Abstract
This case report highlights the complexities of tizanidine withdrawal in a 68-year-old woman with chronic pain. Tizanidine, a widely used imidazole-derived muscle relaxant, poses challenges due to the absence of standardized withdrawal protocols. The patient's presentation included hypertension and tachycardia following a gradual reduction in her outpatient tizanidine dose.
During the de-escalation of tizanidine, the patient experienced withdrawal symptoms, including severe body aches, hypertension, and tachycardia. Management during withdrawal involved a unique approach using a one-time dose of phenobarbital, a measure that allowed the resolution of hemodynamic instability and pain with complete discontinuation of tizanidine. The ultimate decision to transition the patient to methocarbamol and stop taking tizanidine for pain control highlights the importance of individualized care. The patient has responded to this therapy upon follow-up.
Keywords: phenobarbital therapy, addiction, pain, psychiatry, neurology, internal medicine, withdrawal, overdose, tizanidine, toxicology
Introduction
Tizanidine is a widely used imidazole-derived medication for spasticity and chronic pain [1]. It was approved by the FDA in 1996, and it is estimated that in 2021, there will be around 8 million active prescriptions of this medication in the United States [2]. Tizanidine is an alpha-2 agonist at the presynaptic level that works by modulating neurotransmitters (mainly GABA and glutamate), resulting in central analgesia [1], and acting at the imidazoline receptors with inhibition of spinal reflexes and improved spasticity [3].
Due to being a neurotransmitter modulator, tizanidine must be used with caution, as it has the potential for withdrawal [4]. Case reports in the literature suggest that withdrawal from tizanidine is possible. Abruptly stopping this medication, known for its inhibitory effect on the nervous system, can trigger a cascade of catecholamines, leading to symptoms such as hypertension, tachycardia, and spasticity [3-9]. Treatment involves restarting and subsequently de-escalating tizanidine, but patients in withdrawal must be treated using a conservative approach, as overtreatment or restarting tizanidine quickly at high doses can lead to an overdose with catastrophic hemodynamic outcomes like severe and persistent hypotension and bradycardia [10]. There is no standard of care for tizanidine withdrawal. Case reports show that withdrawals often respond to medications such as clonidine, lorazepam, and slowly restarting tizanidine [7,11-13]. To our knowledge, other neurotransmitter modulators have not been studied in the setting of withdrawal and overdose.
Without further ado, we present the case of a 68-year-old woman with chronic pain managed with opioids and tizanidine who arrived at the emergency department hypertensive, tachycardic, and reporting that she had abruptly stopped taking tizanidine, prompting suspicion of withdrawal from this agent.
Case presentation
A 68-year-old woman with a history of chronic pain (managed with opioids and tizanidine 4 mg three times a day), hypertension (on lisinopril), COPD (on 2L of oxygen at home), thrombocytopenia, diastolic heart failure, panniculitis, and a previous osteomyelitis episode in her right lower extremity, was brought in from a facility after a ground level fall, as well as tachycardia, hypertension, tachypnea, nausea, and vomiting. On admission, the patient reported that she had not been taking her tizanidine daily as prescribed. The patient's presenting vital signs can be visualized in Table 1, and the laboratory tests done on admission can be found in Table 2.
Table 1. Comparison of the patient's vital signs on admission with the vital signs upon discharge.
| Variable | On admission | Upon discharge | Reference Range |
| Temperature (in degrees Celsius) | 36.7 | 36.7 | 36.5-37.3 |
| Respiratory Rate (respirations per minute) | 30 | 16 | 12-20 |
| Peripheral Pulse (beats per minute) | 115 | 87 | 60-100 |
| Systolic Blood Pressure (mm of Hg) | 224 | 106 | 90-120 |
| Diastolic Blood Pressure (mm of Hg) | 193 | 88 | 60-80 |
| Oxygen saturation at baseline O2 of 2L (%) | 98 | 95 | 90-100 |
Table 2. Comparison of the patient's laboratory tests on admission with the laboratory tests upon discharge.
| Variable | On admission | Upon discharge | Reference Range |
| Sodium (mg/dL) | 137 | 137 | 135-145 |
| Potassium (mg/dL) | 4.9 | 3.9 | 3.5-5 |
| Chloride (mmol/L) | 106 | 105 | 97-106 |
| CO2 (mmol/L) | 23 | 24 | 23-29 |
| Blood Urea Nitrogen (mg/dL) | 17 | 9 | 6-20 |
| Creatinine (mg/dL) | 0.87 | 0.75 | 0.7-1.2 |
| Random Glucose (mg/dL) | 107 | 87 | 64-100 |
| Anion Gap (mEq/L) | 11 | 8 | 4-12 |
| Hemoglobin (g/dL) | 15 | 15 | 12-16 |
| Hematocrit (%) | 46 | 46 | 36-48 |
| White Blood Cell Count (× 109/L) | 6.3 | 4.8 | 4.5-11 |
| Platelet number (per mcL) | 283,000 | 280,000 | 150,000-450,000 |
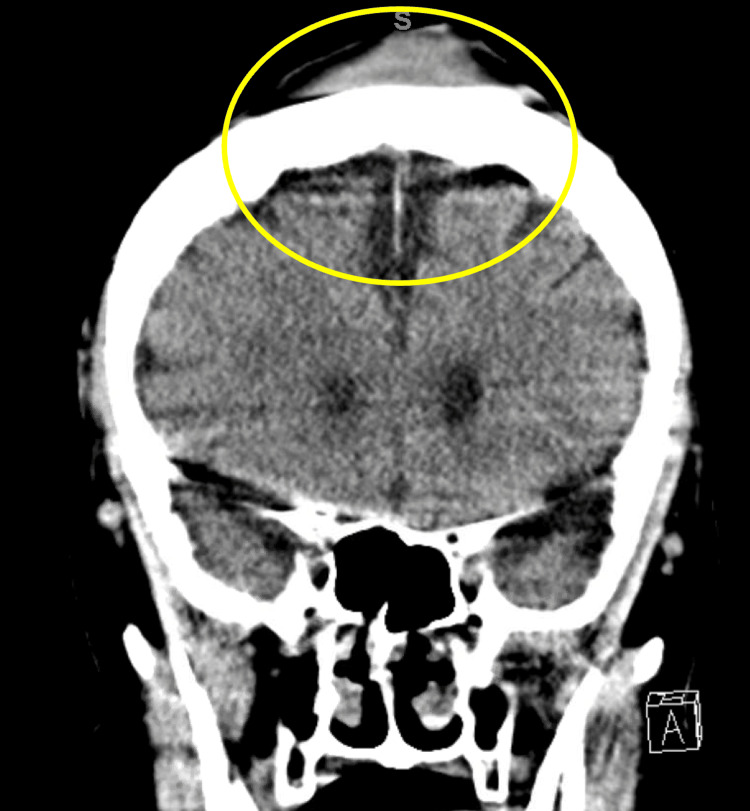
In the emergency department, the patient’s hypertension and tachycardia were resolved with the initiation of her home tizanidine dose of 8 mg three times per day. On the other hand, diagnostic imaging done in the setting of the patient’s fall, including CT scans of the head, CT of the spine, and the abdomen/pelvis, showed no acute abnormalities except for a scalp hematoma that can be visualized in Figure 1.
Figure 1. CT of the head.
The yellow circle highlights the area in which a vertex hematoma can be visualized, likely due to the level-ground fall that the patient sustained. There are no other abnormalities in this study.
The patient was admitted for tizanidine withdrawal. During the de-escalation of the tizanidine dose, especially when she was reduced to twice a day, she experienced withdrawal symptoms of tachycardia, hypertension, and severe pain. The patient received one dose of phenobarbital, considering its effects on glutamate, which she seemed to tolerate well. The resolution of the withdrawal symptoms demonstrates that phenobarbital can aid in tizanidine withdrawal. Vital signs after treatment and laboratory test results upon discharge can be visualized in Tables 1-2.
Despite the above complexities, the patient's condition was resolved, prompting consideration for discharge to a skilled nursing facility (SNF). The patient was given instructions to stop tizanidine and replace it with methocarbamol, a central-acting muscle relaxant, which she had tolerated in the past and had helped control her chronic pain along with her home opioids.
Discussion
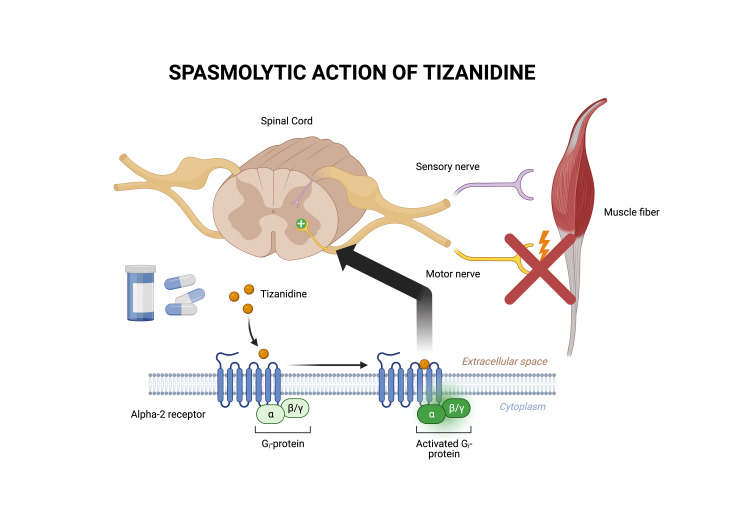
The presented case of tizanidine withdrawal in a 68-year-old woman explores the challenges of managing complications associated with this widely prescribed medication. Tizanidine, an alpha-2 agonist with dual actions on presynaptic neurotransmitters and imidazoline receptors, has been associated with withdrawal symptoms characterized by a cascade of catecholamines, leading to hypertension, tachycardia, and spasticity. The mechanism of action of tizanidine is illustrated in Figure 2.
Figure 2. Mechanism of action of tizanidine.
Diagram depicting the spasmolytic action of tizanidine. The diagram is of the author’s own creation.
Several case reports have shown that tizanidine withdrawal is possible, and they have resolved this complication by various means. The available case reports that we could find about tizanidine withdrawal are compiled in Table 3. One notable aspect of this case is the utilization of phenobarbital in managing the patient's altered hemodynamics. Phenobarbital, a barbiturate, acts as a positive allosteric modulator of the gamma-aminobutyric acid (GABA) receptor, leading to enhanced inhibitory neurotransmission. While tizanidine's primary mechanism of action involves the modulation of GABA and glutamate, the introduction of phenobarbital may have contributed to the resolution of symptoms through its GABAergic effects.
Table 3. Case reports available in the literature depicting tizanidine withdrawal.
| CASE REPORTS DEPICTING TIZANIDINE WITHDRAWAL | ||||
| Author | Year | Case | Strategy implemented | Outcome of the case |
| Morgom et al. [3] | 2023 | Tizanidine withdrawal | Tizanidine taper | Symptoms improved after initiating tizanidine taper |
| Suelt et al. [4] | 2021 | Tizanidine withdrawal | Tizanidine taper | Symptoms improved after initiating tizanidine taper, blood pressure normalized, and agitation stabilized |
| Perez et al. [5] | 2009 | Tizanidine withdrawal | Tizanidine taper | Symptoms improved after initiating tizanidine taper, blood pressure normalized, and agitation stabilized |
| Mörkl et al. [6] | 2015 | Tizanidine withdrawal and Takotsubo cardiomyopathy | Tizanidine taper | Symptoms improved after initiating tizanidine taper |
| Kitta et al. [7] | 2020 | Tizanidine withdrawal | Introduction of clonidine with subsequent tizanidine taper | After establishing clonidine, the off-tapering of tizanidine was much better tolerated by the patient and finally successful |
| Karol et al. [8] | 2011 | Baclofen and Tizanidine withdrawal | Slow taper of tizanidine and baclofen | Delirium and motor disturbances resolved within 24 h of reintroduction of medications |
| Daniels et al. [9] | 2022 | Tizanidine withdrawal | Tizanidine taper | A plan was made to attempt weaning her by 1 mg per dose weekly in the community |
The enhanced GABAergic activity induced by phenobarbital could have mitigated the excessive catecholamine release associated with tizanidine withdrawal [14]. GABA, the principal inhibitory neurotransmitter in the central nervous system, plays a crucial role in regulating sympathetic outflow [14]. By potentiating GABAergic transmission, phenobarbital may have exerted a calming effect on the hyperactive sympathetic response triggered by tizanidine discontinuation, thereby alleviating the patient's hypertensive and tachycardic state [14].
This unique therapeutic approach with phenobarbital offers insights into potential strategies for managing tizanidine withdrawal, emphasizing the importance of understanding the interplay of neurotransmitter systems in the context of withdrawal syndromes. However, it is crucial to note that further research is needed to establish the safety and efficacy of phenobarbital in this specific clinical scenario.
The decision to transition the patient to methocarbamol for pain management also warrants consideration. Methocarbamol, a muscle relaxant with a distinct mechanism of action from tizanidine, was chosen to ensure adequate pain control while minimizing the risk of adverse events. This approach aligns with the principle of individualized care, recognizing the need to tailor treatment strategies to the unique characteristics and tolerances of each patient.
Conclusions
In conclusion, managing tizanidine withdrawal requires a nuanced understanding of the pharmacological interactions involved. The use of phenobarbital in this case provides a compelling avenue for exploration, suggesting that agents affecting GABAergic neurotransmission may play a role in mitigating the hemodynamic consequences of tizanidine withdrawal. Further research is essential to validate and refine these approaches, paving the way for evidence-based guidelines in managing tizanidine-related complications.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Tomas Escobar Gil, Kevin J. McGeorge, Aaron J. Jones
Acquisition, analysis, or interpretation of data: Tomas Escobar Gil, Kevin J. McGeorge, Aaron J. Jones
Drafting of the manuscript: Tomas Escobar Gil, Kevin J. McGeorge
Critical review of the manuscript for important intellectual content: Tomas Escobar Gil, Kevin J. McGeorge, Aaron J. Jones
Supervision: Aaron J. Jones
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.The medical management of spasticity. Abbruzzese G. Eur J Neurol. 2002;9:30–34. doi: 10.1046/j.1468-1331.2002.0090s1030.x. [DOI] [PubMed] [Google Scholar]
- 2.MEPS-HC Data Tools - Medical Expenditure Panel Survey (MEPS) Household Component (HC) [ Jan; 2024 ]. 2024. https://datatools.ahrq.gov/meps-hc/?tab=prescribed-drugs&dash=18 https://datatools.ahrq.gov/meps-hc/?tab=prescribed-drugs&dash=18
- 3.A case of tizanidine withdrawal syndrome: features and management in the emergency department. Morgom M, Sabir DM, Elbashir H, Saeed L, Alamin A, Abuazab Y, Abdelrahman N. Cureus. 2023;15:0. doi: 10.7759/cureus.49248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dependencia a los relajantes musculares- Tizanidina: reporte de un caso [Spanish] Suelt MJ, Arcila JE. https://www.aesed.com/upload/files/v46n2_8_caso-clinico.pdf Revista española de drogodependencias. 2021;46:95–100. [Google Scholar]
- 5.Tizanidine withdrawal syndrome: a case report. Perez OA, Tauquir A, Janosko J, Saleem T, Sarin K. Am J Respir Crit Care Med. 2022;205:1568. [Google Scholar]
- 6.Tizanidine withdrawal symptoms in stress cardiomyopathy [German] Mörkl S, Bengesser SA, Schöggl H, Bayer D, Kapfhammer HP. Fortschr Neurol Psychiatr. 2015;83:170–173. doi: 10.1055/s-0034-1399167. [DOI] [PubMed] [Google Scholar]
- 7.Using clonidine in the treatment of tizanidine abuse and withdrawal: a case report of a patient with somatoform pain disorder. Kitta A, Wippel A, Richwien P, et al. J Subst Use. 2020;25:535–537. [Google Scholar]
- 8.A case of delirium, motor disturbances, and autonomic dysfunction due to baclofen and tizanidine withdrawal: a review of the literature. Karol DE, Muzyk AJ, Preud'homme XA. Gen Hosp Psychiatry. 2011;33:84–82. doi: 10.1016/j.genhosppsych.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Management of tizanidine abuse and withdrawal in a 53-year-old woman: a case report. Daniels NF, Ridwan R, Vasileva AMD. SN Compr Clin Med. 2023;5:1–4. [Google Scholar]
- 10.Retrospective review of Tizanidine (Zanaflex) overdose. Spiller HA, Bosse GM, Adamson LA. J Toxicol Clin Toxicol. 2004;42:593–596. doi: 10.1081/clt-200026978. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed SMI, Salem WA. Tizanidine; the lethal withdrawal. [ Jan; 2024 ]. 2022. https://www.researchgate.net/publication/372910111_Tizanidine_The_Lethal_Withdrawal https://www.researchgate.net/publication/372910111_Tizanidine_The_Lethal_Withdrawal
- 12.Is it prime time for alpha2-adrenocepter agonists in the treatment of withdrawal syndromes? Albertson TE, Chenoweth J, Ford J, Owen K, Sutter ME. J Med Toxicol. 2014;10:369–381. doi: 10.1007/s13181-014-0430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tizanidine may discriminate between imidazoline-receptors and alpha 2-adrenoceptors. Muramatsu I, Kigoshi S. Jpn J Pharmacol. 1992;59:457–459. doi: 10.1254/jjp.59.457. [DOI] [PubMed] [Google Scholar]
- 14.Management of xylazine withdrawal in a hospitalized patient: a case report. Ehrman-Dupre R, Kaigh C, Salzman M, Haroz R, Peterson LK, Schmidt R. J Addict Med. 2022;16:595–598. doi: 10.1097/ADM.0000000000000955. [DOI] [PubMed] [Google Scholar]