Abstract
The authors hereby present a case report of metastasizing pleomorphic adenoma (MPA) of the parotid gland with multiple metachronous cervical lymph node metastases and sternocleidomastoid muscle infiltration. Diagnostic evaluation, surgical management, and follow-up are discussed along with a brief review of the literature.
Keywords: Metastasizing pleomorphic adenoma, Parotid gland tumor, Metastasizing salivary gland tumor
Introduction
Pleomorphic adenoma(PA) is the most common tumor of the salivary glands, with approximately 80% of cases found in the parotid gland [1]. Although PAs are generally considered benign, they can rarely undergo malignant transformation or metastasize [2]. The recent 2017 WHO classification downgraded MPA from “malignant” to “benign epithelial tumor” based on histological appearance but acknowledged its aggressive nature [3].
We present a case of multiple metachronous ipsilateral cervical lymph nodes metastasis and sternocleidomastoid muscle infiltration of the parotid gland PA with a brief review of the literature.
Case Report
A 35-year-old female presented to our Department of Maxillofacial Surgery of Azienda Ospedaliero Universitaria Senese, Siena, Italy, with multiple painless masses in the left neck and parotid gland region.
In 2011, she had surgical enucleation of a left parotid gland mass, confirmed as a pleomorphic adenoma. In 2019, she underwent a partial parotidectomy with facial nerve preservation due to recurrence, which was confirmed as a recurrent PA in the histologic examination.
Physical examination revealed round firm non-tender masses on the left parotid gland and multiple painless neck masses on the left side neck. The Magnetic Resonance(MR) showed left intraparotid and cervical multiple nodules at level II and nodules in contact with the sternocleidomastoid muscle(Fig. 1A,B). These findings were compatible with the diagnosis of recurrent PA. Thus, left total parotidectomy with lymph node dissection of level II and enucleation of the sternocleidomastoid muscle component of the mass were performed.
Fig. 1.
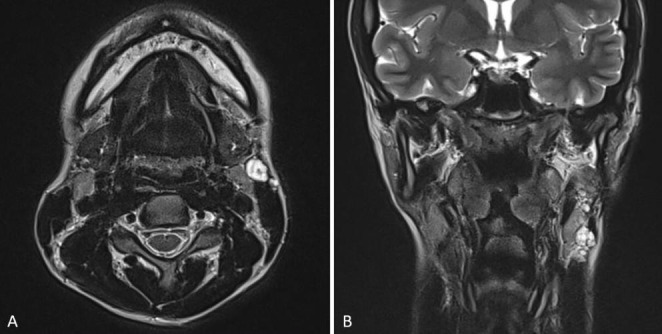
MRI scan (A) axial view: showing multinodular relapse of pleomorphic adenoma of the left parotid gland; (B) coronal view: showing nodules near the upper and lower pole of the left parotid gland and multiple nodules in touch with an anterior and superficial margin of the sternocleidomastoid muscle
Histologic examination of the resected parotid identified a recurrent pleomorphic adenoma(RPA), myxoid subtype. A neck dissection specimen(level II) comprised nine disease-free lymph nodes, two satellite nodules(RPA, myxoid subtype), with no malignant transformation, and confirmed benign mixed epithelial cells consistent with PA in the sternocleidomastoid muscle component. The patient had a good postoperative course, with no complications and facial nerve function was intact(House-Brackmann scale grade I). At the one-year follow-up, no recurrence occurred.
Discussion
MPA is a rare entity that clinically behaves as an indolent malignancy. There are no histological signs to predict MPA, though local recurrence after surgical excision has been shown to be a risk factor [4]. Several hypotheses have been formulated to explain how a histologically benign tumor can produce metastasis. As most reported cases occurred after surgical treatment of primary or recurrent salivary gland lesions, it has been hypothesized that surgical manipulation may result in the displacement of tumor cells and vascular implantation. A hematogenous spread and lymphatic metastatic route have been described [5]. These theories are further supported by the long latency observed between the primary resection of PA and the MPA [2].
Enucleation used to be a common treatment for PA. However, Nourai et al. [2] suggest that due to the increased risk of recurrence and MPA from tumor cell spillage, enucleation should not be the treatment of choice for PA. Indeed, as reported by Reiland et al. [1] total surgical excision, such as parotidectomy should be recommended. Despite these reported data, Czader et al. described cases of MPA without previous surgical treatment of a primary PA, suggesting that seeding of tumor cells at surgery may not be the only mechanism of metastasis [6].
We conducted an English-language literature search using the PUBMED database to identify cases of MPA. We searched for the following keywords: metastasizing/metastasis AND pleomorphic adenoma of the parotid gland. A recent systematic review documented 80 reported cases of MPA since 1942 [4]. We included the results of this previous review, selecting only cases of MPA from the parotid gland with lymph node metastasis, and identified 11 cases from the aforementioned review. The present study expands on the results of this previous review by identifying five additional studies that reported lymph node metastasis in MPA and describing a new case of MPA [7–11] (Table 1).
Table 1.
Overview of included studies
| Investigators | Year | Sex | Age of Primary | Primary site | Age of MPA | Site of MPA |
|---|---|---|---|---|---|---|
| Collina et al. | 1989 | M | 8 | R parotid | 11 | R neck lymph nodes |
| Ferlito et al. | 1991 | F | 42 | R Parotid | 44 | R upper neck |
| Wenig et al. | 1992 | M | 15 | Parotid* | 27 | Cervical lymph nodes |
| Commins et al. | 1995 | M | 23 | L parotid | 41 | L cervical region |
| Chen & Tu | 2000 | F | 29 | L parotid | 51 | L cervical lymph node |
| Hay et al. | 2001 | F | 36 | L parotid | 47 | L lower neck |
| Sebesan et al. | 2007 | M | 33 | L parotid | 61 | L supraclavicular lymph node |
| Rodriguez-Fernandez J | 2008 | F | 54 | L parotid | 57 | Lungs, L cervical lymph nodes |
| Larbcharoensub | 2009 | F | 27 | R parotid | 40 | R cervical lymph nodes |
| 2009 | F | 32 | L parotid | 42 | L cervical lymph nodes | |
| Qureshi et al. | 2009 | NR | NR | Parotid* | 64 | Cervical lymph nodes |
| Soteldo and Aranaga | 2017 | M | 18 | L Parotid | 36 | L cervical lymph nodes |
| Nagarajah et al. | 2017 | F | 40 | R Parotid | 55 | L cervical lymph nodes |
| Wong et al. | 2019 | F | 31 | R Parotid | 61 | R cervical lymph nodes and R infratemporal fossa |
| Wasserman et al. | 2019 | F | 35 | Parotid* | 42 | Peri-parotid limph node |
| M | 34 | Parotid* | 59 | Cervical lymph nodes | ||
| Ko et al. | 2022 | M | 48 | L Parotid | 48 | L cervical lymph nodes |
* The side of the primary site is not mentioned
Cervical lymph nodes represent one of the common sites for MPA(20.1%) [4]. Almost all reported MPA cases have at least one primary PA recurrence before metastatic foci development. Sungchul et al. [10] were the only ones to describe MPA with cervical lymph node involvement, without a history of head and neck surgery. Generally, according to McGarry and Knight, the mean latency period between the primary PA and the recurrence and distant metastasis detection was 16 years and 14.9, respectively [4, 11, 12]. In our case, the metastasis was discovered 12 years after a primary PA and 3 years after its local recurrence. Nevertheless, McGarry et al. [12] describe the only case of MPA with a metastasis infiltrating into the scapula and infraspinatus muscle. To the best of our knowledge, the present study is the first to describe a case of MPA with multiple lymph node metastasis infiltrating the sternocleidomastoid muscle. This local aggressive nature was never described before.
In our opinion, when deciding on the initial therapeutic approach for PA, the likelihood of metastasis should be taken into account, with a preference for superficial parotidectomy. Due to the indolent and asymptomatic nature of its growth, it may be prudent to have an extended follow-up period for patients who have undergone surgical removal of PA, considering the demonstrated delay in the presentation of subsequent metastases. This is especially important for patients with a history of recurrent or incompletely treated PA, as they are at high risk for metastasis.
Although both MRI and Computer Tomography(CT) can be utilized to define the margins and extent of the primary tumor, MRI is undoubtedly better at detecting the development of new metastatic lesions. Thus, MRI best performs follow-up due to its superiority in distinguishing neoplastic tissue from fibrosis in postoperative and post-radiation patterns [13].
The limited number of reported MPA cases makes it difficult to establish the best treatment. The value of radiotherapy seems to be limited in the treatment and prevention of metastatic lesions. Surgery, when possible, appears to be the first option. The total surgical excision of the primary tumor and metastasectomy with wide tissue margins represents the current preferred treatment offering a statistically significant survival advantage [2].
Since is described as a significant morbidity and mortality from distant disease [2], the prevention and adequate treatment of MPA are crucial. In conclusion, MPA is a rare histologically benign but clinically malignant and life-threatening salivary gland tumor. Awareness of these lesions for correct differential diagnosis is important for proper planning and management.
Conceptualization
Lisa Catarzi, Guido Gabriele, Beatrice Pulli, Flavia Cascino, Paolo Gennaro; Methodology: Lisa Catarzi, Beatrice Pulli, Formal analysis and investigation: Lisa Catarzi, Guido Gabriele, Beatrice Pulli; Writing - original draft preparation: Lisa Catarzi, Guido Gabriele, Beatrice Pulli; Writing - review and editing: Lisa Catarzi, Guido Gabriele, Beatrice Pulli, Flavia Cascino, Paolo Gennaro; Supervision: Lisa Catarzi, Guido Gabriele, Paolo Gennaro.
Declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Competing Interests and Funding
The authors have no competing interests to declare that are relevant to the content of this article. The authors did not receive support from any organization for the submitted work. The authors declare they have no financial interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors have equally contributed to the present study.
References
- 1.Reiland MD, Koutlas IG, Gopalakrishnan R, Pearson AG, Basi DL. Metastasizing pleomorphic adenoma presents intraorally: a case report and review of the literature. J Oral Maxillofac Surg. 2012;70(10):e531–e540. doi: 10.1016/j.joms.2012.06.185. [DOI] [PubMed] [Google Scholar]
- 2.Nouraei SA, Ferguson MS, Clarke PM, et al. Metastasizing pleomorphic salivary adenoma. Arch Otolaryngol Head Neck Surg. 2006;132(7):788–793. doi: 10.1001/archotol.132.7.788. [DOI] [PubMed] [Google Scholar]
- 3.Gnepp DR. Tumours of the salivary glands. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organisation classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 245–246. [Google Scholar]
- 4.Knight J, Ratnasingham K. Metastasising pleomorphic adenoma: systematic review. Int J Surg. 2015;19:137–145. doi: 10.1016/j.ijsu.2015.04.084. [DOI] [PubMed] [Google Scholar]
- 5.Chen Ih Tu. Pleomorphic adenoma of the parotid gland metastasizing to the cervical lymph node. Otolaryngol Head Neck Surg. 2000;122(3):455–457. doi: 10.1016/S0194-5998(00)70064-7. [DOI] [PubMed] [Google Scholar]
- 6.Czader M, Eberhart CG, Bhatti N, Cummings C, Westra WH. Metastasizing mixed tumor of the parotid: initial presentation as a solitary kidney tumor and ultimate carcinomatous transformation at the primary site. Am J Surg Pathol. 2000;24(8):1159–1164. doi: 10.1097/00000478-200008000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Nagarajah D, et al. Atypical multiple metastasis of recurrent pleomorphic adenoma. Egypt J Ear Nose Throat Allied Sci. 2017;18:281–283. doi: 10.1016/j.ejenta.2017.05.003. [DOI] [Google Scholar]
- 8.Wong DKC, Muhamad NS, Sobri SS, Amin WAM, Yusof Z. Metastatic pleomorphic adenoma in the infratemporal fossa and neck following total parotidectomy after 30 years. Med J Malaysia. 2019;74(2):85–86. [PubMed] [Google Scholar]
- 9.Wasserman JK, Dickson BC, Smith A, Swanson D, Purgina BM, Weinreb I. Metastasizing Pleomorphic Adenoma: recurrent PLAG1/HMGA2 rearrangements and identification of a Novel HMGA2-TMTC2 Fusion. Am J Surg Pathol. 2019;43(8):1145–1151. doi: 10.1097/PAS.0000000000001280. [DOI] [PubMed] [Google Scholar]
- 10.Ko S, Park KH, Lee JH, Park KN (2022) A case of initially metastasizing pleomorphic adenoma of parotid gland. Rare Tumors. ;14:20363613221130155. Published 2022 Oct 3. 10.1177/20363613221130155 [DOI] [PMC free article] [PubMed]
- 11.Soteldo J, Aranaga N. Metastasizing pleomorphic adenoma of the parotid gland. Ecancermedicalscience. 2017;11:758. doi: 10.3332/ecancer.2017.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGarry JG, Redmond M, Tuffy JB, Wilson L, Looby S. Metastatic pleomorphic adenoma to the supraspinatus muscle: a case report and review of a rare aggressive clinical entity. J Radiol Case Rep. 2015;9(10):1–8. doi: 10.3941/jrcr.v9i10.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarsitano A, Foschini MP, Farneti P, Pasquini E, Marchetti C. Metastasizing benign pleomorphic salivary adenoma: a dramatic case-report and literature review. J Craniomaxillofac Surg. 2014;42(8):1562–1565. doi: 10.1016/j.jcms.2014.01.014. [DOI] [PubMed] [Google Scholar]


