SUMMARY
In addition to their role in promoting feeding and obesity development, hypothalamic arcuate (ARC) agouti-related protein/neuropeptide Y (AgRP/NPY) neurons are widely perceived to be indispensable for maintaining normal feeding and body weight in adults, and consistently, acute inhibition of AgRP neurons is known to reduce short-term food intake. Here, we adopted complementary methods to achieve nearly complete ablation of arcuate AgRP/NPY neurons in adult mice and reported that lesioning arcuate AgRP/NPY neurons in adult mice caused no apparent alterations in ad libitum feeding or body weight. Consistent with previous studies, loss of AgRP/NPY neurons blunted fasting refeeding. Thus, our studies unexpectedly reveal that AgRP/NPY neurons are not required for maintaining ad libitum feeding or body weight homeostasis in adult mice.
INTRODUCTION
Studies from recent decades have established the importance of the melanocortin pathway in body weight regulation in both rodents and humans. Arcuate AgRP/NPY neurons in the melanocortin pathway have emerged as a key regulator in feeding and related behaviors pertinent to environmental adaptation1. Recent optogenetic and chemogenetic studies have demonstrated a compelling role for AgRP neurons in promoting feeding and positive energy balance2,3. Consistently, chronic activation of AgRP neurons through targeted deletion of leptin receptors or expression of neuron-activating channels leads to massive obesity comparable to the body weight increase caused by leptin deficiency4,5. These results collectively show that AgRP neurons are sufficient to drive feeding and obesity development.
In addition to their role in promoting feeding, AgRP neurons have also been widely perceived to be indispensable for maintaining normal feeding and body weight6–8. Acute inhibition of AgRP neurons reduces short-term feeding in mice3,9,10. Based on a mouse model with selective expression of diphtheria toxin receptors (DTRs) in AgRP neurons, whereas the lesion of AgRP neurons by systemic delivery of diphtheria toxin (DTX) produced a little effect in neonates, it caused a rapid arrest in feeding and lethal weight loss in adult mice, which could last up to 2 weeks in surviving obese leptin-deficient mice8,11. Other studies showed that postembryonic partial loss of AgRP neurons led to a lean and hypophagic phenotype6,7. However, recent studies with chronic or chemogenetic inhibition of AgRP neurons showed no or minimal impact on body weight5,12, raising the question of whether AgRP neurons are required for feeding and body weight regulation.
RESULTS
DTX-induced AgRP neuron lesions have no disruptions in ad libitum feeding or body weight
To address this issue, we first employed the DTX-induced AgRP neuron lesion approach in adult mice by using the previously described adult AgRP-DTR mouse strain7,8. Since AgRP and NPY are nearly completely colocalized in the ARC13, we visualized AgRP neurons with GFP expression in AgRP-DTR::NPY-GFP mice. We utilized intracerebroventricular (i.c.v.) delivery of DTX to limit the effects of ablating peripheral AgRP-expressing cells14 (Figure 1A). Due to the potentially toxic effects of DTX15–18, we performed studies with the administration of DTX at doses of 5 ng since it could cause complete lesions of brain neurons with DTR expression19. Indeed, i.c.v. injections of 5 ng DTX in 8- to 10-week-old mice ablated almost all NPY-GFP neurons and AgRP-immunoreactive (AgRP-ir) signals in the paraventricular nucleus (PVN) (Figures 1B, 1C, S1, and S2). The ablation is almost complete since the same i.c.v. injection with a double amount of toxin (10 ng) failed to increase the ablation efficiency (Figure S3). A few neurons retained GFP expression (Figures 1B, 1C, and S1), which is presumably due to NPY expression in a minor number of non-AgRP neurons within the ARC20,21. We also measured individual AgRP transcripts using RNAscope probes against AgRP, which showed a virtual absence of the transcripts, confirming a complete ablation of AgRP neurons (Figures 1D and S4).
Figure 1. DTX-induced AgRP neuron lesions have no disruptions in energy balance.
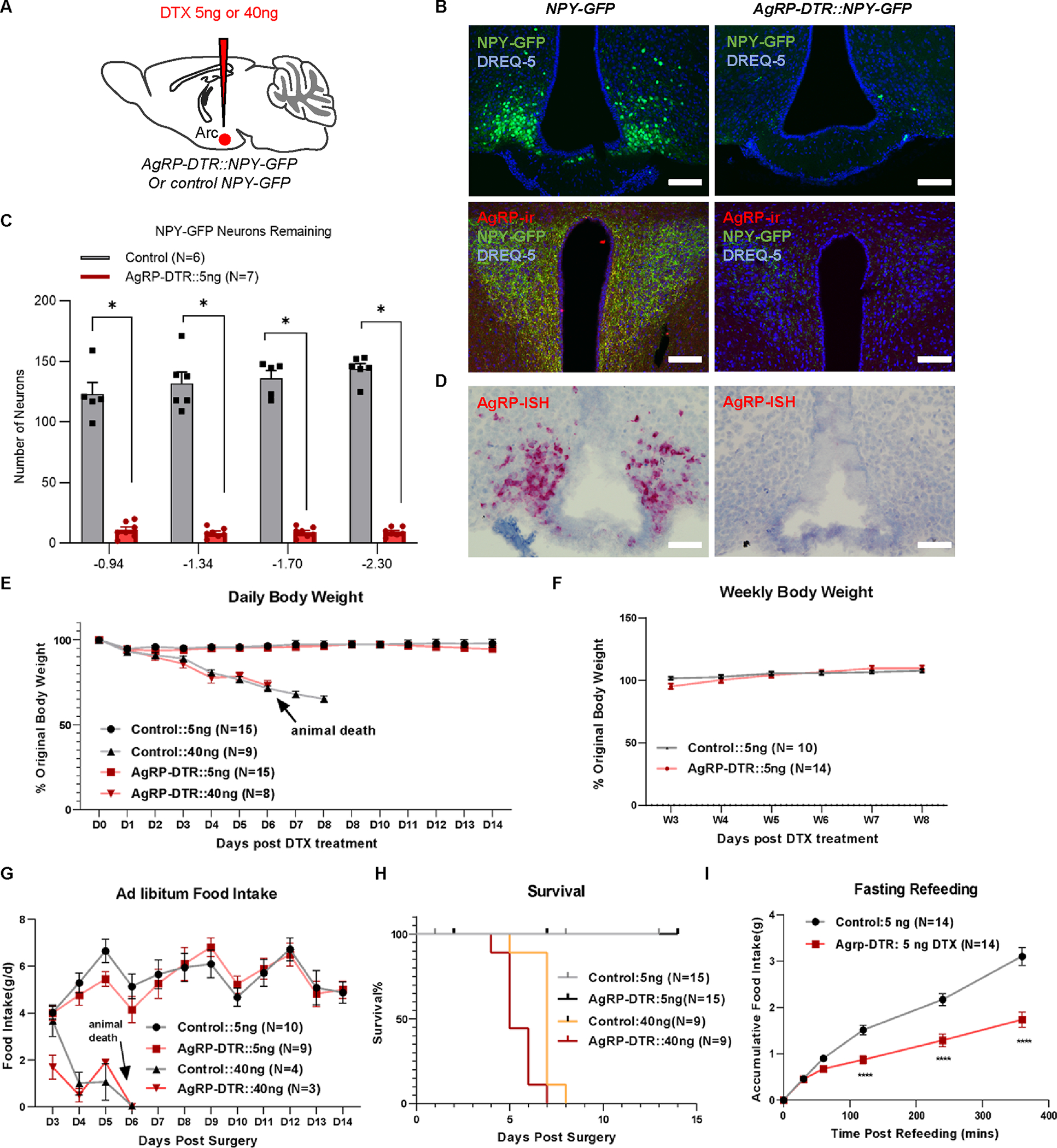
A, Schematic of i.c.v. injections of DTX in mice with the indicated genotypes. B, Representative pictures showing NPY-GFP (green) and nuclear DREQ-5 nucleus signals (blue) in the ARC, and NPY-GFP, nuclear DREQ-5 and AgRP-immunoreactive (-ir) terminals in the PVN of control NPY-GFP (left panels) and AgRP-DTR::NPY-GFP mice (right panels) that received a single i.c.v. injections of 5 ng DTX. Scale bar = 100 μm. C, Quantification of remaining NPY-GFP-positive neurons in the ARC of different Bregma levels from control NPY-GFP and AgRP-DTR::NPY-GFP mice injected with 5 ng DTX at 8 weeks postinjection. Two-way repeated ANOVA followed by Bonferroni’s multiple comparisons; P < 0.0001 between control and AGRP-DTR mice at all Bregma levels. D, Representative pictures showing in situ hybridization (ISH) signals of AgRP in the ARC of control NPY-GFP (left panel) and AgRP-DTR::NPY-GFP mice (right panel). Scale bar = 100 μm. E, Daily percentage of original body weight of control and AgRP-DTR::NPY-GFP male mice with a single i.c.v. injection of 5 ng or 40 ng DTX during the 14 days after DTX injection. Two-way repeated ANOVA followed by Bonferroni’s multiple comparisons; p = 0.4267 between controls and AgRP-DTR mice injected with 5 ng DTX. F, Weekly percentage of original body weight of the control and 5 ng DTX groups 3~8 weeks post injection. Two-way repeated ANOVA followed by Bonferroni’s multiple comparisons; p = 0.6262 between controls and AgRP-DTR mice. G, Daily food intake in control and AgRP-DTR::NPY-GFP male mice with a single i.c.v. injection of 5 ng or 40 ng DTX. Two-way repeated ANOVA followed by Bonferroni’s multiple comparisons; p = 0.1402 between control and AgRP-DTR mice injected with 5 ng DTX. H, Survival curve in control and AgRP-DTR mice that received a single i.c.v. injections of either 5 ng or 40 ng DTX. p = 0.0544 between 5 ng and 40 ng DTX-injected control mice; p > 0.9999 between control and AgRP-DTR mice injected with 5 ng DTX. I, Fasting-refeeding curve. All the fasting-refeeding tests were conducted 2~4 weeks post injection. Two-way repeated ANOVA followed by Bonferroni’s multiple comparisons in control and AgRP-DTR::NPY-GFP mice that received a single i.c.v. injections of 5 ng DTX. Two-way repeated ANOVA followed by Bonferroni’s multiple comparisons; p < 0.0001 between control and AgRP-DTR mice injected with 5 ng DTX. ****p < 0.0001. All data represent the mean ± SEM.
See also Figures S1–S4.
Compared to NPY-GFP littermate controls, AgRP-DTR::NPY-GFP mice showed no changes in daily body weight during the first 2 weeks (Figure 1E) or weekly body weight within 8 weeks (Figure 1F) or in daily ad libitum food intake (Figure 1G) post 5 ng DTX delivery, suggesting that AgRP neurons are not required for ad libitum feeding or energy balance. Surprisingly, i.c.v. injection of 40 ng DTX, which was used in a previous study for the lesion of AgRP neurons8, led to rapid lethal phenotypes with a feeding arrest within the first 10 days post injections in both NPY-GFP and AgRP-DTR::NPY-GFP mice (Figures 1E–1H), suggesting a nonspecific toxic effect. It is important to note that at the time point when feeding was arrested in mice, a significant number of GFP neurons remained in the ARC (Figure S3), suggesting that the observed lethality was not due to AgRP neuron lesions. In fact, it was not until 12 days post-injection that DTX induced a nearly complete lesion (Figure S3). However, 5 ng DTX had no impact on survival in either group (Figure 1H). Of note, we also observed no impact of AgRP neuron lesions on ad libitum feeding or body weight in female AgRP-DTR mice (Figure 2). In line with previous observations22, AgRP lesions caused reduced fasting refeeding (Figure 1I).
Figure 2. DTX mediated lesion of AgRP neurons in female AgRP-DTR mice.
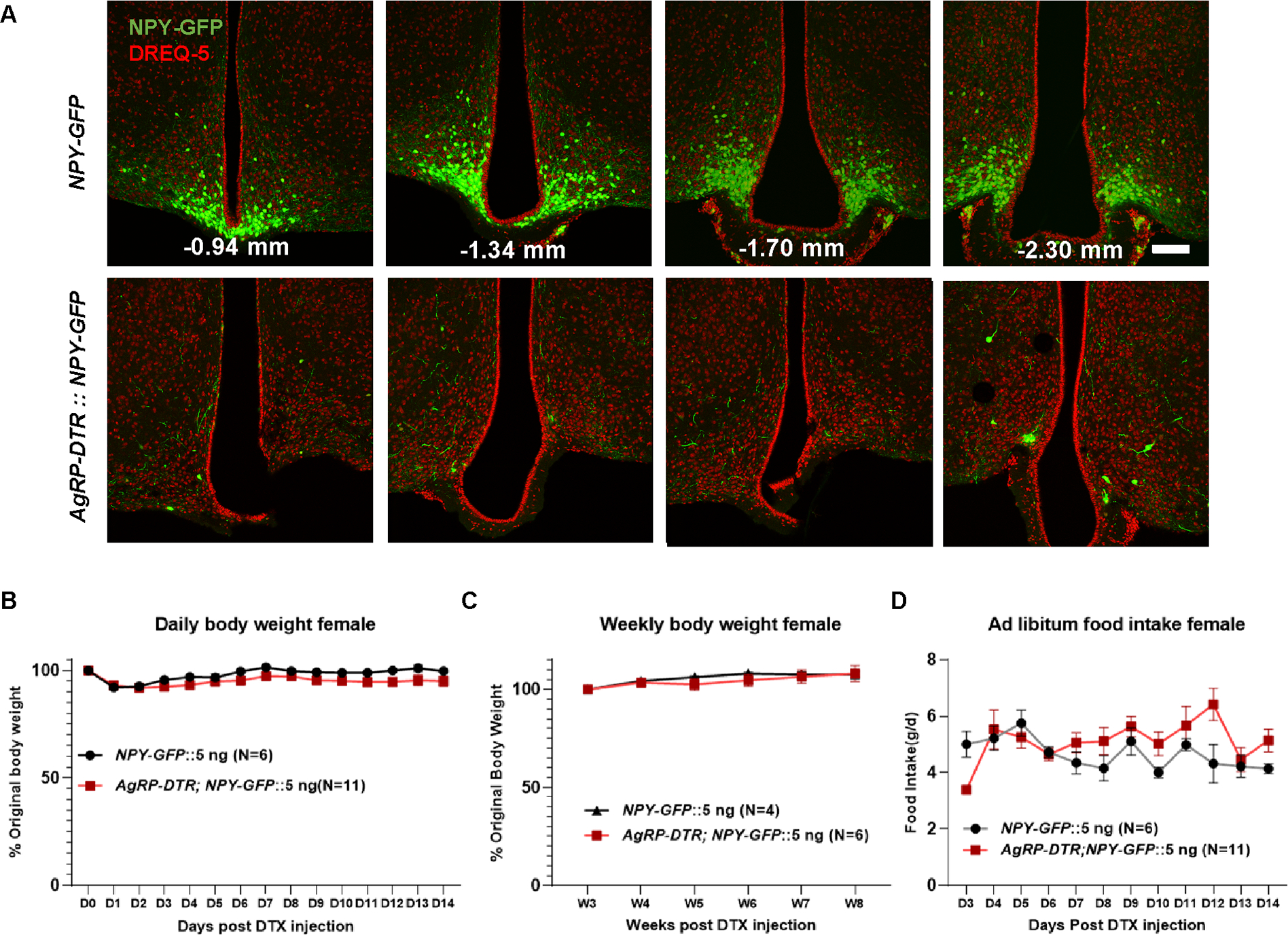
A, Representative pictures showing GFP (green) and DREQ-5 nucleus staining (red) in NPY-GFP and AgRP-DTR::NPY-GFP female mice with single i.c.v injection of 5 ng DTX injections at a series of sections with the indicated Bregma levels in mm. B-D, Comparison in female body weight during the first 14 days (B) and 8 weeks (C) after toxin injections; and daily food intake (D) at the indicated day after toxin injection. Scale bar = 100 μm. Two-way repeated ANOVA followed by Bonferroni’s multiple comparisons: p = 0.1160 (B); p = 0.6557 (C); p = 0.1171 (D). Data was presented as mean ± SEM.
Caspase 3-mediated AgRP neuron lesions do not reduce ad libitum feeding or body weight
To avoid toxicity issues associated with using DTX, we adopted another approach by expressing caspase 3, which is known to induce targeted cell apoptosis23. We bred AgRP-Cre mice with Ai14 reporter mice to track and visualize AgRP neurons (referred to as AgRP-Cre::Ai14 mice). The AAV-flex-taCasp3-TEVp viral vectors were bilaterally injected into the ARC of 8- to 10-week-old AgRP-Cre::Ai14 mice to ablate AgRP neurons (Figure 3A). Sham-injected littermates were used as controls. Expression of caspase 3 effectively ablated ARC AgRP neurons approximately two weeks after viral injection (Figures 3B and S5A). We injected AAV-flex-taCasp3-TEVp into 31 mice of both sexes, among which 19 mice exhibited nearly complete loss of AgRP neurons, remaining only 26–245 neurons (Figures 3C and S5B), as well as less than 1% AgRP expression in downstream areas such as the paraventricular hypothalamic nucleus (PVN), paraventricular thalamic nucleus (PVT), dorsomedial hypothalamic nucleus (DMH), and lateral hypothalamus (LH) compared to control mice (Figures 3B, 3D, and 3E). There were only approximately 100 or fewer remaining AgRP neurons in the ARC of 10 mice. Correlation analysis showed that the body weight changes at weeks 4 and 8 after viral injection were unrelated to the number of remaining AgRP neurons (Figures 3F and 4A). We did not observe any starvation or moribund phenotype in all mice.
Figure 3. Caspase 3-mediated lesion of AgRP neurons in the ARC fails to reduce body weight or food intake in male adult mice.
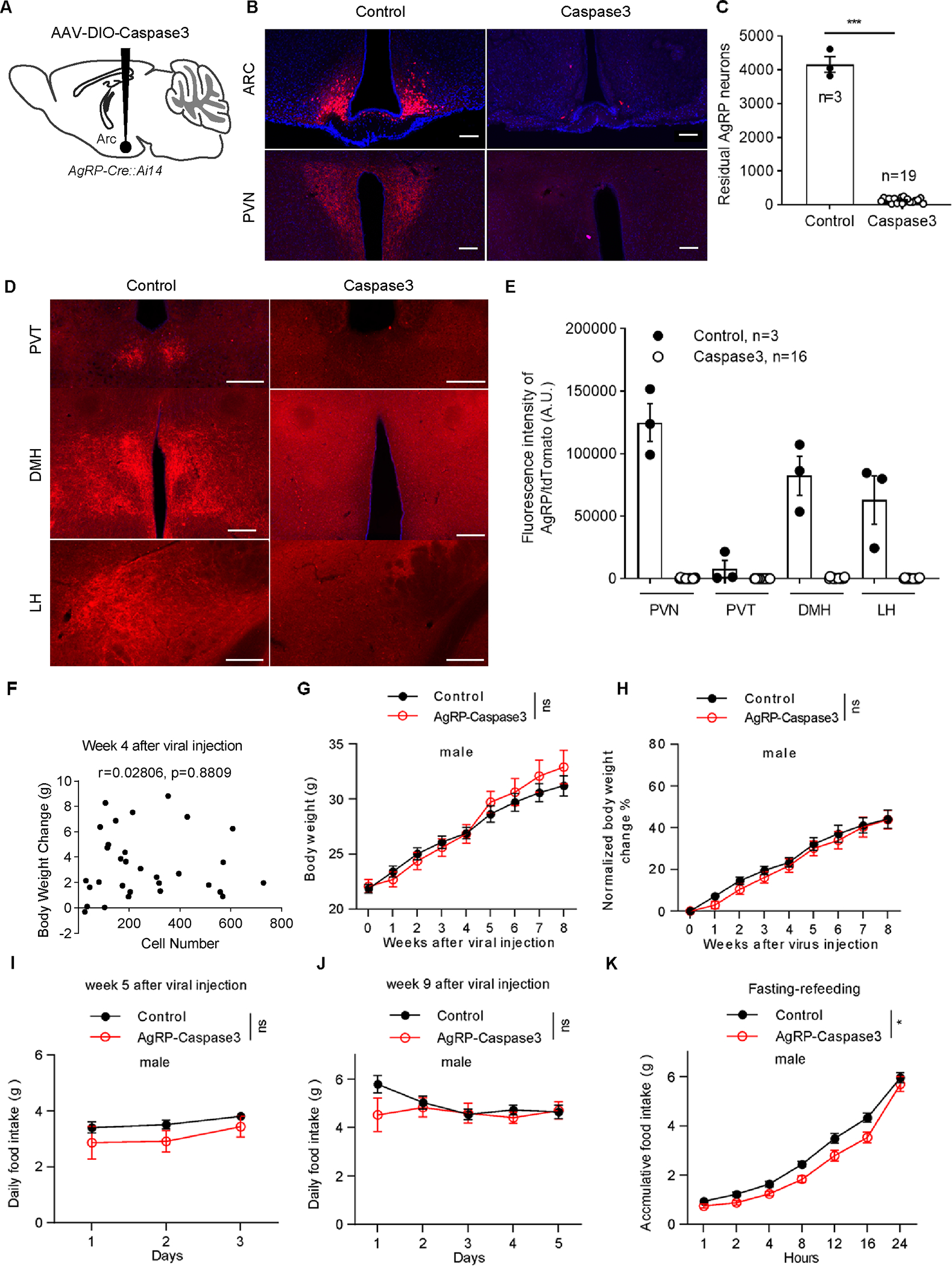
A, Schematic of the virus injection into the ARC of AgRP-Cre::Ai14 mice. B, Ai14 reporter expression (red) and DAPI staining (blue) in mice that received sham (left panels) and AAV-flex-taCasp3-TEVp viral injections (right panels) to bilateral ARC in both ARC (top panels) and one of the major projection sites PVN (bottom panels). Scale bars: 200 μm. C, Comparison of the total numbers of Ai14 reporter-positive neurons in the ARC between the control and AAV-flex-taCasp3-TEVp injection groups. Unpaired Student’s t test. ***p< 0.001. D, E, AgRP/tdTomato terminal signals in projection areas of AgRP neurons. Representative pictures (D) and qualitative results (E). Scale bars: 200 μm. F, The correlation between the number of remaining AgRP neurons and body weight gain at week 4 after viral injection. Each symbol represents an individual mouse. n=31 mice of both sexes. G, H, Comparison of weekly absolute body weight (G) and normalized body weight change (H) between the control mice (n=7 or 15 male mice at different time points) and AAV-flex-taCasp3-TEVp injection groups (n=4 or 11 male mice at different time points) during the 8 weeks after viral delivery. Two-way ANOVA followed by Bonferroni’s multiple comparisons, p = 0.9471 in G, p = 0.4250 in H. ns, not significant. I, J, Daily food intake between the control (n=7 male mice) and AAV-flex-taCasp3-TEVp injection groups (n=6 or 4 male mice) over 3–5 consecutive days at weeks 5 (I) and 9 (J) after viral delivery. Two-way ANOVA followed by Bonferroni’s multiple comparisons, p = 0.2025 in I, p = 0.4476 in J. ns, not significant. K, Fasting-refeeding curve measured at week 9 after viral injections. Two-way repeated ANOVA followed by Bonferroni’s multiple comparisons in control (n=15 mice) and AgRP-ablated mice (n=11), p = 0.0176. *p< 0.05. All data represent the mean ± SEM.
See also Figures S5.
Figure 4. AgRP neuron lesion did not impair body weight gain and ad libitum feeding in female adult mice.
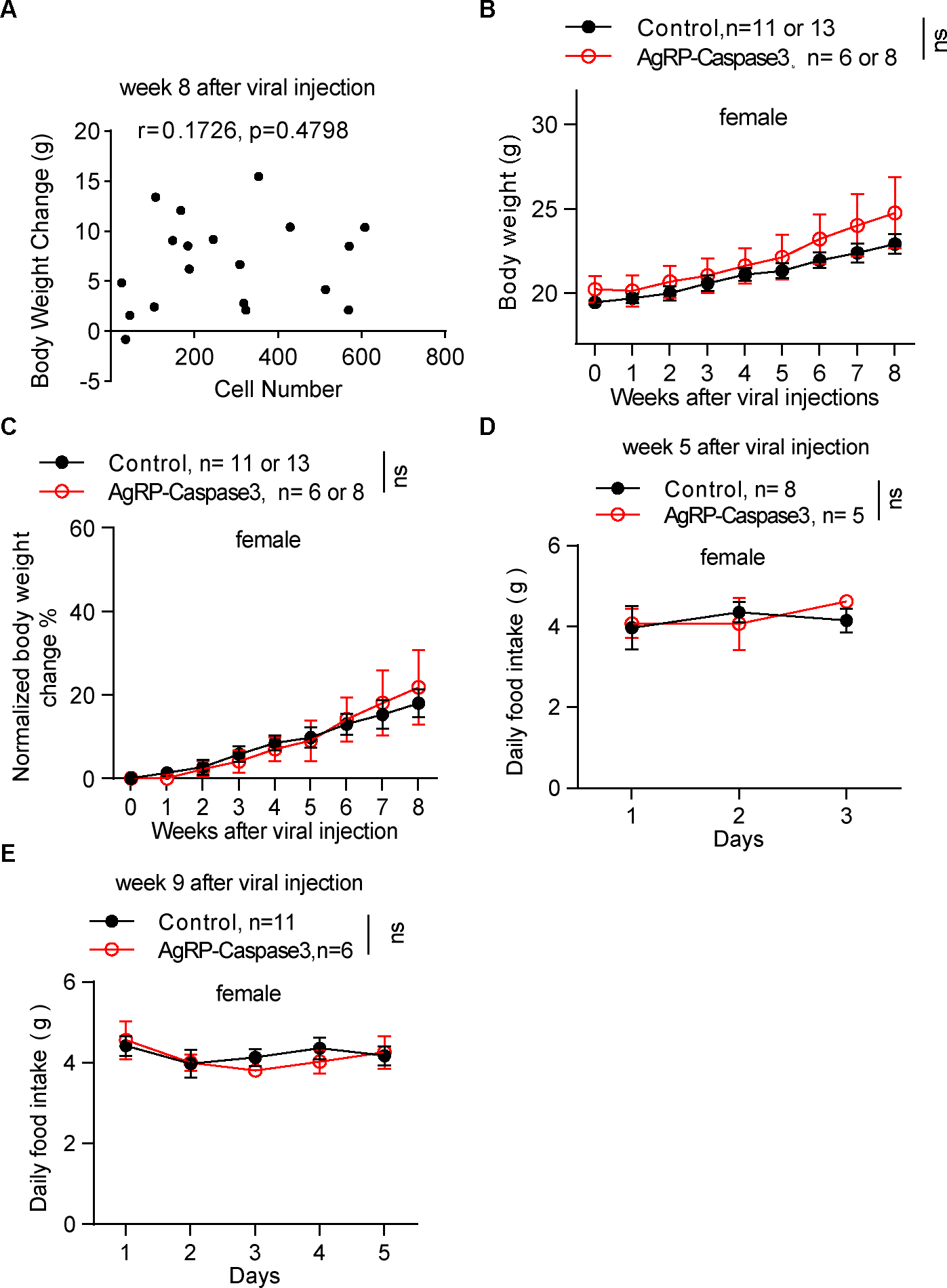
A, The correlation between the numbers of remaining AgRP neurons and bodyweight gain at week 8 after viral injection. Each symbol represents an individual mouse. n=19 mice of both sexes. B, C, Comparison in weekly absolute body weight (B) and normalized body weight change (C) between control and AAV-flex-taCasp3-TEVp injection groups after viral delivery. Two-way ANOVA followed by Bonferroni’s multiple comparisons, p = 0.3388 in B, p = 0.9669 in C. ns, not significant. D, E, Daily food intake between the control and AAV-flex-taCasp3-TEVp injection groups over 3~5 consecutive days at weeks 5 (D) and 9 (E) after viral delivery. Two-way ANOVA followed by Bonferroni’s multiple comparisons, p = 0.7977 in D, p = 0.8118 in E.
All data represent the mean ± SEM.
We compared the body weight change and food intake of 19 completely ablated mice with controls. In corroboration with the results from DTX-induced ablation, caspase 3-mediated ablations showed no effect on body weight over eight weeks in either male or female adult mice (Figures 3G, 3H, 4B, and 4C). We did not observe any significant difference in daily ad libitum food intake at weeks 5 and 9 after viral injections between controls and AgRP-ablated mice (Figures 3I, 3J, 4D, and 4E). Consistent with the DTX-induced ablations, caspase 3-mediated ablations significantly impaired refeeding after a 24 hr fast (Figure 3K). Thus, based on two compelling animal models with AgRP neuron lesions associated with no alterations in ad libitum feeding or body weight, we conclude that AgRP neurons are not indispensable for ad libitum feeding or body weight regulation.
It is unlikely that the maintenance of body weight and ad libitum food intake were due to incomplete ablation. Prior optogenetic work has demonstrated that increased food intake was observed only in mice with more than 300 activated AgRP neurons, whereas there was no feeding stimulation effect in those containing less than 100 activated AgRP neurons2. In our caspase 3-mediated ablation experiments, the number of remaining AgRP neurons in 19 completely ablated mice were less than 300. Even when there were approximately 100 or fewer AgRP neurons left, the mice still exhibited normal body weight gain (Figures 3F and 4A).
It is currently unknown why previous studies with an i.p. or intramuscular (i.m.) injections dose of 40 or 50 μg/kg caused differential effects between the AgRP-DTR and control groups7,8. However, a previous study on AgRP-DTR mice also reported mice death in the control group treated with DTX at a dose of 50 μg/kg8. Other studies reported i.p. administration of DTX at 4 μg/kg or higher doses were highly toxic even in wild-type mice17,18. We found that both AgRP-DTR and control mice injected with 40 ng DTX (i.c.v.) exhibited lethargy and movement difficulty during the first few days after toxin injection (Supplementary Video 1 and Video 2), which may cause difficulty in reaching the food. Using the same strategy as previously employed to administer DTX peripherally (50 μg/kg, i.m.) twice with 2 days apart, we observed no difference in body weight or food intake between AgRP-DTR mice and control mice (Figures S6A, S6E, and S6F). Of note, 3 out of 9 AgRP-DTR mice showed a lethal phenotype (Figure S6G), yet these animals displayed an incomplete ablation of AgRP neurons in the ARC (Figures S6B–S6D), suggesting that the lethal phenotype is not correlated with the extent of AgRP neurons. Thus, it is conceivable that diet conditions, aging, obese states, and strain differences, loss of peripheral AgRP function, as well as defective fast-refeeding by AgRP lesions, may cause differential responses to DTX8,11,15.
DISCUSSION
Our results establish a concept that AgRP neurons are sufficient but not required for ad libitum feeding or body weight maintenance in adult mice. This contrasts with the widely accepted notion that AgRP neurons are mandatory for feeding and body weight maintenance7,8,24. Given the widely perceived critical role of AgRP neurons in feeding and survival, our findings suggest a necessary revision of the neural circuit mechanism, in which AgRP neurons are permissive for feeding and body weight regulation during ad libitum feeding conditions. This notion is consistent with no or little body weight phenotypes from loss of function in AgRP and NPY in neonatal8,25–27 or adult mice26,27, blocking GABA release from AgRP neurons in neonatal28 or adult mice29, or chronic or acute inhibition of AgRP neurons in adult mice5,12. In addition, the previously described lethal starvation phenotype due to AgRP neuronal lesions can be rescued by obesity11, suggesting that the lethal starvation was not due to a specific inhibition mechanism on feeding behaviors, which otherwise should have caused starvation independent of body weight. Given previous results on acute inhibition of AgRP neurons causing a short-term reduction in feeding3,9,10, combined with our current results, acute effects on feeding may not be translated to chronic body weight changes. This notion is supported by the effects of chronic Gq-mediated activation of AgRP neurons in mice30, which did not cause long-lasting obesogenic changes in feeding and body weight. Our results also argue against a widely perceived role for developmental compensation underlying the normal body weight observed in mice with lesions of AgRP neurons from neonatal stages. The concept that AgRP neurons are sufficient but not mandatory for body weight regulation supports our previous observations that ARC GABAergic neurons promote feeding and drive obesity in a redundant mechanism5. This redundant mechanism predicts that while activation of random subsets of ARC GABAergic neurons is sufficient to promote feeding and obesity, inhibition or loss of function of single subsets has no impact on feeding or body weight.
Interestingly, both current and previous studies show that AgRP neurons are necessary to mount appropriate feeding responses to fasting3,5,31. Thus, although AgRP neurons are not required for normal feeding or energy balance in a laboratory setting, these neurons appear to be implicated in stressful conditions such as fasting and may be needed for survival in more challenging natural environments. This notion is also consistent with emerging observations suggesting a role for AgRP neurons in adaptive behaviors to environmental changes22,32–34.
limitations of the study
This study did not examine the impacts of AgRP neuron ablation in adult mice on feeding behavior and body weight maintenance in more challenging feeding paradigms. For example, previous studies have shown impaired adaption to restricted feeding in adult mice after neonatal ablation of AgRP neurons22. However, whether adult ablation of AgRP neurons also affects adaption to such a restricted feeding paradigm is unknown. Likewise, intermittent feeding with variable fasting intervals and switching between different diets are common challenges animals often face under non-laboratory conditions. Studying the potential roles of AgRP neurons in such more natural conditions may add significant novel insights for the field and beyond.
In the present study, we did not examine the potential contributions of other AgRP-expressing cells to normal feeding behavior and bodyweight maintenance. Different from the previous well-established notion that AgRP-expressing cells are only restricted in the hypothalamus, recent studies have shown the existence of AgRP-expressing cells in the pituitary and adrenal glands14,35. Short-term fasting led to an increase in adrenal AgRP expression14, whereas inhibition of AgRP cells in the pituitary caused weight loss35. These findings suggest peripheral AgRP-expressing cells are also involved in regulating feeding behavior. Thus, it is possible that the lethal phenotype led to by i.m. administration of DTX observed in our and previous studies might be contributed by loss of peripheral AgRP-positive cells. It will be of interest to selectively ablate AgRP-expressing cells outside of the hypothalamus and examine its impacts on maintaining normal feeding behavior and body weight in future studies, which may help explain the discrepancy between our and earlier studies.
STAR Methods
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Cheng Zhan (zhancheng@ustc.edu.cn).
Materials availability
All unique/stable reagents generated in this study are available from the lead contact with a completed Materials Transfer Agreement.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Raw data were deposited on Mendeley: doi:10.17632/v9m9vy6ddb.2. Any additional information required to reanalyze the data reported in this work paper is available from the Lead Contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
Mice in DTX injection experiments were housed in the Institute of Molecular Medicine animal facility in Texas, USA. Mice in Caspase 3 experiments were housed at the National Institute of Biological Sciences. All mice were maintained at 21–22°C on a 12 h light/12 h dark cycle with standard pellet chow (Teklad F6 Rodent Diet 8664, 4.05 kcal/g, 12.5% kcal from fat, Harlan Teklad) and water. NPY-GFP (JAX: 006417), Ai14 mice (JAX: 007908), and AgRP-Cre (JAX: 012899) mice were purchased from the Jackson Laboratory. AgRP-Cre::Ai14 mice were generated by crossing AgRP-Cre and Ai14 mice. AgRP-DTR mice were provided by Q. Wu of Baylor College of Medicine. NPY-GFP and AgRP-DTR mice were crossed and bred together to allow GFP expression in AgRP/NPY neurons in the ARC. All experiments were performed on adult mice (8–16 weeks old, both male and female). Behavior experiments were performed from 9 am-5 pm. Mice were euthanized when body weight dropped by >20%, as mandated by the ethics committee. Animal care and procedures were approved by the University of Texas Health Science Center at Houston Institutional Animal Care or the National Institute of Biological Sciences in Beijing following institutional guidelines for the care and use of animals.
METHOD DETAILS
Intracerebroventricular injections
For intracerebroventricular (i.c.v.) injection, DTX (#D0564, Sigma–Aldrich) was delivered to the cerebrospinal fluid in the lateral ventricles. Briefly, mice were anesthetized with a ketamine/xylazine cocktail (100 mg/kg and 10 mg/kg, respectively), and their heads were fixed to a stereotaxic apparatus. DTX was dissolved in saline at different dilution ratios. The concentrations from high to low were 50 ng/μL, 12.5 ng/μL, 6.25 ng/μL and 3.125 ng/μL. Then, 0.4 μL saline or DTX solution was delivered twice through a 0.5 μL syringe (Neuros Model 7000.5 KH, point style 3; Hamilton, Reno, NV, USA) mounted on a motorized stereotaxic injector (Quintessential Stereotaxic Injector; Stoelting, Wood Dale, IL, USA) at a rate of 0.125 μL/min. The coordinates to target the right lateral ventricle were as follows: anteroposterior (AP): 0.0 mm; mediolateral (ML): +1.0 mm; dorsoventral (DV): −2.5 mm. All i.c.v. DTX injections were unilateral since CSF can circulate through the whole brain.
AAV injection
For viral injections, mice were anesthetized with 2,2,2-tribromoethanol (240 mg/kg, IP) dissolved in 2.5% 2-methyl-2-butanol and placed in a stereotaxic holder (RWD Life Science, China). AAV2/9-CAG-DIO-taCaspase3-TEVp-WPRE-pA (Cat#: S0236–9, Tailtool. 2×1012 V. G/ml, 300 nl for each side) was bilaterally injected into the ARC (ARC coordinate AP/ML/DV: −1.46/±0.2/−5.4 mm) of AgRP-Cre::Ai14 mice with pressure (infusion speed 50 nl/minute) (Nanoliter 2000 Injector, WPI).
Intramuscular (i.m.) injection
For i.m. injections, mice were restrained and DTX (#D0564, Sigma–Aldrich) was administered using a 0.5 ml syringe in the thigh muscles. 0.025 μg/μL DTX in saline was delivered to mice at a 50 μg/kg dosage twice, 2 two days apart.
Food intake and body weight measurements
Body weight was measured daily or weekly in group-housed mice after toxin or viral injections. The measurements of food intake were performed when mice were fed standard chow. Before the food intake measurements, all mice were single housed and habituated for two days. Daily food intake was monitored for three or five consecutive days. In fasting-refeeding experiments, mice were single housed and habituated as above and fasted for 18 or 24 hrs. The following morning, mice were refed with standard chow, and food intake was monitored for the following hours.
Histology and imaging
After all body weight data were collected, mice were anesthetized with a ketamine/xylazine cocktail (100 mg/kg and 10 mg/kg, respectively) and subjected to transcardial perfusion. Mice were perfused with 20 ml saline and 20 ml of 10% buffered formalin or 4% paraformaldehyde in PBS with 0.2% picric acid. Brains were dissected and kept in 15 ml tubes filled with 10% buffered formalin for postfixation. After being shaken overnight, brains were switched to 30% sucrose in PBS solution and stored until they sank in the tube. DTX-injected brains were frozen with dry ice and sectioned horizontally into 30 μm slices with a sliding microtome (Leica SM2010 R; Leica Microsystems, Wetzlar, Germany). Cut slices were stored in 0.1% sodium azide in PBS at 4°C before further use. The slices were mounted onto Superfrost Plus Gold microscope slides (FisherScientific, INC). A confocal microscope (Leica TCS SP5, Leica Microsystems, Wetzlar, Germany) with different lenses and lasers was used to image the PVN and ARC regions of slices from DTX-injected brains.
For viral injection experiments, coronal sections (40–50 μm) were cut with a freezing cryostat (Leica CR 1900) and mounted on glass slides. Fluorescent images were acquired and stitched using an automated slider scanner (VS120 virtual slide, Olympus).
In situ hybridization
For DTX-injected brain tissues, brain sections of the ARC were mounted to Superfrost Plus Gold slides (Fisher Scientific, INC). Sections were first air dry at RT for 30 minutes and incubated at 60°C for 30 minutes. After 10 minutes’ retrieval, sections were hybridized with the AgRP probe (targeted region 11–764, access #NM 001271806.1, #400711, Advanced Cell Diagnostic, INC) for 2 h at 40°C and signals were amplified with RNAscope 2.5HD Assay-RED (#322350, Advanced Cell Diagnostic, INC).
QUANTIFICATION AND STATISTICAL ANALYSIS
Imaging Analysis
Imaging analysis was performed with ImageJ (NIH). The number of residual AgRP-tdTomato or NPY-GFP neurons in the ARC was counted manually. For DTX-mediated lesions, neurons were counted as averaged numbers from 4 representative and matched ARC sections. For the caspase 3-mediated lesion, the number of total neurons was counted from consecutive ARC sections. AgRP expression in each terminal region was determined by calculating the fluorescence intensity with the following equation: (mean fluorescence intensity of nucleus - mean fluorescence intensity of background) ×area of nucleus.
Statistical Analysis
All the data were recorded and organized using Excel sheets first and then exported to GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA). For body weight and fasting-refeeding data, two-way ANOVA followed by Sidak’s or Bonferroni’s multiple comparisons were used. For single variable comparisons, such as food intake measurements, unpaired two-tailed Student’s t tests were used. Error bars in all graphs are presented as SEM.
Supplementary Material
Supplemental video 1:
One AgRP-DTR mouse received 40 ng DTX (i.c.v.) injection exhibited lethargy and movement difficulty on day 7 after DTX injection, whereas other AgRP-DTR mice received saline injection behaved normally. Related to Figures 1 and 2.
Supplemental video 2:
A control mouse received 40 ng DTX (i.c.v.) injection exhibited lethargy and movement difficulty on day 7 after DTX injection. Related to Figures 1 and 2.
Key resources table.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| AgRP rabbit polyclonal antibody (mAb) | Phoenix Pharmaceuticals | H-003-57 |
| Alexa Fluor 594-conjugated AffiniPure Donkey (H+L) anti-rabbit immunoglobulin G | Jackson Immunoresearch | 711-585-152 |
| DRAQ5™ Fluorescent Probe | ThermoFisher | 62251 |
| TSA Plus Cyanine 3 | PerkinElmer | NEL753001KT |
| DAPI dihydrochloride | MedChemExpress | HY-D0814 |
| Bacterial and virus strains | ||
| AAV2/9-CAG-DIO-taCaspase3-TEVp-WPRE-pA | Tailtool | S0236-9 |
| Chemicals, peptides, and recombinant proteins | ||
| Diphtheria Toxin from Corynebacterium diphtheriae | Sigma-Aldrich | D0564 |
| Experimental models: Organisms/strains | ||
| B6.129S4-Agrptm2(DTR) Rpa/J | The Jackon Laboratory | 033171 |
| B6.FVB-Tg(Npy-hrGFP)1 Lowl/J | The Jackon Laboratory | 006417 |
| B6;129S6-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J | The Jackon Laboratory | 007908 |
| Agrp-Ires-cre (Agrptm1 (cre)Lowl/J) | The Jackon Laboratory | 012899 |
| Ai14 (B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J) | The Jackon Laboratory | 007914 |
| Software and algorithms | ||
| GraphPad Prism | GraphPad Software | GraphPad Prism 8.0 and 9.0 |
| OlyVIA | Olympus | |
| Zen | Zeiss | |
| Other | ||
| AgRP probe | Advanced Cell Diagnostic | 400711 |
| RNAscope 2.5HD Assay-RED | Advanced Cell Diagnostic | 322350 |
| PicoLab Rodent Diet 5053 | Lab diet | 5053 |
| Rodent chow Diet | SiPeiFu | SPF-F02 |
| Deposited data | ||
| Raw and analyzed data | Mendeley data | doi:10.17632/v9m9vy6ddb.2 |
ACKNOWLEDGMENTS
We acknowledge Dr. Qi Wu for providing AgRP-DTR mice. C.Z. is supported by grants from National Science and Technology Innovation 2030 Major Project of China (2021ZD0203900), National Natural Science Foundation of China (32271063), and Research Funds of Center for Advanced Interdisciplinary Science and Biomedicine of IHM (QYPY20220018). Q.T. is supported by NIH R01 DK136284, R01 DK120858, R01 DK 135212 and R01 DK 131466 (QT), R01DK109934, and DOD W81XWH-19–1-0429 (QT and BRA). Q.T. is the holder of the Cullen Chair in Molecular Medicine at McGovern Medical School. J. Chen is supported by National Natural Science Foundation of China (32100821).
INCLUSION AND DIVERSITY
We support inclusive, diverse, and equitable conduct of research.
Footnotes
DECLEARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Deem JD, Faber CL, and Morton GJ (2022). AgRP neurons: Regulators of feeding, energy expenditure, and behavior. FEBS J 289, 2362–2381. 10.1111/febs.16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aponte Y, Atasoy D, and Sternson SM (2011). AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 14, 351–355. 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, and Lowell BB (2011). Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121, 1424–1428. 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, Bartolome CL, Low CS, Yi X, Chien CH, Wang P, and Kong D (2018). Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature 556, 505–509. 10.1038/s41586-018-0049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu C, Jiang Z, Xu Y, Cai ZL, Jiang Q, Xu Y, Xue M, Arenkiel BR, Wu Q, Shu G, and Tong Q (2020). Profound and redundant functions of arcuate neurons in obesity development. Nat Metab 2, 763–774. 10.1038/s42255-020-0229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bewick GA, Gardiner JV, Dhillo WS, Kent AS, White NE, Webster Z, Ghatei MA, and Bloom SR (2005). Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J 19, 1680–1682. 10.1096/fj.04-3434fje. [DOI] [PubMed] [Google Scholar]
- 7.Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, et al. (2005). Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci 8, 1289–1291. 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 8.Luquet S, Perez FA, Hnasko TS, and Palmiter RD (2005). NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685. 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 9.Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, and Sternson SM (2015). Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185. 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denis RGP, Joly-Amado A, Webber E, Langlet F, Schaeffer M, Padilla SL, Cansell C, Dehouck B, Castel J, Delbes AS, et al. (2017). Palatability Can Drive Feeding Independent of AgRP Neurons. Cell Metab 25, 975. 10.1016/j.cmet.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Wu Q, Whiddon BB, and Palmiter RD (2012). Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility. Proc Natl Acad Sci U S A 109, 3155–3160. 10.1073/pnas.1120501109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uner AG, Kecik O, Quaresma PGF, De Araujo TM, Lee H, Li W, Kim HJ, Chung M, Bjorbaek C, and Kim YB (2019). Role of POMC and AgRP neuronal activities on glycaemia in mice. Sci Rep 9, 13068. 10.1038/s41598-019-49295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn TM, Breininger JF, Baskin DG, and Schwartz MW (1998). Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci 1, 271–272. 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 14.Gupta R, Ma Y, Wang M, and Whim MD (2017). AgRP-Expressing Adrenal Chromaffin Cells Are Involved in the Sympathetic Response to Fasting. Endocrinology 158, 2572–2584. 10.1210/en.2016-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldwich A, Steinkasserer A, Gessner A, and Amann K (2012). Impairment of podocyte function by diphtheria toxin--a new reversible proteinuria model in mice. Lab Invest 92, 1674–1685. 10.1038/labinvest.2012.133. [DOI] [PubMed] [Google Scholar]
- 16.Chapman TJ, and Georas SN (2013). Adjuvant effect of diphtheria toxin after mucosal administration in both wild type and diphtheria toxin receptor engineered mouse strains. J Immunol Methods 400–401, 122–126. 10.1016/j.jim.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerpa V, Gonzalez A, and Richerson GB (2014). Diphtheria toxin treatment of Pet-1-Cre floxed diphtheria toxin receptor mice disrupts thermoregulation without affecting respiratory chemoreception. Neuroscience 279, 65–76. 10.1016/j.neuroscience.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valek L, and Tegeder I (2021). Failure of Diphtheria Toxin Model to Induce Parkinson-Like Behavior in Mice. Int J Mol Sci 22. 10.3390/ijms22179496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xi D, Gandhi N, Lai M, and Kublaoui BM (2012). Ablation of Sim1 neurons causes obesity through hyperphagia and reduced energy expenditure. PLoS One 7, e36453. 10.1371/journal.pone.0036453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, Goldman M, Verstegen AM, Resch JM, McCarroll SA, et al. (2017). A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci 20, 484–496. 10.1038/nn.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen R, Wu X, Jiang L, and Zhang Y (2017). Single-Cell RNA-Seq Reveals Hypothalamic Cell Diversity. Cell Rep 18, 3227–3241. 10.1016/j.celrep.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan K, Knight ZA, and Friedman JM (2014). Ablation of AgRP neurons impairs adaption to restricted feeding. Mol Metab 3, 694–704. 10.1016/j.molmet.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Unger EK, Wells JA, and Shah NM (2013). Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909. 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dietrich MO, and Horvath TL (2009). GABA keeps up an appetite for life. Cell 137, 1177–1179. 10.1016/j.cell.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, et al. (2007). Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 5, 438–449. 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Krashes MJ, Shah BP, Koda S, and Lowell BB (2013). Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab 18, 588–595. 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ste Marie L, Luquet S, Cole TB, and Palmiter RD (2005). Modulation of neuropeptide Y expression in adult mice does not affect feeding. Proc Natl Acad Sci U S A 102, 18632–18637. 10.1073/pnas.0509240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong Q, Ye CP, Jones JE, Elmquist JK, and Lowell BB (2008). Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 11, 998–1000. 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng F, Han Y, Srisai D, Belakhov V, Farias M, Xu Y, Palmiter RD, Baasov T, and Wu Q (2016). New inducible genetic method reveals critical roles of GABA in the control of feeding and metabolism. Proc Natl Acad Sci U S A 113, 3645–3650. 10.1073/pnas.1602049113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ewbank SN, Campos CA, Chen JY, Bowen AJ, Padilla SL, Dempsey JL, Cui JY, and Palmiter RD (2020). Chronic G(q) signaling in AgRP neurons does not cause obesity. Proc Natl Acad Sci U S A 117, 20874–20880. 10.1073/pnas.2004941117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Lin YC, Zimmerman CA, Essner RA, and Knight ZA (2016). Hunger neurons drive feeding through a sustained, positive reinforcement signal. Elife 5. 10.7554/eLife.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel HR, Qi Y, Hawkins EJ, Hileman SM, Elmquist JK, Imai Y, and Ahima RS (2006). Neuropeptide Y deficiency attenuates responses to fasting and high-fat diet in obesity-prone mice. Diabetes 55, 3091–3098. 10.2337/db05-0624. [DOI] [PubMed] [Google Scholar]
- 33.Beutler LR, Corpuz TV, Ahn JS, Kosar S, Song W, Chen Y, and Knight ZA (2020). Obesity causes selective and long-lasting desensitization of AgRP neurons to dietary fat. Elife 9. 10.7554/eLife.55909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deem JD, Faber CL, Pedersen C, Phan BA, Larsen SA, Ogimoto K, Nelson JT, Damian V, Tran MA, Palmiter RD, et al. (2020). Cold-induced hyperphagia requires AgRP neuron activation in mice. Elife 9. 10.7554/eLife.58764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu SM, Ifebi B, Johnson F, Xu A, Ho J, Yang Y, Schwartz G, Jo YH, and Chua S Jr. (2023). The gut signals to AGRP-expressing cells of the pituitary to control glucose homeostasis. J Clin Invest 133. 10.1172/JCI164185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental video 1:
One AgRP-DTR mouse received 40 ng DTX (i.c.v.) injection exhibited lethargy and movement difficulty on day 7 after DTX injection, whereas other AgRP-DTR mice received saline injection behaved normally. Related to Figures 1 and 2.
Supplemental video 2:
A control mouse received 40 ng DTX (i.c.v.) injection exhibited lethargy and movement difficulty on day 7 after DTX injection. Related to Figures 1 and 2.
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Raw data were deposited on Mendeley: doi:10.17632/v9m9vy6ddb.2. Any additional information required to reanalyze the data reported in this work paper is available from the Lead Contact upon request.


