Abstract
Objectives:
To assess the safety and efficacy of the Amplatzer Septal Occluder in the closure of secundum type atrial septal defects.
Background:
The Amplatzer Septal Occluder (ASO; Abbott, St. Paul, MN) is an FDA-approved device for percutaneous closure of secundum type atrial septal defects (ASD). Previous small cohort trials have shown a favorable safety and technical efficacy profile.
Methods:
We conducted a systemic review and meta-analysis of all prospective case series and controlled trials that evaluated the ASO's safety and implant efficacy. The primary endpoint was the technical success rate of implantations. Secondary outcomes included proportions of arrhythmias and embolism specific-adverse events.
Results:
We included a total of 12 studies with 2972 patients. The ratio of device implantation was 2:1 by sex [female: male]. Pooled technical success rate of implantation was 98% (95% CI: 0.968–0.990, P < 0.01). The cumulative adverse event rate was 5.1% (95% CI: 0.035–0.068, P < 0.01), which included arrhythmia and embolism specific adverse event rates of 1.8% (95% CI: 0.007–0.032, P < 0.01) and 0.7% (95% CI: 0.002–0.013, P < 0.01), respectively. Sensitivity analysis did not significantly affect pooled outcomes for success rate and adverse events; both forest plot and Begg's and Egger's regression tests supported symmetricity.
Conclusion:
A high likelihood of technical success can be expected when implanting the ASO in secundum type ASDs. Adverse event rates are expected for one in twenty patients, and thus, our results support the safe use of ASO in secundum type ASDs closure.
Condensed abstract:
The AMPLATZER Septal Occluder is an FDA-approved device for percutaneous closure of secundum type atrial septal defects (ASD). We conducted a systemic review and meta-analysis of all prospective case series and controlled trials that evaluated the ASO's safety and implant efficacy. We included a total of 12 studies with 2972 patients. Pooled technical success rate of implantation was 98% (P < 0.01). The cumulative adverse event rate was 5.1% (P < 0.01), 1.8% (P < 0.01) rate of arrhythmias, and 0.7% (P < 0.01) rate of embolisms. A high likelihood of technical success can be expected with a low rate of adverse events.
Keywords: Amplatzer, Septal occluder, Secundum type atrial septal defect, Congenital heart defect
1. Introduction
Atrial septal defects (ASD) account for about 10% of all congenital cardiac malformations at birth and represent 15% of congenital anomalies managed by clinics in children and adults [1]. Sequalae of ASDs include pulmonary hypertension, arrhythmias, paradoxical embolism, and heart failure [2]. Interventions have been developed to mitigate and prevent sequela of ASDs, which include surgical and transcatheter management. Surgical management had been the de-facto option for decades, even after the introduction of transcatheter closure by Kings and Mills in the 1970's [3,4]. The use of transcatheter atrial septal occluders (ASO) has rapidly increased over the past three decades due to the ease of use and perceived efficacy benefits. Typically, ASOs are designed as self-expandable and self-centering double-sided disks, consisting of nitinol wire mesh, which allows them to be recaptured and repositioned during implantation [5].
Percutaneous closure of atrial septal defects is typically performed in the outpatient setting due to low risks and the likelihood for same day discharge without physical restrictions. Concurrent procedures or significant comorbidities, such as severe pulmonary hypertension, may indicate the need for an inpatient procedure and admission. During the procedure, transthoracic (TTE), transesophageal (TEE), or intracardiac echocardiography (ICE) may be used to confirm device placement. General anesthesia is typically used during the procedure. Newer methods and imaging modalities, however, have allowed for safe implantation with less anesthetic requirements [6,7]. Post-procedure, patients should remain supine for 2 h when the access sheath is removed. Upon discharge, patients are typically prescribed antiplatelet therapy for six months. Antibiotic prophylaxis is also recommended for any dental or invasive procedures during the first six months. Follow-up echocardiography is recommended at six months for confirmation of device placement.
Since its approval by the United States Food and Drug Administration in 2001, multiple studies have shown promising outcomes and the transcatheter approach's superiority compared to high-risk surgical alternative [8,9]. The Amplatzer ASO has strived to supersede previous devices by utilizing smaller-sized devices and implementing self-centering mechanisms [10,11]. We performed a systematic review and meta-analysis of studies reporting the technical success and safety outcomes of the Amplatzer Atrial Septal Occluder as a monotherapy for the management of secundum type atrial septal defects.
2. Methods
2.1. Search strategy
We comprehensively searched PUBMED, EMBASE, and Web of Science from inception until August 2020 for studies reporting outcomes of the Amplatzer ASO in the treatment of secundum type atrial septal defects. The search was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) Epidemiology guidelines. A predefined protocol was used to identify prospective studies reporting the technical success and adverse events associated with the use of the Amplatzer ASD. The keywords “Amplatzer”, “secundum atrial septal defect”, “closure”, and “trial” were used for the identification of relevant studies. The search was restricted to include studies published in the English language of peer-reviewed journals. Two authors independently reviewed titles and abstracts of the studies identified in the primary search; studies were excluded if they did not address the research questions. After, the remaining full-text articles were reviewed to establish the relevancy of the desired information. Discrepancies in the selection of the articles were resolved by consensus after discussion with a co-author. Finally, the bibliography of the chosen articles was manually searched for additional relevant articles and information. Ethical approval was not required as our study would not include protected health information or confidential data about participants and interventions.
2.2. Study selection: Inclusion and exclusion criteria
We included prospective case series, controlled trials, and randomized controlled trials. Study participants included all ages treated using the Amplatzer ASO as monotherapy for secundum type ASDs. Studies with greater than 30 patients that reported the desired endpoints were included. Retrospective studies, studies with 30 patients or fewer, review articles, case reports, comments, editorials, and review articles were excluded. Studies that did not report the desired endpoints were excluded.
2.3. Data extraction
For each of the included studies, we extracted the following data points: first author's name, year published, title of study, country, type of trial, clinical setting, number of patients attempted intervention, number of males, number of females, age in years (mean ± SD), number of devices successfully deployed, number of overall adverse events, number of arrhythmias, and number of embolisms.
2.4. Outcome measures
This analysis's primary outcomes were pooled technical success rate, which was defined as the successful implantation of the Amplatzer ASD. Secondary outcomes included arrhythmia, device embolism, and overall adverse event rates after intervention.
2.5. Statistical methods
Meta-analysis techniques were applied using the random-effects model to establish pooled technical success rates, cumulative adverse events, arrhythmia, and embolism. We assessed heterogeneity using the Cochran Q statistical test and, if heterogeneity was present, I2 was calculated to estimate the variance caused by study heterogeneity versus chance [12,13]. Heterogeneity was categorized as low, moderate, substantial and considerable when I2 values were <30%, 30–60%, 61–75% and >75%, respectively [13,14]. 95% prediction intervals were calculated to estimate the dispersion of effects [12,15].
Pooled estimates were assessed using methods described by DerSimonian and Laird with the random effects model [16]. The Jackson method was used to calculate the confidence interval for pooled estimates [17]. Freeman-Tukey double arcsine transformation was used for synthesizing proportions [18]. Begg's funnel plots were used to ascertain qualitative publication bias, while Egger's test assessed bias quantitatively [19]. All analyses were performed using the “meta” package, version 4.16–2, in R [20].
3. Results
3.1. Search results
One hundred and forty-nine potential articles were preliminarily identified using the abovementioned search strategies. The articles' screening by title and abstract, with the elimination of duplicates, advanced 53 studies for full-text review. A secondary search of the full-text articles excluded 41 additional articles. Ultimately, twelve studies were eligible for inclusion into the meta-analysis, which encompassed 2972 patients [5,21-31]. An illustrative representation of the selection process is presented in Fig. 1.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart summarizing the article selection process.
3.2. Characteristics of the studies
Characteristics of the included studies are presented in Table 1. The mean age of patients 21.1 ± 65.8 years, where 61.7% were female. There was no inter-rater disagreement for the extracted data. Seven studies were prospective case series [21,22,24-27,29], four were controlled trials [5,23,30,31], and one was a randomized controlled trial [28]. Two studies were performed in the United States of America [23,31]. Five of the twelve studies were completed in a single center [22,24-26,29].
Table 1.
Characteristics and description of study features.

|
3.3. Transcatheter implantation
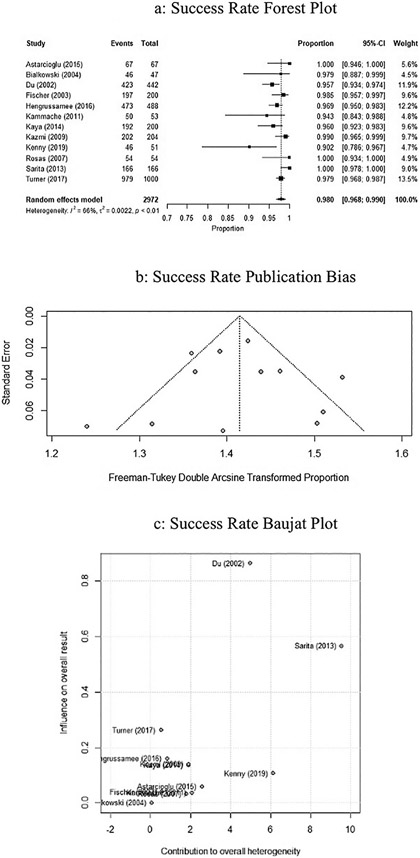
The pooled technical success of the Amplatzer ASD was 98% (95% CI: 0.968–0.990, P < 0.01, I2 = 66.5%), represented by the forest plot in Fig. 2a. Publication bias is represented by the Freeman-Tukey double arcsine transformed proportion in Fig. 2b. Influence of the studies on success rate was performed using a Baujat plot, represented in Fig. 2c. Studies by Saritas, Kenny, and Du contributed a higher heterogeneity than the other nine studies.
Fig. 2.
a. Forrest plot of studies on ASO success rate using random effects modeling. Black boxes represent the individual studies contribution and black lines represent their 95% confidence interval. The black diamond represents the summary effect. b. Funnel plot assessing the bias of studies on success rate. c. Baujat plot assessing the influence of studies on success rate.
3.4. Overall adverse events
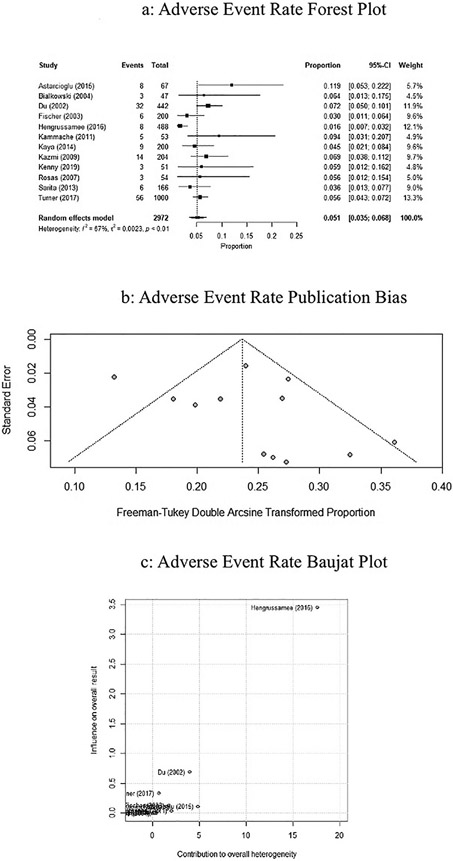
The overall adverse event rate associated with implantation was 5.1% (95% CI: 0.035–0.068, P < 0.01, I2 = 67.3%), and is represented by the forest plot in Fig. 3a. Publication bias is represented by the Freeman-Tukey double arcsine transformed proportion in Fig. 3b. Influence of the studies on success rate was performed using a Baujat plot, represented in Fig. 3c. The Hengrussamee study contributed a higher heterogeneity compared to other studies.
Fig. 3.
a. Forrest plot of studies on ASO adverse event rate using random effects modeling. Black boxes represent the individual studies contribution and black lines represent their 95% confidence interval. The black diamond represents the summary effect. b. Funnel plot assessing the bias of studies on adverse events rate. c. Baujat plot assessing the influence of studies on adverse event rate.
3.5. Adverse events: Arrhythmia
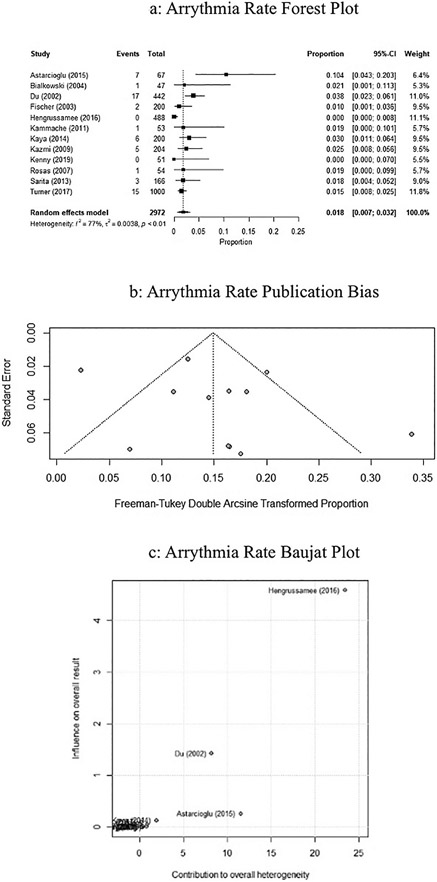
The adverse event rate of arrhythmias associated with implantation was 1.8% (95% CI: 0.007–0.032, P <0.01, I2 = 77.1%), and is represented by the forest plot in Fig. 4a. Publication bias is represented by the Freeman-Tukey double arcsine transformed proportion in Fig. 4b. Influence of the studies on success rate was performed using a Baujat plot, represented in Fig. 4c. The Du, Astarcioglu, Henggrusamee studies contributed a higher heterogeneity compared to other studies.
Fig. 4.
a. Forrest plot of studies on ASO rate of arrhythmias using random effects modeling. Black boxes represent the individual studies contribution and black lines represent their 95% confidence interval. The black diamond represents the summary effect. b. Funnel plot assessing the bias of studies on rates of arrhythmias. c. Baujat plot assessing the influence of studies on the rate of arrhythmias.
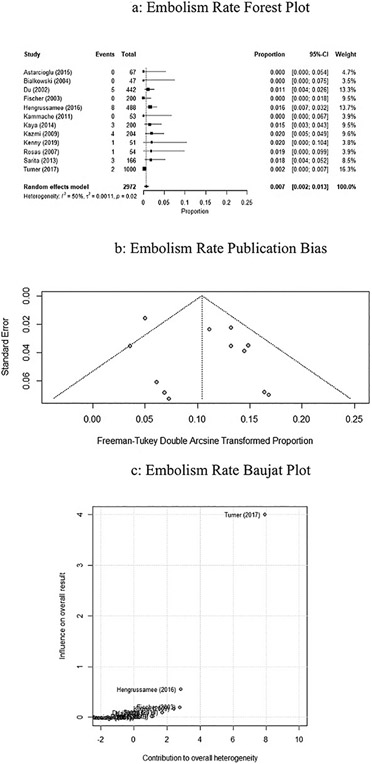
3.6. Adverse events: Embolism
The adverse event rate of arrhythmias associated with device embolism was 0.7% (95% CI: 0.002–0.013, P < 0.01, I2 = 50.1%), and is represented by the forest plot in Fig. 5a. Publication bias is represented by the Freeman-Tukey double arcsine transformed proportion in Fig. 5b. Influence of the studies on success rate was performed using a Baujat plot, represented in Fig. 5c. The Turner study contributed a higher heterogeneity to the analysis compared to other studies.
Fig. 5.
a. Forrest plot of studies on ASO rate of embolisms using random effects modeling. Black boxes represent the individual studies contribution and black lines represent their 95% confidence interval. The black diamond represents the summary effect. b. Funnel plot assessing the bias of studies on the rate of embolisms. c. Baujat plot assessing the influence of studies on the rate of embolisms.
4. Validation of meta-analysis results
4.1. Sensitivity analysis
We conducted a sensitivity analysis to ascertain if any study had a dominant effect on the meta-analysis. For each primary and secondary outcome, we excluded one study at a time to determine its effect on the main summary estimate. Using this analysis, we concluded there were no studies that unduly manipulated the outcomes or heterogeneity.
4.2. Heterogeneity
Q statistics, I2, and Tau2 were calculated independently for the primary and secondary outcomes' heterogeneity. Moderate heterogenicity was found in technical success rate, overall adverse events, and device embolism; substantial heterogenicity was found in the analysis of arrhythmias.
5. Discussion
The Amplatzer ASD is an innovative transcatheter solution for the occlusion of defects in the atrial septal wall. Our meta-analysis assesses the technical success and safety in closing secundum type atrial septal defects. This study included 12 prospectively performed studies, including 2972 patients, which demonstrated a 98% technical success rate and a 5.1% cumulative adverse event rate.
Overall pooled technical success rate, defined as the percentage of implantations completed divided by the implantations attempted, was 98% (95% CI: 0.968–0.990, P < 0.01). Adverse event rates of arrhythmias and embolism were 1.8% (95% CI: 0.007–0.032, P < 0.01) and 0.7% (95% CI: 0.002–0.013, P < 0.01), respectively. Overall adverse events rates were 5.1% (95% CI: 0.035–0.068, P < 0.01). The overall adverse events included any reported event within two years of implantation, which included allergic reactions, new-onset aortic regurgitation, arrhythmia, bleeding, death, effusion, embolism, headache, insertion site hematoma, new-onset mitral regurgitation, pulmonary vein orifice obstruction, retroperitoneal hematoma, thrombus, transient ischemic attack and stroke, and urinary tract disturbance. Individual rates were not calculated for adverse events represented in three studies or fewer studies. One death was noted 1.5 years after the implantation, associated with device embolization and sudden cardiac death [27].
The use of transcatheter devices for secundum type atrial septal occlusion provides secondary and tertiary prevention of potential adverse events, including paradoxical embolism, pulmonary hypertension, arrhythmias, and right heart failure. While previous studies have shown superiority to open heart approaches, the overall success and safety profile has not been significantly examined prospectively for secundum type ASDs [32]. This is of particular interest for practitioners who may contemplate preventative implantation of an atrial septal occluder in patients who are or may become high risk for untreated sequela. Nevertheless, further investigation would be warranted to understand the consequences better.
Percutaneous insertion methods also require further investigation for their influence on adverse events. Typical procedural methods include the use of fluoroscopy with periprocedural TTE, TEE, or ICE. These additional echocardiographic techniques require longer procedure times, additional cost, and may increase complication risks due to additional anesthetic and access requirements. Patent foramen ovale (PFO) closures without periprocedural TEE or ICE have been successful and are supported in the literature [33-35]. Similar techniques have been supported in the closure of ASDs, albeit being limited in power [36]. Future trials may provide insight into this method's feasibility and safety, particularly when limited resources are available.
The strength of this review is as follows: a multi-database systemic literature search using explicitly defined inclusion criteria, redundant review by multiple authors, the inclusion of only prospective studies, detailed extraction of data on technical success and adverse events, arduous evaluation of outcomes, and multiple statistical evaluative methods to establish the validity and negate confounding biases. Furthermore, it was established that the results were mostly of moderate heterogeneity, balanced in potential bias, and not unduly influenced by any single study.
There are limitations to our study, as well. Included studies were not individually representative of the general population, varied in clinical settings, and were conducted primarily outside of the United States of America. Additionally, there was significant heterogeneity in the reporting endpoints for most of the studies, with some studies did not stratifying peri-implantation events from long-term follow-up events. Patient characteristics were unable to be explored due to the lack of individual patient data. These variables would be advantageous in exploring how risk factors influence the adverse rates and how the defect's size and contour influence the success rate. Finally, post-implantation follow-up reporting was missing in most of the studies. Notwithstanding these limitations, our study provides the best assessment of technical success and adverse event rates in treating secundum type atrial septal defects.
6. Conclusion
This systematic review and meta-analysis demonstrates the efficacy and safety of the Amplatzer atrial septal occluder in treating secundum type atrial septal defects. A high likelihood of technical success can be expected when implanting an Amplatzer atrial septal occluder for secundum type ASDs. Adverse events are expected for one in twenty patients over a two-year period, which supports the safe use of the ASO in secundum type ASDs closures.
Abbreviations:
- ASD
atrial septal defect
- ASO
atrial septal occluder
- FDA
Food and Drug Administration
- ICD
intracardiac echocardiography
- PFO
patent foramen ovale
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- SD
standard deviation
- TTE
transthoracic echocardiography
- TEE
transesophageal echocardiography
- CI
confidence interval
Footnotes
CRediT authorship contribution statement
Concept and design: Heaton, Okoh
Acquisition, analysis, or interpretation of data: Heaton, Suh, Ozturk
Statistical analysis: Ozturk
Drafting of the manuscript: Heaton, Okoh, Suh
Critical revision of the manuscript for important intellectual content: Heaton, Okoh
Administrative, technical, or material support: Okoh, Salemi, Waxman, Tayal
Supervision: Salemi, Waxman, Tayal
Declaration of competing interest
The authors report no conflicts of interest regarding the content herein.
References
- [1].Campbell M. Natural history of atrial septal defect. Br Heart J. 1970;32(6):820–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Craig Robert J, Selzer Arthur. Natural history and prognosis of atrial septal defect. Circulation. 1968;37(5):805–15. 10.1161/01.CIR.37.5.805. [DOI] [PubMed] [Google Scholar]
- [3].Mills NL, King TD. Nonoperative closure of left-to-right shunts. J Thorac Cardiovasc Surg. 1976;72(3):371–8. [PubMed] [Google Scholar]
- [4].King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defect: nonoperative closure during cardiac catheterization. JAMA. 1976;235(23):2506–9. 10.1001/jama.1976.03260490024013. [DOI] [PubMed] [Google Scholar]
- [5].Kaya MG, Akpek M, Celebi A, Saritas T, Meric M, Soylu K, et al. A multicentre, comparative study of Cera septal occluder versus AMPLATZER septal occluder in transcatheter closure of secundum atrial septal defects. EuroIntervention. 2014;10(5):626–31. 10.4244/EIJY14M07_04. [DOI] [PubMed] [Google Scholar]
- [6].Calvert PA, Klein AA. Anaesthesia for percutaneous closure of atrial septal defects. Contin Educ Anaesth Crit Care Pain 2008;8(1):16–20. doi:10/bhdxps. [Google Scholar]
- [7].Lipiec P, Miśkowiec D, Peruga JZ, Plewka M, Szymczyk E, Wejner-Mik P, Kupczyńska K, Kasprzak JD. Conscious sedation for transcatheter implantation of atrial septal occluders with two- and three-dimensional transoesophageal echocardiography guidance - a feasibility and safety study. Kardiol Pol 2018;76(2):406–412. doi:10/gjzzp9. [DOI] [PubMed] [Google Scholar]
- [8].Agarwal S, Bajaj NS, Kumbhani DJ, Tuzcu EM, Kapadia SR. Meta-analysis of transcatheter closure versus medical therapy for patent foramen ovale in prevention of recurrent neurological events after presumed paradoxical embolism. J Am Coll Cardiol Intv. 2012;5(7):777–89. 10.1016/j.jcin.2012.02.021. [DOI] [PubMed] [Google Scholar]
- [9].Webb John G, Lukas Altwegg, Boone Robert H, Anson Cheung, Jian Ye, Samuel Lichtenstein, et al. Transcatheter aortic valve implantation. Circulation. 2009;119(23):3009–16. 10.1161/CIRCULATIONAHA.108.837807. [DOI] [PubMed] [Google Scholar]
- [10].Thanopoulos B Vasilios D, Laskari CV, Tsaousis GS, Zarayelyan A, Vekiou A, Papadopoulos GS. Closure of atrial septal defects with the Amplatzer occlusion device: preliminary results. J Am Coll Cardiol. 1998;31(5):1110–6. 10.1016/S0735-1097(98)00039-4. [DOI] [PubMed] [Google Scholar]
- [11].Lock JE, Rome JJ, Davis R, Van Praagh S, Perry SB, Van Praagh R, et al. Transcatheter closure of atrial septal defects. Exp Stud Circ. 1989;79(5):1091–9. 10.1161/01.CIR.79.5.1091. [DOI] [PubMed] [Google Scholar]
- [12].Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kanwal F, White D. “Systematic reviews and meta-analyses” in clinical gastroenterology and hepatology. Clin Gastroenterol Hepatol. 2012;10(11):1184–6. 10.1016/j.cgh.2012.09.019. [DOI] [PubMed] [Google Scholar]
- [14].Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol. 2011;64(12):1294–302. 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- [15].Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- [16].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- [17].Jackson D, Bowden J. Confidence intervals for the between-study variance in random-effects meta-analysis using generalised heterogeneity statistics: should we use unequal tails? BMC Med Res Methodol. 2016;16(1):118. 10.1186/s12874-016-0219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lin L, Xu C. Arcsine-based transformations for meta-analysis of proportions: pros, cons, and alternatives. Health Science Reports. 2020;3(3):e178. 10.1002/hsr2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337(8746):867–72. 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- [20].Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60. 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Astarcioglu MA, Kalcik M, Sen T, Aykan AC, Gokdeniz T, Gursoy OM, et al. Ceraflex versus Amplatzer occluder for secundum atrial septal defect closure. Herz. 2015; 40(2):146–50. 10.1007/s00059-014-4192-0. [DOI] [PubMed] [Google Scholar]
- [22].Bialkowski J, Karwot B, Szkutnik M, Banaszak P, Kusa J, Skalski J. Closure of atrial septal defects in children: surgery versus Amplatzer device implantation. Tex Heart Inst J. 2004;31(3):220–3. [PMC free article] [PubMed] [Google Scholar]
- [23].Du Z-D, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002;39(11):1836–44. 10.1016/S0735-1097(02)018624. [DOI] [PubMed] [Google Scholar]
- [24].Fischer G, Stieh J, Uebing A, Hoffmann U, Morf G, Kramer HH. Experience with transcatheter closure of secundum atrial septal defects using the Amplatzer septal occluder: a single centre study in 236 consecutive patients. Heart. 2003;89(2):199–204. 10.1136/heart.89.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hengrussamee Kriengkrai, Kanaderm Chanikan. TCT-614 Transcatheter closure of secundum-type atrial septal defect with no aortic rim using Amplatzer septal occlude devices and no balloon sizing. J Am Coll Cardiol. 2016;68(18_Supplement): B250–B250. 10.1016/j.jacc.2016.09.754. [DOI] [Google Scholar]
- [26].Kammache I, Mancini J, Ovaert C, Habib G, Fraisse A. Feasibility of transcatheter closure in unselected patients with secundum atrial septal defect, using Amplatzer devices and a modified sizing balloon technique. Catheter Cardiovasc Interv. 2011;78(5):665–74. 10.1002/ccd.23077. [DOI] [PubMed] [Google Scholar]
- [27].Kazmi T, Sadiq M, null Asif-ur-Rehman, Hyder N, Latif F. Intermediate and long-term outcome of patients after device closure of ASD with special reference to complications. J Ayub Med Coll Abbottabad. 2009;21(3):117–21. [PubMed] [Google Scholar]
- [28].Kenny D, Eicken A, Dähnert I, Boudjemline Y, Sievert H, Schneider MB, et al. A randomized, controlled, multi-center trial of the efficacy and safety of the Occlutech figulla flex-II occluder compared to the Amplatzer septal occluder for transcatheter closure of secundum atrial septal defects. Catheter Cardiovasc Interv. 2019;93(2):316–21. 10.1002/ccd.27899. [DOI] [PubMed] [Google Scholar]
- [29].Rosas M, Zabal C, Garcia-Montes J, Buendia A, Webb G, Attie F. Transcatheter versus surgical closure of secundum atrial septal defect in adults: impact of age at intervention. A concurrent matched comparative study. Congenit Heart Dis. 2007;2(3):148–55. 10.1111/j.1747-0803.2007.00091.x. [DOI] [PubMed] [Google Scholar]
- [30].Saritas T, Kaya MG, Lam YY, Erdem A, Akdeniz C, Demir F, et al. A comparative study of Cardi-O-fix septal occluder versus Amplatzer septal occluder in percutaneous closure of secundum atrial septal defects. Catheter Cardiovasc Interv. 2013;82(1):116–21. 10.1002/ccd.23301. [DOI] [PubMed] [Google Scholar]
- [31].Turner DR, Owada CY, Sang CJ, Khan M, Lim DS. Closure of secundum atrial septal defects with the AMPLATZER septal occluder. Circ Cardiovasc Interv. 2017;10(8):e004212. 10.1161/CIRCINTERVENTIONS.116.004212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Atashband A, Lakkis N. First comprehensive analysis of outcomes in adult patients after percutaneous closure of isolated secundum atrial septal defects. Cardiovasc Hematol Agents Med Chem. 2015;13(1):63–9. 10.2174/187152571301150730115936. [DOI] [PubMed] [Google Scholar]
- [33].Fateh-Moghadam S, Steeg M, Dietz R, Bocksch W. Is routine ultrasound guidance really necessary for closure of patent foramen ovale using the Amplatzer PFO occluder? Catheter Cardiovasc Interv. 2009;73(3):361–366. doi:10/fqs986. [DOI] [PubMed] [Google Scholar]
- [34].Hildick-Smith D, Behan M, Haworth P, Rana B, Thomas M. Patent foramen ovale closure without echocardiographic control: use of “standby” intracardiac ultrasound. JACC Cardiovasc Interv 2008;1(4):387–391. doi:10/bgx3mn. [DOI] [PubMed] [Google Scholar]
- [35].Wahl A, Tai T, Praz F, Schwerzmann M, Seiler C, Nedeltchev K, Windecker S, Mattle HP, Meier B. Late results after percutaneous closure of patent foramen ovale for secondary prevention of paradoxical embolism using the amplatzer PFO occluder without intraprocedural echocardiography: effect of device size. JACC Cardiovasc Interv 2009;2(2):116–123. doi:10/fbw4nh. [DOI] [PubMed] [Google Scholar]
- [36].Praz F, Wahl A, Schmutz M, Pfammatter J-P, Pavlovic M, Perruchoud S, et al. Safety, feasibility, and long-term results of percutaneous closure of atrial septal defects using the Amplatzer septal occluder without periprocedural echocardiography. J Invasive Cardiol. 2015;27(3):157–62. [PubMed] [Google Scholar]