Abstract
The different epidermal growth factor (EGF)-related peptides elicit a diverse array of biological responses as the result of their ability to activate distinct subsets of ErbB receptor dimers, leading to the recruitment of different intracellular signaling networks. To specifically examine dimerization-dependent modulation of receptor signaling, we constructed NIH 3T3 cell lines expressing ErbB-1 and ErbB-2 singly and in pairwise combinations with each other ErbB family member. This model system allowed the comparison of EGF-activated ErbB-1 with ErbB-1 activated by Neu differentiation factor (NDF)-induced heterodimerization with ErbB-4. In both cases, ErbB-1 coupled to the adaptor protein Shc, but only when activated by EGF was it able to interact with Grb2. Compared to the rapid internalization of EGF-activated ErbB-1, NDF-activated ErbB-1 showed delayed internalization characteristics. Furthermore, the p85 subunit of phosphatidylinositol kinase (PI3-K) associated with EGF-activated ErbB-1 in a biphasic manner, whereas association with ErbB-1 transactivated by ErbB-4 was monophasic. The signaling properties of ErbB-2 following heterodimerization with the other ErbB receptors or homodimerization induced by point mutation or monoclonal antibody treatment were also analyzed. ErbB-2 binding to peptides containing the Src homology 2 domain of Grb2 or p85 and the phosphotyrosine binding domain of Shc varied according to the mode of receptor activation. Finally, tryptic phosphopeptide mapping of both ErbB-1 and ErbB-2 revealed that receptor phosphorylation is dependent on the dimerization partner. Differential receptor phosphorylation may, therefore, be the basis for the differences in the signaling properties observed.
The ErbB family of receptor tyrosine kinases has four members: epidermal growth factor (EGF) receptor (ErbB-1), ErbB-2, ErbB-3, and ErbB-4. The ErbB receptors are expressed in epithelial, mesenchymal, and neuronal tissue and play fundamental roles during development. Two of the family members, ErbB-1 and ErbB-2, are involved in the development of many types of human cancer (reviewed in references 29 and 44).
A large family of growth factors, the EGF-related peptides, serve as ligands for ErbB receptors (42, 44). The ligands fall into three groups: EGF, amphiregulin (AR), and transforming growth factor α, which bind ErbB-1; betacellulin, epiregulin, and heparin binding EGF-like growth factor, which bind both ErbB-1 and ErbB-4; and Neu differentiation factors (NDFs) or heregulins, which are ligands for ErbB-3 and ErbB-4.
Ligand binding promotes ErbB receptor homo- and heterodimerization. Although no direct ligand for ErbB-2 has been identified, it appears to be the preferred heterodimerization partner of all ErbB proteins (21, 30, 48). Despite the lack of an ErbB-2-specific ligand, homodimerization of this receptor can be achieved by mutating a single amino acid residue in the transmembrane domain (1), leading to constitutive ErbB-2 dimerization and activation. Alternatively, antibody binding to the extracellular domain will also promote ErbB-2 homodimerization (24). The biological responses triggered by ErbB-2 activation vary dramatically, ranging from transformation (1) to monoclonal antibody (MAb)-induced growth inhibition (25, 27) to ligand-induced growth stimulation (3, 22) or apoptosis (12). These diverse responses suggest that there are activation-specific differences in the signaling capacity of ErbB-2. ErbB receptor signaling can be attenuated by the action of phosphatases (51), serine/threonine phosphorylation (14, 15), and ligand-induced internalization of receptors (46). In this respect, heterodimer formation of ErbB receptors may also be of importance since coexpression of ErbB-2 with other family members has been shown to potentiate and prolong signaling (4, 22, 30).
Upon ligand-induced homo- and heterodimerization of ErbB receptors, the receptors autophosphorylate on specific tyrosine residues in their cytoplasmic tails. These phosphorylated tyrosines provide docking sites for Src homology 2 (SH2) and phosphotyrosine binding (PTB) domain-containing proteins, which include Shc, Grb2, and the p85 subunit of phosphatidylinositol kinase (11, 31). This leads to activation of signaling pathways such as the mitogen-activated protein kinase pathway (43) and the S6 kinase cascade (40). Although the four ErbB receptors show a great deal of overlap in the signaling molecules to which they couple, there are some examples of preferential binding. The Cbl protein appears to couple exclusively to ErbB-1 (32), whereas the Csk-homologous kinase binds only to ErbB-2 (53). The ability to heterodimerize expands the signaling diversity of ErbB receptors. Interleukin-3 (IL-3)-dependent Ba/F3 cells engineered to coexpress ErbB-1 and ErbB-4 demonstrated IL-3-independent proliferation in the presence of NDF or EGF. However, neither ligand promoted IL-3-independent proliferation of cells that expressed ErbB-4 or ErbB-1 alone (41). Furthermore, ErbB receptors individually expressed in NIH 3T3 cells did not cause transformation, while various combinations of receptors cooperated to do so (10, 52).
We have previously observed differences in the signaling properties of ErbB-1 directly activated by EGF compared to ErbB-1 activated by NDF-induced heterodimerization with ErbB-3 and ErbB-4. The EGF-activated receptor coupled with Shc and with Cbl, while NDF-activated ErbB-1 associated only with Shc (21). We speculated that these differences may result from differential phosphorylation of the receptor in a homodimer versus a heterodimer. All four ErbB receptors are widely expressed in most human tissues, making analysis of dimer-dependent signaling specificities difficult. To test our hypothesis, we coexpressed single and pairwise combinations of ErbB receptors in a defined cellular context by constructing nine different NIH 3T3-derived cell lines. Due to the roles of ErbB-1 and ErbB-2 in many human cancers, we focused our attention on these two receptors, and our results allow the following conclusions. (i) Coupling of a given receptor to specific intracellular signaling proteins is modulated by the dimerization partner and may indeed originate from differential receptor phosphorylation. (ii) Internalization of an ErbB receptor is influenced by the ligand and its dimerization partner. (iii) The subsets of signaling molecules that couple to an activated receptor undergo time-dependent changes, suggesting that ErbB receptor phosphorylation is not static.
MATERIALS AND METHODS
Antibodies, growth factors, and GST fusion proteins.
Antibodies used were ErbB-1-specific MAbs EGFR1 and 528 and affinity-purified rabbit polyclonal antibody 1005 (Santa Cruz Biotechnology); ErbB-1-specific antiserum 15E (23), ErbB-2-specific MAb FRP5 (24) and polyclonal rabbit antiserum 21N (28); ErbB-3- and ErbB-4-specific affinity-purified rabbit polyclonal antibodies C17 and C18 (Santa Cruz Biotechnology); ErbB-4-specific MAb 111 (8); Grb2-specific rabbit antiserum (Upstate Biotechnology Inc. [UBI]); Shc-specific rabbit immunoglobulin G (UBI); p85-specific antiserum (UBI); and phosphotyrosine-specific MAb (17). Growth factors used were recombinant human EGF (Sigma), recombinant human AR (R&D Systems), and recombinant human EGF-β1-like domain of NDF, which was provided by Amgen (Thousands Oaks, Calif.). Glutathione S-transferase (GST) fusion proteins of the Shc PTB domain and the N-terminal SH2 domain of p85 were a gift from Steve Shoelson (Harvard Medical School, Boston, Mass.). GST-Grb2 SH2 was expressed and purified as described previously (7).
Cell lines and cell culture.
Human ErbB-1 and ErbB-2 were introduced into NIH 3T3 fibroblasts by transfection, giving rise to the clones NE1 and NE2 (5, 35). NIH 3T3 sublines expressing pairwise ErbB receptor combinations were generated by subsequent infection with (i) pBabe-based retroviruses (36) directing the expression of human ErbB-2, ErbB-3, and ErbB-4 in the case of NE1 cells and (ii) pBabe-based retroviruses expressing ErbB-1, ErbB-3, and ErbB-4 in the case of NE2 cells. Cells expressing ErbB-1 were selected with hygromycin (100 μg/ml), cells expressing ErbB-2 were selected with G418 (1 mg/ml), and those expressing ErbB-3 and ErbB-4 were selected with puromycin (2 μg/ml). NIH3.7 cells are an NIH 3T3 clone expressing an oncogenic variant of human ErbB-2 (25). All NIH 3T3 derivative cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum and the appropriate selective antibiotic. Prior to growth factor stimulation, cells were starved for 18 h in serum-free medium, DMEM containing fetuin (1 mg/ml; Sigma) and transferrin (10 μg/ml; Sigma).
Immunoprecipitation, binding assays, and Western blotting.
For analysis of ErbB receptors and associated proteins, cells were solubilized in Triton extraction buffer (50 mM Tris [pH 7.5], 5 mM EGTA, 150 mM NaCl, 1% Triton X-100, 2 mM sodium orthovanadate, 50 mM sodium fluoride, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 1 mM phenylmethylsulfonyl fluoride) for 10 min on ice. The lysates were clarified by centrifugation at 16,000 × g for 15 min. For immunoprecipitations, equal amounts of protein were incubated with specific antibodies for 2 h on ice. Immune complexes were collected with protein A- or protein G-Sepharose (Sigma) and washed three times with extraction buffer and once with TNE buffer (50 mM Tris [pH 7.5], 140 mM NaCl, 5 mM EDTA).
Binding assays were performed by incubating equal amounts of Triton-extracted protein with 5 μg of the specific GST fusion protein in the presence of 0.1% sodium dodecyl sulfate (SDS) for 90 min on ice. Bound proteins were precipitated by the addition of glutathione-Sepharose (Pharmacia) and washed as described above. Precipitated proteins of immunoprecipitations or binding assays were released by boiling in sample buffer and were subjected to SDS polyacrylamide gel electrophoresis (PAGE). The proteins were blotted onto polyvinylidene difluoride membranes. After blocking with 20% horse serum (Gibco BRL) in TTBS (50 mM Tris [pH 7.5], 150 mM NaCl, 0.05% Tween 20), filters were probed with specific antibodies. Proteins were visualized with peroxidase-coupled secondary antibody, using the Amersham ECL (enhanced chemiluminescence) detection system. Stripping of membranes was performed in SDS buffer (62.5 mM Tris [pH 6.8], 2% SDS, 100 mM β-mercaptoethanol) for 30 min at 45°C; membranes were then washed in TTBS and reprobed with the indicated antibodies.
Isolation of internalized ErbB-1.
After growth factor treatment, cells were placed on ice and washed twice with phosphate-buffered saline (PBS). Biotinylation of surface proteins was performed by incubating cells with 3 mg of NHS-SS-Biotin (Pierce) per ml for 30 min on ice. Cells were washed three times (once with PBS, once with PBS containing 50 mM glycine, and once again with PBS) and then lysed in Triton extraction buffer. Immunoprecipitation of ErbB-1 with polyclonal antibody 1005 was performed as described above. After two washes with extraction buffer and two washes with TNE, ErbB-1 immunoprecipitates were released from the beads by boiling for 10 min in TNE containing 0.5% SDS. The supernatant was diluted twofold with extraction buffer, and biotinylated ErbB-1 was removed by using immobilized streptavidin (Pierce). The supernatant which contained the nonbiotinylated intracellular fraction of ErbB-1 was recovered, subjected to SDS-PAGE, and analyzed by Western blotting.
Phosphopeptide mapping.
Cells were deprived of phosphate and serum for 12 h prior to labeling with [32P]orthophosphate (Amersham) for 4 h. After stimulation for 10 min with growth factors, either ErbB-1 was immunoprecipitated with a mixture of MAbs EGFR1 and 528 or ErbB-2 was immunoprecipitated with 21N antiserum. The proteins were resolved by SDS-PAGE; phosphorylated ErbB-1 or ErbB-2 was excised from the gel and washed four times for 1 h with 50% acetonitrile. The gel was air dried and then rehydrated in 100 mM NH4HCO3 containing 5 μg of trypsin (Worthington). After 4 h, a second aliquot of trypsin was added and the digestion continued overnight at 37°C. The peptides were eluted several times by rocking in 50% acetonitrile. Extracted peptides were dried in a SpeedVac concentrator and resuspended in 10 μl of pH 1.9 buffer (2.5% formic acid, 7.8% acetic acid). The sample was spotted onto cellulose thin-layer chromatography (TLC) plates (Merck) and placed in a chromatography tank containing phosphochromatography buffer (37.5% n-butanol, 25% pyridine, 7.5% acetic acid) for 8 to 12 h. The plates were dried and then subjected to electrophoresis in the second dimension in pH 1.9 buffer at 1,000 V for 50 min. Phosphopeptides were detected with a PhosphorImager (Molecular Dynamics).
RESULTS
Expression of ErbB receptors in NIH 3T3 cells.
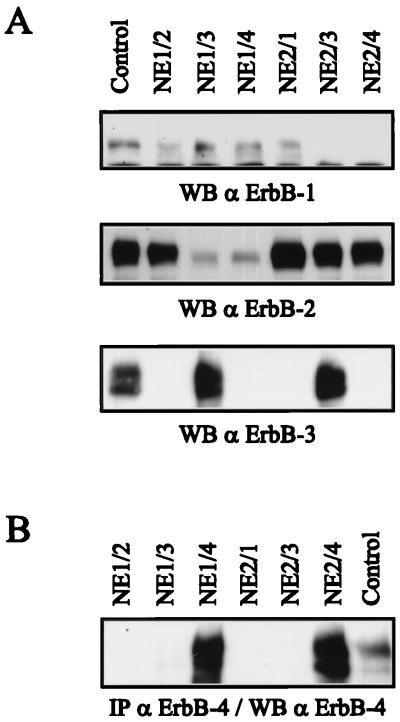
To examine dimerization-dependent differences in ErbB receptor signaling, we generated cell lines coexpressing either ErbB-1 or ErbB-2 with an additional ErbB family member. For these studies, we used NIH 3T3 cells that lack detectable amounts of ErbB-1 and NDF receptors and have a very low level of endogenous ErbB-2. The NE1 clone expresses ErbB-1; the NE2 clone expresses ErbB-2. NE1-derived cell lines coexpressing ErbB-2, ErbB-3, and ErbB-4 were designated NE1/2, NE1/3, and NE1/4, respectively. Likewise, NE2-derived cell lines coexpressing ErbB-1, ErbB-3, and ErbB-4 were designated NE2/1, NE2/3, and NE2/4, respectively. NIH3.7 cells are a clone of NIH 3T3 cells expressing an oncogenic form of ErbB-2 that carries a single amino acid substitution in its transmembrane domain (25). Expression of ErbB receptors in these NIH 3T3 derivatives was analyzed by Western blotting using whole-cell extracts for detection of ErbB-1, ErbB-2, and ErbB-3 (Fig. 1A). In the case of ErbB-4, immunoprecipitation was followed by a Western analysis (Fig. 1B). Cell lines known to express a specific receptor were used as positive controls.
FIG. 1.
Expression of ErbB receptors in NIH sublines. (A) Aliquots of 70 μg of protein extract of the different NIH sublines were subjected to SDS-PAGE (7.5% gel) and analyzed by Western blotting (WB) for the presence of ErbB-1, ErbB-2, and ErbB-3, using 15E antiserum, 21N antiserum, and C17 affinity-purified antibody, respectively. (B) ErbB-4 was immunoprecipitated (IP) with MAb 111 from 1 mg of protein extract. Immune complexes were subjected to SDS-PAGE (7.5% gel) and analyzed by Western blotting using affinity-purified antibody C18. Whole-cell extracts (A) or immunoprecipitated protein (B) from cell lines known to express a specific ErbB receptor were loaded as a positive control. The control cell lines are MCF10A for ErbB-1 and T47D for ErbB-2, ErbB-3, and ErbB-4.
ErbB-1 and ErbB-2 receptor activation in the NIH 3T3 cell lines.
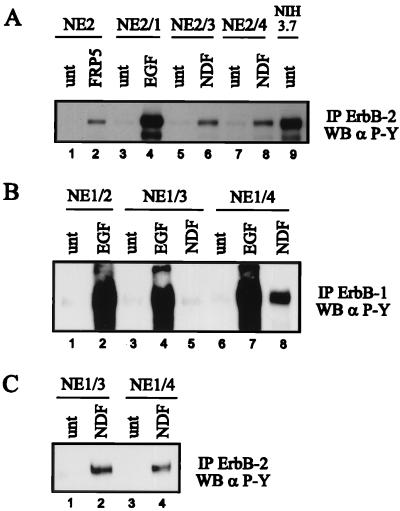
To analyze ligand-induced receptor dimerization and activation, cells were stimulated with EGF or NDF or by MAb-induced dimerization, and the phosphotyrosine content of ErbB receptors was determined following immunoprecipitation with specific antibodies (Fig. 2). In the NE2 derivatives, ErbB-2 was activated by heterodimerization with EGF-activated ErbB-1 (Fig. 2A, lane 4) and with NDF-activated ErbB-3 (Fig. 2A, lane 6) and ErbB-4 (Fig. 2A, lane 8). This is in agreement with previous observations that ErbB-2 readily heterodimerizes with all other ErbB receptors. Treatment of NE2 cells with MAb FRP5, which binds the extracellular domain of the receptor, activated ErbB-2 via antibody-induced homodimer formation (Fig. 2A, lane 2) (24). The kinase activity of the mutated, oncogenic ErbB-2 expressed in NIH3.7 cells was ligand independent due to constitutive dimerization of the receptor (Fig. 2A, lane 9) (6).
FIG. 2.
Ligand-induced tyrosine phosphorylation of ErbB-1 and ErbB-2. The different NIH sublines were starved for 18 h in serum-free medium and were left untreated (unt) or stimulated with the indicated growth factors (1 nM) or MAb FRP5 (10 μg/ml) for 10 min at room temperature. ErbB-1 was immunoprecipitated (IP) with a mixture of ErbB-1-specific MAbs EGFR1 and 528 (B); ErbB-2 was immunoprecipitated with 21N antiserum (A and C). The immune complexes were subjected to SDS-PAGE (7.5% gel) and analyzed by Western blotting (WB) using a phosphotyrosine-specific MAb (α P-Y). (C) Long exposure which enabled detection of tyrosine-phosphorylated endogenous ErbB-2.
Treatment of the NE1 derivatives with EGF led to a strong activation of ErbB-1 (Fig. 2B, lanes 2, 4, and 7), very likely due to ErbB-1 homodimerization. EGF-induced transactivation of endogenous ErbB-2 in the NE1 derivatives and, to a low extent, of ErbB-3 (NE1/3 cells) and ErbB-4 (NE1/4 cells) was also detected (data not shown). We next examined the ability of NDF to transmodulate ErbB-1 in the NE1/3 and NE1/4 sublines. NDF was inefficient in activating ErbB-1 when coexpressed with ErbB-3 (Fig. 2B, lane 5) but led to an increase in the tyrosine phosphorylation of endogenous ErbB-2 (Fig. 2C, lane 2). This finding suggests that ErbB-3/ErbB-2 dimers are favored over ErbB-3/ErbB-1 dimers. In contrast to the NE1/3 cell line, coexpression of ErbB-1 and ErbB-4 enabled NDF to modulate ErbB-1 tyrosine phosphorylation (Fig. 2B, lane 8), in addition to transactivation of ErbB-2 (Fig. 2C, lane 4). Since the low amounts of endogenous ErbB-2 in the NE1/3 subline seemed to interfere with NDF-induced formation of ErbB-3/ErbB-1 dimers, we used the human cell line MDA-MB435, which allowed cell surface ErbB-2 to be removed via expression of the human-specific single-chain antibody scFv-5R (6, 22). The resulting cell line, which expressed endogenous ErbB-3, was then infected with a retrovirus encoding ErbB-1 to provide a cell line expressing exclusively ErbB-1 and ErbB-3. NDF induced ErbB-3/ErbB-1 heterodimers in these cells (38). However, the level of NDF-stimulated tyrosine phosphorylation of the receptors was too low to allow further analysis of the signaling properties of ErbB-1 transmodulated by ErbB-3. Thus, we used the NE1/4 cell line to study NDF-transactivated ErbB-1.
EGF- and NDF-activated ErbB-1 associate with Shc, but only EGF-activated ErbB-1 couples to Grb2.
We have previously observed differences in the signaling properties of EGF- compared to NDF-activated ErbB-1. The EGF-activated receptor coupled with Shc and with Cbl, while NDF-activated ErbB-1 associated only with Shc (21). To expand these studies, we examined the coimmunoprecipitation of the adaptor proteins Shc and Grb2 with ErbB-1 in EGF- and NDF-treated NE1 derivatives. EGF treatment led to a strong association of ErbB-1 with the three isoforms of Shc in all the NE1 derivatives (Fig. 3A, upper panel, lanes 2, 4 and 7). NDF promoted a weaker ErbB-1–Shc interaction in NE1/4 cells (Fig. 3A, lane 8; Fig. 3B, lower panel, lane 4), but no interaction was observed in NE1/3 cells (Fig. 3A, lane 5). This finding parallels the fact that NDF stimulated ErbB-1 tyrosine phosphorylation in NE1/4 but not in NE1/3 cells (Fig. 2B). Interestingly, only ErbB-1 activated by EGF, but not by NDF, associated with the adaptor protein Grb2 (Fig. 3A, lower panel; lanes 2, 4 and 7 compared with lane 8).
FIG. 3.
Ligand-induced ErbB-1 complex formation with Shc and Grb2. NE1 derivatives were serum starved for 18 h and were left untreated (unt) or stimulated with 1 nM EGF, 1 nM NDF, or 100 ng of AR per ml at room temperature. (A) ErbB-1 was immunoprecipitated (IP) with MAbs EGFR1 and 528; immune complexes were subjected to SDS-PAGE (12% gel) and analyzed by Western blotting (WB) with Shc-specific rabbit immunoglobulin G (upper panel). The membrane was stripped and reprobed with Grb2-specific antiserum (lower panel). (B) ErbB-1 was immunoprecipitated with MAbs EGFR1 and 528, and immune complexes were analyzed by Western blotting using a phosphotyrosine-specific MAb (α P-Y; upper panel) and a Shc-specific polyclonal antibody (lower panel). (C) Aliquots of 4 mg of whole-cell extract were incubated with 5 μg of a GST-tagged peptide containing the SH2 domain of Grb2. Protein-peptide complexes were collected by using glutathione-Sepharose, subjected to SDS-PAGE (7.5% gel), and immunoblotted with ErbB-1-specific polyclonal antibody 1005.
To ensure that the lack of Grb2 association with NDF-activated ErbB-1 was not due to the low stoichiometry of tyrosine phosphorylation, we tested the EGF receptor agonist AR. Compared to EGF, AR has a lower affinity for ErbB-1 (45), leading to a less dramatic increase in ErbB-1 phosphotyrosine. In the NE1/4 cells, AR and NDF promoted similar amounts of ErbB-1 tyrosine phosphorylation (Fig. 3B, upper panel, lanes 3 and 4) and similar degrees of Shc/ErbB-1 coupling (Fig. 3B, lower panel, lanes 3 and 4). The ErbB-1/Grb2 association was examined by performing a binding assay with a recombinant GST-Grb2 SH2 fusion protein as an affinity agent for activated ErbB-1. The amount of EGF-activated and AR-activated ErbB-1 bound by the SH2 domain of Grb2 reflected the overall level of ErbB-1 tyrosine phosphorylation (Fig. 3C, lanes 2 and 3). GST alone and a GST-Lck SH2 fusion protein bound no EGF-activated ErbB-1 (not shown). Despite the fact that NDF promoted ErbB-1 tyrosine phosphorylation (Fig. 3B, upper panel, lane 4), the receptor was not bound by GST-Grb2 SH2 (Fig. 3C, lane 4), confirming the results shown in Fig. 3A. The specificity of the binding assay was controlled by reprobing the membrane with the phosphotyrosine-specific MAb. A 175-kDa protein, most likely ErbB-1, represented the most prominent tyrosine-phosphorylated band (data not shown). Taken together, the results from the coimmunoprecipitation experiment and the binding assay demonstrate that NDF is inefficient in creating a direct binding site for Grb2 on ErbB-1.
EGF and NDF lead to differential phosphorylation of ErbB-1.
Treatment of NE1/4 cells with EGF induces mainly ErbB-1 homodimers, while NDF leads to activation of ErbB-1 via ErbB-4/ErbB-1 heterodimer formation. Differences in the signaling properties between NDF- and EGF-activated ErbB-1 with respect to Cbl (21) and Grb2 association (Fig. 3) might be due to differences in the tyrosine residues which are phosphorylated in a homodimer versus a heterodimer. To examine this biochemically, we performed two-dimensional tryptic phosphopeptide mapping of ErbB-1 stimulated by either EGF or NDF. In nonstimulated NE1/4 cells, three major ErbB-1 phosphopeptides were detected (Fig. 4A, a1, a2, and a3). Phosphoamino acid analyses revealed that peptides a1 and a3 contained phosphoserine; peptide a2 contained phosphothreonine. None of the peptides contained phosphotyrosine (data not shown). EGF treatment led to the appearance of four additional phosphopeptides (Fig. 4B, b to e). Phosphoamino acid analysis revealed that peptides d and e contained phosphotyrosine; the level of radioactivity in phosphopeptides b and c, however, was too low to allow phosphoamino acid analysis. NDF treatment of NE1/4 cells resulted in the appearance of only three of these phosphopeptides (Fig. 4C, b, d, and e). While peptide b appeared to be phosphorylated as strongly as in EGF-activated ErbB-1, peptides d and e were labeled to a lower extent than in EGF-activated ErbB-1. In contrast, the phosphothreonine-containing peptide a2 showed a stronger increase in the NDF- compared to the EGF-treated cells. The results show that the sites of ErbB-1 phosphorylation as well as the degree of phosphorylation are dependent on the dimerization partner.
FIG. 4.
Phosphopeptide mapping of EGF- and NDF-activated ErbB-1. NE1/4 cells were deprived of phosphate and serum for 12 h prior to labeling with [32P]orthophosphate for 4 h. Cells were left untreated (A) or stimulated with 1 nM EGF (B) or 1 nM NDF (C) for 10 min at room temperature. ErbB-1 was immunoprecipitated with MAbs EGFR1 and 528 and subjected to SDS-PAGE. In-gel tryptic digestion of 32P-labeled ErbB-1 was performed, and the resulting peptides were extracted. Approximately 1,000 cpm was spotted onto TLC plates and separated by ascending chromatography in the first dimension and electrophoresis at pH 1.9 in the second dimension.
Internalization of NDF-activated ErbB-1 is delayed in comparison to EGF-activated ErbB-1.
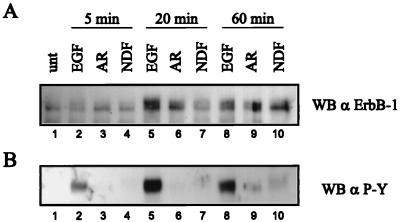
To analyze ligand-induced internalization of ErbB-1 in the NE1/4 cells, cellular surface proteins were biotinylated prior to immunoprecipitation of the receptor. The intracellular pool of ErbB-1 was then isolated by selective removal of the biotinylated surface receptor from the ErbB-1 immunoprecipitate by using immobilized streptavidin. Western analysis with ErbB-1-specific antibody (Fig. 5A) or with a phosphotyrosine-specific MAb (Fig. 5B) revealed that EGF treatment led to rapid internalization of activated ErbB-1. Intracellular levels of ErbB-1 were maximal after 20 min and decreased by 1 h (Fig. 5, lanes 5 and 8), most likely due to degradation of the receptor through the endosomal/lysosmal pathway (46). Likewise, AR treatment of NE1/4 cells resulted in an increase of intracellular ErbB-1. AR promotes a lower degree of ErbB-1 activation; therefore, the amount of internalized ErbB-1 at 20 min was less than that seen with EGF (Fig. 5A, lanes 6 versus 5). After 60 min of AR treatment, a decrease in the intracellular level of ErbB-1 was not observed (Fig. 5A, lanes 9 versus 6), which indicates that AR-stimulated receptor trafficking was not as rapid as in response to EGF. This might be explained by the lower affinity of AR for ErbB-1. In contrast to stimulation with EGF and AR, NDF treatment of NE1/4 cells did not promote a rapid rise of the internal pool of ErbB-1. The amount of intracellular ErbB-1 recovered was comparable to the amount seen in AR-treated cells only after 60 min of NDF treatment (Fig. 5A, lane 10). In addition, phosphotyrosine analysis of the internalized ErbB-1 revealed a reduced electrophoretic mobility of the activated receptor (Fig. 5B, lane 10). This is most likely due to additional serine/threonine phosphorylation, which is in accordance with the results obtained by phosphopeptide mapping of the receptor. In summary, we conclude that compared to stimulation by EGF and AR, the rate of internalization of NDF-activated ErbB-1 is significantly reduced.
FIG. 5.
Internalization of ligand-activated ErbB-1. NE1/4 cells were serum starved for 18 h and were left untreated (unt) or stimulated with 1 nM EGF, 1 nM NDF, or 100 ng of AR per ml for the indicated times at 37°C. Prior to lysis, cell surface proteins were biotinylated for 30 min on ice. ErbB-1 was immunoprecipitated with polyclonal antibody 1005; after release from the beads, biotinylated surface ErbB-1 was removed by using immobilized streptavidin. The supernatant containing the intracellular pool of ErbB-1 protein was recovered, subjected to SDS-PAGE (7.5% gel), and analyzed by Western blotting (WB) with ErbB-1-specific polyclonal antibody 1005 (A) and, after stripping, with phosphotyrosine-specific MAb (α P-Y) (B).
Association of activated ErbB-2 with Grb2, p85, and Shc.
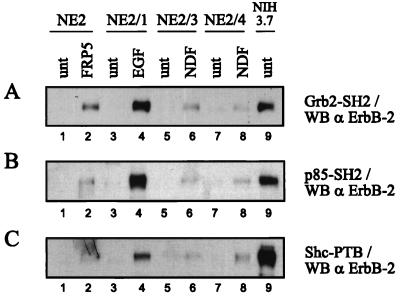
ErbB-2 activated by ligand-induced heterodimerization, constitutive dimerization due to a mutation in the transmembrane domain, or antibody-induced homodimerization was examined for its ability to bind GST fusion proteins containing the SH2 domains of Grb2 and p85 and the PTB domain of Shc. Binding of ErbB-2 demonstrates that these domains are sufficient for interaction and suggests that the tyrosine residues known to serve as recognition sites for the individual proteins are phosphorylated. High levels of ErbB-2 from NIH3.7 cells and from EGF-treated NE2/1 cells were readily bound by each of the three GST fusion proteins tested (Fig. 6A to C, lanes 4 and 9). ErbB-2 from NIH3.7 cells and EGF-treated NE2/1 cells demonstrated similar levels of phosphotyrosine (Fig. 2A, lanes 4 and 9), yet binding of the GST fusion proteins did not reflect merely the content of total phosphotyrosine. Quantification of the filters revealed that GST-Shc PTB associated with the mutated, oncogenic variant of ErbB-2 four times more strongly than with ErbB-2 transactivated by EGF (Fig. 6C, lanes 9 versus 4). ErbB-2 from NDF- and MAb FRP5-treated NE2 derivatives displayed a lower degree of association with the GST fusion proteins (Fig. 6A to C, lanes 2, 6, and 8), reflecting the lower stoichiometry of tyrosine phosphorylation (Fig. 2A). However, ErbB-2 from MAb FRP5-treated cells bound approximately two and four times more GST-Grb2 SH2 than ErbB-2 from, respectively, NDF-treated NE2/3 and NE2/4 cells (Fig. 6A, lanes 2 versus 6 and 8). These results suggest that, dependent on the mode of activation, there are tyrosine residues in ErbB-2 which are preferentially phosphorylated.
FIG. 6.
Binding of Shc PTB, Grb2 SH2, and p85 SH2 to ErbB-2. NE2 derivatives and NIH3.7 cells were serum starved for 18 h. The NE2 derivatives were left untreated (unt) or stimulated with 1 nM EGF, 1 nM NDF, or 10 μg of FRP5 per ml for 10 min at room temperature. NIH3.7 cells were left untreated. Two milligrams of cell lysate was incubated with either the GST-tagged SH2 domain of Grb2 (A), the N-terminal SH2 domain of p85 (B), or the PTB domain of Shc (C). Protein-peptide complexes were precipitated with glutathione-Sepharose, subjected to SDS-PAGE (7.5% gel), and Western blotted (WB) with ErbB-2-specific antiserum 21N.
Phosphopeptide mapping of ErbB-2 reveals dimerization-dependent phosphorylation.
Two-dimensional tryptic phosphopeptide mapping was performed on ErbB-2 from nonstimulated NE2 cells, NIH3.7 cells, and EGF- and NDF-stimulated NE2 derivatives. The maps generated by ligand-activated ErbB-2 were quite complex. In control NE2 cells, three major phosphorylated peptides were detected (Fig. 7A, a1, a2, and a3). Peptides a1 and a2 contained phosphoserine; peptide a3 contained phosphothreonine (not shown). EGF treatment of NE2/1 cells led to the appearance of six additional phosphopeptides (Fig. 7C, b to g). Unfortunately, the level of radioactivity in these peptides was too low to allow phosphoamino acid analysis. ErbB-2 transmodulated by ErbB-3 in NDF-treated NE2/3 cells generated a qualitatively very similar phosphopeptide map (Fig. 7D), while ErbB-2 from NDF-treated NE2/4 cells gave rise to phosphopeptides b to g plus a new phosphopeptide, h (Fig. 7E). Furthermore, two additional phosphopeptides, b′ and b", which may be partial digestion products of b or may represent two novel phosphopeptides, were generated. Constitutively active ErbB-2 from NIH3.7 cells (Fig. 7B) displayed a less complex phosphorylation pattern than ErbB-2 from the ligand-treated cells (Fig. 7C to E). Phosphopeptides b, c, f, and g were evident, although less strongly labeled than in the ligand-induced ErbB-2 receptor, while phosphopeptides d, e, and h were not present. However, compared to ErbB-2 from control cells, there was a dramatic increase in labeling of peptide a1. This appeared to be specific to the ErbB-2 mutant since neither EGF nor NDF promoted an enhancement of this phosphopeptide. Analogous to the results obtained for ErbB-1, tryptic phosphopeptide analysis of ErbB-2 provides biochemical evidence for differential, dimerization-dependent phosphorylation of this receptor.
FIG. 7.
Phosphopeptide mapping of ErbB-2. The indicated cell lines were deprived of phosphate and serum for 12 h prior to labeling with [32P]orthophosphate for 4 h. Cells were left untreated (A and B) or stimulated with 1 nM EGF (C) or 1 nM NDF (D and E) for 10 min at room temperature. ErbB-2 was immunoprecipitated with rabbit antiserum 21N and subjected to SDS-PAGE. In-gel tryptic digestion of 32P-labeled ErbB-2 was performed, and the resulting peptides were extracted. Approximately 500 cpm was spotted onto TLC plates and separated by ascending chromatography in the first dimension and electrophoresis at pH 1.9 in the second dimension.
Kinetics of EGF and NDF activation of ErbB receptors and their association with p85 and Shc.
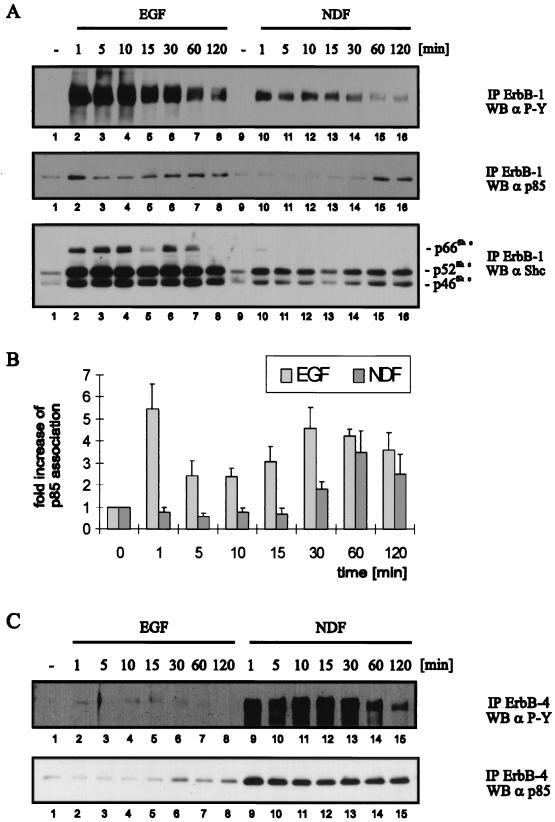
Using EGF- and NDF-treated NE1/4 cells, we next examined the kinetics of association of signaling proteins with ErbB receptors during a 120-min time course. We immunoprecipitated ErbB-1 from ligand-stimulated cells and determined its phosphotyrosine content and the amount of coimmunoprecipitating p85 and Shc. The filters were reprobed with ErbB-1-specific antibody to ensure equal immunoprecipitation of the receptor (data not shown). No ErbB-4 was detected in the ErbB-1 immunoprecipitations (data not shown); therefore, the coimmunoprecitating proteins were specific for ErbB-1 and not brought down by the dimerization partner. Following EGF treatment, tyrosine phosphorylation was maximal between 1 and 10 min and then decreased slowly during 120 min (Fig. 8A, upper panel, lanes 2 to 8). Although NDF-activated ErbB-1 had a lower level of phosphotyrosine, the kinetics were similar to those seen in EGF-treated cells (Fig. 8A, upper panel, lanes 10 to 16). Despite the decrease in ErbB-1 phosphotyrosine, there was a stable association of Shc with the EGF- and NDF-activated receptor throughout the time course (Fig. 8A, lower panel). The amount of Shc associated with ErbB-1 was lower in the NDF-treated cells, likely reflecting the lower stoichiometry of phosphotyrosine on the receptor.
FIG. 8.
Time course of ligand-induced ErbB receptor activation and association with Shc and p85. NE1/4 cells were serum starved for 18 h and, prior to lysis, stimulated with 1 nM EGF or NDF (A and C) for the indicated periods at 37°C. Equal amounts of protein were immunoprecipitated (IP) with ErbB-1-specific MAbs EGFR1 and 528 (A) and ErbB4-specific polyclonal antibody C18 (C), and immune complexes were resolved by SDS-PAGE (8% gel). The membranes were probed by Western blotting (WB) with a phosphotyrosine-specific antibody (α P-Y) (A and C, top panels), with a p85-specific polyclonal antibody (A and C, middle and bottom panels, respectively), and, after stripping, with a Shc-specific polyclonal antibody (A, bottom panel). (B) Quantification of p85 binding was performed with ImageQuant software (Molecular Dynamics). The mean values and standard errors from three independent experiments are represented.
In contrast to Shc, p85 displayed time-dependent changes in its coupling with ErbB-1. A strong association was seen after 1 min of EGF treatment (Fig. 8A, middle panel, lane 2); the interaction declined between 5 and 15 min (lanes 3 to 5) and increased again at 30 to 120 min (lanes 6 to 8). Surprisingly, the association of p85 with ErbB-1 was detected only after 60 min of NDF treatment (Fig. 8A, middle panel, lanes 15 and 16). Although the amount of tyrosine phosphorylation on ErbB-1 at this time was very low (Fig. 8A, upper panel, lanes 15 and 16), the degree of p85 association was as strong as in the EGF-treated cells (Fig. 8A, middle panel, lanes 15 and 16 versus 7 and 8). The inability to detect association of p85 with NDF-activated ErbB-1 at an early time point was not due to its low phosphotyrosine level, since AR-activated ErbB-1, which contained a similar amount of phosphotyrosine, rapidly associated with p85 after ligand stimulation (38). The results from three independent experiments in which the NDF- and EGF-induced association of p85 with ErbB-1 were quantified are shown in Fig. 8B. Intriguingly, phosphatidylinositol kinase activity associated with EGF-activated ErbB-1 was elevated at 1 and 60 min (38).
We then examined the association of ErbB-4 with p85 in NE1/4 cells. Treatment with NDF rapidly induced a strong increase in ErbB-4 phosphotyrosine which decreased between 60 and 120 min (Fig. 8C, upper panel, lanes 9 to 13 versus 14 and 15). Despite this decrease, the association of p85 with ErbB-4 remained constant throughout the time course (Fig. 8C, lower panel, lanes 9 to 15). EGF treatment of NE1/4 cells induced a slight increase in ErbB-4 phosphorylation (Fig. 8C, upper panel, lanes 2 to 8). Interestingly, p85 associated with ErbB-4 transactivated by EGF only after 30 min of treatment, a time at which the phosphotyrosine content of the receptor had started to decrease (Fig. 8C, lanes 6 to 8). In summary, p85 associated continuously with NDF-activated ErbB-4 and displayed biphasic association with EGF-activated ErbB-1. However, the association of p85 with both NDF-activated ErbB-1 and EGF-activated ErbB-4 occurred with a lag of 30 to 60 min. Qualitatively similar kinetics of p85 interaction with EGF- and NDF-activated ErbB-1 were observed in T47D/5R cells (38). Taken together, these results show that the coupling of signaling molecules to a receptor can undergo not only ligand- but also time-dependent changes.
DISCUSSION
The multiplicity of EGF-related ligands combined with their ability to activate different ErbB receptor dimers promotes signal diversification. ErbB receptor dimers have the potential to couple to different signaling pathways dependent on the array of associated phosphotyrosine-binding proteins. The results presented here show that the signaling properties of ErbB receptors depend on the dimerization partner and may originate from the phosphorylation status of the receptor.
The ability of NDF to transactivate ErbB-1 in cells coexpressing ErbB-1 and ErbB-4 (NE1/4 cells) allowed us to compare EGF- and NDF-activated ErbB-1 receptors. One of the major differences was the coimmunoprecipitation of Grb2 with EGF-activated but not NDF-activated ErbB-1. Grb2 can bind the phosphorylated receptor either indirectly via SH2 domain-mediated association with the adaptor protein Shc or directly via SH2 domain-mediated interaction with a specific phosphotyrosine motif on the receptor. A peptide containing the SH2 domain of Grb2 was able to bind EGF-activated ErbB-1. However, it failed to bind ErbB-1 when activated by NDF. We have previously observed that in T47D/5R cells the Cbl protein coupled with EGF- but not with NDF-activated ErbB-1 (21). The SH3 domain of Grb2 can interact with Cbl (20); thus, Grb2 may serve as a link between Cbl and ErbB-1 (34). Therefore, it is reasonable to assume that the failure of NDF-activated ErbB-1 to recruit Cbl is due to its inability to interact with Grb2.
Tryptic phosphopeptide analysis clearly showed that there are ligand-dependent differences in the patterns of ErbB-1 phosphorylation. NDF failed to induce phosphorylation of the phosphotyrosine-containing peptide c. Considering the inability of NDF-activated ErbB-1 to bind Grb2, it is tempting to speculate that this peptide contains a Grb2 binding site. In NDF-activated ErbB-1, two of the tyrosine-containing phosphopeptides (d and e) were less phosphorylated, in accordance with its lower stoichiometry of tyrosine phosphorylation compared to that of EGF-activated ErbB-1. Interestingly, the phosphothreonine-containing peptide a2, also present in nonstimulated control cells, demonstrated increased phosphorylation in response to NDF. This may correlate with the lower electrophoretic mobility of NDF-activated ErbB-1 which we have observed (Fig. 5). Serine/threonine phosphorylation has been implicated in desensitization of ErbB-1 (14, 15), suggesting that EGF- and NDF-activated ErbB-1 may be regulated differently.
Differential phosphorylation of ErbB-1 and coupling to different subsets of signaling molecules might also influence receptor trafficking. Our results show that internalization of ErbB-1 (Fig. 5) and ErbB-2 (38) is determined by both the ligand and the heterodimerization partner. EGF-activated ErbB-1 was rapidly internalized, whereas ErbB-1 activated by NDF showed delayed internalization characteristics. EGF promotes formation of ErbB-1 homodimers which are degraded through the lysosomal pathway (46). EGF remains bound to ErbB-1 even at low pH, which is thought to favor receptor degradation (19). The pH sensitivity of AR- and NDF-bound receptors has not been reported. In comparison with ErbB-1, endocytosis of the other ErbB family members seems to be impaired (2, 39). Therefore, NDF-activated ErbB-1 might display slower internalization due to its heterodimerization with ErbB-4. Interestingly, the adaptor protein Grb2 is required for efficient endocytosis of ErbB-1 (50). Thus, the failure of Grb2 to couple to NDF-activated ErbB-1 might contribute to its slower internalization.
The kinetics of ErbB receptor activation and association with Shc and p85 were examined in NE1/4 cells. In both NDF- and EGF-activated cells, the association of ErbB-1 and Shc was rapid and reflected the overall phosphotyrosine level of the receptor. In contrast to Shc, the association of p85 with ErbB-1 and ErbB-4 showed variations which could not be correlated with receptor phosphorylation. NDF-stimulated ErbB-4 and p85 coimmunoprecipitated throughout the time course, while the association of p85 with both ErbB-1 transactivated by NDF and ErbB-4 transactivated by EGF occurred with a lag of 30 to 60 min. Surprisingly, p85 coimmunoprecipitation was highest when the total phosphotyrosine level in the respective receptor had started to decline. Most intriguing was the biphasic association of p85 with EGF-activated ErbB-1, with one peak occurring after 1 min of EGF treatment and the second peak occurring 30 min later. The mechanism underlying the variation in p85/ErbB-1 binding is unknown. It may be linked to changes of specific phosphorylated tyrosine residues due to continuous phosphatase-mediated dephosphorylation and ligand-induced rephosphorylation events (51). Alternatively, since ErbB-1 is internalized upon ligand binding, there might be preferential binding of signaling molecules to the receptor in different cellular compartments (16, 18). In rat liver, cytosolic ErbB-1 was shown to be hyperphosphorylated and efficiently coupled with Shc and Grb2/Sos (16). Retarded internalization of NDF-activated ErbB-1 may therefore result in prolongation of signaling.
None of the major ErbB-1 autophosphorylation sites are in a consensus p85 binding site. However, additional tyrosine residues are phosphorylated after EGF stimulation. Tyr 920, which is the major Src kinase site in vitro, is a potential p85 binding site (YMIM) (47). Src kinase lies downstream of ErbB-1 (33, 37, 47), making it possible that Src phosphorylation is responsible for the second wave of p85 binding to EGF-activated ErbB-1. The rapid association of p85 with ErbB-1 seen at 1 min, however, may be mediated not by direct binding but via a complex with Grb2. p85 has been shown to associate with both Grb2 (49) and Gab1 (Grb2-associated binder 1) (26). Since NDF-activated ErbB-1 does not interact with Grb2, this might explain the inability of the receptor to rapidly associate with p85. In contrast to ErbB-1, one of the ErbB-4 autophosphorylation sites is in a recognition site for the SH2 domain of p85 (9). Thus, the rapid association of p85 with NDF-activated ErbB-4 is most likely a consequence of the catalytic activity of the receptor.
ErbB-2, the preferred dimerization partner of all other ErbB receptors (21), can be activated by heterodimerization with ErbB-1, ErbB-3, and ErbB-4 or by MAb- and mutation-induced homodimerization. We analyzed ErbB-2 activation by measuring the binding of the receptor to peptides containing the SH2 domains of Grb2 and p85 and the PTB domain of Shc and by tryptic phosphopeptide mapping. The binding sites of the adaptor proteins Grb2 and Shc have been mapped to specific autophosphorylation sites in ErbB-2. However, as with ErbB-1, none of the autophosphorylated tyrosine residues in ErbB-2 provide an optimal binding site for p85. Again, there are potential Src kinase sites in ErbB-2, one of which matches a consensus p85 binding site (Tyr 952 [YMIM]) (47). In general, the level of ErbB-2 bound by the three phosphotyrosine-interacting peptides reflected the total phosphotyrosine content of the activated receptor. However, there were two notable differences. (i) ErbB-2 activated by MAb FRP5 and ErbB-2 activated by NDF displayed similar levels of total phosphotyrosine; however, GST-Grb2 SH2 bound significantly higher amounts of FRP5-activated ErbB-2. (ii) The phosphotyrosine levels in ErbB-2 from NIH3.7 cells and from EGF-treated NE2/1 cells were similar, yet the Shc PTB domain bound four times more constitutively active, mutant ErbB-2 compared to EGF-activated ErbB-2. The tryptic phosphopeptide maps may help explain this result. The patterns of ErbB-2 phosphopeptides generated from EGF-activated and NDF-treated NE2 derivatives were very similar, while the map of the constitutively active ErbB-2 mutant was less complex. Only four (b, c, f, and g) of the seven (b to h) ligand-induced phosphopeptides were present. This might be due to selective activation of phosphorylation sites which contribute to the transforming potential of the ErbB-2 receptor. Specific sites implicated in the negative regulation of the receptor may not be phosphorylated. The Neu receptor with a mutation in one such site, Tyr 1028, displays more Shc binding than the wild-type receptor (13). Thus, the fact that the Shc PTB domain strongly binds the oncogenic ErbB-2 protein may be a consequence of the lack of activation of this negative regulatory site.
In summary, our data demonstrate that the potential to activate signal transduction pathways by an ErbB receptor is dependent on its dimerization partner and may originate from differential receptor phosphorylation. The dimer formed is in turn dependent on the type of ligand and the cell’s complement of ErbB receptors. By extending our studies to additional downstream signaling molecules, we hope to gain more insight into distinct signaling properties of specific ErbB dimers. The use of cell lines expressing defined combinations of ErbB receptors provides a tool to understand in greater detail complex ErbB receptor interactions, and it may help to elucidate why these receptors are important for tumor development.
ACKNOWLEDGMENTS
M.A.O. and D.G.-P. contributed equally to this work.
We thank N. Pullen, P. Dennis, and I. Beuvink for technical advice on phosphopeptide maps and N. Pullen, T. Schaefer, and M. Grob for help with kinase assays. GST-p85 SH2 and GST-Shc PTB proteins were a gift from Steve Shoelson. H. Lane, J. Daly, and H. Kaufmann are acknowledged for critical reading of the manuscript. D.G.-P. was partly supported by a grant from the Basel Cancer League.
REFERENCES
- 1.Bargmann C I, Hung M-C, Weinberg R A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986;45:649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- 2.Baulida J, Kraus M H, Alimandi M, Di Fiore P P, Carpenter G. All ErbB receptors other than the epidermal growth factor are endocytosis impaired. J Biol Chem. 1996;271:5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- 3.Beerli R R, Hynes N E. Epidermal growth factor-related peptides activate distinct subsets of ErbB receptors and differ in their biological activities. J Biol Chem. 1996;271:6071–6076. doi: 10.1074/jbc.271.11.6071. [DOI] [PubMed] [Google Scholar]
- 4.Beerli R R, Graus-Porta D, Woods-Cook K, Chen X, Yarden Y, Hynes N E. Neu differentiation factor activation of ErbB-3 and ErbB-4 is cell specific and displays a differential requirement for ErbB-2. Mol Cell Biol. 1995;15:6496–6506. doi: 10.1128/mcb.15.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beerli R R, Wels W, Hynes N E. Autocrine inhibition of the epidermal growth factor receptor by intracrine expression of a single-chain antibody. Biochem Biophys Res Commun. 1994;204:666–672. doi: 10.1006/bbrc.1994.2511. [DOI] [PubMed] [Google Scholar]
- 6.Beerli R R, Wels W, Hynes N E. Intracellular expression of single chain antibodies reverts ErbB-2 transformation. J Biol Chem. 1994;269:23931–23936. [PubMed] [Google Scholar]
- 7.Braunwalder A F, Weennogle L, Gay B, Lipson K E, Sills M. Application of scintillating microtiter plates to measure phosphopeptide interactions with the Grb2 SH2 binding domain. J Biomol Screening. 1996;1:23–26. [Google Scholar]
- 8.Chen X, Levkowitz G, Tzahar E, Karunagaran D, Lavi S, Ben-Baruch N, Leitner O, Ratzkin B J, Bacus S S, Yarden Y. An immunological approach reveals biological differences between the two NDF/heregulin receptors, ErbB-3 and ErbB-4. J Biol Chem. 1996;271:7620–7629. [PubMed] [Google Scholar]
- 9.Cohen B D, Green J M, Foy L, Fell H P. HER4-mediated biological and biochemical properties in NIH 3T3 cells. J Biol Chem. 1996;271:4813–4818. doi: 10.1074/jbc.271.9.4813. [DOI] [PubMed] [Google Scholar]
- 10.Cohen B D, Kiener P A, Green J M, Foy L, Fell P, Zhang K. The relationship between human epidermal growth-like factor receptor and cellular transformation in NIH3T3 cells. J Biol Chem. 1996;271:30897–30903. doi: 10.1074/jbc.271.48.30897. [DOI] [PubMed] [Google Scholar]
- 11.Cohen G B, Ren R, Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995;80:237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- 12.Daly J M, Jannot C B, Beerli R R, Graus-Porta D, Maurer F G, Hynes N E. Neu differentiation factor induces ErbB-2 down-regulation and apoptosis of ErbB-2 overexpressing breast tumor cells. Cancer Res. 1997;57:3804–3811. [PubMed] [Google Scholar]
- 13.Dankort D L, Wang Z, Blackmore V, Moran M F, Muller W J. Distinct tyrosine autophosphorylation sites negatively and positively modulate Neu-mediated transformation. Mol Cell Biol. 1997;17:5410–5425. doi: 10.1128/mcb.17.9.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis R J, Czech M P. Tumor-promoting phorbol diesters mediate phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1984;259:8545–8549. [PubMed] [Google Scholar]
- 15.Decker S. Effects of epidermal growth factor and 12-O-tetradecanoylphorbol-13-acetate on metabolism of the epidermal growth factor receptor in normal human fibroblasts. Mol Cell Biol. 1984;4:1718–1724. doi: 10.1128/mcb.4.9.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiGuglielmo G M, Baass P C, Ou W-J, Posner B I, Bergeron J J M. Compartmentalization of SHC, GRB2 and mSOS, and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. EMBO J. 1994;13:4269–4277. doi: 10.1002/j.1460-2075.1994.tb06747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Druker B J, Mamon H J, Roberts T M. Oncogenes, growth factors, and signal transduction. N Engl J Med. 1989;321:1283–1391. doi: 10.1056/NEJM198911163212007. [DOI] [PubMed] [Google Scholar]
- 18.Emlet D R, Moscatello D K, Ludlow L B, Wong A J. Subsets of epidermal growth factor receptors during activation and endocytosis. J Biol Chem. 1997;272:4079–4086. doi: 10.1074/jbc.272.7.4079. [DOI] [PubMed] [Google Scholar]
- 19.French A R, Tadaki D K, Niyogi S K, Lauffenburger D A. Intracellular trafficking of epidermal growth factor family ligands is directly influenced by the pH sensitivity of the receptor/ligand interaction. J Biol Chem. 1995;270:4334–4340. doi: 10.1074/jbc.270.9.4334. [DOI] [PubMed] [Google Scholar]
- 20.Fukazawa T, Reedquist K A, Trub T, Soltoff S, Panchamoorthy G, Druker B, Cantley L, Shoelson S E, Band H. The SH2 domain-binding T cell tyrosyl phosphoprotein p120. Demonstration of its identity with the c-cbl protooncogene product and in vivo complexes with Fyn, Grb2, and phosphotidlyinositol 3′-kinase. J Biol Chem. 1995;270:19141–19150. doi: 10.1074/jbc.270.32.19141. [DOI] [PubMed] [Google Scholar]
- 21.Graus-Porta D, Beerli R R, Daly J M, Hynes N E. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graus-Porta D, Beerli R R, Hynes N E. Single-chain antibody-mediated intracellular retention of ErbB-2 impairs Neu differentiation factor and epidermal growth factor signaling. Mol Cell Biol. 1995;15:1182–1191. doi: 10.1128/mcb.15.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gullick W J, Downward D J, Foulkes J G, Waterfield M D. Antibodies to the ATP-binding site of the human epidermal growth factor (EGF) receptor as specific inhibitors of EGF-stimulated protein-tyrosine kinase activity. EMBO J. 1985;4:2869–2877. doi: 10.1111/j.1432-1033.1986.tb09744.x. [DOI] [PubMed] [Google Scholar]
- 24.Harwerth I-M, Wels W, Marte B M, Hynes N E. Monoclonal antibodies against the extracellular domain of the erbB-2 receptor function as partial ligand agonists. J Biol Chem. 1992;267:15160–15167. [PubMed] [Google Scholar]
- 25.Harwerth I-M, Wels W, Schlegel J, Müller M, Hynes N E. Monoclonal antibodies directed to the erbB-2 receptor inhibit in vivo tumour cell growth. Br J Cancer. 1993;68:1140–1145. doi: 10.1038/bjc.1993.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holgado-Madruga M, Emlet D E, Moscatello D K, Godwin A K, Wong A J. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 27.Hudziak R M, Lewis G D, Winget M, Fendly B M, Shepard H M, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989;9:1165–1172. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hynes N E, Gerber H A, Saurer S, Groner B. Overexpression of the c-erbB-2 protein in human breast tumor cell lines. J Cell Biochem. 1989;39:167–173. doi: 10.1002/jcb.240390208. [DOI] [PubMed] [Google Scholar]
- 29.Hynes N E, Stern D F. The biology of erbB-2/neu/HER2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 30.Karunagaran D, Tzahar E, Beerli R R, Chen X, Graus-Porta D, Ratzkin B J, Seger R, Hynes N E, Yarden Y. ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer. EMBO J. 1996;15:254–264. [PMC free article] [PubMed] [Google Scholar]
- 31.Kavanaugh W M, Williams L T. An alternative to SH2 domains for binding tyrosine-phosphorylated proteins. Science. 1994;266:1862–1865. doi: 10.1126/science.7527937. [DOI] [PubMed] [Google Scholar]
- 32.Levkowitz G, Klapper L N, Tzahar E, Freywald A, Sela M, Yarden Y. Coupling of the c-Cbl protooncogene to ErbB-1/EGF-receptor but not to other ErbB proteins. Oncogene. 1996;12:1117–1125. [PubMed] [Google Scholar]
- 33.Luttrell D K, Lee A, Lansing T J, Crosby R M, Jung K D, Willard D, Luther M, Rodriguez M, Berman J, Gilmer T M. Involvement of pp60c-src with two major signaling pathways in human breast cancer. Proc Natl Acad Sci USA. 1994;91:83–87. doi: 10.1073/pnas.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meisner H, Czech M P. Coupling of the proto-oncogene product c-Cbl to the epidermal growth factor receptor. J Biol Chem. 1995;270:25332–25335. doi: 10.1074/jbc.270.43.25332. [DOI] [PubMed] [Google Scholar]
- 35.Messerle K, Schlegel J, Hynes N E, Groner B. NIH/3T3 cells transformed with the activated erbB-2 oncogene can be phenotypecally reverted by a kinase deficient, dominant negative erbB-2 variant. Mol Cell Endocrinol. 1994;105:1–10. doi: 10.1016/0303-7207(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 36.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muthuswamy S K, Muller W J. Direct and specific interaction of c-Src with neu is involved in signaling by the epidermal growth factor receptor. Oncogene. 1995;11:271–279. [PubMed] [Google Scholar]
- 38.Olayioye, M. A. Unpublished data.
- 39.Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin B J, Sela M, Yarden Y. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- 40.Pullen N, Thomas G. The modular phosphorylation and activation of p70S6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- 41.Riese II D J, Bermingham Y, van Raiij T M, Buckley S, Plowman G D, Stern D F. Betacellulin activates the epidermal growth factor receptor and erbB-4 and induces cellular response patterns distinct from those stimulated by epidermal growth factor or neuregulin-β. Oncogene. 1996;12:345–353. [PubMed] [Google Scholar]
- 42.Riese D J, II, Stern D F. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 43.Robinson M J, Cobb M H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 44.Salomon D S, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 45.Shoyab M G, Plowman G, McDonald V L, Bradley J G, Todaro G J. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1989;243:1074–1076. [Google Scholar]
- 46.Sorkin A, Waters C M. Endocytosis of growth factor receptors. Bioessays. 1993;15:375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- 47.Stover D R, Becker M, Liebetanz J, Lydon N B. Src phosphorylation of the epidermal growth factor receptor at novel sites mediates receptor interaction with Src and p85α. J Biol Chem. 1995;270:15591–15597. doi: 10.1074/jbc.270.26.15591. [DOI] [PubMed] [Google Scholar]
- 48.Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin B J, Yarden Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Auger K R, Jarvis L, Shi Y, Roberts T M. Direct association of Grb2 with the p85 subunit of phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:12774–12780. doi: 10.1074/jbc.270.21.12774. [DOI] [PubMed] [Google Scholar]
- 50.Wang Z, Moran M F. Requirement for the adapter protein Grb2 in EGF receptor endocytosis. Science. 1996;272:1935–1939. doi: 10.1126/science.272.5270.1935. [DOI] [PubMed] [Google Scholar]
- 51.Weiss F U, Daub H, Ullrich A. Novel mechanisms of RTK signal generation. Curr Opin Genet Dev. 1997;7:80–86. doi: 10.1016/s0959-437x(97)80113-x. [DOI] [PubMed] [Google Scholar]
- 52.Zhang K, Sun J, Liu N, Wen D, Chang D, Thomason A, Yoshinage S K. Transformation of NIH 3T3 cells by HER3 or HER4 receptors requires the presence of HER1 or HER2. J Biol Chem. 1996;271:3884–3890. [PubMed] [Google Scholar]
- 53.Zrihan-Licht S, Lim J, Keydar I, Sliwkowski M X, Groopman J E, Avraham H. Association of Csk-homologous kinase (CHK) (formerly MATK) with HER-2/ErbB-2 in breast cancer cells. J Biol Chem. 1997;272:1856–1863. doi: 10.1074/jbc.272.3.1856. [DOI] [PubMed] [Google Scholar]