Abstract
Crkl is an adapter protein and phosphotyrosine-containing substrate implicated in transformation by the bcr-abl oncogene and in signaling by cytokines. When phosphorylated, Crkl binds through its Src homology 2 (SH2) domain to other tyrosine phosphoproteins such as paxillin and Cbl. Overexpression of Crkl in fibroblasts induces transformation. Here we examine the role of Crkl in hematopoietic cells and find that overexpression of Crkl confers a signal leading to increased adhesion to fibronectin. In both fibroblasts and hematopoietic cells, individual mutations or deletions of each SH2 and SH3 domain abrogated transformation and adhesion, respectively, indicating that interactions with other proteins such as Cbl and paxillin (SH2 domain) and Abl, Sos, and C3G (N-terminal SH3 domain) are essential for biological activity. In vivo and in vitro tryptic phosphopeptide mapping studies show that Crkl is phosphorylated on multiple tyrosine residues when overexpressed or when activated by Bcr-Abl. Mutation at tyrosine 207, a residue conserved in c-Crk, abrogates all in vivo tyrosine phosphorylation of Crkl. Despite this loss of phosphotyrosine, mutation at this site enhanced Crkl function as measured by complex formation with SH2 binding proteins, signal transduction to Jun Kinase, and fibroblast transformation. These observations implicate Crkl in cellular adhesion and demonstrate that Y207 functions as a negative regulatory site.
Adapters are small molecules composed primarily of protein-protein interaction domains that play a major role in signal transduction by bringing together multiple components of signaling cascades. Many consist entirely of Src homology 2 (SH2) and SH3 domains, which interact with phosphotyrosine residues and proline-rich regions, respectively (27), while others contain additional regions such as phosphotyrosine binding domains (47). One paradigm for adapter function comes from studies of growth factor receptors which become phosphorylated on tyrosine following stimulation. The SH2 or phosphotyrosine binding domain of the adapter binds to phosphotyrosine residues on the receptor, and the SH3 domains mediate interactions with downstream effectors (10). An essential concept in this paradigm is that regulation of the pathway occurs at the level of receptor phosphorylation, which serves as the SH2 or phosphotyrosine binding site for the adapter, rather than at the level of the adapter.
The normal regulation of these signaling cascades can be subverted in cancer cells. One example is the Bcr-Abl tyrosine kinase, which causes chronic myelogenous leukemia (CML) (18). Bcr-Abl is constitutively phosphorylated on many tyrosine residues, one of which binds the SH2 domain of the Grb2 adapter molecule (29). The SH3 domains of Grb2 interact with the guanine nucleotide exchange factor SOS, which activates Ras (7). Bcr-Abl mutants which disrupt the interaction with Grb2 show defects in signal transduction in certain model systems (29), suggesting that adapters play a crucial role in the leukemic phenotype. A search for Bcr-Abl substrates led to the isolation of another adapter named Crkl, which binds to Bcr-Abl and is among the most prominent tyrosine-phosphorylated proteins in CML cells (14, 23, 25, 44). Crkl belongs to the Crk family of adapters and contains a single SH2 domain and two SH3 domains. In addition to its role in CML, Crkl is implicated in signal transduction by integrins, B- and T-cell receptors, and cytokines such as erythropoietin, interleukin-3, stem cell factor, and thrombopoietin (2, 24, 30, 35, 36, 41). In these examples Crkl is part of a multiprotein complex which forms following receptor activation.
While these biochemical studies suggest a role in signal transduction, the precise function of Crkl in these pathways has not been defined. In the case of Bcr-Abl, deletion of the Crkl binding site results in decreased transformation, similar to mutation of the Grb2 binding site. When both deletions are combined in the same molecule, transformation is completely impaired, indicating that Crkl and Grb2 have distinct but complementary functions (40). A comparison of the proteins which bind to Crkl with those that bind to other adapters such as Grb2 and Shc also indicates important differences. For example, the SH2 domain of Crkl binds a set of tyrosine-phosphorylated proteins localized to focal adhesions, such as Cas and paxillin, that are not implicated in Grb2 signal transduction (33, 34). In addition, Crkl is tyrosine phosphorylated by Bcr-Abl in CML cells (14, 23, 25, 44). This raises the possibility that, in contrast to the case for Grb2, phosphorylation of Crkl is a mechanism of adapter regulation. Indeed, phosphorylation of a C-terminal tyrosine residue in the Crkl homolog c-Crk initiates an intramolecular interaction with its own SH2 domain, thereby creating a folded (and presumably inactive) molecule (32). Taken together, these observations demonstrate that Crkl and Grb2 are regulated differently and have nonoverlapping functions.
In an effort to further our understanding of Crkl function and regulation, we have examined the effects of Crkl overexpression in fibroblasts and hematopoietic cells and investigated the structural requirements for Crkl activity. Whereas overexpression of Crkl in fibroblasts causes transformation (40), the primary effect in murine hematopoietic cell lines is enhanced adhesion to fibronectin. We find that mutation of any single SH2 or SH3 domain leads to complete loss of activity in both fibroblasts and hematopoietic cells. The fact that the C-terminal SH3 domain is necessary for Crkl bioactivity distinguishes Crkl from c-Crk proteins, since the homologous domain in c-Crk has an inhibitory function (21, 26). Because constitutive phosphorylation of Crkl is associated with CML, we also examined the effects of phosphorylation on Crkl activity. In vivo and in vitro phosphopeptide mapping data demonstrate that Crkl is phosphorylated on multiple sites when overexpressed or in cells expressing Bcr-Abl. Mutation of tyrosine 207, the primary site for phosphorylation by Bcr-Abl (9), leads to loss of all in vivo Crkl tyrosine phosphorylation yet potentiates binding of Crkl to phosphotyrosine-containing SH2 binding proteins such as paxillin. In addition, loss of Crkl tyrosine phosphorylation appears to enhance signal transduction through Jun kinase (JNK). The same mutation also increases the transforming activity of Crkl in fibroblasts, indicating that Crkl is negatively regulated by phosphorylation at Y207.
MATERIALS AND METHODS
Mutagenesis and plasmids.
PCR-based strategies were utilized to create each point mutation and deletion. The N-terminal primer 5′TCTGACCCGGGAGCCACCATGTCCTCCGCCAGGTTCGAC3′, corresponding to the N terminus of Crkl, and the C-terminal primer 5′ACCGCTCGAGATCGATCAATCACTCGTTTTCATCTGG3′, corresponding to the C terminus of Crkl, were used for the R39L, ΔSH2, W160L, and W275L mutations in addition to the following primers that were used as templates for the indicated mutations and deletions: R39L, R39L a (5′ GGAAGAATCGAGGACGAGGAA3′) and R39L s (5′TTCCTCGTCCTCGATTCTTCC3′); ΔSH2, ΔSH2 a (5′GCGGTTGGGCAGCGAGGCGGAGCGGTCCGA3′) and ΔSH2 s (5′TCGGACCGCTCCGCCTCGCTGCCCAACCGC3′); W160L, W160L a (5′GGCACTCCACAGCGTTCTTC3′) and W160L s (5′GAAGAACAGCTGTGGAGTGCC3′); and W275L, W275L a (5′TTCGCCTTCCAGCTGGCCATT3′) and W275L s (5′AATGGCCAGCTGGAAGGCGAA3′). The PCR products were cloned into either the TA vector (Invitrogen) or pZero-blunt (Invitrogen). Crkl sequences were then cloned into pSRαMSVtkNeoΔHindIIIΔClaI (22) cut with EcoRI and blunted with Klenow fragment or cloned into pSRαMSVtkNEONotI (39) cut with EcoRI and NotI. The Y207F mutant was created by digesting pGEX-KG Crkl (wild type) (25) with XbaI and XhoI, and the Crkl sequence was inserted into pBD3 (11) for single-stranded mutagenesis with the oligonucleotide 5′GCTCATGCTTTCGCTCAAC3′. The Amersham Sculptor mutagenesis system was utilized according to the manufacturer’s instructions. pSRαMSVtkNEO Crkl Y207F was created by digesting pBD3 containing Crkl Y207F with NcoI and XhoI followed by blunt-end ligation into pSRαMSVtkNEOΔHindIIIΔClaI cut with EcoRI and blunted. pSRαMSVtkNEO Crkl (wild type) was made as described previously (40). pGEX-KG Crkl Y207F was made by subcloning the HindIII fragment of pSRαMSVtkNEO Crkl Y207F into pGEX-KG Crkl (wild type) that was digested with HindIII. pSRαMSVtkNEO c-Abl and pSRαMSVtkNEO Bcr-Ablp210 were described previously (28, 38). The double mutants containing Y207F combined with mutations of the SH2 or N-terminal or C-terminal SH3 domain were prepared by swapping HindIII fragments which contain the Y207F mutations with R39L and W160L mutations and by PCR with the W275L primers to create the mutation of the C-terminal SH3 domain in Crkl Y207F.
Protein analysis.
Crkl protein expression was confirmed by lysing cells 48 h after infection in 2× sample buffer (100 mM Tris-Cl [pH 6.8], 4% sodium dodecyl sulfate [SDS], 0.2% bromophenol blue, 20% glycerol, 5% β-mercaptoethanol). Samples were then visualized by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (12.5 to 15% acrylamide) followed by Crkl immunoblotting with Crkl C-terminal antiserum (Santa Cruz Biotechnology). Phosphotyrosine immunoblots were performed with 4G10 antiserum (Upstate Biotechnology). Crkl immunoprecipitations were performed by lysing cells in lysis buffer (150 mM NaCl, 20 mM Tris pH 7.4, 10% glycerol, 1% Nonidet P-40 [NP-40], 1 mM phenylmethylsulfonyl fluoride, 30 μg of aprotinin per ml, 1 mM sodium orthovanadate). Protein concentrations were equalized with the Bio-Rad DC protein assay. Lysates were subjected to immunoprecipitation with 5 μg of Crkl antiserum. Precipitates were analyzed by SDS-PAGE (12.5 to 15% acrylamide) and immunoblotted with phosphotyrosine, Crkl, paxillin, or Abl antiserum and visualized by enhanced chemiluminescence (Amersham). The paxillin antibody was obtained from Transduction Laboratories (Lexington, Ky.). Abl antiserum was previously described (15). JNK assays of retrovirally infected Rat-1 fibroblasts were performed as described previously (40).
Transformation assays.
Retrovirus stocks were created by transient transfection of 293T cells by utilizing calcium phosphate as described previously (22). Rat-1 fibroblasts were infected with retrovirus stocks. Forty-eight hours after infection, cells were counted and plated into a soft agar matrix as previously described (37). Colony formation was detected and measured after 14 days in soft agar.
Phosphopeptide mapping.
In vivo two-dimensional phosphopeptide mapping was performed as follows. 293T cells were transfected with Crkl or Bcr-Ablp210 plasmids, and cells were phosphate labeled overnight with 1 mCi of orthophosphate per ml. Cells were lysed in lysis buffer and immunoprecipitated as described above. Immunoprecipitates were separated by SDS–15% PAGE and transferred to nitrocellulose. Filters were exposed to film, and bands were excised from the nitrocellulose. Trypsin digestion and two-dimensional phosphopeptide mapping were performed as described previously (6). In vitro two-dimensional peptide mapping was performed as follows. One microgram of purified Crkl was incubated with 5 μl of baculovirus-produced full-length Bcr-Ablp210 or Bcr-Abl kinase domain in buffer containing 50 mM Tris (pH 7.5), 1 mM dithiothreitol, and 10 mM MnCl2. The kinase reaction proceded at 30°C for 30 min. Proteins were separated by SDS–15% PAGE and transferred to nitrocellulose. Filters were exposed to film, and bands were excised. Proteins were digested with trypsin, and two-dimensional mapping was performed as described previously (6). Bcr-Ablp210 baculovirus was produced as described previously (4). The Abl kinase domain was purified by lysing Sf9 cells in NP-40 lysis buffer (1% NP-40, 150 mM NaCl, 20 mM Tris [pH 8.0], 10% glycerol) containing 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin per ml, and 1 mM Na3VO4. Lysates were bound to Ni-Sepharose for 6 h to overnight in a batch culture. Beads were spun and washed in 800 mM NaCl–20 mM Tris (pH 6.5)–1% NP-40–10 mM imidizole and loaded onto a column. Protein was eluted with 800 mM NaCl– 20 mM Tris (pH 5.3)–1% NP-40–500 mM imidizole. Fractions were analyzed by Coomassie blue staining of SDS-polyacrylamide gels. Peak fractions were pooled and dialyzed against Tris-buffered saline.
Adhesion assays.
FL5.12 cell lines stably expressing the control vector, wild-type Crkl, or the ΔSH2, W160L, Y207F, or W275L mutant were created by retroviral infection and selection in antibiotic. Six-centimeter-diameter gridded tissue culture dishes (Nunc) were coated with 10 μg of fibronectin or phosphate-buffered saline, and 105 cells were plated and allowed to adhere for 30 min at 37°C. Ten random squares were counted per plate, and the cell number per square millimeter was determined. RGD peptides (Gibco) were added at 0.5 mg/ml.
RESULTS
Crkl increases hematopoietic cell adhesion.
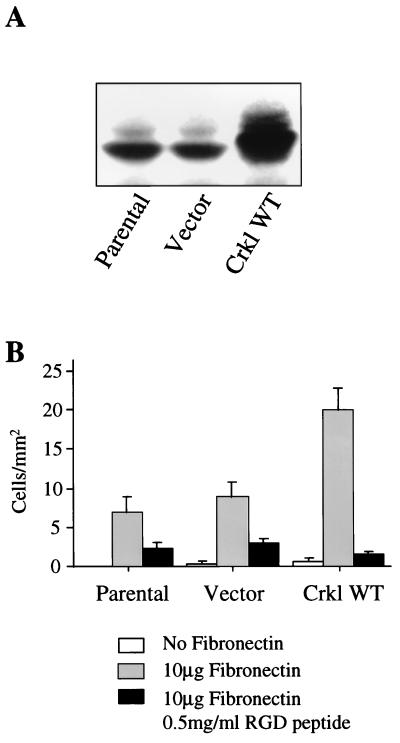
Previous work has shown that overexpression of Crkl transforms fibroblasts, but the effect of Crkl overexpression in hematopoietic cells is unknown. To address this issue, we introduced Crkl by retroviral infection into the cytokine-dependent murine pre-B-cell line FL5.12. As observed previously (40), overexpression of wild-type Crkl resulted in multiple bands which varied in mobility (Fig. 1A). Upon examining these Crkl-overexpressing cell cultures, we noted a pronounced increase in the attachment of these normally nonadherent cells to the bottom of the tissue culture dish. Overexpression of Bcr-Abl has been shown to increase adhesion of hematopoietic cells to extracellular matrix proteins such as fibronectin while also increasing the phosphorylation status of focal adhesion proteins such as paxillin and Cas, which can subsequently interact with Crkl (3, 8, 33, 34). We investigated whether Crkl mediates a similar effect by examining the consequences of Crkl overexpression in a quantitative assay measuring adhesion of hematopoietic cells to fibronectin-coated dishes. In three different murine hematopoietic cell lines (FL5.12, 32D, and BaF3), overexpressed Crkl caused increased adhesion to fibronectin. Results of a representative experiment with FL5.12 cells are shown in Fig. 1B. Adhesion induced by Crkl overexpression was blocked by RGD peptides, which indicates that adhesion is integrin mediated (Fig. 1B). Overexpression of Bcr-Abl also protects hematopoietic cells from apoptosis in response to cytokine withdrawal and allows growth factor-independent proliferation. We tested the ability of wild-type Crkl overexpression to confer similar properties in FL5.12, BaF3, and 32D cells, but we failed to detect any evidence of antiapoptotic or proliferative effects in the absence of interleukin-3 or granulocyte-macrophage colony-stimulating factor (data not shown). These results indicate that Crkl overexpression increases adhesion to hematopoietic cells similarly to Bcr-Abl but does not protect cells from apoptosis after cytokine withdrawal or allow cytokine-independent growth.
FIG. 1.
(A) Stable FL5.12 cell lines were created by retroviral infection followed by antibiotic selection, and expression was analyzed by immunoblotting with polyclonal Crkl antiserum. (B) FL5.12 parental, vector, and wild-type Crkl (Crkl WT) cells were plated onto 6-cm-diameter gridded tissue culture dishes with or without 10 μg of fibronectin and in the absence or presence of 0.5 mg of RGD peptide per ml. The number of cells per square millimeter was determined by light microscopy 30 min after plating. Error bars indicate standard deviations. Similar results were also obtained with the pre-B-cell line BaF3 and the myeloid cell line 32D.
Crkl requires the SH2 domain and both SH3 domains for fibroblast transformation and hematopoietic cell adhesion to fibronectin.
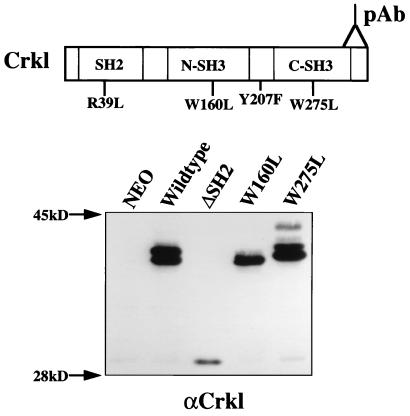
To define the regions of Crkl required for biological activity, we performed structure-function studies of Crkl mutants by using fibroblast transformation and hematopoietic cell adhesion as bioassays. Rat-1 fibroblasts were infected with retroviral constructs containing a deletion of the SH2 domain (amino acids 14 to 64) or point mutations in the SH3 domains of Crkl. A single point mutation in the FLVRES motif of the SH2 domain (Arg to Leu) decreased the stability of the protein (data not shown) and could not be utilized to address SH2 function. The N-terminal and C-terminal SH3 domain mutations were single amino acid changes of tryptophan to leucine at positions 160 (W160L) and 275 (W275L), respectively. Analogous mutations in SH3 domains from other proteins have been shown to disrupt interactions with proline-rich binding proteins (43). Expression of each mutant protein was confirmed by immunoblotting with polyclonal antiserum directed against the C terminus of Crkl (Fig. 2). Overexpression of wild-type Crkl resulted in multiple bands which varied in mobility due to differences in tyrosine phosphorylation (40). The Crkl mutant lacking the SH2 domain migrated in SDS-polyacrylamide gels with a molecular mass of about 30 kDa, consistent with the size of the deletion. Point mutations in either the N-terminal (W160L) or C-terminal (W275L) SH3 domain did not alter the size of Crkl except for subtle variations in the banding pattern.
FIG. 2.
Rat-1 fibroblasts were infected with retrovirus stocks of the indicated plasmids and were lysed in 2× sample buffer 48 h after infection. Lysates were separated by SDS–12.5% PAGE, and proteins were transferred to nitrocellulose. Immunoblotting was performed with Crkl antiserum. Similar expression data were obtained with FL5.12 cells (data not shown).
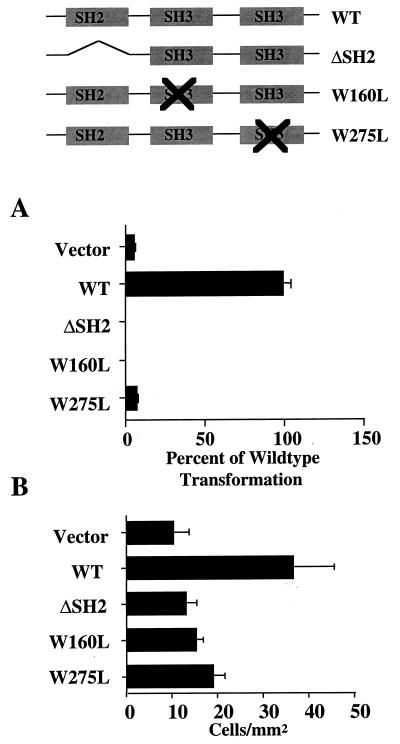
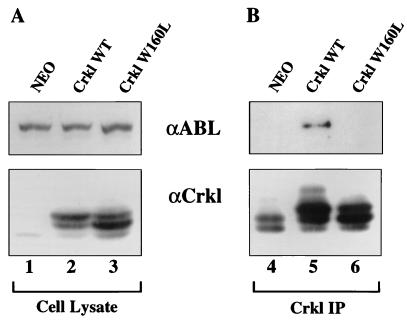
Two days after infection with retrovirus expressing wild-type or mutant Crkl protein, Rat-1 fibroblasts were plated into soft agar to measure anchorage-independent growth. As reported previously, overexpression of wild-type Crkl transformed fibroblasts (Fig. 3A). In contrast, constructs with a deletion of the SH2 domain (Crkl ΔSH2) or a point mutation of the N-terminal SH3 domain (Crkl W160L) did not. Analogous results showing loss of function were obtained with hematopoietic cells by using adhesion as an end point (Fig. 3B). To confirm that the W160L mutation specifically impaired the binding function of the N-terminal SH3 domain, we performed coimmunoprecipitation experiments with the SH3 binding protein c-Abl. As predicted, Crkl W160L failed to bind overexpressed c-Abl in coimmunoprecipitation assays whereas wild-type Crkl did bind c-Abl (Fig. 4). In addition, the inability of Crkl to interact with c-Abl would be predicted to affect the phosphorylation state of Crkl. This is apparent when comparing the Crkl banding patterns in lanes 2 and 3 of Fig. 4 (cell lysate) as well as in lanes 5 and 6 of Fig. 4 (Crkl immunoprecipitation). The results from these mutagenesis studies indicate that the SH2 and N-terminal SH3 domains of Crkl must bind to tyrosine-phosphorylated proteins as well as proline-rich proteins in order to transform cells. Likely candidates based on binding studies are Cbl, paxillin, Hef1, and Cas for the SH2 domain and C3G, Sos, and c-Abl for the SH3 domain (8, 31, 34, 36, 46). We examined the role of the C-terminal SH3 domain by creating a point mutation at amino acid 275 (W275L), analogous to the mutation described previously for the N-terminal SH3 domain (W160L). Similar to the case for the SH2 and N-terminal SH3 domain mutants, Crkl W275L was unable to transform fibroblasts (Fig. 3A) or induce adhesion in hematopoietic cells (Fig. 3B). These results indicate that the C-terminal SH3 domain of Crkl is required for biological activity, whereas the analogous domain in c-Crk II plays an inhibitory role (21, 26).
FIG. 3.
(A) Rat-1 fibroblasts were plated in duplicate into soft agar 48 h after infection. Colonies were counted after 2 to 3 weeks, and results were expressed as percentages of wild-type (WT) transformation, including standard deviations, from three experiments. (B) FL5.12 cell lines expressing the indicated genes were plated onto fibronectin-coated dishes, and cell numbers per square millimeter were determined 30 min after plating.
FIG. 4.
293T cells were cotransfected with wild-type c-Abl and one of the plasmids indicated at the top of the gel. Following lysis, Crkl was immunoprecipitated (IP) with Crkl antiserum (α Crkl) (Santa Cruz Biotechnology). Lysates were separated by SDS–10% PAGE, and proteins were transferred to nitrocellulose. Lanes 1 to 3 show total cell lysate, and lanes 4 to 6 show Crkl immunoprecipitation. (A) Top panel, Abl immunoblot (short exposure indicating comparable amounts of Abl protein); Bottom panel, Crkl immunoblot. (B) Top panel, Abl immunoblot (long exposure showing amount of Abl coimmunoprecipitating with Crkl). Bottom panel, Crkl immunoblot.
Crkl is phosphorylated on multiple tyrosine residues.
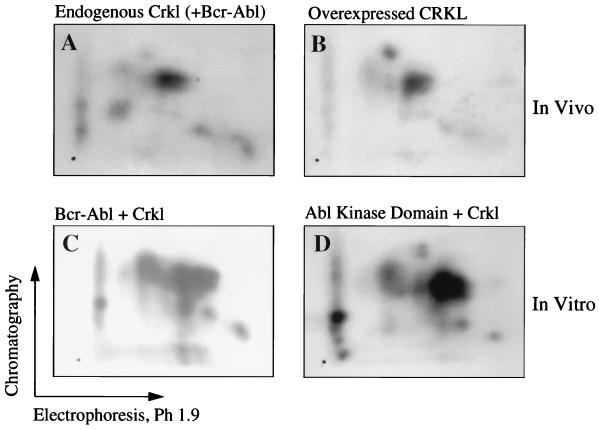
Activation of Crkl by Bcr-Abl or by cytokines is associated with tyrosine phosphorylation. Crkl contains 12 tyrosine residues, of which 5 correspond to the Y-X-X-P motif preferred by the Abl tyrosine kinase (42). The complex banding pattern observed when Crkl is overexpressed or phosphorylated by Bcr-Abl suggests that multiple phosphorylation sites are present. To test this possibility we transfected Bcr-Ablp210 into 293T cells and analyzed endogenous Crkl phosphorylation by phosphopeptide mapping. Trypsin digestion produced at least five distinct spots on the two-dimensional map, indicating that Crkl is phosphorylated at multiple sites (Fig. 5A). Similar results were obtained when Crkl was overexpressed in the absence of Bcr-Abl, although not all spots migrated identically (Fig. 5B). We also examined this issue in vitro by using recombinant Crkl purified from bacteria and recombinant Bcr-Ablp210 produced in baculovirus (4). The phosphopeptide maps generated by this strategy also showed multiple phosphorylation sites, many of which appeared to migrate similarly to spots observed with the in vivo map (Fig. 5C). The results confirm that Crkl is phosphorylated at multiple sites and that Bcr-Abl is directly responsible for these phosphorylations. In addition to a tyrosine kinase domain, Bcr-Abl also contains serine kinase activity contributed by Bcr (20). To examine whether Crkl was phosphorylated by the Abl or Bcr kinase domain, we repeated the in vitro kinase assay with a truncated recombinant protein containing just the Abl kinase domain purified from baculovirus. The resulting map was similar to that obtained with Bcr-Ablp210 (Fig. 5D). The results indicate that Crkl is tyrosine phosphorylated on multiple residues when overexpressed or when activated by Bcr-Abl.
FIG. 5.
(A) Endogenous Crkl was isolated from 293T cells expressing Bcr-Abl, digested with trypsin, and analyzed by two-dimensional phosphopeptide mapping. (B) Crkl overexpressed in 293T cells was digested with trypsin and analyzed similarly. (C) Purified Crkl was incubated with Bcr-Abl produced in baculovirus and then digested with trypsin and analyzed by two-dimensional phosphopeptide mapping. (D) Purified Crkl was incubated with the Abl kinase domain produced in baculovirus and then analyzed by two-dimensional phosphopeptide mapping.
Mutation of tyrosine 207 activates complex formation with paxillin.
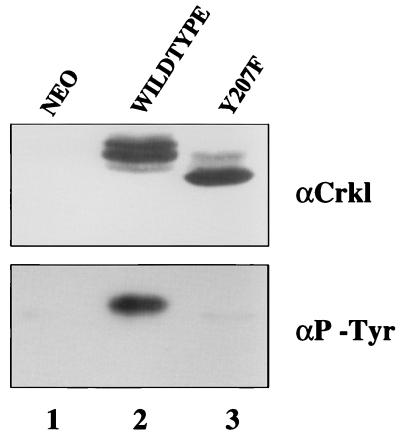
Previous research has implicated Crkl Y207 as a tyrosine phosphorylation site for Bcr-Abl (9). To determine the role of this tyrosine residue in Crkl function, we prepared a retrovirus construct (Crkl Y207F) in which tyrosine 207 was changed to phenylalanine. Immunoblot analysis of Rat-1 cells infected with retrovirus expressing wild-type Crkl or Crkl Y207F showed that the Y207F mutant migrated as a single band with faster mobility than wild-type Crkl (Fig. 6, top). Since the higher-mobility band shift pattern of wild-type Crkl is due to tyrosine phosphorylation (40, 44, 45), this result suggested that Crkl Y207F was not phosphorylated on tyrosine. This hypothesis was confirmed by immunoblot analysis of the same lysates with an antiphosphotyrosine antibody. Crkl Y207F failed to incorporate significant levels of phosphotyrosine compared to wild-type Crkl (Fig. 6, bottom). Therefore, tyrosine 207 is a site for Crkl phosphorylation in vivo and is required for all subsequent phosphorylation events, consistent with results reported previously (9).
FIG. 6.
Rat-1 fibroblasts were infected with retroviruses expressing the indicated proteins. Cells were lysed 48 h after infection, and lysates were immunoprecipitated with Crkl antiserum (α Crkl) (Santa Cruz Biotechnology). Proteins were separated by SDS–12.5% PAGE and transferred to nitrocellulose. Top panel, Crkl immunoblot with Crkl antiserum (Santa Cruz Biotechnology). Bottom panel, phosphotyrosine (P-Tyr) immunoblot with 4G10 antiserum (Upstate Biotechnology).
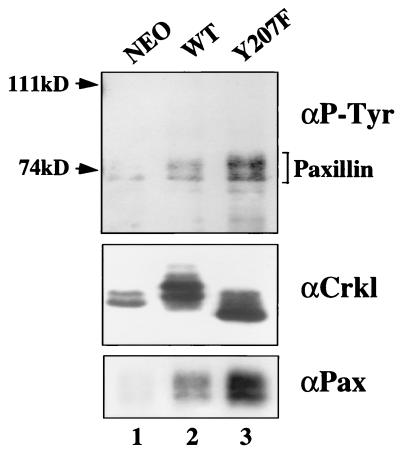
Since phosphorylation of Crkl is associated with growth stimulation by cytokines and leukemic transformation, we anticipated that mutation of Y207, which abrogates all Crkl phosphorylation, would inhibit Crkl function. One consequence of Crkl phosphorylation by Bcr-Abl is complex formation between the Crkl SH2 domain and phosphotyrosine-containing proteins such as paxillin. These interactions are best visualized by phosphotyrosine immunoblotting studies of endogenous Crkl immunoprecipitates and consistently demonstrate the appearance of prominent phosphotyrosine-containing proteins migrating at 68 to 74 kDa (paxillin) in cells expressing Bcr-Abl but not in parental cells (8, 34). To determine the effect of the Y207 mutation on the formation of these complexes, we compared wild-type Crkl and Crkl Y207F in a similar assay. Since we had previously shown that wild-type Crkl becomes phosphorylated when overexpressed and activates many of the same signal transduction pathways as Bcr-Abl (40), we reasoned that similar phosphotyrosine-containing complexes might be observed in wild-type Crkl immunoprecipitates. Indeed, overexpression of wild-type Crkl was sufficient to induce complex formation with phosphotyrosine-containing proteins (Fig. 7, top, compare lanes 1 and 2), similar to results obtained previously in studies of Bcr-Abl. Immunoblot analysis with paxillin antibody confirmed that the 68- to 74-kDa bands are paxillin (Fig. 7, bottom). Next we examined the binding of Crkl Y207F to phosphotyrosine-containing proteins. Surprisingly, mutation of Y207 did not interfere with the ability of Crkl to form complexes with paxillin. In fact, the intensity of the phosphotyrosine signal was consistently stronger with Crkl Y207F than with wild-type Crkl despite comparable levels of protein expression (Fig. 7, lanes 3). Therefore, complex formation between the SH2 domain of Crkl and phosphotyrosine-containing proteins such as paxillin is associated with Crkl phosphorylation, but these complexes can occur in the absence of such phosphorylation if Y207 is mutated. These results raise the possibility that phosphorylation of Y207 is a negative regulatory event, since mutation of this site increases complex formation with SH2 binding proteins. Potential explanations include an increase in levels of phosphotyrosine on paxillin in Y207F-expressing cells or a conformational effect of the Y207F mutation on SH2 domain function.
FIG. 7.
Rat-1 cell lines expressing Neo, wild-type Crkl (WT), or Y207F were lysed, and lysates were immunoprecipitated with Crkl antiserum (αCrkl). Proteins were separated by SDS–10% PAGE. (Top panel) Phosphotyrosine (P-Tyr) immunoblot. (Middle panel) Crkl immunoblot. (Bottom panel) paxillin (Pax) immunoblot.
Crkl Y207 activates the stress kinase pathway and transforms fibroblasts more efficiently than wild-type Crkl.
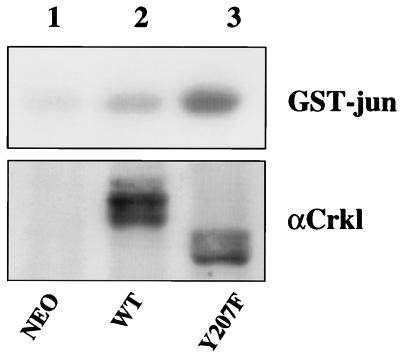
The fact that Crkl Y207F forms complexes with paxillin more efficiently than wild-type Crkl suggests that it may be an activated form of Crkl. We tested this possibility by directly comparing wild-type Crkl and Crkl Y207F in two functional assays. Overexpression of wild-type Crkl activates the stress-activated protein kinase/JNK pathway (40). To compare their relative activities in this assay, Rat-1 fibroblasts were infected with retrovirus stocks expressing wild-type Crkl, Crkl Y207F, or the empty vector (Neo), and JNK immunoprecipitates were analyzed for kinase activity by using glutathione S-transferase–Jun as the substrate. Immunoblot analysis with Crkl antiserum indicated that cells were expressing comparable amounts of Crkl protein (Fig. 8, bottom). In two independent experiments, Crkl Y207F activated JNK more efficiently than wild-type Crkl (Fig. 8, top), consistent with the hypothesis that mutation of Y207 activates Crkl function.
FIG. 8.
Rat-1 fibroblasts were infected with retroviruses expressing the indicated proteins. (Top panel) Cells were lysed 48 h after infection, and endogenous JNK was immunoprecipitated with JNK antiserum (Santa Cruz Biotechnology). In vitro kinase assays were performed with glutathione S-transferase–Jun (GST-jun) as a substrate. Proteins were separated by SDS–12.5% PAGE and exposed for autoradiography (results are representative of three independent experiments). Phosphorimager analysis indicated that JNK was activated twofold over the Neo control and that Y207F was activated fourfold over Neo. (Bottom panel) 1/10 of the cells from the infection were lysed in 2× sample buffer, and proteins were separated by SDS–12.5% PAGE. Proteins were transferred to nitrocellulose, and Crkl protein was detected with Crkl antiserum (αCrkl). WT, wild type.
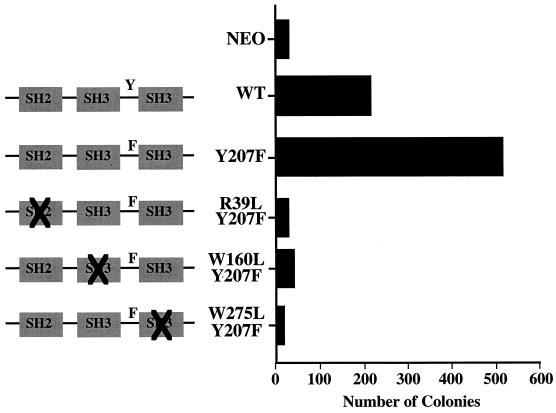
To investigate the effects of the Y207 mutation on Crkl biological activity, cells infected with retrovirus stocks of vector, wild-type Crkl, and Crkl Y207F were examined in the soft-agar colony assay. In four separate experiments with independently derived retrovirus stocks, Crkl Y207F was consistently three to five times more potent at fibroblast transformation than wild-type Crkl (Fig. 9). To determine whether fibroblast transformation by Y207F Crkl is also mediated through complex formation with other proteins, the same deletions or mutations in the SH2 and N-terminal and C-terminal SH3 domains that abrogated transformation of wild-type Crkl were combined independently with the Y207F mutation. Analogous to the case for wild-type Crkl, the SH2, N-terminal SH3, and C-terminal SH3 domains were required for transformation by Crkl Y207F (Fig. 9). Taken together, the results showing enhanced complex formation with the SH2 binding proteins, JNK activation, and fibroblast transformation demonstrate that Y207 functions as a negative regulatory phosphorylation site in Crkl.
FIG. 9.
Rat-1 fibroblasts were infected with the indicated retrovirus stocks and plated in duplicate into soft agar 48 h after infection. Colonies were counted 2 to 3 weeks after plating. Results from one representative experiment of four are shown. WT, wild type.
DISCUSSION
The Crkl adapter protein has been implicated in signal transduction cascades activated by various cytokines and growth factors as well as the Bcr-Abl tyrosine kinase. Identifying the precise role of Crkl in these pathways has been complicated by a lack of assays for Crkl function. We have previously shown that overexpression of Crkl transforms fibroblasts and activates Ras and JNK (40). Our current data show that the primary consequence of Crkl overexpression in hematopoietic cells is enhanced adhesion to fibronectin. We also show that the SH2 domain and both SH3 domains of Crkl are required for Crkl function in fibroblasts and hematopoietic cell lines. Through phosphopeptide mapping studies, we found that Crkl is phosphorylated at multiple sites and that Crkl activity is regulated by phosphorylation at a tyrosine residue (Y207) targeted by the Bcr-Abl kinase. Although phosphorylation of Crkl by Bcr-Abl is associated with its activation, our data suggest that Y207 is a negative regulatory site.
In fibroblasts overexpression of Crkl induces transformation, similar to Bcr-Abl. In hematopoietic cells Bcr-Abl mediates a number of biological effects, including survival after growth factor withdrawal, cytokine-independent proliferation, and increased adhesion to extracellular matrix proteins (3, 19). Our results show that overexpression of Crkl in hematopoietic cells results in adhesion but not cytokine-independent growth or survival; therefore, Crkl can recapitulate some but not all of the signals conferred by Bcr-Abl. These data are consistent with the hypothesis that a primary function of Crkl may be to link signals from growth factor receptors and tyrosine kinases such as Bcr-Abl to the cellular machinery responsible for adhesion.
The mechanism by which Crkl induces adhesion is unknown but must depend on enhanced integrin function, since RGD peptides specifically block the Crkl effect. One potential scenario is that Crkl initiates an “inside-out” signal which modifies focal adhesion structures, possibly through interactions with focal adhesion proteins such as paxillin or Cas. Our observations of increased tyrosine phosphorylation of paxillin and increased complex formation with Crkl in fibroblasts and hematopoietic cells overexpressing Crkl or Bcr-Abl are consistent with this hypothesis. Expression of v-Crk, a relative of Crkl, induces structural and morphological alterations in PC12 cells (1), and c-Crk is implicated in pancreatic carcinoma cell migration (17), consistent with a more general role of the Crk family of adapters in regulating cell adhesion, morphology, and migration. It is of interest that cells from CML patients, which contain a high fraction of phosphorylated Crkl, show integrin-mediated defects in adhesion to bone marrow stroma (5, 16, 48), whereas murine hematopoietic cells reconstituted with Bcr-Abl show increased adhesion to purified extracellular matrix proteins such as fibronectin (3). A mechanistic explanation for these apparently paradoxical results will require further study, with a particular focus on the differences between adhesion to stromal cells and that to purified extracellular matrix components.
The structure-function studies of Crkl demonstrate that the SH2 domain and both SH3 domains are required for biological activity. As discussed above, the requirement for the SH2-mediated binding to focal adhesion proteins such as paxillin, Cas, and Hef1 is consistent with the adhesion phenotype in hematopoietic cells. The requirement of the N-terminal SH3 domain for Crkl function highlights the importance of interactions with tyrosine kinases such as c-Abl and Bcr-Abl and with guanine nucleotide exchange factors such as SOS and C3G (13). Fibroblast transformation by Crkl has previously been shown to require Ras, so it is not surprising that interference with the ability of Crkl to interact with SOS blocks Crkl activity. The fact that Crkl also requires the C-terminal SH3 domain for function is unexpected. In the context of c-Crk, this SH3 domain plays an inhibitory role, since the truncated isoform containing a single SH2 domain and a single SH3 domain (c-Crk I) is transforming, while the full-length protein (c-Crk II) is not (21). To date, there are no known binding partners for this domain as determined from studies of c-Crk or Crkl, but our results suggest that these proteins are important for Crkl function.
Phosphorylation of Crkl is associated with transformation by Bcr-Abl (23, 25, 44) and growth stimulation by cytokines; therefore, a better understanding of the regulation of Crkl by phosphorylation is essential to elucidate its role in these pathways. Previous work demonstrated that residue Y207 in Crkl is phosphorylated by Bcr-Abl (9). Here we show that Crkl is phosphorylated on multiple tyrosine residues and that Y207 is required for higher-order phosphorylation. Surprisingly, we find that mutation of Y207 enhances Crkl function as measured by complex formation with paxillin and activation of downstream signaling pathways. The Y207F mutation also enhanced fibroblast transformation by Crkl, but we failed to see a significant increase in adhesion in hematopoietic cells expressing Y207F over that observed with wild-type Crkl (40a). The reason for distinct responses to Y207F in these cell types is under further investigation but may relate to higher levels of activation when wild-type Crkl is overexpressed in hematopoietic cells versus fibroblasts. Indeed, the fraction of paxillin found in complexes with overexpressed wild-type Crkl is not significantly enhanced by the Y207F mutation in hematopoietic cells (40a). Further activation of Crkl in these cells by mutation of Y207 may not be possible because factors which normally downregulate Crkl function in fibroblasts (i.e., phosphatases that target Y207) may be less active in hematopoietic cells.
How might phosphorylation of Y207 inhibit Crkl function? One possibility is recruitment of an inhibitory molecule. Based on similarities between Y207 in Crkl and Y221 in c-Crk (12), a strong candidate for this inhibitory molecule is the SH2 domain of Crkl itself, which would create an intramolecular interaction and prevent binding to other SH2 binding proteins such as paxillin. While attractive, this model presents an apparent paradox since phosphorylation of Crkl, while leading to an SH2 interaction at Y207 that prevents potential binding of the SH2 domain to other proteins, also leads to binding of the same SH2 domain to phosphotyrosine proteins such as paxillin in cells expressing Bcr-Abl. One resolution of this paradox is that Crkl phosphorylation at residues in addition to Y207 alters the structure of Crkl and allows the SH2 domain access to other proteins. It will be necessary to identify and characterize these secondary phosphorylation sites based on the phosphopeptide mapping data to further address these issues. The details of this regulation have important consequences for understanding aspects of signal transduction by adapter proteins in normal and leukemic cells.
ACKNOWLEDGMENTS
We thank Marc Kaye for excellent technical assistance, Matt Wahl and Owen N. Witte for guidance with phosphopeptide mapping, and Chris Denny, Gerry Weinmaster, and Ke Shuai for critical review of the manuscript.
This work was supported by NRSA Traineeship GM07185 (to K.S.), the American Cancer Society (to C.L.S.), and NIH grant CA32737 (to C.L.S.). C.L.S. is a Scholar of the Leukemia Society of America.
REFERENCES
- 1.Altun-Gultekin Z F, Chandriani S, Bougeret C, Ishizaki T, Narumiya S, de Graaf P, Van Bergan en Henegouwen P, Hanafusa H, Wagner J A, Birge R B. Activation of Rho-dependent cell spreading and focal adhesion biogenesis by the v-Crk adapter protein. Mol Cell Biol. 1998;18:3044–3058. doi: 10.1128/mcb.18.5.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber D L, Mason J M, Fukazawa T, Reedquist K A, Druker B J, Band H, D’Andrea A D. Erythropoietin and interleukin-3 activate tyrosine phosphorylation of CBL and association with CRK adaptor proteins. Blood. 1997;89:3166–3174. [PubMed] [Google Scholar]
- 3.Bazzoni G, Carlesso N, Griffin J D, Hemler M E. Bcr/Abl expression stimulates integrin function in hematopoietic cell lines. J Clin Invest. 1996;98:521–528. doi: 10.1172/JCI118820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhat A, Kolibaba K, Oda T, Ohno-Jones S, Heaney C, Druker B J. Interactions of Cbl with Bcr-Abl and Crkl in Bcr-Abl-transformed myeloid cells. J Biol Chem. 1997;272:16170–16175. doi: 10.1074/jbc.272.26.16170. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia R, Wayner E A, McGlave P B, Verfaillie C M. Interferon-alpha restores normal adhesion of chronic myelogenous leukemia hematopoietic progenitors to bone marrow stroma by correcting impaired beta 1 integrin receptor function. J Clin Invest. 1994;94:384–391. doi: 10.1172/JCI117333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle W J, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–148. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- 7.Chardin P, Camonis J H, Gale N W, van Aelst L, Schlessinger J, Wigler M H, Bar-Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993;260:1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- 8.de Jong R, ten Hoeve J, Heisterkamp N, Groffen J. Crkl is complexed with tyrosine-phosphorylated Cbl in Ph-positive leukemia. J Biol Chem. 1995;270:21468–21471. doi: 10.1074/jbc.270.37.21468. [DOI] [PubMed] [Google Scholar]
- 9.de Jong R, ten Hoeve J, Heisterkamp N, Groffen J. Tyrosine 207 in CRKL is the BCR/ABL phosphorylation site. Oncogene. 1997;14:507–513. doi: 10.1038/sj.onc.1200885. [DOI] [PubMed] [Google Scholar]
- 10.Downward J. The GRB2/Sem-5 adaptor protein. FEBS Lett. 1994;338:113–117. doi: 10.1016/0014-5793(94)80346-3. [DOI] [PubMed] [Google Scholar]
- 11.Druker B J, Roberts T M. Generation of a large library of point mutations in polyoma middle T antigen. Nucleic Acids Res. 1991;19:6855–6861. doi: 10.1093/nar/19.24.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feller S M, Knudsen B, Hanafusa H. c-Abl kinase regulates the protein binding activity of c-Crk. EMBO J. 1994;13:2341–2351. doi: 10.1002/j.1460-2075.1994.tb06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feller S M, Knudsen B, Hanafusa H. Cellular proteins binding to the first Src homology 3 (SH3) domain of the proto-oncogene product c-Crk indicate Crk-specific signaling pathways. Oncogene. 1995;10:1465–1473. [PubMed] [Google Scholar]
- 14.Freed E, Hunter T. A 41-kilodalton protein is a potential substrate for the p210bcr/abl protein-tyrosine kinase in chronic myelogenous leukemia cells. Mol Cell Biol. 1992;12:1312–1323. doi: 10.1128/mcb.12.3.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goga A, Liu X, Hambuch T M, Senechal K, Major E, Berk A J, Witte O N, Sawyers C L. p53 dependent growth suppression by the c-Abl nuclear tyrosine kinase. Oncogene. 1995;11:791–799. [PubMed] [Google Scholar]
- 16.Gordon M, Dowding C, Riley G, Goldman J, Greaves M. Altered adhesive interactions with marrow stroma of hematopoietic progenitor cells in chronic myeloid leukemia. Nature. 1987;328:342–344. doi: 10.1038/328342a0. [DOI] [PubMed] [Google Scholar]
- 17.Klemke R L, Leng J, Molander R, Brooks P C, Vuori K, Cheresh D A. Cas/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurzrock R, Gutterman J U, Talpaz M. The molecular genetics of Philadelphia chromosome positive leukemias. N Engl J Med. 1988;319:990–998. doi: 10.1056/NEJM198810133191506. [DOI] [PubMed] [Google Scholar]
- 19.Laneuville P, Heisterkamp N, Groffen J. Expression of the chronic myelogenous leukemia-associated p210bcr/abl oncoprotein in a murine IL-3 dependent myeloid cell line. Oncogene. 1991;6:275–282. [PubMed] [Google Scholar]
- 20.Maru Y, Witte O N. The BCR gene encodes a novel serine/threonine kinase activity within a single exon. Cell. 1991;67:459–468. doi: 10.1016/0092-8674(91)90521-y. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda M, Tanaka S, Nagata S, Kojima A, Kurata T, Shibuya M. Two species of human CRK cDNA encode proteins with distinct biological activities. Mol Cell Biol. 1992;12:3482–3489. doi: 10.1128/mcb.12.8.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller A J, Young J C, Pendergast A M, Pondel M, Landau N R, Littman D R, Witte O N. Bcr first-exon sequences specifically activate the Bcr-Abl tyrosine kinase oncogene of Philadelphia chromosome-positive human leukemias. Mol Cell Biol. 1991;11:1785–1792. doi: 10.1128/mcb.11.4.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nichols G L, Raines M A, Vera J C, Lacomis L, Tempst P, Golde D W. Identification of CRKL as the constitutively phosphorylated 39-kD tyrosine phosphoprotein in chronic myelogenous leukemia cells. Blood. 1994;84:2912–2918. [PubMed] [Google Scholar]
- 24.Oda A, Miyakawa Y, Druker B J, Ishida A, Ozaki K, Ohashi H, Wakui M, Handa M, Watanabe K, Okamoto S, Ikeda Y. Crkl is constitutively phosphorylated in platelets from chronic myelogenous leukemia patients and inducibly phosphorylated in normal platelets stimulated by thrombopoietin. Blood. 1996;88:4304–4313. [PubMed] [Google Scholar]
- 25.Oda T, Heaney C, Hagopian J R, Okuda K, Griffin J D, Druker B J. Crkl is the major tyrosine-phosphorylated protein in neutrophils from patients with chronic myelogenous leukemia. J Biol Chem. 1994;269:22925–22928. [PubMed] [Google Scholar]
- 26.Ogawa S, Toyoshima H, Kozutsumi H, Hagiwara K, Sakai R, Tanaka T, Hirano N, Mano H, Yazaki Y, Hirai H. The C-terminal SH3 domain of the mouse c-Crk protein negatively regulates tyrosine-phosphorylation of Crk associated p130 in rat 3Y1 cells. Oncogene. 1994;9:1669–1678. [PubMed] [Google Scholar]
- 27.Pawson T, Schlessinger J. SH2 and SH3 domains. Curr Biol. 1993;3:434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- 28.Pendergast A M, Gishizky M L, Havlik M H, Witte O N. SH1 domain autophosphorylation of P210 BCR/ABL is required for transformation but not growth factor independence. Mol Cell Biol. 1993;13:1728–1736. doi: 10.1128/mcb.13.3.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pendergast A M, Quilliam L A, Cripe L D, Bassing C H, Dai Z, Li N, Batzer A, Rabun K M, Der C J, Schlessinger J, Gishizky M L. BCR-ABL-induced oncogenesis is mediated by direct interaction with the SH2 domain of the GRB-2 adaptor protein. Cell. 1993;75:175–185. [PubMed] [Google Scholar]
- 30.Reedquist K A, Fukazawa T, Panchamoorthy G, Langdon W Y, Shoelson S E, Druker B J, Band H. Stimulation through the T cell receptor induces Cbl association with Crk proteins and the guanine nucleotide exchange protein C3G. J Biol Chem. 1996;271:8435–8442. doi: 10.1074/jbc.271.14.8435. [DOI] [PubMed] [Google Scholar]
- 31.Ribon V, Hubbell S, Herrera R, Saltiel A R. The product of the cbl oncogene forms stable complexes in vivo with endogenous Crk in a tyrosine phosphorylation-dependent manner. Mol Cell Biol. 1996;16:45–52. doi: 10.1128/mcb.16.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen M K, Yamazaki T, Gish G D, Kay C M, Pawson T, Kay L E. Direct demonstration of an intramolecular SH2-phosphotyrosine interaction in the Crk protein. Nature. 1995;374:477–479. doi: 10.1038/374477a0. [DOI] [PubMed] [Google Scholar]
- 33.Salgia R, Pisick E, Sattler M, Li J L, Uemura N, Wong W K, Burky S A, Hirai H, Chen L B, Griffin J D. p130CAS forms a signaling complex with the adapter protein CRKL in hematopoietic cells transformed by the BCR/ABL oncogene. J Biol Chem. 1996;271:25198–25203. doi: 10.1074/jbc.271.41.25198. [DOI] [PubMed] [Google Scholar]
- 34.Salgia R, Uemura N, Okuda K, Li J L, Pisick E, Sattler M, de Jong R, Druker B, Heisterkamp N, Chen L B, Groffen J, Griffin J. CRKL links p210BCR/ABL with paxillin in chronic myelogenous leukemia cells. J Biol Chem. 1995;270:29145–29150. doi: 10.1074/jbc.270.49.29145. [DOI] [PubMed] [Google Scholar]
- 35.Sattler M, Salgia R, Shrikhande G, Verma S, Pisick E, Prasad K V, Griffin J D. Steel factor induces tyrosine phosphorylation of CRKL and binding of CRKL to a complex containing c-kit, phosphatidylinositol 3-kinase, and p120(CBL) J Biol Chem. 1997;272:10248–10253. doi: 10.1074/jbc.272.15.10248. [DOI] [PubMed] [Google Scholar]
- 36.Sattler M, Salgia R, Shrikhande G, Verma S, Uemura N, Law S F, Golemis E A, Griffin J D. Differential signaling after beta1 integrin ligation is mediated through binding of CRKL to p120(CBL) and p110(HEF1) J Biol Chem. 1997;272:14320–14326. doi: 10.1074/jbc.272.22.14320. [DOI] [PubMed] [Google Scholar]
- 37.Sawyers C L, Callahan W, Witte O N. Dominant negative myc blocks transformation by ABL oncogenes. Cell. 1992;70:901–910. doi: 10.1016/0092-8674(92)90241-4. [DOI] [PubMed] [Google Scholar]
- 38.Sawyers C L, McLaughlin J, Goga A L, Havlik M, Witte O. The nuclear tyrosine kinase c-Abl negatively regulates cell growth. Cell. 1994;77:121–131. doi: 10.1016/0092-8674(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 39.Sawyers C L, McLaughlin J, Witte O N. Genetic requirement for Ras in the transformation of fibroblasts and hematopoietic cells by the Bcr-Abl oncogene. J Exp Med. 1995;181:307–313. doi: 10.1084/jem.181.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senechal K, Halpern J, Sawyers C L. The CRKL adaptor protein transforms fibroblasts and functions in transformation by the BCR-ABL oncogene. J Biol Chem. 1996;271:23255–23261. doi: 10.1074/jbc.271.38.23255. [DOI] [PubMed] [Google Scholar]
- 40a.Senechal, K., and C. L. Sawyers. Unpublished observations.
- 41.Smit L, van der Horst G, Borst J. Sos, Vav, and C3G participate in B cell receptor-induced signaling pathways and differentially associate with Shc-Grb2, Crk, and Crk-L adaptors. J Biol Chem. 1996;271:8564–8569. doi: 10.1074/jbc.271.15.8564. [DOI] [PubMed] [Google Scholar]
- 42.Songyang Z, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J, Neel B G, Birge R B, Fajardo J E, Chou M M, Hanafusa H, Schaffhausen B, Cantley L C. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka M, Gupta R, Mayer B J. Differential inhibition of signaling pathways by dominant-negative SH2/SH3 adapter proteins. Mol Cell Biol. 1995;15:6829–6837. doi: 10.1128/mcb.15.12.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.ten Hoeve J, Arlinghaus R B, Guo J Q, Heisterkamp N, Groffen J. Tyrosine phosphorylation of CRKL in Philadelphia+ leukemia. Blood. 1994;84:1731–1736. [PubMed] [Google Scholar]
- 45.ten Hoeve J, Kaartinen V, Fioretos T, Haataja L, Voncken J-W, Heisterkamp N, Groffen J. Cellular interactions of CRKL, an SH2-SH3 adaptor protein. Cancer Res. 1994;54:2563–2567. [PubMed] [Google Scholar]
- 46.ten Hoeve J, Morris C, Heisterkamp N, Groffen J. Isolation and chromosomal localization of CRK-L, a human crk-like gene. Oncogene. 1993;8:2469–2474. [PubMed] [Google Scholar]
- 47.van der Geer P, Pawson T. The PTB domain: a new protein module implicated in signal transduction. Trends Biochem Sci. 1995;20:277–280. doi: 10.1016/s0968-0004(00)89043-x. [DOI] [PubMed] [Google Scholar]
- 48.Verfaillie C M, McCarthy J B, McGlave P B. Mechanisms underlying abnormal trafficking of malignant progenitors in chronic myelogenous leukemia. Decreased adhesion to stroma and fibronectin but increased adhesion to the basement membrane components laminin and collagen type IV. J Clin Invest. 1992;90:1232–1241. doi: 10.1172/JCI115985. [DOI] [PMC free article] [PubMed] [Google Scholar]