Abstract
This study focused on optimizing the fermentation-based production of Exopolysaccharides (EPS) from Enterococcus faecium F58 initially isolated from traditional Moroccan Jben, a fresh goat cheese. Using the central composite design, yeast extract, MnSO4, and time affect EPS concentration. The highest experimental and predicted EPS production yields were 2.46 g/L ± 0.38 and 2.86 g/L, respectively. Optimal concentrations of yeast extract (4.46 g/L) and MnSO4 (0.011 g/L) were identified after 26 h at 30 °C. Characterization of EPS was conducted using SEM with EDX, XRD, and FTIR analyses. These tests revealed a specific morphology and an amorphous structure. Additionally, thermogravimetric analysis indicated adequate EPS stability up to 200 °C with anti-adhesion properties against different pathogens. This study offers valuable insights into the optimized production of EPS from Enterococcus faecium F58, which exhibits significant structural and functional properties for various applications in the food and biotechnology industries.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-023-01424-9.
Keywords: Enterococcus faecium F58, Exopolysaccharide, Central composite design, Anti-adherence
Introduction
Over the past few decades, the demand for novel bioactive additives for food production has increased significantly. These additives must possess low toxicity, high efficacy in enhancing functional and sensory properties, and minimal environmental impacts (Mahapatra and Banerjee, 2016). In response to growing consumer awareness, researchers are exploring natural food additives, such as microbial exopolysaccharides (EPS), which are biodegradable, biocompatible, and more environmentally friendly materials than synthetic polymers. Microbial EPS has applications in a diverse range of fields, including tissue engineering, pharmaceuticals, cosmetics, and foods (Alsharabasy et al., 2016; Daba et al., 2021; Korcz and Varga, 2021).
Cheese rheological properties result from the interaction of various components, such as water, fat, protein fibers, precipitated minerals, and microbial cells. Lactic acid bacteria (LAB) are selected for their ability to acidify milk and produce aroma compounds during fermentation. Some LAB are prioritized due to their classification as generally recognized as safe (GRAS) and long history of consumption by humans (Coelho et al., 2022). In the last decade, studies have shown that LAB have various properties, such as the production of antimicrobial compounds called bacteriocins, organic acids, and EPSs (Abarquero et al., 2022; Agriopoulou et al., 2020; Coelho et al., 2022). EPSs facilitate bacterial adhesion and biofilm formation on biotic or abiotic surfaces, making them suitable for surface coating applications that prevent or inhibit bacterial colonization.
Enterococcus faecium is a lactic acid bacteria commonly found in fermented foods and has potential as a probiotic culture (Bhat and Bajaj, 2018). This species is also known for its ability to produce EPS. Previous studies have demonstrated EPS production from various strains of E. faecium (Ayyash et al., 2020; Jia et al., 2019; Elshaghabee et al., 2022). Although xanthan gum, levan, dextran, and pullulan are commonly used as industrial EPS, microbial EPS production remains expensive. Thus, there is growing interest in selecting new microbial strains and optimizing their compositions and culture conditions to achieve maximum EPS production. Nutritional and environmental factors, such as carbon sources, sugar linkage-type, amine sources, inorganic substituents, and polymerization degree, can affect the physical properties and yields of EPS (Sørensen et al., 2022; Hashem et al., 2018 ). Statistical methods, particularly response surface methodology (RSM), can provide a powerful tool in food microbiology for optimizing metabolite production conditions. It enables experimental design, models construction, condition optimization, evaluation of factors on expected responses, and determination of significant interactions between different factors (Khuri and Mukhopadhyay, 2010). Using RSM can reduce the number of experiments needed and help achieve large-scale production of microbial EPS by identifying the main factors influencing EPS production.
To the best of our knowledge, Lactobacillus, Lactococcus, Pediococcus, Leuconostoc, and Streptococcus represent the most widely recognized LAB known to produce EPS. In contrast, Enterococcus species are less well-known in this regard. Consequently, the present study aimed to screen various factors influencing EPS production in E. faecium F58, isolated from goat’s cheese. This was followed by statistical optimization of the formulated medium to enhance EPS production at a laboratory scale. Additionally, we characterized the EPS produced by E. faecium F58 using diverse instrumental analyses and evaluated its application for thermal stability and antibiofilm properties on stainless steel 316 L surfaces.
Materials and methods
Bacterial strain and growth conditions
Enterococcus faecium F58 was employed as the EPS-producing agent in this investigation. This strain was derived from fresh goat cheese (Jben) manufactured without starter culture and was distinguished by its production of enterocins L50A and B active against a diverse range of foodborne and spoilage bacteria. Molecular techniques were employed to identify the bacterium, and to assess its safety traits (Achemchem et al., 2005). Long-term storage was performed at − 23 °C. For subsequent investigations, the strain was cultured twice in de Man, Rogosa and Sharpe broth (MRS, Biokar Diagnostics, France) for 16 h at 30 °C.
Optimization of EPS production using response surface methodology
Sources of carbon and nitrogen for EPS production
The maximum production of EPS was screened by identifying the carbon and amine sources that affect the production (Table 1). A modified MRS broth was prepared with the following components (g/L): MnSO4·H2O (0.05), ammonium citrate (2), K2HPO4 (2), MgSO4·7H2O (0.2), sodium acetate (5), and tween 80 (1 mL/L). The carbon sources (glucose, maltose, lactose, fructose, or sucrose at 30 g/L), and amine sources (yeast extract, meat extract, peptone, or tryptone at 25 g/L) were replaced in the customized medium using one-factor-at-a-time (OFAT) experimental approach through changing one source at a time while maintaining the other components of the customized MRS broth constant (Singh et al., 2017). The pH was adjusted to 6.4. The medium was inoculated with 1% (v/v) of a 16-hours-old inoculum containing bacterial cells with a density of 108 CFU/mL for EPS synthesis.
Table 1.
Effects of carbon and nitrogen sources on cell growth and EPS production of E. faecium F58
| Culture conditions | Growth (OD 620 nm) | EPS (g/L) |
|---|---|---|
| Carbon sources (30 g/L) | ||
| Glucose | 0.73 ± 0.04 | 1.12 ± 0.47 |
| Lactose | 0.50 ± 0.01 | 0.80 ± 0.74 |
| Maltose | 0.68 ± 0.03 | 3.55 ± 0.62 |
| Fructose | 0.46 ± 0.19 | 1.34 ± 0.79 |
| Sucrose | 0.64 ± 0.20 | 1.70 ± 0.52 |
| Nitrogen sources (25 g/L) | ||
| Yeast extract | 1.04 ± 0.21 | 4.00 ± 0.81 |
| Meat extract | 1.17 ± 0.66 | 1.71 ± 0.48 |
| Peptone | 0.33 ± 0.24 | Absence |
| Tryptone sel | No growth | Absence |
Plackett–Burman design (PBD)
To determine the significant factors and culture conditions affecting the EPS yield of E. faecium F58, a Plackett–Burman experimental design was employed. Eleven factors were tested, including tween 80 (X1), MnSO4·H2O (X2), K2HPO4 (X3), MgSO4·7H2O (X4), sodium acetate (X5), yeast extract (X6), maltose (X7), pH (X8), temperature (X9), time (X10), and initial inoculum (X11). Each factor was arranged in the Plackett–Burman matrix with high (+ 1), medium (0), and low level (− 1) tested in 15 trials. All experiments were performed in triplicate, and the mean value ± SD was reported. The statistical analysis was conducted using ordinary one-way ANOVA with Tukey’s multiple comparisons test (p < 0.05).
The results were subjected to analysis by fitting them to a second-order polynomial equation (Eq. 1).
| 1 |
where Y represents the EPS yield, βi is the variables estimates, and Xi is the independent variables.
Central composite design (CCD)
The experimental designs and statistical analysis were conducted using Design-Expert Package 12 (Stat-Ease Inc., Minneapolis, USA). The study evaluated the influences of three independent variables, namely yeast extract (X2), MnSO4 (X3), and time (X10), at five levels (Table 1S, Supplementary data), with 20 experiments including 6 replicates central points (Table 2). The experimental setup comprised 250 mL Erlenmeyer flasks containing 50 mL of media, which was prepared as per the design. The response generated from RSM was expressed through a second-order polynomial equation (Eq. 2):
Table 2.
Results of central composite design of three variables
| Run | Yeast extract (%) | Time (h) | MnSO4 (%) | EPS (g/L) | |
|---|---|---|---|---|---|
| Actual value | Predicted value | ||||
| 1 | 5 | 37 | 0.009 | 3.38 | 3.41 |
| 2 | 5 | 48 | 0.009 | 3.24 | 3.57 |
| 3 | 3.5 | 43 | 0.007 | 2.39 | 2.33 |
| 4 | 3.5 | 22 | 0.007 | 1.67 | 1.10 |
| 5 | 5 | 15 | 0.009 | 2.46 | 2.37 |
| 6 | 5 | 37 | 0.014 | 2.53 | 2.55 |
| 7 | 6.5 | 22 | 0.012 | 5.15 | 4.87 |
| 8 | 3.5 | 22 | 0.012 | 1.63 | 2.01 |
| 9 | 6.5 | 43 | 0.012 | 4.42 | 5.08 |
| 10 | 7.5 | 37 | 0.009 | 6.44 | 6.01 |
| 11 | 5 | 37 | 0.009 | 3.70 | 3.41 |
| 12 | 5 | 37 | 0.005 | 1.28 | 1.44 |
| 13 | 6.5 | 22 | 0.007 | 1.49 | 2.09 |
| 14 | 5 | 37 | 0.009 | 3.44 | 3.41 |
| 15 | 5 | 37 | 0.009 | 3.40 | 3.41 |
| 16 | 6.5 | 43 | 0.007 | 4.30 | 3.96 |
| 17 | 5 | 37 | 0.009 | 3.34 | 3.41 |
| 18 | 3.5 | 43 | 0.012 | 2.47 | 1.59 |
| 19 | 2.5 | 37 | 0.009 | 1.39 | 2.01 |
| 20 | 5 | 37 | 0.009 | 3.32 | 3.41 |
| 2 |
where Y is the predicted response (EPS concentration), β0 is the model constant, Xi and Xj are the coded independent variable, βi, βii, and βij were the linear, quadratic, and interaction coefficient respectively, and k is the number of factors. The experiments were performed in triplicate. To evaluate the optimal medium components, 2D graphical plots were generated to illustrate the mutual interactions between determinative factors, as the response (EPS yield in g/L) was the dependent variable.
To assess the accuracy and performance of the model, we employed determination coefficients (R2), root mean square error (RMSE), and standard error of prediction percentage (SEP%). Specifically, the SEP(%) accounts for the differences in the magnitude order of the response variable. The RMSE and SEP were calculated using the following equations Eqs. (3 and 4):
| 3 |
| 4 |
where obs represents the observed values, pred represents the predicted values, represents the arithmetic mean of observed values, and n indicates the total number of measurements included in the analysis.
Extraction and purification of EPS
After 16 h of incubation of E. faecium F58 (1%) under optimal conditions, 7 mL trichloroacetic acid (TCA) with concentration of 80% was added to the culture and incubated at 30 °C in a shaker incubator at 90 rpm for 40 min to inactivate polysaccharide-degrading enzymes. The resulting precipitate was removed by centrifugation (Centrofriger-BL II, JP SELECTA, Barcelona, Spain) at 4 °C and 5000×g for 30 min. The supernatant was mixed with a two-fold volume of cold 99% ethanol and incubated at 4 °C overnight to precipitate the EPSs. A second centrifugation was carried out at 4 °C and 5000×g for 30 min to collect the EPS pellet, which was then dissolved in ultrapure water. To obtain purified EPS, the sample was subjected to dialysis against ultrapure water for 24 h using a dialysis membrane with Mw cut-off of 12–14 KDa (Sigma Aldrich, USA), and lyophilized at − 50 °C for 24 h. EPS production was estimated using the colorimetric method described by DuBois et al. (1956) with glucose as the standard. In this method, 0.05 mL of 80% phenol solution and 5 mL of 95–98% sulfuric acid were quickly added to 2 mL of EPS suspension after dyalysis. After 10 min of incubation at 20 °C, the samples were incubated at 30 °C for 20–30 min in a water bath, and the optical density was measured at a wavelength of 460 nm using a UV–visible spectrophotometer. The absorbance values obtained were compared with the glucose standard curve, and the control tube consisted of distilled water. For each experiment, three duplicates were conducted.
Characterization of purified EPS
Scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) analysis
The lyophilized EPS sample under the optimal conditions was affixed onto SEM stubs using conductive tape and coated with a thin layer of gold by sputtering. The surface morphology and structure of the EPS were examined using a scanning electron microscope (VEGA3 TESCAN) operated at an acceleration voltage of 10.0 kV. Elemental composition of the EPS was analyzed using SEM equipped with an energy-dispersive X-ray (EDX) spectrometer.
X-ray diffraction analysis (XRD)
To determine the physical properties of the EPS, X-ray diffraction (XRD) analysis was carried out using an EQUINOX 2000 diffractometer. Approximately 10 mg of the dried sample was prepared for analysis. The XRD scan was conducted at various two-theta angles ranging from 10 to 90 °C, using a Cu–Kα (λ = 1.5418 Å) diffractometer.
EPS analysis by fourier transform infrared (FTIR) spectroscopy
The functional groups present in the EPS were analyzed through FTIR spectroscopy using a Vertex 40 DTGS spectrometer. The range of 400–4000 cm−1 was scanned with a resolution of 4 cm−1. To prepare the samples, 1 mg of dried EPS was homogenized with 100 mg of potassium bromide (KBr) powder at room temperature. The resulting mixture was dried at 105 °C for 72 h.
Thermogravimetric analysis (TGA) and differential thermal gravimetry (DTG) analysis
To determine the thermal properties of the EPS sample, a thermal assay using a Q500 V6.7 build 203 instrument was performed. Approximately 3–5 mg of the dried sample was placed in an aluminum tray and then wrapped. An empty tray was used as a reference. The measurements were carried out under a nitrogen atmosphere with a heating rate of 10 °C/min. The weight loss (%) and Deriv. weight (%/°C) were plotted in graphs.
In-vitro anti-adherence activity
The surface utilized in this study was stainless steel 316 L which was initially cut into dimensions of 1 × 1 × 0.2 cm. Prior to use, the surfaces were immersed in 95% ethanol for 15 min followed by washing 3 times with distilled water and autoclaving at 121 °C for 20 min. The antibiofilm activity was conducted following the procedure described by Zanzan et al. (2023a), with slight modifications. Specifically, 10 mL of EPS at a concentration of 2.5 mg/mL was added to the stainless steel surfaces and incubated at 30 °C for 24 h. After incubation, the surfaces were washed 3 times with sterilized distilled water. Next, 10 mL of various pathogenic suspensions (108 CFU/mL) obtained from the Spanish Type Culture Collection (Valencia, Spain) and American Type Culture Collection (Listeria monocytogenes CECT4032, Staphylococcus aureus CECT 976, Pseudomonas aeruginosa ATCC27853, and Escherichia coli ATCC25922) were separately added to the surfaces and incubated at 30 °C for 3 h. The unbound cells were cautiously discarded by washing the surfaces with sterilized distilled water. The colonies that adhered to the surfaces were detached using an ultrasonic bath (POWER SONIC 405, Lab Tech, Namyangju-city, Korea) for 10 min. Subsequently, the viable cells were counted using the serial dilution method using physiological water, where different pathogens were cultured on an appropriate medium: Palcam, Chapman, Cetrimide, and Tryptone Bile X-glucuronide (TBX) agar media (Biokar, France) for the growth of L. monocytogenes, S. aureus, P. aeruginosa, and E. coli respectively. Incubation was carried out for 24 h at 37 °C with triplicates for each strain of pathogenic bacteria, and colonies were counted as cfu/cm2. The control consisted of only pathogenic suspension without the EPS of E. faecium F58 and incubated for 3 h at 30 °C to permit adhesion.
Statistical analysis
The experiments were carried out in triplicate, and the results were expressed as mean ± SD. To determine significant differences an ANOVA analysis was carried out using the Tukey’s HSD test. Differences were considered statistically significant if the associated probability (p) was < 0.05.
Results and discussions
Effect of carbon and amine sources on EPS production
To determine the optimal carbon source for both cell growth and EPS production of E. faecium F58, five different carbon sources (30 g/L) were tested in modified MRS broth instead of glucose (Table 1). Our findings revealed that maltose exhibited the highest carbon source efficiency for EPS production, with a yield of 3.55 g/L. In descending order of EPS yield, sucrose, fructose, glucose, and lactose were found to have yields of 1.70 g/L, 1.34 g/L, 1.12 g/L, and 0.80 g/L, respectively. In contrast, glucose was the most suitable carbon source for cell growth with an optical density of 0.73, demonstrating that the nutritional requirements for cell growth and EPS production differed. Fuso et al. (2023) have reported similar finding, whereby maltose have been identified as suitable for EPS production. However, Rahnama Vosough et al. (2022) and Tilwani et al. (2021) have demonstrated that the sucrose medium confers a significant advantage for the EPS production of three enterococcal strains, specifically E. durans K48, E. faecium T52, E. faecium R114 and E. faecium MC-5.
Furthermore, nitrogen sources (25 g/L) were assessed, and the findings showed that yeast extract was the optimal nitrogen source for EPS production, yielding 4.04 g/L, while meat extract was the preferred nitrogen source for cell growth, with an optical density of 1.169. Yeast extract, which contains amino acids, peptides, carbohydrates, salt, and minimal lipid, provides an optimal environment for microorganisms to produce EPS. Previous studies have also reported the influence of yeast extract as a nitrogen source on EPS production by LAB (Adesulu-Dahunsi et al., 2018).
Optimization of EPS production using response surface methodology
PBD experimental design
The influence of media compositions and cultural conditions on EPS production was determined using the PBD. Optimization of these factors is essential for achieving high EPS productivity. Analysis of the data (Table 2S, Supplementary data) revealed that the p-value were less than 0.05, and the coefficient R2 was 0.98, indicating the significance of the model terms. The PB analysis identified yeast extract, time, and MnSO4 as the significant factors, which were further optimized using a CCD, while the other components were maintained at their usual levels in the MRS broth.
Time was one of the factors that showed a significant influence on EPS production based on the Plackett-Burman screening. However, it is worth noting that the optimal cultivation time for EPS production varies depending on the strain used and the conditions of the culture. Studies have reported that prolonged cultivation time may lead to enzymatic degradation of EPS, resulting in reduced yield (Adesulu-Dahunsi et al., 2018). The decrease in EPS yield could also be attributed to the action of glycohydrolases, such as α and β glucosidases, galactosidases, glucuronidases, and rhamnosidases (Degeest et al., 2002). Yeast extract was identified as a significant factor that had a high impact on EPS produced from Enterococcus faecium MC13 (Kanmani et al., 2013).
Central composite design (CCD)
While evaluating the impact of individual parameters on EPS production, one factor at a time analysis does not consider the interdependence between two or more variables. Therefore, in this study, RSM was utilized to assess the combined influence of the three independent variables mentioned earlier. A single-block design model comprising 20 runs, with each independent variable evaluated at different levels (Table 2), was presented. The EPS yield values were measured in triplicate for each run and compared to the predicted response values obtained from the model, using Design Expert 12 software.
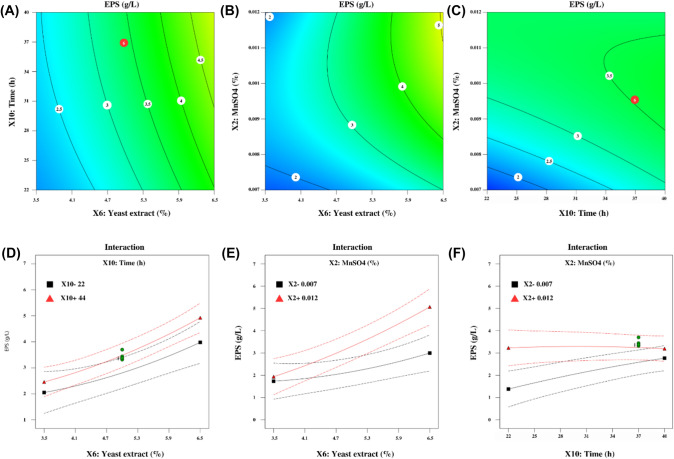
An implementation of a design model was carried out to determine the optimal conditions for the production of EPS with a specific composition and to identify the operational region necessary to achieve the maximum EPS yield. The ANOVA for EPS, presented in Table 3, showed that the fitted model was able to explain 91% of the variability in EPS concentration (R2 value of 0.910). This high R2 value (> 90%) indicates good agreement between the experimental data and the theoretical values predicted by the model (Fig. 1S, Supplementary data) (Niknezhad et al., 2015). The statistical analysis of the second-order model revealed significant prediction of the EPS response, as indicated by the ANOVA p-value (p < 0.05). The model’s performance was further verified by the low values of RMSE and SEP (0.44 and 13.09%, respectively; Table 3), indicating an adequate model fit to the data. Therefore, our study achieved a satisfactory correspondence between the experimental and predicted data. The findings indicate that the chosen model effectively characterizes the impact of yeast extract and MnSO4 concentrations, as well as fermentation time, on EPS production by E. faecium F58 in a batch flask. This relationship can be expressed as a multiple regression equation Eq. (5):
| 5 |
Table 3.
Analysis of variance for quadratic model of exopolysaccharides production
| Source | Sum of Squares | df | Mean square | F-value | p-value |
|---|---|---|---|---|---|
| Model | 2027.53 | 9 | 225.28 | 11.25 | 0.0004* |
| X2: Manganese sulfate | 248.04 | 1 | 248.04 | 12.39 | 0.0055* |
| X6: Yeast extract | 929.62 | 1 | 929.62 | 46.42 | < 0.0001* |
| X10: Time | 141.51 | 1 | 141.51 | 7.07 | 0.0240* |
| X2 × 6 | 114.31 | 1 | 114.31 | 5.71 | 0.0380* |
| X2 × 10 | 96.84 | 1 | 96.84 | 4.84 | 0.0525 |
| X6 × 10 | 14.08 | 1 | 14.08 | 0.70 | 0.4214 |
| X2² | 236.77 | 1 | 236.77 | 11.82 | 0.0063* |
| X6² | 41.28 | 1 | 41.28 | 2.06 | 0.1816 |
| X10² | 7.10 | 1 | 7.10 | 0.36 | 0.5648 |
| R2 | 0.910 | ||||
| R²adjusted | 0.829 | ||||
| RMSE | 0.40 | ||||
| SEP % | 13.09 |
*Significant model terms
The response variable Y represents the predicted EPS production (g/L) and is dependent on coded independent variables, , and corresponding to MnSO4, yeast extract concentrations, and time, respectively. Among the variables examined, MnSO4 , , and the interaction between MnSO4 and yeast extract had a significant (p < 0.05) positive impact on EPS production, confirming the suitability of using second-order polynomial equations. Similarly, various studies showed the effect of yeast extract on EPS production (Adesulu-Dahunsi et al. 2018; Zanzan et al. 2023b). Similarly, results of Amiri et al. (2019) showed that EPS production was significantly increased by increasing incubation time with yeast extract concentration and time significantly affected EPS production from Bifidobacterium animalis subsp. lactis BB12. However, in this study, correlation coefficients between yeast extract and time and MnSO4 and time did not significantly affect EPS production (p > 0.05).
Figure 1 shows contour plots of EPS concentration response surfaces for each factor pair while keeping the remaining factors at their median levels. Notably, the concentration of EPS exhibits an upward trend with increasing levels of MnSO4 and yeast extract, as shown in Fig. 1B, as long as time is maintained at a moderate level. Additionally, Fig. 1A and C demonstrate that increasing fermentation time at a constant medium level of yeast extract or MnSO4 does not significantly affect EPS production.
Fig. 1.
Contour plots and factor interaction plots illustrating the impact of yeast extract, MnSO4, and fermentation time on EPS yield in E. faecium F58. A, D Effect of time and yeast extract concentrations while keeping MnSO4 concentration constant; B, E Effect of MnSO4 and yeast extract concentrations while keeping fermentation time constant; C, F Effect of time and MnSO4 concentrations while keeping yeast extract concentration constant
The relationship between two variables with other variables at zero levels was demonstrated by linear interaction plots. Comparing Fig. 1A–C (contour plots) with Fig. 1D–F (linear plots) illustrates the interdependence of variables. it can be inferred that there is a significant correlation between MnSO4 and yeast extract. As depicted by the differing behavior of the red and black lines in Fig. 1E, the yield of EPS is significantly influenced by yeast extract and MnSO4 concentrations in the range of 2.48–7.52 and 0.0053–0.0137 g/L, respectively. Based on the results, the strain used had a significant impact on the selection of different factors influencing EPS production. Previous studies have demonstrated the main role of components and culture composition on EPS biomass of LAB (Degeest et al., 2002; Du et al., 2017; Hashem et al., 2018).
An experimental validation was conducted to confirm the optimized conditions, resulting in a yield value slightly lower than the predicted value. To validate the results, a confirmation experiment was conducted under the optimized conditions, which consisted of a concentration of 4.46 g/L of yeast extract, 0.011 g/L of MnSO4, and 26 h of fermentation, resulting in an EPS yield of 2.86 g/L. Triplicate verification experiments were carried out to confirm the optimized conditions, which yielded an EPS yield of 2.46 ± 0.38 g/L. Thus, the results obtained from the confirmation experiments were consistent with the predicted values, indicating that the developed quadratic model was both accurate and suitable for EPS production.
Characterization of purified EPS
Scanning electron microscopy and energy dispersive X-ray analysis
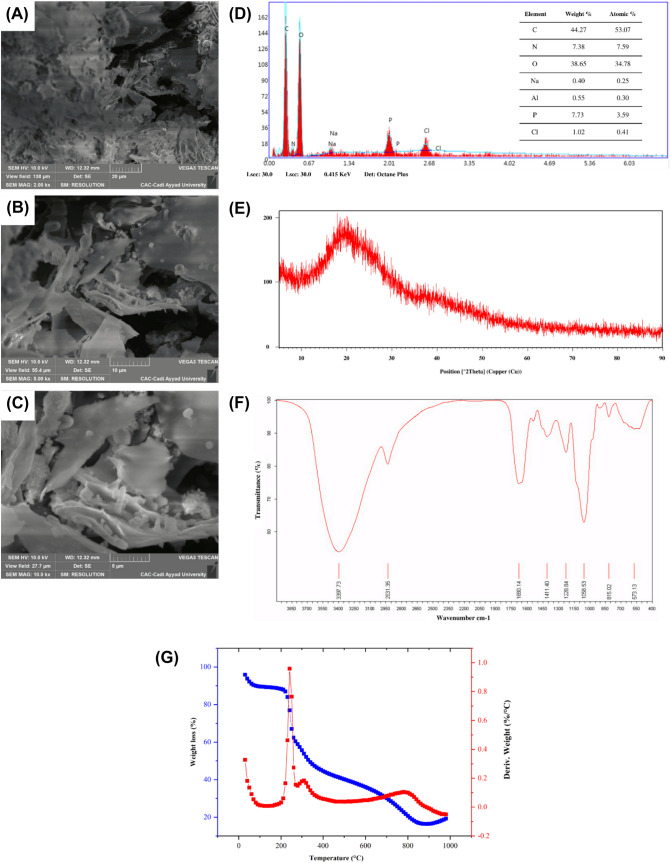
The EPS produced by E. faecium F58 exhibited an irregular shape, compact, porous, and rough surface, as shown in Fig. 2A–C. In the same line Taylan et al. (2019) showed a compact morphology detected with SEM and atomic force microscopy respectively. Previous studies have extensively explored the characteristics of EPS originating from LAB. The findings of these studies have revealed a wide range of structures such as compact and irregular aggregates, which suggest a tendency of the EPS molecules to clump together in a non-uniform manner. Additionally, the EPS have been observed to form porous structures, resembling spongy arrangements, indicating the presence of void spaces within the matrix (Tilwani et al. 2021; Zanzan et al. 2023b). The elemental composition of EPS from E. faecium F58 was analyzed to determine the weight and atomic percentage of different elements (Fig. 2D). The results showed that carbon and oxygen were the most abundant elements in the EPS, accounting for 44.27 and 38.65%, respectively. The presence of carbon indicates carbon ring sugar monomer units with a relative proportion of nitrogen of 7.38% suggesting the presence of amino linkages in the sugar chain. Phosphorus was detected in smaller amounts, at 7.73%. The results were similar in terms of ratios that were close to those reported in the study by Amer et al. (2021) with EPS from Weissella paramesenteroides MN2C2. In addition, Na, Cl, and Al detected in trace with weight reaching 0.40%, 1.02%, 0,55% respectively may be attributed to sample impurities. The EDX analysis confirmed EPS’ organic nature.These findings suggest that differences in the physicochemical composition of EPS may contribute to variations in its morphology and topography (Kanamarlapudi and Muddada, 2017).
Fig. 2.
Scanning electron micrograph of EPS from E. faecium F58. SEM images at 2.00 kx (A), 5.00 kx (B), and 10.0 kx (C) magnifications, along with corresponding EDX spectra (D). XRD pattern (E), FTIR spectrum (F), and TGA/DTG curves (G)
X-ray diffraction analysis (XRD)
The XRD analysis performed on the EPS of E. faecium F58 (Fig. 2E) revealed an amorphous nature of the EPS, with an extremely broad peak near 2θ = ~ 18 °C and an intensity of 200. This is consistent with previous studies that have reported a non-crystalline amorphous nature of EPS from other bacteria, such as Enterococcus hirae KX577639 (Jayamanohar et al., 2018). However, a study by Tilwani et al. (2021) showed a semi-crystalline nature of EPS from Enterococcus faecium MC-5.
FTIR analysis
The FTIR spectrum analysis of the EPS from E. faecium F58 revealed the presence of various functional groups and chemical bonds (Fig. 2F). The broad and intense peak at 3397.73 cm−1 suggested the presence of hydroxyl (O–H) stretching vibration which is common to all polysaccharides (Bhat and Bajaj, 2018). The weak peak at 2931.35 cm−1 indicated C–H stretching vibration of the methyl group, while the band at 1680.14 cm−1 was associated with C=O (carboxyl group) and (N–H) bending of proteins (Banerjee et al., 2018; Venkatesh et al., 2016). The absorption band at 1411.40 cm−1 suggested the presence of C–H and bending bond of NO2 group. The absorption at 1228.84 cm−1 was associated with carboxylic acids and ester groups. The peaks at 1040–1200 cm−1 suggested the presence of carbohydrates, particularly C–O–C and C–O bonds (Choudhuri et al., 2020). Finally, the peaks at 815.02 cm−1 and 573.13 cm−1 indicated α-glucoside linkages and sulfate groups (Venkatesh et al., 2016).
Thermogravimetric analysis (TGA)
TGA is a crucial method for determining the thermal stability and decomposition shape of substrates. The thermal properties of EPSs are important for their commercial applications in the food industry. As shown in Fig. 2G, the TGA curve of EPS from E. faecium F58 was examined dynamically by weight loss versus temperature. The result indicated two phases: the first phase displayed a weight loss of nearly 11.5% from 0 to 120 °C due to the loss of bound water in EPS, which might be attributed to the increased carboxyl content that has a high affinity towards water molecules. The findings were in agreement with a previous study by Tilwani et al. (2021), which reported a marginal decrease in the weight of EPS extracted from E. faecium by around 7.60% within the temperature range of 30 to 110 °C. The second phase involved a degradation of 72.5% with maximum loss at 890 °C, which was due to the depolymerization and destruction of the chemical structure and chemical bonds.
A peak at approximately 225 °C was observed in the DTG curves (Fig. 2G). This value was higher than the peak temperature observed for Streptococcus thermophilus EPS, which was reported as 110.9 °C (Kanamarlapudi and Muddada, 2017). In the food processing industry, treatment temperature often exceeds 150 °C, indicating the need for a higher degradation temperature (Sajna et al., 2013). The EPS derived from E. faecium F58 exhibited significant thermal stability, which is a crucial characteristic for industrial applications, particularly in the dairy industry. Therefore, the EPS produced by E. faecium F58 is a promising candidate for food applications, even at high temperatures.
In-vitro anti-adherence activity
Figure 3 presents the outcomes of the serial dilution technique employed to assess anti-adhesion activity against four pathogenic strains. Pre-treatment of stainless steel 316 L surfaces with EPS led to diverse levels of adhesion rate reduction for distinct pathogenic strains. The anti-adhesion activity against L. monocytogenes, S. aureus, P. aeruginosa, and E. coli exhibited significant differences in comparison to the control.
Fig. 3.
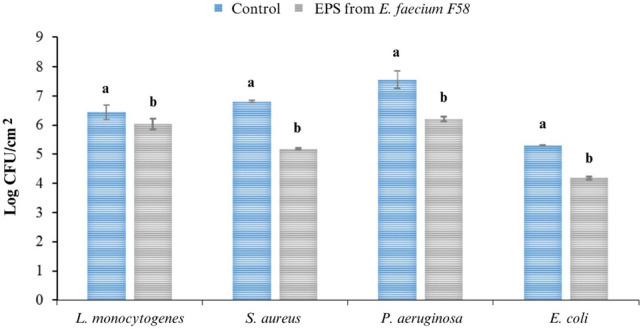
Effect of F58 EPS pretreatment on pathogens adhesion to stainless steel 316 L. Different letters (a, b) indicate significant differences according to the Tukey’s HSD test (p <0.05)
EPS derived from E. faecium F58 displayed moderate anti-adhesion activity against both Gram-positive and Gram-negative bacteria, with the highest reduction observed in S. aureus (24%) and the lowest in L. monocytogenes (6%). These observations align with prior research demonstrating the efficacy of LAB EPSs in impeding biofilm formation in various bacterial strains. For instance, Kanmani et al. (2011) reported that EPS from Streptococcus phocae PI80 substantially inhibited biofilm formation in S. aureus and L. monocytogenes by 51 and 67%, respectively. In another study, Abid et al. (2018) discovered that EPS pre-treatment of microtiter plates diminished biofilm formation in S. aureus ATCC 25,923, E. coli 25,922, and E. faecalis 25,912 following a 6-h exposure.
It is essential to note that EPSs are generally less effective at disrupting pre-existing biofilms (Flemming et al., 2016) and that higher initial EPS concentrations may be necessary for post-treatment activity (Donlan, 2002). The mechanism underlying EPSs’ inhibition of biofilm formation likely involves reducing cell-surface interactions or attenuating cell surface modifications (Hobley et al., 2015). The observed inhibition of biofilm formation and decreased adhesion rates by E. faecium F58-derived EPSs in Fig. 3 could have substantial implications for food safety and public health (Galié et al., 2018). Preventing or reducing biofilm formation on surfaces is crucial for controlling pathogenic bacterial growth and spread in food processing environments, thereby minimizing foodborne illness risks (Gutiérrez et al., 2016).
Moreover, employing LAB-derived EPSs as natural antimicrobial agents offers several advantages over conventional chemical antimicrobials, such as reduced negative impacts on food quality and safety and decreased potential for antimicrobial resistance (Zannini et al., 2016). LAB-derived EPSs could provide a safe, effective, and sustainable alternative to chemical antimicrobials in food processing and preservation applications.
The findings further support the potential utilization of E. faecium F58-derived EPS as an eco-friendly and efficient alternative to chemical antimicrobials in the food industry. The substantial reduction in pathogenic bacteria adhesion rates depicted in Fig. 3 underscores the potential of LAB-derived EPSs to prevent foodborne illnesses and enhance food product safety and quality. However, further research is needed to optimize the application of EPS-based strategies and assess their effectiveness in real-world food processing environments.
In conclusion, this study demonstrates the potential of EPS production from E. faecium F58, with optimal conditions yielding 2.46 ± 0.38 g/L EPS. The purified EPS exhibits a porous structure, ideal for food and cosmetic industry applications as a stabilizing and texturizing agent. Its thermal stability enhances versatility for high-temperature preparations. Additionally, the EPS shows anti-adherence activity against pathogens, suggesting it as a biofilm inhibitor on food industry surfaces. This research highlights the significance of microbial exopolysaccharides and their novel, commercially promising applications.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The author M. Z. was the beneficiary of a scholarship from the National Center for Scientific and Technical Research [Grant Number 18UIZ2016].
Declarations
Conflict of interest
The authors confirm that they have no conflict of interest with respect to the work described in this manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abarquero D, Renes E, Fresno JM, Tornadijo ME. Study of exopolysaccharides from lactic acid bacteria and their industrial applications: A review. International Journal of Food Science & Technology. 2022;57:16–26. doi: 10.1111/ijfs.15227. [DOI] [Google Scholar]
- Abid Y, Casillo A, Gharsallah H, Joulak I, Lanzetta R, Corsaro MM, Attia H, Azabou S. Production and structural characterization of exopolysaccharides from newly isolated probiotic lactic acid bacteria. International Journal of Biological Macromolecules. 2018;108:719–728. doi: 10.1016/j.ijbiomac.2017.10.155. [DOI] [PubMed] [Google Scholar]
- Achemchem F, Martínez-Bueno M, Abrini J, Valdivia E, Maqueda M. Enterococcus faecium F58, a bacteriocinogenic strain naturally occurring in Jben, a soft, farmhouse goat’s cheese made in Morocco. Journal of Applied Microbiology. 2005;99:141–150. doi: 10.1111/j.1365-2672.2005.02586.x. [DOI] [PubMed] [Google Scholar]
- Adesulu-Dahunsi AT, Sanni AI, Jeyaram K. Production, characterization and in vitro antioxidant activities of exopolysaccharide from Weissella cibaria GA44. LWT-Food Science and Technology. 2018;87:432–442. doi: 10.1016/j.lwt.2017.09.013. [DOI] [Google Scholar]
- Agriopoulou S, Stamatelopoulou E, Sachadyn-Król M, Varzakas T. Lactic acid bacteria as antibacterial agents to extend the shelf life of fresh and minimally processed fruits and vegetables: Quality and safety aspects. Microorganisms. 2020;8:952. doi: 10.3390/microorganisms8060952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharabasy AM, Moghannem SA, El-Mazny WN. Physical preparation of alginate/chitosan polyelectrolyte complexes for biomedical applications. Journal of Biomaterials Applications. 2016;30:1071–1079. doi: 10.1177/0885328215613886. [DOI] [PubMed] [Google Scholar]
- Amer MN, Elgammal EW, Atwa NA, Eldiwany AI, Dawoud IE, Rashad FM. Structure elucidation and in vitro biological evaluation of sulfated exopolysaccharide from LAB Weissella paramesenteroides MN2C2. Journal of Applied Pharmaceutical Science. 2021;11:022–031. [Google Scholar]
- Amiri S, Mokarram RR, Khiabani MS, Bari MR, Khaledabad MA. Exopolysaccharides production by Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12: Optimization of fermentation variables and characterization of structure and bioactivities. International Journal of Biological Macromolecules. 2019;123:752–765. doi: 10.1016/j.ijbiomac.2018.11.084. [DOI] [PubMed] [Google Scholar]
- Ayyash M, Stathopoulos C, Abu-Jdayil B, Esposito G, Baig M, Turner MS, Baba AS, Apostolopoulos V, Al-Nabulsi A, Osaili T. Exopolysaccharide produced by potential probiotic Enterococcus faecium MS79: Characterization, bioactivities and rheological properties influenced by salt and pH. Exopolysaccharide produced by potential probiotic Enterococcus faecium MS79: Characterization, bioactivities and rheological properties influenced by salt and pH. LWT-Food Science and Technology. 2020;131:109741. doi: 10.1016/j.lwt.2020.109741. [DOI] [Google Scholar]
- Banerjee A, Rudra SG, Mazumder K, Nigam V, Bandopadhyay R. Structural and functional properties of exopolysaccharide excreted by a novel Bacillus anthracis (Strain PFAB2) of hot spring origin. Indian Journal of Microbiology. 2018;58:39–50. doi: 10.1007/s12088-017-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat B, Bajaj BK. Hypocholesterolemic and bioactive potential of exopolysaccharide from a probiotic Enterococcus faecium K1 isolated from kalarei. Bioresource Technology. 2018;254:264–267. doi: 10.1016/j.biortech.2018.01.078. [DOI] [PubMed] [Google Scholar]
- Choudhuri I, Khanra K, Pariya P, Maity GN, Mondal S, Pati BR, Bhattacharyya N. Structural characterization of an exopolysaccharide isolated from Enterococcus faecalis, and study on its antioxidant activity, and cytotoxicity against HeLa cells. Current Microbiology. 2020;77:3125–3135. doi: 10.1007/s00284-020-02130-z. [DOI] [PubMed] [Google Scholar]
- Coelho MC, Malcata FX, Silva CCG. Lactic acid bacteria in raw-milk cheeses: From starter cultures to probiotic functions. Foods. 2022;11(15):2276. doi: 10.3390/foods11152276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daba GM, Elnahas MO, Elkhateeb WA. Contributions of exopolysaccharides from lactic acid bacteria as biotechnological tools in food, pharmaceutical, and medical applications. International Journal of Biological Macromolecules. 2021;173:79–89. doi: 10.1016/j.ijbiomac.2021.01.110. [DOI] [PubMed] [Google Scholar]
- Degeest B, Mozzi F, De Vuyst L. Effect of medium composition and temperature and pH changes on exopolysaccharide yields and stability during Streptococcus thermophilus LY03 fermentations. International Journal of Food Microbiology. 2002;79:161–174. doi: 10.1016/S0168-1605(02)00116-2. [DOI] [PubMed] [Google Scholar]
- Donlan RM. Biofilms: microbial life on surfaces. Emerging Infectious Diseases. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Du E, Xing DM, Yang A, Jiang EK. Optimization, purification and structural characterization of a dextran produced by L. mesenteroides isolated from Chinese sauerkraut. Carbohydrate Polymers. 2017;174:409–416. doi: 10.1016/j.carbpol.2017.06.084. [DOI] [PubMed] [Google Scholar]
- Elshaghabee FMF, Shawky IM, Elkashef H. Impact of different types of exopolysaccharide-producing adjunct starter cultures on enhancement of Karish cheese properties. Egyptian Journal of Chemistry. 2022;65:677–683. [Google Scholar]
- Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: An emergent form of bacterial life. Nature Reviews Microbiology. 2016;14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- Fuso A, Bancalari E, Castellone V, Caligiani A, Gatti M, Bottari B. Feeding lactic acid bacteria with different sugars: Effect on exopolysaccharides (EPS) production and their molecular characteristics. Foods. 2023;12:215. doi: 10.3390/foods12010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galié S, García-Gutiérrez C, Miguélez EM, Villar CJ, Lombó F. Biofilms in the food industry: Health aspects and control methods. Frontiers in Microbiology. 2018;9:898. doi: 10.3389/fmicb.2018.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez D, Rodríguez-Rubio L, Martínez B, Rodríguez A, García P. Bacteriophages as weapons against bacterial biofilms in the food industry. Frontiers in Microbiology. 2016;7:825. doi: 10.3389/fmicb.2016.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem AM, Gamal AA, Mansour NM, Salama BM, Hassanein NM, Awad GEA, Esawy MA. Optimization of Enterococcus faecalis Esawy KR758759 dextransucrase and evaluation of some dextran bioactivities. Biocatalysis and Agricultural Biotechnology. 2018;15:348–358. doi: 10.1016/j.bcab.2018.06.008. [DOI] [Google Scholar]
- Hobley L, Harkins C, MacPhee CE, Stanley-Wall NR. Giving structure to the biofilm matrix: an overview of individual strategies and emerging common themes. FEMS Microbiology Reviews. 2015;39:649–669. doi: 10.1093/femsre/fuv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayamanohar J, Devi PB, Kavitake D, Rajendran S, Priyadarisini VB, Shetty PH. Characterization of α-d-glucan produced by a probiont Enterococcus hirae KX577639 from feces of south Indian Irula tribals. International Journal of Biological Macromolecules. 2018;118:1667–1675. doi: 10.1016/j.ijbiomac.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Jia K, Tao X, Liu Z, Zhan H, He W, Zhang Z, Zeng Z, Wei H. Characterization of novel exopolysaccharide of Enterococcus faecium WEFA23 from infant and demonstration of its in vitro biological properties. International Journal of Biological Macromolecules. 2019;128:710–717. doi: 10.1016/j.ijbiomac.2018.12.245. [DOI] [PubMed] [Google Scholar]
- Kanamarlapudi SLRK, Muddada S. Characterization of exopolysaccharide produced by Streptococcus thermophilus CC30. BioMed Research International. 2017;2017:4201809. doi: 10.1155/2017/4201809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmani P, kumar RS, Yuvaraj N, Paari KA, Pattukumar V, Arul V. Production and purification of a novel exopolysaccharide from lactic acid bacterium Streptococcus phocae PI80 and its functional characteristics activity in vitro. Bioresource Technology. 2011;102:4827–4833. doi: 10.1016/j.biortech.2010.12.118. [DOI] [PubMed] [Google Scholar]
- Kanmani P, Suganya K, kumar RS, Yuvaraj N, Pattukumar V, Paari KA, Arul V. Synthesis and functional characterization of antibiofilm exopolysaccharide produced by Enterococcus faecium MC13 isolated from the gut of fish. Applied Biochemistry and Biotechnology. 2013;169:1001–1015. doi: 10.1007/s12010-012-0074-1. [DOI] [PubMed] [Google Scholar]
- Khuri AI, Mukhopadhyay S. Response surface methodology. WIREs Computational Statistics. 2010;2:128–149. doi: 10.1002/wics.73. [DOI] [Google Scholar]
- Korcz E, Varga L. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry. Trends in Food Science & Technology. 2021;110:375–384. doi: 10.1016/j.tifs.2021.02.014. [DOI] [Google Scholar]
- Mahapatra S, Banerjee D. Production and structural elucidation of exopolysaccharide from endophytic Pestalotiopsis sp. BC55. International Journal of Biological Macromolecules. 2016;82:182–191. doi: 10.1016/j.ijbiomac.2015.11.035. [DOI] [PubMed] [Google Scholar]
- Niknezhad SV, Asadollahi MA, Zamani A, Biria D, Doostmohammadi M. Optimization of xanthan gum production using cheese whey and response surface methodology. Food Science and Biotechnology. 2015;24:453–460. doi: 10.1007/s10068-015-0060-9. [DOI] [Google Scholar]
- Rahnama Vosough P, Edalatian Dovom MR, Habibi Najafi MB, Javadmanesh A, Mayo B. Biodiversity of exopolysaccharide-producing lactic acid bacteria from Iranian traditional Kishk and optimization of EPS yield by Enterococcus spp. Food Bioscience. 2022;49:101869. doi: 10.1016/j.fbio.2022.101869. [DOI] [Google Scholar]
- Sajna KV, Sukumaran RK, Gottumukkala LD, Jayamurthy H, Dhar KS, Pandey A. Studies on structural and physical characteristics of a novel exopolysaccharide from Pseudozyma sp. NII 08165. International Journal of Biological Macromolecules. 2013;59:84–89. doi: 10.1016/j.ijbiomac.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Singh V, Haque S, Niwas R, Srivastava A, Pasupuleti M, Tripathi CKM. Strategies for fermentation medium optimization: An in-depth review. Frontiers in Microbiology. 2017;7:2087. doi: 10.3389/fmicb.2016.02087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen HM, Rochfort KD, Maye S, MacLeod G, Brabazon D, Loscher C, Freeland B. Exopolysaccharides of lactic acid bacteria: Production, purification and health benefits towards functional food. Nutrients. 2022;14:2938. doi: 10.3390/nu14142938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylan O, Yilmaz MT, Dertli E. Partial characterization of a levan type exopolysaccharide (EPS) produced by Leuconostoc mesenteroides showing immunostimulatory and antioxidant activities. International Journal of Biological Macromolecules. 2019;136:436–444. doi: 10.1016/j.ijbiomac.2019.06.078. [DOI] [PubMed] [Google Scholar]
- Tilwani YM, Lakra AK, Domdi L, Yadav S, Jha N, Arul V. Optimization and physicochemical characterization of low molecular levan from Enterococcus faecium MC-5 having potential biological activities. Process Biochemistry. 2021;110:282–291. doi: 10.1016/j.procbio.2021.08.021. [DOI] [Google Scholar]
- Venkatesh P, Balraj M, Ayyanna R, Ankaiah D, Arul V. Physicochemical and biosorption properties of novel exopolysaccharide produced by Enterococcus faecalis. LWT-Food Science and Technology. 2016;68:606–614. doi: 10.1016/j.lwt.2016.01.005. [DOI] [Google Scholar]
- Zannini E, Waters DM, Coffey A, Arendt EK. Production, properties, and industrial food application of lactic acid bacteria-derived exopolysaccharides. Applied Microbiology and Biotechnology. 2016;100:1121–1135. doi: 10.1007/s00253-015-7172-2. [DOI] [PubMed] [Google Scholar]
- Zanzan M, Achemchem F, Hamadi F, Latrache H, Elmoslih A, Mimouni R. Anti-adherence activity of monomicrobial and polymicrobial food-derived Enterococcus spp. biofilms against pathogenic bacteria. Current Microbiology. 2023;80:216. doi: 10.1007/s00284-023-03326-9. [DOI] [PubMed] [Google Scholar]
- Zanzan M, Ezzaky Y, Achemchem F, Elmoslih A, Hamadi F, Hasnaoui A, Ait-Ali M. Optimisation of thermostable exopolysaccharide production from Enterococcus mundtii A2 isolated from camel milk and its structural characterisation. International Dairy Journal. 2023;147:105718. doi: 10.1016/j.idairyj.2023.105718. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.