Abstract
The proto-oncogenes jun and fos are members of the AP-1 family of transcription factors, which activate transcription of target genes via the tetradecanoyl phorbol acetate response element (TRE). Both jun and fos contain activation domains, but their relative contributions to transcriptional activation of different TREs remain unclear. It is not apparent whether the cellular availability of specific AP-1 members is the major determinant for regulation of TREs or whether other factors including the TRE sequence itself contribute to selectivity. We have identified in the promoter of the rat atrial natriuretic factor (ANF) a novel AP-1 site which is unresponsive to jun homodimers and is inducible only in the presence of c-fos. This activation is potentiated by mitogen-activated protein (MAP) kinase. The jun proteins appear to be required solely to tether c-fos to the promoter, and c-fos mutants lacking putative activation domains abrogate transactivation. Unexpectedly, the oncogenic form of c-fos which diverges most significantly in the carboxy-terminal 50 amino acids is unable to mediate transactivation at this specialized AP-1 site. Mutations within the C terminus of c-fos at serine residues that are phosphorylation targets for growth factors and MAP kinase completely abrogate transactivation and block potentiation by MAP kinase. Using GAL4 fusions, we show that the 90-amino-acid C terminus of c-fos contains autonomous activation domains and that the serine residues are essential for full activity. These results suggest that phosphorylation of the C terminus of c-fos affects its transactivation properties and provide evidence for novel regulatory mechanisms that may contribute to biologic specificities of the AP-1 transcription complex.
One of the earliest cellular responses to many neurotransmitters and growth factors is the induction of the AP-1 transcription complex, which is composed of members of the jun (c-jun, junB, and junD) and fos (c-fos, fosB, fosB2, fra-1, and fra-2) families (3). Expression of the various AP-1 factors is differentially regulated spatially (13, 56) during cell cycle progression (31) and in response to many stimuli (7, 38). Given that the AP-1 proteins have different transcriptional properties as a result of specific activation and repression domains (16, 33, 50) and/or differential posttranslational modifications (reviewed in reference 27), the composition of the AP-1 complex could be critical to its regulatory function.
AP-1 proteins bind specific DNA sequences, termed TREs (tetradecanoyl phorbol acetate [TPA] response elements) that are present within the regulatory regions of many different genes (reviewed in reference 27). jun members bind DNA as homo- or heterodimers, and their affinity for DNA is greatly enhanced by fos proteins (37). Binding by jun homodimers or fos-jun heterodimers produces distinct DNA bending that may result in highly specific protein-protein interactions between AP-1 factors and other promoter-bound transcription complexes (28, 29). Thus, fos proteins may contribute to regulatory specificity at two levels by enhancing association of jun proteins to DNA and altering DNA structure. However, whether fos proteins also contribute to transcriptional activation by the heterodimer remains unclear. Indeed, jun-mediated transactivation of AP-1-dependent promoters requires an N-terminal transactivation domain of c-jun (4, 11) which was reported to be the major contributor to transcriptional stimulation in the context of the jun-fos heterodimer (1). Nevertheless, the c-fos protein contains several transcriptionally active regions, including several autonomous transactivation domains (17, 26, 49, 58), a carboxy-terminal transrepression domain (22, 39, 44), and a region that interacts with the TATA box-binding protein (36). Although these domains are critical for some cellular functions of c-fos such as transformation (55, 58), they may reflect a transcriptional role of fos independent of AP-1 complex (55).
Support for a contribution of c-fos to transactivation of AP-1-dependent promoters came recently from studies using c-fos null cell cultures which provided the first direct evidence for an in vivo function of c-fos in activation of a subset of AP-1-dependent promoters (24, 45). Indeed, two independent groups have analyzed AP-1 binding and transcriptional regulation of several AP-1 target genes in fibroblasts lacking c-fos (12, 24, 45). These studies revealed normal AP-1 DNA binding activity and similar levels of some known AP-1-dependent transcripts; however, other AP-1 target genes were either downregulated (24) or unresponsive to growth factors (24) or UV irradiation (45). These data suggested that AP-1 sites themselves may be divided into subtypes defined by their specificity for certain AP-1 members or by their involvement in basal versus induced transcription.
We have characterized an AP-1 site in the atrial natriuretic factor (ANF) promoter that defines a subclass of AP-1 sites specific for c-fos-containing heterodimers. On this specialized AP-1 site, c-fos appears to be the major contributor to transactivation. Moreover, transactivation by c-fos is enhanced by the mitogen-activated protein kinase (MAPK) and requires a c-fos domain previously associated with transrepression. The data provide evidence for novel regulatory mechanisms that may contribute to biologic specificities of the AP-1 transcription complex.
MATERIALS AND METHODS
Expression vectors.
BamHI-BglII TRE (or mutant) oligonucleotides were inserted into a BamHI site of a vector containing the thymidine kinase (TK109) or the −135 bp ANF (5) promoter in either one or three copies (1× or 3× construct). Expression vectors for the various AP-1 members were previously described (34). The vectors encoding ERK-1 and its kinase-deficient mutant (35) were provided by S. Meloche.
Cell culture and transfections.
HeLa and F9 cells were maintained in Dulbecco modified Eagle medium plus 10 and 15%, respectively, fetal calf serum. Primary cultures of ventricular cardiomyocytes were performed as described previously (5). Transfection of all cell types was by the calcium phosphate precipitation technique. Cells (HeLa and F9) were harvested 30 h after transfection, and luciferase activity or secreted immunoreactive growth hormone (irGH) was assayed as previously described (34). Cardiocytes were maintained for 48 h posttransfection in serum-free synthetic medium, the medium was then changed, and secreted irGH was assayed 48 h later.
Gel shift experiments.
Nuclear extracts were prepared from cell lines and primary cardiocyte cultures as previously described (34). Gel shifts using nuclear extracts and purified or in vitro-translated proteins were carried out according to published protocols (16, 47). Purified c-jun and c-fos proteins were generous gifts of M. Karin, and junD protein was translated in vitro, using rabbit reticulocyte lysate as instructed by the manufacturer (Promega). In supershift experiments, AP-1–TRE complexes were incubated for 1 h at 4°C in the absence or presence of specific AP-1 antibodies. c-fos antibody was purchased from Santa Cruz Biotechnology; c-jun and junD antibodies were a gift of T. Antakly.
Western blotting.
Nuclear extracts prepared from cells overexpressing wild-type or mutant c-fos proteins were used for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting performed according to standard protocols. The various fos proteins were detected by using a commercially available polyclonal antibody (Oncogene Science).
GAL4 fusions.
Oligonucleotides were used to generate by PCR C-terminal fragments of c-fos, corresponding to amino acids 290 to 380, using either wild-type c-fos as a template or one of the serine-to-alanine mutants, serA, serB, or serC. Other oligonucleotides were used to generate fragments missing either the C-terminal transactivation motif (amino acids 320 to 380) or the C-terminal serines (amino acids 290 to 360). These fragments containing XbaI/BamHI ends were cloned in frame into a vector encoding the DNA-binding domain (DBD) of GAL4. The activity of each of these fusions was assessed by using a reporter comprising five tandem copies of a GAL4-binding site adjacent to a minimal elastase promoter.
RESULTS
Identification of an AP-1 site specific for jun-fos heterodimers.
Several members of the AP-1 family of transcription factors are expressed in myocardial cells in response to hormonal or mechanical stimulation (25, 30, 34, 46). Because most of these stimuli lead to cardiac hypertrophy, it has been speculated that AP-1 proteins may be involved in the genetic reprogramming that accompanies cardiac hypertrophy. However, the role of AP-1 proteins in cardiac transcription remains essentially unclear. The ANF gene is a hallmark of the genetic switch associated with trophic stimulation of cardiomyocytes, and the ANF promoter contains an AP-1-like site that was previously shown to interact with similar cardiac nuclear proteins as the well-characterized collagenase AP-1/TRE element (34).
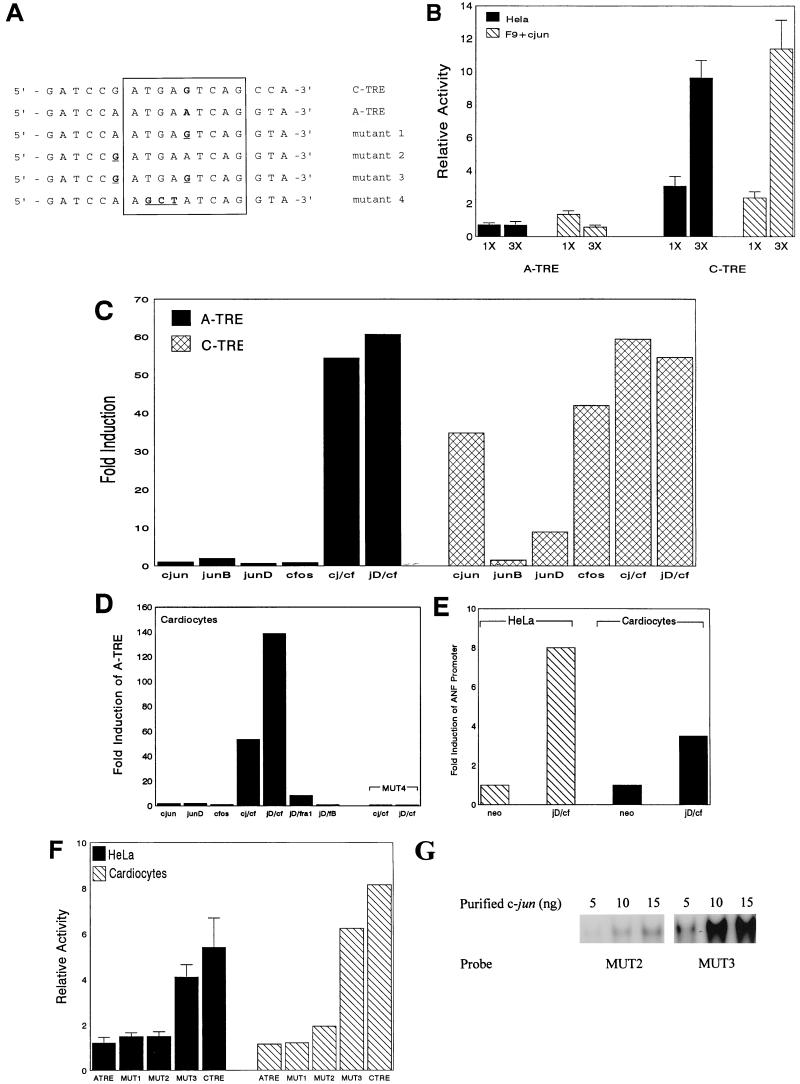
The ANF AP-1 site (A-TRE) is highly homologous to the consensus motif of well-characterized TREs (C-TRE) (Fig. 1A), differing at only the center position of the palindrome (G to A). The activities of the two elements were compared in transfection experiments in HeLa and F9 cells, in the absence (HeLa) or presence (F9) of cotransfected c-jun vector (Fig. 1B). HeLa cells contain abundant AP-1 activity (predominantly c-jun homodimers), while F9 cells are devoid of functional AP-1 (32). In both cases, the transcriptional activity of the C-TRE-containing promoter was substantially induced (Fig. 1B, right). In contrast, the A-TRE did not mediate a similar activation irrespective of its orientation (Fig. 1B and data not shown) indicating that the A-TRE was unresponsive to c-jun homodimers. The lack of response of the A-TRE may be due to its lower affinity for c-jun relative to the C-TRE. Since it is well documented that c-fos enhances the DNA binding affinity of c-jun to AP-1 sites (37), we tested whether c-fos could restore jun inducibility to the A-TRE. In F9 cells, the A-TRE showed no response to various jun members, but cotransfection with either c-fos–c-jun or c-fos–junD resulted in very strong activation of the A-TRE (Fig. 1C). Similar results were obtained for quiescent cardiomyocyte cultures except that the response to c-fos–junD was routinely stronger than that to other AP-1 heterodimers. This was particularly evident for reporter plasmids containing a single copy of the A-TRE (Fig. 1D). As expected, mutation of a half site of the A-TRE core motif (mutant 4 [MUT4]) completely abrogated transactivation by AP-1 heterodimers (Fig. 1D). The A-TRE also responded to c-fos–junD transactivation in the context of its native promoter both in cardiomyocytes and in HeLa cells (Fig. 1E). Given the distinct functional properties of the A-TRE, we examined the effects of point mutations on the activity of the A-TRE (Fig. 1F). In both HeLa cells and cardiomyocytes, conversion of the base pair at the center of the A-TRE motif to that of C-TRE (MUT1) did not alter basal activity. Similarly, conversion of an A to G just 5′ of the core motif (MUT2) produced no change compared to wild-type A-TRE. However, a double mutant (MUT3) of these two sites elevated A-TRE activity to that of the C-TRE in both cell types (Fig. 1E). Together, the data indicate that the A-TRE is activated exclusively by jun-fos heterodimers; the results also suggest that the primary DNA sequence may be an important determinant of TRE selectivity.
FIG. 1.
c-jun does not activate the A-TRE. (A) Sequence comparison of the A- and C-TREs and A-TRE mutants. The core TRE motifs are boxed, the center of the palindrome where the C- and A-TREs diverge is in bold, and mutations of the A-TRE are underlined. (B) Comparison of activity of the A- and C-TREs in HeLa cells and in F9 cells transfected with c-jun. F9 cells were transfected with 3 μg of TRE-human GH reporter and 5 μg of either pRSV-neo (control) or pRSV-c-jun using calcium phosphate precipitation. In HeLa cells, pRSV-neo was used to keep total DNA at 8 μg in both cell types. Results (mean ± standard deviations of six to eight independent determinations) are expressed as fold induction relative to the −135 bp ANF parent vector. (C) The A-TRE is inducible only by heterodimers in F9 cells. Cotransfections of F9 cells with 3× TRE reporter plasmids (3 μg) and various AP-1 vectors (5 μg in total) were performed, and results are expressed as fold induction relative to activity of the respective 3× A-TRE reporter cotransfected with pRSV-neo. Results (n = 2 from a typical experiment of more than six) are expressed as fold induction relative to 3× A-TRE activity in cells cotransfected with pRSV-neo. c-j, c-jun; cf, c-fos; jD, junD. (D) Heterodimers activate the A-TRE in primary cardiocyte cultures. Cardiocytes were transfected with a single-copy A-TRE (or mutant) reporter (3 μg) in the presence of expression vectors for jun-fos (2.5 μg of each), and media were assayed for GH after 48 h. Results are relative to cotransfection with pRSV-neo. The effect of a half-site mutation of the A-TRE (MUT4) is also shown. (E) junD–c-fos activates the ANF promoter. HeLa cells or cardiocytes were transfected with a reporter construct (3 μg) containing 700 bp of the rat ANF promoter in the absence (neo) or presence of junD and c-fos (1 μg of each). (F) Mutation of two base pairs confers basal activity to the A-TRE. HeLa cells and ventricular cardiocytes were transfected with 3 μg of 1× A-TRE (or mutant) expression vectors as shown, and the media were assayed for GH. The sequences of the mutants are shown in Fig. 1A. Results are shown as fold induction relative to the −135 bp ANF parent reporter plasmid. (G) Mutant A-TRE oligonucleotides were incubated with purified c-jun (5 to 15 ng) and subjected to gel shift analysis as described above.
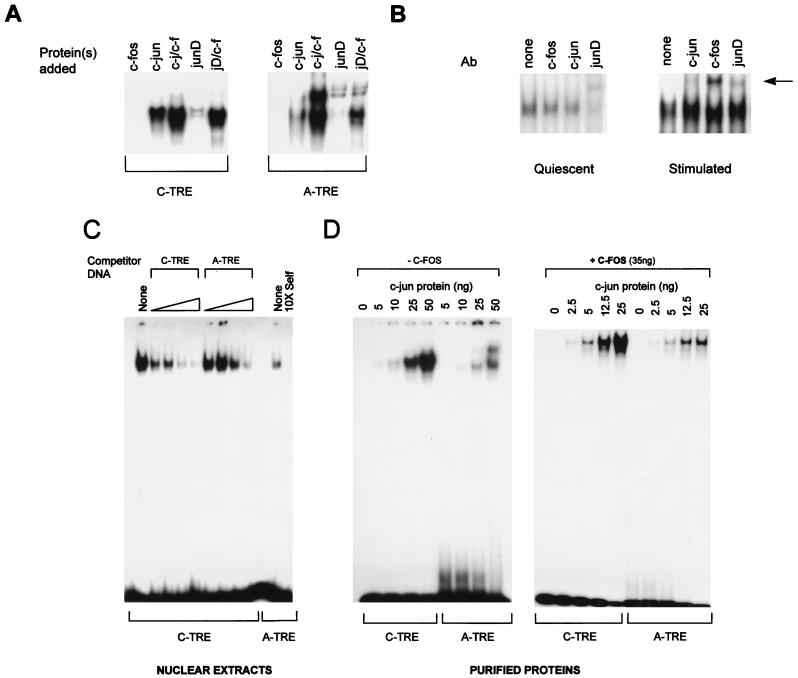
To determine whether the functional differences between the A-TRE and C-TRE were due to differential binding of AP-1 proteins, we analyzed binding of endogenous and purified jun-fos proteins to the A- and C-TRE probes in gel shift experiments (Fig. 2A). As expected, c-fos did not bind either probe alone. Both probes bound c-jun, and the level of binding was increased in the presence of c-fos. junD alone had very low affinity for either probe, but addition of c-fos greatly enhanced its affinity for DNA. The weaker binding of junD than of c-jun to each TRE correlated with other observations suggesting weak affinity of junD homodimers for DNA (41, 52). The ability of the A-TRE to interact with endogenous AP-1 proteins was confirmed in assays using nuclear extracts prepared from quiescent and serum- or TPA-treated cardiomyocytes. As shown in Fig. 2B, in quiescent cardiomyocytes, junD appeared to be the major AP-1 protein bound over the A-TRE; moreover, serum or TPA treatment resulted in the recruitment of c-fos to the A-TRE–AP-1 complex (Fig. 2B, right).
FIG. 2.
(A) In vitro binding of junD to the A-TRE is greatly enhanced in the presence of c-fos protein. Gel shift experiments were used to compare the binding of purified c-fos–c-jun (50 ng of total protein) and in vitro-translated junD (1 μl) to the C- and A-TRE probes. Only the specific AP-1–TRE complexes are shown. (B) Presence of junD in cardiocyte AP-1–A-TRE complexes and recruitment of c-fos to the complex following growth stimulation. Nuclear extracts prepared from quiescent (96 h in serum-free medium) or TPA-treated (100 ng/ml, 45 min) ventricular cardiocytes (10 or 4 μg of protein, respectively) were used in gel shift analyses, in the absence or presence of 1 μl of antibody (Ab) to either c-jun, junD, or c-fos. The supershifted complexes are arrowed. Results are similar when cardiocytes are stimulated with c-fos-inducing agents like serum (not shown). (C) The C-TRE has a higher affinity for AP-1 than the A-TRE. Increasing quantities of unlabeled oligonucleotides were used to compete the binding of HeLa cell nuclear extracts (4 μg) to a C-TRE probe in gel shift analysis. Competitors were at 10-, 25-, 50-, and 100-fold molar excess unless otherwise indicated. Binding on the A-TRE is shown for comparison. (D) The A-TRE has a lower affinity for purified AP-1 homo- or heterodimers. C- and A-TRE probes were analyzed by gel shift assay after incubation with increasing amounts of purified c-jun protein, in the absence (left) or presence (right) of a fixed amount of c-fos (35 ng). The probes were of similar specific activity. The top band seen over the A-TRE is also present in the reticulocyte lysates but not in nuclear extracts from the various cells tested.
Since the two probes exhibited similar binding profiles qualitatively, a more detailed analysis of their relative affinities was carried out by using competition and direct binding assays with HeLa cell nuclear extracts or purified c-jun and c-fos proteins. As shown in Fig. 2C, the A-TRE probe bound significantly less protein in HeLa extracts and was clearly a less efficient competitor of AP-1 binding than the C-TRE. These results were further confirmed by comparing in vitro binding profiles of each TRE to purified c-jun in presence or absence of c-fos (Fig. 2D). The quantitative differences in DNA binding observed in vitro may well contribute to the functional differences between the two TREs in vivo. Indeed, the mutation (MUT3 [Fig. 1]) that increases activity of the A-TRE in HeLa cells and in cardiomyocytes to that of the C-TRE and restores c-jun inducibility also increases binding affinity to c-jun (Fig. 2E). Thus, it appears that the A-TRE is a lower-affinity AP-1 site which might in turn confer an absolute requirement for c-fos since fos-jun heterodimers have greater DNA binding affinity than jun homodimers.
c-fos activation domains are essential for heterodimer-mediated transactivation of the A-TRE.
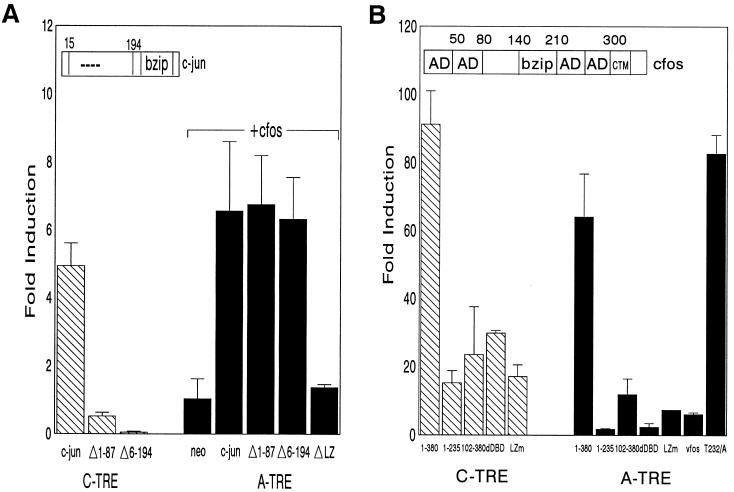
As stated above, c-fos might be required primarily to allow DNA binding of c-jun–c-fos to the lower-affinity A-TRE. To determine whether jun activation domains were functional at this site, we tested the ability of N-terminal c-jun deletions (partially or completely removing activation domains) to activate the A-TRE in the presence of c-fos. For a classical AP-1 site which is inducible by c-jun homodimers (23), deletion of amino acids 1 to 87 or 6 to 194 of c-jun abrogates wild-type activation of TREs (Fig. 3A). In contrast, the A-TRE was not inducible by c-jun alone in F9 cells, and c-jun–c-fos induction of the A-TRE was completely unaffected by removal of 1 to 87 or 6 to 194 amino acids of c-jun (Fig. 3A). This finding suggests that jun activation domains are dispensable for activation of the A-TRE by jun-fos heterodimers. However, as expected, a leucine zipper deletion mutant that has lost the ability to heterodimerize with c-fos was no longer able to mediate transactivation.
FIG. 3.
The C terminus of c-fos is required for activation of the A-TRE. (A) c-jun activation domains are dispensable for heterodimer induction of the A-TRE. F9 cells were cotransfected with A- and C-TRE (3×) reporter plasmids (3 μg) and expression vectors encoding wild-type c-jun (2.5 μg) alone (5 μg in total, using pRSV-neo), or N-terminally deleted c-jun (2.5 μg), in combination (only for A-TRE) with c-fos vector (2.5 μg). The jun mutants correspond to deletions of amino acids 1 to 87 (MUT1) or 6 to 194 (MUT2), or a deletion in the leucine zipper (LZ) of c-jun (MUT3), respectively. Results are shown as fold induction relative to cells cotransfected with pRSV-neo. (B) Effect of c-fos mutants on heterodimer induction of the TREs. F9 cells were cotransfected with 3× A- or C-TRE plasmid (3 μg) and expression vectors encoding c-jun and full-length c-fos (1-380) or c-fos mutant (5 μg in total). The c-fos mutants correspond to a C-terminal deletion (1-235), an N-terminal deletion (102-380), a deletion in the DBD (dDBD), a mutation in the leucine zipper (LZm), or v-fos, the oncogenic counterpart of c-fos (FBJ-v-fos). Also shown is the result obtained in assays using a mutation of Thr 232 to Ala in c-fos, a site previously shown to be a target for a novel c-fos kinase (17). In the c-fos schematic above the data, AD is used to indicate previously identified activation domains. Fold induction is relative to activity of TREs transfected with pRSV-neo, and the data represent the means of two independent experiments done in duplicate.
Next, the contribution of c-fos domains to A-TRE activation was tested in transfection assays using a variety of mutants, in both HeLa and F9 cells (Fig. 3B). As expected, a deletion in the DBD of c-fos or mutation in its leucine zipper resulted in complete loss of inducibility. The activation domains of c-fos are not as well characterized as those in c-jun, but autonomous transactivation domains (HOB1 and -2) within the N- and C-terminal regions have been described (26, 49, 58). Deletion of the first 102 amino acids of c-fos (construct 102-380) reduced activation of the C-TRE by 50% and that of the A-TRE by 5-fold, indicating that an N-terminal activation domain (probably HOB1) is functional on some AP-1 sites. Remarkably, deletion of c-fos carboxy-terminal 145 amino acids (construct 1-235), which among other sites removes the HOB2 activation domain (49), completely abrogated activation of the A-TRE but not the C-TRE. Mutation of Thr 232, which was previously shown to be important for c-fos activation in a heterologous context and was suggested as a target for c-fos kinase (17), had no effect on transactivation. The involvement of an intact C terminus was further emphasized by the inability of v-fos to activate the A-TRE (Fig. 3B). The major difference between c-fos and v-fos resides in the last 50 amino acids, which is a transrepression domain that inhibits transcription including that of c-fos itself (39, 57).
c-fos phosphorylation residues in the C terminus are essential for transactivation.
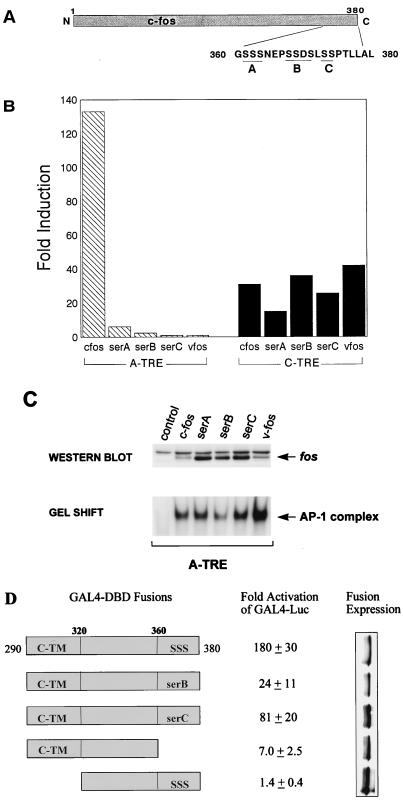
It is known that a cluster of phosphorylatable serine residues in the last 20 amino acids of c-fos are important for transrepression (39), but given the effect of C-terminal deletion on c-fos transactivation at this site, we examined the effect of serine-to-alanine mutations at these sites on A-TRE activation. Figure 4A shows a schematic of the c-fos protein with emphasis on the C-terminal serine residues. The effects of alanine mutations at these sites were tested by transfections in HeLa (not shown) and F9 (Fig. 4B) cells; strikingly, mutation of either the serA, -B, or -C site totally abrogated induction of the A-TRE with little effect on the C-TRE as previously reported. That these mutants were expressed in cells and increased AP-1 binding similarly to wild-type c-fos was confirmed by Western blotting and gel shift analyses of nuclear extracts (Fig. 4C). This finding suggested that the observed effects of alanine mutations resulted in changes at a level distinct from DNA binding in vitro. The transactivating properties of the C terminus of c-fos were further examined in a heterologous context; the wild-type c-fos or c-fos mutated at the last 90 amino acids was fused to the DBD of the yeast transcription factor GAL4 (GAL4 1-147), and the transactivation of a reporter containing tandem GAL4-binding sites was examined with this wild-type C-terminal construct and various mutants. As shown in Fig. 4D, the last 90 amino acids of c-fos contain considerable activation potential; this finding is consistent with that of Funk et al. (20), who recently identified an activation domain (C-TM) within this region of fos. All recombinant proteins were adequately expressed, as evidenced by Western blot analysis (Fig. 4D, right). Deletion of the C-TM identified by Funk et al. (20) abrogated transactivation completely; interestingly, deletion of the last 20 amino acids, which harbor the serine clusters, reduced transactivation 20-fold, indicating that there may be cooperation between these two activation domains. Mutation of either the serB or serC residue also significantly reduced transactivation. Thus, both in the native c-fos protein and in a heterologous context, these serine residues appear to play a major role in transactivation by c-fos.
FIG. 4.
The presence of C-terminal phosphorylation sites in c-fos is critical for activation of the A-TRE. (A) Schematic of the c-fos protein showing positions of the C-terminal serine residues. (B) F9 cells were cotransfected with an A-TRE (3×) or C-TRE (1×) plasmid (3 μg) and expression vectors encoding c-jun in the presence of wild-type or mutant c-fos or v-fos (5 μg in total). The c-fos mutants replace serine with alanine residues in either the A, B, or C phosphorylation site of the c-fos C terminus (39). Data are duplicates from a representative experiment (out of four) and are represented as fold induction over activity obtained using the pRSV-neo control plasmid. (C) c-fos serine mutants are expressed in cells and cause a similar increase in AP-1 binding compared to wild-type c-fos. 293T cells were plated in 100-mm-diameter dishes and transfected with 30 μg of expression vector encoding c-fos or mutant; nuclear extracts (100 μg) prepared from these cells were Western blotted to detect protein levels or used in gel shift experiments (15 μg) to examine AP-1 binding activity. A polyclonal fos antibody was used to detect c-fos and the various mutants. (D) GAL4 fusion experiments implicate a role for the last 20 amino acids of c-fos in transactivation. HeLa cells were cotransfected with a GAL4 reporter plasmid (2 μg) and either a vector encoding GAL4 1-147 (DBD) or one of various GAL4-fos fusions (200 ng) shown. After 36 h in low serum (0.5%), cells were harvested and extracts were assayed for luciferase activity. Results (n = 4, one typical experiment) are expressed as activity relative to the control vector GAL4 1-147.
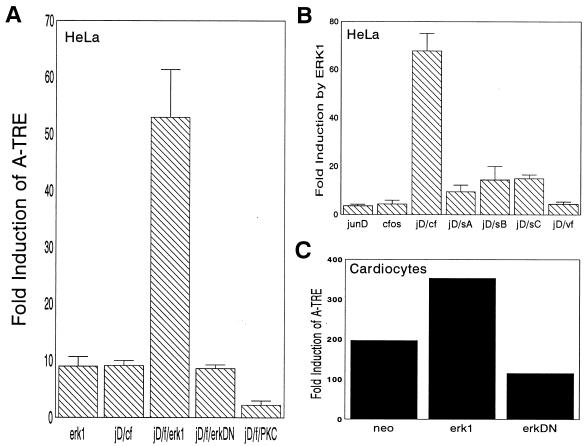
Candidate kinases that have been suggested for these sites include MAPK (2, 14, 51). Given that MAPK activation correlates with ANF induction in hypertrophy (10, 21), we examined whether the p44/ERK-1 kinase could positively affect the ability of c-fos–junD to stimulate the A-TRE. Indeed, we found that ERK-1 but not a kinase-deficient mutant potentiated the activation of the A-TRE by c-fos–junD in HeLa cells (Fig. 5A). The contribution of ERK-1 to basal ANF levels was substantiated by the observation that the ANF promoter was repressed by the ERK-1 kinase-deficient mutant (not shown). None of the other constitutively active kinases that were tested, including protein kinase C (PKC), PKA, and Ca2+/calmodulin-dependent kinase, produced any transactivation of the A-TRE-containing promoters either alone or in combination with junD–c-fos (Fig. 5A and data not shown). Strikingly, the positive effect of ERK-1 was lost when the carboxy-terminal serine residues were mutated (Fig. 5B), and v-fos was unresponsive to ERK-1. That all three mutants were unresponsive to ERK-1 may not be surprising given the evidence of cooperative phosphorylation at these sites (14). Additionally, we found that ERK-1 potentiated the induction of the A-TRE in cardiocytes (Fig. 1C). Collectively, these data support a functional role of MAPK in c-fos-mediated transactivation of specific AP-1 sites.
FIG. 5.
MAPK potentiates c-fos–junD activation of the A-TRE. (A) HeLa cells maintained in 0.5% fetal calf serum were transfected with an A-TRE plasmid (3 μg) and expression vectors encoding p44/ERK-1 (2 μg) or c-fos (cf)–junD (jD) (2 μg in total), or both together. The ERKDN mutant is a dominant negative kinase-deficient ERK-1. The effect of cotransfection of a constitutively activated PKC (β isozyme) is also shown. (B) The ability of MAPK to potentiate junD-fos (f) induction of the A-TRE was also determined on c-fos C-terminal mutants. HeLa cells were transfected with a 3× A-TRE plasmid (3 μg), ERK-1 plasmid (2 μg), and junD/c-fos (or mutant) plasmids (2 μg). sA, sB, and sC, serA, serB, and serC. Except for panel C, where the results from a representative experiment carried out in duplicate are shown, the data presented are the means ± standard deviations of four to six independent determinations. (C) The effect of MAPK on the A-TRE was also tested in ventricular cardiocytes by cotransfection of 1× A-TRE (3 μg) with junD–c-fos (2 μg), in the absence or presence of MAPK vectors (2 μg).
DISCUSSION
c-fos is a multifunctional protein that plays important roles in many cellular processes ranging from differentiation to proliferation, apoptosis, and tumor progression. The molecular mechanisms underlying the effects of c-fos remain largely undefined, although it is widely assumed that they involve transcriptional regulation of target genes. One way c-fos can modulate transcription is via heterodimerization with jun family members and binding TRE/AP-1 sites. However, given that jun homodimers can efficiently bind and transactivate most TREs, the contribution of c-fos to transactivation by a jun-fos heterodimer has been essentially ignored. The work presented here suggests the existence of a subtype of AP-1 sites that is specifically activated by c-fos-containing heterodimers, mainly through c-fos activation domains. The lower affinity of these sites for jun homodimers may provide a first level of discrimination for the c-fos–jun heterodimer which has increased DNA binding affinity. Thus, the sequence of the TRE, including residues outside the core motif, appears to be important in dictating dimer specificity as previously reported for the Myc-Max heterodimer (18).
The strict dependence on c-fos activation domains supports previous structural studies, suggesting that DNA topology around AP-1 sites is differentially affected by jun homodimers and fos-jun heterodimers (28). c-fos transactivation of the specialized AP-1 site mapped to the carboxy-terminal region that was previously shown to be required for c-fos transrepression of its own transcription and that of EGR-1, another immediate-early gene (22, 39). This result was somewhat unexpected since previous studies showed no difference between c-fos and v-fos, which lacks this region, in transactivating classical AP-1 sites (39). Moreover, in assays using chimeric proteins, several transactivation domains have been mapped within the c-fos protein mostly N-terminal to this so-called transrepression domain (17, 26, 49). It is possible, of course, that different transactivation domains are used by c-fos to modulate transcription in an AP-1-independent manner (48). It is also possible that other domains of c-fos contribute to activation in cooperation with the C-terminal region. Sutherland et al. (49) have shown that at least two activation domains of c-fos are required for transactivation, and recent work by Funk et al. (20) also suggests cooperativity between a C- and an N-terminal transactivation domain. The data from the GAL4 fusion studies suggest that the C-TM domain and the serine residues functionally cooperate.
The results of the present study point to a role of MAPK-mediated phosphorylation in c-fos transactivation of AP-1 sites. This finding is consistent with other reports that have documented in vivo association between MAPK and the AP-1 complex (8) and a requirement for MAPK for AP-1 activation in response to some stimuli (19), and it suggests that MAPK targets c-fos in the AP-1 complex. Moreover, previous studies (14) showed that c-fos was phosphorylated in vivo and in vitro by MAPK at serine 374, whereas serine 362 was a target for 90-kDa ribosomal S6 kinase, which can be activated by MAPK (9). Interestingly, it was found that although either kinase functioned by itself, there was marked cooperativity between them which might explain the inhibitory effect of either Ser 374 or Ser 362 mutation on c-fos transactivation. Further studies have also shown that a net negative charge at the extreme C terminus of c-fos augments its transactivation and transformation properties (15). The mechanism by which MAPK alters directly and/or indirectly c-fos function is unclear. MAPK phosphorylation of Ser 374 and Ser 362 has been reported to enhance c-fos stability (15, 40); nuclear extracts prepared from cells expressing wild-type or phosphorylation mutant c-fos proteins show similar AP-1 binding levels (Fig. 5C), suggesting that mechanisms other than those leading to changes in c-fos protein levels must also be implicated. It is possible, for example, that C-terminal phosphorylation favors productive interactions with other transcription factors either directly or through recruitment of coactivators. In this respect, it has been recently shown that c-fos contacts the TATA box-binding protein through a C-terminal domain just upstream of the MAPK phosphorylation sites (36) and interacts with the transcriptional coactivator CREB binding protein via the C terminus of c-fos (6). However, it is not known whether those or other interactions are affected by the MAPK pathway. Notwithstanding these mechanistic uncertainties, the requirement of the carboxy-terminal domain of c-fos, which is divergent in the fos-related protein Fra-1 and is absent in v-fos, is consistent with the inability of these proteins to substitute for c-fos in transactivation, although both form stable DNA-binding complexes over the AP-1 site (Fig. 4B and data not shown). In fact, in this context, v-fos acts as a dominant negative mutant of c-fos. This, in turn, raises the intriguing possibility that the C terminus of c-fos serves two functions essential for normal cells which would be lost in v-fos; i.e., it negatively controls proliferation genes and positively modulates differentiation genes.
Finally, the characterization of the specialized AP-1 site within the ANF promoter and its transactivation by MAPK might be biologically relevant to understanding regulation of cardiac genes in response to stimuli that alter cardiac function. Indeed, stimulation of cardiomyocytes by vasoactive hormones, ischemia, mechanical stretch, etc., is associated with activation both of the MAPK pathway and of the AP-1 complex, and with profound changes in the expression of cardiac genes including the ANF gene (21, 42, 43, 54, 59, 60). In this respect, the finding that mutation of the ANF AP-1 site totally abrogates the in vivo induction of ANF promoter activity in response to pressure overload (53) is especially noteworthy and lends further support for functional significance of the data presented.
ACKNOWLEDGMENTS
We are grateful to M. Chamberland and L. Robitaille for technical assistance and to D. Durocher of the Nemer lab for discussions and suggestions. We thank M. Karin, T. Curran, and T. Antakly for the gift of invaluable reagents.
This work was supported by grants from the Cancer Research Society Inc. and the Medical Research Council of Canada. M.N. is a Scientist of the Medical Research Council of Canada.
REFERENCES
- 1.Abate C, Luk D, Curran T. Transcriptional regulation by Fos and Jun in vitro: interaction among multiple activator and regulatory domains. Mol Cell Biol. 1991;11:3624–3632. doi: 10.1128/mcb.11.7.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abate C, Marshak D R, Curran T. Fos is phosphorylated by p34cdc2, cAMP-dependent protein kinase and protein kinase C at multiple sites clustered within regulatory regions. Oncogene. 1991;6:2179–2185. [PubMed] [Google Scholar]
- 3.Angel P, Karin M. The role of jun, fos and the AP-1 complex in cell proliferation and transformation. Biochim Biophys Acta. 1991;107:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 4.Angel P, Smeal T, Meek J, Karin M. Jun and v-jun contain multiple regions that participate in transcriptional activation in an interdependent manner. New Biol. 1989;1:35–43. [PubMed] [Google Scholar]
- 5.Argentin S, Sun Y-L, Lihrmann I, Schmidt T J, Drouin J, Nemer M. Distal cis-acting promoter sequences mediate glucocorticoid stimulation of cardiac atrial natriuretic factor gene transcription. J Biol Chem. 1991;266:23315–23322. [PubMed] [Google Scholar]
- 6.Bannister A J, Kouzarides T. CBP-induced stimulation of c-Fos activity is abrogated by E1A. EMBO J. 1995;14:4758–4762. doi: 10.1002/j.1460-2075.1995.tb00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartel D P, Sheng M, Lau L F, Greenberg M E. Growth factors and membrane depolarization activate distinct programs of early response gene expression: dissociation of fos and jun induction. Genes Dev. 1989;3:304–313. doi: 10.1101/gad.3.3.304. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein L R, Ferris D K, Colburn N H, Sobel M E. A family of mitogen-activated protein kinase-related proteins interacts in vivo with activator protein-1 transcription factor. J Biol Chem. 1994;269:9401–9404. [PubMed] [Google Scholar]
- 9.Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. . (Review.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogoyevitch M A, Andersson M B, Gillespie-Brown J, Clerk A, Glennon P E, Fuller S J, Sugden P H. Adrenergic receptor stimulation of the mitogen-activated protein kinase cascade and cardiac hypertrophy. Biochem J. 1996;314:115–121. doi: 10.1042/bj3140115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohmann D, Tjian R. Biochemical analysis of transcriptional activation by Jun: differential activity of c- and v-Jun. Cell. 1989;59:709–717. doi: 10.1016/0092-8674(89)90017-2. [DOI] [PubMed] [Google Scholar]
- 12.Brusselbach S, Mohle-Steinlein U, Wang Z Q, Schreiber M, Lucibello F C, Muller R, Wagner E F. Cell proliferation and cell cycle progression are not impaired in fibroblasts and ES cells lacking c-Fos. Oncogene. 1995;10:79–86. [PubMed] [Google Scholar]
- 13.Carrasco D, Bravo R. Tissue-specific expression of the fos-related transcription factor fra-2 during mouse development. Oncogene. 1995;10:1069–1079. [PubMed] [Google Scholar]
- 14.Chen R H, Abate C, Blenis J. Phosphorylation of the c-Fos transrepression domain by mitogen-activated protein kinase and 90-kDa ribosomal S6 kinase. Proc Natl Acad Sci USA. 1993;90:10952–10956. doi: 10.1073/pnas.90.23.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen R H, Juo P C, Curran T, Blenis J. Phosphorylation of c-Fos at the C-terminus enhances its transforming activity. Oncogene. 1996;12:1493–1502. [PubMed] [Google Scholar]
- 16.Deng T, Karin M. JunB differs from c-Jun in its DNA-binding and dimerization domains, and represses c-Jun by formation of inactive heterodimers. Genes Dev. 1993;7:479–490. doi: 10.1101/gad.7.3.479. [DOI] [PubMed] [Google Scholar]
- 17.Deng T, Karin M. c-Fos transcriptional activity stimulated by H-Ras-activated protein kinase distinct from JNK and ERK. Nature. 1994;371:171–175. doi: 10.1038/371171a0. [DOI] [PubMed] [Google Scholar]
- 18.Fisher F, Crouch D H, Jayaraman P S, Clark W, Gillespie D A, Goding C R. Transcription activation by Myc and Max: flanking sequences target activation to a subset of CACGTG motifs in vivo. EMBO J. 1993;12:5075–5082. doi: 10.1002/j.1460-2075.1993.tb06201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost J A, Geppert T D, Cobb M H, Feramisco J R. A requirement for extracellular signal-regulated kinase (ERK) function in the activation of AP-1 by Ha-Ras, phorbol 12-myristate 13-acetate, and serum. Proc Natl Acad Sci USA. 1994;91:3844–3848. doi: 10.1073/pnas.91.9.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funk M, Poensgen B, Graulich W, Jerome V, Muller R. A novel, transformation-relevant activation domain in Fos proteins. Mol Cell Biol. 1997;17:537–544. doi: 10.1128/mcb.17.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillespie-Brown J, Fuller S J, Bogoyevitch M A, Cowley S, Sugden P H. The mitogen-activated protein kinase kinase MEK1 stimulates a pattern of gene expression typical of the hypertrophic phenotype in rat ventricular cardiomyocytes. J Biol Chem. 1995;270:28092–28096. doi: 10.1074/jbc.270.47.28092. [DOI] [PubMed] [Google Scholar]
- 22.Gius D, Cao X, Rauscher III F J, Cohen D R, Curran T, Sukhatme V P. Transcriptional activation and repression by Fos are independent functions: the C terminus represses immediate-early gene expression via CArG elements. Mol Cell Biol. 1990;10:4243–4255. doi: 10.1128/mcb.10.8.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirai S, Bourachot B, Yaniv M. Both Jun and Fos contribute to transcription activation by the heterodimer. Oncogene. 1990;5:39–46. [PubMed] [Google Scholar]
- 24.Hu E, Mueller E, Oliviero S, Papaioannou V E, Johnson R, Spiegelman B M. Targeted disruption of the c-fos gene demonstrates c-fos-dependent and -independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO J. 1994;13:3094–3103. doi: 10.1002/j.1460-2075.1994.tb06608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izumo S, Nadal-Ginard B, Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci USA. 1988;85:339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jooss K U, Funk M, Muller R. An autonomous N-terminal transactivation domain in Fos protein plays a crucial role in transformation. EMBO J. 1994;13:1467–1475. doi: 10.1002/j.1460-2075.1994.tb06401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. . (Review.) [DOI] [PubMed] [Google Scholar]
- 28.Kerppola T K, Curran T. Fos-jun heterodimers and jun homodimers bend DNA in opposite orientations: implications for transcription factor cooperativity. Cell. 1991;66:317–326. doi: 10.1016/0092-8674(91)90621-5. [DOI] [PubMed] [Google Scholar]
- 29.Kerppola T K, Curran T. Selective DNA bending by a variety of bZIP proteins. Mol Cell Biol. 1993;13:5479–5489. doi: 10.1128/mcb.13.9.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komuro I, Kaida T, Shibazaki Y, Kurabayashi M, Katoh Y, Hoh E, Takaku F, Yazaki Y. Stretching cardiac myocytes stimulates protooncogene expression. J Biol Chem. 1990;265:3595–3598. [PubMed] [Google Scholar]
- 31.Kovary K, Bravo R. Expression of different Jun and Fos proteins during the G0-to-G1 transition in mouse fibroblasts: in vitro and in vivo associations. Mol Cell Biol. 1991;11:2451–2459. doi: 10.1128/mcb.11.5.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kryszke M H, Piette J, Yaniv M. Induction of a factor that binds to the polyoma virus A enhancer on differentiation of embryonal carcinoma cells. Nature. 1987;328:254–256. doi: 10.1038/328254a0. [DOI] [PubMed] [Google Scholar]
- 33.Lucibello F C, Slater E P, Jooss K U, Beato M, Müller R. Mutual transrepression of fos and the glucocorticoid receptor: involvement of a functional domain in fos which is absent in fosB. EMBO J. 1990;9:2827–2834. doi: 10.1002/j.1460-2075.1990.tb07471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McBride K, Robitaille L, Tremblay S, Argentin S, Nemer M. Fos/Jun repression of cardiac-specific transcription in quiescent and growth-stimulated myocytes is targeted at a tissue-specific cis element. Mol Cell Biol. 1993;13:600–612. doi: 10.1128/mcb.13.1.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meloche S, Pages G, Pouyssegur J. Functional expression and growth factor activation of an epitope-tagged p44 mitogen-activated protein kinase, p44mapk. Mol Biol Cell. 1992;3:63–71. doi: 10.1091/mbc.3.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metz R, Bannister A J, Sutherland J A, Hagemeier C, O’Rourke E C, Cook A, Bravo R, Kouzarides T. c-Fos-induced activation of a TATA-box-containing promoter involves direct contact with TATA-box-binding protein. Mol Cell Biol. 1994;14:6021–6029. doi: 10.1128/mcb.14.9.6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakabeppu Y, Ryder K, Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988;55:907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- 38.Naranjo J R, Mellstrom B, Auwerx J, Mollinedo F, Sassone-Corsi P. Unusual c-fos induction upon chromaffin PC12 differentiation by sodium butyrate: loss of fos autoregulatory function. Nucleic Acids Res. 1990;18:3605–3610. doi: 10.1093/nar/18.12.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ofir R, Dwarki V J, Rashid D, Verma I M. Phosphorylation of the C terminus of fos protein is required for transcriptional transrepression of the c-fos promoter. Nature. 1990;348:80–82. doi: 10.1038/348080a0. [DOI] [PubMed] [Google Scholar]
- 40.Okazaki K, Sagata N. The Mos/MAP kinase pathway stabilizes c-Fos by phosphorylation and augments its transforming activity in NIH 3T3 cells. EMBO J. 1995;14:5048–5059. doi: 10.1002/j.1460-2075.1995.tb00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryseck R P, Bravo R. c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins. Oncogene. 1991;6:533–542. [PubMed] [Google Scholar]
- 42.Sadoshima J, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J. 1993;12:1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadoshima J, Qiu Z, Morgan J P, Izumo S. Angiotensin II and other hypertrophic stimuli mediated by G protein-coupled receptors activate tyrosine kinase, mitogen-activated protein kinase, and 90-kD S6 kinase in cardiac myocytes. The critical role of Ca(2+)-dependent signaling. Circ Res. 1995;76:1–15. doi: 10.1161/01.res.76.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Sassone-Corsi P, Sisson J C, Verma I M. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988;334:314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- 45.Schreiber M, Baumann B, Cotten M, Angel P, Wagner E F. Fos is an essential component of the mammalian UV response. EMBO J. 1995;14:5338–5349. doi: 10.1002/j.1460-2075.1995.tb00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schunkert H, Jahn L, Izumo S, Apstein C S, Lorell B H. Localization and regulation of c-fos and c-jun protooncogene induction by systolic wall stress in normal and hypertrophied rat hearts. Proc Natl Acad Sci USA. 1991;88:11480–11484. doi: 10.1073/pnas.88.24.11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smeal T, Angel P, Meek J, Karin M. Different requirements for formation of jun:jun and jun:fos complexes. Genes Dev. 1989;3:2091–2100. doi: 10.1101/gad.3.12b.2091. [DOI] [PubMed] [Google Scholar]
- 48.Stein B, Baldwin A S, Jr, Ballard D W, Greene W C, Angel P, Herrlich P. Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 1993;12:3879–3891. doi: 10.1002/j.1460-2075.1993.tb06066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sutherland J A, Cook A, Bannister A J, Kouzarides T. Conserved motifs in Fos and Jun define a new class of activation domain. Genes Dev. 1992;6:1810–1819. doi: 10.1101/gad.6.9.1810. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki T, Okuno H, Yoshida T, Endo T, Nishina H, Iba H. Difference in transcriptional regulatory function between c-Fos and Fra-2. Nucleic Acids Res. 1991;19:5537–5542. doi: 10.1093/nar/19.20.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor L K, Marshak D R, Landreth G E. Identification of a nerve growth factor- and epidermal growth factor-regulated protein kinase that phosphorylates the protooncogene product c-Fos. Proc Natl Acad Sci USA. 1993;90:368–372. doi: 10.1073/pnas.90.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vandel L, Pfarr C M, Huguier S, Loiseau L, Sergeant A, Castellazzi M. Increased transforming activity of JunB and JunD by introduction of an heterologous homodimerization domain. Oncogene. 1995;10:495–507. [PubMed] [Google Scholar]
- 53.von Harsdorf R, Edwards J G, Shen Y T, Kudej R K, Dietz R, Leinwand L A, Nadal Ginard B, Vatner S F. Identification of a cis-acting regulatory element conferring inducibility of the atrial natriuretic factor gene in acute pressure overload. J Clin Invest. 1997;100:1294–1304. doi: 10.1172/JCI119643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Webster K A, Discher D J, Bishopric N H. Induction and nuclear accumulation of fos and jun proto-oncogenes in hypoxic cardiac myocytes. J Biol Chem. 1993;268:16852–16858. [PubMed] [Google Scholar]
- 55.Wick M, Lucibello F C, Muller R. Inhibition of Fos- and Ras-induced transformation by mutant Fos proteins with structural alterations in functionally different domains. Oncogene. 1992;7:859–867. [PubMed] [Google Scholar]
- 56.Wilkinson D G, Bhatt S, Ryseck R P, Bravo R. Tissue-specific expression of c-jun and junB during organogenesis in the mouse. Development. 1989;106:465–471. doi: 10.1242/dev.106.3.465. [DOI] [PubMed] [Google Scholar]
- 57.Wilson T, Treisman R. Fos C-terminal mutations block down-regulation of c-fos transcription following serum stimulation. EMBO J. 1988;7:4193–4202. doi: 10.1002/j.1460-2075.1988.tb03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wisdom R, Verma I M. Transformation by Fos proteins requires a C-terminal transactivation domain. Mol Cell Biol. 1993;13:7429–7438. doi: 10.1128/mcb.13.12.7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Mizuno T, Takano H, Hiroi Y, Ueki K, Tobe K, et al. Mechanical stress activates protein kinase cascade of phosphorylation in neonatal rat cardiac myocytes. J Clin Invest. 1995;96:438–446. doi: 10.1172/JCI118054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao A, Takahashi T, Aoyagi T, Kinugawa K, Kohmoto O, Sugiura S, Serizawa T. Immediate-early gene induction and MAP kinase activation during recovery from metabolic inhibition in cultured cardiac myocytes. J Clin Invest. 1995;96:69–77. doi: 10.1172/JCI118081. [DOI] [PMC free article] [PubMed] [Google Scholar]