Abstract
Introduction
In addition to reduced nectin‐4 expression, the upregulation of ATP‐binding cassette transporters has been suggested as a potential mechanism of resistance to enfortumab vedotin.
Case presentation
A 76‐year‐old man previously treated with platinum‐containing chemotherapy and pembrolizumab for metastatic bladder cancer was administered enfortumab vedotin because of disease progression. Subsequently, metastasectomy was performed for oligometastatic lesions (in the lung and adrenal gland) that exhibited growth during enfortumab vedotin therapy. Immunostaining analysis revealed decreased nectin‐4 expression and elevated MDR1, MRP1, and BCRP expression in the metastatic lesions.
Conclusion
Decreased nectin‐4 expression and increased ATP‐binding cassette transporter expression are potential factors in the development of enfortumab vedotin resistance in urothelial carcinoma. Immunohistochemical evaluation of these proteins may aid in predicting treatment efficacy.
Keywords: ABC transporters, antibody‐drug conjugate, enfortumab vedotin, nectin‐4, urothelial cancer
Abbreviations & Acronyms
- ABC
ATP‐binding cassette
- ADC
antibody‐drug conjugate
- EV
enfortumab vedotin
- FDG
fluorodeoxyglucose
- PET
positron emission tomography
Keynote message.
The expression of ATP‐binding cassette transporters has been suggested as a possible mechanism of enfortumab vedotin resistance.
Lung and adrenal metastases from patients with advanced urothelial cancer who developed resistance to enfortumab vedotin exhibited increased expression of ATP‐binding cassette transporters, including MDR1, MRP1, and BCRP.
Immunohistochemical evaluation of ATP‐binding cassette transporters may aid in predicting the efficacy of enfortumab vedotin.
Introduction
EV has demonstrated efficacy as a third‐line treatment for patients with urothelial cancer who have progressed after platinum‐containing chemotherapy and immune checkpoint inhibitor therapy. 1 Given that EV is an ADC consisting of anti‐nectin‐4 antibody, a decrease in nectin‐4 expression is expected as a potential cause of therapeutic resistance. 2 In addition to the reduced expression of nectin‐4, the expression of ABC transporters, responsible for expelling anticancer drugs from cancer cells, has been identified as an important mechanism of EV resistance in preclinical models. 3 However, there is limited clinical evidence supporting these hypotheses.
We encountered a case of advanced urothelial cancer, in which metastasectomy was performed for oligometastatic lesions after platinum‐containing chemotherapy, immune checkpoint inhibitor (pembrolizumab), and EV therapy. Immunohistochemically, a reduction in nectin‐4 expression and an enhancement in ABC transporter expression were observed in the metastases compared with the primary lesion. This case suggests that decreased nectin‐4 expression and increased ABC transporter expression may contribute to therapeutic resistance to EV.
Case presentation
The patient was a 76‐year‐old male. 5 years before presentation, the patient had received bladder preservation therapy, which included chemoradiotherapy with cisplatin and partial cystectomy, 4 for muscle‐invasive bladder cancer (urothelial cancer, grade 3, pT2). At 2 years after the partial cystectomy, a solitary pulmonary metastasis in the right lung was observed. The patient received six courses of chemotherapy (gemcitabine + cisplatin), resulting in a partial response and subsequent progression, followed by pembrolizumab. Despite the demonstrated efficacy of pembrolizumab for 3 years, lung metastasis progressed and the patient was referred to our hospital for further treatment.
FDG‐PET was performed upon referral to our hospital. The findings revealed a solitary right lung metastasis and mild FDG uptake in the right adrenal gland (Fig. 1a,b). As EV had not yet received approval in Japan, the patient underwent four courses of gemcitabine + paclitaxel therapy as third‐line therapy, resulting in stable disease. Subsequently, EV therapy was initiated following its approval. After seven courses of EV therapy, follow‐up FDG‐PET revealed a reduction in the lung metastasis, but there was an increase in FDG accumulation in the right adrenal gland, leading to the diagnosis of right adrenal metastasis (Fig. 1c,d).
Fig. 1.
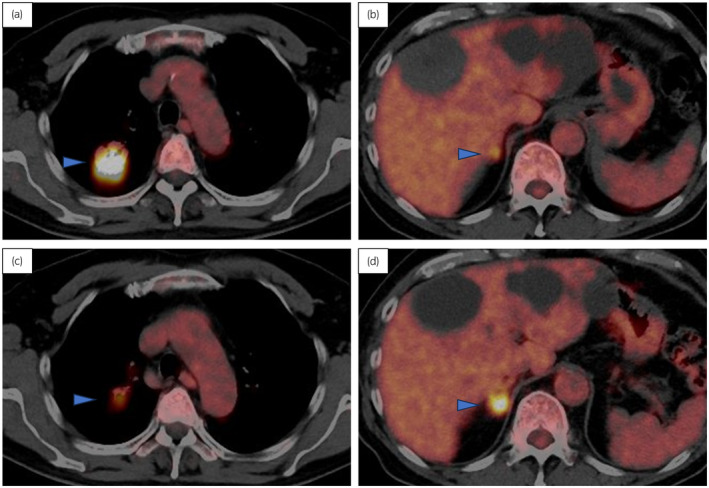
FDG‐PET findings of the right lung metastasis and right adrenal metastasis. (a) Lung metastasis and (b) right adrenal metastasis before EV therapy (c) lung metastasis and (d) right adrenal metastasis after seven courses of EV therapy. The blue arrow heads indicate the metastatic lesion.
Right adrenal metastasis, which was resistant to EV and was the sole progressing lesion, prompted a comprehensive discussion with the patient, leading to the decision to undergo right adrenalectomy. EV therapy was continued after right adrenalectomy; however, the metastatic lesion in the right lung exhibited progression. Consequently, after 6 months, partial resection of the right lung was performed. Although these surgeries resulted in a surgical complete response, multiple lung and liver metastases emerged 5 months later. Re‐administration of EV therapy proved ineffective, and the patient has since been managed with best supportive care.
Immunohistochemical evaluation of the primary bladder tumor, right adrenal metastasis, and lung metastasis was performed after initiating EV therapy and included an assessment of nectin‐4 (1:100, EPR15613‐68, Abcam, Cambridge, UK) and ABC transporter expression (Fig. 2). The ABC transporters evaluated were MDR1 (1:100, EPR10364‐57, Abcam), MRP1 (1:100, EPR21062, Abcam), and BCRP (1:50, BXP‐21, Abcam), all known for their association with anticancer drug resistance. 5 Immunostaining intensity was categorized on a scale from 0 to 3. 6 In the primary lesion, nectin‐4 exhibited high expression (score 3, H‐score 300), while ABC transporters were expressed minimally (score 0). Conversely, in adrenal and lung metastases, nectin‐4 expression was significantly decreased (score 1, H‐score 30), MDR1 exhibited a prominent increase to score 3, and the MRP1 and BCRP levels increased to score 1.
Fig. 2.
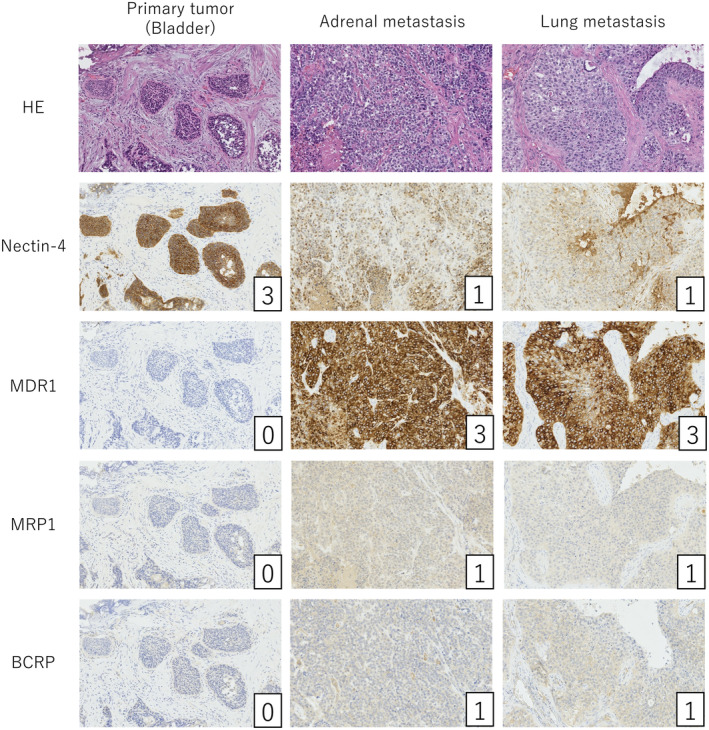
Immunohistochemical analysis for nectin‐4 and ABC transporter proteins in the primary bladder tumor, adrenal, and lung metastases. The numbers in the lower right corner indicate the intensity score of immunostaining.
Discussion
EV is currently approved as the third‐line treatment for advanced urothelial cancer that has developed resistance to cisplatin‐containing chemotherapy and immune checkpoint inhibitors. 1 Moreover, clinical studies have investigated the efficacy of EV in combination with immune checkpoint inhibitors as a first‐line treatment. 7 With the expanding array of therapeutic options for advanced urothelial cancer, there is a growing need for biomarkers that can predict the efficacy of EV. The mechanisms of therapeutic resistance to ADC drugs, including EV, are not well documented; however, two primary mechanisms have been postulated: (i) decreased expression of target antigens and (ii) induced expression of drug efflux transporter proteins. 8 , 9
Nectin‐4 serves as the target antigen for EV, and its low expression is assumed to contribute to therapeutic resistance to EV. 2 Studies have highlighted the heterogeneity in nectin‐4 expression within urothelial cancer, noting a decreased positive rate in muscle‐invasive carcinoma compared with non‐muscle‐invasive carcinoma. 10 Another study reported that variations in nectin‐4 expression rates in different variants of urothelial cancer, with squamous cell carcinoma and adenocarcinoma exhibiting a high positive rate, while sarcomatoid urothelial cancer rarely expresses nectin‐4. 11 Furthermore, it has been reported that nectin‐4 expression is reduced in metastatic lesions compared to primary tumors. 12 These findings underscore the importance of assessing nectin‐4 expression, either in the primary or metastatic lesions, before initiating EV therapy.
ABC transporters, such as MDR1, MRP1, and BCRP, have long been recognized for their significant role in the cellular efflux of traditional antineoplastic agents. 5 Specifically, MDR1 and MRP1 are involved in the mechanism of cisplatin resistance in urothelial cancer. 13 Notably, monomethyl auristatin E, the ADC payload within EV, is also acknowledged as a substrate for ABC transporters, making it potentially susceptible to this mechanism of resistance. 14 Cabaud et al. investigated the mechanism of resistance to EV in a preclinical model using a breast cancer cell line. They demonstrated that MDR1 expression was enhanced in the EV‐resistant cells and that pharmacological MDR1 inhibition restored EV sensitivity. 3 The involvement of increased expression of ABC transporters in resistance to ADC drugs has also been reported in other cancer types. For instance, malignant lymphoma cells resistant to brentuximab vedotin, an ADC‐containing CD30 antibody, exhibit decreased expression of CD30 and increased expression of MDR1. 15 Collectively, these findings suggest that the induction of ABC transporters plays a role in EV resistance in urothelial cancer.
In conclusion, our observations revealed a reduction in nectin‐4 expression and an increase in ABC transporter expression in the metastatic lesions of urothelial cancer that develop resistance to EV therapy. Although the results of the immunohistochemical analyses could not be used for treatment selection in this case, immunohistochemical analysis of nectin‐4 and ABC transporters has the potential to predict the therapeutic efficacy of EV.
Author contributions
Mariko Kotono: Data curation; investigation; writing – original draft. Toshiki Kijima: Conceptualization; data curation; investigation; writing – original draft; writing – review and editing. Atsuko Takada‐Owada: Investigation; methodology. Naoya Okubo: Data curation. Ryo Kurashina: Data curation. Hidetoshi Kokubun: Data curation. Toshitaka Uematsu: Data curation. Kohei Takei: Data curation. Kazuyuki Ishida: Investigation; methodology; supervision; writing – review and editing. Takao Kamai: Conceptualization; supervision; writing – review and editing.
Conflict of interest
The authors have no conflicts of interest to declare.
Approval of the research protocol by an Institutional Reviewer Board
Not applicable.
Informed consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
Registry and the Registration No. of the study/trial
Not applicable.
Acknowledgments
We gratefully acknowledge the technical assistance of Chiaki Satoh and Ayako Shimizu from the Department of Diagnostic Pathology at Dokkyo Medical University.
References
- 1. Powles T, Rosenberg JE, Sonpavde GP et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N. Engl. J. Med. 2021; 384: 1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chu CE, Sjöström M, Egusa EA et al. Heterogeneity in NECTIN4 expression across molecular subtypes of urothelial cancer mediates sensitivity to enfortumab vedotin. Clin. Cancer Res. 2021; 27: 5123–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cabaud O, Berger L, Crompot E et al. Overcoming resistance to anti‐Nectin‐4 antibody‐drug conjugate. Mol. Cancer Ther. 2022; 21: 1227–1235. [DOI] [PubMed] [Google Scholar]
- 4. Kijima T, Tanaka H, Koga F et al. Selective tetramodal bladder‐preservation therapy, incorporating induction chemoradiotherapy and consolidative partial cystectomy with pelvic lymph node dissection for muscle‐invasive bladder cancer: oncological and functional outcomes of 107 patients. BJU Int. 2019; 124: 242–250. [DOI] [PubMed] [Google Scholar]
- 5. Szakács G, Paterson JK, Ludwig JA, Booth‐Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006; 5: 219–234. [DOI] [PubMed] [Google Scholar]
- 6. Wolff AC, Hammond MEH, Allison KH et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J. Clin. Oncol. 2018; 36: 2105–2122. [DOI] [PubMed] [Google Scholar]
- 7. Piombino C, Tonni E, Oltrecolli M et al. Immunotherapy in urothelial cancer: current status and future directions. Expert Rev. Anticancer Ther. 2023; 23: 1141–1155. [DOI] [PubMed] [Google Scholar]
- 8. Loganzo F, Sung M, Gerber HP. Mechanisms of resistance to antibody‐drug conjugates. Mol. Cancer Ther. 2016; 15: 2825–2834. [DOI] [PubMed] [Google Scholar]
- 9. Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody‐drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021; 18: 327–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffman‐Censits JH, Lombardo KA, Parimi V et al. Expression of Nectin‐4 in bladder urothelial carcinoma, in morphologic variants, and nonurothelial histotypes. Appl. Immunohistochem. Mol. Morphol. 2021; 29: 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodler S, Eismann L, Schlenker B et al. Expression of Nectin‐4 in variant Histologies of bladder cancer and its prognostic value‐need for biomarker testing in high‐risk patients? Cancers (Basel) 2022; 14: 4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klümper N, Ralser DJ, Ellinger J et al. Membranous NECTIN‐4 expression frequently decreases during metastatic spread of urothelial carcinoma and is associated with enfortumab vedotin resistance. Clin. Cancer Res. 2023; 29: 1496–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hour TC, Chen J, Huang CY et al. Characterization of chemoresistance mechanisms in a series of cisplatin‐resistant transitional carcinoma cell lines. Anticancer Res. 2000; 20: 3221–3225. [PubMed] [Google Scholar]
- 14. Jackson D, Stover D. Using the lessons learned from the clinic to improve the preclinical development of antibody drug conjugates. Pharm. Res. 2015; 32: 3458–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen R, Hou J, Newman E et al. CD30 downregulation, MMAE resistance, and MDR1 upregulation are all associated with resistance to Brentuximab vedotin. Mol. Cancer Ther. 2015; 14: 1376–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]


