Abstract
SARS COV 2 infection affects primarily the respiratory system and various organs in humans is responsible for higher mortality secondary to cytokine storm during the COVID-19 pandemic. It affects the internal auditory system is responsible for Sensory neural hearing loss in adults as well as children born to COVID-19 infected mothers. This study was aimed to detect the pattern of hearing loss in COVID-19 infected adults and pattern of hearing loss in children born to gestational COVID-19 infected mothers. Fifty asymptomatic RT-PCR COVID-19 infected adults and age, sex matched healthy controls were evaluated for audiological profile using Pure Tone Audiometry (PTA). Children born to COVID-19 infected mothers were tested using Transient product otoacoustic emissions and click-evoked auditory brainstem responses (ABRs) compared with children born to non-COVID mothers. PTA auditory profile of COVID-19 infected adults on day 7 had 30% (16 out of 50) significant high frequency sensory neural hearing impairment for 4000 Hz (p value 0.003), 6000 Hz (p value 0.001), 8000 Hz (p value 0.001). Repeat PTA testing on day 30 showed normal hearing. Whereas in children, 40% (n = 20) born to COVID-19 infected mothers had OAE as “Refer”. BERA (ABRs) testing of that OAE "Refer" children revealed 30% (n = 6) hearing impairment. COVID-19 infection cause transient type of high frequency sensory neural hearing loss in adults. Whereas in children born to COVID-19 infected mothers there is risk of developing permanent, progressive or long-standing transient type of sensory neural hearing loss.
Keywords: SARS COVID-19, Gestational COVID-19, Deafness, Hearing loss
Introduction
The COVID-19 infection caused significant impact of gustatory and auditory systems in addition to respiratory complaints. The affected individuals experienced wide range of symptoms ranging from loss of smell, taste, headache, vertigo, tinnitus and also asymptomatic hearing impairment, at an early stage of infection [1, 2]. The symptomatology appears 2–14 days after the incubation period. The symptoms are mostly mild to moderate severity. The sufferers may recover without any sequelae with immunomodulation following COVID-19 vaccinations. Although rare, COVID-19 infection also known to cause sudden onset of severe neurological complications like Bell’s palsy, encephalitis, ischemic stroke and intracerebral haemorrhage. These neurologic, thromboembolic complications, were identified only at the time of performing autopsies [3, 4]. It has been observed that even severe complications have been reported following COVID vaccination like, Guillain-Barré syndrome, thrombosis with thrombocytopenia syndrome, and myocarditis after COVID-19 vaccinations have been reported [5]. There are many case reports on sensory neural hearing loss in COVID-19 infected adults and children born to COVID-19 infected mothers. The exact mechanism is very poorly understood as the mode of transmission is entirely different.
Among adults the spread of infection from nasal mucosa affects the olfactory nerve, cross the blood brain barrier affect the brain, inner ear and brain stem. Acute otitis media caused by COVID-19 infections can affect the inner ear. As in other viral infections, it may either directly damage the inner ear structures, hair cells, organ of Corti or indirectly it may affect the inner ear, brain stem through host immune mediated mechanisms during a cytokine storm in severe cases [6]. Sensorineural hearing loss (SNHL) pattern is common in COVID-19 infections, which is mostly bilateral, but often unilateral in nature. There are sporadic reports of conductive as well as mixed type of hearing loss have also been reported. The scenario of COVID-19 infection in pregnant mothers is different. COVID-19 virus can affect many organs, by binding to the ACE2 receptor present in cochlea, cochlear nerve and central nervous system [6, 7].
Antenatal mothers exposed to ToRCHES CLAP group of infections during pregnancy may develop deafness of variable degree of severity (Table 1). They can significantly affect the growing foetus. More severe complications occur during first trimester, as they may affect organogenesis [8]. They pregnancy outcome will be variable according to the gestational period. Clinically less severe cases manifest with mild deafness and neurological complications like epilepsy and mental retardation [9, 10]. Very severe cases results in death of newborn. Similarly, the COVID-19 infected mothers have reported complications such as premature rupture of membranes, preterm labour, foetal distress, and foetal loss during the antenatal period. SNHL has been observed in approximately 15% of their children, indicating the possibility of vertical transmission and variable impact. Limited Indian studies and case reports have focused on SNHL among adults and newborns of COVID-19 infected mothers, often detected accidentally during routine screenings. These studies have concluded that the pattern of hearing loss was mostly transient sensory neural type of hearing loss or inconclusive. Furthermore, studies are lacking, to compare the hearing pattern among COVID-19 infected adults and children and also children born to COVID-19 infected mothers [11, 12].
Table 1.
Pattern and severity of sensory neural hearing loss in relation to period of gestation among "ToRCHES CLAP" group of infections during pregnancy
| S. no | Type of infection | Pattern of hearing loss | Gestational period |
|---|---|---|---|
| 1 | Toxoplasmosis | SNHL | 3rd trimester |
| 2 | Rubella | Progressive SNHL | 1st trimester |
| 3 | Cytomegalo virus | Progressive permanent SNHL | 1st trimester |
| 4 | Hepatitis-B virus | Permanent SNHL | Parturition |
| 5 | HSV 1and 2 virus | Permanent SNHL | 3rd trimester |
| 6 | Enterovirus | Sudden severe SNHL | 2nd and 3rd trimester |
| 7 | Syphilis | Progressive irreversible SNHL | Parturition |
| 8 | Chicken pox | Permanent sudden SNHL | Parturition |
| 9 | Lyme disease | Progressive SNHL | 1st trimester |
| 10 | AIDS | Progressive sudden SNHL | Parturition |
| 11 | Parvovirus | SNHL | 1st and 2nd trimester |
Given the persisting threat of the COVID-19 pandemic, including the emergence of new variants with varying severity, the clinical outcome and prognosis may vary. There are occasional clusters of new cases of COVID-19 despite vaccination efforts, among adults become a real threat. This comparative analysis aims to unravel the mechanisms involved in pattern of hearing loss among asymptomatic infected individuals. The findings of this study could aid clinicians in preventing permanent deafness. While addressing psychosocial developmental abnormalities develop later during childhood, all children of asymptomatic COVID-19 infected mothers should undergo periodical screening of hearing function, since birth. According to National Health Mission, newborn hearing screening using OAE were done routinely for all the children born in our hospital and it became a more practical, cost-effective measure, need to be established in a large way and timely intervention by otolaryngologists will play a major role, in bringing down the prevalence of deafness, globally. By shedding light on these mechanisms, we hope to mitigate the long-term impact of COVID-19 on auditory health and overall well-being.
Aim
To study audiological profile in COVID-19- RT PCR positive adult patients and children born to COVID-19 infected mothers, in comparison with age, sex matched controls.
Objectives
To assess the hearing threshold pattern in adults using pure tone audiometry (PTA) among asymptomatic COVID-19 infected study and control group.
To assess the hearing function pattern in children using Otoacoustic Emissions (OAE) and confirmed by Brainstem Evoked Response Audiometry (BERA) among both the study and control group.
To detect the impact of maternal COVID-19 infection in relation to period of gestation among infected mothers and the children born to them.
Methods
The study group consisted of 50 asymptomatic COVID-19 RT-PCR positive adults (Group A) and 50 children born to COVID-19 infected mothers (Group B) with healthy age and sex matched controls were subjected for testing the audiological profile. Adults with the age ranged between 20 and 50 years were included to avoid any age-related hearing affection. All patients were admitted in Govt Cuddalore medical college after obtaining the informed consent to undergo testing based on inclusion and exclusion criteria for both adults and children. Detailed history taking and otological examination were carried out on all subjects before audiological testing and audiometric thresholds were measured using clinical audiometer.
Study Design
Prospective non interventional case control study.
Source of Data
Study to be conducted among the COVID-19 PCR positive in-patients and children born to COVID-19 infected mothers delivered at Government Cuddalore medical college, Chidambaram for the period of 1 year.
Study Period
April 2020 to March 2021for one year period.
Study Population
A total number of fifty asymptomatic COVID-19 RT-PCR positive adults (Group A) and 50 COVID-19 unaffected adults as controls (Group A1). Fifty children born to asymptomatic COVID-19 infected mothers (Group B) and 50 children (Group B1) born to non-COVID mothers with negative RT PCR for COVID-19.
Inclusion Criteria for Study Group
Asymptomatic COVID-19 positive adults confirmed by RT-PCR test within the age of 20–50 years to avoid any age-related hearing affection.
Fifty children born to gestational COVID-19 positive mothers confirmed by RT-PCR test.
Exclusion Criteria in the Study Group
Adults with diabetes, hypertension, inheritable deafness, previous ear diseases, prolonged exposure to loud noise, taken ototoxic drugs and those who failed to come for repeat testing or regular follow up visit and unwilling patients.
Children born with cranio-facial anomalies, pre term babies, birth weight less than 2.5 kg, neonatal jaundice and received blood transfusion. Children born to consanguineous parents, HIV virus infected mothers and those exposed to ToRCHES CLAP group of infection namely Toxoplasmosis, Rubella, Cytomegalo virus, Hepatitis-B virus, Herpes Simplex Virus 1 and 2, Enterovirus, Syphilis, Chicken Pox, Lyme Disease and Parvovirus during pregnancy.
Method of Study
After obtaining clearance from Institutional ethical committee, the study was started in department of ENT, Government Cuddalore Medical College and Hospital. Fifty asymptomatic COVID-19 infected adults (Group A) and 50 children delivered to COVID-19 infected mothers (group B) with appropriate age and sex matched healthy controls adults (Group A1) and children (Group B1) respectively, were enrolled in this study. We have enrolled the children of control group born to COVID-19 unaffected mothers, as per the guidelines of National health Mission, we have screened all the children born in our hospital. A detailed audiological assessment was done for all adult patient with COVID-19 infection on day 7 and day 30.
In addition, those with COVID-19 infected pregnant mothers were enquired about COVID-19 severity status in relation to the period of gestation, and complications like abortions, pre-mature rupture of membranes, foetal distress, emergency cesarean section were obtained. Thorough audiological, otoscopic examination were carried out.
All the COVID-19 infected adult patients of both study and control group were subjected to Pure tone audiometry (PTA), on day 7 and day 30 of infection. Air conduction thresholds were recorded using telephonic TDH39 from 250 to 8000 Hz and the bone conduction thresholds were analyzed using a radio ear B71 bone vibrator for the frequencies between 250 and 4000 Hz. The audiometric thresholds were measured using the modified Hughson-Westlake method and all testing was carried out in a double walled, sound treated room within permissible noise limits. Wilcoxon signed rank test used to analyse the statistical result.
Evaluation of children for hearing function can be obtained by screening them with Otoacoustic Emissions (OAE). Children with result as ‘refer’ would be further screened by Brainstem Evoked Response Audiometry (BERA) to get final result. Otoacoustic Emissions testing can provide detailed information about the status of cochlear and hair cell functions. Brainstem Evoked Response Audiometry (BERA) on the other hand, it allows us to evaluate the normal functioning in the neural pathway involved in the auditory system. For BERA, a Recorder and Medicare System (RMS) Electromyograph EP Mark 2 were used.
Results
Pattern of hearing among COVID-19 infected adults and control group adults:
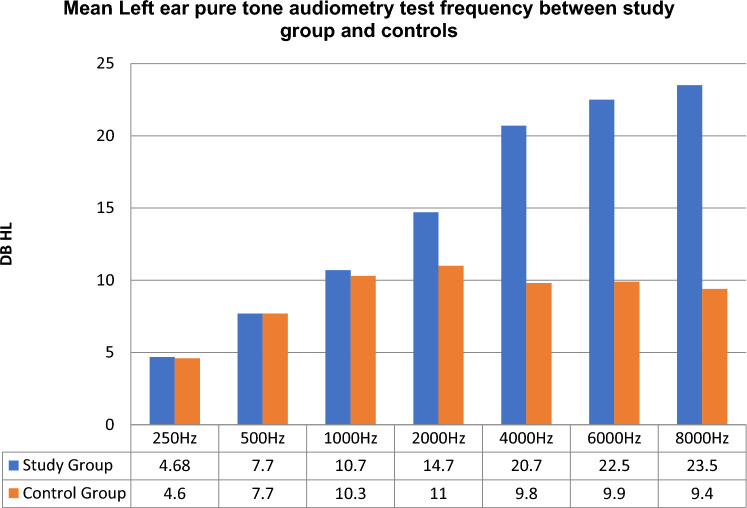
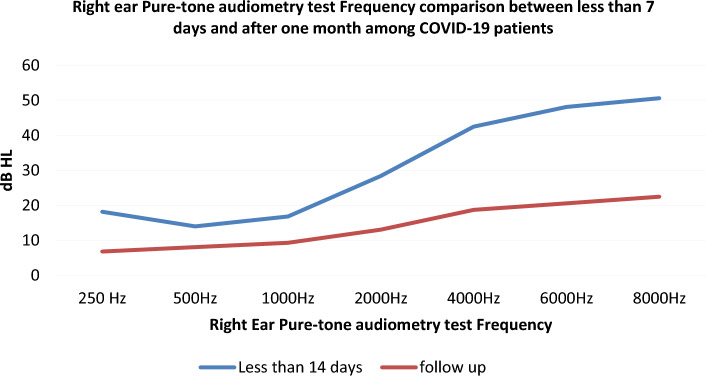
In our study conducted on asymptomatic COVID-19 positive adults, 16 out of 50 (32%) adults in test group (Group A) had significant SNHL at frequencies of 4000 Hz (p = 0.003), 6000 Hz (p = 0.001), 8000 Hz (p = 0.001). This pattern signifies that the infected adults had high frequency sensory neural pattern of hearing loss on initial screening. While repeat assessment of hearing function using PTA, on day 30 for all those initial hearing impaired COVID-19 infected adults (n = 16; 32%), revealed that all of them had a significant improvement on hearing. The hearing pattern become normal (Figs. 1, 2, 3, 4). The normal pattern of hearing pattern after failing at initial screening using PTA, especially among those adults infected with COVID-19 during acute initial stage. This indicate that 32% of COVID-19 infected adults had a of significant sensory neural hearing loss (SNHL) at higher frequencies, during the acute and active stage of the disease. Hence it can be considered as transient high frequency SNHL pattern in COVID-19 infected adults, which may gradually improve over time. The possibility of immune modulation following infection, especially those adults who received COVID vaccination, well in advance. While all 50 control group adults (Group A1) had normal hearing at all frequencies both initial as well as at repeat screening using PTA after 30 days.
Fig. 1.
Audiological evaluation of adults infected with SARS COVID-19 using Pure tone audiometry test
Fig. 2.
Pure tone audiometry results of left ear among COVID-19 infected adults (study group) and controls
Fig. 3.
Pure tone audiometry results of Right ear among COVID-19 infected adults (study group) and controls
Fig. 4.
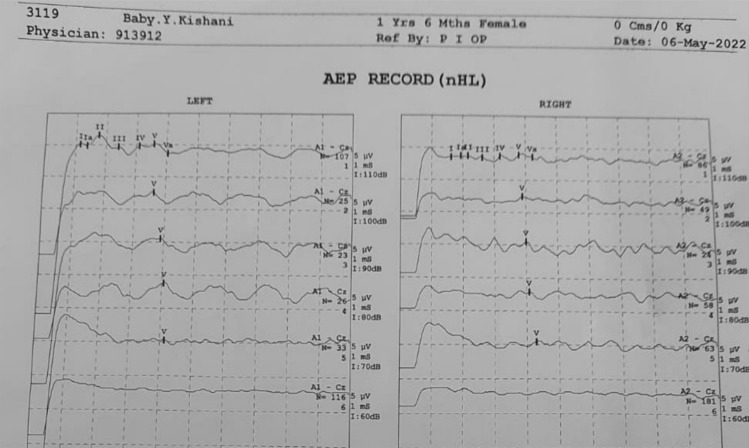
Audiological evaluation OAE in children born to COVID-19 infected mothers during pregnancy
Pattern of hearing among children born to COVID-19 infected mothers and control group children:
All mothers suffering from COVID-19 infection were asymptomatic or had mild course of illness. Out of 50 mothers 35 (70%) acquired COVID-19 during first trimester, 9 (18%) had last trimester, 6 (12%) mothers had COVID-19 in mid trimester. The antenatal period was uneventful. The growth and development was normal in all children in our study group. The hearing function was assessed initially with OAE testing, which was carried out in 50 children. Majority of children were in the age group of 3 months to 1 1/2 years.
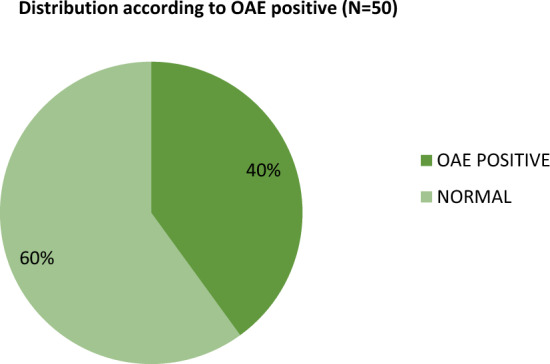
Hearing assessment of 50 children born to COVID-19 infected gestational mothers, nearly 40% children (n = 20) had OAE-1 "refer" (Fig. 5; Table 2). Whereas in the control group 6% children born to COVID-19 unaffected mothers had OAE-1 "refer" (n = 3). The Participants with abnormality was significantly more in the study group than in the control group with p value of less than 0.05.
Fig. 5.

Bar chart showing distribution according to the results of first screening of the both ears with OAE-1
Table 2.
Distribution according to the results of first screening of both ears with OAE-1
| OAE-1 results | Children of COVID-19 infected mothers (n = 50) | Control (n = 50) | X2 | p value | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Refer | 20 | 40 | 3 | 6 | 31.06 | 0.001 |
| Pass | 30 | 60 | 47 | 94 | ||
Among the participants in the study group with abnormality in both right and left ears during the first screening OAE-1, all 100% tested "refer" during the second screening (OAE-2) while in the control group, none were reported as abnormal. The proportion was significantly higher in the study group than in the control group (Fig. 6; Table 2).
Fig. 6.

Bar chart showing distribution according to the results of second screening of the both ears with OAE-2
BERA test was carried out in all the 20 babies with OAE -1 and OAE-2 “refer” (Fig. 7; Table 3). Out of 20 babies nearly 30% (n = 6) had mild, moderate and severe SNHL (Figs. 8, 9, 5). Among the participants in the children of COVID-19 infected mothers’ group, 5% (n = 1) had severe SNHL, 10% (n = 2) had moderate and 15% (n = 3) mild SNHL, and 70% (n = 14) had normal hearing (Table 4). The mothers one severely affected child and two moderately affected children, were tested positive during first trimester. In the control group, BERA testing of all the participants were normal. The occurrence of SNHL was significantly higher in the study group than in the experimental group with P value of less than 0.05.
Fig. 7.

Bar chart showing distribution according to the results of BERA between the groups
Table 3.
Distribution according to the results of second screening of the both ears with OAE-2
| OAE-2 results | Children of COVID-19 infected mothers (n = 20) | Control (n = 3) | X2 | p value | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Refer | 20 | 100 | 0 | 0 | 22 | 0.001 |
| Pass | 0 | 0 | 3 | 100 | ||
Fig. 8.
OAE results of a child born to COVID-19 infected mother
Fig. 9.

Distribution of BERA positivity among and OAE positive children in the study group analysing children born to COVID-19 infected mothers
Table 4.
Distribution according to the results of BERA between the groups
| Hearing impairment According to BERA | Children of COVID-19 infected mothers (n = 20) | Control (n = 50) | X2 | p value | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Normal | 10 | 70 | 50 | 100 | 42.15 | 0.001 |
| Mild | 3 | 15 | 0 | 0 | ||
| Moderate | 2 | 10 | 0 | 0 | ||
| Severe | 1 | 5 | 0 | 0 | ||
Discussion
Our study aimed to investigate the relationship between COVID-19 infection and its impact on the auditory system in both adults and children born to infected mothers, with appropriate age, sex matched controls. The SARS-CoV-2 virus, known for its neurotrophic properties, has been found to invade neural tissues, including the auditory system, leading to SNHL. In our study, we observed various symptoms related to the gustatory and auditory systems in adults during the early stages of COVID-19 infection, such as anosmia, hyposmia, dysgeusia and hearing loss, but had mid symptomatology. Since we have excluded very sick symptomatic cases, seriously ill, hospitalized patients, and to analyze the hearing pattern among asymptomatic cases, we did not come across, sudden onset of SNHL, severe head ache or tinnitus cases clinically. Interestingly, our findings diverged from previous studies, as we did not observe a significant difference in hearing patterns across different age groups. None of our patients had comorbidities such as diabetes or hypertension. This suggests that the impact of COVID-19 on hearing may not be solely dependent on age or pre-existing medical conditions.
Sensory neural hearing loss (SNHL) was the most common pattern of hearing impairment observed in both COVID-19 adults and children born to COVID-19 infected mothers. While some cases presented with severe sudden-onset SNHL, our study focused on milder cases. In this present study, nearly 32% of patients had sensory neural hearing impairment specific higher frequencies, such as 4000 Hz, 6000 Hz, and 8000 Hz, were significantly affected during the early infectious stage of COVID-19 infection. However, on subsequent testing with PTA, we observed a complete recovery of hearing in all previously affected adult patients, suggesting the transient high frequency SNHL was common among adults. Various audiological profile assessment studies have reported the incidental detection of SNHL during routine evaluations of adults and children, suggesting the involvement of the audio-vestibular system in COVID-19 infections, regardless of clinical severity. In our study also the SNHL was detected incidentally.
The exact mechanism of hearing loss in COVID-19 infection remains unclear. Proposed theories include direct infection of the middle ear, crossing of the blood–brain barrier to affect the inner ear, auditory nerve, and brainstem, as well as indirect immune-mediated damage to the outer hair cells of the cochlea [12].
In the case of children vertical transmission of SARS-CoV-2 from infected mothers has been reported, with implications for the auditory system. Our study found that children born to COVID-19-infected mothers exhibited impaired cochlear and auditory brainstem function. This suggests a potential deficit in the medial olivocochlear efferent system in these infants. Although some studies have reported no significant impact on newborn hearing due to maternal SARS-CoV-2 infection, others have observed auditory abnormalities in children born to asymptomatic but infected mothers. The reversible nature of hearing loss observed in our study may be attributed to transient inflammatory changes affecting the organ of Corti and outer hair cells. It is also possible that immune recovery plays a role, especially in vaccinated patients [13].
The impact of hearing on children born to COVID-19 infected mothers differs from its impact on adult hearing. While none of the COVID-19 infected mothers, who were enrolled in the adult study group have experienced hearing loss, but their children exhibited varying degrees of hearing impairment. Initially 40% of children in the study group (B) had both OAE-1 and OAE-2 "refer", while further subjecting all the OAE "refer" cases to BERA testing, nearly 30% (n = 6) had mild moderate and severe SNHL. This highlights the risk of permanent SNHL among children of mothers with COVID-19 infection during the gestational period. Whereas in the control group (Group B1), children born to unaffected mothers, undergone initial OAE-1 screening had "refer" in 6% of cases. While repeating all those three children had normal hearing and had "pass". Early intervention is crucial in preventing hearing loss and related complications in these children.
Our findings highlight the transient but severe impact on cochlear function in adults causing transient high frequency SNHL in COVID-19 infected adults. Whereas in children born to COVID-19 infected mothers, the impact on auditory brainstem causing "permanent sensory neural hearing loss" and also development and maturation will be significantly affected [14]. Further research is warranted to understand the underlying mechanisms and long-term consequences of COVID-19-related hearing loss, in relation to emerging.
Conclusion
COVID-19 can SNHL in both adults and children. Infected adults develop less severe transient high frequency SNHL than children. This transient nature could be attributed to the widespread usage of vaccination that has led to herd immunity, which has reduced the severity of the disease and mortality related to COVID-19. Although vaccine breakthrough infections can occur, the virulence of the virus is decreased when acquiring infection from a vaccinated person than others. In contrast, children of mothers who had gestational COVID-19 may be at risk of developing permanent, progressive, as well as long-standing transient pattern of SNHL than children born to unaffected mothers. It may be due to the high impact of COVID-19 infection on the functioning of the auditory brainstem. There are emerging risk for impaired quality of life and psychosocial development, when they grow older. Early evaluation of hearing function and intervention, as well as timely vaccination, are crucial in preventing future complications.
Funding
Not received any financial assistance.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Approval was obtained from the ethics committee of our university.
Informed consent
Informed consent was obtained from all individual included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Balaji Swaminathan, Email: dryesbee@gmail.com.
Jhansirani Vidyasagar, Email: drjhansient@gmail.com.
References
- 1.Jafari Z, Kolb BE, Mohajerani MH. Hearing loss, tinnitus, and dizziness in COVID-19: a systematic review and meta-analysis. Can J Neurol Sci. 2022;49(2):184–195. doi: 10.1017/cjn.2021.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lechien JR, Chiesa-Estomba CM, De Siati DR. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L, Jin H, Wang M. Neurologic manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeong J, Choi HS. Sudden sensorineural hearing loss after COVID-19 vaccination. Int J Infect Dis. 2021;113:341–343. doi: 10.1016/j.ijid.2021.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celik T, Simsek A, Koca CF, Aydin S, Yasar S. Evaluation of cochlear functions in infants exposed to SARS-CoV-2 intrauterine. Am J Otolaryngol. 2021;42:102982. doi: 10.1016/j.amjoto.2021.102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alan MA, Alan C. Hearing screening outcomes in neonates of SARS-CoV-2 positive pregnant women. Int J Pediatr Otorhinolaryngol. 2021;146:110754. doi: 10.1016/j.ijporl.2021.110754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hon KL, Leung KKY, Leung AKC, Man E, Ip P. Congenital infections in Hong Kong: beyond TORCH. Hong Kong Med J. 2020;26(4):318–322. doi: 10.12809/hkmj208398. [DOI] [PubMed] [Google Scholar]
- 9.Adler SP. Congenital cytomegalovirus screening. Pediatr Infect Dis J. 2005;24(12):1105–1106. doi: 10.1097/00006454-200512000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Veeranna SA, Youngblood PL, Bradshaw L, Marx CG. COVID-19 during pregnancy and its impact on the developing auditory system. Am J Otolaryngol. 2022;43:103484. doi: 10.1016/j.amjoto.2022.103484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buonsenso D, Costa S, Giordano L, Priolo F, Colonna AT, Morini S, et al. Short- and mid-term multidisciplinary outcomes of newborns exposed to SARS-CoV-2 in utero or during the perinatal period: preliminary findings. Eur J Pediatr. 2022;181:1507–1520. doi: 10.1007/s00431-021-04319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mostafa BE, Mostafa A, Fiky LME, Omara A, Teaima A. Maternal COVID-19 and neonatal hearing loss: a multicentric survey. Eur Arch Otorhinolaryngol. 2021;279:3435–3438. doi: 10.1007/s00405-021-07098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oskovi-Kaplan ZA, Ozgu-Erdinc AS, Buyuk GN, Sert-Dinc UY, Ali-Algan C, Demir B, et al. Newborn hearing screening results of infants born to mothers who had COVID-19 disease during pregnancy: a retrospective cohort study. Ear Hear. 2022;43:41–44. doi: 10.1097/AUD.0000000000001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raposo D, Orfão J, Menezes M, Trindade-Soares M, Guimarães A, Freire F. Auditory brainstem response in preterm infants in the neonatal intensive care unit. Otolaryngol Head Neck Surg. 2021;164(4):884–888. doi: 10.1177/0194599820955181. [DOI] [PubMed] [Google Scholar]