Abstract
Introduction
Small cell bladder cancer is a relatively rare tumor, representing <1% of all bladder tumors. Amrubicin monotherapy is used as second‐line treatment for small cell lung cancer in Japan.
Case presentation
A 79‐year‐old woman presented with gross hematuria and was diagnosed with small cell bladder cancer (T2 or higher). Neoadjuvant chemotherapy with etoposide and cisplatin resulted in a partial response. Robot‐assisted radical cystectomy was performed, and radical resection was achieved. As we identified metastasis in the pleura 1 year later, we administered carboplatin and etoposide, which resulted in a partial response. Although pembrolizumab was initiated as maintenance therapy, it was not effective. Amrubicin was given as third‐line therapy, and stable disease was achieved without serious adverse effect for 6 months.
Conclusion
Although there is no established treatment for metastatic small cell bladder cancer, the current case report suggests the effectiveness of amrubicin in this setting.
Keywords: amrubicin, immune checkpoint inhibitors, small cell bladder cancer
Abbreviations & Acronyms
- AMR
amrubicin
- CT
computed tomography
- EP
etoposide and cisplatin
- ICI
immune checkpoint inhibitor
- NCCN
National Comprehensive Cancer Network
- OS
overall survival
- PD
progressive disease
- Pem
pembrolizumab
- PFS
progression‐free survival
- PR
partial response
- ProGRP
pro‐gastrin releasing peptide
- RECIST
Response Evaluation Criteria in Solid Tumors
- SCBC
small cell bladder cancer
- SCLC
small cell lung cancer
- SD
stable disease
Keynote message.
Although there is no established treatment for metastatic small cell bladder cancer, amrubicin may be effective without serious side effects.
Introduction
SCBC is a relatively rare tumor: the prevalence rate of SCBC is estimated to be <1% of all bladder tumors. 1 Due to its rarity, an optimal treatment strategy for SCBC has not been established, and we generally treat metastatic SCBC according to the NCCN guidelines for SCLC. As urologists, we are familiar with the use of combination EP therapy as first‐line treatment for metastatic SCBC. In practice, Davis et al. published the first case report of successful treatment of SCBC using EP over 30 years ago. 2
Although several ICIs are widely used, and preferred regimens for primary therapy of extensive‐stage SCLC that include ICIs are constantly evolving, the optimal second‐line therapy for SCLC remains unclear. 3 AMR, an inhibitor of DNA topoisomerase II, is considered one of the most effective second‐line agents for SCLC, 4 with some studies reporting the efficacy of second‐line AMR for relapsed SCLC. 5 , 6 Nevertheless, prior to 2023, only two case reports had been published regarding the utilization of AMR for metastatic SCBC. 7 , 8
In this report, we present the case of a patient with metastatic SCBC who underwent sequential systematic therapy, including etoposide, Pem, and AMR.
Case presentation
A 79‐year‐old female visited a previous hospital due to macroscopic hematuria. Magnetic resonance imaging and contrast‐enhanced CT revealed a hypervascularity solid mass in the anterior wall of the bladder. The tumor size was 4.5 cm (Fig. 1). No metastatic lesions were observed, including within the upper urinary tract. Cystoscopy showed a single non‐papillary tumor at the anterior of the bladder. Transurethral resection of the bladder tumor was performed, and histopathological specimen findings indicated SCBC. She was referred to our institution for further evaluation and treatment. Blood tests revealed elevated neuron‐specific enolase (26 ng/mL) and proGRP (101 pg/mL) levels. Urine cytology indicated class V malignancy. As we diagnosed SCBC and her TNM stage was cT2N0M0, we decided to administer three courses of EP (cisplatin 73 mg/body and etoposide 91.2 mg/body) as neoadjuvant chemotherapy. CT revealed a (PR; 80% shrinkage) by the Response Evaluation Criteria in Solid Tumors version 1.1. Hence, we performed robot‐assisted radical cystectomy and created an ileal conduit. As the histopathological findings were SCBC ypT2bN0M0, RM0, Ly0, V1 (Fig. 2), we selected surveillance by CT and tumor marker measurement as follow‐up. One year later, CT revealed metastasis in the right pleura, and proGRP levels were markedly elevated to 1856 pg/mL. Based on the NCCN guidelines for SCLC, we decided to readminister etoposide. Since an ileal conduit had been created for urinary diversion, the estimated glomerular filtration rate was relatively low at 35 mL/min/1.73 m2. Carboplatin and etoposide (240 mg/body [AUC5] and 96 mg/body, respectively) were administered. After four courses of this regimen, CT revealed a PR (33% shrinkage) by RECIST ver1.1, and the proGRP level had decreased to 243 pg/mL.
Fig. 1.
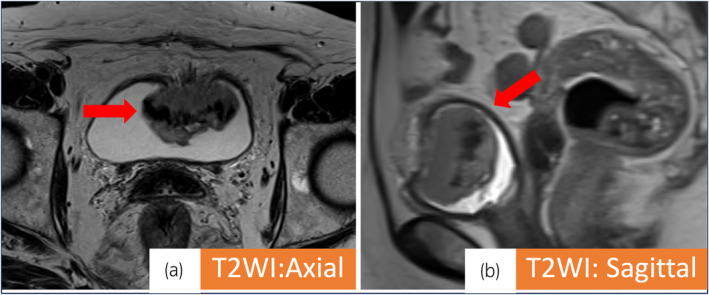
(a, b) (T2 weighted image): Red arrows indicate bladder tumor (4.5 × 2.9 × 4.2 cm). Muscle invasive bladder cancer was suspected.
Fig. 2.
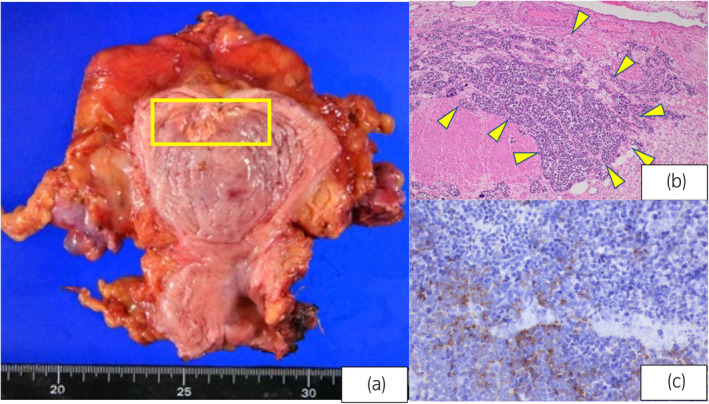
(a) Bladder sample: A shrinking tumor is observed in the yellow square. The tumor extended to the bladder muscle. (b) High N/C ratio, increased chromatin, and diffuse growth of naked nucleated tumor cells without any distinctive structure (hematoxylin–eosin staining ×40). (c) Partially positive areas are observed (chromogranin A staining ×400).
The tumor resumed growing 1 month later. Although we decided to administer Pem as second‐line therapy, CT revealed immune‐confirmed PD after four courses of Pem. There were no adverse events and the best response rate was PD. The proGRP level had increased to 8348 pg/mL.
Since the use of AMR for treating SCBC was not covered by insurance in Japan, we opted to administer AMR every 4 weeks, starting with a dose of 60 mg per body (equivalent to 90% of 40 mg/m2 administered on Days 1–3), as a third‐line therapy after committee approval of its use. As Grade 3 neutropenia occurred in Courses 1 and 5 after about 10 days of administration, AMR dose was reduced to 52 mg/body (80% of 40 mg/m2 on Days 1–3) and 49 mg/body (75% of 40 mg/m2 on Days 1–3) every 4 weeks. Six courses of AMR have been administered without life‐threatening adverse effects, CT revealed SD (12.6% increase), and proGRP levels have gradually decreased to 2866 ng/mL (Fig. 3).
Fig. 3.
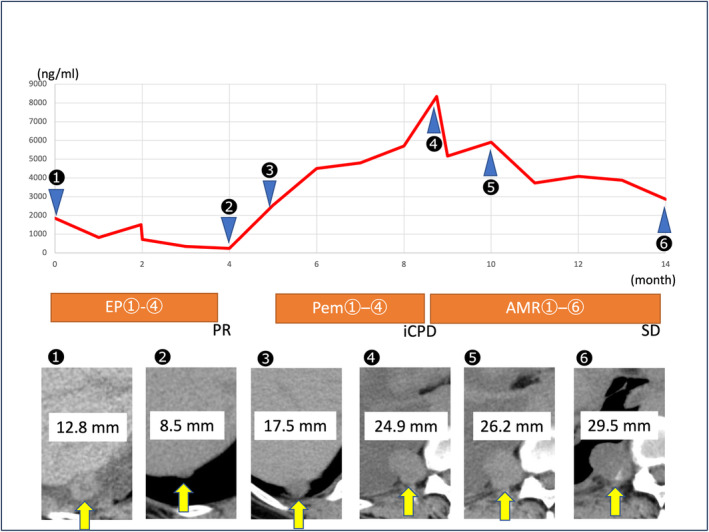
This figure shows the treatment course after recurrence. The red line indicates changes in pro‐gastrin‐releasing peptide (proGRP) level. Yellow arrows indicate metastasis in the right pleura.
Discussion
In this report, we have described a patient with metastatic SCBC after radical cystectomy treated with etoposide, Pem, and AMR, sequentially. SCBC is a rare subtype of bladder tumor with a poor prognosis. A previous study reported that median OS of patients with advanced SCBC was 8.6 months and was only 5.3 months in patients with metastatic disease. 9 As it has been difficult to establish a standardized treatment approach, we usually treat SCBC according to the NCCN guidelines for management of SCLC.
AMR monotherapy has been established as a second‐line chemotherapy regimen in patients with SCLC. 4 A phase‐3 trial evaluating the efficacy of AMR in SCLC reported OS and PFS of 7.5 and 4.1 months, respectively. 10 The most common adverse effect associated with AMR is neutropenia, which can be managed with filgrastim.
Some studies have reported the efficacy and safety of AMR after administration of ICIs in patients with SCLC. These studies also revealed that the incidence of severe toxicities associated with AMR did not increase following treatment with ICIs. 6 , 11 , 12 Moreover, a recent study reported the efficacy of combination Pem and AMR therapy as second‐line treatment for SCLC, including median OS of 10.6 months and median PFS of 4.0 months. 13 We expect that in the near future, SCBC will also be treated with combination ICI and AMR therapy.
As mentioned above, as far as we investigated, only two case reports regarding the use of AMR in patients with metastatic SCBC have been published. 7 , 8 Although these reports could not clearly indicate the efficacy of AMR, the present report revealed a decrease in proGRP level from 8348 to 2866 pg/mL, and a 12.6% increase of the tumor volume. We have administered a total of six courses, and a tumor response of SD has been maintained. Additionally, as this case was a pure small cell carcinoma, it was not precisely a urothelial carcinoma, and therefore, nivolumab adjuvant therapy was not administered. Enfortumab vedotin was not administrated because a previous study revealed that the nectin‐4 staining was negative in SCBC. 14 To the best of our knowledge, this is the first case of SCBC treated sequentially with an ICI and AMR. Further investigation is needed to determine the appropriate use of AMR in patients with metastatic SCBC.
Author contributions
Kazutaka Mitani: Writing – original draft. Ichiro Tsuboi: Writing – review and editing. Gen Tanaka: Writing – review and editing. Saori Yosioka: Writing – review and editing. Shuhei Yokoyama: Writing – review and editing. Yusuke Kobayashi: Writing – review and editing. Hirochika Nakajima: Writing – review and editing. Taichi Nagami: Writing – review and editing. Kohei Ogawa: Writing – review and editing. Koichiro Wada: Supervision; writing – review and editing.
Conflict of interest
The authors declare no conflicts of interest.
Approval of the research protocol by an Institutional Review Board
AMR for metastatic SCBC.
Informed consent
Informed consent was obtained from the patient.
Registry and the Registration No. of the study/trial
No. 2018‐2 (June 24, 2023).
References
- 1. Fischer‐Valuck BW, Rao YJ, Henke LE et al. Treatment patterns and survival outcomes for patients with small cell carcinoma of the bladder. Eur. Urol. Focus 2018; 4: 900–906. [DOI] [PubMed] [Google Scholar]
- 2. Davis MP, Murthy MS, Simon J, Wise H, Minton JP. Successful management of small cell carcinoma of the bladder with cisplatin and etoposide. J. Urol. 1989; 142: 817. [DOI] [PubMed] [Google Scholar]
- 3. National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology bladder cancer. Version 3. 2023. [DOI] [PubMed]
- 4. Shi H, Guo N, Zhao Z et al. Comparison of the second‐line treatments for patients with small cell lung cancer sensitive to previous platinum‐based chemotherapy: a systematic review and Bayesian network analysis. Front. Oncol. 2023; 13: 1154685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uda S, Yamada T, Yoshimura A et al. Clinical impact of amrubicin monotherapy in patients with relapsed small cell lung cancer: a multicenter retrospective study. Transl. Lung Cancer Res. 2022; 11: 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uematsu S, Kitazono S, Tanaka H et al. Clinical efficacy of amrubicin in patients with small cell lung cancer relapse after first‐line treatment including immune checkpoint inhibitors: a retrospective multicenter study (TOPGAN 2021‐01). Thorac. Cancer 2023; 14: 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ota H, Yamaguchi T, Tsuruta M et al. A case of small cell carcinoma of the bladder. Hinyokika Kiyo 2020; 66: 313–317. [DOI] [PubMed] [Google Scholar]
- 8. Naito A, Matsumoto A, Odani K et al. Metastatic small cell carcinoma of the urinary bladder treated with systemic chemotherapy including an AMRUBICIN; a CASE report. Nihon Hinyokika Gakkai Zasshi 2016; 107: 34–38. [DOI] [PubMed] [Google Scholar]
- 9. `Geynisman DM, Handorf E, Wong YN et al. Advanced small cell carcinoma of the bladder: clinical characteristics, treatment patterns and outcomes in 960 patients and comparison with urothelial carcinoma. Cancer Med. 2016; 5: 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. von Pawel J, Jotte R, Spigel DR et al. Randomized phase III trial of amrubicin versus topotecan as second‐line treatment for patients with small‐cell lung cancer. J. Clin. Oncol. 2014; 32: 4012–4019. [DOI] [PubMed] [Google Scholar]
- 11. Nishimura T, Fujimoto H, Fujiwara T et al. Efficacy and safety of amrubicin in small cell carcinoma previously treated with immune checkpoint inhibitors and chemotherapy. Cancers (Basel) 2022; 14: 3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imai H, Nagai Y, Minemura H et al. Efficacy and safety of amrubicin monotherapy after atezolizumab plus carboplatin and etoposide in patients with relapsed small‐cell lung cancer. Invest. New Drugs 2022; 40: 1066–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akamatsu H, Teraoka S, Hayashi H et al. Pembrolizumab plus amrubicin in patients with relapsed SCLC: multi‐institutional, single‐arm phase 2 study. JTO Clin. Res. Rep. 2021; 2: 100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoffman‐Censits JH, Lombardo KA, Parimi V et al. Expression of nectin‐4 in bladder urothelial carcinoma and in morphologic variants and non‐urothelial histotypes. Appl Immunohistochem. Mol. Morphol. 2021; 29: 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]


