Abstract
Allergic rhinitis is among the most common chronic diseases in the world. Obesity can lead to a chronic systemic inflammatory process. In this study, we evaluated the effects of body weight on the response to treatment of allergic rhinitis with nasal corticosteroids. Two groups of patients diagnosed with allergic rhinitis were compared: one composed of obese patients and one composed of normal weight patients. Nasal endoscopy, peak nasal inspiratory flow, quality of life, the VAS, SNOT22, and NOSE-5 questionnaires, and the concentration of nasal cytokines (INF-γ, TNF-ᾳ, IL-4, IL-5, IL-6, and IL-10) through nasal brushing were evaluated before and after treatment with 400 mcg/day nasal beclomethasone. No differences were identified between the groups in nasal endoscopy, peak nasal inspiratory flow, the VAS, SNOT22, and NOSE-5 questionnaires, or in the cytokines INF-γ, TNF-ᾳ, IL-4, IL-5, IL-6, and IL-10 prior to nasal corticosteroid treatment. Both groups showed improvement in the VAS, SNOT-22, and NOSE-5 questionnaires and an increase in peak nasal inspiratory volumes after treatment. In the eutrophic group, there was an increase in INF-γ and IL-5 after treatment. When comparing the variation in cytokines before and after treatment between groups, IL-10 was the cytokine that showed altered behavior dependent on weight. Obesity did not seem to impact nasal symptoms and physiology and presented a similar clinical response to treatment with nasal corticosteroids to normal weight patients. However, obese patients had an impaired anti-inflammatory response during treatment with nasal corticosteroids.
Keywords: Allergic rhinitis, Obesity, Cytokines, Quality of life, Nasal sprays
Introduction
Allergic rhinitis (AR) is among the most common chronic diseases in the world [1]. Treatments range from preventive measures to avoid contact with the allergenic protein to drug treatment. Nasal corticosteroids are the drug treatment of first choice [2, 3].
A portion of patients with allergic rhinitis are refractory to treatment [3]. The literature suggests the use of caution regarding nasal corticosteroids and their use in children due to the dose and body mass of children [4–6]; however, there are few studies relating adult patients with body mass index (BMI), considering that the prevalence of obesity has been increasing [7].
Although obesity may lead to alterations in the respiratory mechanism of the lower airways, which become more intense the higher the degree of obesity, few studies individualize this group and its repercussions on nasal physiology [8–11].
Bhattacharyya demonstrated that an increased body mass index (BMI) was significantly associated with the presence of allergic rhinitis and chronic rhinosinusitis, as both share a predisposition to inflammation [12, 13].
The allergic inflammatory process of the nasal mucosa is regulated by the production of several cytokines, particularly interleukins (IL) IL-4 and IL-5 [14]. Obesity is characterized by low-grade systemic inflammation induced by different inflammatory mediators, such as IL-6, tumor necrosis factor (TNF-ᾳ), and IL-1 [15].
The current study aims to investigate the impact of body weight on the response to topical corticosteroid treatment in patients with allergic rhinitis.
Material and Methods
This prospective, convenience sample, clinical study included 45 patients with allergic rhinitis who voluntarily agreed to participate. The study was approved by the local ethics committee, registration 44937920.5.0000.5505.
Participants were divided into 2 groups according to BMI: an obese group with BMI greater than 30 kg/m2 (22 patients) and a normal weight group with BMI between 18 and 25 kg/m2 (23 patients).
Individuals with a positive prick test for aeroallergens, with nasal irritation complaints, and over 18 years of age were included. The exclusion criteria were the following: immunodeficiencies, ocular hypertension, steroid intolerance, nasal polyposis, severe systemic diseases, or use of corticosteroids and immunomodulators for at least 30 days before the beginning of the research. Patients were evaluated two times by an otorhinolaryngologist who was unaware of the research design: before starting treatment with nasal corticosteroids after a previous period of 30 days without nasal or oral corticosteroids (washout) using only antihistamines in crises if necessary and after 30 days of treatment with nasal corticosteroids.
All participants received 50 mcg nasal beclomethasone with verbal and written guidance on the correct use of the spray, using 400 mcg/day in two daily doses for 30 days.
Prick Test
The prick test contained the following items:
Positive control with histamine;
Negative control with sterile saline;
Test for aeroallergens: Dermatophagoides pteronyssinus, Dermatophagoides farinae, Blomia tropicalis, dog epithelium, cat epithelium, fungi mix (composed of Penicillium notatum, Alternaria alternata, Candida albicans).
The test reading was performed after fifteen minutes with a ruler graduated in mm, and a papule with a mean diameter 3 mm greater than that of the negative control was considered a positive prick test.
Pre- and Posttreatment Assessment
Quality of Life Questionnaires
Peak Inspiratory Flow and Physical Examination
Peak Nasal Inspiratory Flow (PNIF): 3 measurements taken by a Medicate® device, model 3109751 M, with ATS (American Thoracic Society) standard scale ranging from 30 to 370 l/min. The patient took three deep breaths with the device attached to their face. The examiner instructed the patient to inhale deeply using only the nostrils and keep the mouth occluded. The measurements were observed, and if any were taken with the mouth open, the measurement was discarded and repeated after a 30-s pause. Only the largest valid measurements were considered for statistical analysis [21].
Otorhinolaryngological examination and videoendoscopy of the nasal cavity, performed with a 3.2 mm Machida® nasofibroscope, without vasoconstrictors to evaluate the septum deviation, which was divided according to Cottle's classification, and the hypertrophy of inferior nasal turbinates, quantified according to the percentage occupied in the nasal fossa, into grade I 25%, grade II 50%, and grade III 75% or more of the nasal fossa [22]. Obstruction was considered above grade III in both classifications.
Anthropometric measurements of sex, age, height, and weight.
Nasal Cytokines
Nasal brushing was performed in both nostrils with a sterile cervical brush. After collection, the material was homogenized by stirring and centrifuged at 4 °C (3000 rpm/5 min). The supernatant samples were stored at − 80 °C until cytokine measurement. At the end of the collections, the measurement of the substances interferon gamma, (INF-γ) TNF-ᾳ, and interleukins (IL-4, IL-5, IL-6, and IL-10) was performed using the ELISA technique with the commercial "kit" Invtrongen by Thermo Fisher Scientific®. The plates were developed with the addition of the enzymatic substrate tetramethylbenzidine, and the optical density (OD) was determined in a Multiskan SKy spectrophotometer reader (Thermo Fisher Scientific-Waltham) using 450 nm filters. The results obtained were normalized by the total number of proteins, which were quantified by the Bradford method [23].
Statistical Analysis
JAMOVI software (version 2.3 year 2022) was used. The Shapiro‒Wilk test was utilized to assess data normality, and the Levene test was utilized to assess homogeneity of variance. For the comparison between the two independent groups, the Mann‒Whitney test was applied. For dependent samples, the Wilcoxon test was applied. To evaluate possible correlations, Spearman's nonparametric test was applied. When comparing categorical data, Fisher's exact test was used. Data were examined in the 95% confidence range, and the p value was accepted as significant if less than 0.05.
Results
Characteristics of the Study Participants
Of the 45 individuals initially included, one patient in the normal weight group and three in the obese group were lost to follow-up.
The normal weight group presented a median age of 27 years, weight of 56.15 kg, height of 165 cm, and median BMI of 21.20 kg/m2, and 77% were female.
The obese group presented a median age of 38 years, weight of 99.00 kg, height of 165 cm, and BMI of 34.30 kg/m2, and 84% were female.
There was no significant difference between the sexes of the participants (p = 0.839). There was an age difference between the groups (p = 0.036), and the obese group had one patient outliner who was 65 years old.
Results of the Prick Test
The prevalent mites in this sample were Dermatophagoides pteronissinus and Dermatophagoides farinae in both groups. The size of the prick test responses in centimeters was higher for blomia and fungi mix in obese patients, while the other allergens tested showed similar responses between groups (Table 1).
Table 1.
Prick test response to tested allergens between groups
| Prick test | Normal weight N (%) |
Obese N (%) |
Normal weight response in cm Median (minimum–maximum) |
Obese response in cm Median (minimum–maximum) |
p |
|---|---|---|---|---|---|
| N (%) d. pteronyssinus | 22 (100%) | 17 (89%) | 5 (3 -13) | 5 (3–10) | 0.905 |
| N (%) fungi mix | 0 | 2 (22%) | 5 (0–10) | 0 (0–3) | 0.027* |
| N (%) dog | 5 (22%) | 10 (53%) | 0 (0–0) | 2 (0–4) | 0.212 |
| N (%) cat | 5 (22%) | 3(16%) | 0 (0–5) | 0 (0–5) | 0.600 |
| N (%) blomia | 10 (45%) | 19 (100%) | 4 (0–10) | 5 (3–10) | 0.035* |
| N (%) d. farinae | 11 (50%) | 16 (84%) | 3 (0–11) | 5 (0–8) | 0.152 |
N number of patients (percentage)
Questionnaires Applied
Improvements in quality of life were observed in both groups (VAS, NOSE-5, SNOT-22) after using nasal corticosteroids, as shown in Table 2.
Table 2.
Effect of NC treatment on quality of life in eutrophic and obese groups
| Questionnaire | Pre-NC (n = 22) normal weight |
Post-NC (n = 22) normal weight |
p | Pre-NC (n = 19) obese |
Post-NC (n = 19) obese |
p |
|---|---|---|---|---|---|---|
| Median (minimum–maximum) | ||||||
| VAS | 89.5 (43–145) | 23.5 (6–100) | < .001 | 80 (35 -112) | 19 (0–92) | < .001 |
| NOSE | 24 (12–38) | 10 (0–32) | < .001 | 22 (6–40) | 8 (0–28) | < .001 |
| SNOT-22 | 42 (17–88) | 21 (1–72) | < .001 | 49 (22–70) | 23 (3 68) | 0 .001 |
When comparing the results of the questionnaires and PNIF values before and after the treatment between the groups, divided by weight, no significant differences were observed between the groups (Table 3).
Table 3.
Comparison of quality of life questionnaires between pre- and posttreatment groups with NC
| Questionnaires | Pre-NC (n = 22) normal weight | Pre-NC (n = 19) obese |
p | Post-NC (n = 22) normal weight | Post-NC (n = 19) obese |
p |
|---|---|---|---|---|---|---|
| Median (minimum–maximum) | ||||||
| VAS |
89.50 (43–145) |
80.00 (35–112) |
0.094 |
23.50 (6–100) |
19.00 (0–92) |
0.418 |
| NOSE |
24.00 (12–38) |
22.00 (6–40) |
0.501 |
10.00 (0–32) |
8.00 (0–28) |
0.812 |
| SNOT-22 |
42.00 (17–88) |
49.00 (22–70) |
0.434 |
21.00 (1–72) |
23.00 (3–68) |
0.316 |
*Statistically significant; NC nasal corticosteroid
Peak Nasal Inspiratory Flow and Physical Examination
With respect to peak nasal inspiratory flow, both groups presented statistical improvement after treatment (eutrophic p = 0.04 and obese p = 0.02), and there was no significant difference when comparing each moment between groups (first visit p = 0.384, second visit p = 0.145). After treatment, nasal endoscopy showed a decrease in inferior turbinates in normal weight patients (p = 0.033) but not in obese patients (p = 0.160).
Effects of Nasal Corticosteroids on Nasal Secretion Cytokines
There was no difference in the production of cytokines after treatment with NC in the obese group, whereas in the eutrophic group, INF-γ and IL-5 showed an increase after treatment with NC (Table 4).
Table 4.
Comparison of cytokine levels in the nasal mucosa before and after treatment with NC in the eutrophic and obese groups
| Cytokine levels (mg/ml) | Pre-NC (n = 22) normal weight | Post-NC (n = 22) normal weight | p | Pre-NC (n = 19) obese | Post-NC (n = 19) obese | p |
|---|---|---|---|---|---|---|
| Median (minimum–maximum) | ||||||
| IL-4 |
10.99 (6.51–16.03 |
11.62 (7.95–33.60) |
0.068 |
12.35 (5.89–27.54) |
11.86 (7.95–19.49) |
0.809 |
| IL-5 |
36.44 (24.74–111.68) |
48.08 (28.65–109.40) |
0.001* |
39.92 (22.68–65.78) |
39.99 (19.45–125.50) | 0.190 |
| IL-6 |
16.57 (12.90- 31.91) |
17.81 (12.58- 79.70) | 0.166 |
18.59 (12.44–32.62) |
20.05 (7.38–126.04) |
0.164 |
| IL-10 |
20.58 (13.55–57.35) |
22.49 (13.77–46.14) |
0.517 | 22.03 (12.99–68.18) |
21.24 (9.80–282.73) |
0.605 |
| TNF-ᾳ |
36.47 (27.57–65.17) |
44.63 (29.19–81.56) |
0.096 |
39.81 (22.52–66.40) |
41.73 (13.31–104.15) |
0.418 |
| INF-γ |
63.04 (47.67–111.30) |
76.35 (50.81–146.86) |
0.049* |
66.01 (41.42–128.87) |
77.99 (35.34–164.48) |
0.313 |
NC nasal corticosteroid, IL interleukins, IFN interferon, TNF necrosis factor
No significant differences were observed in cytokines in the comparison between groups at baseline and after treatment (Table 5).
Table 5.
Comparisons of cytokine concentrations during each phase between groups
| Cytokine levels (mg/ml) | Pre-NC (n = 22) normal weight | Pre-NC (n = 19) obese | p | Post-NC (n = 22) normal weight | Post-NC (n = 19) obese | p |
|---|---|---|---|---|---|---|
| Median (minimum–maximum) | ||||||
| IL-4 |
10.99 (6.51–16.03 |
12.35 (5.89–27.54) | 0.301 | 11.62 (7.95–33.60) | 11.86 (7.95–19.49) | 0.436 |
| IL-5 | 36.44 (24.74–111.68) | 39.92 (22.68–65.78) | 0.794 | 48.08 (28.65–109.40) | 39.99 (19.45–125.50) | 0.741 |
| IL-6 | 16.57 (12.90- 31.91) |
18.59 (12.44–32.62) |
0.366 | 17.81 (12.58- 79.70) |
20.05 (7.38–126.04) |
0.414 |
| IL-10 |
20.58 (13.55–57.35) |
22.03 (12.99–68.18) | 0.173 |
22.49 (13.77–46.14) |
21.24 (9.80–282.73) |
0.477 |
| TNF-ᾳ |
36.47 (27.57–65.17) |
39.81 (22.52–66.40) |
0.350 |
44.63 (29.19–81.56) |
41.73 (13.31–104.15) |
0.794 |
| INF-γ |
63.04 (47.67–111.30) |
66.01 (41.42–128.87) |
0.918 | 76.35 (50.81–146.86) |
77.99 (35.34–164.48) |
0.772 |
NC nasal corticosteroid, IL interleukins, INF interferon, TNF tumor necrosis factor
Comparisons of cytokine variation (post- vs. pretreatment) between groups showed a difference in IL-10 (Table 6 and Fig. 1).
Table 6.
Comparison between groups of variation in cytokine levels in the nasal mucosa after treatment with corticosteroids
| Variation (Δ) in cytokine levels | Normal weight (n = 22) Median (minimum–maximum) |
Obese (n = 19) Median (minimum–maximum) |
p |
|---|---|---|---|
| Δ IL-4 | 1.29 (− 4.77–20.08) | 0.98 (− 18.84– + 7.65) | 0.371 |
| Δ IL-5 | 10.38 (− 9.09–43.71) | 3.24 (− 19.12–97.69) | 0.270 |
| Δ IL-6 | 2.30 (− 8.54–59.57) | 1.17 (− 9.29–93.42) | 0.846 |
| Δ IL-10 | 2.06 (− 40.77–17.90) | − 3.49 (− 32.92–214.55) | 0.020* |
| Δ TNF-ᾳ | 5.19 (− 18.78–25.58) | 1.70 (− 27.25–37.75) | 0.658 |
| Δ INF-γ | 7.29 (− 31.29–63.28) | 5.90 (− 39.76–46.30) | 0.558 |
*Statistically significant; BMI body mass index, NC nasal corticosteroid, IL interleukins, INF interferon, TNF tumor necrosis factor, Δ: change in interleukin levels (posttreatment minus pretreatment)
Fig. 1.
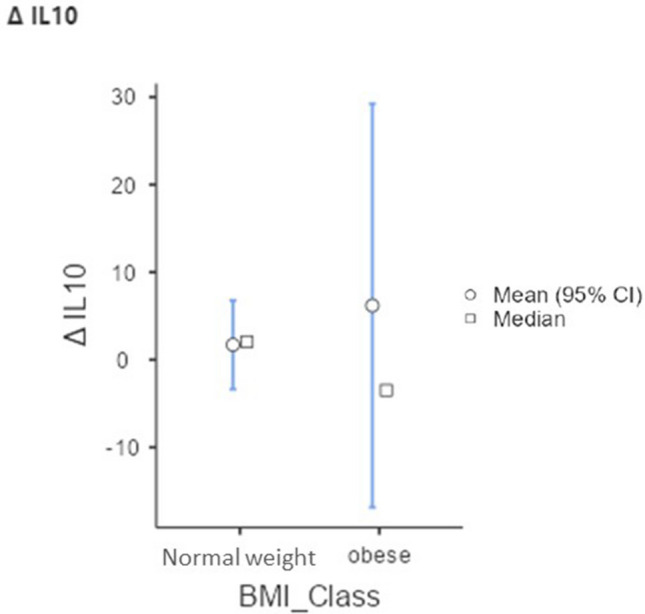
Interleukin 10 (IL-10) variation between the eutrophic and obese groups
Spearman's correlation showed no relationship between BMI and peak inspiratory flow or cytokines evaluated in this study.
Discussion
Allergic rhinitis (AR) represents a global health problem (25), as does obesity (8). Evidence that obesity and AR are related to an inflammatory state may justify a possible association [9–11].
Cytokines produced by Th2 cells (IL-4, IL-5, and IL-13) may be essential for the pathophysiology of allergic disorders, including the production of IgE, the recruitment and activation of mast cells and eosinophils, mucus hypersecretion, subepithelial fibrosis, and tissue remodeling (26), which are all closely associated with the pathophysiology of AR. In addition, previous studies have shown an association between BMI and AR [24, 25].
The three most common mites identified as sensitizers in Brazilian studies are Dermatophagoides pteronyssinus, Dermatophagoides farinae, and Blomia tropicalis [2]. Our study is in agreement with these results, with 95% of participants showing sensitivity to D. pteronyssinus, 70.7% to blomia, and 65.8% to D. farinae. In the study by Soares et al., D. pteronyssinus was the mite most associated with positivity in the prick test (20) in a population whose BMI was not taken into consideration.
Yang et al. conducted a study with the aim of evaluating the association between skin reactivity to histamine and BMI (27), showing that high skin reactivity to histamine was associated with a high BMI [26]. Although our study showed no statistically significant difference between the histamine tests, there was a difference between the response to blomia and fungi mix, with greater erythema in obese patients. Wang et al. concluded that overweight children with allergic asthma had stronger responses to the histamine prick test than normal weight individuals [4, 6].
The mechanism that determines greater erythema in obese patients is still unclear in the literature. It is possibly related to mast cells that are generally involved in both allergic reactions and the inflammatory process of obesity [26]. Therefore, there is a possibility that the association between the response to histamine and BMI may be mediated by the greater number and/or activity of mast cells due to obesity. Another hypothesis is based on the greater blood flow in the skin of obese patients, which could increase histamine reactivity [6].
In the PFIN, there was no significant difference between the studied groups. Both groups, after treatment with nasal corticosteroids, showed an increase in PFIN values (28), demonstrating that obesity apparently does not interfere with the physiology of nasal flow, similar to previous evidence [21, 27].
In the current study, we observed no differences in the evaluated quality of life questions regarding nasal symptoms between obese and eutrophic patients with AR, and regardless of BMI, patients showed improvement in all data evaluated after treatment, demonstrating that obesity does not interfere with the perception of nasal symptoms and that intranasal corticosteroids are effective for the treatment of allergic rhinitis [28–30].
Despite being a subjective test, there were no significant differences in the reduction of edema of the inferior nasal turbinates in normal weight participants after treatment with nasal NC; however, this finding was not reflected in the inspiratory flow or in the symptoms of the obese group [31]. Inflammatory cytokines and chemokines, such as IL-6 and TNF-ᾳ, are secreted by visceral fat into the blood and play a role in inducing a proinflammatory state and systemic inflammation associated with abdominal obesity [32, 33]. Obesity is associated with polarization from macrophage type 2 (M2) to macrophage type 1 (M1) as well as a shift from Th2 to Th1 cells (31). This results in a state of chronic inflammation, with increased production of inflammatory mediators, such as INF-γ, IL6, and TNF-ᾳ [32]. It would be interesting to study the effects of the interaction between the inflammatory process found in the mucosa of individuals with AR (Th2-driven disease) and the systemic inflammatory process (Th1-driven disease) of obesity. However, in this study, when assessing the nasal cytokines before treatment, there were no differences between the groups, contrary to the findings of Salem and Bhattacharyya [13, 32].
Interestingly, normal weight patients showed an increase in IL-5 and INF-γ after clinical treatment with NC, but when comparing the variation in these cytokines (Δ) between groups, no differences were observed, with Δ being a more representative variable for response differences between groups after treatment.
IL-10 was the only cytokine that showed a difference in Δ after clinical treatment between the groups studied, with the obese patients having a negative variation, unlike the normal weight patients. Considering that this cytokine is closely related to the regulatory action of the inflammatory process, it is suggested that obese people have less ability to downregulate the nasal inflammatory process.
In the current study, there was an age difference between groups, but this statistically significant difference does not represent a clinical difference, since the average of the eutrophic group was 27 years and that of the obese group was 38 years, and probably the average of the obese group was higher because of the elderly outlier.
It is important to point out that there was a similar response to nasal NC treatment for nasal symptoms, nasal flow, and the production of most of the nasal cytokines studied in the obese and normal weight groups. This finding is supported by the fact that a difference in cavity area is not expected between obese and eutrophic subjects, thus maintaining the drug concentration distributed in the mucosa. In systemic treatment, however, a lower drug concentration per body area is expected in obese patients when the same drug concentration is used in the treatment, with a higher systemic dose required to achieve a similar therapeutic response to normal weight patients.
Conclusion
The results of the current study suggested no differences in nasal physiology, symptom severity, and nasal inflammatory processes between obese and normal weight individuals, showing that both responded similarly to treatment with nasal corticosteroids in terms of improvement in flow and nasal symptoms. Obese participants presented with a possible impaired anti-inflammatory response during treatment with nasal corticosteroids, but it had no clinical impact.
Funding
This study did not earn any specific grant from funding agencies in the public, commercial or not-for-profit sectors, the author used personal funding.
Declarations
Conflict of interest
None.
Ethical Approval
Ethics Approval was obtained from the ethics committee of Plataforma Brasil from the Universidade São Paulo and from the ethics committee of the university hospital of Universidade São Paulo - UNIFESP.
Informed Consent to Participate
Written informed consent was obtained of the study participant.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Savouré M, Bousquet J, Jaakkola JJK et al (2022) Worldwide prevalence of rhinitis in adults: a review of definitions and temporal evolution. Clin Transl Allergy 12 [DOI] [PMC free article] [PubMed]
- 2.de Rubini NPM, Wandalsen GF, Rizzo MCV, et al. Guia prático sobre controle ambiental para pacientes com rinite alérgica. Arquivos de Asma, Alergia e Imunologia. 2017 doi: 10.5935/2526-5393.20170004. [DOI] [Google Scholar]
- 3.Hellings PW, Fokkens WJ, Bachert C, et al. Positioning the principles of precision medicine in care pathways for allergic rhinitis and chronic rhinosinusitis—A EUFOREA-ARIA-EPOS-AIRWAYS ICP statement. Allergy Eur J Allergy Clin Immunol. 2017;72:1297–1305. doi: 10.1111/all.13162. [DOI] [PubMed] [Google Scholar]
- 4.Kang JW, Lee KH, Hong SC, et al. Histamine skin reactivity increases with body mass index in Korean children. Int J Pediatr Otorhinolaryngol. 2015;79:111–114. doi: 10.1016/j.ijporl.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Lei Y, Yang H, Zhen L. Obesity is a risk factor for allergic rhinitis in children of Wuhan (China) Asia Pac Allergy. 2016;6:101–104. doi: 10.5415/apallergy.2016.6.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Huang Y, Zhang X-L, et al. An analysis of skin prick test reactivity to dust mite in overweight and normal weight children with allergic asthma before and after specific immunotherapy. Zhongguo Dang Dai Er Ke Za Zhi. 2016;18:329–334. doi: 10.7499/j.issn.1008-8830.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Ferreira APS, Szwarcwald CL, Damacena GN. Prevalência e fatores associados da obesidade na população brasileira: estudo com dados aferidos da Pesquisa Nacional de Saúde, 2013. Rev Bras Epidemiol. 2019;22:e190024. doi: 10.1590/1980-549720190024. [DOI] [PubMed] [Google Scholar]
- 8.Stirbulov R (2007) Editorial Repercussões respiratórias da obesidade Respiratory repercussions of obesity [DOI] [PubMed]
- 9.Zhou J, Luo F, Han Y, et al. Obesity/overweight and risk of allergic rhinitis: a meta-analysis of observational studies. Allergy Eur J Allergy Clin Immunol. 2020;75:1272–1275. doi: 10.1111/all.14143. [DOI] [PubMed] [Google Scholar]
- 10.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boulet LP. Obesity and atopy. Clin Exp Allergy. 2015;45:75–86. doi: 10.1111/cea.12435. [DOI] [PubMed] [Google Scholar]
- 12.Steele TO, Mace JC, Deconde AS, et al. Does comorbid obesity impact quality of life outcomes in patients undergoing endoscopic sinus surgery? Int Forum Allergy Rhinol. 2015;5:1085–1094. doi: 10.1002/alr.21599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharyya N. Associations between obesity and inflammatory sinonasal disorders. Laryngoscope. 2013;123:1840–1844. doi: 10.1002/lary.24019. [DOI] [PubMed] [Google Scholar]
- 14.da Silva TM, Guimarães RES, Nascimento E, et al. Análise de citocinas pela RT-PCR em pacientes com rinite alérgica. Rev Bras Otorrinolaringol. 2009;75:24–29. doi: 10.1590/S0034-72992009000100004. [DOI] [Google Scholar]
- 15.Hewagalamulage SD, Lee TK, Clarke IJ, Henry BA. Stress, cortisol, and obesity: a role for cortisol responsiveness in identifying individuals prone to obesity. Domest Anim Endocrinol. 2016;56:S112–S120. doi: 10.1016/j.domaniend.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Caminha GP, De Melo JT, Hopkins C, et al. SNOT-22: psychometric properties and cross-cultural adaptation into the portuguese language spoken in Brazil. Braz J Otorhinolaryngol. 2012;78:34–39. doi: 10.5935/1808-8694.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marambaia PP, Lima MG, Santos KP, et al. Evaluation of the quality of life of patients with chronic rhinosinusitis by means of the SNOT-22 questionnaire. Braz J Otorhinolaryngol. 2013;79:54–58. doi: 10.5935/1808-8694.20130010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins C, Gillett S, Slack R, et al. Psychometric validity of the 22-item Sinonasal outcome test. Clin Otolaryngol. 2009;34:447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 19.Stewart MG, Witsell DL, Smith TL, et al. Development and validation of the nasal obstruction symptom evaluation (NOSE) scale1. Otolaryngol-Head Neck Surg. 2004;130:157–163. doi: 10.1016/j.otohns.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Ubiratan R, Teixeira F, Monteiro Zappelini CE et al., Artigo Original Correlação Entre Peak Flow Nasal Inspiratório e Escala Visual Analógica Pré e Pós Uso de Vasoconstrictor Nasal Peak Flow Inspiratory Nasal and Analogical Visual Scale’s Correlation, Pre and Pos Nasal Vasoconstrictive Nasal Usage
- 21.Starling-Schwanz R, Peake HL, Salome CM, et al. Repeatability of peak nasal inspiratory flow measurements and utility for assessing the severity of rhinitis. Allergy Eur J Allergy Clin Immunol. 2005;60:795–800. doi: 10.1111/j.1398-9995.2005.00779.x. [DOI] [PubMed] [Google Scholar]
- 22.MH C. Rhino-sphyngomanometry: an aid in physical diagnosis. Int Rhinol. 1968;6:7–26. [Google Scholar]
- 23.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 24.Harugop AS, Walia A, Havaldar RR, Mudhol RS. Correlation between allergic rhinitis and body mass index: an observational study. Indian J Otolaryngol Head Neck Surg. 2022;74:1033–1036. doi: 10.1007/s12070-020-02095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han YY, Forno E, Gogna M, Celedón JC. Obesity and rhinitis in a nationwide study of children and adults in the United States. J Allergy Clin Immunol. 2016;137:1460–1465. doi: 10.1016/j.jaci.2015.12.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Gu M. Association of body mass index and season with histamine skin reactivity in Chinese children with allergic rhinitis. Pediatr Neonatol. 2019;60:172–177. doi: 10.1016/j.pedneo.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Demir MG, Ylmaz HB. The relation between body mass index and nasal airflow. J Craniofac Surg. 2015;26:e295–e297. doi: 10.1097/SCS.0000000000001613. [DOI] [PubMed] [Google Scholar]
- 28.Meltzer EO, Jacobs RL, LaForce CF, et al. Safety and efficacy of once-daily treatment with beclomethasone dipropionate nasal aerosol in subjects with perennial allergic rhinitis. Allergy Asthma Proc. 2012;33:249–257. doi: 10.2500/aap.2012.33.3571. [DOI] [PubMed] [Google Scholar]
- 29.Rizzi A, Parrinello G, De Corso E, et al. Mometasone furoate in non-allergic rhinitis: a real-life Italian study. J Pers Med. 2022 doi: 10.3390/jpm12071179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vlaykov AN. Application of SNOT-22 test and visual analogue scale in quality of life evaluation in patients with allergic rhinitis. Folia Med (Plovdiv) 2021;63:337–347. doi: 10.3897/folmed.63.e55256. [DOI] [PubMed] [Google Scholar]
- 31.Moffa A, Giorgi L, Carnuccio L, et al. Comparison of intranasal steroid application using nasal spray and spray-sol to treat allergic rhinitis: a preliminary investigation. J Clin Med. 2023 doi: 10.3390/jcm12103492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salem AM. Th1/Th2 cytokines profile in overweight/obese young adults and their correlation with airways inflammation. J Taibah Univ Med Sci. 2022;17:38–44. doi: 10.1016/j.jtumed.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borges MD, Franca EL, Fujimori M, et al. Relationship between proinflammatory cytokines/chemokines and adipokines in serum of young adults with obesity. Endocr Metab Immune Disord Drug Targets. 2018;18:260–267. doi: 10.2174/1871530318666180131094733. [DOI] [PubMed] [Google Scholar]


