we suggested that initiation of the Y proteins may occur via an IRES-like element positioned somewhere downstream of ATG114/C. However, this turned out to be unlikely for the following reasons.
(i) Y protein initiation in vitro was as sensitive to inhibition by cap analogs as that of C′, P, and C.
(ii) Host protein synthesis is shut off in poliovirus-infected cells due to the selective cleavage of eIF-4G, the largest subunit of the cap-binding eIF-4F complex. However, in cells coinfected with SeV and poliovirus, Y protein synthesis was turned off as efficiently as that of C′, P, and C (reference 15 and unpublished results). Initiation of the Y proteins is clearly cap dependent, both in vitro and in vivo.
(iii) The now-classical assay for identification of an IRES element is to place the prospective sequence between nonoverlapping ORFs of a bicistronic mRNA and to examine the requirement of each ORF for functional eIF-4F (22, 34). Despite repeated attempts, however, we were unable by this test to find the slightest evidence that an IRES was present near ATG183/Y1 and ATG201/Y2 of the P/C mRNA (unpublished results).
(iv) IRESs are generally found only on mRNAs with long 5′ untranslated regions (UTRs) containing multiple, unused upstream ATGs, as well as highly structured regions which can act as barriers to scanning complexes. A notable exception to these rules is cellular Bip, whose translation is thought to be mediated via an IRES even though the ca. 220-nt 5′ UTR contains little obvious secondary structure and no ATGs (27, 28). Likewise, there is no evidence for stable structures within the 5′ end of the SeV P/C mRNA (6a, 17), and all four ATGs (and one ACG) in the 5′ 201 nt of this mRNA are clearly functional.
More recently, some mRNAs have been found to initiate protein synthesis by yet another mechanism, shunting, which combines aspects of both 5′ scanning and internal initiation. Here, scanning complexes enter from the capped 5′ end of the mRNA but then are shunted downstream, bypassing intervening segments of the 5′ UTR, which, like IRESs, include multiple unused ATGs and strong secondary structures which normally block 5′ scanning (13). This shunting is promoted by a cis-acting element thought to be present within the highly structured region of the 5′ UTR (the donor site), which directs the translocating scanning complex to a defined landing or acceptor site, where it resumes its decoding of the mRNA. The list of such mRNAs is still quite limited and includes the pregenome RNAs of the pararetroviruses cauliflower mosaic virus (CaMV) (13) and rice tungro bacilliform virus (RTBV) (14) and the adenovirus late mRNAs (46). This paper reports that the SeV Y proteins are initiated via a shunt which bypasses the C′, P, and C protein start sites and delivers scanning complexes directly to ATG183/Y1 and ATG201/Y2, even though there is no evidence of a highly structured region in the 5′ UTR. Moreover, similar to the IRES of HCV (37) and the shunt of RTBV (14), substitution of normally weak start codons (like ACG) for ATG183 and ATG201 has only a small effect on Y protein synthesis.
MATERIALS AND METHODS
Construction of mutants. (i) Insertion of stem-loops.
The plasmid pGEM-P/Cwt has been previously described (7). At position 23, 53, or 73 of the 5′ UTR, a unique BglII site was introduced by PCR-mediated site-directed mutagenesis. The oligonucleotide 5′ TATAGGATCCCCTTGAGACCTCCGTACCCTGCAGG 3′ (containing eight terminal nucleotides that are self-complementary) was self-annealed and filled in with Klenow fragment. It was then digested with BamHI and cloned into the BglII sites, thereby generating the clones stem-loop23, stem-loop53, and stem-loop73 (the estimated ΔG° for the inserted stem-loop was −54 kcal/mol). Stem-loop1 and stem-loop81 were generated by using the self-annealing oligonucleotide 5′ GATCGGGCGCGTGGTGGCGGCTGCAGCCGCCACCACGCGCCC 3′ (the estimated ΔG° was −54 kcal/mol). The self-annealed oligonucleotide was filled in with Klenow fragment and blunt ligated into the NcoI site at position 81 (stem-loop81) or ligated directly into the BamHI site of the plasmid multiple cloning site (stem-loop1).
(ii) Replacement of 5′ UTR.
For the clone RFXATG81, the 5′ UTR of a plasmid containing a cDNA clone of RFX-AP (9) was PCR amplified with an upstream oligonucleotide containing a SacI site and a downstream oligonucleotide overlapping the RFX-AP initiation codon and containing an NcoI site (. .CCA69TGG. .). The 5′ UTR of the SeV clone ATG81 was removed by SacI/NcoI digestion and replaced by the SacI/NcoI-digested PCR fragment of RFX-AP. For the clone U1ATG81, a pGEM3 clone containing the cDNA of the human U1 snRNA-associating A protein (42) was digested with NcoI (containing the ATG start codon) and PstI (within the downstream region of the polylinker), thereby removing all of the coding region and the 3′ UTR. Into the remaining plasmid backbone was inserted the SeV ATG81 coding region as an NcoI/PstI fragment.
(iii) Spacer mutant.
The sequence between C114 and Y1183 was generated by using two overlapping oligonucleotides containing BamHI sites at their 5′ ends. These oligonucleotides were annealed and then digested with BamHI. The fragment of 66 bp was introduced into the BamHI site of the clone pGEM-P/CBamHI, in which a BamHI site had been introduced between the ATG104/P and ATG114/C start codons at position 106.
(iv) Mutant ACGY1/Y2.
Mutant ACGY1/Y2 was obtained by fusion PCR starting from the SeV pGEM-P/Cwt. The first PCR product was generated by using a T7 primer (positive sense) combined with negative primer A (5′ CGTCGAGGAATCCGATAACGTCCGAGAGCGACTCTC 3′) to introduce ACG codons at positions 183 and 201. The second PCR product was obtained with the negative-sense oligonucleotide PEcoP (see below), complementary to position 1178 on the P/C mRNA, and the positive-sense primer B (5′ GACGTTATCGGATTCCTCGACGCTGTCCTGTCGAG 3′), which overlapped by 22 nt the negative primer A. The fragments from the first and second PCRs were combined in a final fusion PCR. The product of this reaction was digested with SacI (within the upstream polylinker region) and EagI (position 1005 on the P/C mRNA) and then substituted into the SeV P/C backbone.
(v) Insertion of ATG codons within the P ORF.
Insertion of ATG codons within the P ORF was performed by fusion PCR starting from both the SeV P/Cwt and the ATG81 clones. The first PCR product was generated by using a T7 primer (positive sense) combined with a series of negative-sense primers (a, b, c, d, and e [see below]) to introduce an ATG codon at positions 131, 167, 194, 230, and 287. The second PCR product was obtained with the negative-sense oligonucleotide PEcoP, complementary to position 1178 on the P/C mRNA, and a series of positive-sense primers, a′, b′, c′, d′, and e′ (see below), that overlapped by 10 to 12 bp with primers a, b, c, d, and e. The fragments from the first and second PCRs were combined in a final fusion PCR. The product of this reaction was digested with SacI (within the upstream polylinker region) and EagI (position 1005 on the P/C mRNA) and then substituted into the SeV P/C backbone. The primers mentioned above were as follows: T7, 5′ TAATACGACTCACTATAGGG 3′; PEcoP, 5′ GGGCACGTCTTGCAAACAC 3′; a, 5′ CAGAATCCATTTTAAGAATGAAGGC 3′; a′, 5′ TAAAATGGATTCTGAAGTTGAGAGG 3′; b, 5′ GCGACTCCATTCCTCCTGGCGCC 3′; b′, 5′ AGGAATGGAGTCGCTCTCGGATG 3′; c, 5′ GCATCGAGCATTCCGATAACATCCGAGAG 3′; c′, 5′ CGGAATGCTCGATGCTGTCCTGTCG 3′; d, 5′ GTCCCCTCCCATGTCAGTTGGTTCACTCG 3′; d′, 5′ GACATGGGAGGGGACAGAAGC 3′; e, 5′ ATGGGCCATGCCTGGTCCTTGGGGAG 3′; and e′, 5′ AGGCATGGCCCATAGAGCCAAAAG 3′.
(vi) Deletion mutants.
Starting from the clones in which new ATGs had been inserted around the Y start sites (see above), 116 nt from the 5′ end (including the C′, P, and P initiation codons) was deleted by introducing a BamHI site at position 116, digesting the clones with BamHI/PstI, and recloning the fragment into pGEM3.
Cellular expression.
Confluent HeLa or A549 cell monolayers seeded on 5-cm-diameter petri dishes were infected at 2 to 5 PFU/cell with a vaccinia virus recombinant expressing T7 RNA polymerase (vTF7-3 [10]). At 1 h postinfection, the infecting medium was replaced with a transfection mix composed of 0.5 ml of minimal essential medium and 10 μl of transfectASE (39) combined with the various plasmid DNAs as indicated in the figure legends. The cells were incubated at 33°C for 24 h before the monolayer was solubilized in 150 mM NaCl– 50 mM Tris-HCl (pH 7.4)–10 mM EDTA–0.6% Nonidet P-40. Nuclei were removed by pelleting at 12,000 × g for 5 min. Immunoblotting of the cytoplasmic extracts was performed as outlined previously (5) with the disodium 3-(4-methoxyspiro{1,2-dioxetane-3,2′-(5′-chloro)tricyclo[3.3.1.1]decan}-4-yl)phenyl phosphate (CSPD) chemiluminescent substrate (Boehringer). The C/C′ antiserum was raised in rabbits against a bacterial fusion protein. The P monoclonal antibody (1.180) was a gift from Claes Oervell, Stockholm, Sweden (30). Transfection of all P/C plasmid constructs was performed in triplicate, and the Western blots were quantitated by densitometry (Molecular Dynamics).
RESULTS
Evidence for a shunting mechanism.
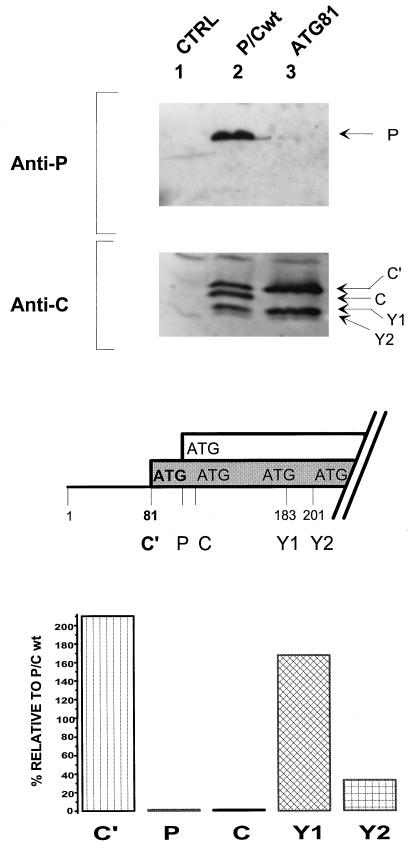
The strongest evidence that ribosome initiation from ATG183/Y1 and ATG201/Y2 does not occur by leaky scanning is that mutation of the 5′-proximal ACG81 start site to ATG (GCCA81TGG [ATG81/C′]) eliminated initiation from the second and third ATG104/P and ATG114/C start codons but had no effect on or increased initiation from ATG183/Y1 and ATG201/Y2. This result was obtained originally by translation in a wheat germ extract and then in cells infected with vaccinia virus recombinants carrying the SeV P/C gene (6). Since we currently use the vaccinia virus-T7 RNA polymerase system (10) for transient cellular expression, it was important to confirm this observation with this system. HeLa cells infected with a vaccinia virus expressing T7 RNA polymerase were transfected with plasmids expressing either P/Cwt or the mutant ATG81/C′ genes. The accumulation of the C, P, C′, Y1, and Y2 proteins was measured by immunoblotting, and the level of each protein expressed by the ATG81/C′ mRNA relative to that expressed by the wild-type (wt) mRNA is shown in Fig. 2. The results are similar to those described previously. There was no evidence of ribosomal scanning to reach ATG104/P and ATG114/C, as indicated by the absence of the P and C proteins. Nevertheless, total Y protein expression (Y1 plus Y2) remained unchanged, with an increase in the levels of Y1 and a decrease in the levels of Y2 (see also Fig. 6).
FIG. 2.
Mutation of ACG81 to ATG81 eliminates leaky scanning but not Y expression. The first initiation codon, ACG81/C′, was changed to ATG, thereby changing the C′ start site context from weak to strong (middle panel). The ATG81/C′ (ATG81) plasmid construct and the parental P/Cwt were transfected into HeLa cells which had been infected with a vaccinia virus recombinant (vTF7-3) expressing T7 RNA polymerase. As a control, cells were also mock transfected (CTRL). At 24 h posttransfection, cytoplasmic extracts were prepared by scraping the cells into lysis buffer. Extracts were resolved on both SDS–10% polyacrylamide gels (for the P protein) and SDS–15% polyacrylamide gels (for the C proteins). Protein expression was then detected by immunoblotting with an anti-P monoclonal antibody and a rabbit anti-C polyclonal antiserum (upper panel), using the CSPD light detection system (Boehringer). The blots were quantitated by densitometry, and expression of the C′, C, P, Y1, and Y2 proteins in the ATG81 construct was plotted as a percentage relative to expression of P/Cwt (lower panel).
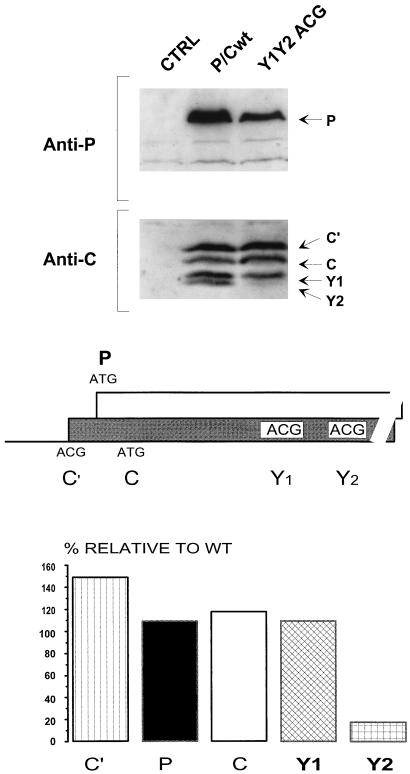
FIG. 6.
The Y proteins can initiate from a non-ATG codon. Both the Y1 and Y2 ATG start codons were changed to ACG (middle panel). This plasmid construct and the parental P/Cwt were transfected into HeLa cells infected with a vaccinia virus recombinant (vTF7-3) expressing T7 RNA polymerase. As a control, cells were also mock transfected. At 24 h posttransfection, cytoplasmic extracts were prepared by scraping the cells into lysis buffer. Expression of the P and C proteins was determined by immunoblotting with an anti-P monoclonal antibody and a polyclonal anti-C antiserum as outlined in the legend to Fig. 2 (upper panel). The blots were quantitated by densitometry, and the level of each protein expressed in the mutant construct was plotted as a percentage relative to that expressed in the parental P/Cwt (lower panel).
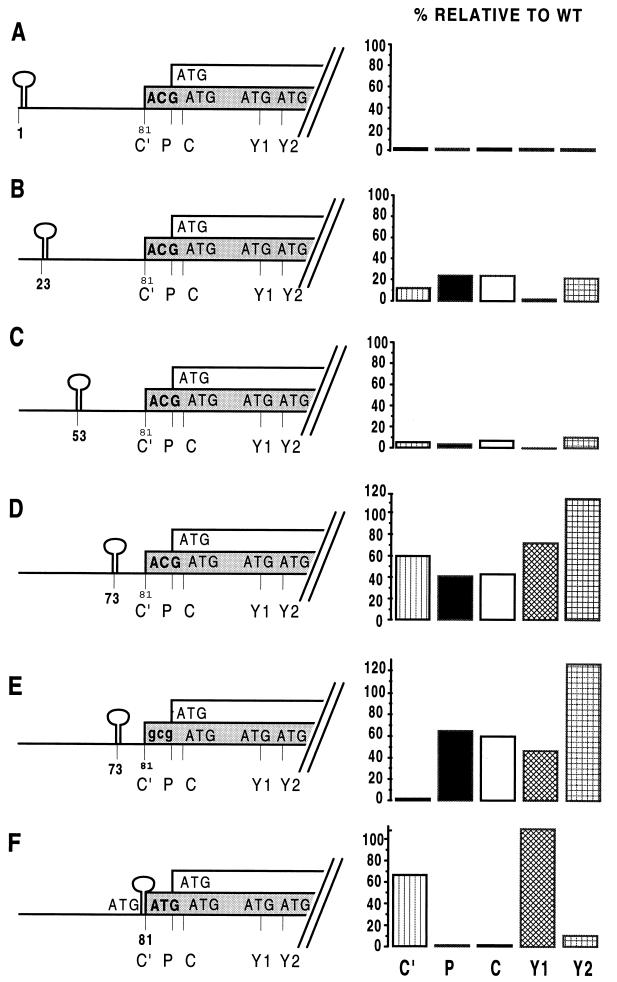
Y protein synthesis is cap dependent, yet it is at least in part scanning independent. This situation is similar to that described for the SeV X protein (5) and initiation on the CaMV 35S RNA (13). In the latter case, a shunt mechanism was shown to operate, in part by experiments in which highly stable stem-loop structures were positioned within the 5′ UTR. Structures with predicted free energies (ΔG) of >−40 kcal/mol have been shown to act as barriers to migrating initiation complexes and to block 5′ scanning (4, 24, 25). We therefore introduced such structures (ΔG > −50 kcal/mol; see Materials and Methods) at positions 1, 23, 53, and 73 within the wt background and at position 81 within the ATG81/C′ background and examined their effect on the relative expression of the products initiated at the five sequential start sites (Fig. 3). Insertion of the stem-loop at the very 5′ end of the mRNA (stem-loop1) blocked all protein expression from the P/C mRNA (Fig. 3A). Since the presence of strong secondary structure near the 5′ end of the mRNA is known to prevent the binding of the scanning complex, this result is consistent with the notion that all initiation (including that at the Y protein ATGs) requires the cap group. When the structural barrier was inserted at either nt 23 or 53, it strongly inhibited initiation from all five start sites (5- to 10-fold for stem-loop23 and 10- to 20-fold for stem-loop53) (Fig. 3B and C), indicating that the scanning complex has to traverse at least the first 53 nt of the UTR before being translocated downstream, if a shunt is operating. When inserted at position 73 (stem-loop73), the barrier appeared to become leaky; i.e., significant levels of expression (40 to 100%, relative to the wt) were observed from all five start sites (Fig. 3D). The scanning complexes were apparently able to bypass the structural barrier in stem-loop73 and then resume scanning at or before ACG81/C′, since mutation of ACG81 to GCG81 (which is not used as an initiation codon) within this construct (stem-loop73/GCG81) eliminated expression of the C′ protein and slightly (but reproducibly) increased that of the P and C proteins (Fig. 3E). The combined expression of the Y proteins (Y1 plus Y2) from both stem-loop73 constructs approached wt levels, with a preferential expression of Y2. Most remarkably, the pattern of expression from the stem-loop81/ATG81 mRNA (Fig. 3F) was rather similar to that of the ATG81 mRNA without the added structural barrier (Fig. 2), in that (i) C′ expression continued (at 67% of the wt level), whereas that of P and C were eliminated, and (ii) Y protein expression also continued and was limited almost entirely to that of Y1. The structural barrier in stem-loop81/ATG81 was introduced as an NcoI cassette into the same site (CCA81TGG) generated when ACG81 was changed to ATG. This insertion leaves ATG codons in good contexts on either side of the stem-loop, both in the C protein ORF. However, only the downstream ATG appeared to be used, because a longer form of the C′ protein was not detected. This could be because the stem-loop structure blocks initiation at the upstream (but not the downstream) ATG or because the shunt effectively causes the upstream site to be bypassed. In any event, these results suggest that the scanning complex can bypass a structural barrier and initiate at ACG81/C′ or ATG81/C′ (at a high frequency) even when this initiation site is found just downstream of the barrier. Since ribosomes on this mRNA also initiate at ATG183/Y1 with high efficiency, they apparently cannot initiate at ATG104/P or ATG114/C because these latter sites are bypassed via the shunt. The results in Fig. 3 also highlight the curious variability in selection of the Y1 and Y2 start codons, an observation noted previously (6).
FIG. 3.
Effect of stable stem-loop structures introduced into the 5′ UTR. Palindromic sequences predicted to form thermodynamically stable stem-loop structures (ΔG > −50 kcal/mol) were inserted into the 5′ UTR of the P/C mRNA at the positions indicated on the left. In constructions A to D the stem-loop was introduced into the P/Cwt background. In construction E, the C′ initiation codon was changed to GCG, and in construction F, the stem-loop was inserted at the NcoI site generated by changing the C′ ACG to ATG (ATG81/C′). This in turn left ATG codons in the C ORF flanking the inserted stem-loop. These clones were expressed in HeLa cells, and C′, P, C, Y1, and Y2 protein expression was determined by immunoblotting (see the legend to Fig. 2). The blots were quantitated by densitometry, and protein expression was plotted as a percentage relative to the levels detected in cells transfected with P/Cwt.
In summary, scanning complexes can efficiently bypass structural barriers placed at either position 73 or 81 and initiate downstream at a high frequency if they are allowed to traverse the first 53 nt of the 5′ UTR in an unimpeded fashion.
The shunt donor site.
Scanning complexes which bypass the highly structured region of shunting mRNAs are thought to disengage from and to reengage the mRNA at specific sites, the shunt donor and acceptor sites. The above results are consistent with a donor site between positions 53 and 73. To more precisely locate this site, deletions were made within the 5′ UTR in the ATG81/C′ background (Δ1-23, Δ1-53, Δ1-73, Δ23-53, Δ23-73, and Δ53-73). We found, however, that none of these deletions (and in particular Δ53-73) prevented expression of the Y proteins (data not shown). This unexpected result led us to examine mRNAs with more radical modifications in the 5′ UTR. We replaced the entire 5′ UTR of the SeV P/C (ATG81) mRNA with that from two cellular mRNAs of similar lengths (the human U1 snRNA-associated A protein and RFX-AP transcription factor [9, 42]), whose translational initiation is assumed to proceed normally via a cap-dependent continuous-scanning mechanism. Fusion with the 68-nt-long 5′ UTR from RFX-A mRNA left the C′ protein ATG in a highly favorable context (. .ACCATGG. .), whereas fusion with the 86-nt-long 5′ UTR of U1-A mRNA left the C′ start codon in a poorer context (. .TCCATGG. .). When both of these chimeric mRNAs were expressed by plasmid transfection, the C′ protein remained at levels equivalent to those of the parental P/C (ATG81/C′) and was apparently insensitive to the context (Fig. 4). There was no evidence of any initiation of the P and C proteins in the RFX-ATG81 construct (Fig. 4, lane 4), whereas traces of C protein (but not P protein) were expressed from the U1-ATG81 construct, suggesting that the ATG81 in the poorer context is a little leaky. Expression of the Y proteins in both chimeric mRNAs was normal. We were thus unable to identify a specific sequence within the 5′ UTR which acts as a shunt donor site in the SeV P/C mRNA.
FIG. 4.
Replacement of the P/C mRNA 5′ UTR does not effect the shunt. Starting from the ATG81/C′ plasmid, the 80-nt 5′ UTR of the P/C mRNA was replaced with the 5′ UTR of the cellular RFX (68 nt) and U1 (86 nt) mRNAs (lower panel). These clones (referred to as RFX-ATG81 and U1-ATG81, respectively), P/Cwt, and ATG81/C′ (ATG81) were transfected into HeLa cells infected with a vaccinia virus recombinant expressing T7 RNA polymerase (vTF7-3). As a control, cells were also mock transfected (CTRL). At 24 h posttransfection, cytoplasmic extracts were prepared by scraping the cells into lysis buffer. Expression of the P and C proteins was determined by immunoblotting with an anti-P monoclonal antibody and a polyclonal anti-C antiserum as outlined in the legend to Fig. 2 (upper panel).
The acceptor site.
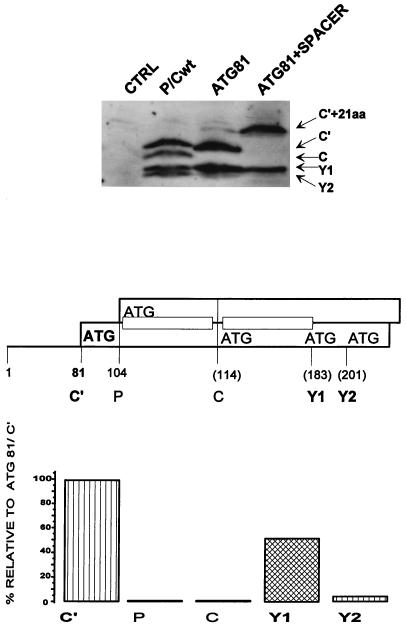
The shunt model posits that the scanning complex, having detached from the upstream region of the 5′ UTR, is translocated to a specific sequence or structure downstream which acts as a shunt acceptor site. To help define the latter element, we duplicated the sequence between ATG114/C and ATG183/Y1 (excluding the ATGs) and inserted this 66-nt sequence as a spacer between the P protein and C protein start sites (via a BamHI site introduced at position 106, …A104TGGATCCAGA114TG…). This was carried out in the ATG81/C′ background, as this 5′-proximal start codon is itself a strong barrier to scanning complexes and thus highlights the shunt. Due to the insertion (Fig. 5), (i) the Y1 and Y2 protein ATGs are displaced downstream by 66 nt, whereas the C protein ATG is now positioned at approximately the same distance from the 5′ end of the mRNA (180 nt) as the Y1 ATG in the wt background (183 nt); (ii) the new C protein ATG114+66/C is now flanked by the same upstream (but not the same downstream) sequences as Y1; and (iii) the length of the C′ protein has been increased by 22 aa.
FIG. 5.
Displacement of the Y start sites downstream. The C, Y1, and Y2 initiation codons were displaced 63 nt downstream by the introduction of a spacer sequence between ATG104/P and ATG114/C within the background ATG81/C′ (middle panel) (see Materials and Methods). This spacer duplicated the sequences between the C and Y1 ATG codons (indicated by the small rectangles in the middle panel). The numbers in parentheses refer to the positions in the P/Cwt background. This clone (referred to as ATG81+SPACER), P/Cwt, and ATG81/C′ (ATG81) were transfected into HeLa cells infected with a vaccinia virus recombinant expressing T7 RNA polymerase (vTF7-3). Cytoplasmic extracts were resolved on an SDS–15% polyacrylamide gel, and C protein expression was determined by immunoblotting with a polyclonal anti-C antiserum by using the CSPD detection system (upper panel). The blot was quantitated by densitometry, and the level of each protein expressed in the ATG81+SPACER construct was plotted as a percentage relative to that expressed in ATG81/C′ (lower panel).
We found that cells transfected with this plasmid continued to express the Y proteins (mostly Y1) at ca. 50% of the level observed in the ATG81 construct (Fig. 5, lanes ATG81 and ATG81+SPACER) and at levels equivalent to those observed in P/Cwt (Fig. 5, lane P/Cwt). Expression from the 5′-proximal ATG81/C′ was normal, except for the decreased mobility of C′ on sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis due to the insertion (Fig. 5, lane ATG81+SPACER). There was also no expression from ATG104/P (not shown) and the displaced ATG114+66/C initiation site (Fig. 5, lane ATG81+SPACER). This result provides evidence that a specific sequence or structure at or near the Y protein ATGs appears to operate as a shunt acceptor site, as it can act even when displaced downstream by 66 nt. Furthermore, the sequence upstream of the Y protein ATGs, which are now also adjacent to the displaced C protein ATG, is insufficient to allow this initiation site to act as a shunt acceptor. This is consistent with our inability to inhibit Y expression by deleting sequences between ATG114/C and ATG183/Y1 (data not shown).
Scanning complexes generally initiate on non-ATG codons only if they are the 5′ proximal start site and are in an otherwise favorable context for initiation (2). For example, inserting an ATG (in either a good or bad context) in the P ORF upstream of ACG81/C′ eliminates all expression of C′ (6). A noticeable exception to this rule was reported for the IRES-directed initiation on HCV genome RNA (37). Although HCV initiation normally starts on an ATG (the third or fifth ATG from the 5′ end, depending on the HCV strain), translation was only slightly compromised when this site was changed to either ATT or CTG. Shortly afterwards, an ATT initiation codon downstream of a number of short ORFs was reported for the badnavirus RTBV, a virus closely related to CaMV (14). Similar to the case for CaMV, scanning complexes were found to reach this initiation codon (>600 nt from the 5′ cap) via a shunt, and the ATT was barely recognized as a start site when reached by a scanning complex. Both groups proposed that efficient translation from these non-ATG codons was favored because both the IRES and the shunt acceptor site positioned the initiation complex directly at these start codons, using sequence elements both upstream and downstream of the initiation codon to form these sites. We therefore changed both ATG183/Y1 and ATG201/Y2 to ACG within the P/Cwt background (P/C-Y1/Y2 ACG), to examine whether non-ATG start codons could similarly function at these downstream sites. When the Y1/Y2 ACG mutant was expressed in vivo, we observed nearly wt expression of all of the P/C translation products. The total Y protein expression (Y1 plus Y2) remained unchanged, but Y1 was increased whereas Y2 was reduced (Fig. 6). This result is all the more impressive considering that the contexts of the Y1 and Y2 start sites are very poor, with neither a purine at position −3, a G at position +4, nor an A or a C at position +5 (. .CGGA183CGTTA. . and . .TCGA201CGCTG. .) (2, 16), whereas in HCV and RTBV the non-ATG initiation codons are in relatively good contexts. The fact that ATG183/Y1 and ATG201/Y2 can be mutated to ACG without significant loss of activity suggests that these initiation codons do not constitute a critical sequence element of the acceptor site. This result is consistent with the notion that the shunt delivers the scanning complex directly to the Y protein start codons by using sequence elements at or near ATG183/Y1 and ATG201/Y2, permitting initiation at a high efficiency independent of the nature and context of these start sites.
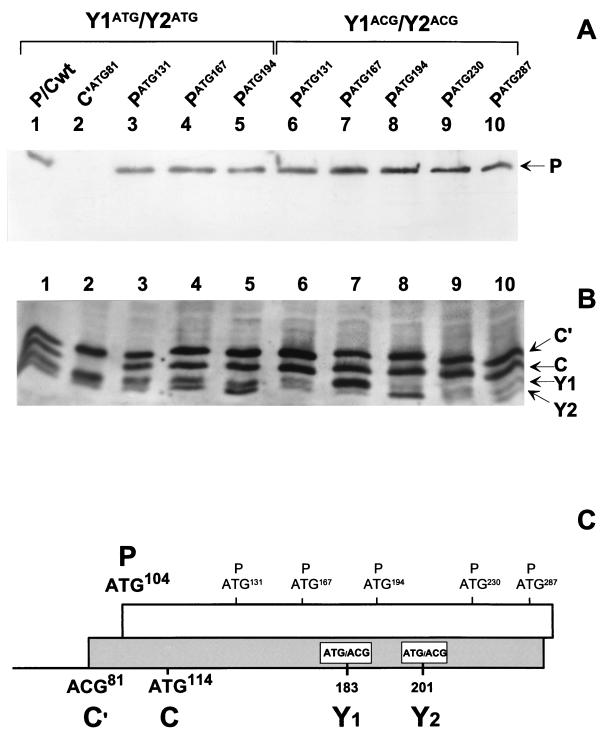
Although the nature of the start codon does not have a major effect on the overall efficiency of Y protein initiation, the context can nevertheless influence the selection of ATG183/Y1 and ATG201/Y2. When the P/Cwt mRNA is translated in vitro, Y2 rather than Y1 is the major protein product (6). However, if the context of the Y1 initiation site is improved by changing the C at position −3 to an A, Y1 becomes the major translation product (data not shown). The shunted initiation complex therefore appears to be able to recognize context elements. We tried to take advantage of this context effect to map the limits of the shunt acceptor site. ATGs in relatively good contexts (i.e., at least with purines at position −3) were introduced into the P ORF at positions 131, 167, 194, 230, and 287 (PATG131, PATG167, PATG194, PATG230, and PATG287, respectively), i.e., upstream of, downstream of, and between the Y1 and Y2 start sites, initially within the ACG183/Y1 and ACG201/Y2 background. The rationale for this approach is that if the new ATG falls within the acceptor site, it should be preferentially selected as a start codon over ACG183/Y1 and ACG201/Y2. This will in turn generate an N-terminally truncated P protein rather than a Y protein. The results from this experiment were, however, somewhat unexpected, as none of the newly introduced ATGs led to a truncated P protein (Fig. 7). In constructs PATG131, PATG230, and PATG287, Y1 and Y2 protein levels were similar to those observed in the parent construct (P/C-Y1/Y2 ACG). In construct PATG167, however, in which the new ATG codon is positioned only 16 nt upstream of ACG183/Y1, Y1 protein expression increased ninefold compared to that observed in the parent construct, whereas Y2 expression remained unchanged (Fig. 7, lane 7). Similarly, insertion of the new ATG between the Y1 and Y2 ACGs (7 nt upstream from ACG201/Y2 and 11 nt downstream from ACG183/Y1), namely, PATG194, resulted in an increased expression of the Y2 protein (fourfold), whereas Y1 expression remained unchanged (Fig. 7, lane 8). The insertion of strong start codons within the P ORF, just upstream (but not downstream) of either the Y1 or Y2 ACG codon, thus enhances initiation from these non-ATGs under conditions in which initiation from the inserted ATGs does not occur. When these new ATG codons were inserted into the P/Cwt background (i.e., Y1/Y2 start on ATG codons), the enhancement of Y protein expression by PATG167 and PATG194 was less apparent, as higher levels of Y1 and Y2 were produced in this background (Fig. 7, lanes 4 and 5), nor was there expression of a truncated P protein. These results appear to map the acceptor site to a region that just spans the two Y protein start codons. They also suggest that within this region the Y initiation sites are privileged even when they are ACG codons flanked by good ATGs.
FIG. 7.
ATGs introduced into the P ORF around the Y start sites are silent. Single ATG start codons in good contexts were introduced at positions 131, 167, and 194 in the P/Cwt clone (Y1ATG/Y2ATG) and at positions 131, 167, 194, 230, and 287 in the construct P/C-Y1ACG/Y2ACG (C). These constructs along with P/Cwt (lanes 1) and ATG81/C′ (C′ATG81) (lanes 2) were expressed in HeLa cells as outlined in the legend to Fig. 2, and protein expression was monitored by immunoblotting with an anti-P monoclonal antibody (A) and a rabbit polyclonal anti-C serum (B).
It was, however, important to determine whether the newly introduced PATGs described above could serve as initiation sites when detected by a linear-scanning complex. Therefore, a BamHI site was introduced just after ATG114/C (…A114TGGATCC… .) in the constructs PATG131, PATG167, PATG194, PATG230, and PATG287, within the ACG183/Y1/ACG201/Y2 background. All sequences upstream of the BamHI site (including the ATG114/C, i.e., nt 1 to 115) were then deleted, and these constructs (referred to as ΔPATG131, ΔPATG167, ΔPATG194, ΔPATG230, and ΔPATG287) were then expressed in vivo. Immunoblotting revealed expression of truncated P proteins from all of these constructs, with migrations consistent with the position of the inserted ATG (Fig. 8). These ATG start sites are therefore efficiently recognized by a continuous-scanning complex.
FIG. 8.
The inserted ATGs are recognized by a linear-scanning complex. A BamHI site was introduced at position 116 in the P/C-Y1ACG/Y2ACG constructs containing single ATG start codons at positions 131, 167, 194, 230, and 287 (Fig. 7). By using this site, all of the sequences upstream of position 116 were deleted, leaving 30 nt of plasmid sequence between the end of the T7 promoter and the start of the P/C gene (lower panel). These clones were expressed in HeLa cells along with P/C-Y1ACG/Y2ACG (P/C ACG Y1/Y2) and a mock-transfected control (see Fig. 2). Expression of full-length P protein (upper panel, lane 2) and N-terminally truncated P protein initiated from the inserted ATGs (referred to as ΔP) was detected by immunoblotting with an anti-P monoclonal antibody recognizing a C-terminal epitope (upper panel).
The enhancement of Y protein expression by the positioning of a cryptic (noninitiating) ATG upstream of the ACG start codon (Fig. 7) is not altogether without precedent. The oligopyrimidine-ATG tandem element is an important cis-acting element in the translation of poliovirus genomic RNA. The ATG moiety of the oligopyrimidine-ATG tandem element is also cryptic and is positioned ca. 150 nt upstream of the ATG initiator codon (43). Likewise, in an HCV IRES-chloramphenicol acetyltransferase construct, the introduction of an out-of-frame ATG codon upstream of the initiator codon increased chloramphenicol acetyltransferase expression sixfold (38). How these elements function during initiation is unclear, but they may act to stabilize the ribosome-mRNA complex. A similar role could be envisaged for the cryptic upstream ATGs observed in this study. This stabilization effect would be observed under conditions in which the acceptor site has been weakened by conversion of the Y ATG start codons to ACG, but this stabilization would be less noticeable in the wt ATG background.
DISCUSSION
Selection of a translational start site on eukaryotic mRNAs generally involves the entry of a scanning complex at the m7GTP-5′ cap, followed by the continuous linear scanning of the RNA to locate the initiation codon. Consistent with this, the vast majority of eukaryotic mRNAs are monocistronic. There are, however, two notable exceptions, (i) cap-independent internal initiation directed by an IRES and (ii) cap-dependent but discontinuous scanning, or ribosomal shunting. Both of these mechanisms can bypass barriers which would otherwise impede initiation at a downstream start site by a continuous scanning process. These barriers can be highly stable secondary structures found within the long 5′ UTRs, as in, e.g., the IRES-directed translation of picornavirus RNAs (21) and the D. antennapedia mRNA (31) and the shunt-mediated translation of CaMV 35S RNA (13) and the adenovirus late mRNAs (46). These long 5′ UTRs often have several potential ATG start sites which have been conserved, at least in the case of the viral systems because they form elements which are important in viral replication (1, 3, 41). IRESs and shunts may also be used to maximize translation under conditions which are not optimal for continuous scanning, when the function of the eIF-4F complex is down-regulated. For example, the D. antennapedia mRNA is expressed during mitotic division early in development, mammalian Bip expression increases during heat shock, and translation of adenovirus mRNAs containing the tripartite leader is promoted during late gene expression (27, 28). These are all conditions in which eIF-4E phosphorylation is low and consequently cap-dependent translation is compromised (for a review, see reference 41). Similarly, picornavirus infection results in the cleavage of eIF-4G, another key component of the eIF-4F complex (21).
In this paper, we have provided evidence that a ribosomal shunt operates in the initiation of the SeV Y1 and Y2 proteins. These initiation sites are the fourth and fifth start codons on the P/C mRNA, and in this instance, it is the upstream ACG81/C′, ATG104/P, and ATG114/C start sites, rather than strong secondary structure, which act as barriers to initiation at ATG183/Y1 and ATG201/Y2 by a strictly linear scanning mechanism. The ribosomal shunt is thus another mechanism used by this viral mRNA to expand its repertoire of gene products, in addition to leaky scanning and mRNA editing. We have no evidence that the shunt is also used to regulate viral gene expression under particular conditions, but this, of course, remains possible (see below).
Ribosomal shunts have so far been described for viral mRNAs, i.e., those of the plant pararetroviruses CaMV and RBTV, adenovirus, and now SeV. We note that several features of the SeV and CaMV shunts are similar and are distinct from that described for adenovirus, as follows.
(i) Discontinuous scanning is thought to occur through cis-acting donor and acceptor sites within the 5′ UTR, with the acceptor site being close to or overlapping the initiation codon (13). Our attempts to locate the shunt donor site by deleting regions within the 5′ UTR failed to identify this element. Indeed, replacement of the entire P/C mRNA 5′ UTR with those from two cellular mRNAs (selected only for the lengths of their 5′ UTRs) also failed to perturb Y protein expression. This suggests that there is no defined sequence or structures within the 5′ UTR required for translocation of the scanning complex to the downstream initiation site. The donor site in CaMV also does not appear to include a specific primary nucleotide sequence but maps to the upstream base of a hairpin structural element (stem I) within the leader (8a, 13, 19). For adenovirus, in contrast, shunting was dependent on precise cis-acting elements within the structured 5′ tripartite leader (46).
(ii) The Y protein start sites could be displaced 66 nt downstream without perturbing the efficiency of the shunt, consistent with the absence of a precise donor site. Similarly, displacement of the CaMV acceptor site downstream by ca. 1,800 nt did not affect the efficiency of this shunt (12). In contrast, for the adenovirus late mRNAs, displacement of the initiating ATG downstream abolished initiation (46). Further, in all adenovirus serotypes, the distance between the end of the tripartite leader and the ATG start codon is always <35 nt, suggesting that the adenovirus shunt has a strict range limit.
(iii) Both the CaMV and SeV shunts, but not that of adenovirus, continue to operate in the presence of functional ATGs upstream. The adenovirus shunt apparently is perturbed by the presence of 80S ribosomes on the mRNA (46).
Irrespective of these differences, both shunts differ from cap-independent IRES-directed initiation in that the preinitiation complex must engage the capped end of the mRNA and scan the beginning of the 5′ UTR before being translocated downstream. Since the SeV shunt does not appear to do this to simply move the preinitiation complex to a defined donor site, this step may be required for the preinitiation complex to acquire (or modify) factors without which it cannot be productively transferred to the acceptor site. An alternative possibility to explain the apparent absence of a precise donor site is that scanning complexes are continually falling off the mRNA as they search for an initiation codon. For the majority of eukaryotic mRNAs whose translation is initiated by continuous scanning, these detached complexes would reenter the mRNA via the 5′ cap and thereby pass unnoticed. When a downstream shunt acceptor site is present, however, they can also bind directly to it and initiate at internal start codons. Such a model predicts that shunting should function in trans, and indeed trans shunting was reported for CaMV (13). Although detachment of the scanning complexes is viewed here as a stochastic process, certain sequence elements within the 5′ UTR, or trans-acting protein factors (see below), may enhance this event. Alternatively, scanning complexes may be translocated directly from the 5′ UTR to the internal acceptor site without detaching from the mRNA, which would constitute simply a shunt without a defined donor site. Once again, this process could be modulated by cis- and trans-acting factors.
The precise nature of the SeV acceptor site remains unresolved. Computer analysis reveals only weak secondary structural elements within the first 300 nt of the mRNA (6a, 17), and it is not impossible that the acceptor site is a linear cis-acting sequence that overlaps the Y initiation codons. We note that the SeV X protein is also expressed by cap-dependent internal initiation at one of two possible ATG codons ca. 1,500 nt from the 5′ end (5). Alignment of the sequences flanking the X and Y start sites reveals no apparent homology, apart from the fact that in each case there are two ATGs separated by five codons. Whether this spacing is an important element of these initiation sites remains unclear. It is also possible that the acceptor site interacts with factors which regulate the efficiency of the shunt. Specific cellular proteins are known to modulate translation of the ferritin mRNA by binding to the iron-responsive element within the 5′ UTR (26, 40), and general RNA binding proteins render translation cap dependent in vitro (44). Likewise, the adenovirus shunt is promoted by one or more late viral gene products (46), and the CaMV shunt is transactivatable by the viral ORF VI protein (11). We have no evidence that an SeV gene product can modulate the shunt. However, Y protein expression changes (relative to expression of the other products of the P/C mRNA) in different cell lines infected with SeV (8) and the ratio between the Y1 and Y2 proteins clearly varies depending on the system of expression used (6). Thus, regulation of the shunt by cellular factors remains possible. Such factors may function by binding to and stabilizing existing weak structures at or near the Y1 and Y2 start codons, thereby facilitating the binding of the preinitiation complex at the acceptor site.
The SeV shunt acceptor site also shares features found in IRES-directed initiation of HCV genome RNA. For both systems the initiator Met-tRNA anticodon interaction is less important than at normal start sites, as the substitution of non-ATG start codons has little effect. Furthermore, these internal start sites must be privileged, as the introduction of out-of-frame ATGs (in good contexts) does not interfere with this initiation. On the contrary, these silent ATGs can in some cases stimulate initiation from the authentic start sites severalfold (Fig. 7) (38). These introduced ATGs, on the other hand, become functional when either the IRES or the shunt is perturbed (in both instances by deleting upstream sequences), indicating that they can be recognized by linear-scanning complexes (38). The HCV IRES has recently been found to bind 40S subunits, forming complexes in which the start codon is placed in the ribosomal P site (36a). These complexes are stabilized by multiple IRES-40S subunit interactions, possibly including the 18S rRNA, such that the canonical initiation factors (eIF-3, eIF-4A, eIF-4B, and eIF-4F) are not required. Our results suggest that the SeV acceptor site can also position the shunted initiation complex directly at the start codon. However, the mechanism by which this occurs remains to be elucidated.
ACKNOWLEDGMENTS
We acknowledge Jean-Baptist Marq for excellent technical assistance and Patrick Linder for careful reading of the manuscript.
This work was supported by the Swiss National Science Foundation.
REFERENCES
- 1.Andino R, Rieckhof G E, Achacosa P L, Baltimore D. Poliovirus RNA synthesis utilises an RNP complex formed around the 5′-end RNA. EMBO J. 1993;12:3587–3598. doi: 10.1002/j.1460-2075.1993.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Böck R, Kolakofsky D. Positions +5 and +6 can be major determinants of the efficiency of non-AUG initiation codons for protein synthesis. EMBO J. 1994;13:3608–3617. doi: 10.1002/j.1460-2075.1994.tb06668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonneville J-M, Hohn T, Pfeiffer P. Reverse transcription in the plant virus, cauliflower mosaic virus. In: Domingo E, Holland J J, Ahlquist P, editors. RNA Genetics. Boca Raton, Fla: CRC Press; 1988. pp. 23–42. [Google Scholar]
- 4.Chevrier D, Vézina C, Bastille J, Linhard C, Sonenberg N, Boileau G. Higher order structures of the 5′-proximal region decrease the efficiency of the porcine pro-opiomelanocortin mRNA. J Biol Chem. 1988;263:902–910. [PubMed] [Google Scholar]
- 5.Curran J, Kolakofsky D. Scanning independent ribosomal initiation of the Sendai virus X protein. EMBO J. 1988;7:2869–2874. doi: 10.1002/j.1460-2075.1988.tb03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curran J, Kolakofsky D. Scanning independent ribosomal initiation of the Sendai virus Y proteins in-vitro and in-vivo. EMBO J. 1989;8:521–526. doi: 10.1002/j.1460-2075.1989.tb03406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Curran, J., and M. M. Pooggin. Unpublished data.
- 7.Curran J A, Boeck R, Kolakofsky D. The Sendai virus P gene expresses both an essential protein and a negative regulator of RNA synthesis by shuffling modules via mRNA editing. EMBO J. 1991;10:3079–3085. doi: 10.1002/j.1460-2075.1991.tb07860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dillon P J, Gupta K C. Expression of five proteins from Sendai virus P/C mRNA in infected cells. J Virol. 1989;63:974–977. doi: 10.1128/jvi.63.2.974-977.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Dominguez D I, Ryabova L A, Pooggin M M, Schmidt-Puchta W, Fütterer J, Hohn T. Ribosome shunting in cauliflower mosaic virus. J Biol Chem. 1998;273:3669–3678. doi: 10.1074/jbc.273.6.3669. [DOI] [PubMed] [Google Scholar]
- 9.Durand B, Sperisen P, Emery P, Barras E, Zufferey M, Mach B, Reith W. RFX-AP, a novel subunit of the RFX DNA binding complex is mutated in MHC class II deficiency. EMBO J. 1997;16:1045–1055. doi: 10.1093/emboj/16.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesises bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fütterer J, Bonneville J-M, Gordon K, deTapia M, Karisson S, Hohn T. Expression from polycistronic cauliflower mosaic virus pregenome RNA. Posttranscriptional control of gene expression. NATO ASI Ser. 1990;H49:349–357. [Google Scholar]
- 12.Fütterer J, Gordon K, Sanfaçon H, Bonneville J-M, Hohn T. Positive and negative control of translation by the leader of cauliflower mosaic virus pregenomic 35S RNA. EMBO J. 1990;9:1697–1707. doi: 10.1002/j.1460-2075.1990.tb08293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fütterer J, Kiss-Lászió Z, Hohn T. Nonlinear ribosome migration on cauliflower mosaic virus 35S RNA. Cell. 1993;73:789–802. doi: 10.1016/0092-8674(93)90257-q. [DOI] [PubMed] [Google Scholar]
- 14.Fütterer J, Potrykus I, Bao Y, Li L, Burns T M, Hull R, Hohn T. Position-dependent ATT initiation during plant pararetrovirus rice tungro bacilliform virus translation. J Virol. 1996;70:2999–3010. doi: 10.1128/jvi.70.5.2999-3010.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giorgi C, Kolakofsky D. Effect of poliovirus superinfection on Sendai virus protein synthesis. J Virol. 1984;52:298–299. doi: 10.1128/jvi.52.1.298-299.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grünert S, Jackson R J. The immediate downstream codon strongly influences the efficiency of utilisation of eukaryotic translation initiation sites. EMBO J. 1994;13:3618–3630. doi: 10.1002/j.1460-2075.1994.tb06669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta K C, Ono E, Xu X. Lack of correlation between Sendai virus P/C mRNA structure and its utilisation of two AUG start sites from alternate reading frames: implications for viral bicistronic mRNAs. Biochemistry. 1996;35:1223–1231. doi: 10.1021/bi9520646. [DOI] [PubMed] [Google Scholar]
- 18.Gupta K C, Patwardhan S. ACG, the initiator codon for a Sendai virus protein. J Biol Chem. 1988;263:8553–8556. [PubMed] [Google Scholar]
- 19.Hemmings-Mieszczak M, Steger G, Hohn T. Regulation of CaMV 35S RNA translation is mediated by a stable hairpin in the leader. RNA. 1998;4:101–111. [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson R J. A comparative view of initiation site selection mechanisms. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 31–70. [Google Scholar]
- 21.Jackson R J, Kaminski A. Internal initiation of translation in eukaryotes: the picornavirus paradigm and beyond. RNA. 1995;1:985–1000. [PMC free article] [PubMed] [Google Scholar]
- 22.Jang S K, Krausslich H G, Nicklin M J, Duke G M, Palmenberg A C, Wimmer E. A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988;62:2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- 24.Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eukaryotic mRNAs. Mol Cell Biol. 1989;9:5134–5142. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozak M. The scanning model for translation—an update. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Latorre P, Cadd T, Itoh M, Curran J, Kolakofsky D. The various Sendai virus C proteins are not functionally equivalent, and exert both positive and negative effects on viral RNA accumulation during the course of infection. J Virol. 1998;72:5984–5993. doi: 10.1128/jvi.72.7.5984-5993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leibold E A, Munro H N. Cytoplasmic protein binds in-vitro to a highly conserved sequence in the 5′ untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci USA. 1988;85:2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindquist S, Petersen R. Selective translation and degradation of heat-shock messenger RNAs in Drosophila. Enzyme. 1990;44:147–166. doi: 10.1159/000468754. [DOI] [PubMed] [Google Scholar]
- 28.Macejak D G, Sarnow P. Internal initiation of translation mediated by the 5′ leader of a cellular mRNA. Nature. 1991;353:90–94. doi: 10.1038/353090a0. [DOI] [PubMed] [Google Scholar]
- 29.Merrick W C, Hershey J W B. The pathway and mechanism of eukaryotic protein synthesis. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 31–70. [Google Scholar]
- 30.Oervell C, Grandien M. The effects of monoclonal antibodies on biological activities of structural proteins of Sendai virus. J Immunol. 1982;129:2779–2787. [PubMed] [Google Scholar]
- 31.Oh S-K, Scott M P, Sarnow P. Homeotic gene Antennapedia mRNA contains 5′-noncoding sequences that confer translational initiation by internal ribosome binding. Genes Dev. 1992;6:1643–1653. doi: 10.1101/gad.6.9.1643. [DOI] [PubMed] [Google Scholar]
- 32.Ohlmann T, Rau M, Pain V M, Morley S. The C-terminal domain of eukaryotic protein synthesis initiation factor (eIF) 4G is sufficient to support cap-independent translation in the absence of eIF4E. EMBO J. 1996;15:1371–1382. [PMC free article] [PubMed] [Google Scholar]
- 33.Patwardhan S, Gupta K C. Translation initiation potential of the 5′ proximal AUGs of the polycistronic P/C mRNA of Sendai virus: a multipurpose vector for site specific mutagenesis. J Biol Chem. 1988;263:4907–4913. [PubMed] [Google Scholar]
- 34.Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- 35.Pestova T V, Hellen C U T, Shatsky I N. Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Mol Cell Biol. 1996;16:6859–6869. doi: 10.1128/mcb.16.12.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pestova T V, Shatsky I N, Hellen C U T. Functional dissection of eukaryotic initiation factor 4F: the 4A subunit and the central domain of the 4G subunit are sufficient to mediate internal entry of 43S preinitiation complexes. Mol Cell Biol. 1996;16:6870–6878. doi: 10.1128/mcb.16.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Pestova T V, Shatsky I N, Fletcher S P, Jackson R J, Hellen C U T. A prokaryotic-like mode of cytoplasmic eukaryotic ribosome binding to the initiation codon during internal translation initiation of hepatitis C and classical swine fever virus RNAs. Genes Dev. 1998;12:67–83. doi: 10.1101/gad.12.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reynolds J E, Kaminski A, Kettinen H J, Grace K, Clarke B E, Carroll A R, Rowlands D J, Jackson R J. Unique features of internal initiation of hepatitis C virus RNA translation. EMBO J. 1995;14:6010–6020. doi: 10.1002/j.1460-2075.1995.tb00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rijnbrand R C A, Abbink T E M, Haasnoot P C J, Spaan W J M, Bredenbeek P J. The influence of AUG codons in the hepatitis C virus 5′ nontranslated region on translation and mapping of the translation initiation window. Virology. 1996;226:47–56. doi: 10.1006/viro.1996.0626. [DOI] [PubMed] [Google Scholar]
- 39.Rose J K, Buonocore I, Whitt M A. A new cationic liposome reagent mediating nearly quantitative transfection of animal cells. BioTechniques. 1991;10:520–525. [PubMed] [Google Scholar]
- 40.Rouault T A, Hentze M W, Caughman S W, Harford J B, Klausner R D. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988;242:1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- 41.Schneider R J. Adenovirus and vaccinia virus translational control. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 575–605. [Google Scholar]
- 42.Sillekens P T G, Habets W J, Beijer R P, van Venraoij W J. cDNA cloning of the human U1 snRNA-associated A protein: extensive homology between U1 and U2 snRNP-specific proteins. EMBO J. 1987;6:3841–3848. doi: 10.1002/j.1460-2075.1987.tb02721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slobodskaya O R, Gmyl A P, Maslova S V, Tolskaya E A, Viktorova E G, Agol V I. Poliovirus neurovirulence correlates with the presence of a cryptic AUG upstream of the initiator codon. Virology. 1996;221:141–150. doi: 10.1006/viro.1996.0360. [DOI] [PubMed] [Google Scholar]
- 44.Svitkin Y V, Ovchinnikov L P, Dreyfuss G, Sonenberg N. General RNA binding proteins render translation cap dependent. EMBO J. 1996;15:7147–7155. [PMC free article] [PubMed] [Google Scholar]
- 45.Vidal S, Curran J, Kolakofsky D. Editing of the Sendai virus P/C mRNA by G insertion occurs during mRNA synthesis via a virus-encoded activity. J Virol. 1990;64:239–246. doi: 10.1128/jvi.64.1.239-246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yueh A, Schneider R J. Selective translation initiation by ribosome jumping in adenovirus-infected and heat-shocked cells. Genes Dev. 1996;10:1557–1567. doi: 10.1101/gad.10.12.1557. [DOI] [PubMed] [Google Scholar]