Abstract
Pierre Robin Sequence (PRS), a rare congenital disorder, is a triad of micrognathia, glossoptosis, and tongue based airway obstruction (TBSO). It may occur as isolated anomaly (iPRS) or as a part of a syndrome (sPRS), like that seen in association with Stickler Syndrome. Approximately 20% of children with PRS have congenital heart diseases. To the best of our knowledge this case of a one-day old infant is the first one to be reported as having two heart defects; patent ductus arteriosus and patent foramen ovale in Pierre Robbin Sequence child.
Keywords: Pierre Robbin Sequence, Type III Stickler Syndrome, Micrognathia, Patent Foramen Ovale
Introduction
Pierre Robin Sequence (PRS), a rare congenital disorder, with probability of occurrence in 1/8500–1/20,000 births, was first addressed in 1923 by Pierre Robin [1, 2]. The standardized and most accepted definition of Pierre Robin Sequence (PRS) consists of a triad of micrognathia, glossoptosis, and tongue based airway obstruction (TBSO), usually associated with cleft palate. Mandibular hypoplasia, which occurs between weeks 7–11 of gestation, results in superior and retro positioning of tongue, preventing palatal shelf fusion and causing micrognathia and glossoptosis [3]. It may occur as isolated anomaly (iPRS) or as a part of a syndrome (sPRS). The incidence of sPRS ranges from 30 to 80%, and this form has a higher incidence of airway obstruction compared to that of iPRS. It is associated with Stickler Syndrome, where patients show flat midface with a depressed nasal bridge, reduced nasal protrusion, anteverted nares and micrognathia [4]. PRS can be managed through both -non operative and operative techniques. Non-operative management includes prone positioning, nasopharyngeal airway and apnea monitoring. Operative management includes tongue lip adhesion, mandibular distraction, and tracheostomy. sPRS requires immediate surgical management, often involving tracheostomy, to achieve a stable airway [5].
Approximately 20% of children with PRS have congenital heart diseases, which may increase the risk of pulmonary hypertension and cor pulmonale and eventually lead to chronic obstructive apnea [6]. A multidisciplinary approach is important early on to address any feeding or breathing difficulties [5].
Not much such cases have been reported in Asia, this is a case of syndromic PRS child born with two heart defects; Patent Ductus Arteriosus (PDA) and Patent Foramen Ovale (PFO).
Case Presentation
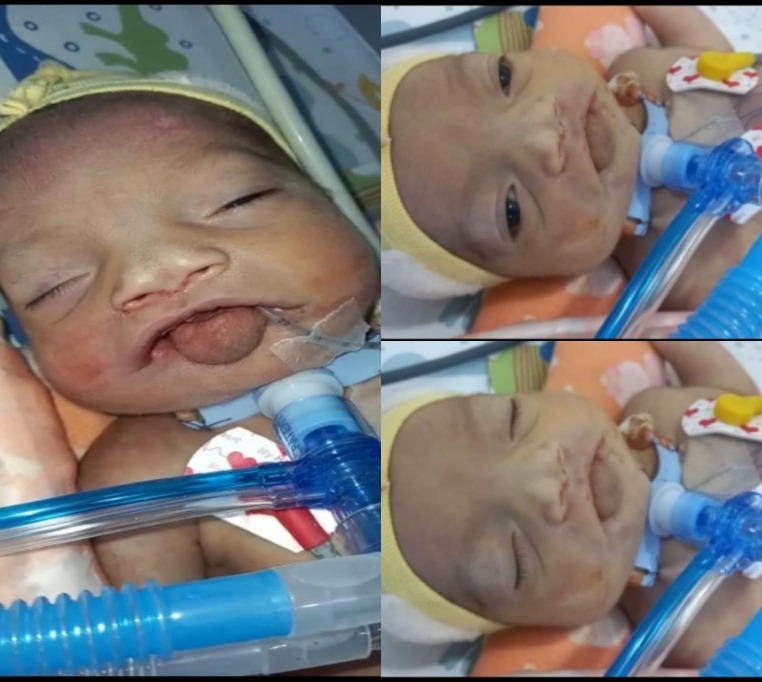
A one-day old female infant, weighing 2.3 kg, born via cesarean section at 35 weeks of gestational age, presented to tertiary care hospital with chief complaints of cyanosis soon after birth, superior ankyloglossia and micrognathia. As per record, the 7month anomaly scan showed evidence of cleft palate and polyhydramnios on antenatal scan. Fetal echocardiogram showed tricuspid valve stenosis, right valve hypoplasia, ventricular and atrial septal defects. Post-natal, spontaneous breathing was absent and infant had poor APGAR score. CPR was done for 3 min for the infant to revive, having heart rate of 100 bpm. Oral intubation was attempted but failed so a trial of nasal intubation was given but upper airway was found obstructed. Therefore, finger guided laryngeal mask airway (LMA) size 1 was placed blindly and 2 L oxygen was given through it, which improved saturation to 94%. The infant underwent post-natal echocardiogram reporting patent ductus arteriosus and patent foramen ovale. Chest x-ray was normal. Upon examination, child had glossoptosis, micrognathia and monophasic inspiratory stridor secondary to upper airway obstruction (thus, labelled as Piere robin sequence), flat midface with depressed nasal bridge, short nose, anteverted nose, hypertelorism but no ocular findings as per ophthalmologists (corresponding to associated Stickler syndrome type III) as shown in Fig. 1. Family history was unremarkable except that her older brother was born with spina bifida.
Fig. 1.
child born with glossoptosis, micrognathia (thus, labelled as Pierre Robbin Sequence), flat midface with depressed nasal bridge, short and anteverted nose, hypertelorism but no ocular findings as per ophthalmologists (corresponding to associated Stickler syndrome type III).
After few hours her condition deteriorated, with saturation dropping to 65%. Umbo was done via LMA, which brought saturation to 95% for a short span of time. She was shifted to operation theater (OT) on urgent basis, where tracheostomy was done via 2.5 size neo trach tube to maintain airway. On giving 5 L oxygen via tracheostomy, saturation was maintained on 99%. After 24 h, she started to gasp and was cyanosed. She was kept on ventilator, CMV mode with PEEP 6 and FiO2 100%, and double ionotropic support. She later developed respiratory acidosis with perfusion rate of 3 s. Frequent episodes of dropping saturation, least recorded below 40%, patient expired, the following day due to cardiac arrest.
Discussion
Pierre Robin Sequence (PRS), previously known as Pierre robin syndrome, first addressed in 1923 by Pierre Robin [1, 2]. Arising in 1 in 8500 to 1 in 20,000 live births, the prevalence of PRS in cleft population as reported in India is about 4.44%, [7] emphasizing the rarity of this disease in Asia.
Known as a sequence and not syndrome, PRS consist of early gestational mandibular hypoplasia (micrognathia) causing protrusion of tongue (i.e. glossoptosis), preventing fusion of palatal shelves posteriorly, leading to tongue based airway obstruction (TBSO). Subtypes are recognized as isolated PRS (iPRS) in 40% and syndromic PRS (sPRS) in 25%, where airway obstruction is more common in sPRS variant as reported in this case as well [8].
Number of syndromes can be associated with PRS, such as velocardiofacial, Treacher Collins and Stickler syndrome (SS) [4]. One third of patients with associated malformation presents with Stickler syndrome (SS), which has ocular and systemic manifestations, along with abnormalities in the production of collagen types II, IX and XI. Divided into six subtypes, sPRS associated with SS is commonly seen in type I, where triad of sPRS is associated with arthropathy, deafness and ocular findings, mainly magalopthalmos and/or high myopia [9].
However, this case is unique as patient had sPRS associated with type III SS instead. Type III SS is also known as non-ocular SS, i.e. patient showing flat midface, depressed nasal bridge, reduced nasal protrusion, anteverted nares, micrognathia, glossoptosis, but no ocular abnormality. Airway obstruction is frequent with sPRS comparatively therefore immediate tracheostomy is often performed to achieve stable airway, as done in our case as well. Despite the triad, about 20% of PRS children has congenital heart disease namely, ventricular septal defects, patent ductus arteriosus, or atrial septal defect [6]. To the best of our knowledge this case is the first one to be reported as having two; patent ductus arteriosus and patent foramen ovale in Pierre Robbin Sequence child. Although the mortality of 10% is significantly associated with sPRS, it is not due to single cardiac anomaly. However, sPRS with more than one heart defect, despite urgent appropriate intervention, can cause cardiac arrest, ultimately reducing the life expectancy of PRS child [10]. Thus it highlight the importance of pre-birth identification and counselling of parents regarding potentially reduce life expectancy of sPRS child.
Acknowledgements
None to declare.
Author’s contribution
All authors contributed to the study conception and design. Material preparation and data collection were performed by Soubia Akhtar, Iqra Shahab and Abdul Rauf Shaikh. The first draft of the manuscript was written by Yumna Afzal. Review and editing was done by Muhammad Wasif and Rahim Dhanani. All authors read and approved the final manuscript.
Funding
The authors did not receive support from any organization for the submitted work.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Approval was obtained from the ethics committee of Dr. Ziauddin Hospital for this specific case report.
Informed consent for participation
Written informed consent was obtained from the parents.
Informed consent for publication
The participant’s parents have consented to the submission of the case report to the journal.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Amin MA, Shawon TA, Shaon NK, Nahin S, Fardous J, Hawlader MD. A case of Pierre Robin syndrome in a child with no soft palate and Complications from Pneumonia in Bangladesh. Clin Case Rep. 2023;11(5):e7350. doi: 10.1002/ccr3.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearl W. Congenital Heart Disease in the Pierre Robin syndrome. Pediatr Cardiol [Internet] 1982;2(4):307–309. doi: 10.1007/BF02426978. [DOI] [PubMed] [Google Scholar]
- 3.Scott AR, Mader NS. Regional variations in the presentation and surgical management of Pierre Robin sequence. Laryngoscope. 2014;124(12):2818–2825. doi: 10.1002/lary.24782. [DOI] [PubMed] [Google Scholar]
- 4.Karempelis P, Hagen M, Morrell N, Roby BB. Associated syndromes in patients with Pierre Robin Sequence. Int J Pediatr Otorhinolaryngol [Internet] 2020;131(109842):109842. doi: 10.1016/j.ijporl.2019.109842. [DOI] [PubMed] [Google Scholar]
- 5.Biskup N, Francis SH. Heart Failure in an infant with Pierre Robin sequence: is there a diagnostic test to aid in treatment planning and monitoring? Cleft Palate Craniofac J [Internet] 2015;52(6):e205–e209. doi: 10.1597/14-265. [DOI] [PubMed] [Google Scholar]
- 6.Javed N, Malik J (2021 Mar-Apr) Pierre Robin sequence with tetralogy of Fallot: an unusual finding. Int J Health Sci (Qassim) 15(2):58–60 PMID: 33708045; PMCID: PMC7934134 [PMC free article] [PubMed]
- 7.Joseph J. Pierre Robin Sequence/Pierre Robin malformation. Asian J Nurs Educ Res. 2016;6(2):265–273. doi: 10.5958/2349-2996.2016.00051.3. [DOI] [Google Scholar]
- 8.Smith MC, Senders CW. Prognosis of airway obstruction and feeding difficulty in the Robin sequence. Int J Pediatr Otorhinolaryngol. 2006;70(2):319–324. doi: 10.1016/j.ijporl.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Snead M Stickler syndrome, ocular-only variants and a key diagnostic role for the ophthalmologist. Eye (London). 2011-11;25:1389 – 400 [DOI] [PMC free article] [PubMed]
- 10.Logjes RJH, Haasnoot M, Lemmers PMA, Nicolaije MFA, van den Boogaard MH, van der Mink AB, Breugem CC. Mortality in Robin sequence: identification of risk factors. Eur J Pediatr. 2018;177(5):781–789. doi: 10.1007/s00431-018-3111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]