Abstract
Background:
In the current era vaccine-associated lymphadenopathy (VAL) is not an uncommon presentation on 18F-FDG PET/CT examinations in patients inoculated with Coronavirus disease 2019 (COVID-19) vaccination. In this study, we are presenting data of VAL on 18F-FDG PET/CT regarding its prevalence, temporal response to vaccination and imaging characteristics of VAL.
Methods:
Seventy-eight (78) consecutive vaccinated breast cancer (BC) patients who had 18FDG PET/CT were retrospectively analyzed. All patients had COVID-19 vaccine shots in contralateral arms and none in breast cancer site axilla (BSA). In 35 patients 18FDG avid nodes were found in vaccine site axilla (VSA). In 25 patients 18FDG avid nodes were found in BSA. Morphological criteria on CT images like size, presence of fatty hila and fat stranding of axillary nodes were analyzed. Metabolic criteria on PET images like SUVmax of nodes and liver as reference were also measured.
Results:
Out of 78 patients, 35 had positive nodes in VSA (45% prevalence) and 25/78 had BSA (33% prevalence). Mean duration of COVID-19 vaccination in each group was 8 ±04 week (non-significant p-value). On CT images, 18FDG avid nodes in VSA were significantly smaller (10 ± 03 mm) and with intact fatty hila without fat stranding than nodes in BSA with loss of fatty hila (25 ±10 mm; p <0.0001). Mean SUVmax of nodes in VSA was significantly lower (2.4 ±1.1) than nodes in BSA (10.2 ±5.5 – p-value <0.0001). Nodes in VSA showed a significant positive linear correlation between size and SUVmax (p-value 0.00001). Similarly, nodes in VSA showed a significant negative linear correlation between duration and SUVmax (p-value 0.00003). In VSA group, 03 patients having SUVmax >2 SD of Hepatic SUVmax were subjected to ultrasound guided fine needle aspiration (FNA) and turned out to be metastatic in nature.
Conclusion:
In COVID-19 vaccinated patients with BC, 18FDG avid nodes in VSA may pose diagnostic challenge. However, morphological (size < 10 mm short axis, intact fatty hila without fat stranding) and metabolic criteria (SUVmax <2.4 with negative correlation with time of inoculation) have higher diagnostic accuracy in resolving the dilemma. Nodes in VSA having SUVmax > 2 SD of hepatic SUVmax should be considered for FNA to rule out possible metastasis.
Key Words: COVID-19 vaccine, vaccine associated lymphadenopathy, vaccine site axilla, FDG PET/CT, breast cancer
Introduction
Since 2020, mass inoculation of COVID1-19 vaccine has resulted in significantly reduced incidence, mortality and morbidity too. However, after vaccine deployment in deltoid, enlargement of axillary, supraclavicular and cervical nodes is quite common (Baden et al., 2021). Lymph nodes that are housing T, B and antigen presenting cells (APC) have an important role in the immune response to vaccination. Once vaccine is injected into the muscle, APC are transported to the regional lymph nodes, and in some cases it may proceed to the next nearest lymphatic chain stations (Irvine et al., 2020). In lymph nodes, peptide antigens of APC provoke (1) cellular response with the formation of cytotoxic T lymphocytes capable of directly killing infected cells and (2) humoral response consists of proliferation and formation of matured B-cells as antibody secreting plasma cells and memory B-cells (Bettini and Locci, 2021). Patients having fluorodeoxyglucose positron emission tomography / computerized tomography (18FDG PET/CT) imaging with history of COVID-19 vaccination, 18FDG avid nodes in ipsilateral axilla or neck is common and poses challenge to interpretation by confounding disease characterization, staging and response assessment. The major impact of vaccine associated lymphadenopathy (VAL) have been describe primarily in context of morphological and metabolic imaging of breast (Edmonds et al., 2021). In recent years, many studies have been published which demonstrated increased 18FDG uptake in post COVID-19 associated lymphadenopathy (Bernstine et al., 2021; Cohen et la., 2021). , The aim of present study was to describe typical and distinctive features of VAL in 18FGD PET/CT imaging in order to increase the confidence of reading nuclear physicians and to mitigate unjustified diagnostic and interventional procedures in breast cancer patients.
Materials and Methods
This was a retrospective study conducted from March 2021 till February 2022 at PET/CT imaging services of Department of Radiology, Aga Khan University Hospital, Karachi, Pakistan. This study was approved by ethical review committee (ERC - 2022-7377-21106). We included 78 consecutive breast cancer patients who were referred for 18-Flourodeoxyglucose positron emission tomography / computerized tomography (18FDG PET/CT) for staging, restaging or response assessment. All patients had COVID-19 vaccine shots in contralateral arms and none in breast cancer site axilla (BSA). Patients not showing 18FDG avid nodes in ipsilateral or contralateral axilla were excluded. Demographic data like age (years), body mass index (kg/m2) and duration of COVID-19 vaccination (weeks) were measured. Morphological criteria on CT images like size (<10 mm in short axis), presence of fatty hila and fat stranding of axillary nodes were analyzed. Metabolic criteria on PET images like maximum standardized uptake value (SUVmax) of positive axillary nodes and liver as reference were also measured.
18FDG PET/CT Imaging: 18FDG PET/CT was performed as per institutional protocol adopted from European Association of Nuclear Medicine (EANM) guidelines (Boellaard et al., 2015). All patients had 4-6 hour fasting (only plain water was allowed) and a fasting blood sugar less than 200 mg% before receiving an intravenous 18FDG dose of 3 MBq/Kg in the uptake room. During uptake period (55 -75 minute) patients were requested to laydown comfortably and allowed to take about 500-1000 ml of plain water. Bladder was emptied prior to call the patient for PET/CT imaging suite equipped with Celesteion, Toshiba, Japan. A low dose CT examination (mid brain to mid-thigh) without intravenous contrast from head to toe followed by acquisition of PET imaging using 3 minute/bed position from toe to head in all patients. CT images were used to measure size (>10 mm short axis as enlarged), presence of fatty hila and fat stranding of axillary nodes. PET images were used to measure maximum standardized uptake value (SUVmax) of 18FDG avid axillary nodes and also liver (right hepatic lobe) as reference.
Statistical Analysis
Commercially available packages Microsoft excel 2010, Medcalc® and statistical package for social sciences (SPSS 19®, IBM, Armonk, New York, US) was used. Continuous variables were described by mean ± standard deviation. Pearson correlation coefficients were analyzed to evaluate linear correlation of node size, duration of vaccine and SUVmax. Statistical significance was defined as P < 0.05.
Results
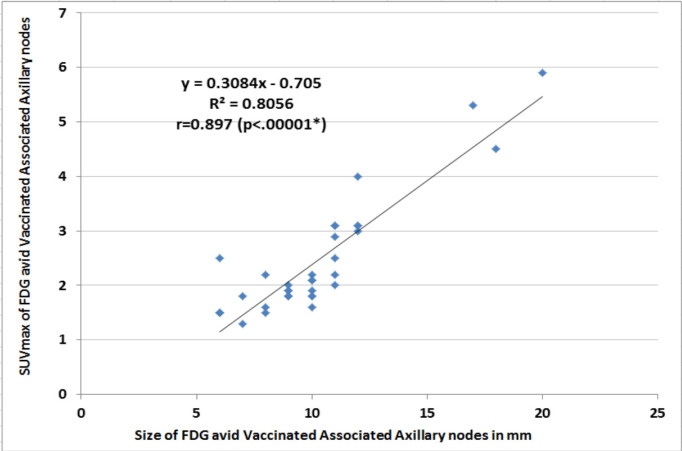
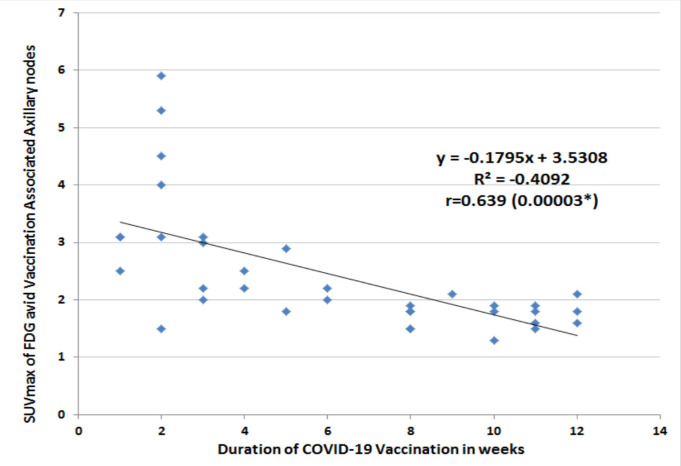
Out of 78 consecutive patients, 35 patients had positive nodes in vaccinated site axilla (VSA; 45% prevalence) and 25/78 had breast cancer axilla (BSA: 33% prevalence). Eighteen patients without 18FDG avid axillary nodes were excluded. No significant difference was seen for age, BMI and duration of vaccination between VSA and BSA groups (non-significant p-values) (Table 1). In 08 patients (23%) 18FDG uptake was seen over deltoid (injection site of vaccine) with positive nodes in VSA group (Double Sign; Figure 1). On CT images, 18FDG avid nodes in VSA group were significantly smaller (10 ± 03 mm), with intact fatty hila and without fat stranding than nodes in BSA group (25 ±10 mm; p <0.0001) with loss of fatty hila and fat stranding as well. Mean SUVmax of nodes in VSA group was significantly lower (2.4 ±1.1) than nodes in BSA group (10.2 ±5.5 – p-value <0.0001) (Table 1). 18FDG avid nodes in VSA group showed a significant positive linear correlation between size and SUVmax (p-value 0.00001) (Figure 2). Similarly, nodes in VSA group showed a significant negative linear correlation between duration and SUVmax (p-value 0.00003) (Figure 3). In VSA group, 03 patients having SUVmax >2 SD of Hepatic SUVmax were subjected to ultrasound guided fine needle aspiration (FNA) and turned out to have metastases (Figure 4).
Table 1.
Demographics of FDG Avid Axillary Nodes in Vaccinated Site and Breast Cancer Site of Axillary Nodes
| Variables | Vaccinated site FDG avid Axillary nodes (n=35) | BC site FDG avid Axillary nodes (n=25) |
Χ2 or t-test values | p value |
|---|---|---|---|---|
| Age in years Mean ± SD | 58 ± 15 | 54 ± 14 | -1.047 | 0.2996 |
| BMI (Kg/m2) Mean ± SD | 31.196 ± 7.377 | 32.871 ± 7.695 | 0.852 | 0.3979 |
| Hepatic SUVmax Mean ± SD | 1.997 ± 0.594 | 2.033 ± 0.585 | 0.233 | -0.8167 |
| SUVmax of FDG avid axillary nodes Mean ± SD | 2.4 ± 1.1 | 10.2 ± 5.5 | 8.19 | <0.0001* |
| Size of FDG avid axillary nodes (mm) Mean ± SD | 10 ± 03 | 25 ± 10 | 0.386 | <0.0001* |
| Duration of COVID-19 vaccination in week Mean ± SD | 06 ± 04 | 06 ± 04 | 0 | 1 |
| Double Sign | 08 (23%) | -- | NA | NA |
*p<0.05; BC, Breast Cancer; SD, Standard Deviation; BMI, Body Mass Index; SUV, Standardized Uptake Value; FDG, Flurodeoxy glucose
Figure 1.
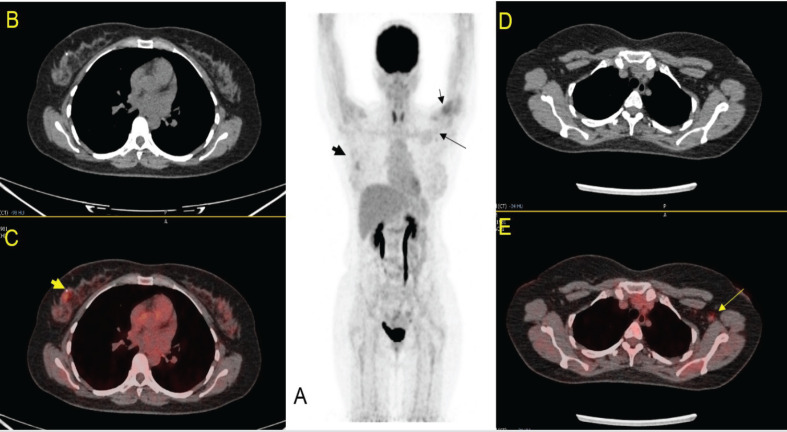
45 Years Female, CA Right Breast for Staging, h/o COVID-19 Vaccine in Left Deltoid within 4 Weeks. Focal 18FDG uptake seen over right breast (thick arrow in 1A & 1C). Double Sign seen on left (left deltoid injection site [small arrow in 1A] and small node in left axilla [long arrow in 1A & 1E]).
Figure 2.
Linear Regrsssion Analysis of SUVmax and Size of FDG Avid Vaccinated Associated Axillary Nodes. *p<0.05
Figure 3.
Linear Regrsssion Analysis of Duration of COVID-19 Vaccination and SUVmax of FDG Avid Vaccinated Associated Axillary Nodes. *p<0.05
Figure 4.
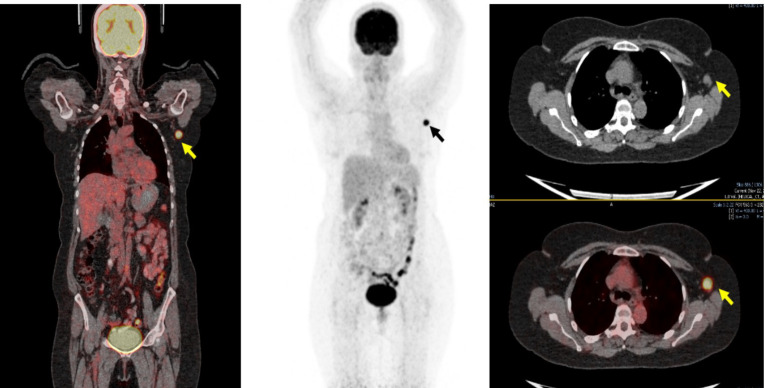
37 Years Female, Right Breat Cancer Survivor with COVID-19 Vaccine in Left Deltoid 04 Weeks back and has had a Survillence 18FDG PET/CT Scan. Solitary enlarged hypermetabolic left axilary node seen (thick arrow) which turned out positive for metastasis on ultrasound guided fine needle aspitaion
Discussion
Studies have highlighted that mRNA vaccines elicit the prominent germinal center response in lymph nodes and formation of matured B-cells and memory B-cells (Bettini and Locci , 2021).3 18FDG positivity in ipsilateral nodes to the vaccine injection site reflects hypermetabolism and cellular proliferation within the involved lymph nodes. A recent study has shown that 18FDG avid nodes in post-vaccinated individuals is a strong predictor of positive serology in these patients (Cohen et al., 2021).6 But appearance of VAL also poses a diagnostic challenge to 18FDG PET/CT readers in staging, restaging and response assessment.
The primary aim of our study was to recognize typical post-COVID-19 vaccinated pattern of 18FDG PET/CT in breast cancer patients. Presence of 18FDG uptake over deltoid muscles and ipsilateral axillary nodes (double sign) was found in about 23% of participants of this study. Double sign is a highly specific imaging pattern which helps the reading physicians to avoid false disease upstaging or further unjustified work-up. Study have shown that 18FDG activity in lymph nodes is associated with immune system activation while deltoid activity is due to post-traumatic inflammatory changes (Bernstine et al., 2021). Incidence of double sign in our study (23%) was in close proximity to 20% what has recently been published (Orevi et al., 2021). Therefore, standalone 18FDG avid axillary nodes (without deltoid uptake) can be a diagnostic challenge in patients without double sign.
In our study incidence of 18FDG avid bilateral axillary nodes was 77% (60/70) and for VSA it was 45%. This incidence is relatively lower than reported by Orevi et al., (2021) as 59% and Skawran et al., (2022) as 54%. The possible explanation of lower incidence in current study could be due to smaller sample size and non-homogeneity in time of scan and date of vaccination in relation with above mentioned published studies.
Mean size of nodes in VSA was significantly smaller than BSA (10 ± 03 Vs 25 ± 10). This is in accordance with study published by Cohen (2021) who found that majority of nodes in VSA were less than 10 mm size criteria of normalcy. Furthermore, we did not find loss of fatty hilum and fat stranding in 18FDG avid nodes in VSA than nodes in BSA. Large body of data show that larger size, round shape, absence of the fatty hilum, and increased cortical thickness (>3 mm) are strong predictors of malignancy (Choi et al., 2009). However, according to a recent review, VAL may have subtle fat stranding also (Brown et al., 2021). While loss of fatty hila in nodes of non-vaccinated axilla is considered to be a strong predictor of metastasis (Chen et al, 2018). Similarly, the metabolic activity (SUVmax) of nodes in VSA was significantly low (mean SUVmax 2.4 ± 1.1). Cohen (2021) has categorized the VAL on basis of SUVmax into Grade-I (SUVmax <2.2), Grade-II (>4 SUVmax ≥2.2) and Grade-III (SUVmax ≥ 4).11 Our finding is in accordance with published study where 78% of VSA nodes had SUVmax <4 (Grad-I,II) (Cohen et al., 2021). Intensity of 18FDG is an indicator of magnitude of immune response in said node and studies have shown higher SUVmax in nodes VSA after booster dose due to higher immune response 9Cohen et al., 2021). Similarly nodes in BSA had significantly higher SUVmax (Grade-III) in our study which reflects the viable tumor burden in these nodes. In VSA group, incidence of malignant node (SUVmax > 2 SD of Hepatic SUVmax) was 8% which is relatively higher than reported 5.1% (Cohen et al., 2021).This discordance could be due to smaller sample size or aggressive tumor behavior in our cohort. However, despite of smaller numerical value, a hot node in VSA (SUVmax > 2 SD of Hepatic SUVmax) must be given due consideration by subsequent ultrasound to observe temporal improvement in it morphology. However, hot nodes in VSA group not showing improvement on subsequent ultrasound must be biopsied to rule out possible metastasis. This notion is also supported by a significant positive correlation between size and metabolic activity in VSA nodes in our study. This is likely due to increase viable tumor load in larger node and possible underestimation of viable disease in normal size node due to partial volume effect (Cysouw et al., 2017). We also observed a significant negative correlation between SUVmax of VSA node(s) and duration of vaccination. This finding is in accordance with most of published studies too (Orevi et al., 2021; Cohen et al., 2021).9, 11
Our study has some important limitations as well. These include its retrospective nature and small sample size. We did not biopsy every node (except 03 hot nodes in VSA) and we did not have follow-up studies as well.
We conclude that in COVID-19 vaccinated patients with BC, enhanced 18FDG uptake on PET/CT by axillary nodes in VSA is quite common and may pose diagnostic dilemma. However, morphological (size < 10 mm, intact fatty hila without fat stranding) and metabolic criteria (SUVmax <2.4 with negative correlation with time of inoculation) have higher diagnostic accuracy in resolving the dilemma. Nodes in VSA having SUVmax > 2 SD of hepatic SUVmax should be considered for FNA to rule out possible metastasis.
Author Contribution Statement
Nosheen Fatima: Interpretation, drafting, final approval. Unaiza Zaman: Conception, Design, Critical revision. Areeba Zaman: Literature search, drafting, critical revision. Rabia Zaman: Drafting, Statistics. Sidra Zaman: Literature search, drafting, critical revision. Maseeh uz Zaman: Conception, interpretation, critical revision, final approval
Acknowledgements
This study is not funded by our institute or any other organization. There is no financial or institutional conflict of interest. The authors acknowledge the editors for their valuable comments to improve the manuscript. This study was approved by ethical review committee of Aga Khan University Hospital (ERC - 2022-7377-21106).
References
- Baden LR, El Sahly HM, Essink B, et al. COVE Study Group Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–16. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstine H, Priss M, Anati T, et al. Axillary lymph nodes hypermetabolism after BNT162b2 mRNA COVID-19 vaccination in cancer patients undergoing 18F-FDG PET/CT: A Cohort Study. Clin Nucl Med. 2021;46:396–401. doi: 10.1097/RLU.0000000000003648. [DOI] [PubMed] [Google Scholar]
- Bettini E, Locci M. SARS-CoV-2 mRNA vaccines: immunological mechanism and beyond. Vaccines. 2021;9:147. doi: 10.3390/vaccines9020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boellaard R, Bolton RD, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2 0. Eur J Nucl Med Mol Imaging. 2015;42:328–54. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Shah S, Dluzewski S, et al. Unilateral axillary adenopathy following COVID-19 vaccination: a multimodality pictorial illustration and review of current guidelines. Clin Radiol. 2021;76:553–8. doi: 10.1016/j.crad.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CF, Zhang YL, Cai ZL, et al. Predictive value of preoperative multidetector‐row computed tomography for axillary lymph nodes metastasis in patients with breast cancer. Front Oncol. 2018;8:666. doi: 10.3389/fonc.2018.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Ko EY, Han BK, et al. High-resolution ultrasonographic features of axillary lymph node metastasis in patients with breast cancer. Breast J. 2009;18:119–22. doi: 10.1016/j.breast.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Cohen D, Hazut KS, Cohen YC, et al. Correlation between BNT162b2 mRNA Covid-19 vaccine-associated hypermetabolic lymphadenopathy and humoral immunity in patients with hematologic malignancy. Eur J Nucl Med Mol Imaging. 2021;8:1–10. doi: 10.1007/s00259-021-05389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Krauthammer SH, Wolf I, Even-Sapir E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed by (18F)FDG PET-CT and relevance to study interpretation. Eur J Nucl Med Mol Imaging. 2021;48:1854–63. doi: 10.1007/s00259-021-05314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysouw MCF, Kramer GM, Schoonmade LJ, et al. Impact of partial-volume correction in oncological PET studies: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2017;44:2105–16. doi: 10.1007/s00259-017-3775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds CE, Zuckerman SP, Conant EF. Management of unilateral axillary lymphadenopathy detected on breast MRI in the era of coronavirus disease (COVID-19) vaccination. AJR. 2021;2021 doi: 10.2214/AJR.21.25604. (published online) [DOI] [PubMed] [Google Scholar]
- Irvine DJ, Aung A, Silva M. Controlling timing and location in vaccines. Adv Drug Deliv Rev. 2020;2020:91–115. doi: 10.1016/j.addr.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orevi M, Chicheportiche A, Ben Haim S. Lessons Learned from Post-COVID-19 Vaccination PET/CT Studies. J Nucl Med. 2021;63:453–60. doi: 10.2967/jnumed.121.262348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skawran S, Gennari AG, Dittli M, et al. (18F)FDG uptake of axillary lymph nodes after COVID-19 vaccination in oncological PET/CT: frequency, intensity, and potential clinical impact. Eur Radiol. 2022;32:508–16. doi: 10.1007/s00330-021-08122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]