Abstract
Aims
Biofilm infections are among the most challenging complications in orthopaedics, as bacteria within the biofilms are protected from the host immune system and many antibiotics. Halicin exhibits broad-spectrum activity against many planktonic bacteria, and previous studies have demonstrated that halicin is also effective against Staphylococcus aureus biofilms grown on polystyrene or polypropylene substrates. However, the effectiveness of many antibiotics can be substantially altered depending on which orthopaedically relevant substrates the biofilms grow. This study, therefore, evaluated the activity of halicin against less mature and more mature S. aureus biofilms grown on titanium alloy, cobalt-chrome, ultra-high molecular weight polyethylene (UHMWPE), devitalized muscle, or devitalized bone.
Methods
S. aureus-Xen36 biofilms were grown on the various substrates for 24 hours or seven days. Biofilms were incubated with various concentrations of halicin or vancomycin and then allowed to recover without antibiotics. Minimal biofilm eradication concentrations (MBECs) were defined by CFU counting and resazurin reduction assays, and were compared with the planktonic minimal inhibitory concentrations (MICs).
Results
Halicin continued to exert significantly (p < 0.01) more antibacterial activity against biofilms grown on all tested orthopaedically relevant substrates than vancomycin, an antibiotic known to be affected by biofilm maturity. For example, halicin MBECs against both less mature and more mature biofilms were ten-fold to 40-fold higher than its MIC. In contrast, vancomycin MBECs against the less mature biofilms were 50-fold to 200-fold higher than its MIC, and 100-fold to 400-fold higher against the more mature biofilms.
Conclusion
Halicin is a promising antibiotic that should be tested in animal models of orthopaedic infection.
Cite this article: Bone Joint Res 2024;13(3):101–109.
Keywords: Halicin, Biofilm, Periprosthetic joint infection, Fracture related infection, Orthopaedic infection, Staphylococcus aureus, Biofilms, Vancomycin, antibiotics, bacteria, ultra-high molecular weight polyethylene (UHMWPE), orthopaedic infections, titanium alloy, Cobalt-Chrome, animal models
Article focus
Does halicin remain antibacterially active against Staphylococcus aureus biofilms grown on different orthopaedically relevant substrates?
How does the activity of halicin against biofilms compare with the activity of vancomycin against biofilms on different orthopaedically relevant substrates?
Key messages
Halicin remains active against S. aureus-Xen36 biofilms grown on different orthopaedically relevant substrates.
Halicin exerts substantially more activity than vancomycin against both less and more mature biofilms.
Strengths and limitations
A strength of this study is that the effects of halicin were measured on S. aureus biofilms grown on multiple orthopaedically relevant substrates, including titanium alloy, cobalt-chrome, ultra-high molecular weight polyethylene, devitalized muscle, and devitalized bone.
A limitation of this study is that only S. aureus biofilms were compared. Future studies will focus on other bacterial biofilms common in orthopaedic infections.
Future studies should test the effects of halicin on biofilms in orthopaedic infection animal models.
Introduction
Bacterial biofilms readily form on orthopaedic implants and musculoskeletal tissues. These biofilm infections are often due to Staphylococcus aureus and are among the most challenging complications in orthopaedics.1-5 Biofilms protect bacteria from both the host immune system and antibiotics.6,7 A primary mechanism responsible for antibiotic tolerance by biofilm bacteria is induction of quiescence, as most conventional antibiotics specifically target proliferating bacteria.7-15 The minimum biofilm eradication concentrations (MBECs) for many antibiotics are substantially higher than the minimum inhibitory concentration (MIC) for planktonic bacteria, and are also substantially higher than the level that is clinically safe for therapeutic use.16-19
Halicin (SU3327), a nitrothiazole compound without structural similarity to any known antibiotic, was recently found to have broad-spectrum activity against planktonic bacteria.20 Halicin was identified by a deep learning artificial intelligence approach and is effective against many bacteria strains including both methicillin-sensitive S. aureus and methicillin-resistant S. aureus.20 Moreover, halicin did not induce resistance in vitro, and was also effective against persister cells in planktonic cultures.20 Because planktonic persister cells are phenotypically similar to bacteria residing in biofilms, halicin might also kill quiescent bacteria within biofilms. Indeed, halicin was recently shown to remain active against S. aureus in both less mature and more mature biofilms grown on polystyrene substrates.18 Similarly, other investigators found that halicin remains active against antimicrobial-resistant S. aureus, Acinetobacter baumannii, and Escherichia coli in immature 24-hour biofilms and mature seven-day biofilms grown on polypropylene substrates.21 More recently, halicin was found to synergize with doxycline to reduce biomass of 24-hour Enterococcus spp. biofilms grown on polymeric substrates.22
Antibiotic susceptibility of bacterial biofilms varies depending on the substrates on which the biofilms are grown.23-26 For example, susceptibility of Staphylococcus spp. biofilms to antibiotics varied up to 250-fold depending on whether biofilms were grown on stainless steel, ultra-high molecular weight polyethylene (UHMWPE), or polymethylmethacrylate (PMMA).23 Moreover, the differences between substrates were substantially dependent on the tested antibiotic.23 Antibiotic susceptibility of biofilms on surrounding tissues such as muscle and bone is perhaps more important to consider, as infected orthopaedic implants are commonly removed surgically, but it is difficult to remove all the necrotic tissue that harbours biofilms.27,28 In that regard, Staphylococcus spp. biofilms grown on devitalized bone were up to 2,500-fold less susceptible to antibiotics than biofilms on PMMA, depending on the antibiotic that was tested.24 Similarly, antibiotic susceptibility of biofilms grown on devitalized muscle or devitalized bone varied between 100-fold less and 100-fold more than biofilms on polystyrene depending on the strain of bacteria that was tested.26 Taken together, the above studies show that it is not possible to predict a priori how biofilm growth on different substrates will affect susceptibility to a specific antibiotic.23-26 This study therefore evaluated effects of halicin against both less mature and more mature S. aureus biofilms grown on orthopaedically relevant substrates, including titanium alloy (Ti6Al4V), Cobalt-Chrome (Co-Cr), UHMWPE, devitalized muscle, and devitalized cortical bone. Vancomycin was used for comparison as an antibiotic known to be affected by biofilm maturity.
Methods
Preparation of orthopaedically relevant substrates
Ti6Al4V discs (medical grade ELI 23, 12.7 mm diameter, Titanium Industries, USA) were autoclaved, sonicated in acetone for 30 minutes, and incubated in alkali ethanol (0.1 N NaOH and 95% ethanol at 32°C, overnight) and then in 25% nitric acid (room temperature, overnight).29 Co-Cr and highly crosslinked UHMWPE discs were manufactured from a knee arthroplasty prosthesis. The Co-Cr discs (11.4 mm diameter, 5.0 mm thickness) were sonicated in alkali ethanol (0.1 N NaOH and 95% ethanol) for 30 minutes, and then incubated in alkali ethanol (0.1 N NaOH and 95% ethanol at 32°C, overnight). The UHMWPE discs (11.0 mm diameter, 5.1 mm thickness) were sonicated in 100% ethanol for 30 minutes, and then incubated in 100% ethanol overnight. Muscle and bone samples were harvested from New Zealand White rabbits. The rabbits were euthanized for other studies, and an ARRIVE checklist is therefore not appropriate for this study. Pieces of rabbit fore limb muscle (biceps brachii, triceps brachii, or brachialis) and cortical bone (humerus, radius, or ulna) without marrow were obtained immediately after euthanasia and stored at -20°C.26 For each experiment, devitalized muscles were cut into approximately 4 mm3 pieces (median 60 g; interquartile range (IQR) 50 to 66), and devitalized cortical bones were cut into pieces approximately 5 mm in length (median 12 g; IQR 10 to 15).
Biofilm growth
A single colony of kanamycin-resistant, methicillin-sensitive S. aureus-Xen36 (Perkin Elmer) was incubated overnight with 200 RPM at 37°C in 3 ml of Luria-Bertani (LB) broth containing kanamycin (200 ug/ml) to avoid confounding growth of other bacteria.30 Overnight cultures were diluted in LB broth without kanamycin to optical density (OD) 600 of 0.07 (approximately 1.5 × 108 CFUs/ml), and were further diluted 200-fold to approximately 6.7 × 105 CFUs/ml in LB without kanamycin. The diluted bacterial suspensions were added to discs (Ti6Al4V, Co-Cr, and UHMWPE) and devitalized tissue pieces (muscle and bone) in 24-well (1.5 ml) or 48-well (1.0 ml) plates, respectively. Plates were then sealed with a gas-permeable membrane (SealMate; Excel Scientific, USA) and incubated at 37°C in a humidified incubator with shaking at 110 RPM for one, three, five, or seven days. For biofilms grown for more than one day, substrates were gently rinsed three times with sterile PBS after the first 24 hours of incubation and then incubated in fresh LB broth without kanamycin (metallic and polymeric discs) or with kanamycin (biological tissues). The broth was changed every 24 hours.18,30,31
Antibiotics
Halicin (Catalogue #3607; Tocris Bioscience, USA) was dissolved to 50 mM in 100% dimethyl sulfoxide (DMSO) and stored at -20°C. For each experiment, the stock was diluted to 10 mM in 100% DMSO followed by serial dilution in 100% DMSO. The serial dilutions were added (1/10 volume) to each experimental well to obtain the indicated final concentrations of halicin in LB broth (Thermo Fisher Scientific, USA) with 10% DMSO. Control wells for halicin experiments therefore also contained LB broth with 10% DMSO. Selected experiments also included halicin groups with final concentrations of 20% DMSO as vehicle or with 10% or 20% Kolliphor HS 15 (Catalogue #42966; Sigma, USA) as vehicle. Kolliphor HS 15 is an amphiphilic solubilizer that is Food and Drug Administration-approved for parenteral administration and has been used as vehicle for halicin in mice.32 Importantly, preliminary experiments found that 10% to 20% DMSO or 10% to 20% Kolliphor HS 15 by themselves had no detectable effect on bacterial viability within the biofilms (Supplementary Figure aa). However, 10% DMSO inhibited the anti-biofilm activity of vancomycin (Supplementary Figure ab). Vancomycin (Sigma) was therefore routinely dissolved to 200 or 800 µM in LB broth without DMSO. For each experiment, vancomycin stock solutions were serially diluted in LB broth without DMSO to obtain the indicated final concentrations. Control wells for vancomycin therefore also contained LB broth without DMSO.
MBEC assay
Biofilms were gently rinsed three times with sterile PBS to remove non-adherent bacteria, then incubated for 20 hours at 37°C in wells containing the indicated concentrations of halicin, vancomycin, or vehicle controls in LB broth without kanamycin. After the antibiotic challenge, biofilms were gently rinsed three times with sterile PBS, transferred to fresh LB broth without kanamycin, and allowed to recover from the antibiotic challenge for 24 hours at 37°C. After the recovery period, biofilms were disrupted by sonication (40 kHz for 30 minutes, BRANSON 3800; Branson Ultrasonics, USA) on a solid stainless steel insert tray in LB broth containing 0.3% Tween-80 (1.5 ml/well in 24-well plates for discs; 1.0 ml/well in 48-well plates for tissue pieces).18 Bacterial viability was assessed by colony counting after serial tenfold dilutions in PBS and incubation on LB agar containing kanamycin (200 ug/ml) at 37°C for 24 hours. Bacterial viability was also assessed by resazurin reduction assays. For these assays, fluorescence was measured (excitation: 530 nm, emission: 595 nm, and gain: 60) with a GENios plus plate reader (Tecan, USA) after incubation for 60 minutes at 37°C with 15 µg/ml of resazurin (Alfa Aesar, USA). Background fluorescence in wells without bacteria was subtracted from experimental fluorescence values. MBECs were defined as the lowest concentration of halicin or vancomycin that induced a ≥ 3 log reduction in CFUs or a ≥ 75% decrease in resazurin reduction.33,34 Medians of four to seven independent experiments for each drug concentration were used to calculate MBECs and are connected by dashed lines in the figures. Symbols in the figures denote the medians for each drug concentration from an independent experiment.
Statistical analysis
PRISM software version 9.4.1 was used for statistical analysis (GraphPad Software, USA). The Mann-Whitney U test was used to compare halicin and vancomycin MBECs. A p-value < 0.05 was considered statistically significant.
Results
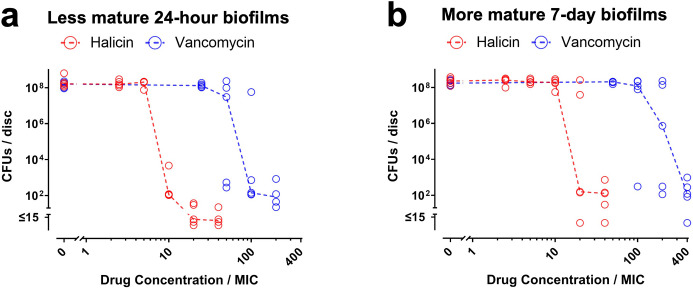
To determine whether halicin and vancomycin remain antibacterially active against S. aureus within biofilms grown on orthopaedically relevant substrates, MBEC results are reported in comparison to the MIC values we previously measured with the same strain of S. aureus (25 µM for halicin and 0.5 µM for vancomycin).18 For the less mature 24-hour and three-day biofilms on Ti6AlV4 discs, halicin’s MBECs, assessed by either CFU assays or resazurin reduction assays, were ten-fold higher than the MIC (red symbols in Figure 1a, in Supplementary Figure ba, and in Supplementary Figure ca). In contrast, vancomycin’s MBECs against those less mature biofilms on Ti6AlV4 discs were 100-fold higher than the MIC (blue symbols in Figure 1a, in Supplementary Figure ba, and in Supplementary Figure ca). For the more mature five-day and seven-day biofilms on Ti6AlV4 discs, halicin’s MBECs were 20-fold higher than the MIC (red symbols in Figure 1b, in Supplementary Figure bb, and in Supplementary Figure cb), while vancomycin’s MBECs were 200-fold higher than the MIC for five-day biofilms and 400-fold higher for seven-day biofilms (blue symbols in Figure 1b, in Supplementary Figure bb, and in Supplementary Figure cb). Since results on the Ti6Al4V discs were similar between the 24-hour and three-day biofilms (Figure 1a, Supplementary Figure ba, and Supplementary Figure ca) and between the five-day and seven-day biofilms (Figure 1b, Supplementary Figure bb, and Supplementary Figure cb), all further experiments focused exclusively on the less mature 24-hour biofilms and the more mature seven-day biofilms.
Fig. 1.
Halicin remains active against Staphylococcus aureus-Xen36 biofilms grown on Ti6Al4V discs. a) Less mature 24-hour biofilms and b) more mature seven-day biofilms on Ti6AI4V discs were exposed to the indicated concentrations of halicin (red symbols) or vancomycin (blue symbols) for 20 hours. Effects on biofilm viability were determined by CFU assays. Dashed lines connect medians of five independent experiments for each drug concentration. Each symbol denotes the median for each drug concentration from an independent experiment, with four Ti6Al4V discs per symbol.
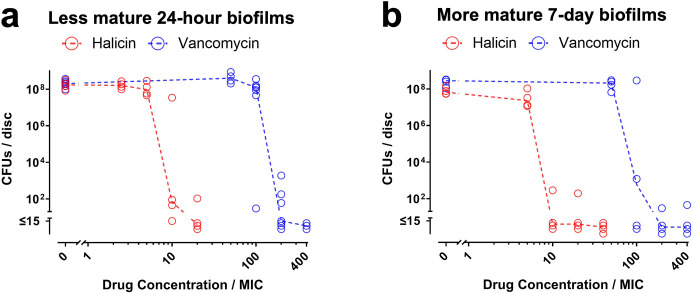
For Co-Cr discs, halicin’s MBECs against less mature 24-hour and more mature seven-day biofilms, assessed by either CFU or resazurin reduction assays, were both ten-fold higher than the MIC (red symbols in Figures 2a to b and in Supplementary Figure da and db). In contrast, vancomycin’s MBEC against the less mature and more mature biofilms were 100-fold to 200-fold higher than the MIC (blue symbols in Figures 2a to b and in Supplementary Figure da and db).
Fig. 2.
Halicin remains active against Staphylococcus aureus-Xen36 biofilms grown on cobalt-chromium (Co-Cr) discs. a) Less mature 24-hour biofilms and b) more mature seven-day biofilms on Co-Cr discs were exposed to the indicated concentrations of halicin (red symbols) or vancomycin (blue symbols) for 20 hours. Effects on biofilm viability were determined by CFU assays. Dashed lines connect medians of four to seven independent experiments for each drug concentration. Each symbol denotes the median for each drug concentration from an independent experiment, with two to three Co-Cr discs per symbol. MIC, minimum inhibitory concentration.
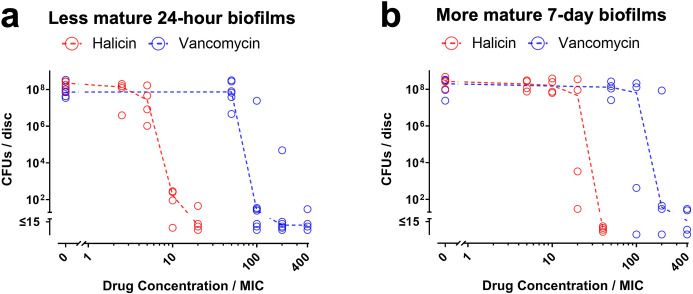
For UHMWPE discs, halicin’s MBEC against less mature 24-hour biofilms measured by CFU assays was ten-fold higher than the MIC (red symbols in Figure 3a), and five-fold higher when measured by resazurin reduction assays (red symbols in Supplementary Figure ea). In contrast, vancomycin’s MBEC against the less mature biofilms was 100-fold higher than the MIC (blue symbols in Figure 3a and in Supplementary Figure ea). For the more mature seven-day biofilms on UHMWPE discs, halicin’s MBEC measured by CFU assays was 40-fold higher than the MIC (red symbols in Figure 3b), and 20-fold higher when measured by resazurin reduction assays (red symbols in Supplementary Figure eb), while vancomycin’s MBEC was 200-fold higher than the MIC (blue symbols in Figure 3b and in Supplementary Figure eb).
Fig. 3.
Halicin remains active against Staphylococcus aureus-Xen36 biofilms grown on ultra-high molecular weight polyethylene (UHMWPE) discs. a) Less mature 24-hour biofilms and b) more mature seven-day biofilms on UHMWPE discs were exposed to the indicated concentrations of halicin (red symbols) or vancomycin (blue symbols) for 20 hours. Effects on biofilm viability were determined by CFU assays. Dashed lines connect medians of four to seven independent experiments for each drug concentration. Each symbol denotes the median for each drug concentration from an independent experiment, with two to three UHMWPE discs per symbol. MIC, minimum inhibitory concentration.
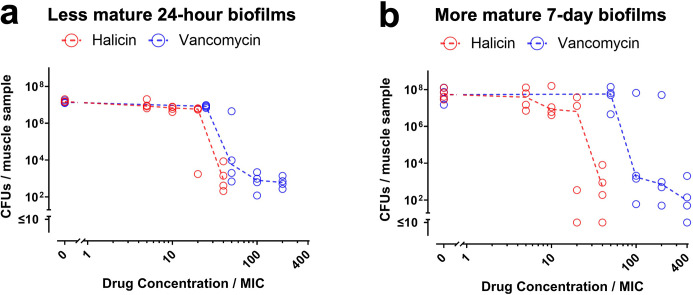
For devitalized muscle, halicin’s MBEC against less mature 24-hour biofilms, assessed by either CFU or resazurin reduction assays, was 40-fold higher than the MIC (blue symbols in Figure 4a and in Supplementary Figure fa). Vancomycin’s MBEC against the less mature biofilms was 50-fold higher than the MIC (blue symbols in Figure 4a and in Supplementary Figure fa). For the more mature seven-day biofilms on muscle samples, halicin’s MBEC measured by CFU assays was 40-fold higher than the MIC (red symbols in Figure 4b), and 20-fold higher when measured by resazurin reduction assays (red symbols in Supplementary Figure fb), while vancomycin’s MBEC was 100-fold higher than the MIC (blue symbols in Figure 4b and in Supplementary Figure fb).
Fig. 4.
Halicin remains active against Staphylococcus aureus-Xen36 biofilms grown on devitalized muscle. a) Less mature 24-hour biofilms and b) more mature seven-day biofilms on muscle samples were exposed to the indicated concentrations of halicin (red symbols) or vancomycin (blue symbols) for 20 hours. Effects on biofilm viability were determined by CFU assays. Dashed lines connect medians of four independent experiments for each drug concentration. Each symbol denotes the median for each drug concentration from an independent experiment, with three muscle samples per symbol.
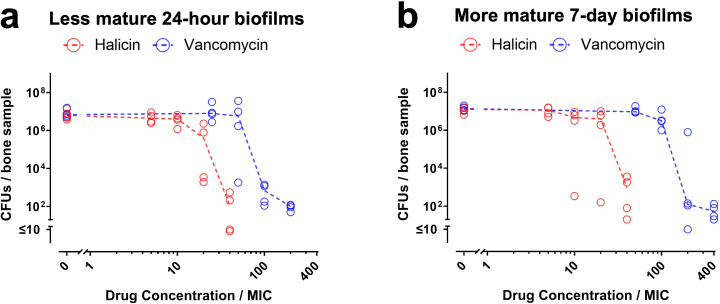
For devitalized cortical bone, halicin’s MBEC against less mature 24-hour biofilms measured by CFU assays was 40-fold higher than the MIC, and 20-fold higher when measured by resazurin reduction assays (red symbols in Figure 5a and in Supplementary Figure ga). In contrast, vancomycin’s MBEC against the less mature biofilms was 100-fold higher than the MIC (blue symbols in Figure 5a and in Supplementary Figure ga). For the more mature seven-day biofilms on cortical bone samples, halicin’s MBEC was 40-fold higher than the MIC (red symbols in Figure 5b and in Supplementary Figure gb), while vancomycin’s MBEC was 200-fold higher than the MIC (blue symbols in Figure 5b and in Supplementary Figure gb).
Fig. 5.
Halicin remains active against Staphylococcus aureus-Xen36 biofilms grown on devitalized cortical bone. a) Less mature 24-hour biofilms and b) more mature seven-day biofilms on bone samples were exposed to the indicated concentrations of halicin (red symbols) or vancomycin (blue symbols) for 20 hours. Effects on biofilm viability were determined by CFU assays. Dashed lines connect medians of four independent experiments for each drug concentration. Each symbol denotes the median for each drug concentration from an independent experiment, with three bone samples per symbol.
MBECs for halicin and vancomycin determined by CFU assays are compared in Table I. Mann-Whitney U test showed that halicin MBECs, expressed as fold change versus MIC, are lower (p < 0.01) than vancomycin MBECs against both the less mature 24-hour biofilms and the more mature seven-day biofilms. MBECs determined by resazurin reduction assays are identical to those in Table I, which were determined by CFU assays, with three exceptions where the halicin MBECs determined by resazurin reduction are two-fold less than those determined by CFU assays (compare red symbols in Figure 3 and Figure 5a, with red symbols in Supplementary Figures ea, eb, and ga).
Table I.
Halicin remains active against Staphylococcus aureus-Xen36 biofilms grown on orthopaedically relevant substrates. Minimal biofilm eradication concentrations from colony-forming unit assays are reported as fold change versus the planktonic minimal inhibitory concentrations, which we previously reported for this strain of S. aureus.18
| Substrate | Less mature 24-hour biofilms MBECs (fold change vs MIC) |
More mature 7-day biofilms MBECs (fold change vs MIC) |
||
|---|---|---|---|---|
| Halicin | Vancomycin | Halicin | Vancomycin | |
| Ti6Al4V | 10× MIC | 100× MIC | 20× MIC | 400× MIC |
| Cobalt-chrome | 10× MIC | 200× MIC | 10× MIC | 100× MIC |
| UHMWPE | 10× MIC | 100× MIC | 40× MIC | 200× MIC |
| Muscle | 40× MIC | 50× MIC | 40× MIC | 100× MIC |
| Bone | 40× MIC | 100× MIC | 40× MIC | 200× MIC |
p-values determined by Mann-Whitney U test were < 0.01, comparing MBECS for halicin to MBECs for vancomycin for both 24-hour and seven-day biofilms.
MBEC, minimum biofilm eradication concentration; MIC, minimal inhibitory concentration; UHMWPE, ultra-high molecular weight polyethylene.
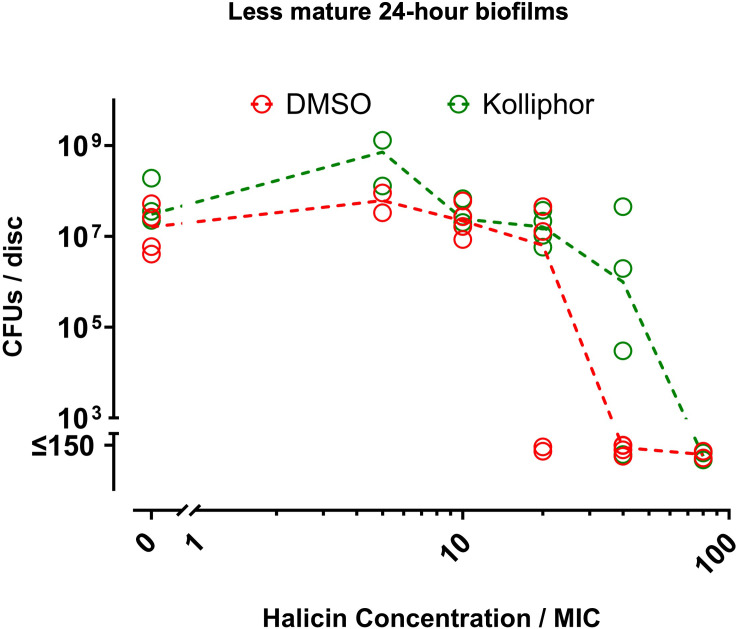
To further assess the translational relevance of our study, we determined whether the antibiofilm effects of halicin depended on the use of DMSO as a vehicle in the previous experiments of this study. For this purpose, halicin with DMSO as vehicle was compared to halicin with Kolliphor HS 15 as vehicle. Kolliphor HS 15 is an amphiphilic solubilizer that is FDA-approved for parenteral administration and has been used as vehicle for halicin in mice.32 The halicin MBEC with DMSO as vehicle was two-fold less than the halicin MBEC with Kolliphor HS 15 vehicle (Figure 6). Thus, halicin continues to exert the majority of its antibiofilm activity regardless of vehicle.
Fig. 6.
Halicin activity against Staphylococcus aureus-Xen 36 biofilms does not depend on DMSO vehicle. Less mature 24-hour biofilms on Ti6Al4V discs were exposed for 20 hours to the indicated concentrations of halicin with either dimethyl sulfoxide (DMSO) (red symbols) or Kolliphor HS 15 (green symbols) as vehicle. Final concentrations of DMSO or Kolliphor HS 15 were 20% in LB broth without kanamycin for groups with halicin at 80 × minimal inhibitory concentration (MIC) and 10% for all other halicin groups. Controls without halicin therefore included groups with 10% or 20% of either DMSO or Kolliphor HS 15 as vehicle. The concentrations of either vehicle did not affect biofilm viability (Supplementary Figure aa). Effects on biofilm viability were determined by colony-forming unit (CFU) assays. Dashed lines connect medians of four independent experiments for each group. Each symbol denotes the median for each group from an independent experiment, with four Ti6Al4V discs per symbol.
Discussion
Previous studies found that halicin kills bacteria in biofilms grown on polystyrene or polypropylene substrates.18,21,22 However, biofilm susceptibility to antibiotics can be altered by growth on different substrates.23-26 This study therefore determined whether halicin remains active against S. aureus biofilms grown on orthopaedically relevant substrates. We found that halicin’s MBECs against both less mature and more mature biofilms are ten-fold to 40-fold higher than its MIC. In contrast, vancomycin’s MBECs are significantly larger: 50-fold to 200-fold higher than its MIC against the less mature biofilms, and 100-fold to 400-fold higher than MIC against the more mature biofilms. The decreased susceptibility to vancomycin confirms biofilm formation and maturation in our study.35,36
Induction of bacterial quiescence is a hallmark of biofilm maturation and a major cause of tolerance to most antibiotics.7-15 Halicin’s effectiveness against bacterial biofilms shows that it remains active against quiescent bacteria, and is consistent with previous findings that similar concentrations of halicin remain active in planktonic cultures of metabolically repressed bacteria and quiescent persister bacteria.20
Our results also show distinct halicin MBECs for the biofilms grown on inorganic substrates compared with the biofilms grown on devitalized tissues. Thus, halicin MBECs against less mature and more mature biofilms grown on discs of Ti6Al4V, Co-Cr, or UHMWPE were ten-fold to 20-fold higher than its MIC. In contrast, halicin MBECs against biofilms on devitalized muscle or devitalized cortical bone were 40-fold higher than its MIC, irrespective of whether the biofilms were grown for 24 hours or seven days. However, the distribution of vancomycin MBECs did not differentiate between inorganic and biological substrates. The different patterns of MBECs for halicin and vancomycin are consistent with the conclusion that MBECs determined on different substrates can vary in an unpredictable manner.23-26
The major limitation of our study is that the clinically achievable serum level of halicin is not known; therefore, it cannot be compared to our measured MBECs. Future pharmacokinetic studies are needed to resolve this question. A second limitation is that we only tested a single strain of bacteria. However, halicin is also effective against biofilms of other S. aureus strains as well as biofilms of A. baumannii, E. coli, and Enterococcus.21,22 Halicin also has broad-spectrum activity against planktonic cultures of many Gram-positive and Gram-negative bacteria, including multidrug-resistant clinical isolates.20,37 Halicin is the first completely novel antibiotic identified by deep-learning artificial intelligence approaches.20 Follow-up studies revealed that halicin has potent broad-spectrum activity, but the antibacterial mechanism of halicin remains uncertain.20 Evidence from planktonic cultures suggests inhibition of bacterial proton motive force as a potential mechanism.20 That mechanism would be consistent with halicin’s activity against planktonic cultures of quiescent persister bacteria and bacteria in biofilms, as both remain dependent on the proton motive force.38 Future studies are needed to determine whether halicin inhibits the proton motive force of bacteria in biofilms. Another limitation of our study is that we did not use bioluminescent techniques to confirm biofilm formation in our cultures. Bioluminescent imaging, however, is primarily advantageous for longitudinal in vivo studies.29,39,40 In contrast, CFU counting is the gold-standard technique for in vitro biofilm studies,41,42 and our resazurin reduction assays provide confirmation in a high-throughput/low-cost setting.18,42 The S. aureus-Xen36 strain is nonetheless advantageous for our in vitro studies, as it also contains a kanamycin-resistance gene cassette.30 Kanamycin can therefore be used to limit confounding growth of other bacteria.30 Importantly, we were careful to exclude kanamycin from the cultures during the antibiotic treatments to avoid potential confounding synergistic effects.
Halicin was previously found to be effective against non-orthopaedic infections in mice.20,22 Those studies demonstrated the feasibility of in vivo halicin treatment for gastrointestinal infections, wound infections, and a subcutaneous abscess biofilm model. In the current study, we found that halicin remains active against S. aureus in biofilms formed on orthopaedically relevant substrates, including Ti6AL4V, Co-Cr, UHMWPE, devitalized muscle, and devitalized bone. We therefore conclude that halicin is a promising antibiotic that should be tested in animal models of orthopaedic infection.
Author contributions
S. Higashihira: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing.
S. J. Simpson: Methodology, Validation, Writing – review & editing.
A. Morita: Data curation, Investigation, Visualization, Writing – review & editing.
J. R. Suryavanshi: Data curation, Investigation, Visualization, Writing – review & editing.
C. J. Arnold: Data curation, Investigation, Writing – review & editing.
R. M. Natoli: Conceptualization, Methodology, Writing – review & editing.
E. M. Greenfield: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding statement
The authors disclose receipt of the following financial or material support for the research, authorship, and/or publication of this article: this project was supported by a Pilot and Feasibility Award within the CDMD NIH/NIDDK (Grant Number P30 DK097512) (to EMG), a Dokkyo Medical University Alumni Association Research Grant (to SH), a grant from the Orthopaedic Research and Education Foundation with funding provided by the John T. Hocker Charitable Foundation in memory of John T. Hocker, MD (Grant Number 22-068) (to JS), a grant from the Orthopaedic Trauma Association (Grant Number 7950) (to JS), and the NIH T32 Comprehensive Musculoskeletal Training Grant (T32AR065971) (to JRS). Co-Cr and highly crosslinked UHMWPE discs were gifted to the authors by Drs Leonard Buller and Evan Deckard (IUSM Department of Orthopaedic Surgery). Muscle and bone tissue from New Zealand White rabbits were gifted to the authors by Drs Uma Sankar and Julian Dilley (IUSM Department of Anatomy, Cell Biology & Physiology).
ICMJE COI statement
E. M. Greenfield reports the Pilot and Feasibility Award within the CDMD NIH/NIDDK (Grant Number P30 DK097512), and materials (Co-Cr and highly crosslinked UHMWPE discs, gifted by Drs. Leonard Buller and Evan Deckard (IUSM Department of Orthopaedic Surgery) and muscle and bone tissue from New Zealand White rabbits gifted by Drs. Uma Sankar and Julian Dilley (IUSM Department of Anatomy, Cell Biology & Physiology), for the purpose of this study. S. Higashihira reports a Dokkyo Medical University Alumni Association Research Grant for this study. J. Suryavanshi reports the following institutional grants: Orthopaedic Research and Education Foundation (No. 22-068), Orthopaedic Trauma Association (No. 7950), and NIH Comprehensive Musculoskeletal Training Grant (T32), all for the purpose of this study.
Data sharing
The data that support the findings for this study are available to other researchers from the corresponding author upon reasonable request.
Acknowledgements
Co-Cr and highly crosslinked UHMWPE discs were gifts of Drs Leonard Buller and Evan Deckard (IUSM Department of Orthopaedic Surgery). Muscle and bone tissue from New Zealand White rabbits were gifted to us from Drs Uma Sankar and Julian Dilley (IUSM Department of Anatomy, Cell Biology & Physiology).
Open access funding
The open access fee for this study was funded by the Indiana University School of Medicine.
Supplementary material
Graphs comparing the effects of DMSO and Kolliphor HS 15 on biofilms, and halicin and vancomycin on biofilms grown on Ti6AL4V, cobalt-chrome, and ultra-high molecular weight polyethylene discs, and devitalized muscle cortical bone.
© 2024 Higashihira et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
Contributor Information
Shota Higashihira, Email: shota.higashihira@gmail.com.
Stefanie J. Simpson, Email: stefsimp@iu.edu.
Akira Morita, Email: akimori@iu.edu.
Joash R. Suryavanshi, Email: jsuryava@iu.edu.
Christopher J. Arnold, Email: arnoldcj@iu.edu.
Roman M. Natoli, Email: rnatoli@iuhealth.org.
Edward M. Greenfield, Email: egreenf@iu.edu.
Data Availability
The data that support the findings for this study are available to other researchers from the corresponding author upon reasonable request.
References
- 1. Davidson DJ, Spratt D, Liddle AD. Implant materials and prosthetic joint infection: the battle with the biofilm. EFORT Open Rev. 2019;4(11):633–639. doi: 10.1302/2058-5241.4.180095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Costerton JW, Montanaro L, Arciola CR. Biofilm in implant infections: its production and regulation. Int J Artif Organs. 2005;28(11):1062–1068. doi: 10.1177/039139880502801103. [DOI] [PubMed] [Google Scholar]
- 3. Metsemakers WJ, Kuehl R, Moriarty TF, et al. Infection after fracture fixation: current surgical and microbiological concepts. Injury. 2018;49(3):511–522. doi: 10.1016/j.injury.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 4. Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27(2):302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McConoughey SJ, Howlin R, Granger JF, et al. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014;9(8):987–1007. doi: 10.2217/fmb.14.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maurice NM, Bedi B, Sadikot RT. Pseudomonas aeruginosa biofilms: host response and clinical implications in lung infections. Am J Respir Cell Mol Biol. 2018;58(4):428–439. doi: 10.1165/rcmb.2017-0321TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5(1):48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 8. Anderl JN, Zahller J, Roe F, Stewart PS. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2003;47(4):1251–1256. doi: 10.1128/AAC.47.4.1251-1256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans DJ, Brown MRW, Allison DG, Gilbert P. Susceptibility of bacterial biofilms to tobramycin: role of specific growth rate and phase in the division cycle. J Antimicrob Chemother. 1990;25(4):585–591. doi: 10.1093/jac/25.4.585. [DOI] [PubMed] [Google Scholar]
- 10. Gimza BD, Cassat JE. Mechanisms of Antibiotic Failure During Staphylococcus aureus Osteomyelitis. Front Immunol. 2021;12:638085. doi: 10.3389/fimmu.2021.638085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35(4):322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 12. Stewart PS, Franklin MJ. Physiological heterogeneity in biofilms. Nat Rev Microbiol. 2008;6(3):199–210. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- 13. Verderosa AD, Totsika M, Fairfull-Smith KE. Bacterial biofilm eradication agents: a current review. Front Chem. 2019;7:824. doi: 10.3389/fchem.2019.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Waters EM, Rowe SE, O’Gara JP, Conlon BP. Convergence of Staphylococcus aureus persister and biofilm research: can biofilms be defined as communities of adherent persister cells? PLoS Pathog. 2016;12(12):e1006012. doi: 10.1371/journal.ppat.1006012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45(4):999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999;37(6):1771–1776. doi: 10.1128/JCM.37.6.1771-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Macià MD, Rojo-Molinero E, Oliver A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin Microbiol Infect. 2014;20(10):981–990. doi: 10.1111/1469-0691.12651. [DOI] [PubMed] [Google Scholar]
- 18. Higashihira S, Simpson SJ, Collier CD, Natoli RM, Kittaka M, Greenfield EM. Halicin Is effective against Staphylococcus aureus biofilms in vitro. Clin Orthop Relat Res. 2022;480(8):1476–1487. doi: 10.1097/CORR.0000000000002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ceri H, Olson M, Morck D, et al. The MBEC Assay System: multiple equivalent biofilms for antibiotic and biocide susceptibility testing. Methods Enzymol. 2001;337:377–385. doi: 10.1016/s0076-6879(01)37026-x. [DOI] [PubMed] [Google Scholar]
- 20. Stokes JM, Yang K, Swanson K, et al. A deep learning approach to antibiotic discovery. Cell. 2020;181(2):475–483. doi: 10.1016/j.cell.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 21. van Gent ME, van der Reijden TJK, Lennard PR, et al. Synergism between the synthetic antibacterial and antibiofilm peptide (SAAP)-148 and halicin. Antibiotics (Basel) 2022;11(5):673. doi: 10.3390/antibiotics11050673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hussain Z, Pengfei S, Yimin L, et al. Study on antibacterial effect of halicin (SU3327) against Enterococcus faecalis and Enterococcus faecium. Pathog Dis. 2022;80(1):ftac037. doi: 10.1093/femspd/ftac037. [DOI] [PubMed] [Google Scholar]
- 23. Naylor PT, Myrvik QN, Gristina A. Antibiotic resistance of biomaterial-adherent coagulase-negative and coagulase-positive staphylococci. Clin Orthop Relat Res. 1990;261(261):126–133. [PubMed] [Google Scholar]
- 24. Webb LX, Holman J, de Araujo B, Zaccaro DJ, Gordon ES. Antibiotic resistance in staphylococci adherent to cortical bone. J Orthop Trauma. 1994;8(1):28–33. doi: 10.1097/00005131-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 25. Gomes LC, Silva LN, Simões M, Melo LF, Mergulhão FJ. Escherichia coli adhesion, biofilm development and antibiotic susceptibility on biomedical materials. J Biomed Mater Res A. 2015;103(4):1414–1423. doi: 10.1002/jbm.a.35277. [DOI] [PubMed] [Google Scholar]
- 26. Badha V, Moore R, Heffernan J, Castaneda P, McLaren A, Overstreet D. Determination of tobramycin and vancomycin exposure required to eradicate biofilms on muscle and bone tissue in vitro. J Bone Jt Infect. 2019;4(1):1–9. doi: 10.7150/jbji.29711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Costerton JW. Biofilm theory can guide the treatment of device-related orthopaedic infections. Clin Orthop Relat Res. 2005;437(437):7–11. doi: 10.1097/00003086-200508000-00003. [DOI] [PubMed] [Google Scholar]
- 28. Osmon DR, Berbari EF, Berendt AR, et al. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):1–10. doi: 10.1093/cid/cis966. [DOI] [PubMed] [Google Scholar]
- 29. Choe H, Narayanan AS, Gandhi DA, et al. Immunomodulatory peptide IDR-1018 decreases implant infection and preserves osseointegration. Clin Orthop Relat Res. 2015;473(9):2898–2907. doi: 10.1007/s11999-015-4301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sosa BR, Niu Y, Turajane K, et al. 2020 John Charnley Award: The antimicrobial potential of bacteriophage-derived lysin in a murine debridement, antibiotics, and implant retention model of prosthetic joint infection. Bone Joint J. 2020;102-B(7_Supple_B):3–10. doi: 10.1302/0301-620X.102B7.BJJ-2019-1590.R1. [DOI] [PubMed] [Google Scholar]
- 31. Koch JA, Pust TM, Cappellini AJ, et al. Staphylococcus epidermidis biofilms have a high tolerance to antibiotics in periprosthetic joint infection. Life (Basel) 2020;10(11):11. doi: 10.3390/life10110253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jang S, Yu L-R, Abdelmegeed MA, Gao Y, Banerjee A, Song B-J. Critical role of c-jun N-terminal protein kinase in promoting mitochondrial dysfunction and acute liver injury. Redox Biol. 2015;6:552–564. doi: 10.1016/j.redox.2015.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wannigama DL, Hurst C, Pearson L, et al. Simple fluorometric-based assay of antibiotic effectiveness for Acinetobacter baumannii biofilms. Sci Rep. 2019;9(1):6300. doi: 10.1038/s41598-019-42353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wannigama DL, Hurst C, Hongsing P, et al. A rapid and simple method for routine determination of antibiotic sensitivity to biofilm populations of Pseudomonas aeruginosa. Ann Clin Microbiol Antimicrob. 2020;19(1):8. doi: 10.1186/s12941-020-00350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen X, Thomsen TR, Winkler H, Xu Y. Influence of biofilm growth age, media, antibiotic concentration and exposure time on Staphylococcus aureus and Pseudomonas aeruginosa biofilm removal in vitro. BMC Microbiol. 2020;20(1):264. doi: 10.1186/s12866-020-01947-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tomizawa T, Nishitani K, Ito H, et al. The limitations of mono- and combination antibiotic therapies on immature biofilms in a murine model of implant-associated osteomyelitis. J Orthop Res. 2021;39(2):449–457. doi: 10.1002/jor.24956. [DOI] [PubMed] [Google Scholar]
- 37. Booq RY, Tawfik EA, Alfassam HA, Alfahad AJ, Alyamani EJ. Assessment of the antibacterial efficacy of halicin against pathogenic bacteria. Antibiotics (Basel) 2021;10(12):1480. doi: 10.3390/antibiotics10121480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hurdle JG, O’Neill AJ, Chopra I, Lee RE. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Microbiol. 2011;9(1):62–75. doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Choe H, Tatro JM, Hausman BS, et al. Staphylococcus aureus and Acinetobacter baumannii inhibit osseointegration of orthopedic implants. Infect Immun. 2022;90(3):e0066921. doi: 10.1128/iai.00669-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pribaz JR, Bernthal NM, Billi F, et al. Mouse model of chronic post-arthroplasty infection: noninvasive in vivo bioluminescence imaging to monitor bacterial burden for long-term study. J Orthop Res. 2012;30(3):335–340. doi: 10.1002/jor.21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Allkja J, van Charante F, Aizawa J, et al. Interlaboratory study for the evaluation of three microtiter plate-based biofilm quantification methods. Sci Rep. 2021;11(1):13779. doi: 10.1038/s41598-021-93115-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Azeredo J, Azevedo NF, Briandet R, et al. Critical review on biofilm methods. Crit Rev Microbiol. 2017;43(3):313–351. doi: 10.1080/1040841X.2016.1208146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings for this study are available to other researchers from the corresponding author upon reasonable request.