Abstract
Aims:
Chemoradiotherapy is the standard treatment for advanced Oropharyngeal squamous cell carcinoma (OPSCC). Upcoming hypofractionation has led to better compliance and non-inferior results in various sites such as breast and prostate cancer etc. This study prospectively compared a dose-intensified schedule in advanced OPSCC with standard hypofractionation.
Materials and methods:
Patients with advanced stage III and IV OPSCC suitable for radical chemoradiotherapy were eligible. Patients were alternatively allocated to both the treatment arms. Arm A planned to receive 64 Gy in 25 fractions (#) with concurrent cisplatin and Arm B received standard fractionation 70 Gy in 35 # with concurrent cisplatin. All patients completed a median follow up of 6 to 18 months. The primary end point was acute toxicity (less than 3 months) and late toxicity at 1 year. Secondary end point was disease free survival and overall survival at 1 year.
Results:
44 patients in arm A and 49 patients in arm B were recruited over 18 months. 34 patients completed full-dose radiotherapy in both arms. Maximum acute toxicity in arm A in terms of skin reaction was Grade II in 47.05% cases and mucositis grade II in 67.6% cases. In arm B grade II skin toxicity was seen in 47.1% and mucositis grade II was seen in 79.4 % cases. Ryle’s tube dependency was seen in 38.2 % cases in arm A and 50% in arm B. Complete response rate at 3 months was equivalent in both arms in Arm A (100%), and in Arm B (96.7%). Disease free survival (DFS), Overall survival (OS) at 3 month, 6 months, and 12 months was comparable.
Conclusions:
64 Gy in 25 fractions with concomitant chemotherapy is tolerable in patients with equivalent results and better compliance. Shorter fractionation schedule is more acceptable and we look forward for more randomized control trials.
Key Words: Oropharynx, chemoradiotherapy, hypofractionation, squamous cell carcinoma
Introduction
Squamous cell carcinoma of head and neck (HNSCC) is being increasingly treated by multimodality approaches combining surgery, radiotherapy, and chemotherapy. Several randomized controlled trials using altered fractionation radiotherapy with or without chemotherapy have shown improvements in loco-regional tumor control as compared with conventional fractionation (Liu et al., 2018; Narvaez et al., 2021). Hypofractionation is done by increasing dose per fraction shortening overall treatment time compared to a conventional protocol. But increasing dose per fraction may result in increase in the incidence of late complications. An audit of hypofractionation data from the United Kingdom 55 Gy in 20 fractions (2.75 Gy/fraction) has been adopted by various centers (James et al., 2003; Williams et al., 2006). This regimen has the theoretical advantage that the treatment is completed beforeaccelerated repopulation becomes a significant radiobiologic factor. During the COVID-19 pandemic in the scarcity of various health and human resources across the world, there has been renewed interest in hypofractionatedchemoradiation and hypofractionated radiation for Head & Neck cancer (Gupta et al., 2020; Huang et al., 2020; Roques and Prestwich, 2020; Thomson et al., 2020) . Shortened radiotherapy schedules may be less vulnerable to treatment breaks (Huang et al., 2020). In the ASTRO-ESTRO consensus statement for the COVID-19 pandemic, while acknowledging a shortage of evidence, there was strong agreement among panelists on the use of hypofractionated radiation alone in locally advanced disease (Williams et al., 2006). There was also agreement to reserve the use of synchronous chemotherapy to standardly fractionationed or hypofractionated radiotherapy. The primary purpose of this paper was to investigate whether the use of different radiotherapy fractionation schedule with chemotherapy was correlated with efficacy, toxicity and overall survival outcomes as compared to conventional chemoradiotherapy.
Materials and Methods
This was a prospective randomized comparative study with two arms, conducted on a cohort of 93 patients diagnosed with Squamous Cell Carcinoma of the oropharynx, who required radiotherapy. Patients were recruited alternatively for the study (Flow diagram 1).
Inclusion criteria
• Histologically proven cases of Squamous Cell Carcinoma of the oropharynx.
• Karnofsky Performance Status > 70.
• Hemoglobin > 10 g/dL.
• Total leukocyte count > 4,000/mm3.
• Platelet count > 100,000/mm3.
• Normal renal and liver function tests.
• Patients provided written informed consent
Exclusion Criteria
• Prior radiation, surgery, or chemotherapy for any disease.
• Poor Karnofsky Performance Status (KPS).
• Any associated medical comorbidity.
• Evidence of metastasis.
Treatment Protocol
Pretreatment Evaluation: Before treatment initiation, a comprehensive evaluation of each patient was conducted, including medical history, physical examination, complete blood count, blood chemistry, CT or MRI of the head and neck, chest radiograph, and ultrasonography of the abdomen.
Simulation
Patients were simulated with a head and neck cast, and CT simulation was performed.
Treatment Planning
Treatment planning was carried out using the Dosisoft treatment planning system.
Treatment Execution
Radiotherapy was administered using the Bhabhatron II machine. The hypofractionation arm (Arm A) received a total dose of 64 Gy delivered in 25 fractions over 5 weeks at 2.56 Gy per fraction. Concurrently, patients received cisplatin (100 mg/m2) on Day 1 and Day 22. This treatment was divided into three phases:
Phase 1: External Beam Radiotherapy (EBRT) - 40.96 Gy in 16 fractions over 3.1 weeks using two parallel opposed fields.
Phase 2: EBRT - 12.8 Gy in 5 fractions over 1 week, targeting high-risk nodal disease after spine sparing to the clinical target volume (CTV).
Phase 3: EBRT - 10.24 Gy in 4 fractions over 0.6 weeks, targeting CTV and CTV-N after spine sparing to the gross tumor volume (GTV).
Conventional Arm (Arm B): Patients in this arm received a total dose of 70 Gy delivered in 35 fractions over 7 weeks at 2 Gy per fraction. They also received cisplatin (100 mg/m2) on Day 1 and Day 22. This treatment was divided into three phases, similar to Arm A.
Assessment and Outcomes
Treatment data, including radiotherapy start and completion dates, chemotherapy details, and toxicity assessments, were recorded weekly during treatment, at 1 month, 3 months, 6 months, and 1 year follow-up.
Overall Survival (OS) and Disease-Free Survival (DFS) were estimated using Kaplan-Meier survival plots.
Follow-up visits were analyzed until October 2022.
OS time was calculated from the date of registration with histopathologically proven diagnosis until the patient’s status at the last follow-up.
DFS was calculated from the end of treatment until the patient’s status at the last follow-up.
Acute toxicity was defined as any adverse effects noted during radiotherapy or within 3 months after completing radiotherapy.
Results
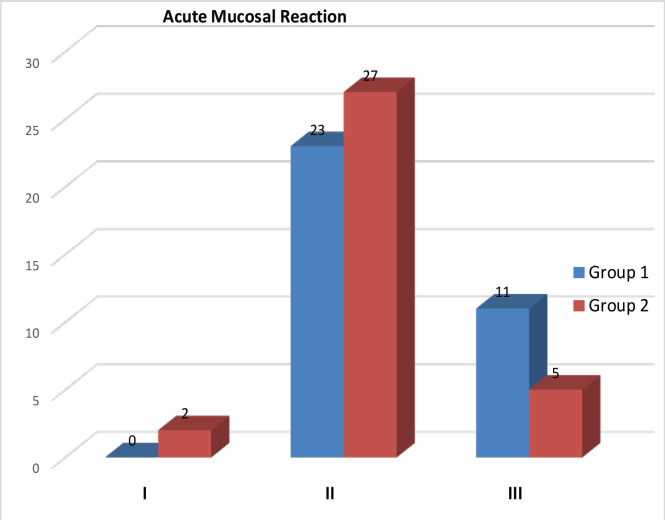
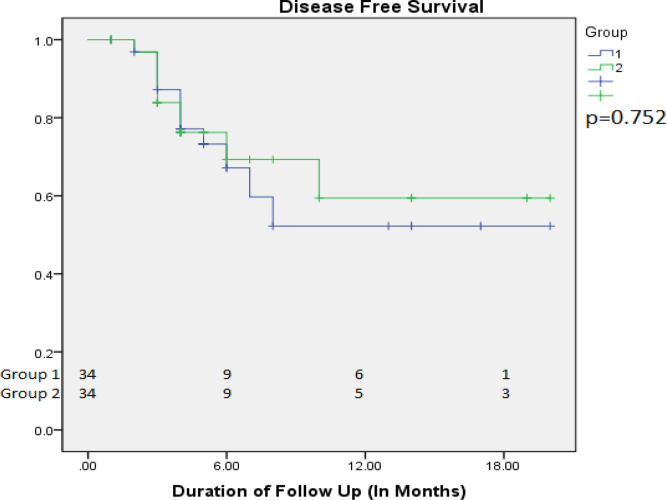
93 patients were recruited over 18 months, randomize in Arm A (44) and in Arm B (49) (Table 1). Out of which 10 (22.7%) and 15 (30.6%) patient in Arm A and Arm B respectively defaulted treatment, excluded from the study. Thirty four patients completed planned dose radiotherapy in both the arms with median treatment time of less than six weeks in Arm A while in Arm B it was more than eight weeks (P <0.001) . All patients completed a median follow up between 6 to 18 months. Grade I, II, III & IV skin reactions 12 (35.29%), 47.05 (10.34%),6 (17.64%),0 in Arm A, and 15 (44.1%),16 (47.1%), 3 (8%), 0 in Arm B patients respectively (Table 2). No grade IV skin toxicity noted in both the arms. In mucosal reaction we noticed Grade I,II,III,IV toxicity in was seen in 0, 23 (67.6 %), 11 (32.4 %), 0, in Arm A and 2 (5.9%), 27 (79.4), 5 (14.7%), 0 in Arm B patients respectively (acute toxicity). Maximum mucositis range lies between grade II and grade III toxicity which was manageable. Recurrence, was seen in 11 (32.35%) and 9 (26.47%) in Arm A and Arm B patients respectively (Figure 1). During treatment Ryles Tube dependency noticed in 13 (38.2%) and 17 (50%) in Arm A and Arm B patients respectively (p value <0.329). Maximum Fibrosis lies in range of grade II 26 (76.5%) and 20 (58.8%) in Arm A and Arm B patients respectively (p value< 0.002) (Figure 2). Maximum dysphagia seen in range of Grade I in 22 (64.7%) patients in both the arms. At last follow up 22 (64.7%) and 21(61.8%) patients were having no evidence of disease clinically in Arm A and Arm B respectively, while one patients shows residual disease at the end of treatment in both the arms, three patients brought dead during follow up in OPD in Arm B during covid-19 pandemic period. Disease free survival (DFS) at 2 months, 6 months with Mean ± SD in Arm A was 96.9 ± 3.1, 67.2 ± 9.5 and in Arm B was 96.8 ± 3.2,69.3 ± 9.8 respectively (p value<0.752) (Figure 3).Overall survival (OS) at 2 months, 6 months and 1 year with Mean ± SD in arm A was 97.1 ± 2.9, 93.8 ± 4.2, 83.4 ± 10.5, and in Arm B was 96.9 ± 3.1, 93.5 ± 4.4, 89.9 ± 5.5 respectively (p value<0.762) (Figure 4).
Table 1.
Demographic Profile of the Patients
| Patient profile | Arm A | Arm B |
|---|---|---|
| No.(%) | No.(%) | |
| Gender | ||
| Male | 32 (94.1) | 33 (97.1) |
| Female | 2 (5.9) | 1 (2.9) |
| Age | ||
| Max range(yrs) | 40-60 | 40-60 |
| Stage(cT) | ||
| cT1 | 0 | 0 |
| cT2 | 1 (2.9) | 0 |
| cT3 | 3 (8.8) | 3 (8.8) |
| cT4 | 30 (88.23) | 31 (91.17) |
| Stage(cN) | ||
| cN0 | 13 (38.2) | 8 (23.5) |
| cN1 | 4 (11.7) | 8 (23.5) |
| cN2a | 3 (8.8) | 2 (5.9) |
| cN2b | 9 (26.47) | 9 (26.47) |
| cN2c | 4 (11.7) | 6 (17.6) |
| cN3a | 0 | 0 |
| cN3b | 1 (2.9) | 1 (2.9) |
| Overall Treatment Time | ||
| Mean range(wks) | <6 | >8 |
| Addictions | ||
| Tobacco | 27 (79.4) | 28 (82.4) |
| Smoking | 32 (94.1) | 33 (97.1) |
| Alcohol | 26 (76.5) | 24 (70.6) |
Table 2.
Toxicity Profile of the Patients
| Toxicity | Arm A | Arm B |
|---|---|---|
| No. (%) | No. (%) | |
| Skin Toxicity | ||
| Grade I | 12 (35.3) | 15 (44.1) |
| Grade II | 16 (47.05) | 16 (47.1) |
| Grade III | 6 (17.64) | 3 (8.8) |
| Grade IV | 0 | 0 |
| Mucosal Toxicity | ||
| Grade I | 0 | 2 (5.9) |
| Grade II | 23 (67.6) | 27 (79.4) |
| Grade III | 11 (32.4) | 5 (14.7) |
| Grade IV | 0 | 0 |
| Ryle Tube Dependancy | ||
| Yes | 13 (38.2) | 17 (50) |
| No | 21 (61.8) | 17 (50) |
| Dysphagia | ||
| Grade I | 22 (64.7) | 22 (64.7) |
| Grade II | 12 (35.3) | 12 (35.3) |
| Grade III | 0 | 0 |
| Grade IV | 0 | 0 |
| Fibrosis | ||
| Grade I | 1 (2.9) | 12 (35.3) |
| Grade II | 26 (76.5) | 20 (58.8) |
| Grade III | 7 (20.6) | 2 (5.9) |
| Grade IV | 0 | 0 |
Figure 1.
Mucosal Reaction in Arm A and Arm B patients
Figure 2.
Fibrosis Lies in Arm A and Arm B patients
Figure 3.
Disease Free Survival (DFS) in Arm A and B
Figure 4.
Overall survival (OS) in Arm A and B
Flow Diagram 1.
Discussion
Altered fractionation has been time tested and has been undoubtly proven to be beneficial in various trials. March meta-analysis has shown a 3.4% OS benefit at 5 years for altered fractionation versus conventional fractionation and mostly for hyperfractionation (Baujat et al., 2010). Concomitant chemotherapy with standard fractionation has shown to offer improved LRC and survival in cancer patients. In another study by Sanghera et al., (2007) 81 patients of SCC of the larynx, oropharynx, oral cavity, and hypopharynx received 55 Gy in 20 fractions with concurrent chemotherapy. They reported 2-year local control rate of 75.4%, OS rate of 71.6% and DFS rate of 68.6% . Another multi-institutional trial of hypofractionated intensity-modulated radiation therapy for early stage oropharyngeal cancer without chemotherapy has shown better tumor control rates and reduced salivary toxicity (Eisbruch et al., 2010). Other trials in head and neck cancer have also shown the feasibility and tolerability of higher hypofractionated doses to the tune of 2.34 to 2.36 Gy/# (Bakst et al., 2011; Lauve et al., 2004). The LRC rate was nearly similar for hypofractionated group in comparison with a conventional group (76% vs. 80%) but in subgroup analysis benefit of hypofractionation was seen. In a study of oropharyngeal carcinoma hypofractionation dose of 64Gy/25# was tested and it was found to be feasible with no greater acute or late toxicity. They categorized patients on basis of smoking and HPV infection (Meade et al., 2018). In our study we used the same fractionation regimen with concurrent chemotherapy. Most of our patients in our study were smokers. The presentation of standardized late toxicity data in published reports remains limited. Comparisons of such toxicity data are hampered by a variety of different grading systems and the subjective nature of some assessments (Trotti and Bentzen, 2004). Detailed late toxicity was given for a small number of late surviving patients in the final report of the 94–01trial performed by the Grouped’OncologieRadiotherapieTete Et Cou. With a median follow-up of 5.5 years for the living patients, they reported the rate of late grade 3/4 toxicity to be as high as 56% in the combined chemoradiationarm (Denis et al., 2004). In a report by Staar et al., (2001) 30% of patients surviving > 2 years remained dependent on a feeding tube, with significantly more patients having swallowing problems in the accelerated chemoradiation arm. Such an increase in late toxicity has not been seen when a reduction in the total dose is made using acceleration in combination with chemotherapy. In addition, Denham et al., (1999) reported that the incidence of a prolonged confluent mucositis, which has been shown to predict for late mucosal reactions, was acceptable. In our study, indicating that increased dose per fraction (2.56 Gy vs. 2 Gy) influenced late radiation-related morbidity. With a lower biologic dose in terms of late reactions compared with 70 Gy in 35 fractions (using α/β ratio of 3 in linear quadratic model), this hypofractionatedschedule was associated with greater long-term toxicity. This, however, needs confirmation through more prospectively collected data, particularly randomized controlled trials, Most of the patients who had loco-regional failure were in the Stage III/IV groups with bulky nodal status at initial presentation. This implies that patients with a large nodal burden are probably less likely to be benefited by hypofractionation. This finding is well-corroborated with MARCH collaborative group meta-analysis, which has shown that the effect of altered fractionation was significantly more pronounced on the primary tumor than on the nodal disease (Baujat et al., 2010). Another factor working in tumor control is tumor hypoxia. Addition of a hypoxic cell radiosensitizer at high doses per fraction is shown to be a potential strategy to obtain similar or greater levels of cell killing than achieved with conventional fractionation (Brizel et al., 1997).Wouters and Brown have previously shown that cells at intermediate oxygen levels are responsible for determining tumor response in conventionally fractionated radiotherapy (Wouters and Brown, 1997). However, decrease in cell killing with increasing dose per fraction is attributed to changes in the effective radiosensitivity (alpha/beta) of tumors with heterogeneous oxygenation, a reduction in interfraction reoxygenation and an increased importance of maximally resistant cells (i.e. the hypoxic fraction) in determining overall dose response as the total dose is delivered in fewer fractions (Carlson et al., 2011). However, as the treatment is completed before the tumor cells enter into the phase of accelerated repopulation, this may result in better cell killing and decreased chance of resistance. Rishi et al., (2013) interpreted that patient with nodal size greater than 2 cm × 2 cm had significantly poor DFS with concomitant boost as compared to conventional chemoradiation (Rishi et al., 2013). We were unable to show a significant difference in OS and DFS. In view of small numbers involved, it would not be correct to draw any definite conclusions regarding local recurrence and survival patterns from this study.
Our study had its share of limitations. It was with a limited number of patients and patient treatment was done by 2D planning. The follow-up of this study was relatively short and prevent us from commenting on the long-term DFS and OS.
In conclusion, the schedule of 64 Gy in 25 fractions with concomitant chemotherapy is tolerable in patients with better compliance and equivalent DFS and OS to standard conventional chemoradiotherapy. Further longer follow up is required for further validation of results. This paper offers some support for the routine use of this regimen in a busy center like ours and we are planning for a larger randomized trial with longer follow up.
Author Contribution Statement
All authors contributed equally in this study.
Acknowledgements
None.
References
- Bakst RL, Lee N, Pfister DG, et al. Hypofractionated dose-painting intensity modulated radiation therapy with chemotherapy for nasopharyngeal carcinoma: a prospective trial. Int J Radiat Oncol Biol Phys. 2011;80:148–53. doi: 10.1016/j.ijrobp.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baujat B, Bourhis J, Blanchard P, et al. Hyperfractionated or accelerated radiotherapy for head and neck cancer. Cochrane Database Syst Rev. 2010;2010:Cd002026. doi: 10.1002/14651858.CD002026.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–9. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- Carlson DJ, Keall PJ, Loo BW Jr, Chen ZJ, Brown JM. Hypofractionation results in reduced tumor cell kill compared to conventional fractionation for tumors with regions of hypoxia. Int J Radiat Oncol Biol Phys. 2011;79:1188–95. doi: 10.1016/j.ijrobp.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham JW, Peters LJ, Johansen J, et al. Do acute mucosal reactions lead to consequential late reactions in patients with head and neck cancer? Radiother Oncol. 1999;52:157–64. doi: 10.1016/s0167-8140(99)00107-3. [DOI] [PubMed] [Google Scholar]
- Denis F, Garaud P, Bardet E, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004;22:69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Eisbruch A, Harris J, Garden AS, et al. Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00-22) Int J Radiat Oncol Biol Phys. 2010;76:1333–8. doi: 10.1016/j.ijrobp.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta T, Ghosh-Laskar S, Agarwal JP. Resource-sparing curative-intent hypofractionated-accelerated radiotherapy in head and neck cancer: More relevant than ever before in the COVID era. Oral Oncol. 2020;111:105045. doi: 10.1016/j.oraloncology.2020.105045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SH, O’Sullivan B, Su J, et al. Hypofractionated radiotherapy alone with 2 4 Gy per fraction for head and neck cancer during the COVID-19 pandemic: The Princess Margaret experience and proposal. Cancer. 2020;126:3426–37. doi: 10.1002/cncr.32968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ND, Robertson G, Squire CJ, et al. A national audit of radiotherapy in head and neck cancer. Clin Oncol (R Coll Radiol) 2003;15:41–6. doi: 10.1053/clon.2002.0198. [DOI] [PubMed] [Google Scholar]
- Lauve A, Morris M, Schmidt-Ullrich R, et al. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas: II--clinical results. Int J Radiat Oncol Biol Phys. 2004;60:374–87. doi: 10.1016/j.ijrobp.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kou C, Bai W, et al. Altered fractionation radiotherapy with or without chemotherapy in the treatment of head and neck cancer: a network meta-analysis. Onco Targets Ther. 2018;11:5465–83. doi: 10.2147/OTT.S172018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade S, Gaunt P, Hartley A, et al. Feasibility of Dose-escalated Hypofractionated Chemoradiation in Human Papilloma Virus-negative or Smoking-associated Oropharyngeal Cancer. Clin Oncol (R Coll Radiol) 2018;30:366–74. doi: 10.1016/j.clon.2018.01.015. [DOI] [PubMed] [Google Scholar]
- Narvaez C, Schild SE, Rades D. Comparison of Conventional Fractionation and Accelerated Fractionation With Concomitant Boost for Radiotherapy of Non-metastatic Stage IV Head-and-Neck Cancer. In Vivo. 2021;35:411–5. doi: 10.21873/invivo.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rishi A, Ghoshal S, Verma R, et al. Comparison of concomitant boost radiotherapy against concurrent chemoradiation in locally advanced oropharyngeal cancers: a phase III randomised trial. Radiother Oncol. 2013;107:317–24. doi: 10.1016/j.radonc.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Roques T, Prestwich R. Head and Neck Cancer and COVID 19. 2020. https://www.rcr.ac.uk/college/coronavirus-covid-19-what-rcr-doing/clinical-information/coronavirus-covid-19-cancer 5/5/2020 .
- Sanghera P, McConkey C, Ho KF, Glaholm J, Hartley A. Hypofractionated accelerated radiotherapy with concurrent chemotherapy for locally advanced squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2007;67:1342–51. doi: 10.1016/j.ijrobp.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Staar S, Rudat V, Stuetzer H, et al. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy--results of a multicentric randomized German trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:1161–71. doi: 10.1016/s0360-3016(01)01544-9. [DOI] [PubMed] [Google Scholar]
- Thomson DJ, Palma D, Guckenberger M, et al. Practice Recommendations for Risk-Adapted Head and Neck Cancer Radiation Therapy During the COVID-19 Pandemic: An ASTRO-ESTRO Consensus Statement. Int J Radiat Oncol Biol Phys. 2020;107:618–27. doi: 10.1016/j.ijrobp.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotti A, Bentzen SM. The need for adverse effects reporting standards in oncology clinical trials. J Clin Oncol. 2004;22:19–22. doi: 10.1200/JCO.2004.10.911. [DOI] [PubMed] [Google Scholar]
- Williams MV, James ND, Summers ET, Barrett A, Ash DV. National survey of radiotherapy fractionation practice in 2003. Clin Oncol (R Coll Radiol) 2006;18:3–14. doi: 10.1016/j.clon.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Wouters BG, Brown JM. Cells at intermediate oxygen levels can be more important than the “hypoxic fraction” in determining tumor response to fractionated radiotherapy. Radiat Res. 1997;147:541–50. [PubMed] [Google Scholar]