Abstract
Human T-cell lymphotropic virus type 1 (HTLV-1) transcriptional activation is mediated by the viral transactivator, Tax, and three 21-bp repeats (Tax response element [TxRE]) located in the U3 region of the viral long terminal repeat (LTR). Each TxRE contains a core cyclic AMP response element (CRE) flanked by 5′ G-rich and 3′ C-rich sequences. The TxRE binds CREB (CRE-binding protein) and Tax to form a ternary complex and confers Tax-dependent transactivation. Recent data indicate that Tax functions as a specific link to connect CREB-binding protein (CBP)/p300 in a phosphorylation-independent manner to CREB/ATF-1 assembled on the viral 21-bp repeats. Glutathione S-transferase pull-down performed with Tax deletion mutants and peptide competition have localized the site in Tax critical for binding CBP/p300 to a highly protease-sensitive region around amino acid residues 81 to 95 (81QRTSKTLKVLTPPIT95) which lies between the domains previously proposed to be important for CREB binding and Tax subunit dimerization. Amino acid residues around the trypsin- and chymotrypsin-sensitive sites (88KVL90) of Tax bear resemblance to those in the kinase-inducible domain of CREB (129SRRPSYRKILNE140) surrounding Ser-133, which undergoes signal-induced phosphorylation to recruit CBP/p300. Site-directed mutagenesis of residues in this domain (R82A, K85A, K88A, and V89A) resulted in proteins which failed to transactivate from the HTLV-1 LTR in vivo. These mutants (K85A, K88A, and V89A) bind CREB with similar affinities as wild-type Tax, yet interaction with CBP/p300 is abrogated in various biochemical assays, indicating that the recruitment of CBP/p300 is crucial for Tax transactivation. A Tax mutant, M47, defective in the COOH-terminal transactivation domain, continued to interact with CBP/p300, suggesting that interactions with additional cellular factors are required for proper Tax function.
Human T-cell lymphotropic virus type 1 (HTLV-1) is the causative agent of adult T-cell leukemia (40, 55) and a neurodegenerative disorder called tropical spastic paraparesis/HTLV-1-associated myelopathy (19, 37). HTLV-1 replication is dependent on the viral transactivator, Tax, and three 21-bp repeat elements, collectively referred to as the Tax response element (TxRE), localized in the U3 region of the proviral long terminal repeat (LTR). Each 21-bp repeat contains a core cyclic AMP (cAMP) response element (CRE) flanked by 5′ G-rich and 3′ C-rich sequences that confer Tax-dependent transactivation and dictate the assembly of Tax, CREB (CRE-binding protein), and the 21-bp repeat into a ternary (3°) complex (38, 46, 53, 56, 57). Tax interacts with CREB via the latter’s basic domain and amino acid residues in its immediate vicinity (2, 39, 54). A cellular homolog of CREB, ATF-1, can also interact with Tax in the form of a CREB/ATF-1 heterodimer or as an ATF-1 homodimer (11, 44, 57). Due to subtle amino acid sequence differences in the ATF-1 basic domain, Tax binds ATF-1 with reduced affinity (2, 57). Like CREB, Tax functions as a dimer (47). Analyses of Tax mutants have suggested that the NH2-terminal domain of Tax is important for CREB bZip (basic domain-leucine zipper) binding, while the midsection and the COOH terminus of Tax are important for subunit dimerization and possibly interaction with basal transcription factors, respectively (1, 23, 42, 47). Several groups have also reported that Tax enhances the dimerization and DNA binding of bZip proteins in vitro (3, 7, 12, 39, 50, 52).
Transcriptional activation via the TxRE, CREB (and/or ATF-1), and Tax is independent of cellular signaling processes. Irrespective of the cell types used, cotransfection of a Tax expression plasmid and a reporter containing the TxRE results in 30- to 100-fold transcriptional induction. This contrasts with cellular somatostatin, proenkephalin, and phosphoenolpyruvate carboxyl kinase genes, whose expression is under CRE control and becomes induced only when the cAMP signaling pathway is activated. Work from many laboratories has indicated that an elevation in the intracellular levels of cAMP, as a consequence of cell surface signaling events, results in activation of protein kinase A, which can phosphorylate a sequence-specific DNA-binding protein, CREB, at amino acid residue Ser-133 (21, 22). Phospho-CREB subsequently recruits a transcriptional coactivator, CBP (CREB-binding protein) (16, 31, 35), or its homolog, p300 (4, 5), to CRE-containing enhancers to augment mRNA transcription (16, 31, 35).
CBP and p300 appear to serve as integrators of numerous cellular signaling processes (26). The massive sizes of CBP and p300 allow them to interact with many cellular transcription factors in a signal-dependent and sometimes mutually exclusive fashion. To date, steroid and retinoid hormone receptors, CREB, c-Jun, c-Myb, Sap-1a, c-Fos, MyoD, p53, Stat-1/2, NF-κB, pp90rsk, TATA-binding protein, and TFIIB have been found to interact with CBP and p300 (see reference 26 for a review). This list will probably continue to grow. The oncoproteins of two DNA tumor viruses, adenovirus E1A and simian virus 40 large T antigen, also target and affect CBP/p300 functions (4, 5, 8). P/CAF (p300/CBP-associated factor), a histone acetyltransferase (HAT) that acetylates histones H3 and H4 but not H2A or H2B, has also been shown to bind to the CBP/p300 domain where E1A binds (51). Finally, in addition to their proposed roles as adaptor or coactivator molecules for transcription, CBP and p300 possess intrinsic HAT activity for all four core histones (9, 36). For this reason, they are suggested to play an important role in changing the nucleosomal structure at or around certain promoters (9, 36). The HAT activities of CBP/p300 and P/CAF presumably release promoter sequences from the repressive effect of the nucleosome and render them accessible to basal transcription factors and RNA polymerase II for transcriptional initiation.
Using fluorescence anisotropy techniques, Kowk et al. have recently shown that HTLV-1 Tax directly binds CBP and p300 (30), results which have been confirmed as well as extended (20). These observations, combined with previous reports that Tax, CREB bZip, and TxRE specifically form a 3° complex, suggests that both CBP and p300 can be recruited to CREB/TxRE in a manner that bypasses signal-induced CREB phosphorylation by interacting with Tax. Thus, via the action of Tax, viral transcription can proceed constitutively without extracellular stimuli. The protein domain in CBP that is important for binding Tax has been localized to amino acid residues 451 to 682 (30). Interestingly, this domain has also been found to be important for binding phosphorylated CREB, c-Jun, c-Myb, and Sap-1a (6, 10, 17, 27, 30).
In this study, we show by electrophoretic mobility shift assay (EMSA) that both the full-length p300 and residues 451 to 682 of CBP (CBP451-682) form stable quaternary (4°) complexes with Tax, CREB bZip, and the HTLV-1 21-bp repeat in vitro. The presence of CBP451-682 or p300 greatly facilitated the formation of a stable, high-order protein-DNA complex on the HTLV-1 21-bp repeat. The 4° complex readily forms on the HTLV-1 21-bp repeat but not on a CRE with nonspecific flanking sequences. While ATF-1 bZip could participate in 4° complex assembly, ATF-2 and ATF-3 failed to do so. Analyses of a series of Tax deletion mutants by glutathione S-transferase (GST) pull-down assays and EMSA, coupled with peptide competition, further revealed Tax amino acid residues 81 to 95 (81QRTSKTLKVLTPPIT95) to be important for CBP/p300 binding. This region lies between the domains for binding CREB bZip and for Tax subunit dimerization (1, 47). Three amino acid residues (88KVL90) in this region constitute sites that are rapidly cleaved by trypsin and chymotrypsin, suggesting that they are highly exposed and accessible to protein-protein interactions. We further report a series of point mutations targeted to this domain in Tax, which result in impairments in LTR transactivation and generate proteins that are defective in the ability to interact with CBP/p300. These data provide direct in vivo evidence in support of the role of CBP/p300 in Tax-mediated transactivation. Importantly, mutations that inactivate the COOH-terminal transactivation domain of Tax did not significantly alter the incorporation of CBP and p300 in vitro, suggesting that 4° complex formation alone might not be sufficient for transcriptional activation. Interactions with additional cellular factors via the COOH-terminal transactivation domain of Tax are likely to be important for efficient LTR-driven gene expression.
MATERIALS AND METHODS
Protein expression and purification.
Expression constructs for wild-type and mutant Tax proteins have been described previously (1, 47, 56, 57). Escherichia coli BL21(DE3) cells transformed with the Tax expression plasmids pET11-TaxH6, pET-K85A, pET-K88A, pET-V89A, pET-L90A, M22 (T130A L131S), and M47 (L319R L320S) were each grown at 37°C in 2 liters of Terrific broth medium containing ampicillin (100 μg/ml) to an A600 of 1.0 to 1.5. Tax expression was then induced with 40 μM IPTG (isopropyl-β-d-thiogalactopyranoside) at room temperature overnight. Cells were harvested and resuspended in 20 ml of 50 mM phosphate-buffered saline (pH 8.0) containing 0.3 M NaCl, 0.25 mM phenylmethylsulfonyl fluoride, 0.5 mM β-mercaptoethanol, and 10 mM imidazole. The cells were ruptured by sonication over an ice-salt bath, using a Branson Sonifier mounted with a microtip. Sonication (four pulses of 1 min each) was carried out at 70% duty cycle. After centrifugation in a Sorvall SS-34 rotor at 16,000 rpm for 50 min at 4°C, the supernatant was mixed with 5 ml of Ni-nitrilotriacetic acid-agarose (Qiagen, Düsseldorf, Germany) at 4°C for at least 2 h. The protein-bound gel matrix was then packed into a column (1.5 by 10 cm) and washed with 40 ml of the same buffer containing 40 mM imidazole. Tax was then eluted with a 60-ml gradient of 80 to 300 mM imidazole. Each fraction was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 12.5 polyacrylamide gel and immunoblotting with an anti-HTLV-1 Tax antibody generated against the C-terminal region of Tax (TaxC-Ab [25]). Fractions containing Tax were dialyzed against buffer D (20 mM HEPES [pH 7.9], 150 mM KCl, 0.2 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol, 20% glycerol) and stored frozen at −80°C. Protein concentrations were determined by the Bio-Rad protein assay, using bovine serum albumin as a standard.
The various GST-bZip constructs have been described elsewhere (2). The GST-CBP451-682 protein, containing the Tax-binding domain (residues 451 to 682) of human CBP, was derived from a GST construct kindly provided by R. H. Goodman (Vollum Institute, Portland, Oreg.) (30). GST fusion proteins were prepared from IPTG-induced E. coli DH5α harboring one of the expression constructs. Cells were lysed by sonication as described above. Bacterial lysates were incubated with glutathione-Sepharose 4B (Pharmacia, Inc.) at 4°C with gentle agitation for 30 min. The protein-bound Sepharose was washed repeatedly, and GST fusion proteins were eluted in reduced glutathione buffer and dialyzed overnight against buffer D at 4°C. Proteins were analyzed by SDS-PAGE (12.5% gel), and fractions were stored at −80°C. Preparation of baculovirus-expressed human p300 will be described elsewhere (49a).
EMSA.
A 46-bp BglII-NcoI fragment containing the promoter-proximal copy of the HTLV-1 21-bp repeat was labeled with [α-32P]dATP by using Klenow enzyme and purified by electrophoresis on a 7.5% polyacrylamide gel. Protein-DNA binding reactions were carried out as described previously (47, 56, 57). The reaction mixtures (typically 8 μl) were electrophoresed on a 5.5% nondenaturing polyacrylamide gel (30:1 acrylamide to bisacrylamide, 18 by 14 cm) in Tris-glycine-EDTA buffer (25 mM Tris, 192 mM glycine, 1 mM EDTA [pH 8.5]) at 200 V and 4°C for 2 to 3 h. The gel was then dried on a piece of Whatman filter paper and autoradiographed. To label the DNA probe that contains the CRE motif with nonspecific flanking sequences, 12.5 pg of the annealed oligonucleotides, 5′-CTAGGATCTTGATGACGCAATACGCCATGGTCGA (where underlining denotes the CRE sequence) and 5′-TTTTCGACCATGGCGTATTGCGTCATCAAGATCCTAG, was incubated with 2.5 U of Klenow enzyme and 2 mM each dC, dT, and dGTP in the presence of 10 μCi of [α-32P]dATP (DuPont/NEN, Inc.). Labeling reaction mixtures were incubated for 30 min at 37°C. The DNA fragment was electrophoresed, resolved in a 7.5% Tris-borate-EDTA gel, and eluted in sterile deionized H2O at 37°C. Three Tax-derived peptides, 76-PSFPTQRTSKTLKVLTPPIT-95, 91-TPPITHTTPNIPPSFLQAMR-110, and 316-PISLLFNEKEADDNDHEPQI-335, were used to compete against the full-length Tax protein for CBP binding.
Derivation of Tax truncation mutants.
All deletion constructs were generated by PCR using Vent polymerase. The DNA sequences of these mutants were confirmed by sequence analyses and subcloned into the pET11d vector via the NcoI and BamHI restriction endonuclease cleavage sites for expression. For the COOH-terminal deletions, a common upstream PCR primer, 5′-CCATGGCCCACTTCCCAGGGTTTGGA, was used. The sequences of the downstream primers are as follows: for Tax1-324, 5′-GGATCCTTATTAATGGTGGTGATGGTGGTGTTTTTCGTTAAAAAGTAGAGA; for Tax1-319, 5′-GGATCCTTATTAATGGTGGTGATGGTGGTGAGAAATGGGGATGTTGGTGTA; and for Tax1-309, 5′-GGATCCTTATTAATGGTGGTGATGGTGGTGAAACAGGAGATGTAAACTATG. The Tax1-244 construct is described in reference 1. Tax NH2-terminal truncations (TaxΔN81 and TaxΔN109) were similarly prepared (34).
Site-directed mutagenesis.
To target alanine substitutions to the CBP-binding domain of HTLV-1 Tax, the Tax coding region was cloned into M13mp19 via HindIII-BamHI sites. Uracil-containing single-stranded template DNA was generated in E. coli CJ236 (dut ung) cells after transfection, plaque purification, and amplification (29). Oligonucleotides containing specific point mutations were kinase treated and annealed to the M13mp19-Tax template, deoxynucleoside triphosphates were added, and replication/ligation reactions were carried out in vitro. The mutagenesis reaction mixture was transformed into E. coli DH5αF′ cells as described above, and mutant phage stocks and replicative forms of DNA were similarly prepared. Mutants were identified by the presence of an engineered Bst98I restriction endonuclease site (K85A, V89A, and L90A) and direct sequencing. Eukaryotic expression plasmids for these mutants were produced by replacing the AccI-SmaI fragment in the RcCMV-M22 Tax construct (45) with the corresponding DNA fragments from the Tax mutants. The NcoI-SmaI fragments from the M13 mutant constructs were subcloned into the pET-HTLV-1 TaxH6 construct for in vitro translation, protein expression, and purification. The following oligonucleotides were used to generate targeted mutations in HTLV-1 Tax: R82A (5′-GGTCTTAGAGGTTGCCTGGGTGGG-3′), K85A (5′-TGGCGGGGTAAGGACCTTAAGGGTCGCAGAGGTTCTCTG-3′), K88A (5′-GGGTAAGGACCGCGAGGGTCTTAG-3′), V89A (5′-GGTAAGGGCCTTAAGGGTCTT-3′), and L90 (5′-TGGCGGGGTAGCGACCTTAAGGGTCTT-3′). The primer used to sequence the Tax-derived mutations was RMT1 (5′-CCAGGTGATGGGGGGGGA-3′).
GST pull-down experiments.
The various Tax expression plasmids were purified via isopycnic banding in CsCl gradients. Individual plasmid DNA was added to a T7 polymerase-rabbit reticulocyte-based in vitro transcription-translation system (TNT system; Promega Corp.) in the presence of the protease inhibitors aprotinin, pepstatin, chymostatin, and antipain (each at 20 ng/μl; Boehringer Mannheim) in a final volume of 50 μl. Proteins were labeled with l-[35S]methionine (DuPont/NEN). Each in vitro translation reaction was carried out for 2 h at 30°C. Protein products were immediately used in GST pull-down experiments. To prepare the affinity matrices, 1 μg of bacterially derived GST-CBP451-682 or GST was preincubated with 60 μl of a 50% slurry of glutathione-Sepharose 4B (Pharmacia) in 1× binding buffer (25 mM HEPES [pH 7.9], 5 mM KCl, 0.5 mM EDTA, 1 mg of bovine serum albumin per ml, 10% glycerol, 0.25 mM dithiothreitol) for 2 to 3 h on ice. The gel matrix was then washed three times with 200 μl of 1× binding buffer. After the final wash, the glutathione-Sepharose charged with the respective GST proteins was resuspended in 30 μl of 1× binding buffer, then mixed with 10 μl of the respective in vitro translation reaction mixture, and incubated overnight at 4°C. Following overnight incubation, 300 μl of 1× binding buffer was added to each reaction and mixed by gentle agitation for 5 min at 4°C. The Sepharose matrix was pelleted by centrifugation at 500 × g for 2 min at 4°C and washed three times. Bound proteins were eluted by resuspending the Sepharose slurry in 12 μl of SDS-PAGE loading buffer and then heating it to 95°C for 2 min. Five microliters from each reaction was loaded to an SDS–12.5% polyacrylamide gel and electrophoresed. The gel was treated with a fixing solution (30% [vol/vol] methanol, 10% [vol/vol] glacial acetic acid), immersed in 25 ml of an autoradiography enhancer solution (DuPont/NEN), dried, and exposed to an X-ray film at −70°C. Bound proteins were quantified by using a Stratagene EAGLE EYE II system.
Transfections and CAT assays.
HeLa cells (2 × 105; American Type Culture Collection) were transfected by Lipofectamine (Gibco-BRL/Life Technologies, Inc.)-mediated DNA transfer in 35-mm-diameter dishes with 1 μg of an HTLV-1 LTR-chloramphenicol acetyltransferase (CAT) reporter construct and 2 μg of either the empty RcCMV vector DNA or a cytomegalovirus (CMV)-based expression vector containing the coding sequence for wild-type HTLV-1 Tax or the Tax-derived mutant CMV-R82A, CMV-K85A, CMV-K88A, CMV-V89A, or CMV-L90A. The transfection was carried out as prescribed by the manufacturer and cells were incubated at 37°C in Dulbecco modified Eagle medium (Gibco-BRL/Life Technologies) supplemented with 10% fetal bovine serum (HyClone Laboratories, Inc.), penicillin G sodium salts (100 U/ml), streptomycin sulfate (100 μg/ml), and 2 mM glutamine. Cells were harvested by scraping and pelleted by centrifugation at 1,250 rpm at 4°C for 5 min. Pellets were washed twice with 2 ml of cold phosphate-buffered saline and centrifuged again as described above. The cells were resuspended in 200 μl of 250 mM Tris-HCl (pH 7.8) and lysed by repeated freeze-thawing. Cellular debris was removed by centrifugation, and proteins were quantitated by the Bradford assay (Bio-Rad, Inc.) and assayed at 560 nm. One hundred micrograms of total cellular protein was incubated with 2 μl of acetyl coenzyme A (5 mM; Sigma Chemical Co.) and 10 μCi of [14C]chloramphenicol (NEC-408A; NEN, DuPont Research Corp.) in a total volume of 200 μl for 5 h at 37°C. The reactions were extracted with 1 ml of cold ethyl acetate (Sigma), dried under vacuum, and resuspended in 35 μl of ethyl acetate. Samples were spotted to thin-layer chromatography plates (Aldrich Chemical Co.) and developed with a 95% chloroform–5% methanol solvent. The plates were exposed to X-ray films (Kodak XAR) overnight at 25°C for autoradiography.
RESULTS
Tax, CBP/p300, and CREB bZip form a stable nucleoprotein complex on the HTLV-1 21-bp repeat.
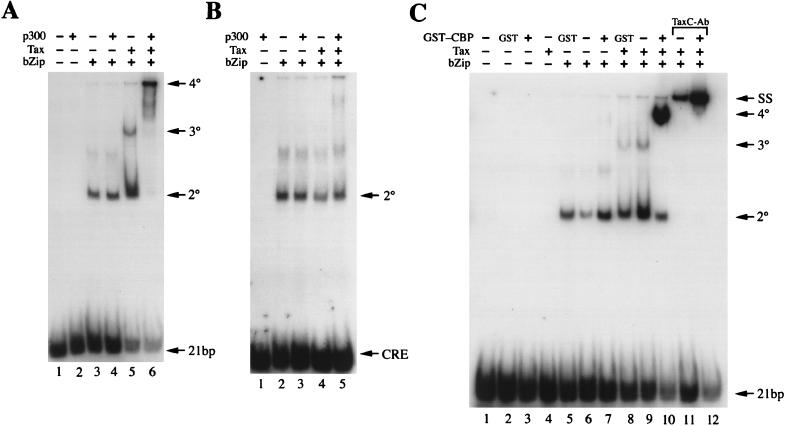
CREB binding to CRE motifs takes place irrespective of the DNA sequence context in which the CRE resides. HTLV-1 Tax, in contrast, selectively binds nucleoprotein complexes formed between CREB and the HTLV-1 21-bp repeat which contain a CRE core flanked by 5′ G-rich and 3′ C-rich sequences (38). Recent fluorescence anisotropy experiments by Kwok et al. have shown that CBP/p300 interacts with Tax and CREB on the 21-bp repeat element (30). These results prompted us to further analyze the CBP/p300-Tax interaction. Because Tax/CREB (or CREB bZip)/HTLV-1 21-bp repeat 3° complex can be readily detected by EMSA (Fig. 1A, lane 5), we similarly tested the formation of a higher-order 4° complex containing CBP (or p300), Tax, CREB bZip, and the HTLV-1 21-bp repeat. Experiments performed with full-length CREB produced data identical to those obtained with the CREB bZip domain (not shown). As expected, baculovirus-derived, full-length p300 alone did not bind the 21-bp repeat (Fig. 1A, lane 2) and had little effect on the binding of CREB bZip to the 21-bp repeat (compare lanes 3 and 4). Addition of Tax and of Tax plus p300 to the binding reactions resulted in the formation of 3° (Fig. 1A, lane 5) and 4° complexes (lane 6), respectively. In the absence of p300, the interaction between Tax and CREB bZip appeared less stable, with a portion of the 3° complex dissociating, as suggested by the trail corresponding to the CREB bZip/probe complex—giving an impression that Tax increased CREB binding to the 21-bp repeat (lane 5). In the presence of p300, however, the overall interactions between Tax and CREB bZip and between CREB bZip and the 21-bp repeat became greatly enhanced. This is evidenced by the conversion of both the binary (2°) and 3° complexes to slower-migrating 4° complexes (lane 6). The majority of the 4° complexes remained at the origin of the gel. A few faster-migrating 4° complexes also appeared; these might be due to proteolysis of p300 (compare lane 6 of Fig. 1A with lane 10 of Fig. 1C, where GST-CBP451-682 was used). In support of our earlier finding that Tax specifically interacts with the CREB/21-bp repeat complex (38), little 3° or 4° complex formation was observed for a CRE probe containing nonspecific flanking sequences, as evidenced by the abundance of unbound probe at the bottom of the gel (Fig. 1A, lanes 5 and 6; Fig. 1B, lanes 4 and 5). The slight amount of 4° complex observed on the nonspecific CRE probe suggests that Tax/CREB can weakly interact with DNA in the absence of the G/C-rich sequences and that p300, at least to some extent, stabilizes this interaction, as no 3° complex was evident on this probe. Kwok et al. have previously shown that amino acid residues 451 to 682 of CBP contain the Tax-binding region (30). Indeed, when the GST-CBP451-682 fusion protein was used in EMSA, it efficiently formed a 4° complex with Tax and CREB bZip on the HTLV-1 21-bp repeat (Fig. 1C, lane 10). The GST protein control had no effect on any of the binding assays (lanes 2, 5, and 8). A peptide antibody generated against 33 amino acid residues in the COOH terminus of Tax (TaxC-Ab [25]) effectively supershifted the 3° and 4° complexes (lanes 11 and 12), indicating the presence of Tax in both complexes. The latter result is of interest because a transactivation domain has been previously localized to the COOH terminus of Tax (1, 42). The fact that the TaxC-Ab did not disrupt 4° complex formation suggests that the CBP-binding domain in Tax might reside in a region(s) other than the transactivation domain.
FIG. 1.
(A) HTLV-1 Tax, CREB bZip, and p300 form a 4° complex with the HTLV-1 21-bp repeat but not with a CRE with nonspecific flanking sequences. Purified Tax, GST-CREB bZip, and p300 were used in EMSAs together with a DNA probe containing the HTLV-1 21-bp repeat (A) or a CRE with nonspecific neighboring sequences (B) as detailed previously (38). Each reaction typically contains 50 ng of GST-CREB bZip, 100 ng of Tax, and 50 ng of p300 in 8 μl. GST-CREB bZip/DNA probe complex (2°), the 3° complex consisting of HTLV-1 Tax, GST-CREB bZip, and the HTLV-1 21-bp repeat, and the 4° complex containing HTLV-1 Tax, GST-CREB bZip, p300, and DNA are labeled. A minor band which migrated between the 2° and 3° complexes (A, lanes 3 and 4; B, lanes 2 to 5) is due to an aggregated form of CREB bZip which possibly originated from the formation of a disulfide linkage between bZip moieties. (C) Residues 451 to 682 of CBP form a 4° complex with Tax, CREB bZip, and the HTLV-1 21-bp repeat. The amounts of proteins used in the binding reactions are the same as for panel A except that 300 ng of GST-CBP451-682 was used instead of p300. Nucleoprotein complexes are labeled as in panel A. Antiserum directed against the C-terminal 33 amino acid residues of Tax (TaxC-Ab) supershifted both the 3° and 4° complexes (SS; lanes 11 and 12). Lanes marked with GST contain the GST protein (300 ng) instead of the GST-CBP451-682 fusion protein.
M22 and M47 Tax mutants and 4° complex formation.
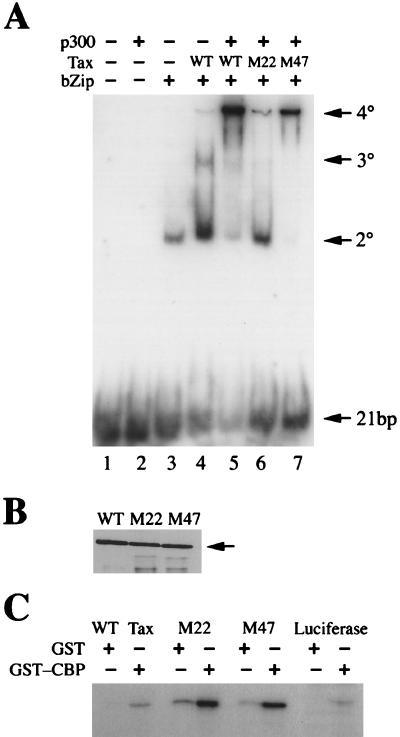
Tax mutants with distinct transactivation phenotypes have been particularly informative in defining the functional domains of Tax (41, 45). M22 (T130A L131S) is a mutant that is partially defective in dimerization (45, 47). As a result of this phenotype, M22 has reduced affinity for CREB and transactivates the HTLV-1 LTR at a level approximately 30% of that observed for wild-type Tax (45, 47). In a COOH-terminal mutant, M47 (L319R L320S), in contrast, HTLV-1 LTR transactivation is completely abrogated, but M47 retains the ability to form dimers and bind CREB (45, 47). For these reasons, we, as well as others, have suggested that the amino acid substitutions in M47 most likely inactivate the transactivation domain of Tax (1, 42). Since the efficient recruitment of cellular transcription factors plays a critical role in transactivation, the M47 mutations may impair the ability of Tax to interact with CBP or p300. Hence, comparable amounts of wild-type, M22, and M47 Tax (Fig. 2B) were analyzed in the presence of p300, CREB bZip, and the HTLV-1 21-bp repeat by EMSA. As shown in Fig. 2A, M22 yielded a low but detectable amount of 4° complex with p300 (lane 6). To our surprise, M47 retained significant capacity to form the 4° complex, albeit at a slightly reduced level compared to wild-type Tax (lanes 5 and 7). While p300 greatly increased the level of the 4° complex that contained wild-type Tax, it had little effect on M22. This result suggests that p300 (or CBP) does not complement the dimerization defect of M22. The 4° complex containing M47 Tax, although formed at an observed level considerably higher than that of the 4° complex containing M22, had no effect on transcription. While the exact biochemical defect for the M47 mutation remains to be defined, it is possible that it lies in the interaction between Tax and basal transcription factors that are recruited to the TATA box. In support of the notion that the M22 and M47 mutations do not directly affect the Tax-p300/CBP interaction, wild-type, M22, and M47 Tax were found to bind immobilized GST-CBP451-682 with comparable affinities (Fig. 2C). Overall, the apparent difference between the interaction of M22 with p300 in a 4° complex, and with the GST-CBP451-682 fusion protein in pull-down experiments, probably reflects the inefficiency with which the M22 mutant forms a 3° complex with the CREB bZip and DNA as a result of its impairment in dimerization (47). Similar defects were also observed when the GST-CBP451-682 fragment was used in EMSA with M22 (not shown). Results obtained from GST pull-down experiments, therefore, are probably more representative of the interaction between CBP and various Tax mutants. The luciferase control did not interact with GST-CBP451-682, and none of the in vitro translation products significantly interacted with GST (Fig. 2C).
FIG. 2.
(A) Quaternary complex formation of Tax mutants M22 and M47. EMSA was performed as described for Fig. 1, using 100 ng each of purified wild-type (WT) Tax, M22, or M47 in the presence or absence of p300; complexes are labeled as in Fig. 1A. (B) Immunoblot of the purified proteins used in EMSA. Proteins were detected by using rabbit TaxC-Ab and a horseradish-peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary antibody; chemiluminescence was used for development. (C) GST-CBP451-682 pull-down of l-[35S]methionine-labeled Tax and Tax mutants M22 and M47. The percentages of bound protein relative to input for wild-type Tax, M22, M47, and luciferase are 1.2, 5.7, 6, and 0.1%, respectively. GST pull-down experiments were performed as described in Materials and Methods, using luciferase as a control.
Quaternary complex formation is specific for CREB/ATF-1 and not other bZip proteins.
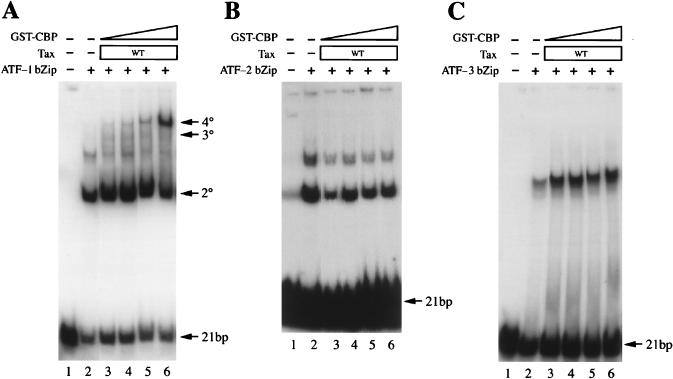
We and others have previously reported that a cellular homolog of CREB, ATF-1 (20, 24), can also interact with Tax in the form of a CREB/ATF-1 heterodimer or as an ATF-1 homodimer (11, 44, 57). Due to amino acid sequence differences (EEAAR in CREB; DDPQL in ATF-1) in the immediate NH2-terminal end of the ATF-1 basic domain, Tax binds ATF-1 with approximately 1/10 the affinity for CREB (2). As shown, ATF-1 bZip also supported 4° complex formation with Tax and the GST-CBP451-682 fusion protein on the 21-bp-repeat probe (Fig. 3A). To achieve comparable levels of 4° complex, however, a higher amount of ATF-1 bZip than of CREB bZip was required. As reported earlier, neither ATF-2 bZip nor ATF-3 bZip interacted with Tax (2, 20); likewise, neither supported 4° complex formation (Fig. 3B and C). These data further strengthen our previous proposal that highly specific protein-protein interactions between Tax and the CREB/ATF-1 subfamily of bZip factors are responsible for Tax-mediated HTLV-1 LTR transactivation (2, 57).
FIG. 3.
ATF-1 bZip (A) but not ATF-2 bZip (B) or ATF-3 bZip (C) forms a 4° complex with HTLV-1 Tax, GST-CBP451-682, and the 21-bp repeat. EMSAs were carried out with 300 ng each of the GST-bZip proteins, Tax, and a probe containing the HTLV-1 21-bp repeat in the presence of increasing amounts of GST-CBP451-682 (80, 160, 240, and 400 ng in lanes 3 to 6 in each panel). Approximately six times more GST–ATF-1 bZip than CREB-bZip is needed to produce the same level of 4° complex. A band migrating between the 2° and 3° complexes was also observed for GST–ATF-1 bZip (A), possibly due to protein aggregation as seen with CREB bZip (Fig. 1A). GST–ATF-2 bZip alone also produced multiple nucleoprotein complexes (B) which may have originated from protein degradation or aggregation. Neither Tax alone or Tax plus GST-CBP451-682 had any effects on these complexes. WT, wild type.
Amino acid residues 81 through 108 in HTLV-1 Tax are critical for the Tax-CBP/p300 interaction.
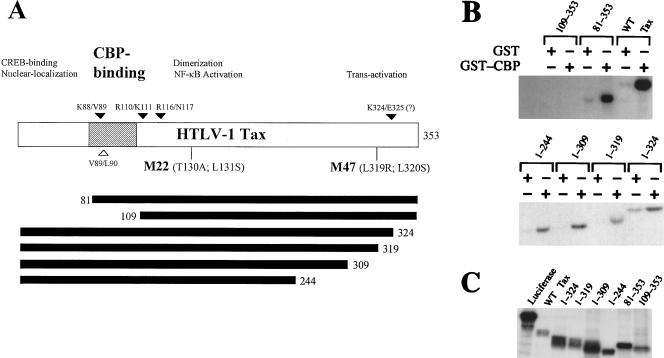
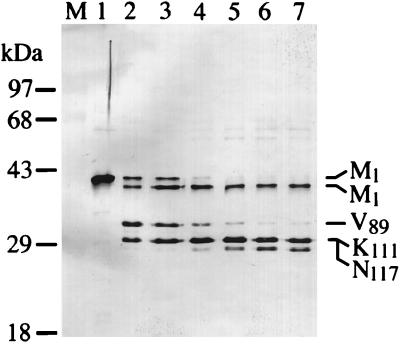
To identify the domain in Tax that is essential for CBP/p300-binding, several NH2- and COOH-terminal deletion mutants of Tax were constructed. These truncations were generated based largely on trypsin or chymotrypsin cleavage sites in Tax that were previously defined (Fig. 4A). In the diagram depicting the domain organization of Tax shown in Fig. 4A, several mutations in Tax that we and others have characterized are included as useful landmarks (1, 23, 42, 45, 47). The various truncations were cloned in the pET11d vector under the control of the bacteriophage T7 promoter. They were translated in vitro and assayed for the ability to interact with GST-CBP451-682 or GST in pull-down experiments. Comparable amounts of in vitro-synthesized proteins were used (Fig. 4C). Deletion of up to 109 amino acid residues from the COOH terminus of Tax did not affect binding to immobilized GST-CBP451-682 (Fig. 4B, lane 1-244). Likewise, deletion of 80 residues from the N terminus of Tax did not alter protein binding. Importantly, removal of 108 residues from the NH2 terminus of Tax completely abolished binding to GST-CBP451-682 (Fig. 4B, top panel), suggesting that amino acids located between positions 81 and 108 of Tax critically contribute to the Tax-CBP/p300 interaction. This region resides between the domains of Tax important for CREB binding and subunit dimerization. Treatment of purified Tax with trypsin resulted in the rapid appearance of protein fragments cleaved between residues K88 and V89, R110 and K111, and R116 and N117 and possibly between K324 and E325. Residues V89 and L90 also become rapidly cleaved by chymotrypsin. The detailed tryptic and chymotrypic map of Tax is described elsewhere (34). Moreover, the kinetics of tryptic cleavage at the K88/V89 site was significantly delayed upon CBP451-682 binding, indicative of protection, compared to digestion of Tax in the absence of CBP451-682 or in the presence of an unrelated control protein (not shown). The greater abundance of the K88/V89 product at the earliest time point of the tryptic reaction (Fig. 5, lane 2) suggests that this site and its neighboring region are highly susceptible to proteolysis.
FIG. 4.
The CBP-binding domain of Tax residues between residues 81 and 108. (A) The deletion mutants of Tax were generated based on the trypsin (solid triangles)- and chymotrypsin (open triangles)-sensitive sites in the full-length Tax molecule. The domain organization of Tax is tentatively outlined, with specific landmarks such as the protease-sensitive sites and the M22 and M47 mutations indicated. (B) Removal of residues 81 to 108 of Tax abrogates binding to GST-CBP451-682. Glutathione-Sepharose was charged either with purified GST or with GST-CBP451-682, washed, and subsequently incubated with approximately equal amounts of wild-type (WT) HTLV-1 Tax or Tax deletion mutants labeled with l-[35S]methionine by in vitro translation. Bound products were eluted and assayed by SDS-PAGE (12.5% gel) followed by fluorography and autoradiography. (C) In vitro-translated wild-type Tax and truncation mutants.
FIG. 5.
The p300/CBP-binding site of Tax is highly exposed and protease sensitive. The first amino acid residue (V89, K111, and N117) of each tryptic fragment is marked. The tryptic fragment (marked M1, like the wild-type Tax) that migrates slightly faster than the full-length Tax retains the native NH2 terminus, indicating that a short peptide has been removed from its COOH terminus. Purified HTLV-1 Tax (8 μg) in 100 μl of 0.5× buffer D (see Materials and Methods) containing 2.5 mM CaCl2 and 0.1 U of trypsin was incubated at 37°C. Twelve-microliter aliquots from the mixture were taken at 0, 1, 2, 3, 5, 10, and 20 min (lanes 1 to 7), and the tryptic reaction was terminated by the addition of 2× SDS sample buffer followed by SDS-PAGE (12% gel). Reaction products were visualized by immunoblotting using the TaxC-Ab and an alkaline phosphatase-conjugated second antibody as previously reported (34, 47).
A peptide spanning residues 76 to 95 of Tax blocks 4° complex formation in vitro.
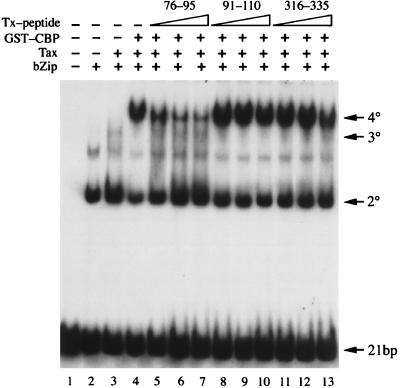
To further delineate the domain(s) in Tax that plays a significant role in binding CBP and p300, synthetic peptides spanning Tax residues 76 to 95, 91 to 110, or 316 to 335 were preincubated with GST-CBP451-682 on ice for 10 min. The mixtures were then added to the binding reactions containing Tax, CREB bZip, and the 21-bp repeat DNA to assess the effects the peptides exert over 4° complex formation. Addition of the peptide spanning residues 76 to 95 in increasing concentrations caused a progressive decrease in the levels of 4° complex observed, with an accompanying increase in the appearance of the 2° and 3° complexes (Fig. 6, lanes 5 to 8). In contrast, the peptides spanning residues 91 to 110 (lanes 9 to 12) or 316 to 335 (lanes 13 to 16) had no effect on the formation of either 3° or 4° complex. These data, in combination with results from the GST pull-down experiments, indicate that the interaction between CBP and amino acid residues located between positions 81 to 95 of Tax is principally responsible for the assembly of a stable 4° nucleoprotein complex.
FIG. 6.
A peptide spanning residues 76 to 95 of Tax blocks Tax binding to GST-CBP451-682. Increasing amounts (100, 200, and 400 ng) of each of three synthetic peptides derived from Tax sequences (residues 76 to 95, 91 to 110, and 316 to 335) were preincubated with GST-CBP451-682 for 10 min on ice prior to the addition of Tax, GST-CREB bZip, and the HTLV-1 21-bp-repeat DNA probe. The binding reactions were carried out as for Fig. 1 and analyzed by EMSA. The peptide sequences are listed in Materials and Methods. Nucleoprotein complexes are labeled on the right.
Targeted mutagenesis of residues in the CBP-binding domain of HTLV-1 Tax.
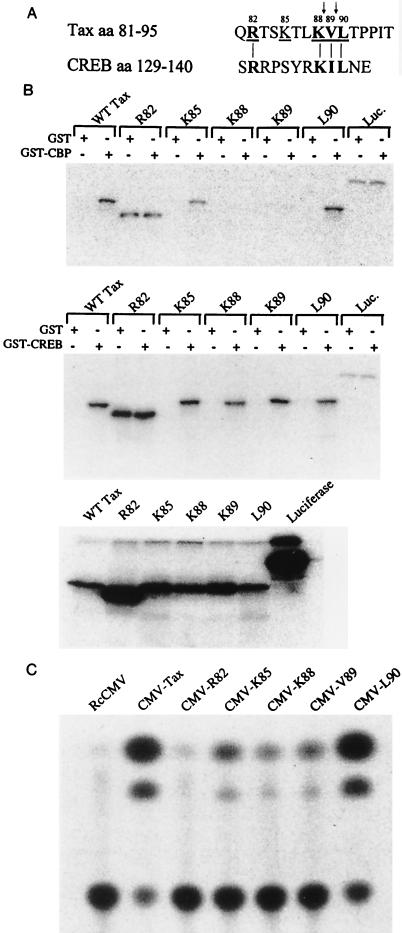
Amino acid residues located between positions 76 and 95 of HTLV-1 Tax bear significant resemblance to those surrounding the kinase-inducible, Ser-133 phosphorylation site in CREB (Fig. 7A). To address the possibility that this region of similarity plays an important role in binding CBP/p300, alanine substitutions were targeted to amino acid residues (R82, K85, K88, V89, and L90) in this region of Tax (Fig. 7A). Mutant proteins were translated and radiolabeled in vitro (Fig. 7B, lower panel) and assayed for the ability to interact with GST-CBP451-682 and GST-CREB. K88A and V89A were incapable of binding GST-CBP451-682 in pull-down experiments (Fig. 7B, top panel), while K85A was partially defective for this interaction. In contrast, L90A bound GST-CBP451-682 with similar affinity as wild-type Tax (Fig. 7B, top). The R82A mutation yielded a faster-migrating Tax species which interacted nonspecifically with either GST-CBP451-682 or GST-CREB, as well as the GST control (Fig. 7B, top and middle panels). We think that this mutation possibly alters the conformation of Tax, and therefore we omitted it from some of the studies described below. None of the mutant proteins except R82A showed any defect in the ability to bind GST-CREB (Fig. 7B, middle panel).
FIG. 7.
Mutation of the CBP-binding domain in HTLV-1 Tax affects CBP binding in vitro and LTR transactivation. (A) Amino acid (aa) sequence comparison, showing similarity between residues in the kinase-inducible domain of CREB and the CBP-binding domain of Tax. Residues in Tax targeted for Ala mutations are underlined and numbered accordingly. The trypsin- and chymotrypsin-sensitive sites are marked with arrows. (B) Top, pull-down of in vitro-translated wild-type (WT) Tax and Tax-derived mutants by purified GST-CBP451-682 or GST proteins; middle, GST-CREB or GST pull-down experiment performed as for the top panel; bottom, in vitro-translated wild-type Tax and CBP-binding domain mutants. (C) HTLV-1 LTR transactivation by wild-type Tax and Tax-derived mutants in HeLa cells. The levels of CAT activation for the CBP-binding-domain mutants relative to the wild-type expression construct are as follows: CMV-Tax, 100%; CMV-R82A, 0%; CMV-K85A, 21.47%; CMV-K88A, 5%; CMV-V89A, 8.8%; and CMV-L90A, 87.63%. Transfections and CAT assays were performed at least in triplicate with similar results. Representative data from a single experiment are shown. Conversion of [14C]chloramphenicol to acetylated forms was measured with a Stratagene EAGLE EYE II system.
Cotransfection of a CMV-based expression vector, encoding wild-type Tax or Tax-derived mutants, with an HTLV-1 LTR-CAT reporter construct permitted assessment of the LTR transactivation potential of these proteins in HeLa cells (Fig. 7C). As expected, R82A did not significantly activate transcription from the HTLV-1 LTR. K85A partially transactivated CAT gene expression, consistent with its partial defect in GST-CBP451-682 binding. The mutants K88A and V89A were impaired in activating HTLV-1 LTR-driven gene expression, whereas L90A displayed wild-type-level transactivation. All of the Tax-derived mutant proteins were efficiently expressed during transient assays in HeLa cells, and stable clones for each have been established (not shown).
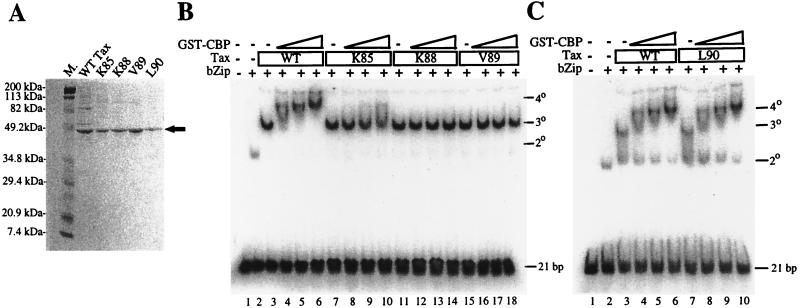
Quaternary and ternary complex formation by wild-type Tax and CBP-binding-domain mutant proteins was also examined in vitro by EMSA, using purified GST-CREB bZip, GST-CBP451-682, and a 32P-labeled DNA probe containing the promoter-proximal HTLV-1 LTR 21-bp-repeat sequence. Wild-type Tax and mutant Tax proteins (K85A, K88A, V89A, and L90A) were purified to near homogeneity for this study (Fig. 8A). All of the CBP-binding-domain mutants formed 3° complexes at levels comparable to that observed for wild-type Tax (Fig. 8B, lanes 3, 7, 11, and 15; Fig. 8C, lanes 3 and 7). As anticipated, the L90A protein showed no obvious defect in 4° complex formation (Fig. 8C; compare lanes 4 to 6 and 8 to 10), consistent with its ability to efficiently transactivate LTR-driven gene expression. K88A and V89A, however, did not significantly interact with GST-CBP451-682, as exemplified by the absence of 4° complexes (Fig. 8B, lanes 12 to 14 and 16 to 18). The K85A protein weakly interacted with GST-CBP451-682 in a 4° complex (Fig. 8B, lanes 8 to 10), in correlation with its partial impairment in transactivation in vivo.
FIG. 8.
CBP-binding-domain mutants of Tax are defective for 4° but not 3° complex formation in vitro. (A) SDS-PAGE (12% gel) analysis of purified wild-type (WT) and mutant Tax proteins (marked by an arrow). Proteins were stained with Coomassie blue. Lane M., size markers. (B) EMSA analysis of wild-type and mutant Tax proteins in the presence or absence of GST-CBP451-682. Nucleoprotein complexes are indicated on the right. (C) The L90A protein displayed no defect in its interaction with GST-CBP451-682 or GST-CREB bZip compared to wild-type Tax.
DISCUSSION
In this study, we show that p300 and CBP interact with HTLV-1 Tax and further stabilize the assembly of a multiprotein complex containing CBP or p300, Tax, and CREB on the HTLV-1 21-bp repeat. The specificity in terms of the bZip proteins (CREB and ATF-1) and the DNA elements (HTLV-1 21-bp repeat and TxRE) that are competent in 4° nucleoprotein complex assembly is a direct reflection of the specificity of the formation of the 3° Tax/CREB/TxRE complex; i.e., Tax is the principal determinant for the specificity of transactivation of the HTLV-1 LTR. These results confirm and extend the previous study by Kowk et al. in which an interaction between Tax and CBP was demonstrated by fluorescence anisotropy and are in agreement with our earlier results and those described recently for studies using similar assays (20, 30). While the inclusion of p300 and CBP into the nucleoprotein complex did not alter the specificity of the Tax-CREB and Tax-CREB-DNA interactions, it did appear to strengthen them. Based on findings from recent biochemical studies, Tax has been implicated in binding the minor groove of the G-rich and C-rich sequences flanking the CRE of the 21-bp repeats (32). It is possible that Tax contact with CREB and/or DNA becomes stabilized as a result of CBP/p300 binding. Consistent with this observation, the KIX sequence located within the Tax-binding domain of CBP has been shown to form a stable 4° complex, but it does not alter Tax-induced DNA footprints in the 5′ or 3′ flanking sequences (20, 32).
Our study indicates that the major site in Tax participating in binding CBP/p300 consists of amino acid residues 81 to 95, which lie between the domains important for CREB binding and Tax dimerization. Point mutations targeted to this sequence negatively affect HTLV-1 LTR transactivation and generate proteins that are partially or completely impaired in the ability to interact with CBP/p300. This region also contains sites that are sensitive to cleavage by trypsin (K88/V89) and chymotrypsin (V89/L90). We think that this portion of Tax is structurally exposed and might function as a flexible joint between the CREB-binding and dimerization domains. Thus, binding of CBP/p300 to this region might order the CREB-binding residues in Tax for optimal contacts with the basic domain of CREB or the G/C-rich sequences. These results indicate that protein-protein and protein-DNA interactions between CBP/p300, Tax, and CREB act to cement a highly stable nucleoprotein complex on the HTLV 21-bp repeats. In essence, Tax functions as an HTLV 21-bp-repeat-specific link between CREB bZip and CBP/p300 and allows HTLV-1 transcription to occur independent of cellular signaling pathways.
The Tax-binding domain of CBP resides in a region from amino acid residues 451 to 682 which has been shown to bind several cellular transcription factors, including CREB, c-Jun, c-Myb, and Sap-1a (see reference 26 for a review; 6, 10, 17, 27, 30, 31). At least some of the pleiotropic effects that Tax exerts on cellular gene expression, including down-regulation of p53 (48, 49) and DNA polymerase β expression (28), could possibly be mediated through a competition with the cellular sequence-specific DNA-binding proteins for a limiting intracellular pool of CBP/p300 or through a direct block of CBP binding to these factors. The amino acid sequence spanning residues 81 to 95 (81QRTSKTLKVLTPPIT95) of Tax contains multiple Ser, Thr, and basic residues (underlined). The kinase-inducible domain (KID), surrounding Ser-133 of CREB (129SRRPSYRKILNE140), also contains multiple basic and polar residues. Several amino acid residues between these two peptides are similar or identical (boldface). The sequence similarity is particularly striking over the three amino acid residues that constitute the trypsin- and chymotrypsin-sensitive sites (88KVL90) of Tax. Supporting the notion that the structure of residues 81 to 95 of Tax mimics or overlaps that of the region surrounding Ser-133 of CREB, mutagenesis targeted to the region surrounding Ser-133 of CREB has found residues 130RRPSY134 and 137IL138, containing the region of similarity to Tax, to be critical for CBP binding (43). Intriguingly, the KID-like domain of Tax contains a lysine residue in the position corresponding to Ser-133 of CREB, suggesting that both the charge and the overall structure of this domain might significantly affect its binding to the KIX domain of CBP/p300. Alternatively, Tax and CREB may bind to different regions of the KIX domain. Experiments are currently in progress to resolve this issue. It should be pointed out that all Tax proteins used in this study were bacterially derived and therefore not phosphorylated. Likewise, the GST-bZip fusion proteins contain only the basic domain-leucine zipper regions of the respective parent molecules and lack the domains for Ser/Thr phosphorylation. Others have reported that phosphorylated CREB supports 4° complex formation with Tax and the KIX domain of CBP on a 21-bp-repeat-containing probe (20, 22); whether CBP additionally contacts CREB under these conditions remains a topic of much speculation. HTLV-1 Tax mutants, defective in CBP/p300-binding, constructed during the course of this study will ultimately allow a resolution of the role of these interactions in the various biological activities of Tax, including T-cell immortalization, induction of apoptosis, and activation or suppression of cellular gene expression. Wagner and Green proposed that the mechanism of transactivation of Tax is via stimulation of dimerization of cellular bZip proteins (50). This hypothesis has not been supported by experimental evidence. Our results described in this report and in earlier papers, as well as reports from other laboratories, indicate that the mechanism of Tax transactivation is its specific binding to CREB/ATF-1 bound to the HTLV-1 21-bp repeat and its recruitment of CBP/p300 through the domain that we have now identified and characterized (2, 11, 20, 23, 30, 32, 42, 44, 47, 56). A study by Low et al. implicates ATF-3 in Tax-mediated activation of the CRE in the human proenkephalin promoter (33). An interaction between Tax and ATF-3 is intriguing, but the role of CREB and ATF-1 in proenkephalin gene expression cannot be ruled out. In fact, the proenkephalin CRE somewhat resembles the HTLV-1 21-bp repeat in that it is flanked by sequences that are G/C rich.
Clearly, for HTLV-1 transactivation, CBP and p300 serve many other important functions besides stabilizing the formation of the 4° complex, as reported here. Recently, both CBP and p300 have been shown to possess intrinsic HAT activity (9, 36) and associate with another distinct HAT, P/CAF (51). The HAT activity of CBP and p300 is expected to play a critical role in changing the nucleosome structure at or near the TATA box to render this region accessible to the basal transcriptional machinery. While the recruitment of CBP/p300 to the HTLV-1 enhancer by Tax is important, we believe that interactions between Tax and basal transcription factors are also likely to play a critical role in consolidating an active initiation complex. This proposal is based on the observation that the M47 mutant Tax, which bears two missense amino acid substitutions in the COOH-terminal domain, can be efficiently incorporated into the 4° complex, yet transactivation of M47 via the TxRE is completely abrogated. A recent study has suggested that the M47 mutant is impaired in CBP but not p300 binding (13). Our results obtained for the same mutant differ from these, as no significant differences in the M47-CBP/p300 interaction were observed between the full-length p300 protein and the GST-CBP451-682 fusion, which contains the Tax-binding domain of CBP. Although it may be argued that the 4° complex containing M47 Tax is qualitatively different from that containing the wild-type Tax, it is possible that the defect in M47 is due to its inability to interact productively with basal transcription factors recruited to the promoter. Indeed, a direct interaction between Tax and basal polymerase II factors has been reported (14, 15, 18). Thus, we think that the role of Tax in transactivation may extend beyond the recruitment of CBP/p300 and the assembly of the CBP/p300-Tax-CREB complex on the HTLV-1 21-bp repeats.
ACKNOWLEDGMENTS
We thank R. Goodman for the gift of the GST-CBP451-682 construct and A. Jerse and F. Grieder for critical reading of the manuscript. Tim Ford is thanked for his contribution in preparing the figures for this study.
This work was supported by grant RO1 CA48709 from the National Institutes of Health.
REFERENCES
- 1.Adya N, Giam C Z. Distinct regions in human T-cell lymphotropic virus type I Tax mediate interactions with activator protein CREB and basal transcription factors. J Virol. 1995;69:1834–1841. doi: 10.1128/jvi.69.3.1834-1841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adya N, Zhao L J, Huang W, Boros I, Giam C Z. Expansion of CREB’s DNA recognition specificity by Tax results from interaction with Ala-Ala-Arg at positions 282-284 near the conserved DNA-binding domain of CREB. Proc Natl Acad Sci USA. 1994;91:5642–5646. doi: 10.1073/pnas.91.12.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson M G, Dynan W S. Quantitative studies of the effect of HTLV-I Tax protein on CREB protein-DNA binding. Nucleic Acids Res. 1994;22:3194–3201. doi: 10.1093/nar/22.15.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arany Z, Newsome D, Oldread E, Livingston D M, Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature. 1995;374:81–84. doi: 10.1038/374081a0. [DOI] [PubMed] [Google Scholar]
- 5.Arany Z, Sellers W R, Livingston D M, Eckner R. E1A-associated p300 and CREB-associated CBP belong to a conserved family of coactivators. Cell. 1994;77:799–800. doi: 10.1016/0092-8674(94)90127-9. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 6.Arias J, Alberts A S, Brindle P, Claret F X, Smeal T, Karin M, Feramisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370:226–229. doi: 10.1038/370226a0. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong A P, Franklin A A, Uittenbogaard M N, Giebler H A, Nyborg J K. Pleiotropic effect of the human T-cell leukemia virus Tax protein on the DNA binding activity of eukaryotic transcription factors. Proc Natl Acad Sci USA. 1993;90:7303–7307. doi: 10.1073/pnas.90.15.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avantaggiati M L, Carbone M, Graessmann A, Nakatani Y, Howard B, Levine A S. The SV40 large T antigen and adenovirus E1A oncoproteins interact with distinct isoforms of the transcriptional co-activator, p300. EMBO J. 1996;15:2236–2248. [PMC free article] [PubMed] [Google Scholar]
- 9.Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 10.Bannister A J, Oehler T, Wilhelm D, Angel P, Kouzarides T. Stimulation of c-Jun activity by CBP: c-Jun residues Ser63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. Oncogene. 1995;11:2509–2514. [PubMed] [Google Scholar]
- 11.Bantignies F, Rousset R, Desbois C, Jalinot P. Genetic characterization of transactivation of the human T-cell leukemia virus type 1 promoter: binding of Tax to Tax-responsive element 1 is mediated by the cyclic AMP-responsive members of the CREB/ATF family of transcription factors. Mol Cell Biol. 1996;16:2174–2182. doi: 10.1128/mcb.16.5.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baranger A M, Palmer C R, Hamm M K, Giebler H A, Brauweiler A, Nyborg J K, Schepartz A. Mechanism of DNA-binding enhancement by the human T-cell leukaemia virus transactivator Tax. Nature. 1995;376:606–608. doi: 10.1038/376606a0. [DOI] [PubMed] [Google Scholar]
- 13.Bex F, Yin M J, Burny A, Gaynor R B. Differential transcriptional activation by human T-cell leukemia virus type I Tax mutants is mediated by distinct interaction with CREB binding protein and p300. Mol Cell Biol. 1998;18:2392–2405. doi: 10.1128/mcb.18.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caron C, Mengus G, Dubrowskaya V, Roisin A, Davidson I, Jalinot P. Human TAF(II)28 interacts with the human T cell leukemia virus type I tax transactivator and promotes its transcriptional activity. Proc Natl Acad Sci USA. 1997;94:3662–3667. doi: 10.1073/pnas.94.8.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caron C, Rousset R, Beraud C, Moncollin V, Egly J M, Jalinot P. Functional and biochemical interaction of the HTLV-I Tax1 transactivator with TBP. EMBO J. 1993;12:4269–4278. doi: 10.1002/j.1460-2075.1993.tb06111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chrivia J C, Kwok R P, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 17.Dai P, Akimaru H, Tanaka Y, Hou D X, Yasukawa T, Kanei-Ishii C, Takahashi T, Ishii S. CBP as a transcriptional coactivator of c-Myb. Genes Dev. 1996;10:528–540. doi: 10.1101/gad.10.5.528. [DOI] [PubMed] [Google Scholar]
- 18.Duvall J F, Kashanchi F, Cvekl A, Radonovich M F, Piras G, Brady J N. Transactivation of the human T-cell lymphotropic virus type 1 Tax1-responsive 21-base-pair repeats requires holo-TFIID and TFIIA. J Virol. 1995;69:5077–5086. doi: 10.1128/jvi.69.8.5077-5086.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calender A, de The G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 20.Giebler H A, Loring J E, van Orden K, Colgin M A, Garrus J E, Escudero K W, Brauweiler A, Nyborg J K. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of tax transactivation. Mol Cell Biol. 1997;17:5156–5164. doi: 10.1128/mcb.17.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez G A, Montminy M R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez G A, Yamamoto K K, Fischer W H, Karr D, Menzel P, Biggs W, Vale W W, Montminy M R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989;337:749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- 23.Goren I, Semmes O J, Jeang K T, Moelling K. The amino terminus of Tax is required for interaction with the cyclic AMP response element binding protein. J Virol. 1995;69:5806–5811. doi: 10.1128/jvi.69.9.5806-5811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hai T W, Liu F, Coukos W J, Green M R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989;3:2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- 25.Hanly S M, Rimsky L T, Malim M H, Kim J H, Hauber J, Duc Dodon M, Le S Y, Maizel J V, Cullen B R, Greene W C. Comparative analysis of the HTLV-I Rex and HIV-1 Rev trans-regulatory proteins and their RNA response elements. Genes Dev. 1989;3:1534–1544. doi: 10.1101/gad.3.10.1534. [DOI] [PubMed] [Google Scholar]
- 26.Janknecht R, Hunter T. Transcription. a growing coactivator network. Nature. 1996;383:22–23. doi: 10.1038/383022a0. [DOI] [PubMed] [Google Scholar]
- 27.Janknecht R, Nordheim A. Regulation of the c-fos promoter by the ternary complex factor Sap-1a and its coactivator CBP. Oncogene. 1996;12:1961–1969. [PubMed] [Google Scholar]
- 28.Jeang K T, Widen S G, Semmes O J, Wilson S H. HTLV-I trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science. 1990;247:1082–1084. doi: 10.1126/science.2309119. [DOI] [PubMed] [Google Scholar]
- 29.Kunkel T A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwok R P, Laurance M E, Lundblad J R, Goldman P S, Shih H, Connor L M, Marriott S J, Goodman R H. Control of cAmp-regulated enhancers by the viral transactivator tax through CREB and the co-activator CBP. Nature. 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 31.Kwok R P, Lundblad J R, Chrivia J C, Richards J P, Bachinger H P, Brennan R G, Roberts S G, Green M R, Goodman R H. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 32.Lenzmeier B A, Giebler H A, Nyborg J K. Human T-cell leukemia virus type I Tax requires direct access to DNA for recruitment of CREB binding protein to the viral promoter. Mol Cell Biol. 1998;18:721–731. doi: 10.1128/mcb.18.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Low K G, Chu H M, Schwartz P M, Daniels G M, Melner M H, Comb M J. Novel interactions between human T-cell leukemia virus type I Tax and activating transcription factor 3 at a cyclic AMP-responsive element. Mol Cell Biol. 1994;14:4958–4974. doi: 10.1128/mcb.14.7.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicot C, Tie F, Giam C-Z. Cytoplasmic forms of human T-cell leukemia virus type 1 Tax induce NF-κB activation. J Virol. 1998;72:6777–6784. doi: 10.1128/jvi.72.8.6777-6784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nordheim A. Transcription factors. CREB takes CBP to tango. Nature. 1994;370:177–178. doi: 10.1038/370177a0. [DOI] [PubMed] [Google Scholar]
- 36.Ogryzko V V, Schiltz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 37.Osame M, Matsumoto M, Usuku K, Izumo S, Ijichi N, Amitani H, Tara M, Igata A. Chronic progressive myelopathy associated with elevated antibodies to human T-lymphotropic virus type I and adult T-cell leukemialike cells. Ann Neurol. 1987;21:117–122. doi: 10.1002/ana.410210203. [DOI] [PubMed] [Google Scholar]
- 38.Paca Uccaralertkun S, Zhao L J, Adya N, Cross J V, Cullen B R, Boros I M, Giam C Z. In vitro selection of DNA elements highly responsive to the human T-cell lymphotropic virus type I transcriptional activator, Tax. Mol Cell Biol. 1994;14:456–462. doi: 10.1128/mcb.14.1.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perini G, Wagner S, Green M R. Recognition of bZIP proteins by the human T-cell leukaemia virus transactivator Tax. Nature. 1995;376:602–605. doi: 10.1038/376602a0. [DOI] [PubMed] [Google Scholar]
- 40.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semmes O J, Jeang K T. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 47 mutant proteins. J Virol. 1992;66:7183–7192. doi: 10.1128/jvi.66.12.7183-7192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semmes O J, Jeang K T. Definition of a minimal activation domain in human T-cell leukemia virus type I Tax. J Virol. 1995;69:1827–1833. doi: 10.1128/jvi.69.3.1827-1833.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shih H M, Goldman P S, De Maggio A J, Hollenberg S M, Goodman R H, Hoekstra M F. A positive genetic selection for disrupting protein-protein interactions: identification of CREB mutations that prevent association with the coactivator CBP. Proc Natl Acad Sci USA. 1996;93:13896–13901. doi: 10.1073/pnas.93.24.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shnyreva M, Munder T. The oncoprotein Tax of the human T-cell leukemia virus type 1 activates transcription via interaction with cellular ATF-1/CREB factors in Saccharomyces cerevisiae. J Virol. 1996;70:7478–7484. doi: 10.1128/jvi.70.11.7478-7484.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith M R, Greene W C. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki T, Fujisawa J I, Toita M, Yoshida M. The trans-activator tax of human T-cell leukemia virus type 1 (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc Natl Acad Sci USA. 1993;90:610–614. doi: 10.1073/pnas.90.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tie F, Adya N, Greene W C, Giam C Z. Interaction of the human T-lymphotropic virus type 1 Tax dimer with CREB and the viral 21-base-pair repeat. J Virol. 1996;70:8368–8374. doi: 10.1128/jvi.70.12.8368-8374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uittenbogaard M N, Armstrong A P, Chiaramello A, Nyborg J K. Human T-cell leukemia virus type 1 Tax protein represses gene expression through the basic helix-loop-helix family of transcription factors. J Biol Chem. 1994;269:22466–22469. [PubMed] [Google Scholar]
- 49.Uittenbogaard M N, Giebler H A, Reisman D, Nyborg J K. Transcriptional repression of p53 by human T-cell leukemia virus type 1 Tax protein. J Biol Chem. 1995;270:28503–28506. doi: 10.1074/jbc.270.48.28503. [DOI] [PubMed] [Google Scholar]
- 49a.Vassilev, A. Unpublished data.
- 50.Wagner S, Green M R. HTLV-I Tax protein stimulation of DNA binding of bZIP proteins by enhancing dimerization. Science. 1993;262:395–399. doi: 10.1126/science.8211160. [DOI] [PubMed] [Google Scholar]
- 51.Yang X J, Ogryzko V V, Nishikawa J, Howard B H, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 52.Yin M J, Gaynor R B. Complex formation between CREB and Tax enhances the binding affinity of CREB for the human T-cell leukemia virus type 1 21-base-pair repeats. Mol Cell Biol. 1996;16:3156–3168. doi: 10.1128/mcb.16.6.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yin M J, Gaynor R B. HTLV-1 21 bp repeat sequences facilitate stable association between tax and CREB to increase CREB binding affinity. J Mol Biol. 1996;264:20–31. doi: 10.1006/jmbi.1996.0620. [DOI] [PubMed] [Google Scholar]
- 54.Yin M J, Paulssen E J, Seeler J S, Gaynor R B. Protein domains involved in both in vivo and in vitro interactions between human T-cell leukemia virus type I Tax and CREB. J Virol. 1995;69:3420–3432. doi: 10.1128/jvi.69.6.3420-3432.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci USA. 1982;79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao L J, Giam C Z. Interaction of the human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator Tax with cellular factors that bind specifically to the 21-base-pair repeats in the HTLV-I enhancer. Proc Natl Acad Sci USA. 1991;88:11445–11449. doi: 10.1073/pnas.88.24.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao L J, Giam C Z. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc Natl Acad Sci USA. 1992;89:7070–7074. doi: 10.1073/pnas.89.15.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]