Summary
Chronotype is linked to adverse health measures and may have important associations with obstructive sleep apnea and blood pressure, but data are limited. This study aimed to determine the separate and combined associations of chronotype with obstructive sleep apnea and blood pressure in a middle‐aged community population. Adults (n = 811) from the Raine Study (female = 59.2%; age mean [range] = 56.6 [42.1–76.6] years) were assessed for chronotype (Morningness–Eveningness Questionnaire), blood pressure and hypertension (doctor diagnosed or systolic blood pressure ≥ 140 mmHg and/or diastolic ≥ 90 mmHg), and obstructive sleep apnea at different in‐laboratory apnea–hypopnea index thresholds (5, 10, 15 events per hr). Linear and logistic regression models examined relationships between chronotype and the presence and severity of obstructive sleep apnea, blood pressure, hypertension, and blood pressure stratified by obstructive sleep apnea severity at above‐mentioned apnea–hypopnea index thresholds. Covariates included age, sex, body mass index, alcohol consumption, smoking, physical activity, sleep duration, anti‐hypertensive medication, insomnia, and depressive symptoms. Most participants were categorised as morning (40%) or intermediate (43%), with 17% meeting criteria for evening chronotypes. Participants with apnea–hypopnea index ≥ 15 events per hr and morning chronotype had higher systolic (9.9 mmHg, p < 0.001) and a trend for higher diastolic blood pressure (3.4 mmHg, p = 0.07) compared with those with an evening chronotype, and higher systolic blood pressure compared with those with an intermediate chronotype (4.8 mmHg, p = 0.03). Across chronotype categories, no differences in systolic or diastolic blood pressure or odds of hypertension were found at apnea–hypopnea index thresholds of ≥ 5 or ≥ 10 events per hr. Among participants with apnea–hypopnea index ≥ 15 events per hr, systolic blood pressure is higher in those with a morning chronotype than evening and intermediate chronotypes. Assessment for morning chronotype may improve risk stratification for hypertension in patients with obstructive sleep apnea.
Keywords: blood pressure, chronotype, community, eveningness, middle‐aged, morningness, sleep apnea
1. INTRODUCTION
Obstructive sleep apnea (OSA) is a prevalent form of sleep‐disordered breathing that is estimated to affect approximately one billion middle‐aged adults worldwide (Benjafield et al., 2019). OSA is characterised by repeated episodes of upper airway collapse resulting in unstable ventilation, intermittent hypoxaemia, cortical arousals, large intrathoracic pressure swings and surges in blood pressure (BP) during sleep (Ryan, 2018). These consequences lead to injurious physiological responses, including oxidative damage, endothelial dysfunction and increased sympathetic activity, which independently or collectively can result in and contribute to hypertension (Chobanian et al., 2003).
Hypertension is an important risk factor for life‐threatening conditions, including coronary artery disease, stroke, heart failure and kidney disease (Rapsomaniki et al., 2014). An estimated 47% of patients with OSA have hypertension (Millman et al., 1991). Dose–response relationships between OSA and both prevalent and incident hypertension have been demonstrated in large population studies (Nieto et al., 2000; Peppard et al., 2000; Young, 1997). These relationships are independent of potential confounding risk factors for hypertension, such as increased body mass index (BMI), older age, male sex, excess alcohol consumption and smoking.
Chronotype is most commonly defined according to behavioural preferences for morning or evening activities, with intermediate types allocated to the middle of the spectrum (Horne & Ostberg, 1976). Recent studies have assessed chronotype as a risk factor for both OSA (Lucassen et al., 2013) and hypertension (Merikanto et al., 2013); however, the interactions between chronotype, OSA and hypertension remain unclear. The most commonly found association is between evening chronotype and higher prevalence of OSA (Lucassen et al., 2013) and hypertension (Merikanto et al., 2013), and other adverse health outcomes (Knutson & von Schantz, 2018). Postulated mechanisms for such associations are that individuals with an evening chronotype experience circadian misalignment, due to difficulties conforming to early work start times or social activities, and have more risky health behaviours, such as smoking, excessive alcohol consumption and physical inactivity (Makarem et al., 2020; Zhang et al., 2019). Other studies have found complex associations between chronotype, sleep quality and health measures; for example, individuals with a morning chronotype were found to have elevated systolic BP and increased obesity in the presence of objectively poor sleep quality (McMahon et al., 2019). In contrast, some studies have failed to show associations between chronotype, OSA and BP or hypertension (Kim et al., 2015; McMahon et al., 2019). OSA impairs sleep quality and is associated with increased BP, yet no study to date has explored the influence of chronotype on the relationship between OSA and hypertension.
This study aimed to determine if chronotype was independently associated with OSA, BP and hypertension. This study also aimed to explore if chronotype influenced the known relationship between OSA and BP and hypertension.
2. METHODS
2.1. Participants
Data for this study were obtained from the Raine Study Generation 1–26‐year follow‐up. The Raine study has been described in detail elsewhere (Dontje et al., 2019); briefly, it is an ongoing community study in Western Australia that enrolled 2900 pregnant women between 1989 and 1991. The mothers (i.e. the 2900 pregnant women) and fathers are termed Generation 1 (Gen1), and their children are termed Generation 2 (Gen2). Gen1 participants underwent a health evaluation between 22 April 2015 and 16 June 2017, at a time when their children (Gen2 participants) were on average 26 years old. A total of 1098 of those currently available for follow‐up took part in the Gen1–26‐year follow‐up. Health evaluations included health and sleep questionnaires, BP measurements and an overnight in‐laboratory polysomnogram (PSG). Participants were excluded from analyses if they had incomplete data for sleep assessments or sociodemographic and health covariates (described below). Written informed consent was obtained from all participants at the time of enrolment, and approval for the study was received from the University of Western Australia Human Research Ethics committee (RA/4/7236 Adult Sleep Study: Prevalence, Phenotype & Genotype of Common Sleep Disorders).
2.2. Study evaluations
2.2.1. Blood pressure
The BP measurements were obtained at three time points over 24 hr: in the afternoon (14:30 hours–18:00 hours) and evening (21:00 hours–22:00 hours) of the PSG, and the following morning (05:30 hours–07:00 hours; see Appendix S1). All measurements were performed using the Dinamap ProCare 100 (GE Medical Systems [Dinamap], UK; calibrated according to service requirements) non‐invasive BP monitor. The correct cuff size was placed on the right arm with the cuff arrow aligned with the brachial artery. The first BP measurement at each time point was excluded, and the remaining BP measurements were used to calculate the average systolic and diastolic BP at each time point. The average systolic and diastolic BP from the afternoon, evening and morning measurements were then averaged to give an overall systolic and diastolic BP average (Unger et al., 2020). Information on hypertension was also determined from a health questionnaire in which participants were asked “has a doctor diagnosed you with high blood pressure?”. Prevalent hypertension was diagnosed as either: (i) “yes” to doctor diagnosed high BP; or (ii) mean systolic BP of ≥ 140 mmHg or diastolic BP of ≥ 90 mmHg.
2.2.2. Polysomnography
Participants underwent a level one in‐laboratory PSG study at the Centre for Sleep Science, The University of Western Australia using the Compumedics Grael sleep‐monitoring system (Grael Compumedics, Abbotsford, Victoria, Australia). Participants were free to go to sleep (i.e. lights out) any time before midnight, and were awoken at or after 06:00 hours. The in‐laboratory PSG evaluation and scoring were carried out by experienced sleep scientists. An apnea was defined as a 90% reduction in airflow for ≥ 10 s, and a hypopnea was defined as ≥ 30% reduction in airflow lasting ≥ 10 s and accompanied by either an arousal or 3% oxygen desaturation (Berry et al., 2012). In the present study, OSA was defined at apnea–hypopnea index (AHI) thresholds of 5, 10 and 15 events per hr. A number of variables were determined from PSG, including: time spent with oxygen saturation under 90% (T90), lowest oxygen saturation, arousal index, periodic limb movement index, percentage of non‐rapid eye movement (NREM) and rapid eye movement (REM), sleep‐onset latency (SOL), wake after sleep onset (WASO) and sleep efficiency (SE).
2.2.3. Chronotype: The Morningness–Eveningness Questionnaire (MEQ)
The MEQ consists of 19 items that aim to assess individual preference for morning or evening (Horne & Ostberg, 1976). Questions relate to sleep and wake time, exercise habits, use of an alarm clock, and most productive work hours. Chronotype was categorised by score ranges recommended in a validation study of a middle‐aged population (Taillard et al., 2004): evening chronotypes 16–52; intermediate chronotypes 53–64; and morning chronotypes 65–86.
2.2.4. Sociodemographic and health measures
Sociodemographic information on age, sex, ethnicity (Caucasian, Aboriginal, Polynesian, Vietnamese, Chinese, Indian, other), annual personal income (Australian Dollars; low, < $31,999; medium, $31,200–$64,999; high, > $65,000) and education (high school or less, training after school, university) was obtained. Height and weight were measured, and BMI calculated. Information on lifestyle habits and the presence of insomnia and depressive symptoms were obtained by questionnaire within the month prior to the overnight PSG. Self‐reported sleep duration was assessed by asking “How many total hours of actual sleep do you usually get (hours)”, and was categorised as short (≤ 6 hr), normal (> 6–8 hr) or long sleepers (> 8 hr). Smoking status was assessed by asking “are you a current smoker?”, and participants were categorised as current smokers or non‐current smokers (i.e. never or past smokers). Daily alcohol consumption was obtained from the Australian version of the food frequency questionnaire (Dietary Questionnaire for Epidemiological Studies [DQES]; Ireland et al., 1994). Alcohol consumption was then used to categorise participants to abstainers (< 1 drink per day), moderate consumers (≥ 1 and ≤ 4 drinks per day) or high consumers (> 4 standard drinks per day). The International Physical Activity Questionnaire (IPAQ) short form was used to determine physical activity, and participants were categorised according to IPAQ guidelines (low, moderate, high activity; The IPAQ Group, 2005). Regular hypnotic use was defined as taking a hypnotic three or more times a week in the last month. All participants who responded “yes” to doing shift work were classified as shift workers. The presence of insomnia and depression symptoms was based on the Insomnia Symptom Questionnaire (ISQ; Okun et al., 2009) and the Depression Anxiety Stress Scale‐21 (DASS‐21; Antony et al., 1998).
2.3. Statistical analyses
Continuous variables were presented as mean (standard deviation, SD) and categorical variables as n (%). Strongly skewed continuous variables were log transformed for multivariable analysis (AHI and T90). Participant demographic and sleep characteristics were compared across the chronotype groups using a one‐way analysis of variance (ANOVA) for continuous variables and Pearson's χ²‐test for categorical variables. Pairwise comparisons were performed for variables with significant differences between chronotypes using unpaired t‐tests and χ 2‐tests for categorical variables.
2.3.1. Logistic regression models
Logistic regression models (odds ratio [OR] and 95% confidence interval [CI]) were performed to investigate: (i) independent association between chronotype and OSA; (ii) independent association between chronotype and hypertension; (iii) interaction between chronotype and AHI on hypertension; and (iv) association between chronotype and hypertension after stratification by OSA severity.
2.3.2. Mean differences between chronotypes
Linear regression models were used to compare the adjusted means and standard errors (R package emmeans; version 1.7.2) of log AHI, logT90, systolic and diastolic BP, between chronotype groups. Tukey's pairwise comparisons of the adjusted mean difference (95% CI) were calculated post hoc. In a separate analysis, using the same linear models as above, an interaction term was entered between log AHI and chronotype. This analysis was repeated for diastolic and systolic BP across chronotypes stratified by OSA severity (using threshold AHI values of 5, 10 and 15 events per hr to define OSA). In supplementary analyses we defined OSA severity based on T90. T90 severity was dichotomised as below (low) or above (high) the median.
2.3.3. Covariates
Covariates considered for models with BP and hypertension outcomes were age, sex, BMI, alcohol consumption, depressive symptoms, insomnia symptoms, self‐reported physical activity, self‐reported sleep duration, smoking status, prescribed BP medication and AHI. For models with the outcome of AHI and T90, the same covariates were considered except AHI and BP medication. Backward elimination (R package olsrr and blorr; Chatterjee & Hadi, 2012), was used to select sociodemographic and health covariates for statistical models (Zhang, 2016), and covariates were excluded if multicollinearity existed with another variable. Multicollinearity was assessed using variance inflation factors (values above 10 were considered high; O'brien, 2007). A p‐value of ≤ 0.05 was considered statistically significant. All analyses were performed in RStudio (RStudio Team 2018, Boston, MA, USA) and R version 4.0.4 (R Core Teams 2021, Vienna, Austria). Tables and graphs were produced using R packages Tidyverse (version 1.3.0), gtsummary (version 1.3.7) and flextable (version 0.6.6).
3. RESULTS
3.1. Participants and study flow
There were 1098 participants in the Gen1–26‐year follow‐up, and 1026 of them completed the MEQ (Figure 1). Of these participants, a total of 911 individuals had PSG and BP data to diagnose OSA and hypertension; however, of these, seven participants did not have BP measurements from all three time points (afternoon, evening, morning), thus the average BP was calculated from available measures. A further 100 were excluded due to missing sociodemographic or health covariate information required for multivariable models. Therefore, the total study sample analysed comprised 811 participants. A comparison of sociodemographic and health characteristics of the included versus excluded participants showed slightly higher income and education attainment in the included (Table S1).
FIGURE 1.
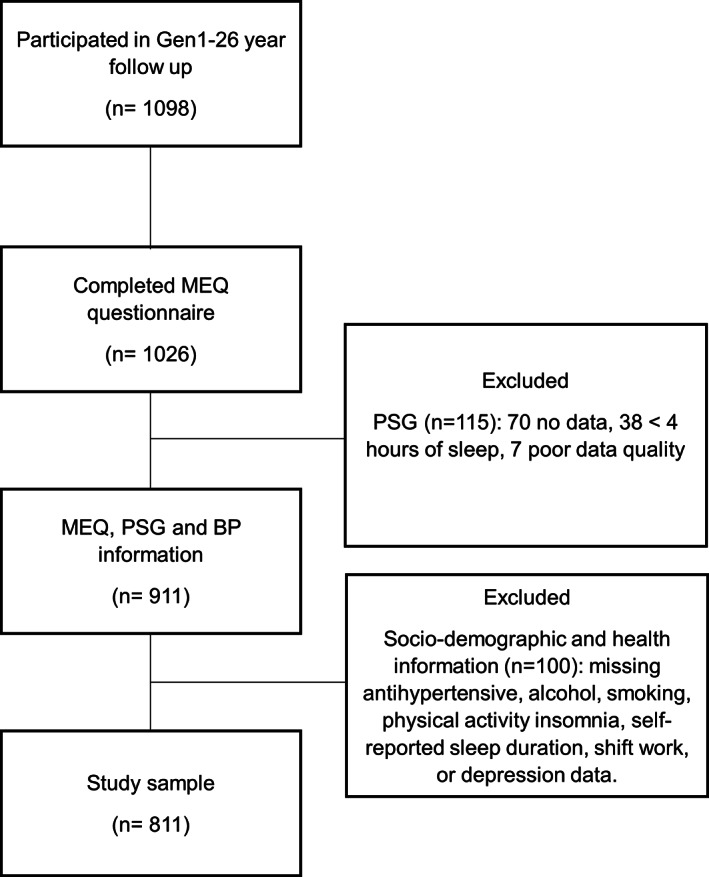
Participant flow diagram. BP, blood pressure; MEQ, Morningness–Eveningness Questionnaire; PSG, polysomnogram
3.2. Sociodemographic and health characteristics
Differences in sex, age, shift work, smoking status, physical activity and depression symptoms were observed between chronotypes (Table 1). In pairwise comparisons, relative to morning and intermediate chronotypes, evening chronotypes were more likely to be female (p = 0.008, p = 0.016, respectively), younger in age (p < 0.001, p = 0.001), engage in shift work (p = 0.006, p = 0.006), smoke (p < 0.001, p < 0.001) and report depressive symptoms (p < 0.001, p = 0.013). Participants with a morning chronotype showed higher self‐reported physical activity levels compared with those with an intermediate (p = 0.019) and evening chronotype (p = 0.05).
TABLE 1.
Sociodemographic, health and sleep characteristics of participants
| Chronotype | ||||
|---|---|---|---|---|
| Characteristic | Morning, N = 326 a | Evening, N = 138 a | Intermediate, N = 347 a | p‐Value b |
| Sex | 0.014 | |||
| Male | 142 (44%) | 41 (30%) | 148 (43%) | |
| Female | 184 (56%) | 97 (70%) | 199 (57%) | |
| Age, years | 57.0 (5.4) | 55.1 (5.7) | 56.9 (5.5) | 0.002 |
| Ethnicity | 0.4 | |||
| Caucasian | 305 (94%) | 126 (91%) | 312 (90%) | |
| Aboriginal | 2 (0.6%) | 0 (0%) | 2 (0.6%) | |
| Polynesian | 2 (0.6%) | 1 (0.7%) | 0 (0%) | |
| Vietnamese | 0 (0%) | 1 (0.7%) | 2 (0.6%) | |
| Chinese | 10 (3.1%) | 3 (2.2%) | 12 (3.5%) | |
| Indian | 6 (1.8%) | 6 (4.3%) | 17 (4.9%) | |
| Other | 0 (0%) | 1 (0.7%) | 2 (0.6%) | |
| Unknown | 1 | 0 | 0 | |
| Education | 0.8 | |||
| Education: High school or less | 77 (24%) | 28 (20%) | 77 (22%) | |
| Education: Training after school | 120 (37%) | 59 (43%) | 130 (38%) | |
| Education: University | 126 (39%) | 50 (36%) | 139 (40%) | |
| Unknown | 3 | 1 | 1 | |
| Income | 0.10 | |||
| Low | 83 (26%) | 48 (35%) | 79 (23%) | |
| Middle | 88 (28%) | 40 (29%) | 103 (30%) | |
| High | 144 (46%) | 50 (36%) | 159 (47%) | |
| Unknown | 11 | 0 | 6 | |
| Shift work (Yes) | 34 (10%) | 28 (20%) | 36 (10%) | 0.005 |
| Smoking (Yes) | 29 (8.9%) | 29 (21%) | 24 (6.9%) | < 0.001 |
| Alcohol | 0.7 | |||
| Low | 101 (31%) | 48 (35%) | 118 (34%) | |
| Moderate | 167 (51%) | 64 (46%) | 177 (51%) | |
| High | 58 (18%) | 26 (19%) | 52 (15%) | |
| BMI, kg m−2 b | 28.2 (5.3) | 29.3 (6.8) | 28.1 (5.3) | 0.075 |
| Physical activity | 0.040 | |||
| Low | 71 (22%) | 43 (31%) | 108 (31%) | |
| Moderate | 113 (35%) | 49 (36%) | 110 (32%) | |
| High | 142 (44%) | 46 (33%) | 129 (37%) | |
| Hypertension (Yes) | 86 (26%) | 40 (29%) | 94 (27%) | 0.8 |
| Depression symptoms (Yes) | 37 (11%) | 34 (25%) | 51 (15%) | 0.001 |
Abbreviations: BMI, body mass index.
Note: n (%); mean (SD).
Pearson's χ²‐test; one‐way ANOVA.
3.3. Sleep‐related measurements
Differences in sleep characteristics between chronotypes are summarised in Table 2. In pairwise comparisons, relative to morning and intermediate chronotypes, evening chronotypes had longer SOL (p < 0.001, p = 0.029, respectively), increased percentage of NREM sleep (p = 0.002, p = 0.003) and reduced percentage of REM sleep (p = 0.003, p = 0.003), more insomnia symptoms (p < 0.001, p < 0.001), poorer self‐reported sleep quality (p < 0.001, p = 0.003), and were more likely to use hypnotics (p < 0.001, p = 0.006) regularly.
TABLE 2.
Sleep‐related characteristics of participants
| Chronotype | ||||
|---|---|---|---|---|
| Variable | Morning, N = 326 a | Intermediate, N = 347 a | Evening, N = 138 a | p‐Value b |
| Insomnia symptoms (Yes) | 39 (12%) | 42 (12%) | 34 (25%) | < 0.001 |
| Sleepiness (ESS) | 6.4 (3.7) | 6.0 (3.4) | 6.0 (3.5) | 0.3 |
| Unknown | 0 | 1 | 1 | |
| Sleep quality (PSQI) | 5.1 (3.0) | 5.6 (3.3) | 6.6 (3.4) | < 0.001 |
| Unknown | 7 | 9 | 1 | |
| Hypnotic medication (Yes) | 37 (11%) | 64 (18%) | 42 (30%) | < 0.001 |
| Unknown | 0 | 1 | 0 | |
| Self‐reported sleep duration | 0.061 | |||
| < 6 hr | 74 (23%) | 96 (28%) | 33 (24%) | |
| 6–8 hr | 232 (71%) | 222 (64%) | 87 (63%) | |
| > 8 hr | 20 (6.1%) | 29 (8.4%) | 18 (13%) | |
| PSG total sleep time, hr | 6.1 (0.8) | 6.1 (0.8) | 5.9 (0.8) | 0.047 |
| AHI, events per hr | 13.1 (14.8) | 14.8 (16.5) | 13.6 (15.1) | 0.4 |
| T90, min | 3.3 (13.5) | 5.4 (23.9) | 7.7 (31.7) | 0.13 |
| SE, % | 80.7 (14.1) | 82.0 (19.7) | 81.2 (22.1) | 0.7 |
| SOL, min | 12.9 (12.9) | 17.2 (17.1) | 21.0 (19.6) | < 0.001 |
| WASO, min | 72.1 (38.5) | 70.7 (35.3) | 70.3 (36.7) | 0.8 |
| NREM, % of total sleep | 80.8 (6.3) | 80.9 (5.9) | 82.9 (6.2) | 0.002 |
| REM, % of total sleep | 19.1 (6.3) | 19.1 (5.9) | 17.1 (6.2) | 0.002 |
| Periodic leg movements, events per hr | 9.3 (16.4) | 10.5 (19.3) | 8.5 (16.2) | 0.5 |
| Arousal Index, events per hr | 11.0 (12.9) | 11.3 (13.0) | 12.5 (14.1) | 0.3 |
| Lowest oxygen saturation, % | 85.6 (7.3) | 84.6 (7.8) | 85.2 (7.7) | 0.4 |
| Unknown | 0 | 1 | 1 | |
Abbreviations: AHI, apnea–hypopnea index; ESS, Epworth Sleepiness Scale; NREM, non‐rapid eye movement; PSG, polysomnography; PSQI, Pittsburgh Sleep Quality Index; REM, rapid eye movement; SE, sleep efficiency; SOL, sleep‐onset latency; T90, time SpO2 < 90% saturation; WASO, wake after sleep onset.
Mean (SD).
One‐way ANOVA.
3.4. Chronotype and OSA
There were no differences between chronotypes in the presence of OSA across the AHI thresholds of 5, 10 and 15 events per hr. Similarly, the adjusted mean log AHI and log T90 (Table 3) were not different according to chronotype.
TABLE 3.
Independent associations between chronotype and the presence of OSA and OSA severity
| AHI ≥ 5 events per hr | AHI ≥10 events per hr | AHI ≥ 15 events per hr | Log AHI | Log T90 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | OR (95% CI) | p‐Value | OR (95% CI) | p‐Value | OR (95% CI) | p‐Value | Adjusted means (SE) b | Adjusted means (SE) b |
| Chronotype | |||||||||
| Morning | 326 | 2.3 (0) | 0.7 (0.1) | ||||||
| Intermediate a | 347 | 1.2 (0.8, 1.8) | 0.3 | 1.0 (0.7, 1.4) | 0.9 | 1.2 (0.8, 1.7) | 0.3 | 2.4 (0) | 0.8 (0.1) |
| Evening a | 138 | 1.0 (0.7 1.7) | 0.9 | 1.3 (0.8, 2.0) | 0.3 | 0.9 (0.6, 1.6) | 0.8 | 2.3 (0.1) | 0.8 (0.1) |
Note: The presence of OSA was defined as an AHI of ≥ 5, ≥ 10 and ≥ 15 events per hr. Variables assessed as potential covariates for models were age, sex, BMI, alcohol, smoking, physical activity, self‐reported sleep duration, insomnia and depression. Covariates were excluded if not significant (p > 0.05) or multicollinearity existed.
Abbreviations: AHI, apnea–hypopnea index; CI, confidence interval; OR, odds ratio; SE, standard error; T90, time spent with arterial oxygen saturation < 90%.
No significant difference in odds of OSA was found between evening and intermediate chronotypes, when evening was the reference group, across all three cut‐offs (p > 0.05).
Adjusted means are not significantly different between three chronotype groups in pairwise comparisons (p > 0.05).
3.5. Chronotype, BP and hypertension
The adjusted mean systolic and diastolic BP and odds of prevalent hypertension were not different between chronotypes (Table 4). However, participants with OSA had increased odds of hypertension and AHI was significantly associated with increased systolic and diastolic BP (Table S2).
TABLE 4.
Independent associations between chronotype and the presence of hypertension and measured BP
| Hypertension | Systolic BP | Diastolic BP | |||
|---|---|---|---|---|---|
| Characteristic | N | OR (95% CI) | p‐Value | Adjusted means (SE) b | Adjusted means (SE) b |
| Chronotype | |||||
| Morning | 326 | Reference | 124.0 (0.8) | 72.4 (0.5) | |
| Intermediate a | 347 | 0.9 (0.6, 1.4) | 0.6 | 122.6 (0.8) | 71.6 (0.5) |
| Evening a | 138 | 0.9 (0.5, 1.6) | 0.8 | 122.3 (1.2) | 71.2 (0.7) |
Note: Variables assessed as potential covariates for models were age, sex, BMI, alcohol, smoking, physical activity, self‐reported sleep duration, depression, insomnia, AHI and anti‐hypertensive medication. Covariates were excluded if not significant (p > 0.05) or multicollinearity existed.
Abbreviations: BP, blood pressure; CI, confidence interval; OR, odds ratio; SE, standard error.
Comparison of evening and intermediate groups with evening as the reference was not significantly different (p > 0.05).
Adjusted systolic and diastolic BP means were not significantly different between three chronotype groups (p > 0.05).
3.6. Chronotype interaction and stratification by OSA on BP and hypertension
An interaction term between AHI and chronotype for linear models and an interaction between OSA and chronotype for logistic models was not significant (p > 0.05). In stratified analyses, morning chronotypes in the stratum AHI ≥ 15 events per hr had higher systolic BP than intermediate and evening chronotypes by 4.8 mmHg (p = 0.03) and 9.9 mmHg (p < 0.001), respectively (Figure 2). In the stratum with AHI ≥ 15 events per hr, a trend for higher diastolic BP was found in morning chronotypes relative to evening chronotypes (3.4 mmHg, p = 0.07). No differences in systolic or diastolic BP were observed between chronotypes among participants with OSA defined by AHI thresholds of 5 and 10 events per hr (Table S3). In logistic modelling, the odds of hypertension were not different between chronotypes across the OSA strata (Table S3). Additionally, stratification by OSA, according to oxygenation (T90), revealed no differences in BP and prevalent hypertension (Tables S4 and S5, respectively).
FIGURE 2.
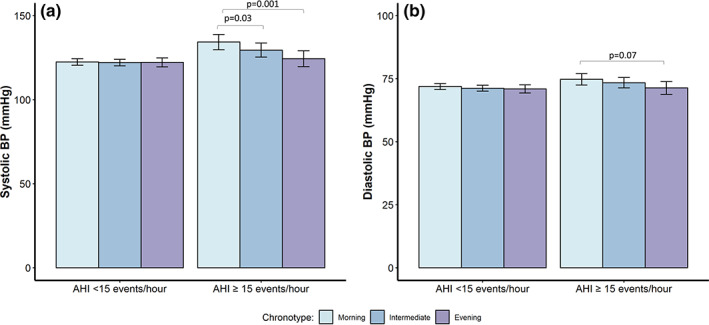
Adjusted systolic and diastolic blood pressure (BP) across chronotype groups in participants with an apnea–hypopnea index (AHI) < 15 and an AHI ≥ 15 events per hr. (a, b) Systolic and diastolic BP, respectively. Error bars reflect 95% confidence interval (CI) of standard error of the mean. Variables assessed as potential covariates for models were age, sex, body mass index (BMI; kg m−2), alcohol, smoking, physical activity, self‐reported sleep duration, depressive symptoms, insomnia symptoms, AHI, and anti‐hypertensive medication. Covariates were excluded if p‐value > 0.05 or multicollinearity existed
4. DISCUSSION
This study used cross‐sectional data from a large, well‐characterised community population to add to the limited information on the relationships between chronotype, OSA, BP and hypertension. Participants in this cohort were middle‐aged, identified as predominantly Caucasian and, apart from slightly increased socio‐economic status, are representative of the general Australian population based on sociodemographic factors and BMI (Dontje et al., 2019; McArdle et al., 2022). The main study findings were that chronotype was not associated with increased odds of OSA or hypertension. We extend previous work by showing that participants with an AHI ≥ 15 events per hr who were morning chronotypes had higher systolic BP compared with those with an evening and intermediate chronotype, independent of potential confounders. Hence, chronotype may modify the relationship between OSA and BP.
4.1. Chronotype and OSA
Our study showed no independent association between chronotype and OSA. The few prior studies examining the relationship between chronotype, assessed by the MEQ, and OSA have reported inconsistent findings. The community sample studied in the present investigation is comparable to a community study by Kim et al. (2015), albeit the latter were a slightly younger cohort of multi‐ethnic individuals (mean age 40 years, range 20–80 years, n = 856). Our findings are largely consistent with the Kim et al. (2015) study, which also found no association between chronotype and OSA (diagnosed by in‐laboratory PSG) after adjusting for BMI and age.
Our findings differ from two previous publications that examined the relationship between chronotype and OSA. In a highly selected sample of participants who were obese and short sleepers (n = 119; BMI 30–55 kg m−2; < 6 hr sleep duration; age range, 18–50 years), Lucassen et al. (2013) found that “eveningness” was independently predictive of the frequency of obstructive breathing events, assessed using a level 3 home sleep study. Elevated levels of plasma adrenocorticotrophic hormone and urinary adrenaline and noradrenaline levels were also found in evening chronotypes, suggesting elevated sympathetic activity, a feature consistent with OSA. In contrast, in a USA multi‐ethnic community study of women (n = 506; mean age 37 ± 16 years), those with an intermediate chronotype were twice as likely to be at risk for OSA, assessed using the Berlin Questionnaire (Netzer et al., 1999), compared with individuals with a morning chronotype (Makarem et al., 2020).
The explanation for these discrepancies could reflect several factors, including differences in characteristics of the samples and the different methods used to diagnose OSA. Age (Diaz‐Morales & Parra‐Robledo, 2018; Paine et al., 2006) and, in some studies, sex (Adan & Natale, 2002; Mongrain et al., 2005) and ethnicity (Malone et al., 2016) have been shown to influence chronotype. General population samples, as in the current and the Kim et al. (2015) study found no associations, whereas associations were found in selected samples (Lucassen et al., 2013; Makarem et al., 2020). Indeed, in the Kim et al. (2015) study, stratified analyses found morning and evening chronotypes had significantly higher AHI scores in the sub‐group with a BMI > 26.8 kg m−2 and age > 42 years relative to intermediate chronotypes. Both the current and Kim et al. (2015) study used gold‐standard in‐laboratory PSG to diagnose OSA, whereas Lucassen et al. (2013) and Makarem et al. (2020) used limited sleep studies and questionnaires, respectively.
4.2. Chronotype and BP
Our study showed no independent association between chronotype and BP or hypertension. Similar results were found when we used a “high‐normal” BP threshold (i.e. systolic BP > 130 mmHg and diastolic BP > 85 mmHg) in a secondary analysis (Table S6). A number of studies have demonstrated associations between evening chronotype and poor cardiovascular health and all‐cause mortality, compared with other chronotypes (Knutson & von Schantz, 2018; Makarem et al., 2020; Merikanto et al., 2013). Evening chronotypes report unhealthy behavioural patterns that are postulated to contribute to these worse health outcomes (Makarem et al., 2020; Maukonen et al., 2016; Nauha et al., 2020). In the current study we confirm that a range of adverse health and lifestyle characteristics, including depression, insomnia, regular hypnotic medication use and shift work, were associated with evening chronotype. Although some of these behavioural characteristics could predispose individuals to increased BP and hypertension, we did not confirm the latter associations. Consistent with our findings, two USA‐based studies, one of young healthy participants (n = 430) by McMahon et al. (2019) and the other of women aged 20–79 years (n = 506) by Makarem et al. (2020) reported no association between chronotype and BP. Of note, in the study by McMahon et al. (2019), chronotype was determined using mid‐sleep time from actigraphy rather than the MEQ and participants with hypertension were excluded.
By contrast, a large general community cohort (n = 4589) found evening chronotypes had 1.3‐fold increased odds of prevalent self‐reported hypertension compared with morning chronotypes (Merikanto et al., 2013). Counterintuitively, BP measured between 07:00 hours and 01:00 hours was lower in the evening relative to morning chronotypes. The authors indicated that timing of BP measurement was similar across the chronotypes and unlikely to explain this apparent inconsistency.
Considering the above studies together, and the findings of the present study, it is unclear whether chronotype is linked to BP and hypertension. Large and well‐characterised cohorts may be required to better understand whether chronotype contributes to adverse outcomes by its effects on BP, and whether there are susceptible demographic sub‐groups.
4.3. Chronotype interaction and stratification by OSA on BP/hypertension
The known relationships between AHI and BP (Young, 1997) and OSA and hypertension (Peppard et al., 2000) were confirmed in our cohort. We investigated, for the first time, whether chronotype modifies this relationship between OSA, BP and hypertension. As there were no prior studies of this relationship to guide our choice of appropriate AHI thresholds, we explored three commonly used thresholds (≥ 5, ≥ 10 and ≥ 15 events per hr). While there were no statistically significant interactions between chronotype and OSA on BP in the whole cohort, there was an association between chronotype and OSA in the stratified analysis. Specifically, in the stratum of AHI ≥ 15 events per hr, we found morning chronotypes had higher systolic BP compared with those with evening and intermediate chronotypes, and there was a trend to higher diastolic BP in those with morning compared with evening chronotype. We re‐examined the data using an AHI threshold of 30 events per hr in secondary analysis, due to a smaller sample size, and found systolic BP in morning chronotypes was 15 mmHg (p = 0.0008) less relative to evening chronotypes (Table S3). These differences in BP, between 5 and 10 mmHg on average, may be of clinical significance given that a 5 mmHg reduction in BP is associated with a 10% decrease in cardiovascular risk (Rahimi et al., 2021). Therefore, morning chronotypes with co‐existing moderately severe OSA may be at higher risk of increased BP, and could benefit from education about this risk and focused efforts to treat their OSA. The negative finding in the logistic regression modelling, using hypertension as an outcome, may relate to a lack of statistical power relative to the use of a continuous measure (BP). Similarly, when high‐normal BP was used as an outcome, using the threshold of systolic BP > 130 mmHg and diastolic BP > 85 mmHg, in secondary analysis, the results remained unchanged (Table S7).
The underlying mechanisms for higher BP in morning chronotypes are unclear. It could be hypothesised that individuals with a morning chronotype may have more REM sleep than other chronotypes, due to less curtailment of REM at the end of the sleep period secondary to social constraints, such as work commitments. Sympathetic activity and BP responses are more pronounced in REM sleep, and this effect is augmented in the presence of OSA (Somers et al., 1993). In the large community‐based Wisconsin Sleep Cohort Study, Mokhlesi et al. (2014) found REM AHI to be associated with increased odds for incident hypertension, while NREM AHI was not.
It is also possible that the higher BP in morning chronotypes may relate to cortisol secretion. Morning chronotypes have been reported to have a more pronounced cortisol response to waking from sleep compared with evening chronotypes (Kudielka et al., 2006; Randler & Schaal, 2010), which might predispose morning chronotypes to augmented haemodynamic responses in the presence of significant OSA. McMahon et al. (2019) found that morning chronotypes had greater adverse metabolic and BP changes in the presence of sleep disruption, and suggested this may be mediated by higher cortisol, in keeping with our findings.
It is important to consider whether methodological differences may account for different findings across the studies. For example, Merikanto et al. (2013) measured BP in the morning and thus postulated that at this time circadian factors may have resulted in morning chronotypes having higher BP relative to evening chronotypes. Of note, in the present study, measures of BP were reported as the average of measures taken in the morning, afternoon and evening, which should minimise any circadian influence on our study outcome (Smolensky et al., 2017).
4.4. Strengths and limitations
Strengths of this study include the use of a large, well‐characterised community population of middle‐aged adults. Study participants completed a validated MEQ, gold‐standard in‐laboratory PSG to diagnose OSA, multiple BP measurements at consistent times over a 24‐hr period, and validated health and lifestyle questionnaires. Potential confounding was addressed by statistical adjustment for important demographic and health variables.
Despite our moderately large sample size, individuals with extreme morning and evening chronotypes, who may be more susceptible to the effects of chronotype on OSA or hypertension, might be underrepresented in our study. Furthermore, chronotype was measured using the MEQ, which is a subjective measure of daily timing preferences and is therefore subject to bias (Roenneberg, 2015). However, the MEQ is the most well‐studied and validated method for evaluating chronotype and used by chronobiologists, facilitating comparisons with prior studies (Adan & Natale, 2002). Finally, our study utilised a cross‐sectional design, which limits our ability to make causal inferences.
5. CONCLUSION
This large community‐based study did not find independent associations between chronotype and OSA, or between chronotype and BP and hypertension. However, morning chronotypes with moderate–severe OSA had higher systolic BP than other chronotypes. These findings suggest that chronotype could have a modulating effect on the well‐established relationship between OSA and hypertension. Consideration of chronotype in the clinical setting may aid in risk stratification of patients with OSA. Further studies are required to confirm these findings and to understand potential mechanism(s) responsible for higher BP in morning chronotypes with moderate–severe OSA. Such knowledge could lead to the development of new treatment strategies for improving BP outcomes for patients with OSA.
AUTHOR CONTRIBUTIONS
Kelly Sansom contributed to study design, performed statistical analysis, interpreted data and drafted the manuscript. Amy Reynolds, Jennifer Walsh, Kathleen Maddison, Bhajan Singh and Peter Eastwood were involved in study design, interpretation of results, and drafting and revising of the manuscript. Nigel McArdle contributed to study conception and design, analysis, and interpretation of data, drafting and revising of the manuscript. Satvinder S. Dhaliwal participated in statistical analysis and interpretation of data.
CONFLICT OF INTEREST
Kelly Sansom, Bhajan Singh and Satvinder S. Dhaliwal have no conflicts to declare. Amy Reynolds has received research funding support within the last 5 years from Compumedics, Vanda Pharmaceuticals, the Flinders Foundation, Sydney Trains, Safework SA, and both Merck, Sharp & Dohme (Australia) Pty Ltd and Carers Australia through funding awarded by the Sleep Health Foundation. She also discloses receiving speaker fees for work related to her research from the Sleep Health Foundation, Teva Pharmaceuticals and Sealy Australia. Nigel McArdle, Jennifer Walsh and Kathleen Maddison have received research funding support within the last 5 years from Nyxoah Pty Ltd (Mont‐Saint Guibert, Belgium), Zelira Therapeutics Pty Ltd (Australia) and Incannex Healthcare (Australia). Peter Eastwood has received research funding support within the last 5 years from Nyxoah Pty Ltd (Mont‐Saint Guibert, Belgium) and Zelira Therapeutics Pty Ltd (Australia).
Supporting information
APPENDIX S1 Supporting Information.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the Raine Study participants and their families for their ongoing participation in the study, and the Raine Study team for study co‐ordination and data collection. The authors also thank the National Health and Medical Research Council (NHMRC) for their long‐term contribution to funding the study over the last 30 years. The core management of the Raine Study is funded by The University of Western Australia, Curtin University, Telethon Kids Institute, Women and Infants Research Foundation, Edith Cowan University, Murdoch University, The University of Notre Dame Australia and the Raine Medical Research Foundation. The authors would also like to acknowledge the specific funding bodies for the Raine Study Gen1–26‐year follow‐up: NHMRC (Eastwood et al., ID 1084947), The Centre for Sleep Science, School of Anatomy, Physiology & Human Biology, The University of Western Australia, and the Lions Eye Institute. Open access publishing facilitated by The University of Western Australia, as part of the Wiley ‐ The University of Western Australia agreement via the Council of Australian University Librarians.
Sansom, K. , Reynolds, A. , Dhaliwal, S. S. , Walsh, J. , Maddison, K. , Singh, B. , Eastwood, P. , & McArdle, N. (2023). Cross‐sectional interrelationships between chronotype, obstructive sleep apnea and blood pressure in a middle‐aged community cohort. Journal of Sleep Research, 32(3), e13778. 10.1111/jsr.13778
DATA AVAILABILITY STATEMENT
We are willing to share data from this study, according to current Raine study data sharing rules. The Raine Study holds a rich and detailed collection of data gathered over 30 years for the purpose of health and well‐being research. The informed consent provided by each participant does not permit individual‐level data to be made available in the public domain (i.e., a public data repository). However de‐identified analytic data sets are available to all researchers for original research or auditing of published findings. All data access is managed through established Raine Study procedures which require data handlers to agree to a code of conduct, outlined in the Raine Study Researcher Engagement Policy, that includes safeguards to protect the identity of participants. Details of the data access processes, and code of conduct are available on the Raine Study website (www.rainestudy.org.au).
REFERENCES
- Adan, A. , & Natale, V. (2002). Gender differences in morningness‐eveningness preference. Chronobiology International, 19(4), 709–720. 10.1081/cbi-120005390 [DOI] [PubMed] [Google Scholar]
- Antony, M. M. , Bieling, P. J. , Cox, B. J. , Enns, M. W. , & Swinson, R. P. (1998). Psychometric properties of the 42‐item and 21‐item versions of the depression anxiety stress scales in clinical groups and a community sample. Psychological Assessment, 10(2), 176–181. 10.1037/1040-3590.10.2.176 [DOI] [Google Scholar]
- Benjafield, A. V. , Ayas, N. T. , Eastwood, P. R. , Heinzer, R. , Ip, M. S. M. , Morrell, M. J. , Nunez, C. M. , Patel, S. R. , Penzel, T. , Pepin, J. L. , Peppard, P. E. , Sinha, S. , Tufik, S. , Valentine, K. , & Malhotra, A. (2019). Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature‐based analysis. The Lancet Respiratory Medicine, 7(8), 687–698. 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry, R. B. , Budhiraja, R. , Gottlieb, D. J. , Gozal, D. , Iber, C. , Kapur, V. K. , Marcus, C. L. , Mehra, R. , Parthasarathy, S. , Quan, S. F. , Redline, S. , Strohl, K. P. , Davidson Ward, S. L. , Tangredi, M. M. , & American Academy of Sleep Medicine . (2012). Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of Sleep and associated events. Deliberations of the Sleep apnea definitions task force of the American Academy of Sleep medicine. Journal of Clinical Sleep Medicine, 8(5), 597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee, S. , & Hadi, A. S. (2012). Regression analysis by example (5th ed.). Wiley. [Google Scholar]
- Chobanian, A. V. , Bakris, G. L. , Black, H. R. , Cushman, W. C. , Green, L. A. , Izzo, J. L., Jr. , Jones, D. W. , Materson, B. J. , Oparil, S. , Wright, J. T., Jr. , Roccella, E. J. , Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung , & Blood Institute; National High Blood Pressure Education Program Coordinating Committee . (2003). The seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. Journal of the American Medical Association, 289(19), 2560–2572. 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- Diaz‐Morales, J. F. , & Parra‐Robledo, Z. (2018). Age and sex differences in Morningness/Eveningness along the life span: A cross‐sectional study in Spain. Journal of Genetic Psychology, 179(2), 71–84. 10.1080/00221325.2018.1424706 [DOI] [PubMed] [Google Scholar]
- Dontje, M. L. , Eastwood, P. , & Straker, L. (2019). Western Australian pregnancy cohort (Raine) study: Generation 1. British Medical Journal Open, 9(5), e026276. 10.1136/bmjopen-2018-026276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne, J. A. , & Ostberg, O. (1976). A self‐assessment questionnaire to determine morningness‐eveningness in human circadian rhythms. International Journal of Chronobiology, 4(2), 97–110. https://www.ncbi.nlm.nih.gov/pubmed/1027738 [PubMed] [Google Scholar]
- Ireland, P. , Jolley, D. , Giles, G. , O'Dea, K. , Powles, J. , Rutishauser, I. , Wahlqvist, M. L. , & Williams, J. (1994). Development of the Melbourne FFQ: A food frequency questionnaire for use in an Australian prospective study involving an ethnically diverse cohort. Asia Pacific Journal of Clinical Nutrition, 3(1), 19–31. https://www.ncbi.nlm.nih.gov/pubmed/24351203 [PubMed] [Google Scholar]
- Kim, L. J. , Coelho, F. M. , Hirotsu, C. , Bittencourt, L. , Tufik, S. , & Andersen, M. L. (2015). Is the chronotype associated with obstructive sleep apnea? Sleep & Breathing, 19(2), 645–651. 10.1007/s11325-014-1070-1 [DOI] [PubMed] [Google Scholar]
- Knutson, K. L. , & von Schantz, M. (2018). Associations between chronotype, morbidity and mortality in the UK biobank cohort. Chronobiology International, 35(8), 1045–1053. 10.1080/07420528.2018.1454458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka, B. M. , Federenko, I. S. , Hellhammer, D. H. , & Wust, S. (2006). Morningness and eveningness: The free cortisol rise after awakening in "early birds" and "night owls". Biological Psychology, 72(2), 141–146. 10.1016/j.biopsycho.2005.08.003 [DOI] [PubMed] [Google Scholar]
- Lucassen, E. A. , Zhao, X. , Rother, K. I. , Mattingly, M. S. , Courville, A. B. , de Jonge, L. , Csako, G. , Cizza, G. , & Sleep Extension Study Group . (2013). Evening chronotype is associated with changes in eating behavior, more sleep apnea, and increased stress hormones in short sleeping obese individuals. Public Library of Science One, 8(3), e56519. 10.1371/journal.pone.0056519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarem, N. , Paul, J. , Giardina, E. V. , Liao, M. , & Aggarwal, B. (2020). Evening chronotype is associated with poor cardiovascular health and adverse health behaviors in a diverse population of women. Chronobiology International, 37(5), 673–685. 10.1080/07420528.2020.1732403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone, S. K. , Patterson, F. , Lu, Y. , Lozano, A. , & Hanlon, A. (2016). Ethnic differences in sleep duration and morning‐evening type in a population sample. Chronobiology International, 33(1), 10–21. 10.3109/07420528.2015.1107729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maukonen, M. , Kanerva, N. , Partonen, T. , Kronholm, E. , Konttinen, H. , Wennman, H. , & Mannisto, S. (2016). The associations between chronotype, a healthy diet and obesity. Chronobiology International, 33(8), 972–981. 10.1080/07420528.2016.1183022 [DOI] [PubMed] [Google Scholar]
- McArdle, N. , Reynolds, A. C. , Hillman, D. , Moses, E. , Maddison, K. , Melton, P. , & Eastwood, P. (2022). Prevalence of common sleep disorders in a middle‐aged community sample. Journal of Clinical Sleep Medicine, 18(6), 1503–1514. 10.5664/jcsm.9886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon, D. M. , Burch, J. B. , Youngstedt, S. D. , Wirth, M. D. , Hardin, J. W. , Hurley, T. G. , Blair, S. N. , Hand, G. A. , Shook, R. P. , Drenowatz, C. , Burgess, S. , & Hebert, J. R. (2019). Relationships between chronotype, social jetlag, sleep, obesity and blood pressure in healthy young adults. Chronobiology International, 36(4), 493–509. 10.1080/07420528.2018.1563094 [DOI] [PubMed] [Google Scholar]
- Merikanto, I. , Lahti, T. , Puolijoki, H. , Vanhala, M. , Peltonen, M. , Laatikainen, T. , Vartiainen, E. , Salomaa, V. , Kronholm, E. , & Partonen, T. (2013). Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiology International, 30(4), 470–477. 10.3109/07420528.2012.741171 [DOI] [PubMed] [Google Scholar]
- Millman, R. P. , Redline, S. , Carlisle, C. C. , Assaf, A. R. , & Levinson, P. D. (1991). Daytime hypertension in obstructive sleep apnea. Prevalence and contributing risk factors. Chest, 99(4), 861–866. 10.1378/chest.99.4.861 [DOI] [PubMed] [Google Scholar]
- Mokhlesi, B. , Finn, L. A. , Hagen, E. W. , Young, T. , Hla, K. M. , Van Cauter, E. , & Peppard, P. E. (2014). Obstructive sleep apnea during REM sleep and hypertension. Results of the Wisconsin sleep cohort. American Journal of Respiratory and Critical Care Medicine, 190(10), 1158–1167. 10.1164/rccm.201406-1136OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongrain, V. , Carrier, J. , & Dumont, M. (2005). Chronotype and sex effects on sleep architecture and quantitative sleep EEG in healthy young adults. Sleep, 28(7), 819–827. 10.1093/sleep/28.7.819 [DOI] [PubMed] [Google Scholar]
- Nauha, L. , Jurvelin, H. , Ala‐Mursula, L. , Niemela, M. , Jamsa, T. , Kangas, M. , & Korpelainen, R. (2020). Chronotypes and objectively measured physical activity and sedentary time at midlife. Scandinavian Journal of Medicine and Science in Sports, 30(10), 1930–1938. 10.1111/sms.13753 [DOI] [PubMed] [Google Scholar]
- Netzer, N. C. , Stoohs, R. A. , Netzer, C. M. , Clark, K. , & Strohl, K. P. (1999). Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Annals of Internal Medicine, 131(7), 485–491. 10.7326/0003-4819-131-7-199910050-00002 [DOI] [PubMed] [Google Scholar]
- Nieto, F. J. , Young, T. B. , Lind, B. K. , Shahar, E. , Samet, J. M. , Redline, S. , D'Agostino, R. B. , Newman, A. B. , Lebowitz, M. D. , & Pickering, T. G. (2000). Association of sleep‐disordered breathing, sleep apnea, and hypertension in a large community‐based study. Sleep Heart health study. Journal of the American Medical Association, 283(14), 1829–1836. 10.1001/jama.283.14.1829 [DOI] [PubMed] [Google Scholar]
- O'brien, R. M. (2007). A caution regarding rules of thumb for variance inflation factors. Quality & Quantity, 41(5), 673–690. 10.1007/s11135-006-9018-6 [DOI] [Google Scholar]
- Okun, M. L. , Kravitz, H. M. , Sowers, M. F. , Moul, D. E. , Buysse, D. J. , & Hall, M. (2009). Psychometric evaluation of the insomnia symptom questionnaire: A self‐report measure to identify chronic insomnia. Journal of Clinical Sleep Medicine, 5(1), 41–51. https://www.ncbi.nlm.nih.gov/pubmed/19317380 [PMC free article] [PubMed] [Google Scholar]
- Paine, S. J. , Gander, P. H. , & Travier, N. (2006). The epidemiology of morningness/eveningness: Influence of age, gender, ethnicity, and socioeconomic factors in adults (30‐49 years). Journal of Biological Rhythms, 21(1), 68–76. 10.1177/0748730405283154 [DOI] [PubMed] [Google Scholar]
- Peppard, P. E. , Young, T. , Palta, M. , & Skatrud, J. (2000). Prospective study of the association between sleep‐disordered breathing and hypertension. New England Journal of Medicine, 342(19), 1378–1384. 10.1056/NEJM200005113421901 [DOI] [PubMed] [Google Scholar]
- Rahimi, K. , Bidel, Z. , Nazarzadeh, M. , Copland, E. , Canoy, D. , Ramakrishnan, R. , Pinho‐Gomes, A.‐C. , Woodward, M. , Adler, A. , Agodoa, L. , Algra, A. , Asselbergs, F. W. , Beckett, N. S. , Berge, E. , Black, H. , Brouwers, F. P. J. , Brown, M. , Bulpitt, C. J. , Byington, R. P. , … Davis, B. R. (2021). Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: An individual participant‐level data meta‐analysis. The Lancet, 397(10285), 1625–1636. 10.1016/s0140-6736(21)00590-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randler, C. , & Schaal, S. (2010). Morningness‐eveningness, habitual sleep‐wake variables and cortisol level. Biological Psychology, 85(1), 14–18. 10.1016/j.biopsycho.2010.04.006 [DOI] [PubMed] [Google Scholar]
- Rapsomaniki, E. , Timmis, A. , George, J. , Pujades‐Rodriguez, M. , Shah, A. D. , Denaxas, S. , White, I. R. , Caulfield, M. J. , Deanfield, J. E. , Smeeth, L. , Williams, B. , Hingorani, A. , & Hemingway, H. (2014). Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life‐years lost, and age‐specific associations in 1.25 million people. Lancet, 383(9932), 1899–1911. 10.1016/S0140-6736(14)60685-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg, T. (2015). Having trouble typing? What on earth is chronotype? Journal of Biological Rhythms, 30(6), 487–491. 10.1177/0748730415603835 [DOI] [PubMed] [Google Scholar]
- Ryan, S. (2018). Mechanisms of cardiovascular disease in obstructive sleep apnoea. Journal of Thoracic Disease, 10(Suppl 34), S4201–S4211. 10.21037/jtd.2018.08.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolensky, M. H. , Hermida, R. C. , & Portaluppi, F. (2017). Circadian mechanisms of 24‐hour blood pressure regulation and patterning. Sleep Medicine Reviews, 33, 4–16. 10.1016/j.smrv.2016.02.003 [DOI] [PubMed] [Google Scholar]
- Somers, V. K. , Dyken, M. E. , Mark, A. L. , & Abboud, F. M. (1993). Sympathetic‐nerve activity during sleep in normal subjects. New England Journal of Medicine, 328(5), 303–307. 10.1056/NEJM199302043280502 [DOI] [PubMed] [Google Scholar]
- Taillard, J. , Philip, P. , Chastang, J. F. , & Bioulac, B. (2004). Validation of Horne and Ostberg morningness‐eveningness questionnaire in a middle‐aged population of French workers. Journal of Biological Rhythms, 19(1), 76–86. 10.1177/0748730403259849 [DOI] [PubMed] [Google Scholar]
- The IPAQ Group . (2005). Guidelines for data processing and analysis of the international physical activity questionnaire (IPAQ). http://www.ipaq.ki.se
- Unger, T. , Borghi, C. , Charchar, F. , Khan, N. A. , Poulter, N. R. , Prabhakaran, D. , Ramirez, A. , Schlaich, M. , Stergiou, G. S. , Tomaszewski, M. , Wainford, R. D. , Williams, B. , & Schutte, A. E. (2020). 2020 International Society of Hypertension global hypertension practice guidelines. Journal of Hypertension, 38(6), 982–1004. 10.1097/HJH.0000000000002453 [DOI] [PubMed] [Google Scholar]
- Young, T. (1997). Population‐based study of sleep‐disordered breathing as a risk factor for hypertension. Archives of Internal Medicine, 157(15), 1746–1752. 10.1001/archinte.1997.00440360178019 [DOI] [PubMed] [Google Scholar]
- Zhang, Z. (2016). Model building strategy for logistic regression: Purposeful selection. Annals of Translational Medicine, 4(6), 111. 10.21037/atm.2016.02.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Cajochen, C. , & Khatami, R. (2019). Social jetlag and Chronotypes in the Chinese population: Analysis of data recorded by wearable devices. Journal of Medical Internet Research, 21(6), e13482. 10.2196/13482 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1 Supporting Information.
Data Availability Statement
We are willing to share data from this study, according to current Raine study data sharing rules. The Raine Study holds a rich and detailed collection of data gathered over 30 years for the purpose of health and well‐being research. The informed consent provided by each participant does not permit individual‐level data to be made available in the public domain (i.e., a public data repository). However de‐identified analytic data sets are available to all researchers for original research or auditing of published findings. All data access is managed through established Raine Study procedures which require data handlers to agree to a code of conduct, outlined in the Raine Study Researcher Engagement Policy, that includes safeguards to protect the identity of participants. Details of the data access processes, and code of conduct are available on the Raine Study website (www.rainestudy.org.au).


