During my university studies in Munich, Germany, I explored Zoology, Biochemistry, Parasitology, and Immunology to focus on tumor biology and melanoma formation in my Diploma and PhD projects in Judy Johnson's lab. She encouraged, guided, and provided maximal freedom for scientific thinking and all basic methods.
Cell specification and the plasticity of cell fate in response to surrounding signals and the resulting precise gene activation/repression mechanisms remain my strong interest. At the end of my PhD I came to three major conclusions: first, we cannot fully understand a pathological situation without knowing in depth about the normal genesis of cells along development; second, we need to study molecular mechanisms in vivo to avoid cell lineage artifacts; and third, we need to simplify things by using less complex but informative model organisms that can reveal evolutionarily conserved concepts.
For my post‐doc, I chose Xenopus as an in vivo model at UC Irvine (Prof. Ken Cho lab) and Caltech Pasadena (Prof. Scott Fraser lab) to reveal conserved molecular players in embryonic signaling, notably that both Drosophila and Xenopus Dishevelled (Dsh) can mediate Wnt signaling in Xenopus secondary axis (Spemann's Organizer) formation (Rothbächer et al., 1995; Rothbächer et al., 2000). We also showed that non‐canonical planar cell polarity signaling via Dsh controls gastrulation in vertebrates (Wallingford et al., 2000) while the canonical ß‐catenin from Hydra could induce complete secondary axes upon mRNA injection in Xenopus embryos (Hobmayer et al., 2000).
During my postdoc time, my daughter was born and taught me the true miracles of life, also straightening out my priorities and my efficiency. Together, we thereafter moved to Marseille, France.
At that time tunicates (ascidians) were being established in Patrick Lemaire's lab at the Marseille Institute of Developmental Biology as a simpler chordate developmental model, and I soon realized that ascidians could give access to many questions that were rather difficult to address in Xenopus. As invertebrate chordates, their larvae resemble an evolutionary prototype for vertebrates! Transparency, few and large cells, and an invariant developmental lineage seemed truly amazing, in addition to techniques like electroporation en masse to allow for functional genomics in synchronized embryos. Here, I learned and co‐developed many tools for Ciona functional genomics and I worked in collaboration with this lab for around 10 years while publishing my independent research work. Here, I also obtained the “habilitation to direct research” and supervised doctoral candidates. Discovering the earliest zygotic events and the regulatory DNA (enhancer) level of maternally activated target genes was my main interest in ascidians, and we revealed for example, that a GATA factor is required to specify ectodermal cells, and its range of action is restricted by ß‐catenin (Rothbächer et al., 2007).
In 2012, I established an independent tunicate research group at the University of Innsbruck, Austria. I have a high teaching load but enjoy mentoring young researchers, and also encourage them to develop new experimental approaches in Ciona (Kari et al., 2016). We continued studying the repressive mechanism of ß‐catenin on GATA (Oda‐Ishii et al., 2016), which is reminiscent of opposite wnt/ß‐catenin signaling in C. elegans (Murgan et al., 2015). In parallel, inspired by neighboring groups and the technical possibilities offered by Ciona, I designed a new project aimed at using our knowledge of bioadhesion in ascidians to develop biomimetic glues (Davey et al., 2021). Primarly conducted by a female reseacher, Fan Zeng, first as a Ph.D. student and currently as a postdoc in my lab (Figure 1), we described in detail the cells within the Ciona sensory adhesive papillae (Figure 2a,b; Zeng, Wunderer, Salvenmoser, Ederth, et al., 2019) as well as adhesive components and markers of the adhesive material that these cells secrete at the time of larval settlement (Zeng, Wunderer, Salvenmoser, Hess, et al., 2019), and collaborated with another group on the specification of papillar cells (Johnson et al., 2023). Presently, we are determining the molecular composition of the larval glue in three ascidians and performed Phallusia long‐read genome sequencing to resolve repeated regions (unpublished). Through novel CRISPR technology, Ph.D. candidate Alessandro Pennati (Figure 1) identified and characterized conserved cis‐regulation of tail sensory neuron genes (Figure 2c,d) (Papadogiannis et al., 2022). He is now working on an additional repressive mechanism that further refines binary cell fate choice. Sadly, our work on ascidians is increasingly affected by the warming seas, notably by a seemingly shortened reproductive season in both the Northern Altlantic and the Mediterranean Sea.
FIGURE 1.

The Innsbruck Tunicate lab today: from left to right, Alessandro Pennati (PhD candidate), David Feldmann and Luca Ciampa (Master students), Ute Rothbächer (group leader), Fan Zeng (postdoc) and Laura Kaczmarek (rotation student).
FIGURE 2.
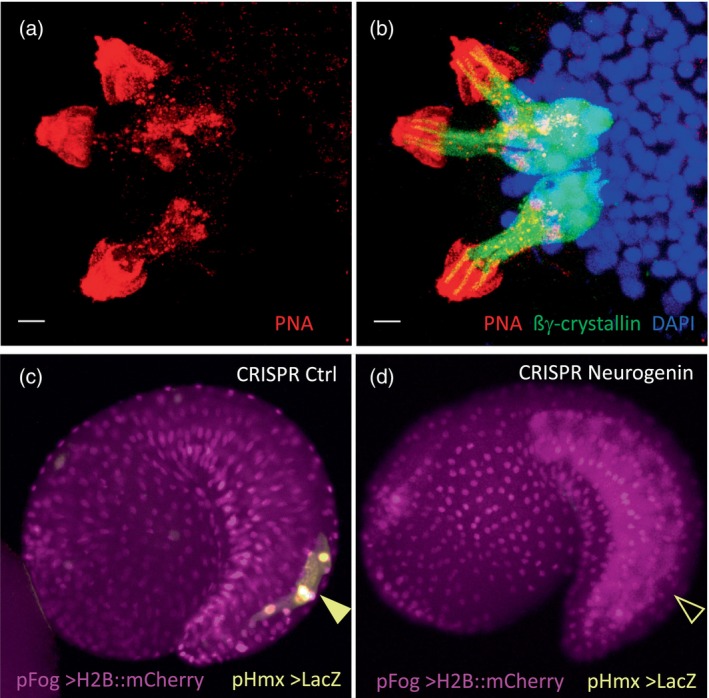
(a,b) Three sensory adhesive papillae of a Ciona larva show collocytes and the anterior hyaline cap (to the left) containing adhesive material marked by peanut agglutinin (PNA, red), while (b) axial columnar cells (ACCs) are marked by an anti‐ßγ‐crystallin (green) antibody (Zeng, Wunderer, Salvenmoser, Hess, et al., 2019). Scale bars 5 μm. (c) A control CRISPR electroporated Ciona tadpole shows normal expression of the Hmx cis‐regulatory region driving expression of the reporter gene lacZ in bipolar tail neurons (BTNs), (d) CRISPR knockout of Neurogenin abolishes expression of Hmx that is required for bipolar tail neuron specification, a sensory cell population that shares Hmx gene expression with vertebrate cranial sensory ganglia of placodal origin. The conservation of cis‐regulatory elements suggests a shared evolutionary ancestry of these cell types (Papadogiannis et al., 2022).
ACKNOWLEDGMENTS
Prof. Marianne Bronner remains a true female role model and I am also grateful for encouragement by my parents, Prof. Christophe Marcelle (my daughter's father), Prof. Ken Cho, Dr. Patrick Lemaire and Prof. Bert Hobmayer, and support from Boehringer Ingelheim Fonds and CNRS, France. Our work was funded by the University of Innsbruck (UIBK) and the Austrian ÖAW, TWF/TNF and FWF agencies and the EU and UIBK cofunded international PhD program ARDRE as well as EU COST actions for Bioadhesion or Marine stem cells (Maristem). Special thanks to previous lab members Dr. Willi Kari, Dr. Johannes Will, M.Sc. Milos Jakobi and the courageous female colleagues Dr. Fan Zeng and M.Sc. Barbara Ritter, both raising two kids, the latter working as a freelance scientific writer. Many unlisted scientists, colleagues and students have contributed or were/are an important source of inspiration. Animal work was fostered by EMBRC France, EU‐Assemble+ and Prof. Loriano Ballarin at Padova University.
Rothbächer, U. (2023). Ascidian gene regulation and bioadhesion. genesis, 61(6), e23572. 10.1002/dvg.23572
REFERENCES
- Davey, P. A. , Power, A. M. , Santos, R. , Bertemes, P. , Ladurner, P. , Palmowski, P. , Clarke, J. , Flammang, P. , Lengerer, B. , Hennebert, E. , Rothbächer, U. , Pjeta, R. , Wunderer, J. , Zurovec, M. , & Aldred, N. (2021). Omics‐based molecular analyses of adhesion by aquatic invertebrates. Biological Reviews of the Cambridge Philosophical Society, 96(3), 1051–1075. 10.1111/brv.12691 [DOI] [PubMed] [Google Scholar]
- Hobmayer, B. , Rentzsch, F. , Kuhn, K. , Happel, C. , von Laue, C. , Snyder, P. , Rothbächer, U. , & Holstein, T. (2000). WNT signalling molecules act in axis formation in the diploblastic metazoan hydra . Nature, 407(6801), 186–189. 10.1038/35025063 [DOI] [PubMed] [Google Scholar]
- Johnson, C. J. , Razy‐Krajka, F. , Zeng, F. , Piekarz, K. M. , Biliya, S. , Rothbächer, U. , & Stolfi, A. (2023). Specification of distinct cell types in a sensory‐adhesive organ for metamorphosis in the Ciona larva. bioRxiv, 2023.2005.2002.539060. 10.1101/2023.05.02.539060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kari, W. , Zeng, F. , Zitzelsberger, L. , Will, J. , & Rothbächer, U. (2016). Embryo microinjection and electroporation in the chordate Ciona intestinalis . JoVE Journal of Visualized Experiments, 116, 54313. 10.3791/54313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgan, S. , Kari, W. , Rothbächer, U. , Iche‐Torres, M. , Melenec, P. , Hobert, O. , & Bertrand, V. (2015). Atypical transcriptional activation by TCF via a Zic transcription factor in C. Elegans neuronal precursors. Developmental Cell, 33(6), 737–745. 10.1016/j.devcel.2015.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda‐Ishii, I. , Kubo, A. , Kari, W. , Suzuki, N. , Rothbächer, U. , & Satou, Y. (2016). A maternal system initiating the zygotic developmental program through combinatorial repression in the ascidian embryo. PLoS Genetics, 12(5), e1006045. 10.1371/journal.pgen.1006045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadogiannis, V. , Pennati, A. , Parker, H. J. , Rothbächer, U. , Patthey, C. , Bronner, M. E. , & Shimeld, S. M. (2022). Hmx gene conservation identifies the origin of vertebrate cranial ganglia. Nature, 605(7911), 701–705. 10.1038/s41586-022-04742-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbächer, U. , Bertrand, V. , Lamy, C. , & Lemaire, P. (2007). A combinatorial code of maternal GATA, Ets and beta‐catenin‐TCF transcription factors specifies and patterns the early ascidian ectoderm. Development, 134(22), 4023–4032. 10.1242/dev.010850 [DOI] [PubMed] [Google Scholar]
- Rothbächer, U. , Laurent, M. N. , Blitz, I. L. , Watabe, T. , Marsh, L. J. , & Cho, K. W. Y. (1995). Drosophila Dishevelled functionally mimics the Wnt signal in Xenopus . Developmental Biology, 170(2), 771. [DOI] [PubMed] [Google Scholar]
- Rothbächer, U. , Laurent, M. N. , Deardorff, M. A. , Klein, P. S. , Cho, K. W. Y. , & Fraser, S. E. (2000). Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO Journal, 19(5), 1010–1022. 10.1093/emboj/19.5.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford, J. , Rowning, B. , Vogeli, K. , Rothbächer, U. , Fraser, S. , & Harland, R. (2000). Dishevelled controls cell polarity during Xenopus gastrulation. Nature, 405(6782), 81–85. 10.1038/35011077 [DOI] [PubMed] [Google Scholar]
- Zeng, F. , Wunderer, J. , Salvenmoser, W. , Ederth, T. , & Rothbächer, U. (2019). Identifying adhesive components in a model tunicate. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 374(1784), 20190197. 10.1098/rstb.2019.0197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, F. , Wunderer, J. , Salvenmoser, W. , Hess, M. W. , Ladurner, P. , & Rothbächer, U. (2019). Papillae revisited and the nature of the adhesive secreting collocytes. Developmental Biology, 448(2), 183–198. 10.1016/j.ydbio.2018.11.012 [DOI] [PubMed] [Google Scholar]


