Abstract
Introduction
The efficacy of olaparib for treatment‐related neuroendocrine prostate cancer is unknown. Here, we report a case of treatment‐related neuroendocrine prostate cancer with a BRCA2 mutation that was treated with olaparib with 1‐year efficacy.
Case presentation
A 75‐year‐old man initially diagnosed with prostate adenocarcinoma developed treatment‐related neuroendocrine prostate cancer after 10‐year androgen deprivation therapy. Despite the initial temporary effects of etoposide and carboplatin, the patient experienced prostate bed tumor recurrence 1 year after chemotherapy cessation. FoundationOne® detected a BRCA2 gene mutation, and olaparib was initiated after repeating one chemotherapy course using the same chemotherapeutic agents. The patient received olaparib with sustained tumor regression for 1 year without severe side effects.
Conclusion
Olaparib may be the treatment of choice for treatment‐related neuroendocrine prostate cancer in patients with BRCA mutations.
Keywords: BRCA2, castration‐resistant, neuroendocrine tumor, poly(ADP‐ribose) polymerase inhibitors, prostate cancer
Abbreviations & Acronyms
- ABI
abiraterone
- ADT
androgen‐deprivation therapy
- AWD
alive with disease
- BCL
bicalutamide
- CAB
combined androgen blockade
- CBDCA
carboplatin
- CBZ
cabazitaxel
- CD
cancer death
- CDDP
cisplatin
- CRPC
castration‐resistant prostate cancer
- DTX
docetaxel
- ENZ
enzalutamide
- ETP
etoposide
- GOS
goserelin
- HRD
homologous recombination deficiency
- HRR
homologous recombination repair
- LPR
leuprolide
- N/A
not available
- NED
no evidence of disease
- NEPC
neuroendocrine prostate cancer
- NSE
neuron‐specific enolase
- PSA
prostate‐specific antigen
- PARP
poly(ADP‐ribose) polymerase
- proGRP
pro‐gastrin‐releasing peptide
- RP
radical prostatectomy
- RT
radiation therapy
- t‐NEPC
treatment‐related neuroendocrine prostate cancer
Keynote message.
A 75‐year‐old man initially diagnosed with prostate adenocarcinoma developed treatment‐related neuroendocrine prostate cancer 10 years after androgen deprivation therapy was initiated. Chemotherapy with etoposide and carboplatin was effective; however, the patient experienced prostate bed tumor recurrence 1 year after chemotherapy. FoundationOne® detected BRCA2 gene mutation, and olaparib has been used with sustained tumor regression for 1 year.
Introduction
PARP is critical in DNA damage repair. Olaparib, its selective inhibitor, exploits synthetic lethality against CRPC with HRD. 1 t‐NEPC, a CRPC status after androgen deprivation therapy (ADT), is characterized by either low or absent androgen receptor expression, small‐cell carcinoma morphology, and expression of neuroendocrine markers. 2
In most cases with t‐NEPC, the efficacy of chemotherapy is limited, and the prognosis is extremely poor. 3 Mutations in HRR genes, including breast cancer gene (BRCA) mutation, are rare in t‐NEPC, 4 and the efficacy of olaparib for t‐NEPC remains unclear. Here, we report a case of t‐NEPC with a BRCA2 mutation that was treated with sustained tumor regression for 1 year.
Case presentation
In 2008, a 64‐year‐old man with a serum PSA level of 6.5 ng/mL and a family history of breast and prostate cancers was diagnosed as having cT3N0M0 prostate cancer. Prostate biopsy revealed adenocarcinoma with a Gleason score of 4 + 5. The patient underwent a prostatectomy 3 months after receiving neoadjuvant hormonal therapy. One year after surgery, salvage ADT was introduced for biochemical recurrence, and the PSA level was <0.02 ng/mL. In 2015, the patient progressed to non‐metastatic CRPC, with elevated PSA levels and local recurrence in the pelvic floor. The disease was controlled with salvage radiotherapy (74 Gy/37 Fr) to the pelvic floor, with decreased serum PSA levels. NSE and proGRP levels were 12.5 ng/mL (normal: <16.3 ng /mL) and 53.8 ng/mL (normal: <67 pg/mL), respectively, at the end of salvage radiotherapy.
In 2019, the PSA levels decreased to 0.001 ng/mL. However, NSE and proGRP levels increased to 31.8 ng/mL and 65.8 pg/mL, respectively, despite low PSA levels. Imaging revealed a resurgence of the pelvic floor tumor and mediastinal and pelvic lymph node metastases (Fig. 1a,b). Biopsy of the pelvic floor tumor revealed small malignant cells with a high nuclear‐to‐cytoplasmic ratio, and frequent mitotic figures were arranged in diffuse sheets (Fig. 1c,d). Immunohistochemical analysis showed that the tumor was positive for synaptophysin, CD56, and chromogranin A but negative for PSA (Fig. 1e–g). Based on the appearance of tumor cells and positive findings for neuroendocrine markers, the recurrent tumor was pathologically diagnosed as small‐cell NEPC and clinically diagnosed as t‐NEPC. Adenocarcinoma components were not detected.
Fig. 1.
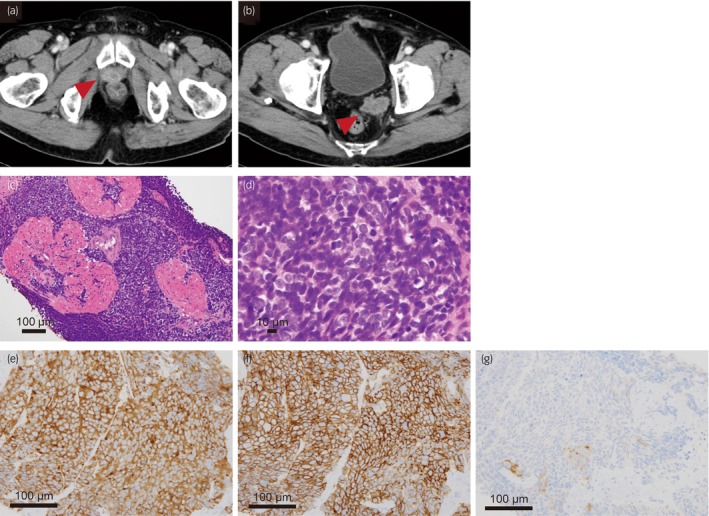
Computed tomography image when the patient was diagnosed with t‐NEPC (a, b) and microscopic findings of the tumor (c–g). (c) Small, clustered cells with a high nuclear‐to‐cytoplasmic ratio and no glandular pattern are observed (hematoxylin and eosin staining: ×20). (d) There are frequent mitotic figures (hematoxylin and eosin staining: ×100). (e) The tumor cells are positive for synaptophysin, (f) CD56, and (g) chromogranin A, partially (×200).
The clinical course after NEPC diagnosis is shown in Figure 2. Four‐month chemotherapy with ETP and CBDCA resulted in a complete response. However, in 2021, the pelvic floor tumor recurred again (Fig. 3). We restarted ETP and CBDCA chemotherapy, but the patient discontinued because he experienced delirium. At that time, the FoundationOne® genomic test on the biopsy specimen of the pelvic floor tumor diagnosed as NEPC revealed a BRCA2 gene mutation and some variants of uncertain significance (Table S1). A single‐site analysis with peripheral blood was performed to confirm the pathogenic variant identified in FoundationOne®; the patient harbored a BRCA2 germline mutation. Therefore, olaparib was administered as a fifth‐line treatment for prostate cancer. The proGRP level decreased, and the tumor diminished in size, indicating stable disease following the revised Response Evaluation Criteria in Solid Tumors version 1.1. 5 However, the proGRP level gradually increased after 1 year of treatment with olaparib and 15 months after initiating olaparib, the pelvic floor tumor showed regrowth, indicating progressive disease. The patient continued olaparib for 40 months after t‐NEPC diagnosis because of a slow increase in tumor size and minimal side effects.
Fig. 2.
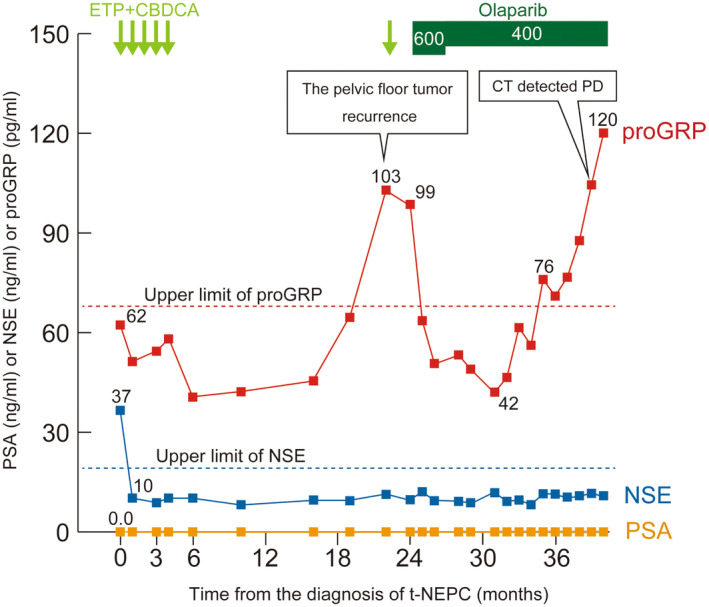
The clinical course after the diagnosis of t‐NEPC. Olaparib resulted in decreased proGRP level and tumor reduction.
Fig. 3.
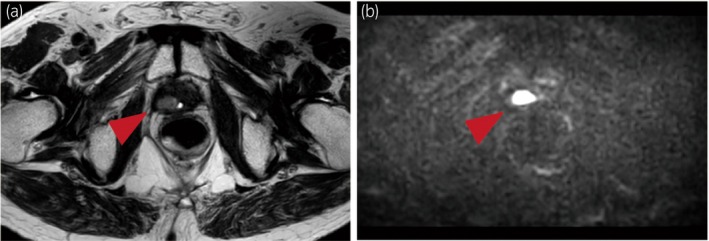
Magnetic resonance imaging of recurrent pelvic floor tumor before olaparib administration. The tumors show faintly high signal intensity on T2‐weighted images and are diffusion‐weighted image‐positive.
Discussion
To our knowledge, this is the eighth t‐NEPC case treated with olaparib, and the rarity of our case is due to the relatively long‐term disease control with olaparib. Low serum PSA levels, positive neuroendocrine markers, and an aggressive clinical course characterize t‐NEPC. 3 , 6 Our patient experienced rapid local progression and distant lymph node metastasis with low PSA levels and was diagnosed with t‐NEPC after a 10‐year ADT. de novo NEPC at the initial diagnosis of prostate cancer is very rare; 7 however, the incidence of t‐NEPC in CRPC is considered high because of the widely used ADT and androgen receptor axis‐targeted agents. 8 Aggarwal et al. reported that 17% of patients with CRPC had histologic neuroendocrine features in biopsies of metastatic sites. 4
In reports of t‐NEPC genomic alteration, MYCN and AURKA amplifications were detected in 65% of patients with primary prostate cancer who developed t‐NEPC. 9 Loss of function in TP53 or RB1 is not observed in a few t‐NEPC cases. 10 These genomic features may be deeply involved in the development of t‐NEPC; 3 however, we did not observe these gene mutations in our patient, indicating there might be other genomic or epigenetic alterations that trigger t‐NEPC arising from initial adenocarcinoma. 11 t‐NEPCs often present poorer prognosis than common prostate adenocarcinoma. 4 Following the National Comprehensive Cancer Network guidelines version 1.2023, the standard treatment for NEPC is chemotherapy with ETP and platinum‐based drugs such as CDDP. t‐NEPCs are initially sensitive to chemotherapy; tumors soon develop resistance, and median overall survival is approximately 7 months. 3 , 12 Therefore, more effective treatment options are required. Recently, several cases of t‐NEPC treated with olaparib have been reported. The clinical features of seven previously reported cases and the present case are summarized in Table 1. 13 , 14 , 15 , 16 , 17 , 18 Three patients exhibited a partial response to olaparib. However, in most cases, the efficacy of olaparib in treating t‐NEPC was observed only for a short duration (<6 months). In contrast, in our case, olaparib provided >1‐year efficacy with stable t‐NEPC. Regarding ovarian cancer, platinum resistance is related to olaparib resistance. 19 In our patient, platinum‐based chemotherapy was still effective, and olaparib was initiated before the tumor acquired platinum resistance. This suggests that olaparib can be successfully used to treat t‐NEPC before chemotherapy or as an early‐line treatment.
Table 1.
Clinical features of t‐NEPC cases treated with olaparib
| No. | Author | At the diagnosis of prostate adenocarcinoma | Time to NEPC | At the diagnosis of t‐NEPC | Outcome | Survival from NEPC diagnosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, year | PSA, ng/mL | Gleason score | Tumor stage | Treatment | PSA, ng/mL | NSE, ng/mL | proGRP, pg/mL | Sites of organ metastasis | BRCA mutation | Treatment | |||||
| 1 | Turina, et al. 2019 13 | N/A | 9.23 | 4 + 4 | pT3bN0 |
Local treatment: RP 1st line: ADT 2nd line: ENZ |
73 week | 9.93 | N/A | N/A | Liver, bladder |
BRCA2 Copy number loss |
1st line: ETP + CBDCA maintenance therapy: olaparib | NED | 51 week |
| 2 | Wu et al. 2020 14 | 63 | 55.13 | 4 + 4 | cT4N1M1b |
1st line: GOS + BCL 2nd line: ABI + BCL |
7 month | 1.5 | 212.9 | 976.2 | Liver, lung | BRCA1 |
1st line: olaparib+RT 2nd line: ETP + CDDP |
CD | 5 month |
| 3 | Pandya et al. 2021 15 | 65 | 95 | 4 + 4 | M1b | LPR + ABI | 16 month | 0.5 | 824 | N/A | Liver |
BRCA2 (Ser1882*) |
1st line: ETP + CBDCA 2nd line: olaparib 3rd line: pembrolizumab 4th line: platinum‐based chemotherapy |
CD | 18 month |
| 4 | Naiki et al. 2022 16 | 63 | 20.3 | 4 + 3 | cT2N1M1a | Surgical castration+ABI | 10 month | N/A | 27.4 | N/A | Liver, bone |
BRCA2 (H1223fs*9) |
1st line: ETP + CDDP 2nd line: amrubicin 3rd line: olaparib |
CD | 10 month |
| 5 | Miyazawa Y, et al. 2022–2 cases 17 | 70 | 40.8 | 4 + 5 | cT3bN1M1b | CAB+RT(prostate) | 36 month | <0.01 | 171 | N/A | Liver, bone | BRCA2 |
1st line: ETP + CDDP 2nd line: ENZ 3rd line: olaparib |
AWD | N/A |
| 6 | 78 | 15.2 | 4 + 4 | cT3bN0M0 |
Local treatment: RP 1st line: ADT 2nd line: BCL 3rd line: ENZ 4th line: DTX 5th line: CBZ |
N/A | N/A | N/A | N/A | Bladder | BRCA2 |
1st line: ETP + CBDCA 2nd line: olaparib |
AWD | N/A | |
| 7 | Kaitsumaru M, et al. 2023 18 | 67 | 29.99 | 5 + 5 | M1b |
1st line: LPR + ENZ 2nd line: DTX |
20 month | 0.19 | 211 | 53.5 | Liver, bone |
BRCA1 (deletion of intron 3–7) |
1st line: olaparib | CD | 6 month |
| 8 | The present case | 64 | 6.5 | 4 + 5 | cT3N0M0 |
Local treatment: RP 1st line: ADT 2nd line: salvage RT |
132 month | 0.001 | 31.8 | 65.8 | None |
BRCA2 (D427fs*3) |
1st line: ETP + CBDCA 2nd line: olaparib |
AWD | 40 month |
Conclusion
We report a case of t‐NEPC treated with olaparib that achieved a 1‐year stable disease. Additional cases are required to clarify the ideal treatment strategy for t‐NEPC; however, olaparib may be the treatment of choice for this aggressive disease.
Author contributions
Riko Ikeda: Writing – original draft. Yoh Matsuoka: Supervision; writing – review and editing. Masaharu Inoue: Supervision. Ayataka Ishikawa: Supervision. Kiwamu Akagi: Supervision. Yukio Kageyama: Supervision.
Conflict of interest
The authors declare no conflict of interest.
Approval of the research protocol by an Institutional Reviewer Board
Not applicable.
Informed consent
Informed consent was obtained from the patient. using the opt‐out method. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3079702/).
Registry and the Registration No. of the study/trial
Not applicable.
Supporting information
Table S1. Gene alterations in our case.
Acknowledgments
We would like to thank Editage (www.editage.com) for the English language editing.
References
- 1. de Bono J, Mateo J, Fizazi K et al. Olaparib for metastatic castration‐resistant prostate cancer. N. Engl. J. Med. 2020; 382: 2091–2102. [DOI] [PubMed] [Google Scholar]
- 2. Zhang Y, Zheng D, Zhou T et al. Androgen deprivation promotes neuroendocrine differentiation and angiogenesis through CREB‐EZH2‐TSP1 pathway in prostate cancers. Nat. Commun. 2018; 9: 4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akamatsu S, Inoue T, Ogawa O, Gleave ME. Clinical and molecular features of treatment‐related neuroendocrine prostate cancer. Int. J. Urol. 2018; 25: 345–351. [DOI] [PubMed] [Google Scholar]
- 4. Aggarwal R, Huang J, Alumkal JJ et al. Clinical and genomic characterization of treatment‐emergent small‐cell neuroendocrine prostate cancer: a multi‐institutional prospective study. J. Clin. Oncol. 2018; 36: 2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 6. Spetsieris N, Boukovala M, Patsakis G, Alafis I, Efstathiou E. Neuroendocrine and aggressive‐variant prostate cancer. Cancers 2020; 12: 3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beltran H, Tagawa ST, Park K et al. Challenges in recognizing treatment‐related neuroendocrine prostate cancer. J. Clin. Oncol. 2012; 30: e386–e389. [DOI] [PubMed] [Google Scholar]
- 8. Yao J, Liu Y, Liang X et al. Neuroendocrine carcinoma as an independent prognostic factor for patients with prostate cancer: a population‐based study. Front. Endocrinol. 2021; 12: 778758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mosquera JM, Beltran H, Park K et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment‐related neuroendocrine prostate cancer. Neoplasia 2013; 15: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beltran H, Prandi D, Mosquera JM et al. Divergent clonal evolution of castration‐resistant neuroendocrine prostate cancer. Nat. Med. 2016; 22: 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen J, Shi M, Chuen Choi SY et al. Genomic alterations in neuroendocrine prostate cancer: a systematic review and meta‐analysis. BJUI Compass. 2023; 4: 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang HT, Yao YH, Li BG, Tang Y, Chang JW, Zhang J. Neuroendocrine prostate cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis‐a systematic review and pooled analysis. J. Clin. Oncol. 2014; 32: 3383–3390. [DOI] [PubMed] [Google Scholar]
- 13. Turina CB, Coleman DJ, Thomas GV, Fung AW, Alumkal JJ. Molecular testing identifies determinants of exceptional response and guides precision therapy in a patient with lethal, treatment‐emergent neuroendocrine prostate cancer. Cureus 2019; 11: e5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu Y, Gao Y, Dou X, Yue J. Metastatic castration‐resistant prostate cancer with neuroendocrine transformation and BRCA 1 germ‐line mutation: a case report and literature review. Onco. Targets. Ther. 2020; 13: 8049–8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pandya D, Shah M, Kaplan F et al. Treatment‐emergent neuroendocrine prostate cancer with a germline BRCA2 mutation: identification of a candidate reversion mutation associated with platinum/PARP‐inhibitor resistance. Cold Spring Harb. Mol. Case Stud. 2021; 7: a005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Naiki T, Naiki‐Ito A, Kawai T et al. A case of metastatic treatment‐emergent small cell/neuroendocrine prostate cancer with BRCA2 mutation diagnosed by liver biopsy. IJU Case Rep. 2022; 5: 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miyazawa Y, Shimizu T, Sekine Y et al. Two cases of CRPC with BRCA mutation treated by olaparib after favorable response to cisplatin. IJU Case Rep. 2023; 6: 37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaitsumaru M, Shiota M, Takamatsu D et al. Interstitial pneumonia after regression by olaparib for neuroendocrine prostate cancer with BRCA1 mutation: a case report. Int. Cancer Conf. J. 2023; 12: 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fong PC, Yap TA, Boss DS et al. Poly(ADP)‐ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum‐free interval. J. Clin. Oncol. 2010; 28: 2512–2519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Gene alterations in our case.


