Abstract
The effects of acute stress on memory encoding are complex, and we do not yet know all of the conditions that can determine whether stress at encoding improves or impairs memory. Recent work has found that changing contexts between encoding and stress can abolish the effects of post-encoding stress on memory, suggesting that context may play an important role in the effects of stress on memory. However, the role of context in the effects of stress on memory encoding is not yet known. We addressed this gap by examining the effects of context on the influence of acute stress on memory encoding. In a 2×2 experimental design, participants (N=103) completed either a stressor (i.e., Socially Evaluated Cold Presser Test) or control task (i.e., warm water control) before completing a memory encoding task, which occurred in either in the same room as or a different room from the stressor or control task. Memory retrieval was tested for each participant within the context that they completed the encoding task. We found that, relative to nonstressed (i.e., control) participants, stressed participants who switched contexts prior to encoding showed better memory for both negative and neutral images. In contrast, when the stressor or control task occurred in the same room as memory encoding, stress had no beneficial effect on memory. These results highlight the importance of the ongoing context as a determinant of the effects of stress on memory encoding and present a challenge to current theoretical accounts of stress and memory.
Keywords: stress, episodic memory, context, memory encoding, acute stress, recognition memory
1. Introduction
Acute stress can impact episodic memory (i.e., memory for specific events one has personally experienced, such as the last thing you said to another person) in a variety of ways (McCullough et al., 2015; Ritchey et al., 2017; Shields et al., 2017; Wolf et al., 2016). For example, stress induced either by social manipulations, such as having to give a public presentation, or physical manipulations, such as submersing an arm in ice water, disrupts the ability to retrieve episodic memories (Roozendaal, 2002). In contrast, when stress occurs shortly after learning (i.e., post-encoding stress), it generally leads to an increase in subsequent memory for the previously learned material (Cahill et al., 2003; McCullough & Yonelinas, 2013; Smeets et al., 2008)—particularly when both the encoding event and post-encoding stress occur within the same context (e.g., the same room) (Sazma et al., 2019; Shields et al., 2017).
In contrast to the above, the effects of stress on memory encoding (i.e., when stress occurs prior to or during, and thus influences, the initial exposure to information later remembered) are less clear (compare, for example, Goldfarb et al., 2019; Quaedflieg et al., 2013; Wiemers et al., 2019; Zoladz et al., 2011, 2013). For example, some studies have found that stress prior to or during encoding improves memory (e.g., Maheu et al., 2005; Payne et al., 2007), whereas other studies have found that stress prior to or during encoding has no effect or impairs memory (e.g., Payne et al., 2007; Smeets et al., 2007).
Although there are a number of factors that could contribute to opposing effects of stress on memory encoding (e.g., Shields et al., 2017, 2022), one factor that has not been experimentally examined is whether the context of the stressor relative to encoding plays a critical role. Although post-encoding stress benefits memory only when the stressor occurs in the same context as the encoding task (Sazma et al., 2019; Shields et al., 2017), and changing contexts between encoding and retrieval can modulate the effects of stress on memory encoding (Schwabe et al., 2009; Wiemers et al., 2014; see also Pützer & Wolf, 2021), as far as we are aware, no published study has experimentally tested the effect of changing the stressor context on memory encoding, holding the context constant between encoding and retrieval. That is, as with post-encoding stress, pre-encoding stress may only benefit memory for the items that are encoded in the same but not different context as the stressor.
Determining the role of context in the effects of stress on memory encoding could have important implications for current theories of stress and memory. For example, beneficial effects of post-encoding stress have been taken to suggest that stress facilitates the consolidation of recently encoded events (e.g., Cahill & McGaugh, 1996; McGaugh, 2000). That is, the release of stress hormones is thought to facilitate the stabilization of recently encoded memories after their initial encoding. The finding that post-encoding stress preferentially benefits memory only for study events that share the same context as the stressor is not predicted by these accounts, and suggests that post-encoding stress affects memory via some context-dependent mechanism (Sazma et al., 2019; Shields et al., 2017). For example, it has been proposed that because the stressor is quite memorable, it increases memory for any events that share the same episodic context, and thus increases memory for the events that occurred just prior to the stressor. However, no experimental work to date has examined whether such a context-specific mechanism plays a role in determining the effects of stress on memory encoding. It is possible that, as with post-encoding stress effects, the stressor may be well remembered which will lead to an increase in memory for subsequent events that occur in the same context as the stressor but not for items that occur in a different context. Alternatively, stress may facilitate the consolidation of memories that are encoded for a short period after the stressor, regardless of the episodic context.
The current study was designed to determine the effects of pre-encoding stress on recognition memory for negative and neutral images when the stressor (or control task) and study events (i.e., the encoding task) occurred in either the same or differing contexts. This experimental design allowed us to determine whether context played a moderating role in the effects of pre-encoding stress on memory. Based on studies of post-encoding stress (Sazma et al., 2019; Shields et al., 2017; see also Trammel & Clore, 2013), we expected that pre-encoding stress would benefit memory only when the stressor context and the encoding context were the same, and that no stress benefits would be observed when the contexts changed. Although stress generally has similar effects on negative and neutral materials (Shields et al., 2017), some studies have suggested that stress effects may be larger for negative materials (e.g., Cahill et al., 2003). For this reason, we included both negative and neutral materials to assess whether any potential effects of context differed by the material’s valence. We expected overall memory to be better for negative than neutral items (Kensinger, 2009), but did not have any strong a priori predictions about how context would interact with stress and emotion.
We collected saliva to assay cortisol in order to verify the stress manipulation. Saliva was collected before the manipulation, 15 minutes after the manipulation, and immediately prior to the memory test during the second session. Cortisol is a stress-responsive hormone that influences memory (Roozendaal, 2002). To account for cortisol’s diurnal rhythm (Baum & Grunberg, 1995; Ryan et al., 2016), all participants were tested in the afternoon to evening.
Finally, we assessed memory using a recognition test in which participants were instructed to respond “recollect” if they were able to vividly remember qualitative details surrounding the item seen during the study phase. If the participant was unable to recollect the item, they were instructed to respond with their own memory confidence ranging on a 1–5 scale from low to high confidence. This assessment is designed to provide an unbiased measure of memory sensitivity through the analysis receiver operating characteristics (Macmillan & Creelman, 2004) and to determine the degree to which recognition relies on recollection or familiarity-based responses (Yonelinas, 2002). Although acute stress can impact both recollection and familiarity (e.g., McCullough, et al., 2015), how changes in context impact the effects of encoding stress on recollection and familiarity is unknown.
2. Method
2.1. Participants
107 (66.0% female) undergraduates (Mage = 19.78) from UC Davis participated in this study for course credit as compensation for their time. We chose this sample size to mimic our recent study that manipulated both stress and context during the post-encoding period (Sazma et al., 2019). We excluded individuals who were left-handed, smoked, or took antidepressant medication, anti-anxiety medication, or hormonal contraceptives (Sazma et al., 2019; Shields, 2020). Additionally, one day prior to participating, we emailed each participant instructions to avoid eating one hour before the study and to additionally avoid caffeine, alcohol, and cardiovascular exercise at least four hours before participating. Participants were randomly assigned to stress condition (Stress, Control) and context condition (Same, Different) in a 2×2 design. Four participants were removed from memory analyses due to memory performance at or below chance. All procedures were approved by the University of California Davis Institutional Review Board.
2.2. Stimuli
We used a set of 248 pictures (largely drawn from the International Affective Photo Series, or, IAPS; Lang et al., 1997) that we have used previously (McCullough & Yonelinas, 2013; Sharot et al., 2004; Sharot & Yonelinas, 2008; Yonelinas et al., 2011) in this study’s memory encoding and retrieval tasks. Half of these pictures were negative in emotional valence, and the other half were neutral. Prior work (Sharot et al., 2007) has shown expected differences in emotional valence between neutral and negative images, t(10) = 14.23, p < .0001, and that the neutral images are less arousing than the negative images, t(11) = 10.67, p < .0001. Eight of the 248 images were used for encoding or recognition instructions or practice. During the encoding session, participants viewed 120 of the images (60 negative). During the recognition task, participants then saw the 120 previously encoded images as well as 120 new images during recognition memory testing. During both encoding and recognition, images were presented in a randomized order and were counterbalanced across participants using two lists (i.e., participants saw one of two lists at encoding but both lists at retrieval, with the unseen list at retrieval as lures, we have done previously; see Sazma et al., 2019; Sazma, 2023; Sharot & Yonelinas, 2008; Shields et al., 2019; Wiemers et al., 2019).
2.3. Materials and Procedure
All participants were tested separately between 12 and 7 pm. Upon arrival, participants filled out an informed consent form, a demographics and health behavior form, and a brief anxiety and current stress questionnaire. Hours of sleep and physical activity within the past 24 hours were assessed via the health behavior form. Although participants were reminded of all instructions, including those for caffeine and alcohol, 24 hours prior to their study timeslot, compliance with the caffeine and alcohol instructions was not verified. Once the forms were completed, participants were given 10 minutes to relax and acclimate to the environment. Participants then provided a baseline (i.e., pre-manipulation) saliva sample, and subsequently completed either the Socially Evaluated Cold Presser Task (Schwabe et al., 2008; SECPT) or a control equivalent. For the SECPT, participants were instructed to hold their nondominant hand in a bucket of ice water (0–3 °C) for as long as they were able to, up to 3 minutes. They were also told that their facial expressions were being recorded by a web camera attached to the computer they were facing, and that they should attempt to maintain a neutral expression. In the control task, participants were asked to hold their nondominant hand in a bucket of room-temperature water (19–22 °C) for up to 3 minutes, but participants were not instructed to maintain a neutral expression, nor was there a web camera attached to the computer. After the SECPT or control task, participants were given a towel and one minute to dry off their arm. Participants then rated the stressfulness of that task (i.e., the SECPT or control task) on a scale that ranged from 1 (low stress) to 10 (high stress).
Following the stress or control manipulation, participants then underwent a 10-minute transition period where they either remained in the same room (i.e., same context condition) or changed contexts (i.e., different context condition). The transition period was 10 minutes because it took approximately that long to change buildings, rooms, and overall context, and we note that 10 minutes is within the predicted boundary of 17 minutes necessary for observing improved memory performance as a function of pre-encoding stress (Shields et al., 2017). Context was manipulated at multiple levels (e.g., spatial, social), as participants who changed contexts were informed that the experiment had come to an end and that they would begin a new experiment in a new location. These participants were then led to an adjacent building where they were introduced to a new research assistant to begin the encoding task. Starting rooms were counterbalanced and were visually distinct: one room was small and square with a single computer and desk with white walls, while the other room—in a different building—was rectangular with 5 computers lining the back wall and had off-yellow walls. The participants who were in the same context condition remained in the same room for the entire duration of the study and waited in their seats for 10 minutes to equate timing with the context change condition. At the end of the transition period, participants provided a second (i.e., post-manipulation) saliva sample and then began the encoding task.
Participants incidentally encoded 120 pictures (60 negative, 60 neutral) by rating them each on their visual complexity (defined as how “busy” an image appeared to be) on 1–6 scale from low- to high-complexity. We have used this set of pictures and protocol in prior work (e.g., Sazma et al., 2019; Sharot & Yonelinas, 2008; Shields et al., 2019; Wiemers et al., 2019). Requiring visual complexity judgments functions to maintain participants’ attention while they view the images; we did not analyze participants’ visual complexity ratings. Pictures were presented for 800ms each, which was followed by visual complexity rating scale which remained on the screen for a 2000ms. Participants were then shown a fixation cross for 500ms before viewing the next picture. These procedures have been used previously and were selected to ensure memory performance was not affected by floor or ceiling effects (McCullough et al., 2015). Participants were then given an unrelated diet questionnaire that took 20 minutes.
48 hours after encoding, participants returned to the room where they performed the encoding task for free recall and recognition memory tests. They were given a 5-minute acclimation period to readjust to the room before they provided a third saliva sample (i.e., session two baseline) to both ensure that there were no residual condition effects on stress hormone levels and establish a baseline for the memory tasks. Free recall was assessed by having participants write down the details of any of the pictures they could remember. In line with previous studies, the number of recalled items was quite low, and so was not analyzed (Sazma et al., 2019). After the free recall test, participants performed a recognition memory test where they would view a mixture of all 120 pictures they saw during incidental encoding as well as 120 new pictures they had not seen before for a total of 240 pictures. The new pictures were similar to the encoding pictures in that they were also comprised of 60 negative and 60 neutral images that were counterbalanced across participants as either the encoding or new retrieval images (as described above). Participants were instructed to view each picture and judge how strongly they remembered it. Participants rated their memory strength on a 1–5+R (i.e., recollect) scale, where a rating of 1 was low/no memory for the presented item and 5 was strong memory for an item. A rating of “recollect” was given if the participant was able to recollect additional encoding details surrounding the image (e.g., “I remember looking to check what time it was when I saw this image.”). If a participant was unable to recollect details of the encoding event, they were asked to use the 1–5 scale. This distinction of “recollect” and 1–5 was made clear to the participant and required a comprehension check before beginning the encoding task. The recognition memory test was self-paced, so participants had as long as they needed to make a judgment, however the image would only appear on the screen for the first 1500 ms of a trial while the rating scale remained on the screen until a judgment was made. A fixation cross was presented for 500ms between each rating.
2.4. Data Analysis
Recognition memory performance was measured by calculating the area under curve (AUC) of the Receiver Operating Characteristics (ROC; Macmillan & Creelman, 2004) for each participant, and separately for negative and neutral items. AUC provides an estimate of an individual’s ability to separate old from new items while also accounting for response bias; it is calculated via the trapezoidal rule for the area under the curve (Pollack & Norman, 1964). In particular, where hits and false alarms are cumulative proportions at each criterion (organized from R to 1; n = number of criteria, which is 6 in this study):
Additionally, we estimated recollection and familiarity processes within recognition memory. Recollection describes a hippocampus-dependent threshold process within recognition memory wherein recognition is accompanied by contextually bound information; familiarity describes a continuous signal-detection process supporting a general “sense of knowing,” which is thought to be supported by the perirhinal cortex (e.g., Yonelinas, 2002; Yonelinas & Parks, 2007; Eichenbaum et al., 2007). Formally, these processes are estimated via hit and false alarm cumulative proportions (organized as above) using the following equations at each response criterion , where is the cumulative normal distribution function:
Discrepancies (i.e., squared errors) between model-predicted hits and false alarms and observed hits and false alarms at each criterion were summed and then minimized via the Nelder-Mead simplex algorithm to provide recollection and familiarity estimates for each participant. Mathematically, recollection is the Y-axis value when the fitted ROC curve has the value of X=0, and familiarity describes the distance between nonrecollected memory distributions for new and old items—familiarity is thus equivalent to d’ within the fitted model. For additional details, see Yonelinas (1994).
The effects of the manipulations on memory outcomes and cortisol were examined via fully factorial restricted error maximum likelihood mixed-model ANOVAs. The effects of the manipulations on cortisol were examined with Stress (i.e., stress, no stress) and Context (i.e., same context between stress and encoding, different context between stress and encoding) as between-subjects factors, with Time (i.e., pre-manipulations, post-manipulations, pre-retrieval) as a within-subjects factor, nesting observations within participants (i.e., random intercept by participant). The effects of the manipulations on memory AUC were examined with Stress (i.e., stress, no stress) and Context (i.e., same context between stress and encoding, different context between stress and encoding) as between-subjects factors, with Image Valence (i.e., negative, neutral) as a within-subjects factor, nesting observations within participants. The effects of the manipulations on recollection and familiarity were examined in nearly the same model as AUC, with the exception that Recognition Parameter (i.e., recollection, familiarity) was included as an additional within-subjects factor. Covarying pre-retrieval cortisol did not influence any of the memory results.
All analyses were conducted in R version 4.3.1, and linear mixed effect models were fit using the lmerTest package (Bates et al., 2015; Kuznetsova et al., 2017). Estimated marginal means and standard errors were derived via the emmeans package (Lenth, 2020).
Results
Manipulation Checks
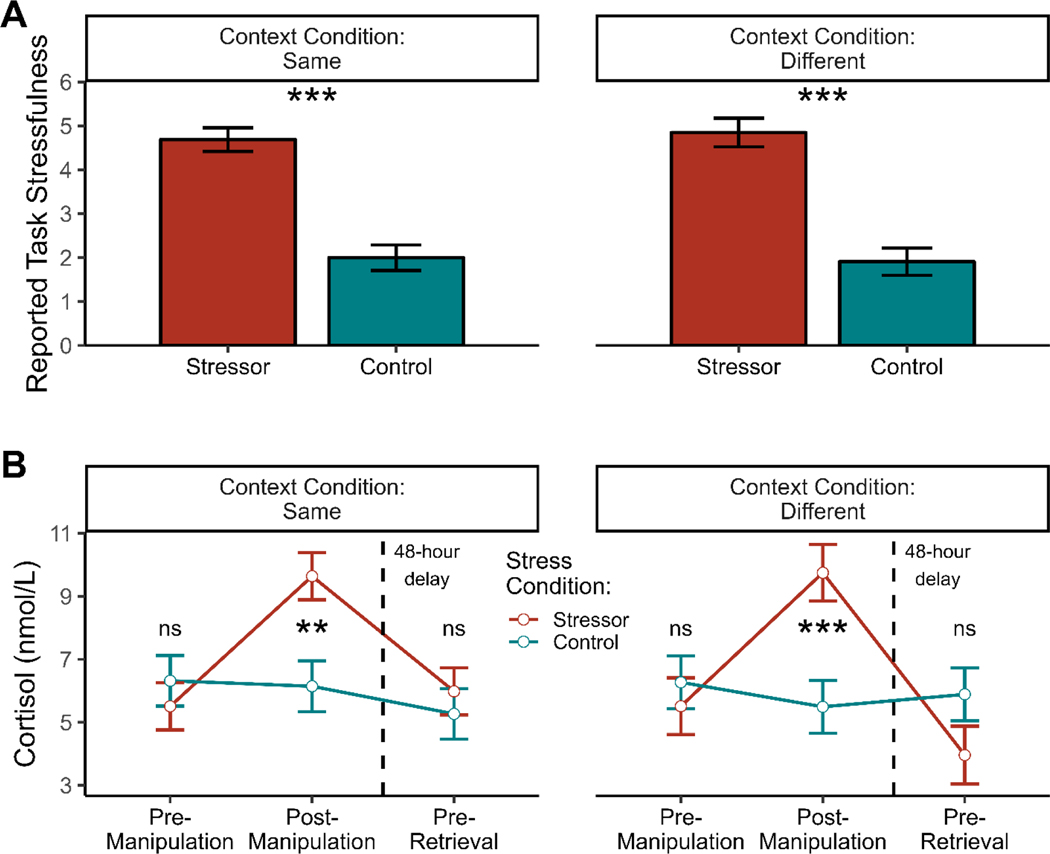
We first examined whether our stress manipulation successfully induced stress. As expected, we found that participants’ reports of the stressfulness of the task they had just completed (i.e., the SECPT or control task, depending upon randomly assigned condition) was higher in the stress condition (M = 4.76, SE = 0.20), than in the control condition (M = 1.96, SE = 0.20), t(94) = 9.51, p < .001, and this stress effect was not moderated by context change condition, p = .676 (Figure 1a).
Figure 1.
Manipulation checks. Participants in the stress condition reported more task stressfulness from the SECPT than participants in the control condition reported from their task, and participants in the stress condition showed greater cortisol responses as well. Context did not interact with either of these effects; the stress and control conditions did not differ in preretrieval cortisol in either the same-context condition (p = .123) or the different-context condition (p = .514). Controlling for pre-retrieval cortisol numerically strengthened the memory results, but it did not alter any inferences. Depicted means and standard errors are estimated marginal means and standard errors.
Similarly, we observed a significant Stress × Time interaction in changes in cortisol, F(2, 185.2) = 15.82, p < .001 (see Figure 1b). Probing this, we found that prior to the manipulation (i.e., at baseline), participants in the stress and control conditions did not differ in cortisol (M = 5.51 nmol/L and M = 6.29 nmol/L, respectively), p = .232. In contrast, after stress exposure, the stress group exhibited significantly higher salivary cortisol levels than controls (M = 9.69 nmol/L and M = 5.82 nmol/L, respectively), p < .001. Pre-retrieval, the stress and control conditions again no longer differed in cortisol (M = 4.97 nmol/L and M = 5.58 nmol/L, respectively), p = .467. Importantly, there was no three-way Stress × Time × Context three-way interaction (i.e., changing context after the stress manipulation did not influence the effect of the stress manipulation on cortisol), p = .166, illustrating that stress led to comparable increases in cortisol in both context conditions.
Stress and Memory
The average recognition memory ROCs for the stress and non-stress control groups are presented in Figure 2. As illustrated in Figure 2, stress improved memory for both negative and neutral items when stress occurred in a different context from memory encoding, but stress did not improve memory when it occurred in the same context as memory encoding. In particular, with memory area under the curve as the outcome, we observed a significant Stress × Context interaction, F(1, 99.0) = 4.69, p = .033, indicating that the effects of stress on memory were dependent on whether the stressor or control task occurred in the same context as or a different context from the memory encoding task. Probing this interaction, we found that stress improved recognition when the stressor/control task and the encoding task occurred in different contexts, t(99.0) = −2.18, p = .032, whereas no significant stress effect was observed when the stressor or control task and the encoding task occurred in the same context, t(99.0) = 0.79, p = .429. Although image valence did not moderate this stress by context interaction effect, p = .925, there was a main effect of image valence on recognition memory performance, F(1, 99.0) = 14.36, p <. 001, indicating that recognition was greater for negative than the neutral items.
Figure 2.
Recognition memory ROCs for the stress and non-stress control groups, for neutral (left panels) and negative items (right panels), when the stressor occurred in the same context as the encoding task (top panels) or in a different context (bottom panels). Stress improved memory only when it occurred in a different context than the encoding task.
Further exploration of this memory effect examined whether the observed change in memory was differentially related to either recollection or familiarity. In this model, we observed a Stress × Context × Recognition Parameter interaction, F(1, 297.0) = 4.64, p = .032. Probing this, we found that stress improved familiarity relative to the control task within the different-context condition, t(195.0) = 2.98, p = .003, whereas stress did not influence familiarity in the same-context condition, t(195.0) = 0.32, p = .748, nor did stress influence recollection in either context condition, ps > .839. Thus, the observed effects of stress on recognition seem to be preferentially driven by effects on familiarity. These specific effects on recollection versus familiarity are depicted within observed data in Figure 2, depicted within estimate predictions in Figure 3, and provided in Table 1.
Figure 3.
Parameter-estimated ROCs by condition. Stress improved familiarity (represented in the overall “bend” of the curve in A) only when it occurred in a different context than the encoding task. The interactive effect of stress and context on recollection (the Y-intercept location in the zoomed-in graph, panel B) was less pronounced, perhaps because stress numerically enhanced recollection regardless of context condition.
Table 1.
Raw memory means (SDs) by experimental condition.
| Condition | AUC (Negative) | AUC (Neutral) | Recollection (Negative) | Recollection (Neutral) | Familiarity (Negative) | Familiarity (Neutral) |
|---|---|---|---|---|---|---|
|
| ||||||
| Same Context/Control | 0.84 (0.09) | 0.82 (0.08) | 0.15 (0.17) | 0.08 (0.11) | 1.46 (0.67) | 1.48 (0.52) |
| Same Context/Stress | 0.81 (0.13) | 0.80 (0.12) | 0.14 (0.11) | 0.13 (0.15) | 1.47 (0.77) | 1.39 (0.73) |
| Different Context/Control | 0.81 (0.10) | 0.77 (0.10) | 0.14 (0.16) | 0.11 (0.13) | 1.32 (0.70) | 1.21 (0.69) |
| Different Context/Stress | 0.87 (0.06) | 0.83 (0.07) | 0.17 (0.15) | 0.13 (0.15) | 1.66 (0.56) | 1.54 (0.58) |
Note: AUC = area under the curve, which is a model-free, nonparametric measure of overall memory performance. SD = standard deviation. Recollection and familiarity were estimated using the fitting procedure described in Method. Values are provided for both neutral and negative encoding materials.
Notably, there was no main effect of context change on either memory area under the curve (AUC) or model estimates, ps > .990. Similarly, no-stress (nonstress control) participants who changed contexts did not show significantly poorer memory than no-stress participants who remained in the same context, p = .121, nor did their model estimates differ, ps > .054.
Discussion
The current study investigated the effects of socially evaluated cold pressor stress on the encoding of negative and neutral pictures under conditions in which the stressor or control task occurred in either the same context as or a different context from the encoding task. Overall, stress enhanced recognition memory performance when the stressor occurred in a different spatial and psychological context from the encoding event, but, surprisingly, stress had no significant effect on memory when it occurred in the same context as the encoding event. Additionally, although participants’ memory for negative pictures was better than their memory for neutral pictures, stress effects on memory did not differ by picture valence—consistent with prior meta-analytic work on stress shortly prior to encoding (Shields et al., 2017).. Together, these results indicate that the effects of stress on memory encoding depend upon the context in which the stressor and the encoding events occur.
The current study is the first to experimentally test how context change influences the effects of stress on memory encoding. Prior work has shown that the effects of stress on encoding differ as a function of various factors—such as the relevance of the information to the stressor and the delay between stress and encoding (see Shields et al., 2017, 2022; Wolf, 2012). The current study is the first to show that the effects of stress on encoding are dependent on another factor: the stressor’s context. This finding is consistent with recent work showing that the effects of post-encoding stress on memory also depend critically on the stressor’s context (Sazma et al., 2019; Shields et al., 2017). Importantly however, the pre-encoding stress benefits observed in the current study were only observed when the stress context mismatched the study context, whereas the post-encoding stress benefits previously reported were only observed when the stress context matched the study context, suggesting that the context-dependent effects of stress on encoding and post-encoding processes are different. The theoretical implications of this difference are considered below.
The current results also showed that recognition memory was better for the emotionally negative items than it was for neutral items, which is a finding that is consistent with a large body of prior work (for reviews Kensinger, 2009; Yonelinas & Ritchey, 2015). Moreover, the finding that stress had similar effects on both negative and neutral materials is also consistent with some previous pre-encoding stress studies that have indicated comparable effects of stress on emotional and neutral materials (e.g., Goldfarb et al., 2019; for review see Shields et al., 2017). In addition to examining overall recognition accuracy, we also examined parameter estimates of recollection and familiarity-based responses. The parameter estimates suggested that stress numerically increased estimates of both recollection and familiarity when the stressor occurred in a different context from the encoding task, but the context-dependent effects of stress on memory encoding were stronger in familiarity than in recollection (see McCullough et al., 2015; see also Sazma et al., 2019; Shields et al., 2019). This finding is consistent with prior work on stress prior to encoding that occurred in a different context, which has also found improvements in familiarity more-so than recollection (Kamp et al., 2019). However, a selective improvement in familiarity was not one of our a priori predictions. Therefore, although stress in a different context from learning significantly improved familiarity relative to the control condition, we do not make strong claims about specific effects of stress on recognition subprocesses.
As described above, we did not observe an interaction with the emotional valence of items, but an interesting albeit nonsignificant pattern within our data is that, relative to stressed participants who stayed in the same context for encoding, stressed participants who changed contexts prior to encoding had a nonsignificantly larger improvement in memory for negative items than neutral items, whereas the opposite pattern was apparent in control participants. This general pattern is consistent with some prior work finding that stress may, in some cases, preferentially increases memory encoding of highly arousing negative information (e.g., Goldfarb et al., 2019; Luo et al., 2018), though our lack of significant difference in observed stress effects by valence is consistent with a meta-analysis (Shields et al., 2017).
Theoretical Implications
Why might stress shortly prior to encoding enhance memory for information that was encoded in a different context, but not for information that was encoded in the same context as the stressor? Perhaps the most straightforward prediction—indeed, one made by both consolidation and contextual binding theories—was that pre-encoding stress would benefit memory most when the stressor context matched the encoding context. The observation that pre-encoding stress in the current study enhanced memory only when the stressor occurred in a different context from the study event is the opposite of what is seen in studies of post-encoding stress (Sazma et al., 2019; Shields et al., 2017). A context enhancement account explanation can explain context-dependent post-encoding stress effects on memory, but it is quite clear that such an account does not explain the pre-encoding stress effects that we observed. The current results also present a challenge to simple consolidation accounts, as they provide no mechanism to explain the context-specific nature of the memory benefits.
We interpret the existing results as suggesting that stress has both beneficial and disruptive effects on memory encoding that are differentially impacted by factors such as the experimental context. The specific processes that are differentially affected by stress are not yet clear, but there are a number of possibilities. For example, a ‘dual mode’ account of stress has been proposed (Schwabe et al., 2012) in which stress generally benefits memory encoding by facilitating a glucocorticoid-dependent cellular consolidation process acting on memory for recent events, but these beneficial effects can be offset by a noradrenergic-dependent process that shifts attentional and encoding-related resources toward the stressor itself, resulting in a reduction in memory for events or stimuli that are unrelated to the stressor (also see Leblanc, 2009; Mather & Sutherland, 2011). Although this account does not explicitly address the role of context, if changing contexts reduces the detrimental effects of stress on attention, this could explain why we only observed beneficial effects of stress on encoding in the different context condition. This idea is supported by work showing that locus coeruleus neurons, which are the source of hippocampal norepinephrine, change in tonic and phasic activity as a function of changing contexts (e.g., Bouret & Sara, 2005; Grella et al., 2019). That is, changing contexts may reduce the effects of stress on central noradrenergic activity, which would otherwise impair memory for context-irrelevant information, and thus leave only memory-beneficial glucocorticoid activity to influence memory encoding. In other words, when context remains constant, stress may narrow attention onto the stressor, thereby decreasing the likelihood of successful encoding of stress-unrelated information, while simultaneously increasing the consolidation of any information that is successfully encoded—resulting in an overall null effect of stress on memory for stressor-unrelated information. In contrast, when context changes between stress and encoding, stress-induced glucocorticoid activity would still facilitate the consolidation of the studied pictures, but the context change would abolish any narrowing effect of stress on attention, thus producing an overall beneficial effect of stress on memory.
This ‘dual mode’ account of the current results is obviously post hoc and there are a number of alternatives that should be considered. For example, the beneficial effects of stress on encoding may not involve consolidation per se, but rather an increase in vigilance or arousal induced by the stressor that leads to a general increase in memory encoding, which can then be offset by the attention narrowing processes proposed above. Alternatively, it may be that it is the abrupt change in context itself that may disrupt the negative impact of stress on attentional processes by leading to a ‘network reset’ (Bouret & Sara, 2005) that restores noradrenergic activity and cognitive resources to tonic levels upon changing contexts. This restoration of noradrenergic activity could reduce any potential impairing effects of stress on subsequent encoding. Future studies directly testing these post hoc accounts will be needed, but the current results indicate that any such account for stress will need to explain the critical role that ongoing context plays in producing these effects.
A caveat to the above is that the locus coeruleus results have been examined with manipulations of spatial context, whereas our study cannot determine which of the many aspects of context that we manipulated (e.g., spatial, social, psychological, etc.) were either necessary or sufficient to influence memory. As in our past work with post-encoding stress (Sazma et al., 2019), we manipulated all forms of context except temporal context. As such, we do not make claims about what form(s) of context were responsible for these effects. This study represents an important first step in understanding the role of context in stress effects on memory encoding, and in doing so it paves the way for future research to probe aspects of context in order to determine which contextual features are most important in the effects of stress on memory.
Limitations
Although this study has several strengths, including the use of a well-validated stressor task and a well-validated memory paradigm, it has a number of limitations that should be noted. First, we did not assess attentional processes independent of memory encoding, and it is possible that changing contexts affected attentional processes that influenced memory encoding. Therefore, it is unclear whether the effects of stress and context that we observed on memory encoding are due to effects on encoding processes per se or if they might be due to other memory processes, such as those underpinning retention or consolidation. Future work could address this issue via eyetracking, or by manipulating the attentional demands of the encoding to determine if the effects of stress and context on memory encoding are influenced by attentional demands of the encoding task. Second, we did not assess whether the effects we observed differed as a function of the relevance of the encoded materials to the stressor. If our extension of the dual mode model to explain these results is correct, we may find memory for stress-relevant items to be enhanced regardless of context. Third, it is not yet clear whether the current results would generalize to other types of stressors. For example, pain-based stressors (e.g., cold pressor) do not show the typical time-dependent modulation of stress on encoding, whereas nonpain-based stressors (e.g., Trier Social Stress Test) do (Shields et al., 2017). As such, it is possible that we would have observed a different pattern of results had we manipulated stress using a nonpainful task. Fourth, we did not verify compliance with caffeine and alcohol instructions, entailing that our stress effects may have been noisier than is typical. However, random assignment to both stress/control and context conditions prevents this limitation from altering our critical result (i.e., that a context change altered the effect of stress on memory encoding). Fifth, as mentioned above, in order to increase the extent to which a participant viewed the context as changed in the context change condition, we changed both spatial (i.e., the room) and psychological (i.e., different research assistant, supposedly a different study) contexts in the context change condition. Future studies will also be needed to determine which aspects of the experimental context are most critical in moderating the observed stress effects on memory. Changes in the physical environment such as spatial location likely play a critical role, but other aspects of context, such as the presence of different people (social context), as well as accompanying changes in emotional and cognitive states (mental context) may also play an important role (Newtson & Engquist, 1976; Zacks et al., 2001). Finally, although changing contexts between the control water task and encoding did not significantly worsen memory in control participants, it is possible that the difference between context conditions would have been significant with more participants. Our data could be taken to suggest, for example, that changing contexts was a highly salient event, which hurt memories not encoded within a highly salient context themselves. Future work should attempt to determine the extent to which prior nonstressful but highly salient information might reduce subsequent memory, and examine to what extent stress following the nonstressful salient information but prior to encoding might mitigate those effects.
Conclusion
Although the effects of stress on memory encoding are complex, they are gradually becoming clearer. In this study, we examined how stressor context might contribute to the effects of stress on memory encoding by experimentally manipulating both stress and context. In line with prior work on post-encoding stress, our data indicate that context plays an important role in the effects of stress on memory encoding. In particular, contrary to what would have been expected from the post-encoding stress literature, we found that stress induced by the socially evaluated cold pressor enhanced memory encoding only when that stressor occurred in a different context from the encoding task. These findings thus suggest complex relations among stress and context, potentially dependent upon other factors (e.g., stressor type), in their effects on memory. Nonetheless, our results highlight the importance of considering context in the effects of stress on memory and, in doing so, challenge existing theories of how stress affects memory encoding. If you were stressed recently, we hope that you read this paper in a different context.
Footnotes
Author Note:
Cameron Riddell is now at Don’t Use This Code, New York City, New York, USA.
Data and syntax are available at https://osf.io/zxvj6/?view_only=4066ee16e24e472f83157ae6ccc8feca
Cameron Riddell: Conceptualization, Methodology, Investigation, Formal Analysis, Writing – Original Draft, Visualization, Project Administration, Data Curation
Andrew Yonelinas: Conceptualization, Methodology, Investigation, Writing – Review & Editing, Supervision, Funding Acquisition, Resources
Grant Shields: Conceptualization, Methodology, Formal Analysis, Writing – Review & Editing, Visualization, Data Curation
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, & Clarke G. (2014). Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neuroscience and Biobehavioral Reviews, 38, 94–124. 10.1016/j.neubiorev.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10(6), 410–422. 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker BM, & Walker SC. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Baum A, & Grunberg N. (1995). Measurement of stress hormones. Measuring Stress: A Guide for Health and Social Scientists. https://psycnet.apa.org/record/1998-07054-007 [Google Scholar]
- Bouret S, & Sara SJ. (2005). Network reset: A simplified overarching theory of locus coeruleus noradrenaline function. Trends in Neurosciences, 28, 574–582. 10.1016/j.tins.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, & Le K. (2003). Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning & Memory, 10(4), 270–274. 10.1101/lm.62403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, & McGaugh JL. (1996). Modulation of memory storage. Current Opinion in Neurobiology, 6(2), 237–242. 10.1016/S0959-4388(96)80078-X [DOI] [PubMed] [Google Scholar]
- Chiu YC, & Egner T. (2015). Inhibition-induced forgetting: When more control leads to less memory. Psychological Science, 26(1), 27–38. 10.1177/0956797614553945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb EV, Tompary A, Davachi L, & Phelps EA. (2019). Acute stress throughout the memory cycle: Diverging effects on associative and item memory. Journal of Experimental Psychology: General, 148(1), 13–29. 10.1037/xge0000472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp SM, Endemann R, Domes G, & Mecklinger A. (2019). Effects of acute psychosocial stress on the neural correlates of episodic encoding: Item versus associative memory. Neurobiology of Learning and Memory, 157, 128–138. 10.1016/j.nlm.2018.12.006 [DOI] [PubMed] [Google Scholar]
- Kensinger EA. (2009). Remembering the details: Effects of emotion. Emotion Review, 1(2), 99–113. 10.1177/1754073908100432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB. (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert, &, Greenwald M, Dhman A, Vaid D, Hamm A, Cook E, Bertron A, Petry M, Bruner R, Mcmanis M, Zabaldo D, Martinet S, Cuthbert S, Ray D, Koller K, Kolchakian M, & Hayden S. (1997). International Affective Picture System (IAPS): Technical Manual and Affective Ratings. [Google Scholar]
- Leblanc VR. (2009). The effects of acute stress on performance: Implications for health professions education. Academic Medicine, 84(SUPPL. 10), 25–33. 10.1097/ACM.0b013e3181b37b8f [DOI] [PubMed] [Google Scholar]
- Lennartsson A-K, Kushnir MM, Bergquist J, Billig H, & Jonsdottir IH. (2012). Sex steroid levels temporarily increase in response to acute psychosocial stress in healthy men and women. International Journal of Psychophysiology, 84, 246–253. 10.1016/j.ijpsycho.2012.03.001 [DOI] [PubMed] [Google Scholar]
- Lenth R. (2020). emmeans: Estimated marginal means, aka least-squares means. https://cran.r-project.org/package=emmeans
- Luo Y, Fernández G, Hermans E, Vogel S, Zhang Y, Li H, & Klumpers F. (2018). How acute stress may enhance subsequent memory for threat stimuli outside the focus of attention: DLPFC-amygdala decoupling. NeuroImage, 171, 311–322. 10.1016/j.neuroimage.2018.01.010 [DOI] [PubMed] [Google Scholar]
- Macmillan N, & Creelman D. (2004). Detection theory: A user’s guide. [Google Scholar]
- Maheu FS, Collicutt P, Kornik R, Moszkowski R, & Lupien SJ. (2005). The perfect time to be stressed: A differential modulation of human memory by stress applied in the morning or in the afternoon. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 29(8), 1281–1288. 10.1016/j.pnpbp.2005.08.012 [DOI] [PubMed] [Google Scholar]
- Marsland AL, Walsh C, Lockwood K, & John-Henderson NA. (2017). The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain, Behavior, and Immunity, 64, 208–219. 10.1016/j.bbi.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, & Sutherland MR. (2011). Arousal-biased competition in perception and memory. Perspectives on Psychological Science, 6(2), 114–133. 10.1177/1745691611400234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough AM, Ritchey M, Ranganath C, & Yonelinas A. (2015). Differential effects of stress-induced cortisol responses on recollection and familiarity-based recognition memory. Neurobiology of Learning and Memory, 123, 1–10. 10.1016/j.nlm.2015.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough AM, & Yonelinas AP. (2013). Cold-pressor stress after learning enhances familiarity-based recognition memory in men. Neurobiology of Learning and Memory, 106, 11–17. 10.1016/j.nlm.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. (2000). Memory - A century of consolidation. Science, 287(5451), 248–251. 10.1126/science.287.5451.248 [DOI] [PubMed] [Google Scholar]
- Mehta PH, & Josephs RA. (2010). Testosterone and cortisol jointly regulate dominance: Evidence for a dual-hormone hypothesis. Hormones and Behavior, 58(5), 898–906. 10.1016/j.yhbeh.2010.08.020 [DOI] [PubMed] [Google Scholar]
- Newtson D, & Engquist G. (1976). The perceptual organization of ongoing behavior. Journal of Experimental Social Psychology, 12(5), 436–450. 10.1016/0022-1031(76)90076-7 [DOI] [Google Scholar]
- Nielson KA, & Arentsen TJ. (2012). Memory modulation in the classroom: Selective enhancement of college examination performance by arousal induced after lecture. Neurobiology of Learning and Memory, 98(1), 12–16. [DOI] [PubMed] [Google Scholar]
- Payne JD, Jackson ED, Hoscheidt S, Ryan L, Jacobs WJ, & Nadel L. (2007). Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learning and Memory, 14(12), 861–868. 10.1101/lm.743507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pützer A, & Wolf OT. (2021). Odours as context cues of emotional memories–The role of semantic relatedness. Acta Psychologica, 219, 103377. 10.1016/j.actpsy.2021.103377 [DOI] [PubMed] [Google Scholar]
- Quaedflieg CWEM, Schwabe L, Meyer T, & Smeets T. (2013). Time dependent effects of stress prior to encoding on event-related potentials and 24h delayed retrieval. Psychoneuroendocrinology, 38(12), 3057–3069. 10.1016/j.psyneuen.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Ritchey M, McCullough AM, Ranganath C, & Yonelinas AP. (2017). Stress as a mnemonic filter: Interactions between medial temporal lobe encoding processes and post-encoding stress. Hippocampus, 27(1), 77–88. 10.1002/hipo.22674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B. (2002). Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiology of Learning and Memory, 78, 578–595. 10.1006/nlme.2002.4080 [DOI] [PubMed] [Google Scholar]
- Ryan R, Booth S, Spathis A, Mollart S, & Clow A. (2016). Use of salivary diurnal cortisol as an outcome measure in randomised controlled trials: A systematic review. Annals of Behavioral Medicine, 50(2), 210–236. 10.1007/s12160-015-9753-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazma MA, Cervantes S, & Siegel J. (2023). Exploring the absence of episodic memory benefits from post-encoding emotional arousal and novelty manipulations in humans. Preprint retrieved from https://psyarxiv.com/w7a4f/
- Sazma MA, McCullough AM, Shields GS, & Yonelinas AP. (2019). Using acute stress to improve episodic memory: The critical role of contextual binding. Neurobiology of Learning and Memory, 158(December 2018), 1–8. 10.1016/j.nlm.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Böhringer A, Chatterjee M, & Schachinger H. (2008). Effects of pre-learning stress on memory for neutral, positive and negative words: Different roles of cortisol and autonomic arousal. Neurobiology of Learning and Memory, 90(1), 44–53. 10.1016/j.nlm.2008.02.002 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Böhringer A, & Wolf OT. (2009). Stress disrupts context-dependent memory. Learning & Memory, 16, 110–113. 10.1101/lm.1257509 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Haddad L, & Schachinger H. (2008). HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology, 33(6), 890–895. 10.1016/j.psyneuen.2008.03.001 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Joëls M, Roozendaal B, Wolf OT, & Oitzl MS. (2012). Stress effects on memory: An update and integration. Neuroscience and Biobehavioral Reviews, 36(7), 1740–1749. 10.1016/j.neubiorev.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Sharot T, Delgado MR, & Phelps EA. (2004). How emotion enhances the feeling of remembering. Nature Neuroscience, 7(12), 1376–1380. 10.1038/nn1353 [DOI] [PubMed] [Google Scholar]
- Sharot T, Verfaellie M, & Yonelinas AP. (2007). How emotion strengthens the recollective experience: a time-dependent hippocampal process. PLoS ONE, 2(10), e1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, & Yonelinas AP. (2008). Differential time-dependent effects of emotion on recollective experience and memory for contextual information. Cognition, 106(1), 538–547. 10.1016/j.cognition.2007.03.002 [DOI] [PubMed] [Google Scholar]
- Shields GS. (2020). Stress and cognition: A user’s guide to designing and interpreting studies. Psychoneuroendocrinology, 112, 104475. 10.1016/j.psyneuen.2019.104475 [DOI] [PubMed] [Google Scholar]
- Shields GS, Dunn TM, Trainor BC, & Yonelinas AP. (2019). Determining the biological associates of acute cold pressor post-encoding stress effects on human memory: The role of salivary interleukin-1β. Brain, Behavior, and Immunity, 81, 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Hunter CL, & Yonelinas AP. (2022). Stress and memory encoding: What are the roles of the stress-encoding delay and stress relevance? Learning & Memory, 29(2), 48–54. 10.1101/lm.053469.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Lam JCW, Trainor BC, & Yonelinas AP. (2016). Exposure to acute stress enhances decision-making competence: Evidence for the role of DHEA. Psychoneuroendocrinology, 67, 51–60. 10.1016/j.psyneuen.2016.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, McCullough AM, & Yonelinas AP. (2017). The effects of acute stress on episodic memory: A meta-analysis and integrative review. Psychological Bulletin, 143(6), 636–675. 10.1037/bul0000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, & Yonelinas AP. (2016). The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. In Neuroscience and Biobehavioral Reviews (Vol. 68, pp. 651–668). 10.1016/j.neubiorev.2016.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets T, Giesbrecht T, Jelicic M, & Merckelbach H. (2007). Context-dependent enhancement of declarative memory performance following acute psychosocial stress. Biological Psychology, 76(1–2), 116–123. 10.1016/J.BIOPSYCHO.2007.07.001 [DOI] [PubMed] [Google Scholar]
- Smeets Tom, Jelicic M, & Merckelbach H. (2005). Stress-induced cortisol responses, sex differences, and false recollections in a DRM paradigm. Biological Psychology, 72(2), 164–172. 10.1016/j.biopsycho.2005.09.004 [DOI] [PubMed] [Google Scholar]
- Smeets Tom, Otgaar H, Candel, & Wolf OT. (2008). True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology, 33(10), 1378–1386. 10.1016/j.psyneuen.2008.07.009 [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JRR, Chen J, Beverly JL, & Johnson RW. (2006). Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. Journal of Neuroscience, 26(42), 10709–10716. 10.1523/JNEUROSCI.3376-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T, & Risbrough V. (2012). Pharmacological treatment of PTSD – Established and new approaches. Neuropharmacology, 62(2), 617–627. 10.1016/J.NEUROPHARM.2011.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma MV, Kirschbaum C, Wolf JM, & Rohleder N. (2012). Acute stress responses in salivary alpha-amylase predict increases of plasma norepinephrine. Biological Psychology, 91, 342–348. 10.1016/j.biopsycho.2012.07.008 [DOI] [PubMed] [Google Scholar]
- Wiemers US, Hamacher-Dang TC, Yonelinas AP, & Wolf OT. (2019). Pre-encoding stress induced changes in perceived stress, blood pressure and cortisol are differentially associated with recollection and familiarity. Brain and Cognition. 10.1016/j.bandc.2018.03.013 [DOI] [PubMed] [Google Scholar]
- Wiemers US, Sauvage MM, & Wolf OT. (2014). Odors as effective retrieval cues for stressful episodes. Neurobiology of Learning and Memory, 112, 230–236. 10.1016/j.nlm.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Wirkner J, Weymar M, Löw A, & Hamm AO. (2013). Effects of pre-encoding stress on brain correlates associated with the long-term memory for emotional scenes. PLoS ONE, 8(9), e68212. 10.1371/journal.pone.0068212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT, Atsak P, de Quervain DJ, Roozendaal B, & Wingenfeld K. (2016). Stress and memory: A selective review on recent developments in the understanding of stress hormone effects on memory and their clinical relevance. Journal of Neuroendocrinology, 28(8). 10.1111/jne.12353 [DOI] [PubMed] [Google Scholar]
- Wolf Oliver T. (2012). Immediate recall influences the effects of pre-encoding stress on emotional episodic long-term memory consolidation in healthy young men. Stress, 15(3), 272–280. 10.3109/10253890.2011.622012 [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. (1994). Receiver-operating characteristics in recognition memory: Evidence for a dual-process model. Journal of Experimental Psychology: Learning, Memory, and Cognition, 20(6), 1341–1354. 10.1037/0278-7393.20.6.1341 [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. (2002). The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language, 46(3), 441–517. 10.1006/jmla.2002.2864 [DOI] [Google Scholar]
- Yonelinas AP, Parks CM, Koen JD, Jorgenson J, & Mendoza SP. (2011). The effects of post-encoding stress on recognition memory: Examining the impact of skydiving in young men and women. Stress, 14(2), 136–144. 10.3109/10253890.2010.520376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, & Ritchey M. (2015). The slow forgetting of emotional episodic memories: An emotional binding account. Trends in Cognitive Sciences, 19(5), 259–267. 10.1016/j.tics.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM, Braver TS, Sheridan MA, Donaldson DI, Snyder AZ, Ollinger JM, Buckner RL, & Raichle ME. (2001). Human brain activity time-locked to perceptual event boundaries. Nature Neuroscience, 4(6), 651–655. 10.1038/88486 [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Clark B, Warnecke A, Smith L, Tabar J, & Talbot JN. (2011). Pre-learning stress differentially affects long-term memory for emotional words, depending on temporal proximity to the learning experience. Physiology & Behavior, 103, 467–476. 10.1016/j.physbeh.2011.01.016 [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Warnecke AJ, Woelke SA, Burke HM, Frigo RM, Pisansky JM, Lyle SM, & Talbot JN. (2013). Pre-learning stress that is temporally removed from acquisition exerts sex-specific effects on long-term memory. Neurobiology of Learning and Memory, 100, 77–87. 10.1016/j.nlm.2012.12.012 [DOI] [PubMed] [Google Scholar]