Abstract
A family of structurally related cyclin‐dependent protein kinases (CDKs) drives many aspects of eukaryotic cell function. Much of the literature in this area has considered individual members of this family to act primarily either as regulators of the cell cycle, the context in which CDKs were first discovered, or as regulators of transcription. Until recently, CDK7 was the only clear example of a CDK that functions in both processes. However, new data points to several “cell‐cycle” CDKs having important roles in transcription and some “transcriptional” CDKs having cell cycle‐related targets. For example, novel functions in transcription have been demonstrated for the archetypal cell cycle regulator CDK1. The increasing evidence of the overlap between these two CDK types suggests that they might play a critical role in coordinating the two processes. Here we review the canonical functions of cell‐cycle and transcriptional CDKs, and provide an update on how these kinases collaborate to perform important cellular functions. We also provide a brief overview of how dysregulation of CDKs contributes to carcinogenesis, and possible treatment avenues.
This article is categorized under:
RNA Interactions with Proteins and Other Molecules > RNA‐Protein Complexes
RNA Processing > 3′ End Processing
RNA Processing > Splicing Regulation/Alternative Splicing
Keywords: CDK, cell cycle, cyclin, kinase, transcription
A family of structurally related cyclin‐dependent protein kinases (CDKs) drives many aspects of eukaryotic cell function. Much of the literature in this area has considered individual members of this family to act primarily either as regulators of the cell cycle or transcription, but there is increasing evidence of overlap.
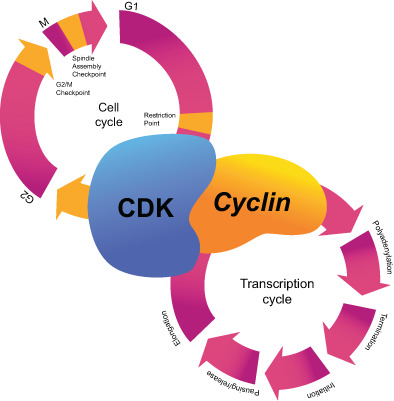
1. INTRODUCTION
Cyclin‐dependent kinases (CDKs) are a conserved eukaryotic family of heterodimeric serine/threonine protein kinases, whose catalytic activity is entirely dependent on association with a specialized regulatory cyclin subunit and the phosphorylation status of the CDK activating domain, the T‐loop (Brown et al., 1999; Malumbres, 2014; Pines, 1995; Wood & Endicott, 2018). CDKs have been implicated in numerous processes in the cell, the most prominent being the cell cycle and the transcriptional cycle (Lim & Kaldis, 2013; Palmer & Kaldis, 2020) (Figures 1 and 2). These kinases function through dynamic and generally reversible phosphorylation of a wide range of targets, and 20 proteins belonging to this family (CDK1 to CDK20) have been identified in metazoans (Kalra et al., 2017; Malumbres et al., 2009; Marak et al., 2020; Roskoski, 2019) (Tables 1, 4, and 7). They can be further subdivided based on amino acid sequence and function into eight sub‐families (Cao et al., 2014; J. Liu & Kipreos, 2000; Malumbres, 2014; Figure 3).
FIGURE 1.
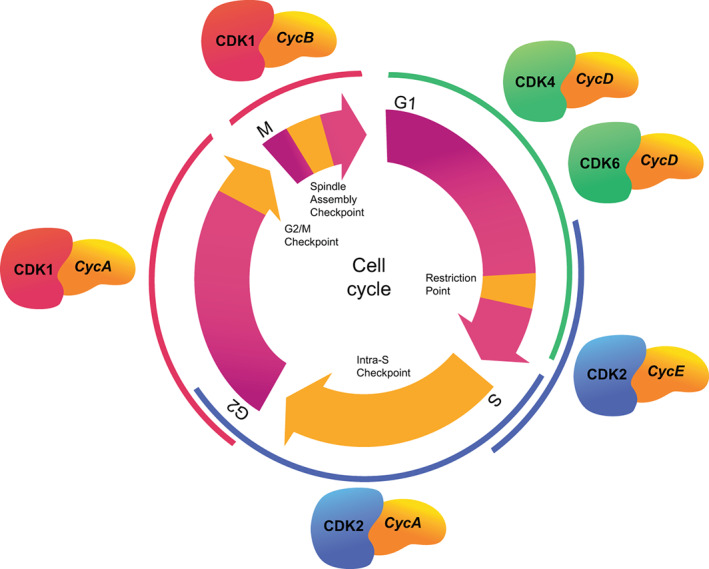
CDKs in the mammalian cell cycle. Different CDK‐cyclin complexes drive the individual phases of the cell cycle. Chromosomal DNA is replicated during the synthesis (S) phase; replicated chromosomes are segregated to daughter nuclei in M phase (mitosis). Gap phases G1 and G2 of variable duration may separate M from S and S from M, respectively. Mitogen‐dependent cells become committed to a new cycle and escape the requirement for mitogen signaling at the restriction (R) point in G1. In addition, progression through the cell cycle may be delayed by the presence of abnormal DNA structures at the point of DNA replication origin firing (the intra‐S checkpoint) or at the G2/M transition (the G2/M checkpoint), or by unattached chromosomes at the spindle attachment checkpoint in mitosis.
FIGURE 2.
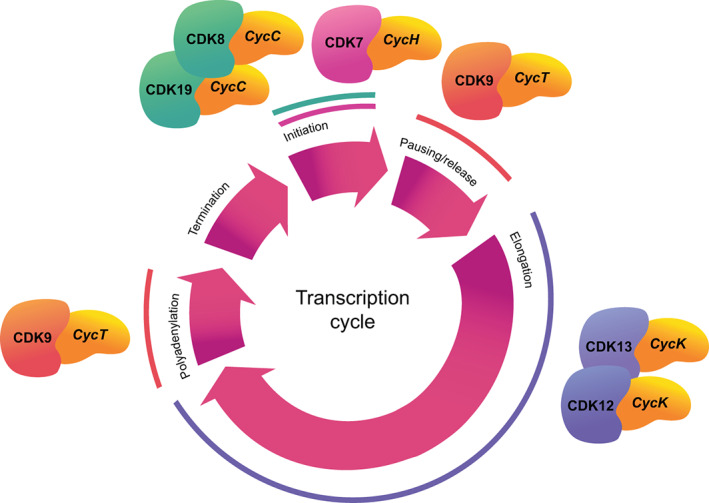
CDKs in transcription of mammalian protein‐coding genes. Individual CDK‐cyclin complexes are implicated in driving the sequential stages of transcription—initiation, pausing and release, elongation, polyadenylation and termination. The replication‐dependent histone genes have a specialized RNA 3′ processing signal rather than a poly(A) site but the cycle is thought to be similar (Marzluff & Koreski, 2017). The transcriptional CDKs influence transcription by phosphorylating the carboxy‐terminal domain of RNA polymerase II (pol II) and other members of the elongation complex. Additionally, CDK8/CDK19 form part of the mediator complex, which can promote or repress transcription.
TABLE 1.
Cell‐cycle CDKs in metazoans.
| Cyclin‐dependent kinase | Binding partner | Roles in cell cycle | Roles in transcription | Selected additional roles |
|---|---|---|---|---|
| CDK1 | Cyclin A, B | Orchestrates mitosis and determines its timing (Maryu & Yang, 2022; Santamaría et al., 2007;Satyanarayana et al., 2008; Satyanarayana & Kaldis, 2009b) |
Phosphorylates factors in cell‐cycle transcriptional programs (Akoulitchev & Reinberg, 1998; Chymkowitch & Enserink, 2013; Cisek & Corden, 1989; Enserink & Chymkowitch, 2022; Gavet & Pines, 2010a, 2010b; Kobor & Greenblatt, 2002) Maintains pluripotency of embryonic stem cells (mouse) (Michowski et al., 2020) Inhibits neuronal differentiation by phosphorylating Ngn2 (Ali et al., 2011) Phosphorylates of splicing and polyadenylation factors (in cell cycle‐dependent manner) (Colgan et al., 1998; Okamoto et al., 1998) |
Regulates mitochondrial bioenergetics (B. Xie et al., 2019) Upregulates mRNA translation (Haneke et al., 2020) Functions in DNA damage response and DNA repair (Palmer & Kaldis, 2020) |
| CDK2 | Cyclin A, E |
Participates in G1/S transition (Satyanarayana et al., 2008; Satyanarayana & Kaldis, 2009b) Orchestrates S phase events, such as initiation of replication (Coverley et al., 2000) |
Targets transcriptional repressor Rb (Dynlacht, 1997; Hydbring et al., 2016) Inhibits FOXO1 to promote cell survival (H. Huang et al., 2006) Activates ELK4 to stimulate cell transformation (Peng et al., 2016) Stimulates transcription during HIV‐1 infection (Agbottah et al., 2006; Deng et al., 2002; Nekhai et al., 2002; Rice, 2018) Targets chromatin‐modifying proteins and general transcription factors (e.g., DOT1L, GTF2I) (Chi et al., 2020) Inhibits neuronal differentiation by phosphorylating Ngn2 (Ali et al., 2011) |
Maintains pluripotent neural progenitor cell pool (Caillava et al., 2011) Functions in DNA damage response and DNA repair (Satyanarayana & Kaldis, 2009a) |
| CDK4 | Cyclin D | Governs progression through R‐point (Malumbres & Barbacid, 2005; Satyanarayana & Kaldis, 2009b) |
Targets transcriptional repressors (Rb, p107, p130) and Smad transcription factors (Cobrinik, 2005; Dynlacht, 1997; Ezhevsky et al., 1997; Goel et al., 2018; Hydbring et al., 2016) Activates FOXM1 to protect against senescence (Anders et al., 2011) Activates c‐Jun to form AP‐1 transcription complexes (Vanden Bush & Bishop, 2011) |
|
| CDK6 | Cyclin D | Governs progression through R‐point (Malumbres & Barbacid, 2005; Satyanarayana & Kaldis, 2009b) |
Targets transcriptional repressors (Rb, p107, p130) and Smad transcription factors (Cobrinik, 2005; Dynlacht, 1997; Ezhevsky et al., 1997; Goel et al., 2018; Hydbring et al., 2016) Activates FOXM1 to protect against senescence (Anders et al., 2011) Negatively regulates differentiation (Grossel & Hinds, 2006; Matushansky et al., 2000; Urbach & Witte, 2019) Activates NF‐κB (Buss et al., 2012; Handschick et al., 2014) Upregulates expression of pro‐angiogenic VEGF‐A (Kollmann et al., 2013) |
Acts as sensitizer for apoptosis (MacKeigan et al., 2005) |
TABLE 4.
Transcriptional CDKs in metazoans.
| Cyclin‐dependent kinase | Binding partner | Roles in cell cycle | Roles in transcription | Selected additional roles |
|---|---|---|---|---|
| CDK7 | Cyclin H |
CAK activity (for CDK1/2/4/6) (Bisteau et al., 2013; Fisher, 2005, 2012; Larochelle et al., 1998, 2007; Merrick et al., 2008; Schachter & Fisher, 2013) Required for cell cycle progression (Ganuza et al., 2012; Larochelle et al., 1998, 2007; Olson et al., 2019; Wallenfang & Seydoux, 2002) |
CAK activity (for CDK9/12/13) (Fisher, 2012; Larochelle et al., 2012; Rimel et al., 2020) Part of TFIIH complex, important for early steps of pol II transcription (Fisher, 2005; Glover‐Cutter et al., 2009; Larochelle et al., 2012; Maldonado & Reinberg, 1995; Rimel & Taatjes, 2018; Roy et al., 1994) CTD kinase (S5, S7) (Akhtar et al., 2009; Fisher, 2005; Glover‐Cutter et al., 2009; Pinhero et al., 2004; Roy et al., 1994; Wallenfang & Seydoux, 2002) |
Implicated in DNA damage and repair pathways (Rimel & Taatjes, 2018) |
| CDK8 | Cyclin C |
Inhibits CAK activity (Akoulitchev et al., 2000; Szilagyi & Gustafsson, 2013) Promotes cell‐cycle commitment through β‐catenin pathway (Firestein et al., 2008; Szilagyi & Gustafsson, 2013) Promotes G2/M transition (Philip et al., 2018; Xu et al., 2015) Promotes cell cycle arrest at R point through p21 activation (Donner et al., 2007; Porter et al., 2012; Szilagyi & Gustafsson, 2013) Maintains quiescence of VPC in C. elegans (Clayton et al., 2008) |
Part of mediator complex (Dannappel et al., 2019; Fant & Taatjes, 2019; Galbraith et al., 2010; Luyties & Taatjes, 2022; Poss et al., 2013; D. Wu, Zhang, et al., 2021) CTD kinase (S5) (Pinhero et al., 2004; Rickert et al., 1999) Can upregulate and downregulate transcription (Galbraith et al., 2010) Inhibits TFIIH to downregulate transcription (Akoulitchev et al., 2000) Promotes expression of hypoxia‐inducible genes after recruitment by HIF1A (Galbraith et al., 2013) |
Acts as sensitiser for apoptosis (MacKeigan et al., 2005) Regulates Myc to maintain stem cell pluripotency (Adler et al., 2012) Essential for early embryogenesis (mouse) (Westerling et al., 2007) Implicated in DNA damage and repair pathways (Poss et al., 2016) |
| CDK19 | Cyclin C | Part of mediator complex (Dannappel et al., 2019; Fant & Taatjes, 2019; Luyties & Taatjes, 2022) | Implicated in DNA damage and repair pathways (Poss et al., 2016) | |
| CDK9 | Cyclin T |
Necessary for cell cycle recovery after replication stress (D. S. Yu et al., 2010) Upregulates cell proliferation through PABIR1 and PCNP (Tellier et al., 2022) Gets recruited to chromatin to promote progression through G1 (Anshabo et al., 2021; Cai et al., 2006; Storch & Cordes, 2016; Yang et al., 2008) |
Part of P‐TEFb complex, important for pol II release from the promoter‐proximal pause and elongation (Anshabo et al., 2021; Bacon & D'Orso, 2019; Egloff, 2021; Jonkers & Lis, 2015; Luo et al., 2012; Parua et al., 2018) CTD kinase (S2, T4, S5, S7) (Eick & Geyer, 2013; Heidemann et al., 2013; Pinhero et al., 2004; Zaborowska et al., 2016) Targets transcriptional repressor Rb (Graña et al., 1994; Simone et al., 2002; Storch & Cordes, 2016) |
Implicated in DNA damage and repair pathways (Anshabo et al., 2021) |
| CDK12 | Cyclin K |
Phosphorylates cyclin E1 to stimulate formation of pre‐replicative complex, therefore controlling G1/S progression (Lei et al., 2018; S. Liang et al., 2020; Manavalan et al., 2019) Controls translation of mitotic‐regulator gene mRNAs (Choi et al., 2019) Promotes G2/M transition (Blazek et al., 2011; Geng et al., 2019; H. R. Chen et al., 2017; Schecher et al., 2017) Downregulates G2/M transition in breast cancer cells (Quereda et al., 2019) |
Required for efficient elongation and pre‐mRNA 3′ end formation (Bösken et al., 2014; Choi et al., 2020; Eifler et al., 2015; Fan et al., 2020; Lui et al., 2018; Quereda et al., 2019; Tellier et al., 2020) CTD kinase (S2, S5) (Bartkowiak et al., 2010; Blazek et al., 2011; Bösken et al., 2014; Cheng et al., 2012; Tellier et al., 2020) Implicated in regulation of splicing (H. H. Chen et al., 2006; K. Liang, Gao, et al., 2015; S. Liang et al., 2020; Panzeri et al., 2013) Inhibition severely affects expression of long DNA Damage Response (DDR) genes (Blazek et al., 2011; Choi et al., 2020; Dubbury et al., 2018; Krajewska et al., 2019) Negatively regulates differentiation (Dai et al., 2012) |
Implicated in DNA damage response (Blazek et al., 2011; H. R. Chen et al., 2017; Choi et al., 2020; Dubbury et al., 2018; Fan et al., 2020; Juan et al., 2015; Krajewska et al., 2019; K. Liang, Gao, et al., 2015; S. Liang et al., 2020; Manavalan et al., 2019; Quereda et al., 2019; Tellier et al., 2020) Promotes neurogenesis (H. R. Chen et al., 2017) Essential for early embryogenesis (mouse) (Juan et al., 2015) |
| CDK13 | Cyclin K |
Promotes G2/M transition in gastric cancer cells (Z. Wu, Wang, et al., 2021) Downregulates G2/M transition in breast cancer cells (Quereda et al., 2019) |
Required for efficient elongation and pre‐mRNA 3′ end formation (Fan et al., 2020; K. Liang, Gao, et al., 2015; Quereda et al., 2019) CTD kinase (S2, S5) (Greifenberg et al., 2016) Regulates expression of snoRNA genes (K. Liang, Gao, et al., 2015) Implicated in regulation of splicing (H. H. Chen et al., 2007; K. Liang, Gao, et al., 2015; Panzeri et al., 2013) Activates RNA surveillance pathway (Insco et al., 2023) Negatively regulates differentiation (Dai et al., 2012) Increases splicing of HIV‐1 mRNA (Berro et al., 2008) |
Implicated in DNA damage response (Fan et al., 2020) Implicated in growth signaling pathways (Fan et al., 2020; Greifenberg et al., 2016) Promotes cell survival in breast cancer cells (Quereda et al., 2019) Promotes neurogenesis (H. R. Chen et al., 2017) |
TABLE 7.
Additional CDKs in metazoans.
| Cyclin‐dependent kinase | Binding partner | Roles in cell cycle | Roles in transcription | Selected additional roles |
|---|---|---|---|---|
| CDK3 | Cyclin A, C, E | Promotes G0/G1 and G1/S transitions (Ren & Rollins, 2004; Sage, 2004; Satyanarayana & Kaldis, 2009b; Teo et al., 2022; van den Heuvel & Harlow, 1993; Ye et al., 2001) |
Targets transcriptional repressor Rb (Hofmann & Livingston, 1996; Ren & Rollins, 2004) Phosphorylates c‐Jun and ATF1 to simulate cell transformation (Cho et al., 2009; Zheng et al., 2008) |
Promotes apoptosis (Meikrantz & Schlegel, 1996) Promotes EMT (Lu et al., 2016) |
| CDK5 | p35, p38 |
Blocks cell cycle in postmitotic neurons (Cicero & Herrup, 2005; Zhang et al., 2008) Promotes proliferation in neuroendocrine thyroid cancer (Pozo et al., 2013) |
Implicated in transcriptional programs for neuronal differentiation (Cicero & Herrup, 2005) Activates STAT3 (A. K. Y. Fu et al., 2004) Inhibits MEF2 to promote apoptosis (Gong et al., 2003) Activates mSds3 to promote histone acetylation (Z. Li, David, et al., 2004) Activates p53 and Rb (Lee et al., 1997; Pozo et al., 2013; Zhang et al., 2002) |
Implicated in a wide range of neuronal processes (Chae et al., 1997; Cicero & Herrup, 2005; Cruz & Tsai, 2004; Maestre et al., 2008; Nikolic et al., 1996; Ohshima et al., 1996; Pao & Tsai, 2021; Tanaka et al., 2001) Regulates circadian rhythm through phosphorylation of CLOCK and PER2 (Brenna et al., 2019; Kwak et al., 2013; Pao & Tsai, 2021) Protects against mitochondrial dysfunction and oxidative stress (Pao & Tsai, 2021; Qu et al., 2007; K. H. Sun et al., 2008) Implicated in DNA damage response (Kim et al., 2008; W. Liu, Li, et al., 2017) |
| CDK10 | Cyclin M | Promotes G2/M transition (S. Li et al., 1995) |
Phosphorylates ETS2, leading to silencing of the MAPK pathway (Guen et al., 2013; Kasten & Giordano, 2001) Promotes transcription of 20E‐inducible genes (W. Liu et al., 2014) |
Regulates cilium biogenesis and degradation (Guen et al., 2016, 2018; Windpassinger et al., 2017) Regulates the cytoskeleton and actin dynamics (Guen et al., 2016, 2018) |
| CDK11 | Cyclin L |
Participates in G2/M transition and cytokinesis (primarily CDK11p58 isoform) (An et al., 2020; Barna et al., 2008; Hu et al., 2007; Loyer & Trembley, 2020; Petretti et al., 2006; Wilker et al., 2007; Yokoyama et al., 2008) Required for transcription of replication‐dependent histone genes during S phase (Gajdušková et al., 2020) |
Associates with multiple transcription elongation factors (Loyer & Trembley, 2020; Trembley et al., 2002, 2003) Links transcription with RNA processing events (Loyer & Trembley, 2020; Trembley et al., 2002, 2003) Implicated in regulation of splicing (Hluchý et al., 2022; Hu et al., 2003; Loyer et al., 2005; Loyer & Trembley, 2020; Shin & Manley, 2004) CTD kinase in vitro (S2) for replication‐dependent histone genes (Gajdušková et al., 2020) Involved in processing and polyadenylation of HIV‐1 transcripts (Pak et al., 2015; Rice, 2018) |
Part of autophagy machinery (Wilkinson et al., 2011) Represses estrogen and vitamin D receptor pathways (Chi et al., 2009; Wang et al., 2009) Promotes apoptosis (Ariza et al., 1999; Beyaert et al., 1997; Lahti et al., 1995; J. Shi et al., 2003, 2009; L. Shi et al., 1994; Tang et al., 1998) Essential for early embryogenesis (mouse) (T. Li, Inoue, et al., 2004) |
| CDK14 | Cyclin B, Y | Stimulates Wnt/β‐catenin signaling pathway to drive cell cycle progression (Gu et al., 2015; Niehrs & Acebron, 2012; Ou‐Yang et al., 2017; T. Sun et al., 2014) | Upregulates phosphorylation of Rb to deactivate it (L. Chen et al., 2019) |
Implicated in tumor cell migration and EMT (L. Chen et al., 2019; Gu et al., 2015; Ou‐Yang et al., 2017; Pang et al., 2007; Zhu et al., 2016) Promotes axon regeneration (Hisamoto et al., 2021) Implicated in glucose homeostasis (Tang et al., 2006) |
| CDK15 | Cyclin Y | Promotes cell proliferation through β‐catenin/MEK–ERK pathway in colorectal cancer (C. Huang et al., 2022) |
Protects against tumor cell migration (S. Li, Dai, et al., 2019) Downregulates apoptosis (Park et al., 2014) |
|
| CDK16 | Cyclin Y, p35 | Promotes cell proliferation (Gillani et al., 2022; X. Li et al., 2022; Yanagi et al., 2014) | Deactivates p27 and p53 tumor suppressors (J. Xie et al., 2018; Yanagi et al., 2014) |
Involved in terminal differentiation in spermatogenesis (Mikolcevic, Rainer, & Geley, 2012; Mikolcevic, Sigl, et al., 2012) Implicated in neural outgrowth (Graeser et al., 2002; Mokalled et al., 2010) Functions in vesicular trafficking (Y. Liu et al., 2006; Ou et al., 2010; Palmer et al., 2005; Shehata et al., 2019) Implicated in brain development (Cole, 2009; Fu et al., 2011; Le Bouffant et al., 2000; Mokalled et al., 2010; Shehata et al., 2015, 2019) Suppresses apoptosis (Gillani et al., 2022; Yanagi & Matsuzawa, 2015) Implicated in glucose homeostasis (X. Y. Chen et al., 2012; Tang et al., 2006) Promotes myogenesis (Shimizu et al., 2014) |
| CDK17 | Cyclin Y | Phosphorylates Histone 1 (Hirose et al., 1997) |
Promotes Alzheimer pathology (Chaput et al., 2016) Implicated in neuronal development (Hirose et al., 2000; Yamochi et al., 2001) Downregulates autophagy (Leonardi et al., 2019) |
|
| CDK18 | Cyclin A |
Induces cell cycle arrest in glioblastoma cells (Naumann et al., 2005) Required for S phase progression (Barone et al., 2016) |
Promotes Alzheimer pathology, promotes phosphorylation of tau (Chaput et al., 2016; Herskovits & Davies, 2006) Downregulates autophagy (Leonardi et al., 2019) Promotes ATR‐dependent homologous recombination (Ning et al., 2019) Prevents accumulation of DNA damage and genome instability (Barone et al., 2016, 2018) Involved in reorganization of the actin cytoskeleton (Matsuda et al., 2014) |
|
| CDK20 | Cyclin H |
Possible CAK activity (for CDK2) (X. An et al., 2010; Y. Liu, Kung, et al., 2004; Tian et al., 2012; questioned by Wohlbold et al., 2006; Wu et al., 2009) Promotes G1/S transition, and to a lesser extent G2/M (Mok et al., 2018) Promotes progression through the cell cycle by cyclin D upregulation (Wu et al., 2009) and cyclin E (X. An et al., 2010) Stimulates Wnt/β‐catenin/TCF signaling pathway to drive cell cycle progression (Feng et al., 2011) |
Upregulates EZH2 and H3K27me3 (Feng et al., 2015) |
Phosphorylates MAK‐related kinase/intestinal cell kinase (MRK/ICK) to suppress apoptosis (Z. Fu et al., 2023) Regulates ciliogenesis, and Hedgehog signaling downstream (Snouffer et al., 2017; Y. Yang et al., 2013) Promotes cell growth and survival in different contexts (Lai et al., 2020) Promotes the establishment of an immunosuppressive tumor microenvironment (Mok et al., 2018; Zhou et al., 2018) |
FIGURE 3.
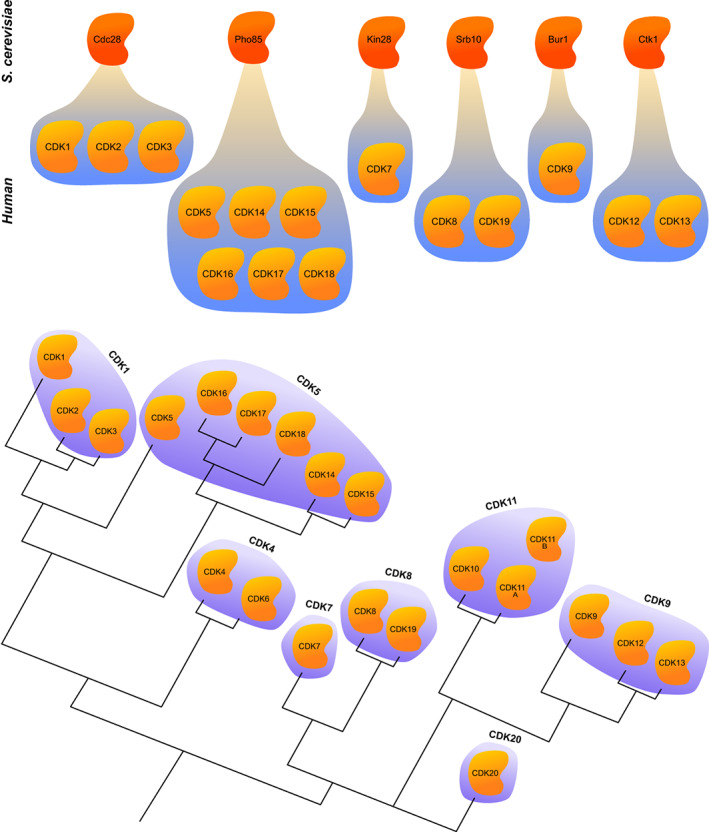
Evolution of CDKs. (a) Saccharomyces cerevisiae possesses the archetypal cell‐cycle CDK Cdc28, as well as Pho85, and four transcriptional CDKs—Kin28, Srb10, Bur1, and Ctk1. These kinases have 15 human CDK homologues. It is important to note that not all of the human CDKs have budding yeast homologues. (b) The relationships between human CDKs, based on their amino acid sequences, group them into eight subfamilies.
CDKs in general are relatively long‐lived proteins and in most cases their levels do not markedly fluctuate through the cell cycle. However, the levels of their cyclin partners can vary over a wide range (Evans et al., 1983; Malumbres, 2014; Pines, 1995). Due to the crucial role of the CDK‐cyclin complexes in governing cell behavior, dysregulation of their activity often contributes to disease. As CDKs are involved in guarding both uncontrolled proliferation and genomic instability, they have become increasingly appealing as therapeutic targets in anticancer treatment (Malumbres & Barbacid, 2009).
2. CDKs DRIVE THE CELL CYCLE
The orderly replication and segregation of chromosomes during the cell cycle is governed by the sequential activation and inactivation of distinct CDK‐cyclin complexes. Three CDK‐dependent cell cycle transitions have been identified: the initiation of chromosomal DNA replication—in the S phase of the cell cycle (DNA synthesis), the initiation of mitosis—during M phase (usually followed by cell division), and cell cycle commitment in the G1 phase (Gap1), which in mammals is termed the restriction (R) point (Figure 1).
The orchestration of mitosis by CDK1 together with cyclin B is an essential aspect of eukaryotic cell cycle control conserved from fungi and plants to vertebrates. The universality of this function is underscored by the fact that human CDK1 was first identified through its capacity to complement loss of Cdc2 activity in fission yeast (Lee & Nurse, 1987). Interestingly, genetic inactivation of CDK1 in yeast and mammals not only blocks entry into mitosis but also de‐represses DNA replication, leading to endoreduplication of nuclear DNA (Hayles et al., 1994; Itzhaki et al., 1997). Germ‐line deletion of CDK1 or cyclin B in mice is associated with very early embryonic lethality (Santamaría et al., 2007), whereas tissue‐specific deletion of CDK1 in hepatocytes results in mitotic arrest, endoreduplication and cellular hypertrophy (Diril et al., 2012). In mammals, the essential role of CDK1 is supplemented by three “interphase” CDKs—CDK2, CDK4, CDK6 (Figure 1). These four kinases can bind to 10 individual cyclins, which in turn belong to four classes (A‐, B‐, D‐, and E‐cyclins) (Malumbres & Barbacid, 2009). Progression through the R point, beyond which cells have reduced dependency on mitogenic signaling, is attributed to activation of CDK4/6‐cyclin D complexes, while CDK2‐cyclin A/E activity is important for initiation of DNA replication (Malumbres, 2014). Each of the cell cycle CDKs is susceptible to inhibition by proteins of the Cip/Kip and/or Ink4 families, providing an additional level of regulation (Jeffrey et al., 2000; Kiyokawa & Koff, 1997; Lim & Kaldis, 2013; Pavletich, 1999; Sherr & Roberts, 1999). Further, all CDKs share two inhibitory phosphorylation sites in their ATP‐binding pocket, which are targeted by negative regulator kinases, and an activating T‐loop motif (Gould & Nurse, 1989; Lim & Kaldis, 2013; Loyer et al., 2005; Norbury et al., 1991).
Yeast CDK1 (originally named Cdc28 in budding yeast and Cdc2 in fission yeast) sequentially partners different cyclin proteins and can support all cell cycle functions (Nurse, 1990). Similarly, mouse development can proceed until mid‐gestation with CDK1 alone when CDKs 2, 4, and 6 are absent (Malumbres & Barbacid, 2009; Santamaría et al., 2007; Satyanarayana et al., 2008; Satyanarayana & Kaldis, 2009a, 2009b). The core cell cycle machinery is therefore conserved throughout eukaryotic evolution, despite the appearance of additional cell‐cycle CDKs in more recent evolutionary time (Tables 1, 2, 3).
TABLE 2.
Cell‐cycle CDKs in S. cerevisiae.
| Cyclin‐dependent kinase | Binding partner | Roles in cell cycle | Roles in transcription | Selected additional roles |
|---|---|---|---|---|
| Cdc28 | Cln1‐3, Clb1‐6 | Orchestrates mitosis and determines its timing; executes ‘START’ control in G1 and the initiation of DNA replication (Enserink & Kolodner, 2010; Hartwell et al., 1973; Mendenhall & Hodge, 1998) |
Phosphorylates factors in cell‐cycle transcriptional programs (Archambault et al., 2004; R. J. Cho, Huang, et al., 2001; Cosma et al., 2001; Darieva et al., 2003; De Bruin et al., 2004; Enserink & Chymkowitch, 2022; Jans et al., 1995; Kõivomägi et al., 2011; Moll et al., 1991; O'Conalláin et al., 1999; Pic‐Taylor et al., 2004; Reynolds et al., 2003; Ubersax et al., 2003; Wittenberg & Reed, 2005) CTD kinase (T4, S5) (Chymkowitch & Enserink, 2013; Kõivomägi et al., 2021; Nemec et al., 2019) Recruits the proteasome to the promoter regions of certain genes (Morris et al., 2003; Yu et al., 2005). Maintains transcription of highly expressed housekeeping genes (e.g., PMA1) (Chymkowitch et al., 2012) Increases transcription through activating NuA4 leading to increased Lys14 acetylation on Htz1 (Fiedler et al., 2009) Recruits the proteasome to promoter regions of specific genes (Morris et al., 2003; V. P. C. C. Yu et al., 2005) |
Implicated in bud morphogenesis (Enserink & Kolodner, 2010; Lew & Reed, 1993) Antagonizes pheromone signaling in G1 (Enserink & Kolodner, 2010) Governs genome stability and DNA repair pathways (Enserink & Kolodner, 2010) |
TABLE 3.
Cell‐cycle CDKs in S. pombe.
| Cyclin‐dependent kinase | Binding partner | Roles in cell cycle | Roles in transcription | Selected additional roles |
|---|---|---|---|---|
| Cdc2 | Cdc13, Cig1, Cig2, Puc1, Pas1, Pch1, Rem1 | Governs cell cycle commitment at START in G1; orchestrates mitosis and determines its timing (Beach et al., 1982; Coudreuse & Nurse, 2010; Fisher & Nurse, 1996; Gutiérrez‐Escribano & Nurse, 2015; Martín‐Castellanos et al., 1996, 2000; Nurse, 1975; Nurse & Bissett, 1981; Reymond et al., 1993) |
Required for Cdc10/Sct1 transcription complex formation (Connolly et al., 1997) Inhibits Ste11 (Kjærulff et al., 2007) |
3. TRANSCRIPTIONAL CDKs DRIVE THE TRANSCRIPTION CYCLE
In eukaryotes, transcription of protein‐coding genes by RNA polymerase II (pol II) can be characterized as a cycle of initiation, pausing, elongation, and termination (Figure 2). Each of these phases requires a different set of transcriptional machinery‐associated factors (Buratowski, 2009; Shandilya & Roberts, 2012; Svejstrup, 2004; Zaborowska et al., 2016). The best‐characterized transcriptional (t)CDKs to date are CDK7, CDK8, CDK9, CDK12, CDK13, and the CDK8 paralogue CDK19 (Table 4). These CDKs not only coordinate the transcription cycle, but also regulate co‐transcriptional processes, such as 5′ end capping, splicing, 3′ end cleavage and polyadenylation, termination, and regulation of the chromatin landscape (Chou et al., 2020; Fisher, 2017; Svejstrup, 2004; Zaborowska et al., 2016). One well‐studied target of the tCDKs is the carboxy‐terminal domain (CTD) of the largest pol II subunit, RPB1. In humans, the CTD comprises 52 heptad repeats with the consensus motif Tyr1Ser2Pro3Thr4Ser5Pro6Ser7, with the serine and threonine residues subject to dynamic and reversible phosphorylation by the tCDKs (Bartkowiak & Greenleaf, 2011; Galbraith et al., 2019; Zaborowska et al., 2016) to produce patterns of phosphorylation termed the CTD code (Buratowski, 2003). CTD phosphorylation plays a major role in the recruitment of transcription and RNA processing factors at the right point of the transcription cycle (Harlen & Churchman, 2017; Meinhart et al., 2005; Zaborowska et al., 2016). However, each tCDK has a range of other targets, which are in the process of being fully characterized (Fan et al., 2020; Krajewska et al., 2019; Larochelle et al., 2012; Rimel et al., 2020; Sansó et al., 2016; Tellier et al., 2020, 2022).
In contrast to cell‐cycle CDKs, tCDKs usually have a single cyclin partner and are recruited to the transcriptional machinery as part of larger protein complexes (Galbraith et al., 2019) (Tables 4, 5, 6). For example, CDK7 associates with cyclin H and MAT1 to form the ternary kinase module of the TFIIH complex, a general transcription factor (TF) required for early steps of RNA pol II transcription (Glover‐Cutter et al., 2009; Maldonado & Reinberg, 1995; Rimel & Taatjes, 2018; Roy et al., 1994). CDK8, together with its paralogue CDK19, form the mediator complex kinase module through binding to cyclin C, MED12 and MED13 (Luyties & Taatjes, 2022). Together, this complex can regulate pol II activity through direct interaction with the transcriptional machinery, with activating or repressing functions depending on the context (Dannappel et al., 2019; Fant & Taatjes, 2019; Luyties & Taatjes, 2022; Parua & Fisher, 2020; D. Wu, Zhang, et al., 2021). Further, CDK9 and cyclin T make up positive transcription elongation factor b (P‐TEFb), which associates with a variety of transcription factors and coactivators, and forms part of the Super Elongation Complex (Bacon & D'Orso, 2019; Egloff, 2021; Luo et al., 2012). P‐TEFb kinase activity releases pol II from a promoter‐proximal pause into productive elongation by phosphorylating negative elongation factors associated with pol II (Jonkers & Lis, 2015; Parua et al., 2018; Parua & Fisher, 2020; Zaborowska et al., 2014). Finally, the most recent kinases described as bona fide tCDKs are CDK12 and CDK13. CDK12 is required for efficient elongation and pre‐mRNA 3′ end formation through recruitment of elongation and polyadenylation factors (Bösken et al., 2014; S. H. Choi et al., 2020; Greenleaf, 2019; Lui et al., 2018; Tellier et al., 2020). Although both bind to cyclin K and are redundant for some functions, including CTD phosphorylation and the regulation of the DNA damage response pathway (Fan et al., 2020; Greenleaf, 2019; Krajewska et al., 2019), the exact role(s) of CDK13 in transcription is less clear. As its individual role is being gradually characterized, it is becoming clearer that despite their structural similarities, the function of CDK13 is distinct from CDK12 and they can affect different sets of genes (Fan et al., 2020; Greifenberg et al., 2016; K. Liang, Gao, et al., 2015). However, both CDK12 and CDK13 have been shown to regulate splicing and to phosphorylate the CTD on Ser2 and Ser5 (H. H. Chen et al., 2007; H. H. Chen et al., 2006; Galbraith et al., 2019; Panzeri et al., 2013; Zaborowska et al., 2016). Recently, CDK13 was also found to play a critical role in the nuclear RNA surveillance pathway (Insco et al., 2023).
TABLE 5.
Transcriptional CDKs in S. cerevisiae.
| Cyclin‐dependent kinase | Binding partner | Roles in cell cycle | Roles in transcription | Selected additional roles |
|---|---|---|---|---|
| Kin28 | Ccl1 |
Essential for proliferation (Simon et al., 1986) Binds to SBF promoters and can rescue cell cycle defects caused by Cln3 depletion (Kõivomägi et al., 2021) Creates autoregulatory loop with Srb10 to govern meiosis (Ohkuni & Yamashita, 2000) |
Part of TFIIH complex, important for early steps of pol II transcription (Cismowski et al., 1995; Feaver et al., 1994; Svejstrup et al., 1996) Upregulates transcription (Hengartner et al., 1998; Valay et al., 1995) CTD kinase (S5, S7) (Akhtar et al., 2009; Hengartner et al., 1998; Komarnitsky et al., 2000) Important for recruitment of mRNA processing machinery (e.g., for 5′ capping) (Komarnitsky et al., 2000; Rodriguez et al., 2000; Schroeder et al., 2000) Primes CTD for Bur1 recruitment (Qiu et al., 2009, 2012) Stimulates mediator disassociation from preinitiation complex leading to promoter escape (Y. Liu, Wu, & Galaktionov, 2004; Wong et al., 2014) |
Participates in nucleotide excision repair (Bhatia et al., 1996) |
| Srb10 | Srb11 |
Represses meiotic genes in response to glucose by decreasing their mRNA stability (Surosky et al., 1994) Creates autoregulatory loop with Kin28 to govern meiosis (Ohkuni & Yamashita, 2000) Coordinates entry into stationary phase (Chang et al., 2001) |
Part of mediator complex (Carlson, 1997) Downregulates transcription, e.g., through interaction with Gcn4 (Carlson, 1997; Chi et al., 2001; Hengartner et al., 1998; Holstege et al., 1998; Kuchin & Carlson, 1998; Rosonina et al., 2012) Upregulates transcription (Andrau et al., 2006; Galbraith et al., 2010; Y. Liu, Wu, & Galaktionov, 2004; X. Zhu et al., 2006) CTD kinase (S5) (Hengartner et al., 1998; Liao et al., 1995) Phosphorylates and decreases stability of Ste12 in normal nitrogen conditions (Nelson et al., 2003) Represses a‐specific genes in α cells (Wahi & Johnson, 1995) Activates Sip4 in nonfermentable carbon sources (Vincent et al., 2001) |
Participates in glucose repression (Kuchin et al., 1995) Coordinates nutrient starvation response (Holstege et al., 1998) |
| Bur1 | Bur2 | Required for activation of Sch9 leading to progression through G1 (Jin et al., 2022) |
Required for efficient elongation (Keogh et al., 2003; Murray et al., 2001; Wood et al., 2005; Wood & Shilatifard, 2006; Yao et al., 2000) CTD kinase (S2, S5, S7) (Y. Liu et al., 2009; Murray et al., 2001; Qiu et al., 2009; Tietjen et al., 2010) Phosphorylates Spt5 to recruit PAF1 (Y. Liu et al., 2009; Qiu et al., 2012) Represses SUC2 basal promoter (Prelich & Winston, 1993) Regulates epigenetic histone modifications, e.g., through PAF recruitment (Chu et al., 2006, 2007; Laribee et al., 2005; Y. Liu et al., 2009; Wood et al., 2005) Modulates co‐transcriptional splicing (Maudlin & Beggs, 2021) |
Governs DNA damage and replication stress pathways (Clausing et al., 2010) Implicated in lengthening of telomeres (Connelly et al., 2022) Essential for cell growth (Irie et al., 1991; Winzeler et al., 1999) Suppresses mating pheromone hyperadaptivity (Irie et al., 1991) |
| Ctk1 | Ctk2, Ctk3 | Role unclear—deletion results in cell cycle defects (Chymkowitch et al., 2012) |
Required for efficient elongation (Jona et al., 2001; J. M. Lee & Greenleaf, 1997; Murray et al., 2001) Regulates co‐transcriptional processes such as mRNA 3′‐end processing, polyadenylation, and nuclear export of mRNA (Ahn et al., 2004; Hurt et al., 2004; Skaar & Greenleaf, 2002) Promotes release of basal transcription factors from pol II (Ahn et al., 2009) CTD kinase (S2) (E. J. Cho, Kobor, et al., 2001; J. M. Lee & Greenleaf, 1989, 1991) Downregulates CTD phosphorylation during logarithmic phase growth, but upregulates during diauxic phase (Patturajan et al., 1999) Important for transcription termination of small non‐coding RNAs (Lenstra et al., 2013) Upregulates H3K36me3 by interaction with Set2 and Spt6 (Dronamraju & Strahl, 2014; Xiao et al., 2003; Youdell et al., 2008) Implicated in glucose‐dependent transcriptional regulation (van Driessche et al., 2005) |
Implicated in cell growth (J. M. Lee & Greenleaf, 1991) Required for translation initiation and elongation (Coordes et al., 2015; Röther & Sträßer, 2007) Implicated in transcription by RNA pol I and synthesis of rRNA (Bouchoux et al., 2004; Grenetier et al., 2006) Implicated in DNA damage response (Ostapenko & Solomon, 2003) |
TABLE 6.
Transcriptional CDKs in S. pombe.
| Cyclin‐dependent kinase | Binding partner | Roles in cell cycle | Roles in transcription | Selected additional roles |
|---|---|---|---|---|
| Mc6 | Mcs2 |
CAK activity (for Cdc2) (Buck et al., 1995; Damagnez et al., 1995; Lee et al., 1999; Saiz & Fisher, 2002) Inhibition impairs cytokinesis (Buck et al., 1995; Saiz & Fisher, 2002; Viladevall et al., 2009) |
Part of TFIIH complex, important for early steps of pol II transcription (Booth et al., 2018; Spåhr et al., 2003; Viladevall et al., 2009) CTD kinase (S5, S7) (Amour et al., 2012; Booth et al., 2018; Viladevall et al., 2009) Recruits P‐TEFb to pol II by priming the CTD, leading to mRNA capping (Amour et al., 2012; Viladevall et al., 2009) Controls transcription of cell‐cycle periodic genes through interactions with Sep1 (Lee et al., 2005) |
Essential for growth (Damagnez et al., 1995; Molz et al., 1989; Saiz & Fisher, 2002) |
| Srb10 | Srb11 | Controls entry into mitosis by phosphorylating Fkh2 (Banyai et al., 2014; Szilagyi et al., 2012) |
Part of mediator complex (Borggrefe et al., 2002; Samuelsen et al., 2003; Spåhr et al., 2003) Downregulates transcription in vitro (Spåhr et al., 2003) CTD kinase in vitro (S2, S5) (Borggrefe et al., 2002) Blocks mediator‐pol II interactions (Elmlund et al., 2006; Samuelsen et al., 2003) Acts as a global regulator of mitotic transcription (Banyai et al., 2014) |
Downregulates expression of adhesins (Linder et al., 2008; Samuelsen et al., 2003) |
| SpCDK9 | Pch1 | Inhibition impairs cytokinesis (Viladevall et al., 2009) |
Part of P‐TEFb complex, important for efficient elongation (Amour et al., 2012; Bartkowiak & Greenleaf, 2011; Guiguen et al., 2007; Parua et al., 2018; Viladevall et al., 2009) Regulates an early elongation checkpoint (Booth et al., 2018; Guiguen et al., 2007; Pei et al., 2003; Viladevall et al., 2009) CTD kinase (S2, S5, marginally S7) (Amour et al., 2012; Guiguen et al., 2007; Pei & Shuman, 2003) Phosphorylates Spt5 elongation factor (Amour et al., 2012; Booth et al., 2018; Parua et al., 2018; Pei & Shuman, 2003) Couples transcription to mRNA capping through its association with Pct1 and Pcm1 (Amour et al., 2012; Guiguen et al., 2007; Pei et al., 2003, 2006; Viladevall et al., 2009) Downregulates PP1 isoform Dis2 to create a termination switch (Parua et al., 2018) Upregulates histone H2B mono‐ubiquitylation (Sansó et al., 2012) |
Essential for growth (Bimbó et al., 2005; Guiguen et al., 2007; Pei & Shuman, 2003) Implicated in DNA damage response (Gerber et al., 2008) |
| Lsk1 | Lsc1 |
Upregulates the Septation Initiation Network to promote cytokinesis (Karagiannis et al., 2005; Karagiannis & Balasubramanian, 2007) Upregulates meiosis via stress‐responsive MAPK pathway (Coudreuse et al., 2010; Sukegawa et al., 2011) |
Deletion causes minimal changes in transcription (Booth et al., 2018) CTD kinase (S2) (Booth et al., 2018; Karagiannis & Balasubramanian, 2007; Viladevall et al., 2009) |
Downregulates sexual differentiation through interaction with Ste11 (Coudreuse et al., 2010; Sukegawa et al., 2011) |
4. OTHER ROLES FOR CDKs
CDKs are implicated in a variety of cellular processes in addition to the cell or transcription cycles. This is true both for metazoans and yeast, with CDK1 homologues alone implicated in cell morphogenesis and polarity, genome stability, telomere maintenance, and pheromone signaling in Saccharomyces cerevisiae, and mitochondrial bioenergetics, and positive regulation of mRNA translation in humans (Enserink & Kolodner, 2010; Haneke et al., 2020; Xie et al., 2019). CDKs have also been recently found to mediate inflammatory responses in mammals (Sundar et al., 2021). They also govern parts of the DNA damage response pathways, including DNA repair and damage checkpoint signaling (Hydbring et al., 2016; Palmer & Kaldis, 2020). Interestingly, CDKs may even perform functions independent of their kinase activity: CDK6 was demonstrated to upregulate the expression of p16INK4a and VEGF‐A, while the budding yeast CDK1 homologue—Cdc28—has been suggested to act as an adaptor protein during the recruitment of the proteasome (Kollmann et al., 2013; V. P. C. C. Yu et al., 2005).
A novel and intriguing area of research focuses on the roles of CDKs in stem cell biology (Jirawatnotai et al., 2020), which may also uncover new ways in which CDKs are implicated in cancer. For example, there are similarities between undifferentiated stem cells and malignant progenitor cells which are relatively undifferentiated but which give rise to more differentiated progeny within the tumor. CDK8 is implicated in cancer cell de‐differentiation and helps to maintain stem cell pluripotency due to regulation of the Myc proto‐oncogene (Adler et al., 2012; Peyressatre et al., 2015). Similarly, CDK2 seems to play a role in maintaining a pluripotent neural progenitor cell pool (Caillava et al., 2011; Chi et al., 2020). Relevant to this, CDK2 knockout mice are viable, but their neural progenitor cells display impaired proliferation in adults (Satyanarayana & Kaldis, 2009b).
5. LESS‐STUDIED CDKs
Additional CDKs have less well‐understood functions (Tables 7, 8, 9). However, it is worth noting that most of them have reported roles in both cell cycle and transcription (Chou et al., 2020; Kasten & Giordano, 2001; Lim & Kaldis, 2013; Loyer & Trembley, 2020; Malumbres & Barbacid, 2005, 2009; Trembley et al., 2003; Zheng et al., 2008) (Tables 7, 8, 9). For instance, apart from its involvement in transcription and splicing (Dickinson et al., 2002; Hluchý et al., 2022; Hu et al., 2003; Loyer & Trembley, 2020; Malumbres & Barbacid, 2009; Trembley et al., 2003), CDK11 is required for transcription of replication‐dependent histone genes during S phase, and depletion of this kinase induces accumulation of cells in G1 (Gajdušková et al., 2020). A separate CDK11p58 isoform functions at the G2/M transition (Hu et al., 2007; Petretti et al., 2006). The roles of these kinases emphasize that CDKs are versatile and not always easily pigeonholed.
TABLE 8.
Additional CDKs in S. cerevisiae.
| Cyclin‐dependent kinase | Binding partner | Roles in cell cycle | Roles in transcription | Selected additional roles |
|---|---|---|---|---|
| Pho85 | Pcl1, Pcl2, Pho80, Clg1, Pcl5–10 |
Required for G1 progression and cell cycle commitment in the absence of Cln1 and Cln2 (Espinoza et al., 1994; Measday et al., 1994, 1997) Phosphorylates Sic1 leading to its degradation and G1/S transition (Nishizawa et al., 1998) Phosphorylates Rim15 to prevent cells from entering G0 (Huang et al., 2007) Establishes mitotic spindle in M and interacts with spindle assembly checkpoint genes MAD1 and BUB3 (Daniel et al., 2006) |
Deactivates Pho4 in high phosphate conditions (Huang et al., 2007; Lenburg & O'Shea, 1996) Phosphorylates Gcn4p to target it for degradation in high amino acid conditions (Carroll & O'Shea, 2002; Huang et al., 2007; Meimoun et al., 2000; Shemer et al., 2002) Phosphorylates Rim101 for export from the nucleus to regulate alkali stress response (Nishizawa et al., 2010) Phosphorylates Crz1 in high calcium conditions (Huang et al., 2007; Sopko et al., 2006) |
Downregulates Gsy2 glycogen synthase (Carroll & O'Shea, 2002; Huang et al., 1998, 2007) Implicated in bud morphogenesis (Carroll & O'Shea, 2002; Huang et al., 2007; Moffat & Andrews, 2004) Downregulates autophagy (Wang et al., 2001) Regulates the actin cytoskeleton (J. Lee et al., 1998) |
TABLE 9.
Additional CDKs in S. pombe.
| Cyclin‐dependent kinase | Binding partner | Roles in cell cycle | Roles in transcription | Selected additional roles |
|---|---|---|---|---|
| Pef1 | Pas1, Clg1, Psl2 |
Promotes pre‐meiotic DNA replication (Matsuda et al., 2021) Upregulates cohesin binding during the cell cycle (Birot et al., 2020) |
Activates Res2p‐Cdc10p on MCB genes (Tanaka & Okayama, 2000) |
Regulates sexual differentiation through upregulation of Ste11 and balancing the TORC1 pathway and autophagy (Matsuda et al., 2020) Downregulates cellular lifespan (Chen et al., 2013) |
| CDK11 | Lcp1 | Upregulates assembly of the mediator complex (Drogat et al., 2012) |
6. STRUCTURE OF CDKs AND CYCLINS
Throughout evolution, the CDK family has maintained some important features among its members. A protein kinase can be classified as a CDK based on its structural similarities with the canonical CDKs, including the presence of a PSTAIRE‐like cyclin‐binding element in the catalytic domain (Lim & Kaldis, 2013; Loyer & Trembley, 2020; Malumbres, 2014; Malumbres et al., 2009; Pines, 1995). Cyclins, by comparison, share more limited amino acid sequence similarity (Wood & Endicott, 2018). They were first identified by and named after their marked cell cycle‐dependent degradation on exit from mitosis and subsequent resynthesis (Evans et al., 1983). It is now clear that this cyclical behavior is a feature of a minority of cyclins; members of the family are more generally identified through one or two repeats of a “cyclin box” motif which assumes a fold comprising five alpha helices (Pines, 1995; Wood & Endicott, 2018).
The structural characterization of the CDK family began with studies on the CDK2‐cyclin A complex in various activational states and was used as an overarching model for CDK activation and regulation (Echalier et al., 2010; Wood & Endicott, 2018). It has now become apparent that the CDK‐cyclin complexes are highly variable in their 3D organization, and that this original standard is not applicable to many of the family members (Peissert et al., 2020). In addition, the size of CDKs ranges from 297 amino acid residues in CDK1 to 1512 residues in the case of CDK13 (Kohoutek & Blazek, 2012; Malumbres, 2014; Malumbres & Barbacid, 2005; Marqués et al., 2000).
7. CELL CYCLE OR TRANSCRIPTION: A FALSE CDK DICHOTOMY
7.1. Half a century of CDKs
The archetypal CDKs were discovered in the 1970s and 1980s, through genetic analysis of the yeast cell cycle (Hartwell et al., 1973; Morgan, 1995; Nurse et al., 1976), while cyclins were first identified through investigations of cell cycle‐regulated protein synthesis in sea urchin embryos (Evans et al., 1983). With the realization that the mitosis‐promoting activity of CDK1 depends on its association with cyclins, the term “cyclin‐dependent kinase” was first coined in 1991, at which point it was widely assumed that other enzymes of this class might also be involved in cell cycle regulation (Draetta et al., 1989; Labbe et al., 1989; Malumbres & Barbacid, 2005; Nurse, 1990). With the ever‐increasing availability of cDNA and ultimately whole‐genome sequences, it quickly became apparent that there is a multiplicity of CDKs, even in single‐celled eukaryotes. Budding yeast have 6 CDKs and 23 cyclins, while mammals have at least 20, along with a complement of ~30 distinct cyclins (Cao et al., 2014; Ercan et al., 2021; D. Huang et al., 2007; King et al., 1996; J. Liu & Kipreos, 2000; Malumbres, 2014; Tables 1, 2, 3, 4, 5, 6, 7, 8, 9).
Parallel but independent lines of investigation led to the identification of CDK‐cyclin complexes involved in transcriptional regulation, notably CDK7‐cyclin H in TFIIH (Akoulitchev et al., 1995; Drapkin & Reinberg, 1994; Shiekhattar et al., 1995) and CDK9‐cyclin in T/P‐TEFb (Graña et al., 1994; Marshall et al., 1996; J. Peng et al., 1998; Peterlin & Price, 2006). These distinct routes to the identification of cell‐cycle CDKs on the one hand and tCDKs on the other inevitably suggested a binary distinction between CDKs based on these broad biological functions, but it is becoming increasingly clear that such a classification is overly simplistic, as discussed below.
7.2. Regulation of transcription through the cell cycle
During the cell cycle, specific transcriptional programs ensure its directionality and proper timing, creating an oscillating pattern of gene expression (Gottesfeld & Forbes, 1997; Johnson & Holland, 1965; Ramos‐Alonso et al., 2023; Segil et al., 1996; Zaret, 2014). A significant change occurs at the onset of M phase, when transcription is largely, although not completely, silenced. This mitotic repression of all RNA polymerases is a well‐documented phenomenon (Gottesfeld & Forbes, 1997; Johnson & Holland, 1965; Parsons & Spencer, 1997). The main driving force behind mitotic silencing is the loss of chromatin accessibility, which is achieved through chromatin condensation during prophase, and/or through a range of histone modifications (Gottesfeld & Forbes, 1997; Ramos‐Alonso et al., 2023). The second essential feature of transcriptional repression during mitosis is the displacement of transcription factors from chromatin, including even the actively engaged pol II (Gottesfeld & Forbes, 1997; K. Liang, Woodfin, et al., 2015; Timmers & Verrijzer, 2017).
Mitotic silencing requires the hyperphosphorylation of pol II, and release of the general factors TFIID and TFIIH from core promoters (Loyer et al., 2005; Loyer & Trembley, 2020; Segil et al., 1996). Thus, the phosphorylation status of pol II not only changes through the transcriptional cycle, but with the cell cycle as well. However, a low‐level transcriptional program remains active during mitosis, keeping a subset of genes ready for re‐entry into interphase (Palozola et al., 2017; Timmers & Verrijzer, 2017; Zaret, 2014). Thus, some general transcription factors, including TFIID, can be retained on some genes, in a process known as mitotic “bookmarking” (Y. Liu, Pelham‐Webb, et al., 2017; Teves et al., 2016). General transcription is subsequently re‐established in telophase (Gottesfeld & Forbes, 1997). With the clear need for communication between the cell and transcription cycles to ensure they occur in the right order, the emerging evidence of interconnected functions of the cell‐cycle and tCDKs could point to their key role in coupling these processes.
7.3. Cell‐cycle CDKs with direct transcriptional roles—CDK1 as a tCDK?
Progression through the consecutive stages of the cell cycle requires activation of distinct transcriptional programs; factors expressed at a particular stage of the cycle will activate processes and proteins important for the next phase, thus ensuring unidirectionality. The “cell‐cycle” CDKs play therefore important roles in transcriptional control. For example, in yeast the archetypal cell‐cycle kinase CDK1 activates transcriptional programs required for maintaining the directionality of the cell cycle (Chymkowitch et al., 2012; Cosma et al., 2001; Enserink & Kolodner, 2010). Depending on the cell‐cycle stage, budding yeast Cdc28 communicates with distinct complexes and proteins to perform its function. Although the direct transcriptional effects of Cdc28 still need to be fully characterized, it is known that, for instance, in G2 Cdc28 stimulates the expression of CLB2 cluster of genes through phosphorylation of forkhead transcription factor Fkh2 and rate‐limiting transcriptional transactivator Ndd1 (Cho, Huang, et al., 2001; Darieva et al., 2003; Pic‐Taylor et al., 2004; Reynolds et al., 2003; Wittenberg & Reed, 2005), while in M phase it regulates the MCM, SIC1, and MAT gene clusters by phosphorylating transcription factors such as Swi5, and Ace2 (Archambault et al., 2004; Cho, Kobor, et al., 2001; Jans et al., 1995; Moll et al., 1991; O'Conalláin et al., 1999). However, the best characterized impact of Cdc28 on transcription is during G1; here Cdc28 acts on genes bound by the transcription factors Mlu1‐box binding factor (MBF) and Swi4/6‐dependent box‐binding factor (SBF), which encode proteins involved in DNA repair and cell cycle progression (Enserink & Kolodner, 2010). Cdc28 recruits pol II, TFIIB, and TFIIH to promoter regions of these genes through phosphorylation of Whi5, which then dissociates from SBF leading to activation of genes responsible for cell cycle entry (Cosma et al., 2001; De Bruin et al., 2004; Enserink & Kolodner, 2010). Notably, Cdc28 is able to directly phosphorylate the pol II CTD at S5 at the SBF target genes, through a positive feedback loop with Kin28 (TFIIH; Enserink & Chymkowitch, 2022; Kõivomägi et al., 2021; Ubersax et al., 2003). However, in mutant yeast cells which do not express S phase or mitotic cyclins, almost 70% of cell cycle‐regulated genes are still activated on time, suggesting an independent transcriptional oscillator functions alongside Cdc28 (Haase & Reed, 1999; Orlando et al., 2008; Simmons Kovacs et al., 2008).
Apart from regulation of the cell cycle‐dictated transcriptional programs, there is growing evidence of the involvement of Cdc28 in governing basal transcription. In S. cerevisiae, Cdc28 activity regulates a number of highly expressed housekeeping genes, such as PMA1 (Chymkowitch et al., 2012; Enserink & Chymkowitch, 2022; Serrano et al., 1982) by phosphorylating the S5 residue of pol II to upregulate transcription and promote the recruitment of capping factors. Again, it is able to perform this role through a mutual priming system with Kin28 (Chymkowitch et al., 2012; Cosma et al., 2001; Enserink & Chymkowitch, 2022; Kõivomägi et al., 2011). Cdc28 may phosphorylate the CTD not only at the S5 residue, but also at T4 in vivo and S2 in vitro (Nemec et al., 2019). Additionally, Cdc28 can regulate transcriptional processes in a more indirect manner; for instance, it phosphorylates the NuA4 chromatin modifier leading to increased Lys14 acetylation on histone Htz1 and increased transcription (Enserink & Kolodner, 2010; Fiedler et al., 2009).
Intriguingly, there appears to be a marked difference in the effect of Cdc28 on the transcriptional machinery in budding yeast and the effect of CDK1 on transcription in metazoans. In S. cerevisiae, the onset of M‐phase does not induce a dramatic shutdown of transcription; this may reflect the limited extent of mitotic chromatin condensation in this organism, which is also unusual by comparison with many other eukaryotes in that the key mitotic event of microtubular spindle assembly overlaps with S phase. Cdc28 usually serves to upregulate transcription, while CDK1 has a seemingly opposite function in human cells. The reasons behind this are not yet fully understood, but a possible explanation is that so far CDK1 studies were focused mainly on the transcriptional silencing that occurs during the M phase of the mammalian cell cycle, while the transcriptional actions of CDK1 in interphase have been largely overlooked (Enserink & Chymkowitch, 2022). However, it is important to note that the evolutionary ancestor of Cdc28 evolved into CDK1, CDK2, and CDK3 in metazoans, which complicates any direct functional comparison of Cdc28 and CDK1 (Figure 3).
It is clear that in human cells, as in yeast, CDK1 engages primarily in the control of cell‐cycle transcriptional programs to regulate cell division. For instance, during interphase, the CDK1‐cyclin A complex phosphorylates E2F to induce transcription of S‐phase genes, and its inhibition leads to E2F transcription factor‐dependent cell death (Shapiro, 2006). Although the CDK1‐cyclin B complex is thought to be mostly cytoplasmic during interphase, effects on transcription may be carried out by CDK1 partnered with a different cyclin, or simply indirectly (Gavet & Pines, 2010a, 2010b; Maryu & Yang, 2022). However, the best characterized transcriptional role of CDK1 is that of transcriptional silencing during M phase of the metazoan cell cycle. Unlike in budding yeast, where mutual phosphorylation between Cdc28 and Kin28 upregulates transcription, in human cells, CDK1 inhibits CDK7 through phosphorylation of the Ser164 residue in its T‐loop, therefore suppressing TFIIH‐dependent phosphorylation of the CTD (Akoulitchev & Reinberg, 1998; Cisek & Corden, 1989; Guo & Stiller, 2004; Kobor & Greenblatt, 2002; Long et al., 1998; Loyer & Trembley, 2020). Other direct mitotic phosphorylation targets of CDK1 include several general transcription factors, including TBP and TBP‐associated factors (Enserink & Chymkowitch, 2022; Long et al., 1998). CDK1 has also been shown to phosphorylate the pol II CTD in vitro (Enserink & Chymkowitch, 2022; Gebara et al., 1997; Xu et al., 2003; Xu & Manley, 2004; Zhang & Corden, 1991). However, it is not yet clear whether CDK1 phosphorylates the pol II CTD in vivo. It is worth mentioning that the first‐reported classical biochemical purification of a CTD kinase identified CDK1 (Chymkowitch & Enserink, 2013; Cisek & Corden, 1989; Pines, 1995).
Intriguingly, an unexpectedly high level of CDK1‐cyclin A activity in mouse embryonic stem cells phosphorylates multiple chromatin‐associated proteins to maintain the stem cell epigenetic landscape, and its inhibition leads to stem cell differentiation (Michowski et al., 2020). It remains to be seen if hyperactive CDK1 is similarly involved in the maintenance of cancer stem cell populations, but this discovery clearly underlines that cell‐cycle CDKs can regulate functions previously attributed purely to tCDKs (Sánchez‐Martínez et al., 2019). It also serves as further example of the involvement of CDKs in stem cell biology (see Section 4). In prostate cancer, ABCC5‐bound CDK1 has been found to directly phosphorylate the AR transcription factor to stimulate its activity, further highlighting that CDK1 might possess non‐mitotic transcriptional roles in different cellular contexts (Ji et al., 2021). CDK1 may also carry out cell cycle‐dependent phosphorylation of splicing factors and the polyadenylation machinery (Colgan et al., 1998; Okamoto et al., 1998). It is even conceivable that the key role of ancestral CDK1 lay in transcriptional regulation, and that functions of CDK1 in the more direct regulation of the mitotic apparatus evolved more recently (Chymkowitch & Enserink, 2013; Enserink & Chymkowitch, 2022).
7.4. The relationship of interphase cell‐cycle CDKs to transcription
The transcriptional roles of interphase CDKs in metazoans are also becoming increasingly evident. Progression through the R point requires CDK4/6 to phosphorylate the retinoblastoma protein (Rb), which is a broad‐specificity transcriptional repressor, and Rb‐related pocket proteins p107 and p130 (Dynlacht, 1997). In addition, substrates of CDK4/6 include other transcription factors, such as Smads (Anders et al., 2011; Cobrinik, 2005; Goel et al., 2018; Hydbring et al., 2016; Malumbres & Barbacid, 2001). CDK4/6‐dependent phosphorylation maintains activity of the Forkhead Box M1 (FOXM1) transcription factor, thereby preventing cells from entering senescence (Anders et al., 2011). CDK4 was further found to phosphorylate c‐Jun to form active AP‐1 transcription complexes in non‐dividing immune cells (Vanden Bush & Bishop, 2011). CDK6 is also implicated in influencing transcription during angiogenesis by upregulating expression of pro‐angiogenic VEGF‐A; and in pro‐inflammatory signaling where it phosphorylates and so activates the p65 subunit of NF‐κB (Handschick et al., 2014; Kollmann et al., 2013). Even further, CDK6 was found to phosphorylate and inactivate transcription factors which drive cell differentiation, for example in osteoblast and osteoclast cells or during neurogenesis (Grossel & Hinds, 2006; Urbach & Witte, 2019).
The CDK2‐cyclin E complex has several transcriptional targets including Rb and the transcription factor ELK4. Activation of ELK4 through phosphorylation leads to an increase in c‐fos expression, which facilitates malignant transformation in, for example, melanoma development (C. Peng et al., 2016). CDK2 also promotes cell survival in response to DNA damage through phosphorylation of the pro‐apoptotic TF FOXO1, which then relocates to the cytoplasm (H. Huang et al., 2006). Intriguingly, it has been found that CDK2 is recruited during interphase to stimulate transcription during human immunodeficiency virus 1 (HIV‐1) infection (Agbottah et al., 2006; Nekhai et al., 2002; Rice, 2018). To sustain HIV‐1 transcriptional elongation, CDK2 binds and phosphorylates the viral transactivator protein Tat, which in turn activates CDK7 leading to pol II clearance of the HIV‐1 proviral promoter. Tat also stimulates the recruitment of CDK9, and together these kinases phosphorylate the pol II CTD, which allows for the cell cycle‐dependent expression of HIV‐1 (Deng et al., 2002; Nekhai et al., 2002). CDK2 may also be involved in phosphorylation of Hepadnavirus core protein C‐terminal domain during human hepatitis B infection (Ludgate et al., 2012). Although CDK2 can phosphorylate the pol II CTD in vitro, it is unclear if it phosphorylates the pol II CTD in vivo outside of HIV‐1 infection (Guo & Stiller, 2004; Malumbres, 2014; Palancade & Bensaude, 2003). A recent study used the analogue‐sensitive kinase (AS) approach, which utilizes bulky ATP analogues carrying a transferable thiophosphate (Larochelle et al., 2006, 2007; Schachter & Fisher, 2013), to identify nuclear targets of CDK2 (Chi et al., 2020). Notably, several identified substrates were chromatin‐modifying proteins, such as the histone demethylase LSD1 and the histone methyltransferase DOT1L, in addition to several transcription factors, like the general TF GTF2I, or BCL11A and AF9, which are linked to cancer. This study implicates CDK2 directly in transcription regulation, which may provide an additional link to cell cycle control.
7.5. tCDKs with cell cycle roles
As the cell‐cycle CDKs clearly have a profound influence on transcription, it is logical to consider the reciprocal effect of tCDKs on the cell cycle. A good example of a CDK with dual roles is CDK7 (Table 4), which despite being traditionally classified as a tCDK, was initially identified as the metazoan CDK‐activating kinase (CAK), required for activation of both CDK1 and CDK2 by phosphorylating the “T‐loop” region in a way that is essential for their activity (Fisher, 2005). More recently, CDK7 was also shown to activate CDK4/6 (Schachter & Fisher, 2013) and tCDKs CDK9, 12 and 13 (Fisher, 2012; Larochelle et al., 2012; Rimel et al., 2020). Interestingly, when its central role in transcriptional regulation was first uncovered, its status as a CAK was questioned (Fisher, 2005; Harper et al., 1998). This may be due to the fact that its budding yeast homologue, Kin28, does not perform this function (Fisher, 2005, 2019; Malumbres, 2014). Not only do S. cerevisiae cells possess a specialized CAK responsible for activating CDK1 (and Kin28), but also they have a separate CAK which activates the tCDKs including the CDK9 homologue Bur1 (Espinoza et al., 1998; Fisher, 2019; Ostapenko & Solomon, 2005; Yao & Prelich, 2002). In multicellular organisms, however, CDK7 is indispensable for cell proliferation and development. For example, CDK7 activity is required for cell division in Drosophila and C. elegans (Larochelle et al., 1998; Wallenfang & Seydoux, 2002). In addition, CDK7 deficiency in mice leads to early‐embryonic lethality and premature aging of adult tissues with high proliferative ability, such as skin or intestinal epithelium (Ganuza et al., 2012). In human cells expressing the AS version of CDK7, CDK7 was shown to be essential for G1 phase progression, DNA replication and mitotic entry through activation of CDK4/6, CDK2, and CDK1, respectively (Bisteau et al., 2013; Larochelle et al., 2007; Schachter & Fisher, 2013). Similarly, inhibition of CDK7 with the selective inhibitor YKL‐5‐124 increased the number of cells in G1 and G2, with a concomitant decrease of cells in S phase (Olson et al., 2019). While CDK7 is considered constitutively active and stably expressed during the cell cycle, several mechanisms explain the timely and specific activation of the cell‐cycle CDKs. Firstly, mitogenic signals can trigger CDK7 T‐loop phosphorylation leading to an increase activity toward CDK4, which in turn phosphorylates Rb to promote R point transition (Schachter & Fisher, 2013). In addition, CDK7 has a preference for cyclin‐associated CDK1 but for the monomer of CDK2, which is likely to mediate the sequential activation of CDK2 followed by CDK1 (Larochelle et al., 2007; Merrick et al., 2008, 2011). Other factors, such as post‐translational modifications of cyclin H or MAT1, as well as association with its partners in TFIIH, are likely to account for CDK7 substrate specificity during the cell cycle (Akoulitchev et al., 2000; Rimel et al., 2020; Schneider et al., 2002).
Further, CDK8‐cyclin C can inhibit CAK activity through phosphorylation of cyclin H in vitro (Malumbres & Barbacid, 2005; Szilagyi & Gustafsson, 2013). CDK8 also targets p21, a broad‐specificity CDK inhibitor protein that represses cell cycle progression following exposure to a variety of stresses, including activation of the p53 tumor suppressor (Donner et al., 2007; Szilagyi & Gustafsson, 2013). CDK8 acts as a co‐activator of WAF1, the gene encoding p21, promoting cell‐cycle arrest at the R point and p21, in turn, directly stimulates the activity of CDK8, creating a potential positive feedback loop (Porter et al., 2012). Conversely, as a part of the mediator complex, CDK8 activates the β‐catenin transcriptional program, which promotes cell cycle commitment (Firestein et al., 2008; Szilagyi & Gustafsson, 2013). Although there is no evidence that CDK8/19 can influence the G2/M transition in metazoans, the CDK8 homologue in fission yeast phosphorylates Fkh2, which controls a cluster of genes expressed at the onset of mitosis (Buck et al., 2004; Szilagyi et al., 2012). CDK8 has also been implicated in G1 cell cycle commitment in budding yeast, where the mediator complex is recruited to the SBF‐controlled genes by Swi5 transcription factor, before they are acted upon by the Cdc28 (Bhoite et al., 2001; Cosma et al., 2001; Kishi et al., 2008; Szilagyi & Gustafsson, 2013). The implication of CDK8 in the restriction point is further highlighted by its role in maintaining quiescence of vulval precursor cells in C. elegans, which start performing superfluous cell divisions after loss of the mediator complex (Clayton et al., 2008; Szilagyi & Gustafsson, 2013).
Despite CDK12/13 being typically classified as tCDKS, similarly to CDK7, they were first identified during cDNA screens for cell‐cycle regulators (Ko et al., 2001; Kohoutek & Blazek, 2012; Marqués et al., 2000). Similarly, the gene encoding their cyclin partner, cyclin K, was first identified through its ability to complement G1 cyclin gene deletion mutants in S. cerevisiae (Edwards et al., 1998). The pattern of Cyclin K expression also correlates positively with proliferative capacity (Dai et al., 2012; Lei et al., 2018; Xiang et al., 2014). More recently, the knockdown of either CDK12 or cyclin K in different human cell lines was shown to induce cell cycle arrest in G1 by preventing assembly of the pre‐replicative complex (Lei et al., 2018). In particular, CDK12‐cyclin K complex was found to phosphorylate cyclin E1 in G1 to restrict its ability to interact with CDK2, thereby favoring formation of the pre‐replicative complex. A peak of CDK12 expression in early G1 adds further weight to this discovery (Bertoli et al., 2013; Manavalan et al., 2019). Whether CDK12/cyclin K implication in G1 progression is direct or transcriptional is, however, still under debate. A chemical genetic approach identified CDK12 activity as critical for G1 to S progression in HCT116 cells, but this was shown to depend on its function in activating RNA pol II processivity on key DNA replication genes (Manavalan et al., 2019). Interestingly, depletion of CDK12 in human cells also causes a G2/M arrest, but this may reflect a specific requirement for CDK12 activity for effective transcription of long DNA‐damage response genes (Blazek et al., 2011; Dubbury et al., 2018; Geng et al., 2019; Krajewska et al., 2019; S. Liang et al., 2020; Tellier et al., 2020). CDK12 was also shown to control translation of mitotic regulatory gene mRNAs in human U2OS cells (S. H. Choi et al., 2019). Specifically, CDK12 directly phosphorylates the translation repressor 4E‐BP1, in cooperation with the mTORC1 kinase, to promote its dissociation from the 5′ end of target mRNAs. In comparison, the involvement of CDK13 in the cell cycle is not as well‐understood. However, in gastric cancer cells, which experience increased cell proliferation due to overexpression of the HMGA2 protein, Gene Ontology analysis indicated CDK13 as a cell‐cycle related target of HMGA2 (Z. Wu, Wang, et al., 2021). Rapid cell proliferation in these cells is thought to rely on shortening of the S phase and speeding up progression through the G2/M transition. Joint inhibition of CDK13 and HMGA2 could therefore be antiproliferative (Z. Wu, Wang, et al., 2021). Intriguingly, in breast cancer cells, upregulation of genes involved in S and G2/M progression was caused by inhibiting CDK12/13, further highlighting the role of these kinases in these stages of the cell cycle, and indicating that such effects may be cell type‐dependent (Quereda et al., 2019).
The possible cell‐cycle functions of CDK9 remain elusive. CDK9 levels can oscillate throughout the cell cycle, while cyclin T levels stay relatively constant (Kiernan et al., 2001). However, this is not always the case (Garriga et al., 2003), arguing that CDK9 does not play a major role in cell cycle regulation. In some cell types, such as T cells, signals which induce cell cycle entry can also upregulate cyclin T expression (Garriga et al., 1998; Herrmann et al., 1998), suggesting that it is not the cell cycle but cell activation state that regulates the CDK9‐cyclin T complex (H. Liu & Herrmann, 2005). However, depletion of CDK9 in non‐small cell lung, and head and neck squamous cell carcinoma cell lines induces cell cycle delay with an accumulation of cells in G1 and a corresponding decrease in S phase cells (Cai et al., 2006; Storch & Cordes, 2016). In addition, RNAi‐mediated knockdown of CDK9 in Drosophila cells leads to cell cycle arrest in G1 (Anshabo et al., 2021; Yang et al., 2008). Further, CDK9 phosphorylates the Rb protein in vitro and in vivo, which could be linked to the decrease of D‐type cyclins and increase in E‐type cyclins observed after downregulation of this kinase (Graña et al., 1994; Simone et al., 2002; Storch & Cordes, 2016). CDK9 activity was also found necessary for cell cycle recovery after replication stress (D. S. Yu et al., 2010). Recent phosphoproteomic analysis also identified phosphorylation targets of CDK9 that are implicated in the cell cycle, such as FAM122A (PABIR1), which is involved in blocking the G2/M transition, and PCNP, which promotes proliferation (Tellier et al., 2022). Although not as direct as the effects of cell‐cycle CDKs on transcription, tCDKs can therefore influence the cell cycle, to an the extent which is still being uncovered.
8. CDKs AND DISEASE
With CDKs implicated in virtually all cellular processes, it is unsurprising that mutation or dysregulation of these kinases can cause a wide range of diseases. Their involvement extends from defects in proliferation, through ischaemia, to rare congenital disorders (Colas, 2020; Łukasik et al., 2021). For example, CDK4 is thought to play a role in the development of some neurodegenerative diseases (Greene et al., 2007; Icreverzi et al., 2015; Łukasik et al., 2021; Mcshea et al., 1997; Sanphui et al., 2013), while CDK5 is specifically implicated in Alzheimer disease, cardiovascular disorders, and diabetes (Arif, 2012; Cicenas & Valius, 2011; Łukasik et al., 2021; Malhotra et al., 2021). As noted above, CDKs can also be co‐opted to help with the replication of viral genomes (Yan et al., 2022). Accordingly, a range of specific and potent CDK inhibitors have been developed, both for clinical and research purposes. Several dozen small‐molecule CDK inhibitors are now being used or tested as treatments for a range of diseases (Abdelmalak et al., 2022; Cicenas & Valius, 2011; Goel et al., 2020; Jhaveri et al., 2021; Marak et al., 2020; Mughal et al., 2023; Roskoski, 2019; Sánchez‐Martínez et al., 2019; Zhang et al., 2021).
8.1. Cell‐cycle CDKs contribute to tumorigenesis
The unscheduled cell proliferation that characterizes tumorigenesis is in many cases attributable to decreased requirement for authentic mitogen signaling and so loss of stringent R point control. The first indications that dysregulation of interphase CDK‐cyclin complexes might contribute to this aspect of tumor development came from observations of chromosomal translocations involving the CCND1 gene, which encodes cyclin D1, in a human parathyroid carcinoma and, most notably, B cell lymphomas (Hsi et al., 1996; Lesage et al., 2005; H. Liu, Wang, & Epner, 2004; Shane, 2001; Thomázy et al., 2002; Vasef et al., 1999; Zhao et al., 2014). A causative role for the resulting cyclin D1 overexpression in tumorigenesis was supported by findings from mouse models in which tissue‐specific promoter‐driven transgenic expression of cyclin D1 led to a high frequency of tumor formation in the corresponding tissue (Fantl et al., 1995; Sicinski et al., 1995). Furthermore, tumorigenesis driven by oncogenes such as Erb‐B2 in transgenic mice was blocked by the simultaneous genetic knock‐out of CDK4 or cyclin D1, or by expression of a mis‐sense mutant form of cyclin D1 that prevents activation of the bound CDK (Landis et al., 2006; Q. Yu et al., 2001, 2006). In familial human melanoma the predisposing alleles include loss‐of‐function mutations in CDKN2A, which encodes the p16 CDK inhibitor, and mis‐sense mutations in CDK4 which result in failure of p16 inhibition of the mutant kinase (Zuo et al., 1996). Thus, cyclin D1, normally expressed in a stringently mitogen‐dependent manner, can be activated pathologically as a result of oncogenic mutations in components of mitogen signaling pathways, and constitutive CDK4/6‐cyclin D1 activity can drive constitutive cell‐cycle commitment as tumors develop. The most widely‐mutated genes across all tumor types include CDKN2A and RB1, most frequently loss‐of‐function, and CCND1, which usually increases in copy number (Aaltonen et al., 2020). As cyclin D1 ablation attenuates tumor growth and activation of cellular senescence, therapeutic targeting of cyclin D1‐associated CDK activity may be therapeutically useful in human cancers (Y. J. Choi et al., 2012).
The process of developing successful CDK inhibitors has been challenging, and many have not progressed beyond early‐stage clinical trials (Asghar et al., 2015; Chohan et al., 2018) largely because they act as competitive inhibitors of ATP binding to CDKs, which are just one sub‐group of some 518 protein kinases in the human proteome. The multiplicity of protein kinases and the limited availability of active, pure preparations of these enzymes have meant that the specificity of even those CDK inhibitors that have been approved for use in the clinic is unclear (see, e.g., Fry et al., 2004). These small‐molecule inhibitors may therefore have biologically significant impacts on multiple protein kinases, and perhaps other ATP‐dependent processes, in patients. There is therefore an urgent need for allosteric CDK inhibitors with increased specificity (Marak et al., 2020; Sánchez‐Martínez et al., 2019).
Despite these caveats, the ATP‐competitive inhibitors palbociclib, ribociclib, and abemaciclib show some specificity for CDK4/CDK6 inhibition in vitro and inhibit the proliferation of cancer cell lines, inducing G1 arrest in an Rb‐dependent manner (Finn et al., 2009). These inhibitors were approved by the U.S. Food and Drug Administration as a therapy against hormone receptor‐positive advanced breast cancer, in conjunction with endocrine agents (Finn et al., 2016; Sánchez‐Martínez et al., 2019). Ribociclib and abemaciclib confer measurable overall survival benefits (Im et al., 2019; Sledge et al., 2020) and they are now being tested in combination with other drugs, immuno‐ and chemotherapy, as well as against other types of cancer (Bonelli et al., 2019; Lynce et al., 2018; Spring et al., 2019). However, evidence is lacking that the clinical responses seen were due to inhibition of CDK4/6 in patients' tumors.
Although CDK2 is not required for proliferation of brain or connective tissue, its inhibition in glioblastoma and osteosarcoma cell lines causes decrease in proliferation of transformed cells (Malumbres & Barbacid, 2009). In this context, it is notable that amplification of the gene encoding the CDK2 partner cyclin E1, is even more common than CCND1 amplification across numerous human tumor types (Aaltonen et al., 2020). Overexpressed cyclin E1 may be redundant with cyclin D1 in driving R point transit and may contribute to genomic instability by promoting re‐replication of chromosomal DNA.
In line with its role in ensuring developmental viability, and its nonredundant role in the cell cycle, CDK1 is one of the least mutated CDKs in cancer (Asghar et al., 2015; Otto & Sicinski, 2017; Peyressatre et al., 2015). In tumors, CDK1 activity is often dysregulated through indirect mutations in DNA damage response pathways, or loss of CDK inhibitors (Asghar et al., 2015). However, there is growing evidence that CDK1 can act as a driver of cancer development and progression (Otto & Sicinski, 2017; Sofi et al., 2022). Overexpression of CDK1 has been found to be a biomarker in a number of different types of cancer, including lung, pancreas and sarcomas (M. Li et al., 2020; Q. Li, Zhang, et al., 2019; Piao et al., 2019; Yamamura et al., 2020). Similarly, overexpression of its partner cyclin B is often associated with poor prognosis in, for instance, breast cancer patients (Agarwal et al., 2010; Winters et al., 2001). Inhibition of CDK1 can induce apoptosis in several types of malignancy (Ongkeko et al., 1995; Otto & Sicinski, 2017) and could prove effective against cancer stem cells, particularly in gliomas and pancreatic cancers (Sánchez‐Martínez et al., 2019). In the absence of evidence to the contrary, it is even possible that clinical responses to CDK inhibition are due, in part at least, to off‐target inhibition of CDK1.
8.2. tCDKs are dysregulated in cancer
As malignant cells generally rely on a higher transcriptional output than healthy cells, often relying on oncogenic transcription factors or super‐enhancers, it is not surprising that dysregulation of key tCDKs is implicated in a range of cancers. As a result, tCDKs are currently seen as important pharmaceutical targets and biomarkers of cancer, with several tCDK inhibitors currently in clinical trials for cancer treatment (Franco & Kraus, 2015; Galbraith et al., 2019; Parua & Fisher, 2020; Sánchez‐Martínez et al., 2019).
The dual actions of the highly related kinases CDK8/CDK19 (Table 4) have led them to be implicated in cancer development as both drivers or suppressors of tumorigenesis. Inhibition of both kinases, whose catalytic domains share 94% amino acid sequence identity, by cortistatin A leads to decreased proliferation of malignant cells (Chou et al., 2020; Pelish et al., 2015). In addition, overexpression of CDK8 can contribute to the development of melanoma, as well as colorectal, gastric and breast cancers (Chohan et al., 2018; Peyressatre et al., 2015). In prostate cancer cells, inhibition of CDK8/19 leads to premature G1/S transition and cell death (Nakamura et al., 2018). However, CDK8 can also inhibit tumor growth, for instance in the case of endometrial cancer. It was shown that ectopic expression of CDK8 inhibited proliferation in the KLE cancer cell line, and that it could even block growth of a mouse tumor model in vivo (Gu et al., 2013). In addition to cortistatin A, a number of CDK8/CDK19 inhibitors are currently under development but none has yet reached the clinic (Galbraith et al., 2019).
Interest in designing a specific CDK9 inhibitor began when it was discovered that pan‐CDK inhibitors, for instance flavopiridol, potently inhibit this kinase. CDK9 is hyperactivated in a number of cancers, particularly hematological malignancies (Chohan et al., 2018; Galbraith et al., 2019). The involvement of CDK9 in lymphoma development is also connected to its role in maintaining the expression of short‐lived anti‐apoptotic mRNAs, such as MCL1. It is further recruited to chromatin by various cancer‐dependent transcription factors and super‐enhancers (Galbraith et al., 2019). Because of its central role in transcription elongation, CDK9 remains a high‐priority target, with a number of inhibitors currently undergoing clinical trials (Galbraith et al., 2019).
Like CDK8/CDK19, CDK12, and CDK13 have been shown to have both tumor‐permissive and suppressive functions. CDK12 is recognized as a significant player in genomic instability of cancers and its loss‐of‐function mutations have been implicated in advanced ovarian and breast malignancies (Cheng et al., 2022; Chohan et al., 2018; Galbraith et al., 2019; H. Liu et al., 2021). On the other hand, its overexpression is associated with HER2‐positive breast cancers and increased levels of ERBB2 (Quereda et al., 2019). Importantly, depletion of CDK12 preferentially decreases the expression of DNA damage response (DDR) genes (Blazek et al., 2011; Manavalan et al., 2019; S. H. Choi et al., 2020; Dubbury et al., 2018; Krajewska et al., 2019). This allows for sensitization of the tumor to synthetic‐lethal therapy, for instance with poly‐ADP ribose polymerase (PARP) inhibitors (Chou et al., 2020; Parua & Fisher, 2020). Despite being less studied, CDK13 has also been found to be amplified in some primary liver and colon cancers (Galbraith et al., 2019). Although a clinical inhibitor of CDK12 or CDK13 is still in the works, there currently exists a range of research‐grade tools used to inhibit these kinases. Further, as seen with the example of PARP inhibitors, CDK12/CDK13 inhibitors can synergize with other therapeutic agents (Galbraith et al., 2019; Niu et al., 2022; Quereda et al., 2019; Tadesse et al., 2020). Preliminary data suggest triple‐negative breast cancer as a potential therapeutic target for CDK12/13 inhibition, which would depend on suppressing the DNA damage response pathway and leading to sensitization of malignant cells to apoptosis (Hopkins & Zou, 2019; Quereda et al., 2019; Tadesse et al., 2020).
Perhaps the biggest success in therapeutic targeting of tCDKs is seen in the example of CDK7, with multiple selective inhibitors currently in Phase I/II clinical trials (Kovalová et al., 2023; Sava et al., 2020). The dual actions of CDK7, which participates in both regulation of transcription and the cell cycle, have made it an important player in tumorigenesis. Its overactivation is associated with gastric, breast, ovarian, and liver cancers, and it has been suggested that the malignant cells show greater dependency on elevated CDK7 levels than healthy cells (Galbraith et al., 2019; Sava et al., 2020; Wang et al., 2015). THZ1 is a covalent CDK inhibitor, and although it primarily targets CDK7, it also has some anti‐CDK12/CDK13 activity (Sánchez‐Martínez et al., 2019). Encouragingly, treating cells advanced breast cancer cells with THZ1 leads to apoptotic cell death (Franco & Kraus, 2015; Wang et al., 2015). Several other groups also showed that selective inhibition of CDK7 leads to cell cycle arrest, apoptosis, and decreased transcription levels, especially of genes associated with super‐enhancers (Galbraith et al., 2019; Sava et al., 2020). Importantly, inhibition of CDK7 shows promise in treating cancers with heightened transcription, even without the presence of any apparent oncogenic driver mutations (Wang et al., 2015). Additionally, CDK7 inhibitors show promise of synergistic treatment, for example with pro‐apoptotic agents (He et al., 2022; Kalan et al., 2017). In the same manner, colorectal cancer cells treated with the antimetabolite 5‐fluorouracil became sensitized to reversible or covalent CDK7 inhibitors, and showed increased rates of apoptotic cell death (Kalan et al., 2017).
9. CONCLUSION
The picture emerging from the field of CDK biology is one of great complexity. The multitasking CDKs can be seen as a versatile group of kinases, which help to receive, interpret, and act on a variety of endogenous and exogenous signals. The redundancy between individual family members and low selectivity of inhibitors has made investigation of CDK roles challenging. Examining the direct, primary actions of these kinases was further complicated by the use of long‐term inhibition approaches, such as siRNA‐mediated knockdown. Despite this, their molecular functions are now coming more sharply into focus. In cancer, tumors acquire dependencies on processes guarded by the CDKs, which may become rate‐limiting for tumor growth and progression. As the involvement of CDK deregulation in diseases becomes clearer, more therapeutic opportunities arise.
It is also increasingly apparent that a binary distinction between cell‐cycle and transcriptional CDKs is inappropriate. Rather, the involvement of several CDKs in both aspects of biology suggests an evolutionarily ancient role in the co‐ordination of cell proliferation and gene expression.
AUTHOR CONTRIBUTIONS
Aleksandra J. Pluta: Conceptualization (lead); writing – original draft (lead); writing – review and editing (lead). Cécilia Studniarek: Writing – original draft (supporting); writing – review and editing (supporting). Shona Murphy: Conceptualization (equal); funding acquisition (lead); supervision (equal); writing – review and editing (lead). Chris J. Norbury: Conceptualization (equal); supervision (equal); writing – review and editing (equal).
FUNDING INFORMATION
This work was supported by Wellcome Trust Investigator Grant 210641/Z/18/Z to SM.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
RELATED WIREs ARTICLES
ACKNOWLEDGMENTS
We would like to apologize to those not cited due to space constraints. We would like to thank all cited and uncited authors for their contributions to the field of CDK biology.
Pluta, A. J. , Studniarek, C. , Murphy, S. , & Norbury, C. J. (2024). Cyclin‐dependent kinases: Masters of the eukaryotic universe. WIREs RNA, 15(1), e1816. 10.1002/wrna.1816
Edited by: Alexandra Moreira, Associate Editor and Jeff Wilusz, Editor‐in‐Chief
Contributor Information
Shona Murphy, Email: shona.murphy@path.ox.ac.uk.
Chris J. Norbury, Email: chris.norbury@path.ox.ac.uk.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Aaltonen, L. A. , Abascal, F. , Abeshouse, A. , Aburatani, H. , Adams, D. J. , Agrawal, N. , Ahn, K. S. , Ahn, S.‐M. , Aikata, H. , Akbani, R. , Akdemir, K. C. , Al‐Ahmadie, H. , Al‐Sedairy, S. T. , Al‐Shahrour, F. , Alawi, M. , Albert, M. , Aldape, K. , Alexandrov, L. B. , Ally, A. , … ICGC/TCGA Pan‐Cancer Analysis of Whole Genomes Consortium . (2020). Pan‐cancer analysis of whole genomes. Nature, 578(7793), 82–93. 10.1038/s41586-020-1969-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmalak, M. , Singh, R. , Anwer, M. , Ivanchenko, P. , Randhawa, A. , Ahmed, M. , Ashton, A. W. , Du, Y. , Jiao, X. , & Pestell, R. (2022). The renaissance of CDK inhibitors in breast cancer therapy: An update on clinical trials and therapy resistance. Cancers, 14(21), 5388. 10.3390/CANCERS14215388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler, A. S. , McCleland, M. L. , Truong, T. , Lau, S. , Modrusan, Z. , Soukup, T. M. , Roose‐Girma, M. , Blackwood, E. M. , & Firestein, R. (2012). CDK8 maintains tumor dedifferentiation and embryonic stem cell pluripotency. Cancer Research, 72(8), 2129–2139. 10.1158/0008-5472 [DOI] [PubMed] [Google Scholar]
- Agarwal, R. , Myhre, S. , Carey, M. , Overgaard, J. , Alsner, J. , Stemke‐hale, K. , Lluch, A. , Richard, M. , Kuo, W. L. , Sorlie, T. , Sahin, A. , Valero, V. , Gray, J. W. , Mills, G. B. , & Bryan, T. (2010). Integrative analysis of cyclin protein levels identifies cyclin B1 as a classifier and predictor of outcomes in breast cancer. Clinical Cancer Research, 15(11), 3654–3662. 10.1158/1078-0432.CCR-08-3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbottah, E. , Deng, L. , Dannenberg, L. O. , Pumfery, A. , & Kashanchi, F. (2006). Effect of SWI/SNF chromatin remodeling complex on HIV‐1 tat activated transcription. Retrovirology, 3(1), 1–19. 10.1186/1742-4690-3-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, S. H. , Keogh, M. C. , & Buratowski, S. (2009). Ctk1 promotes dissociation of basal transcription factors from elongating RNA polymerase II. The EMBO Journal, 28(3), 205–212. 10.1038/EMBOJ.2008.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, S. H. , Kim, M. , & Buratowski, S. (2004). Phosphorylation of serine 2 within the RNA polymerase II C‐terminal domain couples transcription and 3′ end processing. Molecular Cell, 13(1), 67–76. 10.1016/S1097-2765(03)00492-1 [DOI] [PubMed] [Google Scholar]
- Akhtar, M. S. , Heidemann, M. , Tietjen, J. R. , Zhang, D. W. , Chapman, R. D. , Eick, D. , & Ansari, A. Z. (2009). TFIIH kinase places bivalent marks on the carboxy‐terminal domain of RNA polymerase II. Molecular Cell, 34(3), 387–393. 10.1016/j.molcel.2009.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoulitchev, S. , & Reinberg, D. (1998). The molecular mechanism of mitotic inhibition of TFIIH is mediated by phosphorylation of CDK7. Genes & Development, 12(22), 3541–3550. 10.1101/GAD.12.22.3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoulitchev, S. , Chuikov, S. , & Reinberg, D. (2000). TFIIH is negatively regulated by cdk8‐containing mediator complexes. Nature, 407(6800), 102–106. 10.1038/35024111 [DOI] [PubMed] [Google Scholar]
- Akoulitchev, S. , Mäkelät, T. P. , Weinberg, R. A. , & Reinberg, D. (1995). Requirement for TFIIH kinase activity in transcription by RNA polymerase II. Nature, 377(6549), 557–560. 10.1038/377557a0 [DOI] [PubMed] [Google Scholar]
- Ali, F. , Hindley, C. , McDowell, G. , Deibler, R. , Jones, A. , Kirschner, M. , Guillemot, F. , & Philpott, A. (2011). Cell cycle‐regulated multi‐site phosphorylation of Neurogenin 2 coordinates cell cycling with differentiation during neurogenesis. Development, 138(19), 4267–4277. 10.1242/DEV.067900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amour, C. V. S. , Sansó, M. , Bösken, C. A. , Lee, K. M. , Larochelle, S. , Zhang, C. , Shokat, K. M. , Geyer, M. , & Fisher, R. P. (2012). Separate domains of fission yeast Cdk9 (P‐TEFb) are required for capping enzyme recruitment and primed (Ser7‐phosphorylated) Rpb1 carboxyl‐terminal domain substrate recognition. Molecular and Cellular Biology, 32(13), 2372–2383. 10.1128/MCB.06657-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, S. , Kwon, O. S. , Yu, J. , & Jang, S. K. (2020). A cyclin‐dependent kinase, CDK11/p58, represses cap‐dependent translation during mitosis. Cellular and Molecular Life Sciences, 77(22), 4693–4708. 10.1007/S00018-019-03436-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, X. , Ng, S. S. , Xie, D. , Zeng, Y. X. , Sze, J. , Wang, J. , Chen, Y. C. , Chow, B. K. C. , Lu, G. , Poon, W. S. , Kung, H. f. , Wong, B. C. Y. , & Lin, M. C. m. (2010). Functional characterisation of cell cycle‐related kinase (CCRK) in colorectal cancer carcinogenesis. European Journal of Cancer, 46(9), 1752–1761. 10.1016/j.ejca.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Anders, L. , Ke, N. , Hydbring, P. , Choi, Y. J. , Widlund, H. R. , Chick, J. M. , Zhai, H. , Vidal, M. , Gygi, S. P. , Braun, P. , & Sicinski, P. (2011). A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell, 20(5), 620–634. 10.1016/j.ccr.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrau, J. C. , van de Pasch, L. , Lijnzaad, P. , Bijma, T. , Koerkamp, M. G. , van de Peppel, J. , Werner, M. , & Holstege, F. C. P. (2006). Genome‐wide location of the coactivator mediator: Binding without activation and transient Cdk8 interaction on DNA. Molecular Cell, 22(2), 179–192. 10.1016/j.molcel.2006.03.023 [DOI] [PubMed] [Google Scholar]
- Anshabo, A. T. , Milne, R. , Wang, S. , & Albrecht, H. (2021). CDK9: A comprehensive review of its biology, and its role as a potential target for anti‐cancer agents. Frontiers in Oncology, 11, 1573. 10.3389/FONC.2021.678559/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault, V. , Chang, E. J. , Drapkin, B. J. , Cross, F. R. , Chait, B. T. , & Rout, M. P. (2004). Targeted proteomic study of the cyclin‐Cdk module. Molecular Cell, 14(6), 699–711. 10.1016/J.MOLCEL.2004.05.025 [DOI] [PubMed] [Google Scholar]
- Arif, A. (2012). Extraneuronal activities and regulatory mechanisms of the atypical cyclin‐dependent kinase Cdk5. Biochemical Pharmacology, 84(8), 985–993. 10.1016/J.BCP.2012.06.027 [DOI] [PubMed] [Google Scholar]
- Ariza, M. E. , Broome‐Powell, M. , Lahti, J. M. , Kidd, V. J. , & Nelson, M. A. (1999). Fas‐induced apoptosis in human malignant melanoma cell lines is associated with the activation of the p34cdc2‐related PITSLRE protein kinases. Journal of Biological Chemistry, 274(40), 28505–28513. 10.1074/JBC.274.40.28505 [DOI] [PubMed] [Google Scholar]
- Asghar, U. , Witkiewicz, A. K. , Turner, N. C. , & Knudsen, E. S. (2015). The history and future of targeting cyclin‐dependent kinases in cancer therapy. Nature Reviews Drug Discovery, 14(2), 130–146. 10.1038/nrd4504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon, C. W. , & D'Orso, I. (2019). CDK9: A signaling hub for transcriptional control. Transcription, 10(2), 57–75. 10.1080/21541264.2018.1523668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banyai, G. , Lopez, M. D. , Szilagyi, Z. , & Gustafsson, C. M. (2014). Mediator can regulate mitotic entry and direct periodic transcription in fission yeast. Molecular and Cellular Biology, 34(21), 4008–4018. 10.1128/MCB.00819-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barna, M. , Pusic, A. , Zollo, O. , Costa, M. , Kondrashov, N. , Rego, E. , Rao, P. H. , & Ruggero, D. (2008). Suppression of Myc oncogenic activity by ribosomal protein haploinsufficiency. Nature, 456(7224), 971–975. 10.1038/nature07449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone, G. , Arora, A. , Ganesh, A. , Abdel‐Fatah, T. , Moseley, P. , Ali, R. , Chan, S. Y. , Savva, C. , Schiavone, K. , Carmell, N. , Myers, K. N. , Rakha, E. A. , Madhusudan, S. , Collis, S. J. , Barone, G. , Arora, A. , Ganesh, A. , Abdel‐Fatah, T. , Moseley, P. , … Collis, S. J. (2018). The relationship of CDK18 expression in breast cancer to clinicopathological parameters and therapeutic response. Oncotarget, 9(50), 29508–29524. 10.18632/ONCOTARGET.25686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone, G. , Staples, C. J. , Ganesh, A. , Patterson, K. W. , Bryne, D. P. , Myers, K. N. , Patil, A. A. , Eyers, C. E. , Maslen, S. , Skehel, J. M. , Eyers, P. A. , & Collis, S. J. (2016). Human CDK18 promotes replication stress signaling and genome stability. Nucleic Acids Research, 44(18), 8772. 10.1093/NAR/GKW615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkowiak, B. , & Greenleaf, A. L. (2011). Phosphorylation of RNAPII: To P‐TEFb or not to P‐TEFb? Transcription, 2(3), 115–119. 10.4161/TRNS.2.3.15004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkowiak, B. , Liu, P. , Phatnani, H. P. , Fuda, N. J. , Cooper, J. J. , Price, D. H. , Adelman, K. , Lis, J. T. , & Greenleaf, A. L. (2010). CDK12 is a transcription elongation‐associated CTD kinase, the metazoan ortholog of yeast Ctk1. Genes & Development, 24(20), 2303–2316. 10.1101/GAD.1968210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach, D. , Durkacz, B. , & Nurse, P. (1982). Functionally homologous cell cycle control genes in budding and fission yeast. Nature, 300(5894), 706–709. 10.1038/300706A0 [DOI] [PubMed] [Google Scholar]
- Berro, R. , Pedati, C. , Kehn‐Hall, K. , Wu, W. , Klase, Z. , Even, Y. , Genevière, A.‐M. , Ammosova, T. , Nekhai, S. , & Kashanchi, F. (2008). CDK13, a new potential human immunodeficiency virus type 1 inhibitory factor regulating viral mRNA splicing. Journal of Virology, 82(14), 7155–7166. 10.1128/JVI.02543-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoli, C. , Skotheim, J. M. , & de Bruin, R. A. M. (2013). Control of cell cycle transcription during G1 and S phases. Nature Reviews Molecular Cell Biology, 14(8), 518–528. 10.1038/nrm3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyaert, R. , Kidd, V. J. , Cornelis, S. , van de Craen, M. , Denecker, G. , Lahti, J. M. , Gururajan, R. , Vandenabeele, P. , & Fiers, W. (1997). Cleavage of PITSLRE kinases by ICE/CASP‐1 and CPP32/CASP‐3 during apoptosis induced by tumor necrosis factor. Journal of Biological Chemistry, 272(18), 11694–11697. 10.1074/JBC.272.18.11694 [DOI] [PubMed] [Google Scholar]
- Bhatia, P. K. , Wang, Z. , & Friedberg, E. C. (1996). DNA repair and transcription. Current Opinion in Genetics & Development, 6(2), 146–150. 10.1016/S0959-437X(96)80043-8 [DOI] [PubMed] [Google Scholar]
- Bhoite, L. T. , Yu, Y. , & Stillman, D. J. (2001). The Swi5 activator recruits the mediator complex to the HO promoter without RNA polymerase II. Genes & Development, 15(18), 2457–2469. 10.1101/GAD.921601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimbó, A. , Jia, Y. , Siew, L. P. , Karuturi, R. K. M. , den Elzen, N. , Peng, X. , Zheng, L. , O'Connell, M. , Liu, E. T. , Balasubramanian, M. K. , & Liu, J. (2005). Systematic deletion analysis of fission yeast protein kinases. Eukaryotic Cell, 4(4), 799. 10.1128/EC.4.4.799-813.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birot, A. , Tormos‐Pérez, M. , Vaur, S. , Feytout, A. , Jaegy, J. , Gil, D. A. , Vazquez, S. , Ekwall, K. , & Javerzat, J. P. (2020). The CDK Pef1 and protein phosphatase 4 oppose each other for regulating cohesin binding to fission yeast chromosomes. eLife, 9, e50556. 10.7554/ELIFE.50556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisteau, X. , Paternot, S. , Colleoni, B. , Ecker, K. , Coulonval, K. , de Groote, P. , Declercq, W. , Hengst, L. , & Roger, P. P. (2013). CDK4 T172 phosphorylation is central in a CDK7‐dependent bidirectional CDK4/CDK2 interplay mediated by p21 phosphorylation at the restriction point. PLoS Genetics, 9(5), e1003546. 10.1371/JOURNAL.PGEN.1003546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazek, D. , Kohoutek, J. , Bartholomeeusen, K. , Johansen, E. , Hulinkova, P. , Luo, Z. , Cimermancic, P. , Ule, J. , & Peterlin, B. M. (2011). The cyclin K/Cdk12 complex maintains genomic stability via regulation of expression of DNA damage response genes. Genes & Development, 25(20), 2158–2172. 10.1101/GAD.16962311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli, M. , La Monica, S. , Fumarola, C. , & Alfieri, R. (2019). Multiple effects of CDK4/6 inhibition in cancer: From cell cycle arrest to immunomodulation. Biochemical Pharmacology, 170, 113676. 10.1016/j.bcp.2019.113676 [DOI] [PubMed] [Google Scholar]
- Booth, G. T. , Parua, P. K. , Sansó, M. , Fisher, R. P. , & Lis, J. T. (2018). Cdk9 regulates a promoter‐proximal checkpoint to modulate RNA polymerase II elongation rate in fission yeast. Nature Communications, 9(1), 1–10. 10.1038/s41467-018-03006-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borggrefe, T. , Davis, R. , Erdjument‐Bromage, H. , Tempst, P. , & Kornberg, R. D. (2002). A complex of the Srb8, ‐9, ‐10, and ‐11 transcriptional regulatory proteins from yeast. Journal of Biological Chemistry, 277(46), 44202–44207. 10.1074/jbc.M207195200 [DOI] [PubMed] [Google Scholar]
- Bösken, C. A. , Farnung, L. , Hintermair, C. , Schachter, M. M. , Vogel‐Bachmayr, K. , Blazek, D. , Anand, K. , Fisher, R. P. , Eick, D. , & Geyer, M. (2014). The structure and substrate specificity of human Cdk12/Cyclin K. Nature Communications, 5, 3505. 10.1038/NCOMMS4505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchoux, C. , Hautbergue, G. , Grenetier, S. , Carles, C. , Riva, M. , & Goguel, V. (2004). CTD kinase I is involved in RNA polymerase I transcription. Nucleic Acids Research, 32(19), 5851–5860. 10.1093/NAR/GKH927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenna, A. , Olejniczak, I. , Chavan, R. , Ripperger, J. A. , Langmesser, S. , Cameroni, E. , Hu, Z. , de Virgilio, C. , Dengjel, J. , & Albrecht, U. (2019). Cyclin‐dependent kinase 5 (CDK5) regulates the circadian clock. eLife, 8, e50925. 10.7554/ELIFE.50925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, N. R. , Noble, M. E. M. , Lawrie, A. M. , Morris, M. C. , Tunnah, P. , Divita, G. , Johnson, L. N. , & Endicott, J. A. (1999). Effects of phosphorylation of threonine 160 on cyclin‐dependent kinase 2 structure and activity. Journal of Biological Chemistry, 274(13), 8746–8756. 10.1074/jbc.274.13.8746 [DOI] [PubMed] [Google Scholar]
- Buck, V. , Ng, S. S. , Ruiz‐Garcia, A. B. , Papadopoulou, K. , Bhatti, S. , Samuel, J. M. , Anderson, M. , Millar, J. B. A. , & McInerny, C. J. (2004). Fkh2p and Sep1p regulate mitotic gene transcription in fission yeast. Journal of Cell Science, 117(23), 5623–5632. 10.1242/JCS.01473 [DOI] [PubMed] [Google Scholar]
- Buck, V. , Russell, P. , & Millar, J. B. A. (1995). Identification of a cdk‐activating kinase in fission yeast. EMBO Journal, 14(24), 6173–6183. 10.1002/J.1460-2075.1995.TB00308.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratowski, S. (2003). The CTD code. Nature Structural & Molecular Biology, 10(9), 679–680. 10.1038/nsb0903-679 [DOI] [PubMed] [Google Scholar]
- Buratowski, S. (2009). Progression through the RNA polymerase II CTD cycle. Molecular Cell, 36(4), 541–546. 10.1016/J.MOLCEL.2009.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss, H. , Handschick, K. , Jurrmann, N. , Pekkonen, P. , Beuerlein, K. , Müller, H. , Wait, R. , Saklatvala, J. , Ojala, P. M. , Schmitz, M. L. , Naumann, M. , & Kracht, M. (2012). Cyclin‐dependent kinase 6 phosphorylates NF‐κB P65 at serine 536 and contributes to the regulation of inflammatory gene expression. PLoS One, 7(12), 51847. 10.1371/JOURNAL.PONE.0051847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, D. , Latham, V. M. , Zhang, X. , & Shapiro, G. I. (2006). Combined depletion of cell cycle and transcriptional cyclin‐dependent kinase activities induces apoptosis in cancer cells. Cancer Research, 66(18), 9270–9280. 10.1158/0008-5472.CAN-06-1758 [DOI] [PubMed] [Google Scholar]
- Caillava, C. , Vandenbosch, R. , Jablonska, B. , Deboux, C. , Spigoni, G. , Gallo, V. , Malgrange, B. , & van Evercooren, A. B. (2011). Cdk2 loss accelerates precursor differentiation and remyelination in the adult central nervous system. Journal of Cell Biology, 193(2), 397–407. 10.1083/jcb.201004146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, L. , Chen, F. , Yang, X. , Xu, W. , Xie, J. , & Yu, L. (2014). Phylogenetic analysis of CDK and cyclin proteins in premetazoan lineages. BMC Evolutionary Biology, 14, 10. 10.6070/H4RF5S05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, M. (1997). Genetics of transcriptional regulation in yeast: Connections to the RNA polymerase II CTD. Annual Review of Cell and Developmental Biology, 13, 1–23. 10.1146/ANNUREV.CELLBIO.13.1.1 [DOI] [PubMed] [Google Scholar]
- Carroll, A. S. , & O'Shea, E. K. (2002). Pho85 and signaling environmental conditions. Trends in Biochemical Sciences, 27(2), 87–93. 10.1016/S0968-0004(01)02040-0 [DOI] [PubMed] [Google Scholar]
- Chae, T. , Kwon, Y. T. , Bronson, R. , Dikkes, P. , En, L. , & Tsai, L. H. (1997). Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron, 18(1), 29–42. 10.1016/S0896-6273(01)80044-1 [DOI] [PubMed] [Google Scholar]
- Chang, Y. W. , Howard, S. C. , Budovskaya, Y. V. , Rine, J. , & Herman, P. K. (2001). The rye mutants identify a role for Ssn/Srb proteins of the RNA polymerase II holoenzyme during stationary phase entry in Saccharomyces cerevisiae . Genetics, 157(1), 17–26. 10.1093/GENETICS/157.1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput, D. , Kirouac, L. , Stevens, S. M., Jr. , Padmanabhan, J. , Chaput, D. , Kirouac, L. , Stevens, S. M., Jr. , & Padmanabhan, J. (2016). Potential role of PCTAIRE‐2, PCTAIRE‐3 and P‐histone H4 in amyloid precursor protein‐dependent Alzheimer pathology. Oncotarget, 7(8), 8481–8497. 10.18632/ONCOTARGET.7380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, B. R. , Li, Y. , Eisenstatt, J. R. , & Runge, K. W. (2013). Identification of a lifespan extending mutation in the Schizosaccharomyces pombe cyclin gene clg1+ by direct selection of long‐lived mutants. PLoS One, 8(7), e69084. 10.1371/JOURNAL.PONE.0069084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. H. , Wong, Y. H. , Geneviere, A. M. , & Fann, M. J. (2007). CDK13/CDC2L5 interacts with L‐type cyclins and regulates alternative splicing. Biochemical and Biophysical Research Communications, 354(3), 735–740. 10.1016/J.BBRC.2007.01.049 [DOI] [PubMed] [Google Scholar]
- Chen, H. R. , Juan, H. C. , Wong, Y. H. , Tsai, J. W. , & Fann, M. J. (2017). Cdk12 regulates neurogenesis and late‐arising neuronal migration in the developing cerebral cortex. Cerebral Cortex, 27(3), 2289–2302. 10.1093/CERCOR/BHW081 [DOI] [PubMed] [Google Scholar]
- Chen, H. H. , Wang, Y.‐C. , & Fann, M.‐J. (2006). Identification and characterization of the CDK12/cyclin L1 complex involved in alternative splicing regulation. Molecular and Cellular Biology, 26(7), 2736–2745. 10.1128/MCB.26.7.2736-2745.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Wang, Y. , Jiang, W. , Ni, R. , Wang, Y. , & Ni, S. (2019). CDK14 involvement in proliferation migration and invasion of esophageal cancer. Annals of Translational Medicine, 7(22), 681. 10.21037/ATM.2019.11.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. Y. , Gu, X. T. , Saiyin, H. , Wan, B. , Zhang, Y. J. , Li, J. , Wang, Y. L. , Gao, R. , Wang, Y. F. , Dong, W. P. , Najjar, S. M. , Zhang, C. Y. , Ding, H. F. , Liu, J. O. , & Yu, L. (2012). Brain‐selective kinase 2 (BRSK2) phosphorylation on PCTAIRE1 negatively regulates glucose‐stimulated insulin secretion in pancreatic β‐cells. Journal of Biological Chemistry, 287(36), 30368–30375. 10.1074/jbc.M112.375618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, L. , Zhou, S. , Zhou, S. , Shi, K. , Cheng, Y. , Cai, M. C. , Ye, K. , Lin, L. , Zhang, Z. , Jia, C. , Xiang, H. , Zang, J. , Zhang, M. , Yin, X. , Li, Y. , Di, W. , Zhuang, G. , & Tan, L. (2022). Dual inhibition of CDK12/CDK13 targets both tumor and immune cells in ovarian cancer. Cancer Research, 82(19), 3588–3602. 10.1158/0008-5472.CAN-22-0222/ [DOI] [PubMed] [Google Scholar]
- Cheng, S.‐W. G. , Kuzyk, M. A. , Moradian, A. , Ichu, T.‐A. , Chang, V. C.‐D. , Tien, J. F. , Vollett, S. E. , Griffith, M. , Marra, M. A. , & Morin, G. B. (2012). Interaction of cyclin‐dependent kinase 12/CrkRS with cyclin K1 is required for the phosphorylation of the C‐terminal domain of RNA polymerase II. Molecular and Cellular Biology, 32(22), 4691–4704. 10.1128/MCB.06267-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi, Y. , Carter, J. H. , Swanger, J. , Mazin, A. V. , Moritz, R. L. , & Clurman, B. E. (2020). A novel landscape of nuclear human CDK2 substrates revealed by in situ phosphorylation. Science Advances, 6(16), 1–11. 10.1126/sciadv.aaz9899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi, Y. , Hong, Y. , Zong, H. , Wang, Y. , Zou, W. , Yang, J. , Kong, X. , Yun, X. , & Gu, J. (2009). CDK11p58 represses vitamin D receptor‐mediated transcriptional activation through promoting its ubiquitin‐proteasome degradation. Biochemical and Biophysical Research Communications, 386(3), 493–498. 10.1016/J.BBRC.2009.06.061 [DOI] [PubMed] [Google Scholar]
- Chi, Y. , Huddleston, M. J. , Zhang, X. , Young, R. A. , Annan, R. S. , Carr, S. A. , & Deshaies, R. J. (2001). Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin‐dependent kinase. Genes & Development, 15(9), 1078–1092. 10.1101/GAD.867501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, E. J. , Kobor, M. S. , Kim, M. , Greenblatt, J. , & Buratowski, S. (2001). Opposing effects of Ctk1 kinase and Fcp1 phosphatase at Ser 2 of the RNA polymerase II C‐terminal domain. Genes & Development, 15(24), 3319–3329. 10.1101/GAD.935901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, R. J. , Huang, M. , Campbell, M. J. , Dong, H. , Steinmetz, L. , Sapinoso, L. , Hampton, G. , Elledge, S. J. , Davis, R. W. , & Lockhart, D. J. (2001). Transcriptional regulation and function during the human cell cycle. Nature Genetics, 27(1), 48–54. 10.1038/83751 [DOI] [PubMed] [Google Scholar]
- Cho, Y. Y. , Tang, F. , Yao, K. , Lu, C. , Zhu, F. , Zheng, D. , Pugliese, A. , Bode, A. M. , & Dong, Z. (2009). Cyclin‐dependent kinase‐3‐mediated c‐Jun phosphorylation at Ser63 and Ser73 enhances cell transformation. Cancer Research, 69(1), 272–281. 10.1158/0008-5472.CAN-08-3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chohan, T. A. , Qayyum, A. , Rehman, K. , Tariq, M. , & Akash, M. S. H. (2018). An insight into the emerging role of cyclin‐dependent kinase inhibitors as potential therapeutic agents for the treatment of advanced cancers. Biomedicine and Pharmacotherapy, 107, 1326–1341. 10.1016/j.biopha.2018.08.116 [DOI] [PubMed] [Google Scholar]
- Choi, S. H. , Kim, S. , & Jones, K. A. (2020). Gene expression regulation by CDK12: A versatile kinase in cancer with functions beyond CTD phosphorylation. Experimental & Molecular Medicine, 52(5), 762–771. 10.1038/S12276-020-0442-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, S. H. , Martinez, T. F. , Kim, S. , Donaldson, C. , Shokhirev, M. N. , Saghatelian, A. , & Jones, K. A. (2019). CDK12 phosphorylates 4E‐BP1 to enable mTORC1‐dependent translation and mitotic genome stability. Genes and Development, 33(7‐8), 418–435. 10.1101/GAD.322339.118/-/DC1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, Y. J. , Li, X. , Hydbring, P. , Sanda, T. , Stefano, J. , Christie, A. L. , Signoretti, S. , Look, A. T. , Kung, A. L. , von Boehmer, H. , & Sicinski, P. (2012). The requirement for cyclin D function in tumor maintenance. Cancer Cell, 22(4), 438. 10.1016/J.CCR.2012.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, J. , Quigley, D. A. , Robinson, T. M. , Feng, F. Y. , & Ashworth, A. (2020). Transcription‐associated cyclin‐dependent kinases as targets and biomarkers for cancer therapy. Cancer Discovery, 10(3), 351–370. 10.1158/2159-8290.CD-19-0528 [DOI] [PubMed] [Google Scholar]
- Chu, Y. , Simic, R. , Warner, M. H. , Arndt, K. M. , & Prelich, G. (2007). Regulation of histone modification and cryptic transcription by the Bur1 and Paf1 complexes. The EMBO Journal, 26(22), 4646–4656. 10.1038/SJ.EMBOJ.7601887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, Y. , Sutton, A. , Sternglanz, R. , & Prelich, G. (2006). The BUR1 cyclin‐dependent protein kinase is required for the normal pattern of histone methylation by SET2. Molecular and Cellular Biology, 26(8), 3029–3038. 10.1128/MCB.26.8.3029-3038.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chymkowitch, P. , & Enserink, J. M. (2013). The cell cycle rallies the transcription cycle Cdc28/Cdk1 is a cell cycle‐regulated transcriptional CDK. Transcription, 4(1), 3–6. 10.4161/trns.22456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chymkowitch, P. , Eldholm, V. , Lorenz, S. , Zimmermann, C. , Lindvall, J. M. , Bjørås, M. , Meza‐Zepeda, L. A. , & Enserink, J. M. (2012). Cdc28 kinase activity regulates the basal transcription machinery at a subset of genes. Proceedings of the National Academy of Sciences of the United States of America, 109(26), 10450–10455. 10.1073/PNAS.1200067109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicenas, J. , & Valius, M. (2011). The CDK inhibitors in cancer research and therapy. Journal of Cancer Research and Clinical Oncology, 137(10), 1409–1418. 10.1007/s00432-011-1039-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero, S. , & Herrup, K. (2005). Cyclin‐dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. The Journal of Neuroscience, 25(42), 9658. 10.1523/JNEUROSCI.1773-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek, L. J. , & Corden, J. L. (1989). Phosphorylation of RNA polymerase by the murine homologue of the cell‐cycle control protein cdc2. Nature, 339, 679–684. 10.1038/339679a0 [DOI] [PubMed] [Google Scholar]
- Cismowski, M. J. , Laff, G. M. , Solomon, M. J. , & Reed, S. I. (1995). KIN28 encodes a C‐terminal domain kinase that controls mRNA transcription in Saccharomyces cerevisiae but lacks Cyclin‐dependent kinase‐activating kinase (CAK) activity. Molecular and Cellular Biology, 15(6), 2983–2992. 10.1128/MCB.15.6.2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausing, E. , Mayer, A. , Chanarat, S. , Müller, B. , Germann, S. M. , Cramer, P. , Lisby, M. , & Strässer, K. (2010). The transcription elongation factor Bur1‐Bur2 interacts with replication protein A and maintains genome stability during replication stress. Journal of Biological Chemistry, 285(53), 41665–41674. 10.1074/jbc.M110.193292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton, J. E. , van den Heuvel, S. J. L. , & Saito, R. M. (2008). Transcriptional control of cell‐cycle quiescence during C. elegans development. Developmental Biology, 313(2), 603–613. 10.1016/J.YDBIO.2007.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobrinik, D. (2005). Pocket proteins and cell cycle control. Oncogene, 24(17), 2796–2809. 10.1038/sj.onc.1208619 [DOI] [PubMed] [Google Scholar]
- Colas, P. (2020). Cyclin‐dependent kinases and rare developmental disorders. Orphanet Journal of Rare Diseases, 15(1), 1–14. 10.1186/S13023-020-01472-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, A. R. (2009). PCTK proteins: The forgotten brain kinases? Neurosignals, 17(4), 288–297. 10.1159/000231895 [DOI] [PubMed] [Google Scholar]
- Colgan, D. F. , Murthy, K. G. K. , Zhao, W. , Prives, C. , & Manley, J. L. (1998). Inhibition of poly(A) polymerase requires p34(cdc2)/cyclin B phosphorylation of multiple consensus and non‐consensus sites. EMBO Journal, 17(4), 1053–1062. 10.1093/EMBOJ/17.4.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly, C. J. , Vidal‐Cardenas, S. , Goldsmith, S. , & Greider, C. W. (2022). The Bur1 cyclin‐dependent kinase regulates telomere length in Saccharomyces cerevisiae . Yeast (Chichester, England), 39(3), 177. 10.1002/YEA.3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly, T. , Caligiuri, M. , & Beach, D. (1997). The Cdc2 protein kinase controls Cdc10/Sct1 complex formation. Molecular Biology of the Cell, 8(6), 1105–1115. 10.1091/MBC.8.6.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coordes, B. , Brünger, K. M. , Burger, K. , Soufi, B. , Horenk, J. , Eick, D. , Olsen, J. V. , & Sträßer, K. (2015). Ctk1 function is necessary for full translation initiation activity in Saccharomyces cerevisiae. Eukaryotic Cell, 14(1), 86–95. 10.1128/EC.00106-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosma, M. P. , Panizza, S. , & Nasmyth, K. (2001). Cdk1 triggers association of RNA polymerase to cell cycle promoters only after recruitment of the mediator by SBF. Molecular Cell, 7(6), 1213–1220. 10.1016/S1097-2765(01)00266-0 [DOI] [PubMed] [Google Scholar]
- Coudreuse, D. , & Nurse, P. (2010). Driving the cell cycle with a minimal CDK control network. Nature, 468(7327), 1074–1079. 10.1038/nature09543 [DOI] [PubMed] [Google Scholar]
- Coudreuse, D. , van Bakel, H. , Dewez, M. , Soutourina, J. , Parnell, T. , Vandenhaute, J. , Cairns, B. , Werner, M. , & Hermand, D. (2010). A gene‐specific requirement of RNA polymerase II CTD phosphorylation for sexual differentiation in S. pombe . Current Biology, 20(12), 1053–1064. 10.1016/j.cub.2010.04.054 [DOI] [PubMed] [Google Scholar]
- Coverley, D. , Pelizon, C. , Trewick, S. , & Laskey, R. A. (2000). Chromatin‐bound Cdc6 persists in S and G2 phases in human cells, while soluble Cdc6 is destroyed in a cyclin A‐cdk2 dependent process. Journal of Cell Science, 113(11), 1929–1938. 10.1242/JCS.113.11.1929 [DOI] [PubMed] [Google Scholar]
- Cruz, J. C. , & Tsai, L. H. (2004). A Jekyll and Hyde kinase: Roles for Cdk5 in brain development and disease. Current Opinion in Neurobiology, 14(3), 390–394. 10.1016/J.CONB.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Dai, Q. , Lei, T. , Zhao, C. , Zhong, J. , Tang, Y. Z. , Chen, B. , Yang, J. , Li, C. , Wang, S. , Song, X. , Li, L. , & Li, Q. (2012). Cyclin K‐containing kinase complexes maintain self‐renewal in murine embryonic stem cells. The Journal of Biological Chemistry, 287(30), 25344–25352. 10.1074/JBC.M111.321760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damagnez, V. , Pamäkelä, T. , & Cottarel, G. (1995). Schizosaccharomyces pombe Mop1‐Mcs2 is related to mammalian CAK. The EMBO Journal, 14(24), 6164–6172. 10.1002/J.1460-2075.1995.TB00307.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel, J. A. , Keyes, B. E. , Ng, Y. P. Y. , Freeman, C. O. , & Burke, D. J. (2006). Diverse functions of spindle assembly checkpoint genes in Saccharomyces cerevisiae . Genetics, 172(1), 53–65. 10.1534/GENETICS.105.046441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannappel, M. V. , Sooraj, D. , Loh, J. J. , & Firestein, R. (2019). Molecular and in vivo functions of the CDK8 and CDK19 kinase modules. Frontiers in Cell and Developmental Biology, 6, 171. 10.3389/FCELL.2018.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darieva, Z. , Pic‐Taylor, A. , Boros, J. , Spanos, A. , Geymonat, M. , Reece, R. J. , Sedgwick, S. G. , Sharrocks, A. D. , & Morgan, B. A. (2003). Cell cycle‐regulated transcription through the FHA domain of Fkh2p and the coactivator Ndd1p. Current Biology, 13(19), 1740–1745. 10.1016/j.cub.2003.08.053 [DOI] [PubMed] [Google Scholar]
- De Bruin, R. A. M. , McDonald, W. H. , Kalashnikova, T. I. , Yates, J. , & Wittenberg, C. (2004). Cln3 activates G1‐specific transcription via phosphorylation of the SBF bound repressor Whi5. Cell, 117(7), 887–898. 10.1016/j.cell.2004.05.025 [DOI] [PubMed] [Google Scholar]
- Deng, L. , Ammosova, T. , Pumfery, A. , Kashanchi, F. , & Nekhai, S. (2002). HIV‐1 tat interaction with RNA polymerase II C‐terminal domain (CTD) and a dynamic association with CDK2 induce CTD phosphorylation and transcription from HIV‐1 promoter. Journal of Biological Chemistry, 277(37), 33922–33929. 10.1074/jbc.M111349200 [DOI] [PubMed] [Google Scholar]
- Dickinson, L. A. , Edgar, A. J. , Ehley, J. , & Gottesfeld, J. M. (2002). Cyclin L is an RS domain protein involved in pre‐mRNA splicing. Journal of Biological Chemistry, 277(28), 25465–25473. 10.1074/jbc.M202266200 [DOI] [PubMed] [Google Scholar]
- Diril, M. K. , Koumar, C. , Padmakumar, V. C. , Du, T. , & Wasser, M. (2012). Cyclin‐dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re‐replication but not for liver regeneration. Proceedings of the National Academy of Sciences of the United States of America, 109(10), 3826–3831. 10.1073/pnas.1115201109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner, A. J. , Szostek, S. , Hoover, J. M. , & Espinosa, J. M. (2007). CDK8 is a stimulus‐specific positive coregulator of p53 target genes. Molecular Cell, 27(1), 121–133. 10.1016/J.MOLCEL.2007.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta, G. , Luca, F. , Westendorf, J. , Brizuela, L. , Ruderman, J. , & Beach, D. (1989). cdc2 protein kinase is complexed with both Cyclin A and B: Evidence for proteolytic inactivation of MPF. Cell, 56(5), 829–838. 10.1016/0092-8674(89)90687-9 [DOI] [PubMed] [Google Scholar]
- Drapkin, R. , & Reinberg, D. (1994). The multifunctional TFIIH complex and transcriptional control. Trends in Biochemical Sciences, 19(11), 504–508. 10.1016/0968-0004(94)90139-2 [DOI] [PubMed] [Google Scholar]
- Drogat, J. , Migeot, V. , Mommaerts, E. , Mullier, C. , Dieu, M. , van Bakel, H. , & Hermand, D. (2012). Cdk11‐CyclinL controls the assembly of the RNA polymerase II mediator complex. Cell Reports, 2(5), 1068–1076. 10.1016/j.celrep.2012.09.027 [DOI] [PubMed] [Google Scholar]
- Dronamraju, R. , & Strahl, B. D. (2014). A feed forward circuit comprising Spt6, Ctk1 and PAF regulates Pol II CTD phosphorylation and transcription elongation. Nucleic Acids Research, 42(2), 870–881. 10.1093/NAR/GKT1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbury, S. J. , Boutz, P. L. , & Sharp, P. A. (2018). CDK12 regulates DNA repair genes by suppressing intronic polyadenylation. Nature, 564(7734), 141–145. 10.1038/S41586-018-0758-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynlacht, B. D. (1997). Regulation of transcription by proteins that control the cell cycle. Nature, 389(6647), 149–152. 10.1038/38225 [DOI] [PubMed] [Google Scholar]
- Echalier, A. , Endicott, J. A. , & Noble, M. E. M. (2010). Recent developments in cyclin‐dependent kinase biochemical and structural studies. Biochimica et Biophysica Acta (BBA) – Proteins and Proteomics, 1804(3), 511–519. 10.1016/J.BBAPAP.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Edwards, M. C. , Wong, C. , & Elledge, S. J. (1998). Human cyclin K, a novel RNA polymerase II‐associated cyclin possessing both carboxy‐terminal domain kinase and Cdk‐activating kinase activity. Molecular and Cellular Biology, 18(7), 4291–4300. 10.1128/MCB.18.7.4291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff, S. (2021). CDK9 keeps RNA polymerase II on track. Cellular and Molecular Life Sciences, 78(14), 5543–5567. 10.1007/s00018-021-03878-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick, D. , & Geyer, M. (2013). The RNA polymerase II carboxy‐terminal domain (CTD) code. Chemical Reviews, 113(11), 8456–8490. 10.1021/CR400071F [DOI] [PubMed] [Google Scholar]
- Eifler, T. T. , Shao, W. , Bartholomeeusen, K. , Fujinaga, K. , Jäger, S. , Johnson, J. R. , Luo, Z. , Krogan, N. J. , & Peterlin, B. M. (2015). Cyclin‐dependent kinase 12 increases 3′ end processing of growth factor‐induced c‐FOS transcripts. Molecular and Cellular Biology, 35(2), 468–478. 10.1128/MCB.01157-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmlund, H. , Baraznenok, V. , Lindahl, M. , Samuelsen, C. O. , Koeck, P. J. B. , Holmberg, S. , Hebert, H. , & Gustafsson, C. M. (2006). The cyclin‐dependent kinase 8 module sterically blocks mediator interactions with RNA polymerase II. Proceedings of the National Academy of Sciences of the United States of America, 103(43), 15788–15793. 10.1073/PNAS.0607483103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink, J. M. , & Chymkowitch, P. (2022). Cell cycle‐dependent transcription: The cyclin dependent kinase Cdk1 is a direct regulator of basal transcription machineries. International Journal of Molecular Sciences, 23(3), 1293. 10.3390/ijms23031293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink, J. M. , & Kolodner, R. D. (2010). An overview of Cdk1‐controlled targets and processes. Cell Division, 5, 11. 10.1186/1747-1028-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan, D. P. , Chrétien, F. , Chakravarty, P. , Flynn, H. R. , Snijders, A. P. , & Uhlmann, F. (2021). Budding yeast relies on G1 cyclin specificity to couple cell cycle progression with morphogenetic development. Science Advances, 7(23), 7–11. 10.1126/SCIADV.ABG0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza, F. H. , Farrell, A. , Nourse, J. L. , Chamberlin, H. M. , Gileadi, O. , & Morgan, D. O. (1998). Cak1 is required for Kin28 phosphorylation and activation in vivo. Molecular and Cellular Biology, 18(11), 6365–6373. 10.1128/MCB.18.11.6365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza, F. H. , Ogas, J. , Herskowitz, I. , & Morgan, D. O. (1994). Cell cycle control by a complex of the cyclin HCS26 (PCL1) and the kinase PHO85. Science (New York, NY), 266(5189), 1388–1391. 10.1126/SCIENCE.7973730 [DOI] [PubMed] [Google Scholar]
- Evans, T. , Rosenthal, E. T. , Youngblom, J. , Distel, D. , & Hunt, T. (1983). Cyclin: A protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell, 33(2), 389–396. 10.1016/0092-8674(83)90420-8 [DOI] [PubMed] [Google Scholar]
- Ezhevsky, S. A. , Nagahara, H. , Vocero‐Akbani, A. M. , Gius, D. R. , Wei, M. C. , & Dowdy, S. F. (1997). Hypo‐phosphorylation of the retinoblastoma protein (pRb) by cyclin D: Cdk4/6 complexes results in active pRb. Proceedings of the National Academy of Sciences of the United States of America, 94(20), 10699–10704. 10.1073/PNAS.94.20.10699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, Z. , Devlin, J. R. , Hogg, S. J. , Doyle, M. A. , Harrison, P. F. , Todorovski, I. , Cluse, L. A. , Knight, D. A. , Sandow, J. J. , Gregory, G. , Fox, A. , Beilharz, T. H. , Kwiatkowski, N. , Scott, N. E. , Vidakovic, A. T. , Kelly, G. P. , Svejstrup, J. Q. , Geyer, M. , Gray, N. S. , … Johnstone, R. W. (2020). CDK13 cooperates with CDK12 to control global RNA polymerase II processivity. Science Advances, 6(18), eaaz5041. 10.1126/SCIADV.AAZ5041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fant, C. B. , & Taatjes, D. J. (2019). Regulatory functions of the mediator kinases CDK8 and CDK19. Transcription, 10(2), 76–90. 10.1080/21541264.2018.1556915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl, V. , Stamp, G. , Andrews, A. , Rosewell, I. , & Dickson, C. (1995). Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes & Development, 9(19), 2364–2372. 10.1101/GAD.9.19.2364 [DOI] [PubMed] [Google Scholar]
- Feaver, W. J. , Svejstrup, J. Q. , Henry, N. L. , & Kornberg, R. D. (1994). Relationship of CDK‐activating kinase and RNA polymerase II CTD kinase TFIIH/TFIIK. Cell, 79(6), 1103–1109. 10.1016/0092-8674(94)90040-X [DOI] [PubMed] [Google Scholar]
- Feng, H. , Cheng, A. S. L. , Tsang, D. P. , Li, M. S. , Go, M. Y. , Cheung, Y. S. , Zhao, G. J. , Ng, S. S. , Lin, M. C. , Yu, J. , Lai, P. B. , To, K. F. , & Sung, J. J. Y. (2011). Cell cycle‐related kinase is a direct androgen receptor‐regulated gene that drives β‐catenin/T cell factor‐dependent hepatocarcinogenesis. The Journal of Clinical Investigation, 121(8), 3159–3175. 10.1172/JCI45967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, H. , Yu, Z. , Tian, Y. , Lee, Y. Y. , Li, M. S. , Go, M. Y. Y. , Cheung, Y. S. , Lai, P. B. S. , Chan, A. M. L. , To, K. F. , Chan, H. L. Y. , Sung, J. J. Y. , & Cheng, A. S. L. (2015). A CCRK‐EZH2 epigenetic circuitry drives hepatocarcinogenesis and associates with tumor recurrence and poor survival of patients. Journal of Hepatology, 62(5), 1100–1111. 10.1016/j.jhep.2014.11.040 [DOI] [PubMed] [Google Scholar]
- Fiedler, D. , Braberg, H. , Mehta, M. , Chechik, G. , Cagney, G. , Mukherjee, P. , Silva, A. C. , Shales, M. , Collins, S. R. , van Wageningen, S. , Kemmeren, P. , Holstege, F. C. P. , Weissman, J. S. , Keogh, M. C. , Koller, D. , Shokat, K. M. , & Krogan, N. J. (2009). Functional organization of the S. cerevisiae phosphorylation network. Cell, 136(5), 952–963. 10.1016/j.cell.2008.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, R. S. , Dering, J. , Conklin, D. , Kalous, O. , Cohen, D. J. , Desai, A. J. , Ginther, C. , Atefi, M. , Chen, I. , Fowst, C. , Los, G. , & Slamon, D. J. (2009). PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor‐positive human breast cancer cell lines in vitro. Breast Cancer Research, 11(5), 1–13. 10.1186/bcr2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, R. S. , Martin, M. , Rugo, H. S. , Jones, S. , Im, S.‐A. , Gelmon, K. , Harbeck, N. , Lipatov, O. N. , Walshe, J. M. , Moulder, S. , Gauthier, E. , Lu, D. R. , Randolph, S. , Diéras, V. , & Slamon, D. J. (2016). Palbociclib and letrozole in advanced breast cancer. New England Journal of Medicine, 375(20), 1925–1936. 10.1056/nejmoa1607303 [DOI] [PubMed] [Google Scholar]
- Firestein, R. , Bass, A. J. , Kim, S. Y. , Dunn, I. F. , Silver, S. J. , Guney, I. , Freed, E. , Ligon, A. H. , Vena, N. , Ogino, S. , Chheda, M. G. , Tamayo, P. , Finn, S. , Shrestha, Y. , Boehm, J. S. , Jain, S. , Bojarski, E. , Mermel, C. , Barretina, J. , … Hahn, W. C. (2008). CDK8 is a colorectal cancer oncogene that regulates β‐catenin activity. Nature, 455(7212), 547–551. 10.1038/nature07179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, D. L. , & Nurse, P. (1996). A single fission yeast mitotic cyclin B p34cdc2 kinase promotes both S‐phase and mitosis in the absence of G1 cyclins. The EMBO Journal, 15(4), 850–860. 10.1002/J.1460-2075.1996.TB00420.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, R. P. (2005). Secrets of a double agent: CDK7 in cell‐cycle control and transcription. Journal of Cell Science, 118(22), 5171–5180. 10.1242/jcs.02718 [DOI] [PubMed] [Google Scholar]
- Fisher, R. P. (2012). The CDK network: Linking cycles of cell division and gene expression. Genes and Cancer, 3(11‐12), 731–738. 10.1177/1947601912473308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, R. P. (2017). CDK regulation of transcription by RNAP II: Not over 'til it's over? Transcription, 8(2), 81–90. 10.1080/21541264.2016.1268244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, R. P. (2019). Cdk7: A kinase at the core of transcription and in the crosshairs of cancer drug discovery. Transcription, 10(2), 47–56. 10.1080/21541264.2018.1553483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco, H. L. , & Kraus, W. L. (2015). No driver behind the wheel? Targeting transcription in cancer. Cell, 163(1), 28–30. 10.1016/j.cell.2015.09.013 [DOI] [PubMed] [Google Scholar]
- Fry, D. W. , Harvey, P. J. , Keller, P. R. , Elliott, W. L. , Meade, M. A. , Trachet, E. , Albassam, M. , Zheng, X. X. , Leopold, W. R. , Pryer, N. K. , & Toogood, P. L. (2004). Specific inhibition of cyclin‐dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Molecular Cancer Therapeutics, 3(11), 1427–1438. 10.1158/1535-7163.1427.3.11 [DOI] [PubMed] [Google Scholar]
- Fu, A. K. Y. , Fu, W. Y. , Ng, A. K. Y. , Chien, W. W. Y. , Ng, Y. P. , Wang, J. H. , & Ip, N. Y. (2004). Cyclin‐dependent kinase 5 phosphorylates signal transducer and activator of transcription 3 and regulates its transcriptional activity. Proceedings of the National Academy of Sciences of the United States of America, 101(17), 6728–6733. 10.1073/PNAS.0307606100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, W. Y. , Cheng, K. , Fu, A. K. Y. , & Ip, N. Y. (2011). Cyclin‐dependent kinase 5‐dependent phosphorylation of Pctaire1 regulates dendrite development. Neuroscience, 180, 353–359. 10.1016/j.neuroscience.2011.02.024 [DOI] [PubMed] [Google Scholar]
- Fu, Z. , Larson, K. A. , Chitta, R. K. , Parker, S. A. , Turk, B. E. , Lawrence, M. W. , Kaldis, P. , Galaktionov, K. , Cohn, S. M. , Shabanowitz, J. , Hunt, D. F. , & Sturgill, T. W. (2023). Identification of Yin‐Yang regulators and a phosphorylation consensus for male germ cell‐associated kinase (MAK)‐related kinase. Molecular and Cellular Biology, 26(22), 8639–8654. 10.1128/MCB.00816-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdušková, P. , Ruiz de los Mozos, I. , Rájecký, M. , Hluchý, M. , Ule, J. , & Blazek, D. (2020). CDK11 is required for transcription of replication‐dependent histone genes. Nature Structural & Molecular Biology, 27(5), 500–510. 10.1038/s41594-020-0406-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith, M. D. , Allen, M. A. , Bensard, C. L. , Wang, X. , Schwinn, M. K. , Qin, B. , Long, H. W. , Daniels, D. L. , Hahn, W. C. , Dowell, R. D. , & Espinosa, J. M. (2013). XHIF1A employs CDK8‐mediator to stimulate RNAPII elongation in response to hypoxia. Cell, 153(6), 1327. 10.1016/j.cell.2013.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith, M. D. , Bender, H. , & Espinosa, J. M. (2019). Therapeutic targeting of transcriptional cyclin‐dependent kinases. Transcription, 10(2), 118–136. 10.1080/21541264.2018.1539615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith, M. D. , Donner, A. J. , & Espinosa, J. M. (2010). CDK8: A positive regulator of transcription. Transcription, 1(1), 4–12. 10.4161/TRNS.1.1.12373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganuza, M. , Sáiz‐Ladera, C. , Cañamero, M. , Gómez, G. , Schneider, R. , Blasco, M. A. , Pisano, D. , Paramio, J. M. , Santamaría, D. , & Barbacid, M. (2012). Genetic inactivation of Cdk7 leads to cell cycle arrest and induces premature aging due to adult stem cell exhaustion. The EMBO Journal, 31(11), 2498–2510. 10.1038/EMBOJ.2012.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriga, J. , Bhattacharya, S. , Calbó, J. , Marshall, R. M. , Truongcao, M. , Haines, D. S. , & Graña, X. (2003). CDK9 is constitutively expressed throughout the cell cycle, and its steady‐state expression is independent of SKP2. Molecular and Cellular Biology, 23(15), 5165. 10.1128/MCB.23.15.5165-5173.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriga, J. , Peng, J. , Parreño, M. , Price, D. H. , Henderson, E. E. , & Graña, X. (1998). Upregulation of cyclin T1/CDK9 complexes during T cell activation. Oncogene, 17(24), 3093–3102. 10.1038/sj.onc.1202548 [DOI] [PubMed] [Google Scholar]
- Gavet, O. , & Pines, J. (2010a). Activation of cyclin B1‐Cdk1 synchronizes events in the nucleus and the cytoplasm at mitosis. Journal of Cell Biology, 189(2), 247–259. 10.1083/JCB.200909144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavet, O. , & Pines, J. (2010b). Progressive activation of CyclinB1‐Cdk1 coordinates entry to mitosis. Developmental Cell, 18(4), 533–543. 10.1016/j.devcel.2010.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebara, M. M. , Sayre, M. H. , & Corden, J. L. (1997). Phosphorylation of the carboxy‐terminal repeat domain in RNA polymerase II by cyclin‐dependent kinases is sufficient to inhibit transcription. Journal of Cellular Biochemistry, 64(3), 390–402. [DOI] [PubMed] [Google Scholar]
- Geng, M. , Yang, Y. , Cao, X. , Dang, L. , Zhang, T. , & Zhang, L. (2019). Targeting CDK12‐mediated transcription regulation in anaplastic thyroid carcinoma. Biochemical and Biophysical Research Communications, 520(3), 544–550. 10.1016/J.BBRC.2019.10.052 [DOI] [PubMed] [Google Scholar]
- Gerber, H. B. , Pikman, Y. , & Fisher, R. P. (2008). The CDK‐activating kinase (CAK) Csk1 is required for normal levels of homologous recombination and resistance to DNA damage in fission yeast. PLoS One, 3(1), e1492. 10.1371/JOURNAL.PONE.0001492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillani, S. Q. , Reshi, I. , Nabi, N. , Nisa, M. U. , Sarwar, Z. , Bhat, S. , Roberts, T. M. , Higgins, J. M. G. , & Andrabi, S. (2022). PCTAIRE1 promotes mitotic progression and resistance against antimitotic and apoptotic signals. Journal of Cell Science, 135(3), jcs258831. 10.1242/JCS.258831/VIDEO-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover‐Cutter, K. , Larochelle, S. , Erickson, B. , Zhang, C. , Shokat, K. , Fisher, R. P. , & Bentley, D. L. (2009). TFIIH‐associated Cdk7 kinase functions in phosphorylation of C‐terminal domain Ser7 residues, promoter‐proximal pausing, and termination by RNA polymerase II. Molecular and Cellular Biology, 29(20), 5455. 10.1128/MCB.00637-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel, B. , Tripathi, N. , Bhardwaj, N. , & Jain, S. K. (2020). Small molecule CDK inhibitors for the therapeutic management of cancer. Current Topics in Medicinal Chemistry, 20(17), 1535–1563. 10.2174/1568026620666200516152756 [DOI] [PubMed] [Google Scholar]
- Goel, S. , DeCristo, M. J. , McAllister, S. S. , & Zhao, J. J. (2018). CDK4/6 inhibition in cancer: Beyond cell cycle arrest. Trends in Cell Biology, 28(11), 911–925. 10.1016/j.tcb.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, X. , Tang, X. , Wiedmann, M. , Wang, X. , Peng, J. , Zheng, D. , Blair, L. A. C. , Marshall, J. , & Mao, Z. (2003). Cdk5‐mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity‐induced apoptosis. Neuron, 38(1), 33–46. 10.1016/S0896-6273(03)00191-0 [DOI] [PubMed] [Google Scholar]
- Gottesfeld, J. M. , & Forbes, D. J. (1997). Mitotic repression of the transcriptional machinery. Trends in Biochemical Sciences, 22(6), 197–202. 10.1016/S0968-0004(97)01045-1 [DOI] [PubMed] [Google Scholar]
- Gould, K. L. , & Nurse, P. (1989). Tyrosine phosphorylation of the fission yeast CDC2+ protein kinase regulates entry into mitosis. Nature, 342(6245), 39–45. 10.1038/342039a0 [DOI] [PubMed] [Google Scholar]
- Graeser, R. , Gannon, J. , Poon, R. Y. C. , Dubois, T. , Aitken, A. , & Hunt, T. (2002). Regulation of the CDK‐related protein kinase PCTAIRE‐1 and its possible role in neurite outgrowth in Neuro‐2A cells. Journal of Cell Science, 115(17), 3479–3490. 10.1242/JCS.115.17.3479 [DOI] [PubMed] [Google Scholar]
- Graña, X. , De Luca, A. , Sang, N. , Fu, Y. , Claudio, P. P. , Rosenblatt, J. , Morgan, D. O. , & Giordano, A. (1994). PITALRE, a nuclear CDC2‐related protein kinase that phosphorylates the retinoblastoma protein in vitro. Proceedings of the National Academy of Sciences of the United States of America, 91(9), 3834–3838. 10.1073/PNAS.91.9.3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene, L. A. , Liu, D. X. , Troy, C. M. , & Biswas, S. C. (2007). Cell cycle molecules define a pathway required for neuron death in development and disease. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease, 1772(4), 392–401. 10.1016/J.BBADIS.2006.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf, A. L. (2019). Human CDK12 and CDK13, multi‐tasking CTD kinases for the new millenium. Transcription, 10(2), 91–110. 10.1080/21541264.2018.1535211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greifenberg, A. K. , Hönig, D. , Pilarova, K. , Düster, R. , Bartholomeeusen, K. , Bösken, C. A. , Anand, K. , Blazek, D. , & Geyer, M. (2016). Structural and functional analysis of the Cdk13/Cyclin K complex. Cell Reports, 14(2), 320–331. 10.1016/J.CELREP.2015.12.025 [DOI] [PubMed] [Google Scholar]
- Grenetier, S. , Bouchoux, C. , & Goguel, V. (2006). CTD kinase I is required for the integrity of the rDNA tandem array. Nucleic Acids Research, 34(17), 4996–5006. 10.1093/NAR/GKL493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossel, M. J. , & Hinds, P. W. (2006). Beyond the cell cycle: A new role for cdk6 in differentiation. Journal of Cellular Biochemistry, 97(3), 485–493. 10.1002/jcb.20712 [DOI] [PubMed] [Google Scholar]
- Gu, W. , Wang, C. , Li, W. , Hsu, F. N. , Tian, L. , Zhou, J. , Yuan, C. , Xie, X. J. , Jiang, T. , Addya, S. , Tai, Y. , Kong, B. , & Ji, J. Y. (2013). Tumor‐suppressive effects of CDK8 in endometrial cancer cells. Cell Cycle, 12(6), 987–999. 10.4161/CC.24003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, X. , Wang, Y. , Wang, H. , Ni, Q. , Zhang, C. , Zhu, J. , Huang, W. , Xu, P. , Mao, G. , & Yang, S. (2015). Upregulated PFTK1 promotes tumor cell proliferation, migration, and invasion in breast cancer. Medical Oncology, 32(7), 195. 10.1007/S12032-015-0641-8 [DOI] [PubMed] [Google Scholar]
- Guen, V. J. , Edvardson, S. , Fraenkel, N. D. , Fattal‐Valevski, A. , Jalas, C. , Anteby, I. , Shaag, A. , Dor, T. , Gillis, D. , Kerem, E. , Lees, J. A. , Colas, P. , & Elpeleg, O. (2018). A homozygous deleterious CDK10 mutation in a patient with agenesis of corpus callosum, retinopathy, and deafness. American Journal of Medical Genetics. Part A, 176(1), 92–98. 10.1002/AJMG.A.38506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guen, V. J. , Gamble, C. , Flajolet, M. , Unger, S. , Thollet, A. , Ferandin, Y. , Superti‐Furga, A. , Cohen, P. A. , Meijer, L. , & Colas, P. (2013). CDK10/cyclin M is a protein kinase that controls ETS2 degradation and is deficient in STAR syndrome. Proceedings of the National Academy of Sciences of the United States of America, 110(48), 19525–19530. 10.1073/PNAS.1306814110/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guen, V. J. , Gamble, C. , Perez, D. E. , Bourassa, S. , Zappel, H. , Gärtner, J. , Lees, J. A. , & Colas, P. (2016). Star syndrome‐associated CDK10/Cyclin M regulates actin network architecture and ciliogenesis. Cell Cycle, 15(5), 678–688. 10.1080/15384101.2016.1147632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiguen, A. , Soutourina, J. , Dewez, M. , Tafforeau, L. , Dieu, M. , Raes, M. , Vandenhaute, J. , Werner, M. , & Hermand, D. (2007). Recruitment of P‐TEFb (Cdk9‐Pch1) to chromatin by the cap‐methyl transferase Pcm1 in fission yeast. The EMBO Journal, 26(6), 1552–1559. 10.1038/SJ.EMBOJ.7601627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Z. , & Stiller, J. W. (2004). Comparative genomics of cyclin‐dependent kinases suggest co‐evolution of the RNAP II C‐terminal domain and CTD‐directed CDKs. BMC Genomics, 5, 69. 10.1186/1471-2164-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez‐Escribano, P. , & Nurse, P. (2015). A single cyclin–CDK complex is sufficient for both mitotic and meiotic progression in fission yeast. Nature Communications, 6(1), 1–13. 10.1038/ncomms7871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase, S. B. , & Reed, S. I. (1999). Evidence that a free‐running oscillator drives G1 events in the budding yeast cell cycle. Nature, 401(6751), 394–397. 10.1038/43927 [DOI] [PubMed] [Google Scholar]
- Handschick, K. , Beuerlein, K. , Jurida, L. , Bartkuhn, M. , Müller, H. , Soelch, J. , Weber, A. , Dittrich‐Breiholz, O. , Schneider, H. , Scharfe, M. , Jarek, M. , Stellzig, J. , Schmitz, M. L. , & Kracht, M. (2014). Cyclin‐dependent kinase 6 is a chromatin‐bound cofactor for NF‐κB‐dependent gene expression. Molecular Cell, 53(2), 193–208. 10.1016/J.MOLCEL.2013.12.002 [DOI] [PubMed] [Google Scholar]
- Haneke, K. , Schott, J. , Lindner, D. , Hollensen, A. K. , Damgaard, C. K. , Mongis, C. , Knop, M. , Palm, W. , Ruggieri, A. , & Stoecklin, G. (2020). CDK1 couples proliferation with protein synthesis. Journal of Cell Biology, 219(3), e201906147. 10.1083/JCB.201906147/133706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlen, K. M. , & Churchman, L. S. (2017). The code and beyond: Transcription regulation by the RNA polymerase II carboxy‐terminal domain. Nature Reviews. Molecular Cell Biology, 18(4), 263–273. 10.1038/NRM.2017.10 [DOI] [PubMed] [Google Scholar]
- Harper, J. W. , Elledge, S. J. , & McLean, M. (1998). The role of Cdk7 in CAK function, a retro‐retrospective. Genes & Development, 12(3), 285–289. 10.1101/gad.12.3.285 [DOI] [PubMed] [Google Scholar]
- Hartwell, L. H. , Mortimer, R. K. , Culotti, J. , & Culotti, M. (1973). Genetic control of the cell division cycle in yeast: Genetic analysis of CDC mutants. Genetics, 74(2), 267–286. 10.1093/GENETICS/74.2.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayles, J. , Fisher, D. , Woollard, A. , & Nurse, P. (1994). Temporal order of S phase and mitosis in fission yeast is determined by the state of the p34cdc2‐mitotic B cyclin complex. Cell, 78(5), 813–822. 10.1016/S0092-8674(94)90542-8 [DOI] [PubMed] [Google Scholar]
- He, L. , Arnold, C. , Thoma, J. , Rohde, C. , Kholmatov, M. , Garg, S. , Hsiao, C. , Viol, L. , Zhang, K. , Sun, R. , Schmidt, C. , Janssen, M. , MacRae, T. , Huber, K. , Thiede, C. , Hébert, J. , Sauvageau, G. , Spratte, J. , Fluhr, H. , … Pabst, C. (2022). CDK7/12/13 inhibition targets an oscillating leukemia stem cell network and synergizes with venetoclax in acute myeloid leukemia. EMBO Molecular Medicine, 14(4), e14990. doi: 10.15252/EMMM.202114990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann, M. , Hintermair, C. , Voß, K. , & Eick, D. (2013). Dynamic phosphorylation patterns of RNA polymerase II CTD during transcription. Biochimica et Biophysica Acta (BBA) – Gene Regulatory Mechanisms, 1829(1), 55–62. 10.1016/J.BBAGRM.2012.08.013 [DOI] [PubMed] [Google Scholar]
- Hengartner, C. J. , Myer, V. E. , Liao, S. M. , Wilson, C. J. , Koh, S. S. , & Young, R. A. (1998). Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin‐dependent kinases. Molecular Cell, 2(1), 43–53. 10.1016/S1097-2765(00)80112-4 [DOI] [PubMed] [Google Scholar]
- Herrmann, C. H. , Carroll, R. G. , Wei, P. , Jones, K. A. , & Rice, A. P. (1998). Tat‐associated kinase, TAK, activity is regulated by distinct mechanisms in peripheral blood lymphocytes and promonocytic cell lines. Journal of Virology, 72(12), 9881–9888. 10.1128/JVI.72.12.9881-9888.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits, A. Z. , & Davies, P. (2006). The regulation of tau phosphorylation by PCTAIRE 3: Implications for the pathogenesis of Alzheimer's disease. Neurobiology of Disease, 23(2), 398–408. 10.1016/J.NBD.2006.04.004 [DOI] [PubMed] [Google Scholar]
- Hirose, T. , Kawabuchi, M. , Tamaru, T. , Okumura, N. , Nagai, K. , & Okada, M. (2000). Identification of tudor repeat associator with PCTAIRE 2 (Trap). European Journal of Biochemistry, 267(7), 2113–2121. 10.1046/J.1432-1327.2000.01218.X [DOI] [PubMed] [Google Scholar]
- Hirose, T. , Tamaru, T. , Okumura, N. , Nagai, K. , & Okada, M. (1997). PCTAIRE 2, a Cdc2‐related serine/threonine kinase, is predominantly expressed in terminally differentiated neurons. European Journal of Biochemistry, 249(2), 481–488. 10.1111/J.1432-1033.1997.T01-1-00481.X [DOI] [PubMed] [Google Scholar]
- Hisamoto, N. , Sakai, Y. , Ohta, K. , Shimizu, T. , Li, C. , Hanafusa, H. , & Matsumoto, K. (2021). CDK14 promotes axon regeneration by regulating the noncanonical Wnt Signaling pathway in a kinase‐independent manner. Journal of Neuroscience, 41(40), 8309–8320. 10.1523/JNEUROSCI.0711-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hluchý, M. , Gajdušková, P. , Ruiz de los Mozos, I. , Rájecký, M. , Kluge, M. , Berger, B. T. , Slabá, Z. , Potěšil, D. , Weiß, E. , Ule, J. , Zdráhal, Z. , Knapp, S. , Paruch, K. , Friedel, C. C. , & Blazek, D. (2022). CDK11 regulates pre‐mRNA splicing by phosphorylation of SF3B1. Nature, 609(7928), 829–834. 10.1038/s41586-022-05204-z [DOI] [PubMed] [Google Scholar]
- Hofmann, F. , & Livingston, D. M. (1996). Differential effects of cdk2 and cdk3 on the control of pRb and E2F function during G1 exit. Genes & Development, 10(7), 851–861. 10.1101/GAD.10.7.851 [DOI] [PubMed] [Google Scholar]
- Holstege, F. C. P. , Jennings, E. G. , Wyrick, J. J. , Lee, T. I. , Hengartner, C. J. , Green, M. R. , Golub, T. R. , Lander, E. S. , & Young, R. A. (1998). Dissecting the regulatory circuitry of a eukaryotic genome. Cell, 95(5), 717–728. 10.1016/S0092-8674(00)81641-4 [DOI] [PubMed] [Google Scholar]
- Hopkins, J. L. , & Zou, L. (2019). Induction of BRCAness in triple‐negative breast cancer by a CDK12/13 inhibitor improves chemotherapy. Cancer Cell, 36(5), 461–463. 10.1016/J.CCELL.2019.10.012 [DOI] [PubMed] [Google Scholar]
- Hsi, E. D. , Zukerberg, L. R. , Yang, W.‐I. , & Arnold, A. (1996). Cyclin D1/PRAD1 expression in parathyroid adenomas: An immunohistochemical study. The Journal of Clinical Endocrinology & Metabolism, 81(5), 1736–1739. 10.1210/JCEM.81.5.8626826 [DOI] [PubMed] [Google Scholar]
- Hu, D. , Mayeda, A. , Trembley, J. H. , Lahti, J. M. , & Kidd, V. J. (2003). CDK11 complexes promote pre‐mRNA splicing. Journal of Biological Chemistry, 278(10), 8623–8629. 10.1074/jbc.M210057200 [DOI] [PubMed] [Google Scholar]
- Hu, D. , Valentine, M. , Kidd, V. J. , & Lahti, J. M. (2007). CDK11(p58) is required for the maintenance of sister chromatid cohesion. Journal of Cell Science, 120(14), 2424–2434. 10.1242/JCS.007963 [DOI] [PubMed] [Google Scholar]
- Huang, C. , Du, R. , Jia, X. , Liu, K. , Qiao, Y. , Wu, Q. , Yao, N. , Yang, L. , Zhou, L. , Liu, X. , Xiang, P. , Xin, M. , Wang, Y. , Chen, X. , Kim, D. J. , Dong, Z. , & Li, X. (2022). CDK15 promotes colorectal cancer progression via phosphorylating PAK4 and regulating β‐catenin/MEK‐ERK signaling pathway. Cell Death and Differentiation, 29(1), 14. 10.1038/S41418-021-00828-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, D. , Friesen, H. , & Andrews, B. (2007). Pho85, a multifunctional cyclin‐dependent protein kinase in budding yeast. Molecular Microbiology, 66(2), 303–314. 10.1111/J.1365-2958.2007.05914.X [DOI] [PubMed] [Google Scholar]
- Huang, D. , Moffat, J. , Wilson, W. A. , Moore, L. , Cheng, C. , Roach, P. J. , & Andrews, B. (1998). Cyclin partners determine Pho85 protein kinase substrate specificity In vitro and In vivo: Control of glycogen biosynthesis by Pcl8 and Pcl10. Molecular and Cellular Biology, 18(6), 3289–3299. 10.1128/MCB.18.6.3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Regan, K. M. , Lou, Z. , Chen, J. , & Tindall, D. J. (2006). CDK2‐dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science, 314(5797), 294–297. 10.1126/SCIENCE.1130512/ [DOI] [PubMed] [Google Scholar]
- Hurt, E. , Luo, M. J. , Röther, S. , Reed, R. , & Sträßer, K. (2004). Cotranscriptional recruitment of the serine‐arginine‐rich (SR)‐like proteins Gbp2 and Hrb1 to nascent mRNA via the TREX complex. Proceedings of the National Academy of Sciences of the United States of America, 101(7), 1858–1862. 10.1073/PNAS.0308663100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hydbring, P. , Malumbres, M. , & Sicinski, P. (2016). Non‐canonical functions of cell cycle cyclins and cyclin‐dependent kinases. Nature Reviews Molecular Cell Biology, 17(5), 280–292. 10.1038/nrm.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icreverzi, A. , de la Cruz, A. F. A. , Walker, D. W. , & Edgar, B. A. (2015). Changes in neuronal CycD/Cdk4 activity affect aging, neurodegeneration, and oxidative stress. Aging Cell, 14(5), 896–906. 10.1111/ACEL.12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im, S.‐A. , Lu, Y.‐S. , Bardia, A. , Harbeck, N. , Colleoni, M. , Franke, F. , Chow, L. , Sohn, J. , Lee, K.‐S. , Campos‐Gomez, S. , Villanueva‐Vazquez, R. , Jung, K.‐H. , Chakravartty, A. , Hughes, G. , Gounaris, I. , Rodriguez‐Lorenc, K. , Taran, T. , Hurvitz, S. , & Tripathy, D. (2019). Overall survival with Ribociclib plus endocrine therapy in breast cancer. New England Journal of Medicine, 381(4), 307–316. 10.1056/NEJMOA1903765/ [DOI] [PubMed] [Google Scholar]
- Insco, M. L. , Abraham, B. J. , Dubbury, S. J. , Kaltheuner, I. H. , Dust, S. , Wu, C. , Chen, K. Y. , Liu, D. , Bellaousov, S. , Cox, A. M. , Martin, B. J. E. , Zhang, T. , Ludwig, C. G. , Fabo, T. , Modhurima, R. , Esgdaille, D. E. , Henriques, T. , Brown, K. M. , Chanock, S. J. , … Zon, L. I. (2023). Oncogenic CDK13 mutations impede nuclear RNA surveillance. Science, 380(6642), eabn7625. 10.1126/SCIENCE.ABN7625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie, K. , Nomoto, S. , Miyajima, I. , & Matsumoto, K. (1991). SGV1 encodes a CDC28/cdc2‐related kinase required for a Gα subunit‐mediated adaptive response to pheromone in S. cerevisiae . Cell, 65(5), 785–795. 10.1016/0092-8674(91)90386-D [DOI] [PubMed] [Google Scholar]
- Itzhaki, J. E. , Gilbert, C. S. , & Porter, A. C. G. (1997). Construction by gene targeting in human cells of a “conditional” CDC2 mutant that rereplicates its DNA. Nature Genetics, 15(3), 258–265. 10.1038/NG0397-258 [DOI] [PubMed] [Google Scholar]
- Jans, D. A. , Moll, T. , Nasmyth, K. , & Jans, P. (1995). Cyclin‐dependent kinase site‐regulated signal‐dependent nuclear localization of the SW15 yeast transcription factor in mammalian cells. The Journal of Biological Chemistry, 270(29), 17064–17067. 10.1074/JBC.270.29.17064 [DOI] [PubMed] [Google Scholar]
- Jeffrey, P. D. , Tong, L. , & Pavletich, N. P. (2000). Structural basis of inhibition of CDK‐cyclin complexes by INK4 inhibitors. Genes & Development, 14(24), 3115–3125. 10.1101/GAD.851100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri, K. , Burris, H. A. , Yap, T. A. , Hamilton, E. , Rugo, H. S. , Goldman, J. W. , Dann, S. , Liu, F. , Wong, G. Y. , Krupka, H. , & Shapiro, G. I. (2021). The evolution of cyclin dependent kinase inhibitors in the treatment of cancer. Expert Review of Anticancer Therapy, 21(10), 1105–1124. 10.1080/14737140.2021.1944109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, G. , He, S. , Huang, C. , Gong, Y. , Li, X. , & Zhou, L. (2021). Upregulation of atp binding cassette subfamily c member 5 facilitates prostate cancer progression and enzalutamide resistance via the cdk1‐mediated ar ser81 phosphorylation pathway. International Journal of Biological Sciences, 17(7), 1613–1628. 10.7150/ijbs.59559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, Y. , Jin, N. , Oikawa, Y. , Benyair, R. , Koizumi, M. , Wilson, T. E. , Ohsumi, Y. , & Weisman, L. S. (2022). Bur1 functions with TORC1 for vacuole‐mediated cell cycle progression. EMBO Reports, 23(4), e53477. 10.15252/EMBR.202153477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirawatnotai, S. , Dalton, S. , & Wattanapanitch, M. (2020). Role of cyclins and cyclin‐dependent kinases in pluripotent stem cells and their potential as a therapeutic target. Seminars in Cell and Developmental Biology, 107, 63–71. 10.1016/j.semcdb.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, T. C. , & Holland, J. J. (1965). Ribonucleic acid and protein synthesis IN mitotic HELA cells. Journal of Cell Biology, 27(3), 565–574. 10.1083/JCB.27.3.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jona, G. , Wittschieben, B. O. , Svejstrup, J. Q. , & Gileadi, O. (2001). Involvement of yeast carboxy‐terminal domain kinase I (CTDK‐I) in transcription elongation in vivo. Gene, 267(1), 31–36. 10.1016/S0378-1119(01)00389-4 [DOI] [PubMed] [Google Scholar]
- Jonkers, I. , & Lis, J. T. (2015). Getting up to speed with transcription elongation by RNA polymerase II. Nature Reviews. Molecular Cell Biology, 16(3), 167–177. 10.1038/NRM3953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan, H. C. , Lin, Y. , Chen, H. R. , & Fann, M. J. (2015). Cdk12 is essential for embryonic development and the maintenance of genomic stability. Cell Death & Differentiation, 23(6), 1038–1048. 10.1038/cdd.2015.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalan, S. , Amat, R. , Schachter, M. M. , Kwiatkowski, N. , Abraham, B. J. , Liang, Y. , Zhang, T. , Olson, C. M. , Larochelle, S. , Young, R. A. , Gray, N. S. , & Fisher, R. P. (2017). Activation of the p53 transcriptional program sensitizes cancer cells to Cdk7 inhibitors. Cell Reports, 21(2), 467–481. 10.1016/J.CELREP.2017.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra, S. , Joshi, G. , Munshi, A. , & Kumar, R. (2017). Structural insights of cyclin dependent kinases: Implications in design of selective inhibitors. European Journal of Medicinal Chemistry, 142, 424–458. 10.1016/j.ejmech.2017.08.071 [DOI] [PubMed] [Google Scholar]
- Karagiannis, J. , & Balasubramanian, M. K. (2007). A Cyclin‐dependent kinase that promotes cytokinesis through modulating phosphorylation of the Carboxy terminal domain of the RNA pol II Rpb1p sub‐unit. PLoS One, 2(5), e433. 10.1371/JOURNAL.PONE.0000433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis, J. , Bimbó, A. , Rajagopalan, S. , Liu, J. , & Balasubramanian, M. K. (2005). The nuclear kinase Lsk1p positively regulates the septation initiation network and promotes the successful completion of cytokinesis in response to perturbation of the actomyosin ring in Schizosaccharomyces pombe . Molecular Biology of the Cell, 16(1), 358–371. 10.1091/MBC.E04-06-0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten, M. , & Giordano, A. (2001). Cdk10, a Cdc2‐related kinase, associates with the Ets2 transcription factor and modulates its transactivation activity. Oncogene, 20(15), 1832–1838. 10.1038/sj.onc.1204295 [DOI] [PubMed] [Google Scholar]
- Keogh, M.‐C. , Podolny, V. , & Buratowski, S. (2003). Bur1 kinase is required for efficient transcription elongation by RNA polymerase II. Molecular and Cellular Biology, 23(19), 7005–7018. 10.1128/MCB.23.19.7005-7018.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan, R. E. , Emiliani, S. , Nakayama, K. , Castro, A. , Labbé, J. C. , Lorca, T. , Nakayama, K. , & Benkirane, M. (2001). Interaction between cyclin T1 and SCFSKP2 targets CDK9 for ubiquitination and degradation by the proteasome. Molecular and Cellular Biology, 21(23), 7956–7970. 10.1128/MCB.21.23.7956-7970.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D. , Frank, C. L. , Dobbin, M. M. , Tsunemoto, R. K. , Tu, W. , Peng, P. L. , Guan, J. S. , Lee, B. H. , Moy, L. Y. , Giusti, P. , Broodie, N. , Mazitschek, R. , Delalle, I. , Haggarty, S. J. , Neve, R. L. , Lu, Y. M. , & Tsai, L. H. (2008). Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron, 60(5), 803–817. 10.1016/j.neuron.2008.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, R. W. , Deshaies, R. J. , Peters, J.‐M. , Kirschner, M. W. , King, R. W. , Peters, J. , & Kirschner, M. W. (1996). How proteolysis drives the cell cycle. Science, 274(5293), 1652–1659. 10.1126/SCIENCE.274.5293.1652 [DOI] [PubMed] [Google Scholar]
- Kishi, T. , Ikeda, A. , Koyama, N. , Fukada, J. , & Nagao, R. (2008). A refined two‐hybrid system reveals that SCFCdc4‐dependent degradation of Swi5 contributes to the regulatory mechanism of S‐phase entry. Proceedings of the National Academy of Sciences of the United States of America, 105(38), 14497–14502. 10.1073/PNAS.0806253105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa, H. , & Koff, A. (1997). Roles of cyclin‐dependent kinase inhibitors: Lessons from knockout mice. Current Topics in Microbiology and Immunology, 227, 105–120. 10.1007/978-3-642-71941-7_5 [DOI] [PubMed] [Google Scholar]
- Kjærulff, S. , Andersen, N. R. , Borup, M. T. , & Nielsen, O. (2007). Cdk phosphorylation of the Ste11 transcription factor constrains differentiation‐specific transcription to G1. Genes & Development, 21(3), 347. 10.1101/GAD.407107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, T. K. , Kelly, E. , & Pines, J. (2001). CrkRS: A novel conserved Cdc2‐related protein kinase that colocalises with SC35 speckles. Journal of Cell Science, 114(14), 2591–2603. 10.1242/JCS.114.14.2591 [DOI] [PubMed] [Google Scholar]
- Kobor, M. S. , & Greenblatt, J. (2002). Regulation of transcription elongation by phosphorylation. Biochimica et Biophysica Acta (BBA) – Gene Structure and Expression, 1577(2), 261–275. 10.1016/S0167-4781(02)00457-8 [DOI] [PubMed] [Google Scholar]
- Kohoutek, J. , & Blazek, D. (2012). Cyclin K goes with Cdk12 and Cdk13. Cell Division, 7(1), 1–10. 10.1186/1747-1028-7-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kõivomägi, M. , Swaffer, M. P. , Turner, J. J. , Marinov, G. , & Skotheim, J. M. (2021). G1 cyclin‐Cdk promotes cell cycle entry through localized phosphorylation of RNA polymerase II. Science, 374(6565), 347–351. 10.1126/SCIENCE.ABA5186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kõivomägi, M. , Valk, E. , Venta, R. , Iofik, A. , Lepiku, M. , Balog, E. R. M. , Rubin, S. M. , Morgan, D. O. , & Loog, M. (2011). Cascades of multisite phosphorylation control Sic1 destruction at the onset of S phase. Nature, 480(7375), 128–131. 10.1038/nature10560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann, K. , Heller, G. , Schneckenleithner, C. , Warsch, W. , Scheicher, R. , Ott, R. G. , Schäfer, M. , Fajmann, S. , Schlederer, M. , Schiefer, A. I. , Reichart, U. , Mayerhofer, M. , Hoeller, C. , Zöchbauer‐Müller, S. , Kerjaschki, D. , Bock, C. , Kenner, L. , Hoefler, G. , Freissmuth, M. , … Sexl, V. (2013). A kinase‐independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell, 24(2), 167–181. 10.1016/J.CCR.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarnitsky, P. , Cho, E. J. , & Buratowski, S. (2000). Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes & Development, 14(19), 2452–2460. 10.1101/GAD.824700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalová, M. , Baraka, J. P. , Mik, V. , Jorda, R. , Luo, L. , Shao, H. , & Kryštof, V. (2023). A patent review of cyclin‐dependent kinase 7 (CDK7) inhibitors (2023). Expert Opinion on Therapeutic Patents, 33(2), 67–87. 10.1080/13543776.2023.2195547 [DOI] [PubMed] [Google Scholar]
- Krajewska, M. , Dries, R. , Grassetti, A. V. , Dust, S. , Gao, Y. , Huang, H. , Sharma, B. , Day, D. S. , Kwiatkowski, N. , Pomaville, M. , Dodd, O. , Chipumuro, E. , Zhang, T. , Greenleaf, A. L. , Yuan, G. C. , Gray, N. S. , Young, R. A. , Geyer, M. , Gerber, S. A. , & George, R. E. (2019). CDK12 loss in cancer cells affects DNA damage response genes through premature cleavage and polyadenylation. Nature Communications, 10(1), 1–16. 10.1038/s41467-019-09703-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchin, S. , & Carlson, M. (1998). Functional relationships of Srb10‐Srb11 kinase, carboxy‐terminal domain kinase CTDK‐I, and transcriptional corepressor Ssn6‐Tup1. Molecular and Cellular Biology, 18(3), 1163–1171. 10.1128/MCB.18.3.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchin, S. , Yeghiayan, P. , & Carlson, M. (1995). Cyclin‐dependent protein kinase and cyclin homologs SSN3 and SSN8 contribute to transcriptional control in yeast. Proceedings of the National Academy of Sciences, 92(9), 4006–4010. 10.1073/PNAS.92.9.4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak, Y. , Jeong, J. , Lee, S. , Park, Y. U. , Lee, S. A. , Han, D. H. , Kim, J. H. , Ohshima, T. , Mikoshiba, K. , Suh, Y. H. , Cho, S. , & Park, S. K. (2013). Cyclin‐dependent kinase 5 (Cdk5) regulates the function of CLOCK protein by direct phosphorylation. Journal of Biological Chemistry, 288(52), 36878–36889. 10.1074/jbc.M113.494856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe, J. C. , Capony, J. P. , Caput, D. , Cavadore, J. C. , Derancourt, J. , Kaghad, M. , Lelias, J. M. , Picard, A. , & Doree, M. (1989). MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO Journal, 8(10), 3053–3058. 10.1002/j.1460-2075.1989.tb08456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti, J. M. , Xiang, J. , Heath, L. S. , Campana, D. , & Kidd, V. J. (1995). PITSLRE protein kinase activity is associated with apoptosis. Molecular and Cellular Biology, 15(1), 1–11. 10.1128/MCB.15.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, L. , Shin, G. Y. , & Qiu, H. (2020). The role of cell cycle regulators in cell survival—Dual functions of Cyclin‐dependent kinase 20 and p21Cip1/Waf1. International Journal of Molecular Sciences, 21(22), 1–14. 10.3390/IJMS21228504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis, M. W. , Pawlyk, B. S. , Li, T. , Sicinski, P. , & Hinds, P. W. (2006). Cyclin D1‐dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell, 9(1), 13–22. 10.1016/J.CCR.2005.12.019 [DOI] [PubMed] [Google Scholar]
- Laribee, R. N. , Krogan, N. J. , Xiao, T. , Shibata, Y. , Hughes, T. R. , Greenblatt, J. F. , & Strahl, B. D. (2005). BUR kinase selectively regulates H3 K4 trimethylation and H2B ubiquitylation through recruitment of the PAF elongation complex. Current Biology, 15(16), 1487–1493. 10.1016/j.cub.2005.07.028 [DOI] [PubMed] [Google Scholar]
- Larochelle, S. , Amat, R. , Glover‐Cutter, K. , Sansó, M. , Zhang, C. , Allen, J. J. , Shokat, K. M. , Bentley, D. L. , & Fisher, R. P. (2012). Cyclin‐dependent kinase control of the initiation‐to‐elongation switch of RNA polymerase II. Nature Structural & Molecular Biology, 19(11), 1108–1115. 10.1038/NSMB.2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle, S. , Batliner, J. , Gamble, M. J. , Barboza, N. M. , Kraybill, B. C. , Blethrow, J. D. , Shokat, K. M. , & Fisher, R. P. (2006). Dichotomous but stringent substrate selection by the dual‐function Cdk7 complex revealed by chemical genetics. Nature Structural & Molecular Biology, 13(1), 55–62. 10.1038/NSMB1028 [DOI] [PubMed] [Google Scholar]
- Larochelle, S. , Merrick, K. A. , Terret, M. E. , Wohlbold, L. , Barboza, N. M. , Zhang, C. , Shokat, K. M. , Jallepalli, P. V. , & Fisher, R. P. (2007). Requirements for Cdk7 in the assembly of Cdk1/cyclin B and activation of Cdk2 revealed by chemical genetics in human cells. Molecular Cell, 25(6), 839–850. 10.1016/J.MOLCEL.2007.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle, S. , Pandur, J. , Fisher, R. P. , Salz, H. K. , & Suter, B. (1998). Cdk7 is essential for mitosis and for in vivo Cdk‐activating kinase activity. Genes & Development, 12(3), 370–381. 10.1101/GAD.12.3.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouffant, F. , Le Minter, P. , Traiffort, E. , Ruat, M. , & Sladeczek, F. (2000). Multiple subcellular localizations of PCTAIRE‐1 in brain. Molecular and Cellular Neuroscience, 16(4), 388–395. 10.1006/MCNE.2000.0881 [DOI] [PubMed] [Google Scholar]
- Lee, J. M. , & Greenleaf, A. L. (1989). A protein kinase that phosphorylates the C‐terminal repeat domain of the largest subunit of RNA polymerase II. Proceedings of the National Academy of Sciences, 86(10), 3624–3628. 10.1073/PNAS.86.10.3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. M. , & Greenleaf, A. L. (1991). CTD kinase large subunit is encoded by CTK1, a gene required for normal growth of Saccharomyces cerevisiae. Gene Expression, 1(2), 149. [PMC free article] [PubMed] [Google Scholar]
- Lee, J. M. , & Greenleaf, A. L. (1997). Modulation of RNA polymerase II elongation efficiency by C‐terminal heptapeptide repeat domain kinase I. Journal of Biological Chemistry, 272(17), 10990–10993. 10.1074/jbc.272.17.10990 [DOI] [PubMed] [Google Scholar]
- Lee, J. , Colwill, K. , Aneliunas, V. , Tennyson, C. , Moore, L. , Ho, Y. , & Andrews, B. (1998). Interaction of yeast Rvs167 and Pho85 cyclin‐dependent kinase complexes may link the cell cycle to the Actin cytoskeleton. Current Biology, 8(24), 1310–1321. 10.1016/s0960-9822(07)00561-1 [DOI] [PubMed] [Google Scholar]
- Lee, K. M. , Miklos, I. , Du, H. , Watt, S. , Szilagyi, Z. , Saiz, J. E. , Madabhushi, R. , Penkett, C. J. , Sipiczki, M. , Bähler, J. , & Fisher, R. P. (2005). Impairment of the TFIIH‐associated CDK‐activating kinase selectively affects cell cycle‐regulated gene expression in fission yeast. Molecular Biology of the Cell, 16(6), 2734–2745. 10.1091/MBC.E04-11-0982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K. M. , Saiz, J. E. , Barton, W. A. , & Fisher, R. P. (1999). Cdc2 activation in fission yeast depends on Mcs6 and Csk1, two partially redundant Cdk‐activating kinases (CAKs). Current Biology, 9(8), 441–444. 10.1016/S0960-9822(99)80194-8 [DOI] [PubMed] [Google Scholar]
- Lee, K. Y. , Helbing, C. C. , Choi, K. S. , Johnston, R. N. , & Wang, J. H. (1997). Neuronal Cdc2‐like kinase (Nclk) binds and phosphorylates the retinoblastoma protein. Journal of Biological Chemistry, 272(9), 5622–5626. 10.1074/jbc.272.9.5622 [DOI] [PubMed] [Google Scholar]
- Lee, M. G. , & Nurse, P. (1987). Complementation used to clone a human homologue of the fission yeast cell cycle control gene CDC2. Nature, 327(6117), 31–35. 10.1038/327031A0 [DOI] [PubMed] [Google Scholar]
- Lei, T. , Zhang, P. , Zhang, X. , Xiao, X. , Zhang, J. , Qiu, T. , Dai, Q. , Zhang, Y. , Min, L. , Lei, Q. , Yin, R. , Ding, P. , Li, N. , Qu, Y. , Mu, D. , Qin, J. , Zhu, X. , Xiao, Z. X. , & Li, Q. (2018). Cyclin K regulates prereplicative complex assembly to promote mammalian cell proliferation. Nature Communications, 9(1), 1876. 10.1038/S41467-018-04258-W [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenburg, M. , & O'Shea, E. (1996). Signaling phosphate starvation. Trends in Biochemical Sciences, 21(10), 383–387. 10.1016/S0968-0004(96)10048-7 [DOI] [PubMed] [Google Scholar]
- Lenstra, T. L. , Tudek, A. , Clauder, S. , Xu, Z. , Pachis, S. T. , Van Leenen, D. , Kemmeren, P. , Steinmetz, L. M. , Libri, D. , & Holstege, F. C. P. (2013). The role of Ctk1 kinase in termination of small non‐coding RNAs. PLoS One, 8(12), e80495. 10.1371/JOURNAL.PONE.0080495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi, M. , Perna, E. , Tronnolone, S. , Colecchia, D. , & Chiariello, M. (2019). Activated kinase screening identifies the IKBKE oncogene as a positive regulator of autophagy. Autophagy, 15(2), 312–326. 10.1080/15548627.2018.1517855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage, D. , Troussard, X. , & Sola, B. (2005). The enigmatic role of cyclin D1 in multiple myeloma. International Journal of Cancer, 115(2), 171–176. 10.1002/IJC.20907 [DOI] [PubMed] [Google Scholar]
- Lew, D. J. , & Reed, S. I. (1993). Morphogenesis in the yeast cell cycle: Regulation by Cdc28 and cyclins. Journal of Cell Biology, 120(6), 1305–1320. 10.1083/JCB.120.6.1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. , He, F. , Zhang, Z. , Xiang, Z. , & Hu, D. (2020). CDK1 serves as a potential prognostic biomarker and target for lung cancer. Journal of International Medical Research, 48(2), 300060519897508. 10.1177/0300060519897508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Zhang, L. , Jiang, J. , Zhang, Y. , Wang, X. , Zhang, Q. , Wang, Y. , Liu, C. , & Li, F. (2019). CDK1 and CCNB1 as potential diagnostic markers of rhabdomyosarcoma: Validation following bioinformatics analysis. BMC Medical Genomics, 12(1), 1–13. 10.1186/s12920-019-0645-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Dai, X. , Gong, K. , Song, K. , Tai, F. , & Shi, J. (2019). PA28α/β promote breast cancer cell invasion and metastasis via Down‐regulation of CDK15. Frontiers in Oncology, 9, 1283. 10.3389/FONC.2019.01283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Maclachlan, T. K. , De Luca, A. , Claudio, P. P. , Condorelli, G. , & Giordano, A. (1995). The cdc2‐related kinase, PISSLRE, is essential for cell growth and acts in G2 phase of the cell cycle1. Cancer Research, 55, 3992–3995. [PubMed] [Google Scholar]
- Li, T. , Inoue, A. , Lahti, J. M. , & Kidd, V. J. (2004). Failure To proliferate and mitotic arrest of CDK11p110/p58‐null mutant mice at the blastocyst stage of embryonic cell development. Molecular and Cellular Biology, 24(8), 3188–3197. 10.1128/MCB.24.8.3188-3197.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Li, J. , Xu, L. , Wei, W. , Cheng, A. , Zhang, L. , Zhang, M. , Wu, G. , & Cai, C. (2022). CDK16 promotes the progression and metastasis of triple‐negative breast cancer by phosphorylating PRC1. Journal of Experimental and Clinical Cancer Research, 41(1), 1–18. 10.1186/S13046-022-02362-W [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , David, G. , Hung, K. W. , DePinho, R. A. , Fu, A. K. Y. , & Ip, N. Y. (2004). Cdk5/p35 phosphorylates mSds3 and regulates mSds3‐mediated repression of transcription. The Journal of Biological Chemistry, 279(52), 54438–54444. 10.1074/JBC.M411002200 [DOI] [PubMed] [Google Scholar]
- Liang, K. , Gao, X. , Gilmore, J. M. , Florens, L. , Washburn, M. P. , Smith, E. , & Shilatifard, A. (2015). Characterization of human cyclin‐dependent kinase 12 (CDK12) and CDK13 complexes in C‐terminal domain phosphorylation, gene transcription, and RNA processing. Molecular and Cellular Biology, 35(6), 928–938. 10.1128/MCB.01426-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, K. , Woodfin, A. R. , Slaughter, B. D. , Unruh, J. R. , Box, A. C. , Rickels, R. A. , Gao, X. , Haug, J. S. , Jaspersen, S. L. , & Shilatifard, A. (2015). Mitotic transcriptional activation: Clearance of actively engaged pol II via transcriptional elongation control in mitosis. Molecular Cell, 60(3), 435–445. 10.1016/j.molcel.2015.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, S. , Hu, L. , Wu, Z. , Chen, Z. , Liu, S. , Xu, X. , & Qian, A. (2020). CDK12: A potent target and biomarker for human cancer therapy. Cell, 9(6), 1483. 10.3390/CELLS9061483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, S. M. , Zhang, J. , Jeffery, D. A. , Koleske, A. J. , Thompson, C. M. , Chao, D. M. , Viljoen, M. , van Vuuren, H. J. J. , & Young, R. A. (1995). A kinase–cyclin pair in the RNA polymerase II holoenzyme. Nature, 374(6518), 193–196. 10.1038/374193a0 [DOI] [PubMed] [Google Scholar]
- Lim, S. , & Kaldis, P. (2013). Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development (Cambridge), 140(15), 3079–3093. 10.1242/dev.091744 [DOI] [PubMed] [Google Scholar]
- Linder, T. , Rasmussen, N. N. , Samuelsen, C. O. , Chatzidaki, E. , Baraznenok, V. , Beve, J. , Henriksen, P. , Gustafsson, C. M. , & Holmberg, S. (2008). Two conserved modules of Schizosaccharomyces pombe mediator regulate distinct cellular pathways. Nucleic Acids Research, 36(8), 2489–2504. 10.1093/NAR/GKN070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , & Herrmann, C. H. (2005). Differential localization and expression of the Cdk9 42k and 55k isoforms. Journal of Cellular Physiology, 203(1), 251–260. 10.1002/JCP.20224 [DOI] [PubMed] [Google Scholar]
- Liu, H. , Liu, K. , & Dong, Z. (2021). Targeting CDK12 for cancer therapy: Function, mechanism, and drug discovery. Cancer Research, 81(1), 18–26. 10.1158/0008-5472.CAN-20-2245 [DOI] [PubMed] [Google Scholar]
- Liu, H. , Wang, J. , & Epner, E. M. (2004). Cyclin D1 activation in B‐cell malignancy: Association with changes in histone acetylation, DNA methylation, and RNA polymerase II binding to both promoter and distal sequences. Blood, 104(8), 2505–2513. 10.1182/BLOOD-2004-02-0483 [DOI] [PubMed] [Google Scholar]
- Liu, J. , & Kipreos, E. T. (2000). Evolution of cyclin‐dependent kinases (CDKs) and CDK‐activating kinases (CAKs): Differential conservation of CAKs in yeast and metazoa. Molecular Biology and Evolution, 17(7), 1061–1074. [DOI] [PubMed] [Google Scholar]
- Liu, W. , Cai, M. J. , Wang, J. X. , & Zhao, X. F. (2014). In a nongenomic action, steroid hormone 20‐Hydroxyecdysone induces phosphorylation of Cyclin‐dependent kinase 10 to promote gene transcription. Endocrinology, 155(5), 1738–1750. 10.1210/EN.2013-2020 [DOI] [PubMed] [Google Scholar]
- Liu, W. , Li, J. , Song, Y. S. , Li, Y. , Jia, Y. H. , & Zhao, H. D. (2017). Cdk5 links with DNA damage response and cancer. Molecular Cancer, 16(1), 1–9. 10.1186/S12943-017-0611-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Cheng, K. , Gong, K. , Fu, A. K. Y. , & Ip, N. Y. (2006). Pctaire1 phosphorylates N‐ethylmaleimide‐sensitive fusion protein. Journal of Biological Chemistry, 281(15), 9852–9858. 10.1074/jbc.m513496200 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Kung, C. , Fishburn, J. , Ansari, A. Z. , Shokat, K. M. , & Hahn, S. (2004). Two cyclin‐dependent kinases promote RNA polymerase II transcription and formation of the scaffold complex. Molecular and Cellular Biology, 24(4), 1721–1735. 10.1128/MCB.24.4.1721-1735.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Pelham‐Webb, B. , Di Giammartino, D. C. , Li, J. , Kim, D. , Kita, K. , Saiz, N. , Garg, V. , Doane, A. , Giannakakou, P. , Hadjantonakis, A. K. , Elemento, O. , & Apostolou, E. (2017). Widespread mitotic bookmarking by histone marks and transcription factors in pluripotent stem cells. Cell Reports, 19(7), 1283–1293. 10.1016/J.CELREP.2017.04.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Warfield, L. , Zhang, C. , Luo, J. , Allen, J. , Lang, W. H. , Ranish, J. , Shokat, K. M. , & Hahn, S. (2009). Phosphorylation of the transcription elongation factor Spt5 by yeast Bur1 kinase stimulates recruitment of the PAF complex. Molecular and Cellular Biology, 29(17), 4852–4863. 10.1128/MCB.00609-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Wu, C. , & Galaktionov, K. (2004). p42, a novel cyclin‐dependent kinase‐activating kinase in mammalian cells. Journal of Biological Chemistry, 279(6), 4507–4514. 10.1074/jbc.M309995200 [DOI] [PubMed] [Google Scholar]
- Long, J. J. , Leresche, A. , Kriwacki, R. W. , & Gottesfeld, J. M. (1998). Repression of TFIIH transcriptional activity and TFIIH‐associated cdk7 kinase activity at mitosis. Molecular and Cellular Biology, 18(3), 1467–1476. 10.1128/MCB.18.3.1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyer, P. , & Trembley, J. H. (2020). Roles of CDK/Cyclin complexes in transcription and pre‐mRNA splicing: Cyclins L and CDK11 at the cross‐roads of cell cycle and regulation of gene expression. Seminars in Cell & Developmental Biology, 107, 36–45. 10.1016/J.SEMCDB.2020.04.016 [DOI] [PubMed] [Google Scholar]
- Loyer, P. , Trembley, J. H. , Katona, R. , Kidd, V. J. , & Lahti, J. M. (2005). Role of CDK/cyclin complexes in transcription and RNA splicing. Cellular Signalling, 17(9), 1033–1051. 10.1016/J.CELLSIG.2005.02.005 [DOI] [PubMed] [Google Scholar]
- Lu, J. , Zhang, Z. L. , Huang, D. , Tang, N. , Li, Y. , Peng, Z. , Lu, C. , Dong, Z. , Tang, F. , Lu, J. , Zhang, Z. L. , Huang, D. , Tang, N. , Li, Y. , Peng, Z. , Lu, C. , Dong, Z. , & Tang, F. (2016). Cdk3‐promoted epithelial‐mesenchymal transition through activating AP‐1 is involved in colorectal cancer metastasis. Oncotarget, 7(6), 7012–7028. 10.18632/ONCOTARGET.6875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludgate, L. , Ning, X. , Nguyen, D. H. , Adams, C. , Mentzer, L. , & Hu, J. (2012). Cyclin‐dependent kinase 2 phosphorylates S/T‐P sites in the hepadnavirus core protein C‐terminal domain and is incorporated into viral capsids. Journal of Virology, 86(22), 12237–12250. 10.1128/JVI.01218-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui, G. Y. L. , Grandori, C. , & Kemp, C. J. (2018). CDK12: An emerging therapeutic target for cancer. Journal of Clinical Pathology, 71(11), 957–962. 10.1136/JCLINPATH-2018-205356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łukasik, P. , Załuski, M. , & Gutowska, I. (2021). Cyclin‐dependent kinases (CDK) and their role in diseases development—review. International Journal of Molecular Sciences, 22(6), 2935. 10.3390/IJMS22062935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, Z. , Lin, C. , & Shilatifard, A. (2012). The super elongation complex (SEC) family in transcriptional control. Nature Reviews. Molecular Cell Biology, 13(9), 543–547. 10.1038/NRM3417 [DOI] [PubMed] [Google Scholar]
- Luyties, O. , & Taatjes, D. J. (2022). The mediator kinase module: An interface between cell signaling and transcription. Trends in Biochemical Sciences, 47(4), 314–327. 10.1016/J.TIBS.2022.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynce, F. , Shajahan‐Haq, A. N. , & Swain, S. M. (2018). CDK4/6 inhibitors in breast cancer therapy: Current practice and future opportunities. Pharmacology and Therapeutics, 191, 65–73. 10.1016/j.pharmthera.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKeigan, J. P. , Murphy, L. O. , & Blenis, J. (2005). Sensitized RNAi screen of human kinases and phosphatases identifies new regulators of apoptosis and chemoresistance. Nature Cell Biology, 7(6), 591–600. 10.1038/ncb1258 [DOI] [PubMed] [Google Scholar]
- Maestre, C. , Delgado‐Esteban, M. , Gomez‐Sanchez, J. C. , Bolaños, J. P. , & Almeida, A. (2008). Cdk5 phosphorylates Cdh1 and modulates cyclin B1 stability in excitotoxicity. The EMBO Journal, 27(20), 2736–2745. 10.1038/EMBOJ.2008.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado, E. , & Reinberg, D. (1995). News on initiation and elongation of transcription by RNA polymerase II. Current Opinion in Cell Biology, 7(3), 352–361. 10.1016/0955-0674(95)80090-5 [DOI] [PubMed] [Google Scholar]
- Malhotra, N. , Gupta, R. , & Kumar, P. (2021). Pharmacological relevance of CDK inhibitors in Alzheimer's disease. Neurochemistry International, 148, 105115. 10.1016/J.NEUINT.2021.105115 [DOI] [PubMed] [Google Scholar]
- Malumbres, M. (2014). Cyclin‐dependent kinases. Genome Biology, 15(6), 1–10. 10.1186/gb4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres, M. , & Barbacid, M. (2001). To cycle or not to cycle: A critical decision in cancer. Nature Reviews. Cancer, 1(3), 222–231. 10.1038/35106065 [DOI] [PubMed] [Google Scholar]
- Malumbres, M. , & Barbacid, M. (2005). Mammalian cyclin‐dependent kinases. Trends in Biochemical Sciences, 30(11), 630–641. 10.1016/j.tibs.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Malumbres, M. , & Barbacid, M. (2009). Cell cycle, CDKs and cancer: A changing paradigm. Nature Reviews Cancer, 9(3), 153–166. 10.1038/nrc2602 [DOI] [PubMed] [Google Scholar]
- Malumbres, M. , Harlow, E. , Hunt, T. , Hunter, T. , Lahti, J. M. , Manning, G. , Morgan, D. O. , Tsai, L. H. , & Wolgemuth, D. J. (2009). Cyclin‐dependent kinases: A family portrait. Nature Cell Biology, 11(11), 1275–1276. 10.1038/ncb1109-1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavalan, A. P. C. , Pilarova, K. , Kluge, M. , Bartholomeeusen, K. , Rajecky, M. , Oppelt, J. , Khirsariya, P. , Paruch, K. , Krejci, L. , Friedel, C. C. , & Blazek, D. (2019). CDK12 controls G1/S progression by regulating RNAPII processivity at core DNA replication genes. EMBO Reports, 20(9), e47592. 10.15252/EMBR.201847592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marak, B. N. , Dowarah, J. , Khiangte, L. , & Singh, V. P. (2020). A comprehensive insight on the recent development of cyclic dependent kinase inhibitors as anticancer agents. European Journal of Medicinal Chemistry, 203, 112571. 10.1016/j.ejmech.2020.112571 [DOI] [PubMed] [Google Scholar]
- Marqués, F. , Moreau, J. L. , Peaucellier, G. , Lozano, J. C. , Schatt, P. , Picard, A. , Callebaut, I. , Perret, E. , & Genevière, A. M. (2000). A new subfamily of high molecular mass CDC2‐related kinases with PITAI/VRE motifs. Biochemical and Biophysical Research Communications, 279(3), 832–837. 10.1006/BBRC.2000.4042 [DOI] [PubMed] [Google Scholar]
- Marshall, N. F. , Peng, J. , Xie, Z. , & Price, D. H. (1996). Control of RNA polymerase II elongation potential by a novel carboxyl‐terminal domain kinase. Journal of Biological Chemistry, 271(43), 27176–27183. 10.1074/JBC.271.43.27176 [DOI] [PubMed] [Google Scholar]
- Martín‐Castellanos, C. , Blanco, M. A. , De Prada, J. M. , & Moreno, S. (2000). The puc1 cyclin regulates the G1 phase of the fission yeast cell cycle in response to cell size. Molecular Biology of the Cell, 11(2), 543–554. 10.1091/MBC.11.2.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín‐Castellanos, C. , Labib, K. , & Moreno, S. (1996). B‐type cyclins regulate G1 progression in fission yeast in opposition to the p25rum1 cdk inhibitor. The EMBO Journal, 15(4), 839–849. 10.1002/J.1460-2075.1996.TB00419.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryu, G. , & Yang, Q. (2022). Nuclear‐cytoplasmic compartmentalization of cyclin B1‐Cdk1 promotes robust timing of mitotic events. Cell Reports, 41(13), 111870. 10.1016/J.CELREP.2022.111870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluff, W. F. , & Koreski, K. P. (2017). Birth and death of histone mRNAs. Trends in Genetics, 33(10), 745–759. 10.1016/J.TIG.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda, S. , Kikkawa, U. , & Nakashima, A. (2021). The S. pombe CDK5 orthologue Pef1 cooperates with three cyclins, Clg1, Pas1 and Psl1, to promote pre‐meiotic DNA replication. Biomolecules, 11(1), 89. 10.3390/BIOM11010089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda, S. , Kikkawa, U. , Uda, H. , & Nakashima, A. (2020). The S. pombe CDK5 ortholog Pef1 regulates sexual differentiation through control of the TORC1 pathway and autophagy. Journal of Cell Science, 133(17), jcs247817. 10.1242/JCS.247817/266500/AM [DOI] [PubMed] [Google Scholar]
- Matsuda, S. , Kominato, K. , Koide‐Yoshida, S. , Miyamoto, K. , Isshiki, K. , Tsuji, A. , & Yuasa, K. (2014). PCTAIRE kinase 3/cyclin‐dependent kinase 18 is activated through association with cyclin A and/or phosphorylation by protein kinase A. The Journal of Biological Chemistry, 289(26), 18387–18400. 10.1074/JBC.M113.542936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matushansky, I. , Radparvar, F. , & Skoultchi, A. I. (2000). Reprogramming leukemic cells to terminal differentiation by inhibiting specific cyclin‐dependent kinases in G. Proceedings of the National Academy of Sciences of the United States of America, 97(26), 14317–14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudlin, I. E. , & Beggs, J. D. (2021). Conditional depletion of transcriptional kinases Ctk1 and Bur1 and effects on co‐transcriptional spliceosome assembly and pre‐mRNA splicing. RNA Biology, 18(S2), 782–793. 10.1080/15476286.2021.1991673/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcshea, A. , Harris, P. L. R. , Webster, K. R. , Wahl, A. F. , & Smith, M. A. (1997). Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer's disease. The American Journal of Pathology, 150(6), 1933. [PMC free article] [PubMed] [Google Scholar]
- Measday, V. , Moore, L. , Ogas, J. , Tyers, M. , & Andrews, B. (1994). The PCL2 (ORFD)‐PHO85 cyclin‐dependent kinase complex: A cell cycle regulator in yeast. Science (New York, N.Y.), 266(5189), 1391–1395. 10.1126/SCIENCE.7973731 [DOI] [PubMed] [Google Scholar]
- Measday, V. , Moore, L. , Retnakaran, R. , Lee, J. , Donoviel, M. , Neiman, A. M. , & Andrews, B. (1997). A family of cyclin‐like proteins that interact with the Pho85 cyclin‐dependent kinase. Molecular and Cellular Biology, 17(3), 1212–1223. 10.1128/MCB.17.3.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikrantz, W. , & Schlegel, R. (1996). Suppression of apoptosis by dominant negative mutants of cyclin‐dependent protein kinases. Journal of Biological Chemistry, 271(17), 10205–10209. 10.1074/jbc.271.17.10205 [DOI] [PubMed] [Google Scholar]
- Meimoun, A. , Holtzman, T. , Weissman, Z. , McBride, H. J. , Stillman, D. J. , Fink, G. R. , & Kornitzer, D. (2000). Degradation of the transcription factor Gcn4 requires the kinase Pho85 and the SCF(CDC4) ubiquitin‐ligase complex. Molecular Biology of the Cell, 11(3), 915–927. 10.1091/MBC.11.3.915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhart, A. , Kamenski, T. , Hoeppner, S. , Baumli, S. , & Cramer, P. (2005). A structural perspective of CTD function. Genes & Development, 19(12), 1401–1415. 10.1101/GAD.1318105 [DOI] [PubMed] [Google Scholar]
- Mendenhall, M. D. , & Hodge, A. E. (1998). Regulation of Cdc28 Cyclin‐dependent protein kinase activity during the cell cycle of the yeast Saccharomyces cerevisiae. Microbiology and Molecular Biology Reviews, 62(4), 1191–1243. 10.1128/MMBR.62.4.1191-1243.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick, K. A. , Larochelle, S. , Zhang, C. , Allen, J. J. , Shokat, K. M. , & Fisher, R. P. (2008). Distinct activation pathways confer cyclin‐binding specificity on Cdk1 and Cdk2 in human cells. Molecular Cell, 32(5), 662–672. 10.1016/J.MOLCEL.2008.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick, K. A. , Wohlbold, L. , Zhang, C. , Allen, J. J. , Horiuchi, D. , Huskey, N. E. , Goga, A. , Shokat, K. M. , & Fisher, R. P. (2011). Switching Cdk2 on or off with small molecules to reveal requirements in human cell proliferation. Molecular Cell, 42(5), 624–636. 10.1016/J.MOLCEL.2011.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michowski, W. , Chick, J. M. , Chu, C. , Kolodziejczyk, A. , Wang, Y. , Suski, J. M. , Abraham, B. , Anders, L. , Day, D. , Dunkl, L. M. , Li Cheong Man, M. , Zhang, T. , Laphanuwat, P. , Bacon, N. A. , Liu, L. , Fassl, A. , Sharma, S. , Otto, T. , Jecrois, E. , … Sicinski, P. (2020). Cdk1 controls global epigenetic landscape in embryonic stem cells. Molecular Cell, 78(3), 459–476. 10.1016/j.molcel.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolcevic, P. , Rainer, J. , & Geley, S. (2012). Orphan kinases turn eccentric. Cell Cycle, 11(20), 3758–3768. 10.4161/CC.21592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolcevic, P. , Sigl, R. , Rauch, V. , Hess, M. W. , Pfaller, K. , Barisic, M. , Pelliniemi, L. J. , Boesl, M. , & Geley, S. (2012). Cyclin‐dependent kinase 16/PCTAIRE kinase 1 is activated by cyclin Y and is essential for spermatogenesis. Molecular and Cellular Biology, 32(4), 868–879. 10.1128/MCB.06261-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat, J. , & Andrews, B. (2004). Late‐G1 cyclin–CDK activity is essential for control of cell morphogenesis in budding yeast. Nature Cell Biology, 6(1), 59–66. 10.1038/ncb1078 [DOI] [PubMed] [Google Scholar]
- Mok, M. T. , Zhou, J. , Tang, W. , Zeng, X. , Oliver, A. W. , Ward, S. E. , & Cheng, A. S. (2018). CCRK is a novel signalling hub exploitable in cancer immunotherapy. Pharmacology & Therapeutics, 186, 138–151. 10.1016/J.PHARMTHERA.2018.01.008 [DOI] [PubMed] [Google Scholar]
- Mokalled, M. H. , Johnson, A. , Kim, Y. , Oh, J. , & Olson, E. N. (2010). Myocardin‐related transcription factors regulate the Cdk5/Pctaire1 kinase cascade to control neurite outgrowth, neuronal migration and brain development. Development, 137(14), 2365–2374. 10.1242/DEV.047605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll, T. , Tebb, G. , Surana, U. , Robitsch, H. , & Nasmyth, K. (1991). The role of phosphorylation and the CDC28 protein kinase in cell cycle‐regulated nuclear import of the S. cerevisiae transcription factor SWI5. Cell, 66(4), 743–758. 10.1016/0092-8674(91)90118-I [DOI] [PubMed] [Google Scholar]
- Molz, L. , Booher, R. , Young, P. , & Beach, D. (1989). CDC2 and the regulation of mitosis: Six interacting mcs genes. Genetics, 122(4), 773–782. 10.1093/GENETICS/122.4.773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, D. O. (1995). Principles of CDK regulation. Nature, 374, 131–134. [DOI] [PubMed] [Google Scholar]
- Morris, M. C. , Kaiser, P. , Rudyak, S., Baskerville, C., Watson, M. H., & Reed, S. I. (2003). Cks1‐dependent proteasome recruitment and activation of CDC20 transcription in budding yeast. Nature, 423(6943), 1009–1013. 10.1038/nature01720 [DOI] [PubMed] [Google Scholar]
- Mughal, M. J. , Bhadresha, K. , & Kwok, H. F. (2023). CDK inhibitors from past to present: A new wave of cancer therapy. Seminars in Cancer Biology, 88, 106–122. 10.1016/J.SEMCANCER.2022.12.006 [DOI] [PubMed] [Google Scholar]
- Murray, S. , Udupa, R. , Yao, S. , Hartzog, G. , & Prelich, G. (2001). Phosphorylation of the RNA polymerase II carboxy‐terminal domain by the Bur1 cyclin‐dependent kinase. Molecular and Cell Biology, 21(13), 4089–4096. 10.1128/MCB.21.13.4089-4096.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, A. , Nakata, D. , Kakoi, Y. , Kunitomo, M. , Murai, S. , Ebara, S. , Hata, A. , & Hara, T. (2018). CDK8/19 inhibition induces premature G1/S transition and ATR‐dependent cell death in prostate cancer cells. Oncotarget, 9(17), 13474. 10.18632/ONCOTARGET.24414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann, U. , Huang, H. , Wolburg, H. , Wischhusen, J. , Weit, S. , Ohgaki, H. , & Weller, M. (2005). PCTAIRE3: A putative mediator of growth arrest and death induced by CTS‐1, a dominant‐positive p53‐derived synthetic tumor suppressor, in human malignant glioma cells. Cancer Gene Therapy, 13(5), 469–478. 10.1038/sj.cgt.7700917 [DOI] [PubMed] [Google Scholar]
- Nekhai, S. , Zhou, M. , Fernandez, A. , Lane, W. S. , Lamb, N. J. C. , Bradyr, J. , & Kumar, A. (2002). HIV‐1 Tat‐associated RNA polymerase C‐terminal domain kinase, CDK2, phosphorylates CDK7 and stimulates Tat‐mediated transcription. Biochemical Journal, 364(3), 649–657. 10.1042/BJ20011191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, C. , Goto, S. , Lund, K. , Hung, W. , & Sadowski, I. (2003). Srb10/Cdk8 regulates yeast filamentous growth by phosphorylating the transcription factor Ste12. Nature, 421(6919), 187–190. 10.1038/nature01243 [DOI] [PubMed] [Google Scholar]
- Nemec, C. M. , Singh, A. K. , Ali, A. , Tseng, S. C. , Syal, K. , Ringelberg, K. J. , Ho, Y. H. , Hintermair, C. , Ahmad, M. F. , Kar, R. K. , Gasch, A. P. , Akhtar, M. S. , Eick, D. , & Ansari, A. Z. (2019). Noncanonical CTD kinases regulate RNA polymerase II in a gene‐class‐specific manner. Nature Chemical Biology, 15(2), 123–131. 10.1038/s41589-018-0194-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs, C. , & Acebron, S. P. (2012). Mitotic and mitogenic Wnt signalling. EMBO Journal, 31(12), 2705–2713. 10.1038/EMBOJ.2012.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic, M. , Dudek, H. , Kwon, Y. T. , Ramos, Y. F. M. , & Tsai, L. H. (1996). The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes & Development, 10(7), 816–825. 10.1101/GAD.10.7.816 [DOI] [PubMed] [Google Scholar]
- Ning, J. F. , Stanciu, M. , Humphrey, M. R. , Gorham, J. , Wakimoto, H. , Nishihara, R. , Lees, J. , Zou, L. , Martuza, R. L. , Wakimoto, H. , & Rabkin, S. D. (2019). Myc targeted CDK18 promotes ATR and homologous recombination to mediate PARP inhibitor resistance in glioblastoma. Nature Communications, 10(1), 1–18. 10.1038/s41467-019-10993-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa, M. , Kawasumi, M. , Fujino, M. , & Toh‐e, A. (1998). Phosphorylation of Sic1, a cyclin‐dependent kinase (Cdk) inhibitor, by Cdk including Pho85 kinase is required for its prompt degradation. Molecular Biology of the Cell, 9(9), 2393–2405. 10.1091/MBC.9.9.2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa, M. , Tanigawa, M. , Hayashi, M. , Maeda, T. , Yazaki, Y. , Saeki, Y. , & Toh‐e, A. (2010). Pho85 kinase, a cyclin‐dependent kinase, regulates nuclear accumulation of the Rim101 transcription factor in the stress response of Saccharomyces cerevisiae . Eukaryotic Cell, 9(6), 943–951. 10.1128/EC.00247-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, T. , Li, K. , Jiang, L. , Zhou, Z. , Hong, J. , Chen, X. , Dong, X. , He, Q. , Cao, J. , Yang, B. , & Zhu, C. L. (2022). Noncovalent CDK12/13 dual inhibitors‐based PROTACs degrade CDK12‐Cyclin K complex and induce synthetic lethality with PARP inhibitor. European Journal of Medicinal Chemistry, 228, 114012. 10.1016/J.EJMECH.2021.114012 [DOI] [PubMed] [Google Scholar]
- Norbury, C. , Blow, J. , & Nurse, P. (1991). Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. The EMBO Journal, 10(11), 3321–3329. 10.1002/J.1460-2075.1991.TB04896.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse, P. (1975). Genetic control of cell size at cell division in yeast. Nature, 256(5518), 547–551. 10.1038/256547a0 [DOI] [PubMed] [Google Scholar]
- Nurse, P. (1990). Universal control mechanism regulating cell cycle timing of M‐phase. Nature, 344, 503–508. 10.1038/344503a0 [DOI] [PubMed] [Google Scholar]
- Nurse, P. , & Bissett, Y. (1981). Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature, 292(5823), 558–560. 10.1038/292558a0 [DOI] [PubMed] [Google Scholar]
- Nurse, P. , Thuriaux, P. , & Nasmyth, K. (1976). Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe . MGG Molecular & General Genetics, 146(2), 167–178. 10.1007/BF00268085 [DOI] [PubMed] [Google Scholar]
- O'Conalláin, C. , Doolin, M. T. , Taggart, C. , Thornton, F. , & Butler, G. (1999). Regulated nuclear localisation of the yeast transcription factor Ace2p controls expression of chitinase (CTS1) in Saccharomyces cerevisiae . Molecular & General Genetics: MGG, 262(2), 275–282. 10.1007/S004380051084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuni, K. , & Yamashita, I. (2000). A transcriptional autoregulatory loop for KIN28‐CCL1 and SRB10‐SRB11, each encoding RNA polymerase II CTD kinase‐cyclin pair, stimulates the meiotic development of S. cerevisiae . Yeast, 16(9), 829–846. [DOI] [PubMed] [Google Scholar]
- Ohshima, T. , Ward, J. M. , Huh, C. G. , Longenecker, G. , Veeranna, A. , Pant, H. C. , Brady, R. O. , Martin, L. J. , & Kulkarni, A. B. (1996). Targeted disruption of the cyclin‐dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proceedings of the National Academy of Sciences of the United States of America, 93(20), 11173. 10.1073/PNAS.93.20.11173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, Y. , Onogi, H. , Honda, R. , Yasuda, H. , Wakabayashi, T. , Nimura, Y. , & Hagiwara, M. (1998). CDC2 kinase‐mediated phosphorylation of splicing factor SF2/ASF. Biochemical and Biophysical Research Communications, 249(3), 872–878. 10.1006/BBRC.1998.9247 [DOI] [PubMed] [Google Scholar]
- Olson, C. M. , Liang, Y. , Leggett, A. , Park, W. D. , Li, L. , Mills, C. E. , Elsarrag, S. Z. , Ficarro, S. B. , Zhang, T. , Düster, R. , Geyer, M. , Sim, T. , Marto, J. A. , Sorger, P. K. , Westover, K. D. , Lin, C. Y. , Kwiatkowski, N. , & Gray, N. S. (2019). Development of a selective CDK7 covalent inhibitor reveals predominant cell‐cycle phenotype. Cell Chemical Biology, 26(6), 792–803.e10. 10.1016/J.CHEMBIOL.2019.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongkeko, W. , Ferguson, D. J. P. , Harris, A. L. , & Norbury, C. (1995). Inactivation of Cdc2 increases the level of apoptosis induced by DNA damage. Journal of Cell Science, 108(8), 2897–2904. 10.1242/JCS.108.8.2897 [DOI] [PubMed] [Google Scholar]
- Orlando, D. A. , Lin, C. Y. , Bernard, A. , Wang, J. Y. , Socolar, J. E. S. , Iversen, E. S. , Hartemink, A. J. , & Haase, S. B. (2008). Global control of cell‐cycle transcription by coupled CDK and network oscillators. Nature, 453(7197), 944–947. 10.1038/nature06955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapenko, D. , & Solomon, M. J. (2003). Budding yeast CTDK‐I is required for DNA damage‐induced transcription. Eukaryotic Cell, 2(2), 274–283. 10.1128/EC.2.2.274-283.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapenko, D. , & Solomon, M. J. (2005). Phosphorylation by Cak1 regulates the C‐terminal domain kinase Ctk1 in Saccharomyces cerevisiae. Molecular and Cellular Biology, 25(10), 3906–3913. 10.1128/MCB.25.10.3906-3913.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto, T. , & Sicinski, P. (2017). Cell cycle proteins as promising targets in cancer therapy. Nature Reviews Cancer, 17(2), 93–115. 10.1038/nrc.2016.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou‐Yang, J. , Huang, L. H. , & Sun, X. X. (2017). Cyclin‐dependent kinase 14 promotes cell proliferation, migration and invasion in ovarian cancer by inhibiting Wnt signaling pathway. Gynecologic and Obstetric Investigation, 82(3), 230–239. 10.1159/000447632 [DOI] [PubMed] [Google Scholar]
- Ou, C. Y. , Poon, V. Y. , Maeder, C. I. , Watanabe, S. , Lehrman, E. K. , Fu, A. K. Y. , Park, M. , Fu, W. Y. , Jorgensen, E. M. , Ip, N. Y. , & Shen, K. (2010). Two cyclin‐dependent kinase pathways are essential for polarized trafficking of presynaptic components. Cell, 141(5), 846–858. 10.1016/j.cell.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak, V. , Eifler, T. T. , Jäger, S. , Krogan, N. J. , Fujinaga, K. , & Peterlin, B. M. (2015). CDK11 in TREX/THOC regulates HIV mRNA 3′ end processing. Cell Host and Microbe, 18(5), 560–570. 10.1016/j.chom.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palancade, B. , & Bensaude, O. (2003). Investigating RNA polymerase II carboxyl‐terminal domain (CTD) phosphorylation. European Journal of Biochemistry, 270(19), 3859–3870. 10.1046/J.1432-1033.2003.03794.X [DOI] [PubMed] [Google Scholar]
- Palmer, K. J. , Konkel, J. E. , & Stephens, D. J. (2005). PCTAIRE protein kinases interact directly with the COPII complex and modulate secretory cargo transport. Journal of Cell Science, 118(17), 3839–3847. 10.1242/JCS.02496 [DOI] [PubMed] [Google Scholar]
- Palmer, N. , & Kaldis, P. (2020). Less‐well known functions of cyclin/CDK complexes. Seminars in Cell and Developmental Biology, 107, 54–62. 10.1016/j.semcdb.2020.04.003 [DOI] [PubMed] [Google Scholar]
- Palozola, K. C. , Donahue, G. , Liu, H. , Grant, G. R. , Becker, J. S. , Cote, A. , Yu, H. , Raj, A. , & Zaret, K. S. (2017). Mitotic transcription and waves of gene reactivation during mitotic exit. Science, 358(6359), 119–122. 10.1126/SCIENCE.AAL4671/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang, E. Y. T. , Bai, A. H. C. , To, K. F. , Sy, S. M. H. , Wong, N. L. Y. , Lai, P. B. S. , Squire, J. A. , & Wong, N. (2007). Identification of PFTAIRE protein kinase 1, a novel cell division cycle‐2 related gene, in the motile phenotype of hepatocellular carcinoma cells. Hepatology (Baltimore, MD), 46(2), 436–445. 10.1002/HEP.21691 [DOI] [PubMed] [Google Scholar]
- Panzeri, V. , Pieraccioli, M. , Cesari, E. , de la Grange, P. , & Sette, C. (2013). CDK12/13 promote splicing of proximal introns by enhancing the interaction between RNA polymerase II and the splicing factor SF3B1. Nucleic Acids Research, 1, 13–14. 10.1093/NAR/GKAD258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao, P. C. , & Tsai, L. H. (2021). Three decades of Cdk5. Journal of Biomedical Science, 28(1), 79. 10.1186/S12929-021-00774-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, M. H. , Kim, S. Y. , Kim, Y. J. , & Chung, Y. H. (2014). ALS2CR7 (CDK15) attenuates TRAIL induced apoptosis by inducing phosphorylation of survivin Thr34. Biochemical and Biophysical Research Communications, 450(1), 129–134. 10.1016/J.BBRC.2014.05.070 [DOI] [PubMed] [Google Scholar]
- Parsons, G. G. , & Spencer, C. A. (1997). Mitotic repression of RNA polymerase II transcription is accompanied by release of transcription elongation complexes. Molecular and Cellular Biology, 17(10), 5791–5802. 10.1128/MCB.17.10.5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parua, P. K. , & Fisher, R. P. (2020). Dissecting the Pol II transcription cycle and derailing cancer with CDK inhibitors. Nature Chemical Biology, 16(7), 716–724. 10.1038/s41589-020-0563-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parua, P. K. , Booth, G. T. , Sansó, M. , Benjamin, B. , Tanny, J. C. , Lis, J. T. , & Fisher, R. P. (2018). A Cdk9–PP1 switch regulates the elongation–termination transition of RNA polymerase II. Nature, 558(7710), 460–464. 10.1038/s41586-018-0214-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patturajan, M. , Conrad, N. K. , Bregman, D. B. , & Corden, J. L. (1999). Yeast carboxyl‐terminal domain kinase I positively and negatively regulates RNA polymerase II carboxyl‐terminal domain phosphorylation. Journal of Biological Chemistry, 274(39), 27823–27828. 10.1074/jbc.274.39.27823 [DOI] [PubMed] [Google Scholar]
- Pavletich, N. P. (1999). Mechanisms of cyclin‐dependent kinase regulation: Structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. Journal of Molecular Biology, 287(5), 821–828. 10.1006/JMBI.1999.2640 [DOI] [PubMed] [Google Scholar]
- Pei, Y. , & Shuman, S. (2003). Characterization of the Schizosaccharomyces pombe Cdk9/Pch1 protein kinase. Journal of Biological Chemistry, 278(44), 43346–43356. 10.1074/jbc.m307319200 [DOI] [PubMed] [Google Scholar]
- Pei, Y. , Du, H. , Singer, J. , St. Amour, C. , Granitto, S. , Shuman, S. , & Fisher, R. P. (2006). Cyclin‐dependent kinase 9 (Cdk9) of fission yeast is activated by the CDK‐activating kinase Csk1, overlaps functionally with the TFIIH‐associated kinase Mcs6, and associates with the mRNA cap methyltransferase Pcm1 in vivo. Molecular and Cellular Biology, 26(3), 777–788. 10.1128/MCB.26.3.777-788.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei, Y. , Schwer, B. , & Shuman, S. (2003). Interactions between fission yeast Cdk9, its cyclin partner Pch1, and mRNA capping enzyme Pct1 suggest an elongation checkpoint for mRNA quality control. Journal of Biological Chemistry, 278(9), 7180–7188. 10.1074/jbc.M211713200 [DOI] [PubMed] [Google Scholar]
- Peissert, S. , Schlosser, A. , Kendel, R. , Kuper, J. , & Kisker, C. (2020). Structural basis for CDK7 activation by MAT1 and Cyclin H. Proceedings of the National Academy of Sciences of the United States of America, 117(43), 26739–26748. 10.1073/PNAS.2010885117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelish, H. E. , Liau, B. B. , Nitulescu, I. I. , Tangpeerachaikul, A. , Poss, Z. C. , Da Silva, D. H. , Caruso, B. T. , Arefolov, A. , Fadeyi, O. , Christie, A. L. , Du, K. , Banka, D. , Schneider, E. V. , Jestel, A. , Zou, G. , Si, C. , Ebmeier, C. C. , Bronson, R. T. , Krivtsov, A. V. , … Shair, M. D. (2015). Mediator kinase inhibition further activates super‐enhancer‐associated genes in AML. Nature, 526(7572), 273–276. 10.1038/nature14904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, C. , Zeng, W. , Su, J. , Kuang, Y. , He, Y. , Zhao, S. , Zhang, J. , Ma, W. , Bode, A. M. , Dong, Z. , & Chen, X. (2016). Cyclin dependent kinase 2 (CDK2) is a key mediator for EGF‐induced cell transformation mediated through the ELK4/c‐Fos signaling pathway. Oncogene, 35(9), 1170. 10.1038/ONC.2015.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, J. , Zhu, Y. , Milton, J. T. , & Price, D. H. (1998). Identification of multiple cyclin subunits of human P‐TEFb. Genes & Development, 12(5), 755. 10.1101/GAD.12.5.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin, B. M. , & Price, D. H. (2006). Controlling the elongation phase of transcription with P‐TEFb. Molecular Cell, 23(3), 297–305. 10.1016/J.MOLCEL.2006.06.014 [DOI] [PubMed] [Google Scholar]
- Petretti, C. , Savoian, M. , Montembault, E. , Glover, D. M. , Prigent, C. , & Giet, R. (2006). The PITSLRE/CDK11p58 protein kinase promotes centrosome maturation and bipolar spindle formation. EMBO Reports, 7(4), 418–424. 10.1038/SJ.EMBOR.7400639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyressatre, M. , Prével, C. , Pellerano, M. , & Morris, M. C. (2015). Targeting cyclin‐dependent kinases in human cancers: From small molecules to peptide inhibitors. Cancers, 7(1), 179–237. 10.3390/cancers7010179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip, S. , Kumarasiri, M. , Teo, T. , Yu, M. , & Wang, S. (2018). Cyclin‐dependent kinase 8: A new hope in targeted cancer therapy? Journal of Medicinal Chemistry, 61(12), 5073–5092. 10.1021/ACS.JMEDCHEM.7B00901 [DOI] [PubMed] [Google Scholar]
- Piao, J. , Zhu, L. , Sun, J. , Li, N. , Dong, B. , Yang, Y. , & Chen, L. (2019). High expression of CDK1 and BUB1 predicts poor prognosis of pancreatic ductal adenocarcinoma. Gene, 701, 15–22. 10.1016/j.gene.2019.02.081 [DOI] [PubMed] [Google Scholar]
- Pic‐Taylor, A. , Darieva, Z. , Morgan, B. A. , & Sharrocks, A. D. (2004). Regulation of cell cycle‐specific gene expression through cyclin‐dependent kinase‐mediated phosphorylation of the Forkhead transcription factor Fkh2p. Molecular and Cellular Biology, 24(22), 10036–10046. 10.1128/MCB.24.22.10036-10046.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines, J. (1995). Cyclins and cyclin‐dependent kinases: A biochemical view. Biochemical Journal, 308(3), 697–711. 10.1042/bj3080697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinhero, R. , Liaw, P. , Bertens, K. , & Yankulov, K. (2004). Three cyclin‐dependent kinases preferentially phosphorylate different parts of the C‐terminal domain of the large subunit of RNA polymerase II. European Journal of Biochemistry, 271(5), 1004–1014. 10.1111/J.1432-1033.2004.04002.X [DOI] [PubMed] [Google Scholar]
- Porter, D. C. , Farmaki, E. , Altilia, S. , Schools, G. P. , West, D. K. , Chen, M. , Chang, B. D. , Puzyrev, A. T. , Lim, C. U. , Rokow‐Kittell, R. , Friedhoff, L. T. , Papavassiliou, A. G. , Kalurupalle, S. , Hurteau, G. , Shi, J. , Baran, P. S. , Gyorffy, B. , Wentland, M. P. , Broude, E. V. , … Roninson, I. B. (2012). Cyclin‐dependent kinase 8 mediates chemotherapy‐induced tumor‐promoting paracrine activities. Proceedings of the National Academy of Sciences of the United States of America, 109(34), 13799–13804. 10.1073/PNAS.1206906109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss, Z. C. , Ebmeier, C. C. , & Taatjes, D. J. (2013). The mediator complex and transcription regulation. Critical Reviews in Biochemistry and Molecular Biology, 48(6), 575–608. 10.3109/10409238.2013.840259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss, Z. C. , Ebmeier, C. C. , Odell, A. T. , Tangpeerachaikul, A. , Lee, T. , Pelish, H. E. , Shair, M. D. , Dowell, R. D. , Old, W. M. , & Taatjes, D. J. (2016). Identification of mediator kinase substrates in human cells using cortistatin A and quantitative phosphoproteomics. Cell Reports, 15(2), 436–450. 10.1016/j.celrep.2016.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo, K. , Castro‐Rivera, E. , Tan, C. , Plattner, F. , Schwach, G. , Siegl, V. , Meyer, D. , Guo, A. , Gundara, J. , Mettlach, G. , Richer, E. , Guevara, J. A. , Ning, L. , Gupta, A. , Hao, G. , Tsai, L. H. , Sun, X. , Antich, P. , Sidhu, S. , … Bibb, J. A. (2013). The role of Cdk5 in neuroendocrine thyroid cancer. Cancer Cell, 24(4), 499–511. 10.1016/j.ccr.2013.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelich, G. , & Winston, F. (1993). Mutations that suppress the deletion of an upstream activating sequence in yeast: Involvement of a protein kinase and histone H3 in repressing transcription in vivo. Genetics, 135(3), 665–676. 10.1093/GENETICS/135.3.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, H. , Hu, C. , & Hinnebusch, A. G. (2009). Phosphorylation of the Pol II CTD by KIN28 enhances BUR1/BUR2 recruitment and Ser2 CTD phosphorylation near promoters. Molecular Cell, 33(6), 752–762. 10.1016/j.molcel.2009.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, H. , Hu, C. , Gaur, N. A. , & Hinnebusch, A. G. (2012). Pol II CTD kinases Bur1 and Kin28 promote Spt5 CTR‐independent recruitment of Paf1 complex. The EMBO Journal, 31(16), 3494–3505. 10.1038/EMBOJ.2012.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, D. , Rashidian, J. , Mount, M. P. , Aleyasin, H. , Parsanejad, M. , Lira, A. , Haque, E. , Zhang, Y. , Callaghan, S. , Daigle, M. , Rousseaux, M. W. C. , Slack, R. S. , Albert, P. R. , Vincent, I. , Woulfe, J. M. , & Park, D. S. (2007). Role of Cdk5‐mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson's disease. Neuron, 55(1), 37–52. 10.1016/j.neuron.2007.05.033 [DOI] [PubMed] [Google Scholar]
- Quereda, V. , Bayle, S. , Vena, F. , Frydman, S. M. , Monastyrskyi, A. , Roush, W. R. , & Duckett, D. R. (2019). Therapeutic targeting of CDK12/CDK13 in triple‐negative breast cancer. Cancer Cell, 36(5), 545–558. 10.1016/J.CCELL.2019.09.004 [DOI] [PubMed] [Google Scholar]
- Ramos‐Alonso, L. , Holland, P. , Le Gras, S. , Zhao, X. , Jost, B. , Bjørås, M. , Barral, Y. , Enserink, J. M. , & Chymkowitch, P. (2023). Mitotic chromosome condensation resets chromatin to safeguard transcriptional homeostasis during interphase. Proceedings of the National Academy of Sciences, 120(4), e2210593120. 10.1073/PNAS.2210593120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, S. , & Rollins, B. J. (2004). Cyclin C/Cdk3 promotes Rb‐dependent G0 exit. Cell, 117(2), 239–251. 10.1016/S0092-8674(04)00300-9 [DOI] [PubMed] [Google Scholar]
- Reymond, A. , Marks, J. , & Simanis, V. (1993). The activity of S.pombe DSC‐1‐like factor is cell cycle regulated and dependent on the activity of p34(cdc2). EMBO Journal, 12(11), 4325–4334. 10.1002/J.1460-2075.1993.TB06117.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, D. , Shi, B. J. , McLean, C. , Katsis, F. , Kemp, B. , & Dalton, S. (2003). Recruitment of Thr 319‐phosphorylated Ndd1p to the FHA domain of Fkh2p requires Clbkinase activity: A mechanism for CLB cluster gene activation. Genes & Development, 17(14), 1789–1802. 10.1101/GAD.1074103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, A. P. (2018). Roles of CDKs in RNA polymerase II transcription of the HIV‐1 genome. Transcription, 10(2), 111–117. 10.1080/21541264.2018.1542254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert, P. , Corden, J. L. , & Lees, E. (1999). Cyclin C/CDK8 and cyclin H/CDK7/p36 are biochemically distinct CTD kinases. Oncogene, 18(4), 1093–1102. 10.1038/sj.onc.1202399 [DOI] [PubMed] [Google Scholar]
- Rimel, J. K. , & Taatjes, D. J. (2018). The essential and multifunctional TFIIH complex. Protein Science, 27(6), 1018–1037. 10.1002/PRO.3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimel, J. K. , Poss, Z. C. , Erickson, B. , Maas, Z. L. , Ebmeier, C. C. , Johnson, J. L. , Decker, T. M. , Yaron, T. M. , Bradley, M. J. , Hamman, K. B. , Hu, S. , Malojcic, G. , Marineau, J. J. , White, P. W. , Brault, M. , Tao, L. , DeRoy, P. , Clavette, C. , Nayak, S. , … Taatjes, D. J. (2020). Selective inhibition of CDK7 reveals high‐confidence targets and new models for TFIIH function in transcription. Genes & Development, 34(21‐22), 1452–1473. 10.1101/GAD.341545.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, C. R. , Cho, E.‐J. , Keogh, M.‐C. , Moore, C. L. , Greenleaf, A. L. , & Buratowski, S. (2000). Kin28, the TFIIH‐associated carboxy‐terminal domain kinase, facilitates the recruitment of mRNA processing machinery to RNA polymerase II. Molecular and Cellular Biology, 20(1), 104–112. 10.1128/MCB.20.1.104-112.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski, R. (2019). Cyclin‐dependent protein serine/threonine kinase inhibitors as anticancer drugs. Pharmacological Research, 139, 471–488. 10.1016/j.phrs.2018.11.035 [DOI] [PubMed] [Google Scholar]
- Rosonina, E. , Duncan, S. M. , & Manley, J. L. (2012). Sumoylation of transcription factor Gcn4 facilitates its Srb10‐mediated clearance from promoters in yeast. Genes & Development, 26(4), 350–355. 10.1101/GAD.184689.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röther, S. , & Sträßer, K. (2007). The RNA polymerase II CTD kinase Ctk1 functions in translation elongation. Genes & Development, 21(11), 1409–1421. 10.1101/GAD.428407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, R. , Adamczewski, J. P. , Seroz, T. , Vermeulen, W. , Tassan, J. P. , Schaeffer, L. , Nigg, E. A. , Hoeijmakers, J. H. J. , & Egly, J. M. (1994). The MO15 cell cycle kinase is associated with the TFIIH transcription‐DNA repair factor. Cell, 79(6), 1093–1101. 10.1016/0092-8674(94)90039-6 [DOI] [PubMed] [Google Scholar]
- Sage, J. (2004). Cyclin C makes an entry into the cell cycle. Developmental Cell, 6(5), 607–608. 10.1016/S1534-5807(04)00137-6 [DOI] [PubMed] [Google Scholar]
- Saiz, J. E. , & Fisher, R. P. (2002). A CDK‐activating kinase network is required in cell cycle control and transcription in fission yeast. Current Biology, 12(13), 1100–1105. 10.1016/S0960-9822(02)00903-X [DOI] [PubMed] [Google Scholar]
- Samuelsen, C. O. , Baraznenok, V. , Khorosjutina, O. , Spåhr, H. , Kieselbach, T. , Holmberg, S. , & Gustafsson, C. M. (2003). TRAP230/ARC240 and TRAP240/ARC250 mediator subunits are functionally conserved through evolution. Proceedings of the National Academy of Sciences of the United States of America, 100(11), 6422–6427. 10.1073/PNAS.1030497100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Martínez, C. , Lallena, M. J. , Sanfeliciano, S. G. , & de Dios, A. (2019). Cyclin dependent kinase (CDK) inhibitors as anticancer drugs: Recent advances (2015–2019). Bioorganic and Medicinal Chemistry Letters, 29(20), 126637. 10.1016/j.bmcl.2019.126637 [DOI] [PubMed] [Google Scholar]
- Sanphui, P. , Pramanik, S. K. , Chatterjee, N. , Moorthi, P. , Banerji, B. , & Biswas, S. C. (2013). Efficacy of cyclin dependent kinase 4 inhibitors as potent neuroprotective agents against insults relevant to Alzheimer's disease. PLoS One, 8(11), e78842. 10.1371/JOURNAL.PONE.0078842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansó, M. , Lee, K. M. , Viladevall, L. , Jacques, P. É. , Pagé, V. , Nagy, S. , Racine, A. , St. Amour, C. V. , Zhang, C. , Shokat, K. M. , Schwer, B. , Robert, F. , Fisher, R. P. , & Tanny, J. C. (2012). A positive feedback loop links opposing functions of P‐TEFb/Cdk9 and histone H2B Ubiquitylation to regulate transcript elongation in fission yeast. PLoS Genetics, 8(8), e1002822. 10.1371/JOURNAL.PGEN.1002822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansó, M. , Levin, R. S. , Lipp, J. J. , Wang, V. Y. F. , Greifenberg, A. K. , Quezada, E. M. , Ali, A. , Ghosh, A. , Larochelle, S. , Rana, T. M. , Geyer, M. , Tong, L. , Shokat, K. M. , & Fisher, R. P. (2016). P‐TEFb regulation of transcription termination factor Xrn2 revealed by a chemical genetic screen for Cdk9 substrates. Genes & Development, 30(1), 117–131. 10.1101/GAD.269589.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaría, D. , Barrière, C. , Cerqueira, A. , Hunt, S. , Tardy, C. , Newton, K. , Cáceres, J. F. , Dubus, P. , Malumbres, M. , & Barbacid, M. (2007). Cdk1 is sufficient to drive the mammalian cell cycle. Nature, 448(7155), 811–815. 10.1038/nature06046 [DOI] [PubMed] [Google Scholar]
- Satyanarayana, A. , & Kaldis, P. (2009a). A dual role of Cdk2 in DNA damage response. Cell Division, 4, 2–5. 10.1186/1747-1028-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana, A. , & Kaldis, P. (2009b). Mammalian cell‐cycle regulation: Several CDKs, numerous cyclins and diverse compensatory mechanisms. Oncogene, 28(33), 2925–2939. 10.1038/onc.2009.170 [DOI] [PubMed] [Google Scholar]
- Satyanarayana, A. , Berthet, C. , Lopez‐Molina, J. , Coppola, V. , Tessarollo, L. , & Kaldis, P. (2008). Genetic substitution of Cdk1 by Cdk2 leads to embryonic lethality and loss of meiotic function of Cdk2. Development, 135(20), 3389–3400. 10.1242/dev.024919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sava, G. P. , Fan, H. , Coombes, R. C. , Buluwela, L. , & Ali, S. (2020). CDK7 inhibitors as anticancer drugs. Cancer and Metastasis Reviews, 39(3), 805–823. 10.1007/s10555-020-09885-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter, M. M. , & Fisher, R. P. (2013). The CDK‐activating kinase Cdk7: Taking yes for an answer. Cell Cycle, 12(20), 3239–3240. 10.4161/CC.26355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecher, S. , Walter, B. , Falkenstein, M. , Macher‐Goeppinger, S. , Stenzel, P. , Krümpelmann, K. , Hadaschik, B. , Perner, S. , Kristiansen, G. , Duensing, S. , Roth, W. , & Tagscherer, K. E. (2017). Cyclin K dependent regulation of Aurora B affects apoptosis and proliferation by induction of mitotic catastrophe in prostate cancer. International Journal of Cancer, 141(8), 1643–1653. 10.1002/IJC.30864 [DOI] [PubMed] [Google Scholar]
- Schneider, E. , Kartarius, S. , Schuster, N. , & Montenarh, M. (2002). The cyclin H/cdk7/Mat1 kinase activity is regulated by CK2 phosphorylation of cyclin H. Oncogene, 21(33), 5031–5037. 10.1038/sj.onc.1205690 [DOI] [PubMed] [Google Scholar]
- Schroeder, S. C. , Schwer, B. , Shuman, S. , & Bentley, D. (2000). Dynamic association of capping enzymes with transcribing RNA polymerase II. Genes & Development, 14(19), 2435–2440. 10.1101/GAD.836300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segil, N. , Guermah, M. , Hoffmann, A. , Roeder, R. G. , & Heintz, N. (1996). Mitotic regulation of TFIID: Inhibition of activator‐dependent transcription and changes in subcellular localization. Genes & Development, 10(19), 2389–2400. 10.1101/GAD.10.19.2389 [DOI] [PubMed] [Google Scholar]
- Serrano, R. , Kielland‐Brandt, M. C. , & Fink, G. R. (1982). Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+‐ and Ca2+‐ATPases. Nature, 29, 3509–3513. 10.1038/319689a0 [DOI] [PubMed] [Google Scholar]
- Shandilya, J. , & Roberts, S. G. E. (2012). The transcription cycle in eukaryotes: From productive initiation to RNA polymerase II recycling. Biochimica et Biophysica Acta, 1819(5), 391–400. 10.1016/J.BBAGRM.2012.01.010 [DOI] [PubMed] [Google Scholar]
- Shane, E. (2001). Parathyroid carcinoma. The Journal of Clinical Endocrinology & Metabolism, 86(2), 485–493. 10.1210/JCEM.86.2.7207 [DOI] [PubMed] [Google Scholar]
- Shapiro, G. I. (2006). Cyclin‐dependent kinase pathways as targets for cancer treatment. Journal of Clinical Oncology, 24(11), 1770–1783. 10.1200/JCO.2005.03.7689 [DOI] [PubMed] [Google Scholar]
- Shehata, S. N. , Deak, M. , Collodet, C. , Spiegl, S. , Geley, S. , Sumpton, D. , & Sakamoto, K. (2019). Identification of novel PCTAIRE‐1/CDK16 substrates using a chemical genetic screen. Cellular Signalling, 59, 53–61. 10.1016/J.CELLSIG.2019.03.012 [DOI] [PubMed] [Google Scholar]
- Shehata, S. N. , Deak, M. , Morrice, N. A. , Ohta, E. , Hunter, R. W. , Kalscheuer, V. M. , & Sakamoto, K. (2015). Cyclin Y phosphorylation‐ and 14‐3‐3‐binding‐dependent activation of PCTAIRE‐1/CDK16. Biochemical Journal, 469(3), 409–420. 10.1042/BJ20150486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer, R. , Meimoun, A. , Holtzman, T. , & Kornitzer, D. (2002). Regulation of the transcription factor Gcn4 by Pho85 cyclin PCL5. Molecular and Cellular Biology, 22(15), 5395–5404. 10.1128/MCB.22.15.5395-5404.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr, C. J. , & Roberts, J. M. (1999). CDK inhibitors: Positive and negative regulators of G1‐phase progression. Genes & Development, 13(12), 1501–1512. 10.1101/GAD.13.12.1501 [DOI] [PubMed] [Google Scholar]
- Shi, J. , Feng, Y. , Goulet, A. C. , Vaillancourt, R. R. , Sachs, N. A. , Hershey, J. W. , & Nelson, M. A. (2003). The p34cdc2‐related cyclin‐dependent kinase 11 interacts with the p47 subunit of eukaryotic initiation factor 3 during apoptosis. Journal of Biological Chemistry, 278(7), 5062–5071. 10.1074/JBC.M206427200 [DOI] [PubMed] [Google Scholar]
- Shi, J. , Hershey, J. W. B. , & Nelson, M. A. (2009). Phosphorylation of the eukaryotic initiation factor 3f by cyclin‐dependent kinase 11 during apoptosis. FEBS Letters, 583(6), 971–977. 10.1016/J.FEBSLET.2009.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, L. , Nishioka, W. K. , Th'ng, J. , Bradbury, E. M. , Litchfield, D. W. , & Greenberg, A. H. (1994). Premature p34cdc2 activation required for apoptosis. Science, 263(5150), 1143–1145. 10.1126/SCIENCE.8108732 [DOI] [PubMed] [Google Scholar]
- Shiekhattar, R. , Mermelstein, F. , Fisher, R. P. , Drapkin, R. , Brian, D. , Wessling, H. C. , Morgan, D. O. , & Reinberg, D. (1995). Cdk‐activating kinase complex is a component of human transcription factor TFIIH. Nature, 374(6519), 283–287. 10.1038/374283A0 [DOI] [PubMed] [Google Scholar]
- Shimizu, K. , Uematsu, A. , Imai, Y. , & Sawasaki, T. (2014). Pctaire1/Cdk16 promotes skeletal myogenesis by inducing myoblast migration and fusion. FEBS Letters, 588(17), 3030–3037. 10.1016/J.FEBSLET.2014.05.060 [DOI] [PubMed] [Google Scholar]
- Shin, C. , & Manley, J. L. (2004). Cell signalling and the control of pre‐mRNA splicing. Nature Reviews Molecular Cell Biology, 5(9), 727–738. 10.1038/nrm1467 [DOI] [PubMed] [Google Scholar]
- Sicinski, P. , Donaher, J. L. , Parker, S. B. , Li, T. , Fazeli, A. , Gardner, H. , Haslam, S. Z. , Bronson, R. T. , Elledge, S. J. , & Weinberg, R. A. (1995). Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell, 82(4), 621–630. 10.1016/0092-8674(95)90034-9 [DOI] [PubMed] [Google Scholar]
- Simmons Kovacs, L. A. , Orlando, D. A. , & Haase, S. B. (2008). Transcription networks and cyclin/CDKs: The yin and yang of cell cycle oscillators. Cell Cycle, 7(17), 2626–2629. 10.4161/CC.7.17.6515 [DOI] [PubMed] [Google Scholar]
- Simon, M. , Seraphin, B. , & Faye, G. (1986). KIN28, a yeast split gene coding for a putative protein kinase homologous to CDC28. The EMBO Journal, 5(10), 2697. 10.1002/J.1460-2075.1986.TB04553.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone, C. , Bagella, L. , Bellan, C. , & Giordano, A. (2002). Physical interaction between pRb and cdk9/cyclinT2 complex. Oncogene, 21(26), 4158–4165. 10.1038/sj.onc.1205511 [DOI] [PubMed] [Google Scholar]
- Skaar, D. A. , & Greenleaf, A. L. (2002). The RNA polymerase II CTD kinase CTDK‐I affects pre‐mRNA 3′ cleavage/polyadenylation through the processing component Pti1p. Molecular Cell, 10(6), 1429–1439. 10.1016/S1097-2765(02)00731-1 [DOI] [PubMed] [Google Scholar]
- Sledge, G. W. , Toi, M. , Neven, P. , Sohn, J. , Inoue, K. , Pivot, X. , Burdaeva, O. , Okera, M. , Masuda, N. , Kaufman, P. A. , Koh, H. , Grischke, E. M. , Conte, P. , Lu, Y. , Barriga, S. , Hurt, K. , Frenzel, M. , Johnston, S. , & Llombart‐Cussac, A. (2020). The effect of Abemaciclib plus Fulvestrant on overall survival in hormone receptor‐positive, ERBB2‐negative breast cancer that progressed on endocrine therapy‐MONARCH 2: A randomized clinical trial. JAMA Oncology, 6(1), 116–124. 10.1001/JAMAONCOL.2019.4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snouffer, A. , Brown, D. , Lee, H. , Walsh, J. , Lupu, F. , Norman, R. , Lechtreck, K. , Ko, H. W. , & Eggenschwiler, J. (2017). Cell cycle‐related kinase (CCRK) regulates ciliogenesis and hedgehog signaling in mice. PLoS Genetics, 13(8), e1006912. 10.1371/JOURNAL.PGEN.1006912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi, S. , Mehraj, U. , Qayoom, H. , Aisha, S. , Almilaibary, A. , Alkhanani, M. , & Mir, M. A. (2022). Targeting cyclin‐dependent kinase 1 (CDK1) in cancer: Molecular docking and dynamic simulations of potential CDK1 inhibitors. Medical Oncology, 39(9), 1–15. 10.1007/S12032-022-01748-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopko, R. , Huang, D. , Preston, N. , Chua, G. , Papp, B. , Kafadar, K. , Snyder, M. , Oliver, S. G. , Cyert, M. , Hughes, T. R. , Boone, C. , & Andrews, B. (2006). Mapping pathways and phenotypes by systematic gene overexpression. Molecular Cell, 21(3), 319–330. 10.1016/J.MOLCEL.2005.12.011 [DOI] [PubMed] [Google Scholar]
- Spåhr, H. , Khorosjutina, O. , Baraznenok, V. , Linder, T. , Samuelsen, C. O. , Hermand, D. , Mäkelä, T. P. , Holmberg, S. , & Gustafsson, C. M. (2003). Mediator influences Schizosaccharomyces pombe RNA polymerase II‐dependent transcription in vitro. Journal of Biological Chemistry, 278(51), 51301–51306. 10.1074/jbc.M306750200 [DOI] [PubMed] [Google Scholar]
- Spring, L. M. , Wander, S. A. , Zangardi, M. , & Bardia, A. (2019). CDK 4/6 inhibitors in breast cancer: Current controversies and future directions. Current Oncology Reports, 21(3), 25. 10.1007/s11912-019-0769-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch, K. , & Cordes, N. (2016). The impact of CDK9 on radiosensitivity, DNA damage repair and cell cycling of HNSCC cancer cells. International Journal of Oncology, 48(1), 191–198. 10.3892/IJO.2015.3246 [DOI] [PubMed] [Google Scholar]
- Sukegawa, Y. , Yamashita, A. , & Yamamoto, M. (2011). The fission yeast stress‐responsive MAPK pathway promotes meiosis via the phosphorylation of pol II CTD in response to environmental and feedback cues. PLoS Genetics, 7(12), e1002387. 10.1371/JOURNAL.PGEN.1002387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, K. H. , de Pablo, Y. , Vincent, F. , & Shah, K. (2008). Deregulated Cdk5 promotes oxidative stress and mitochondrial dysfunction. Journal of Neurochemistry, 107(1), 265–278. 10.1111/J.1471-4159.2008.05616.X [DOI] [PubMed] [Google Scholar]
- Sun, T. , Co, N. N. , & Wong, N. (2014). PFTK1 interacts with cyclin Y to activate non‐canonical Wnt signaling in hepatocellular carcinoma. Biochemical and Biophysical Research Communications, 449(1), 163–168. 10.1016/J.BBRC.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Sundar, V. , Vimal, S. , Sai Mithlesh, M. S. , Dutta, A. , Tamizhselvi, R. , & Manickam, V. (2021). Transcriptional cyclin‐dependent kinases as the mediators of inflammation‐a review. Gene, 769, 145200. 10.1016/J.GENE.2020.145200 [DOI] [PubMed] [Google Scholar]
- Surosky, R. T. , Strich, R. , & Esposito, R. E. (1994). The yeast UME5 gene regulates the stability of meiotic mRNAs in response to glucose. Molecular and Cellular Biology, 14(5), 3446–3458. 10.1128/MCB.14.5.3446-3458.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svejstrup, J. Q. (2004). The RNA polymerase II transcription cycle: Cycling through chromatin. Biochimica et Biophysica Acta – Gene Structure and Expression, 1677(1‐3), 64–73. 10.1016/j.bbaexp.2003.10.012 [DOI] [PubMed] [Google Scholar]
- Svejstrup, J. Q. , Vichi, P. , & Egly, J. (1996). The multiple roles of transcription/repair factor TFIIH. Trends in Biochemical Sciences, 21(9), 346–350. 10.1016/S0968-0004(96)10046-3 [DOI] [PubMed] [Google Scholar]
- Szilagyi, Z. , & Gustafsson, C. M. (2013). Emerging roles of Cdk8 in cell cycle control. Biochimica et Biophysica Acta (BBA) – Gene Regulatory Mechanisms, 1829(9), 916–920. 10.1016/J.BBAGRM.2013.04.010 [DOI] [PubMed] [Google Scholar]
- Szilagyi, Z. , Banyai, G. , Lopez, M. D. , McInerny, C. J. , & Gustafsson, C. M. (2012). Cyclin‐dependent kinase 8 regulates mitotic commitment in fission yeast. Molecular and Cellular Biology, 32(11), 2099–2109. 10.1128/MCB.06316-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse, S. , Duckett, D. R. , & Monastyrskyi, A. (2020). The promise and current status of CDK12/13 inhibition for the treatment of cancer. Future Medicinal Chemistry, 13(2), 117–141. 10.4155/FMC-2020-0240 [DOI] [PubMed] [Google Scholar]
- Tanaka, K. , & Okayama, H. (2000). A Pcl‐like cyclin activates the Res2p‐Cdc10p cell cycle “start” transcriptional factor complex in fission yeast. Molecular Biology of the Cell, 11(9), 2845. 10.1091/MBC.11.9.2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, T. , Veeranna, A. , Ohshima, T. , Rajan, P. , Amin, N. D. , Cho, A. , Sreenath, T. , Pant, H. C. , Brady, R. O. , & Kulkarni, A. B. (2001). Neuronal cyclin‐dependent kinase 5 activity is critical for survival. Journal of Neuroscience, 21(2), 550–558. 10.1523/JNEUROSCI.21-02-00550.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, D. , Gururajan, R. , & Kidd, V. J. (1998). Phosphorylation of PITSLRE p110 isoforms accompanies their processing by Caspases during Fas‐mediated cell death. Journal of Biological Chemistry, 273(26), 16601–16607. 10.1074/JBC.273.26.16601 [DOI] [PubMed] [Google Scholar]
- Tang, X. , Guilherme, A. , Chakladar, A. , Powelka, A. M. , Konda, S. , Virbasius, J. V. , Nicoloro, S. M. C. , Straubhaar, J. , & Czech, M. P. (2006). An RNA interference‐based screen identifies MAP4K4/NIK as a negative regulator of PPARγ, adipogenesis, and insulin‐responsive hexose transport. Proceedings of the National Academy of Sciences of the United States of America, 103(7), 2087–2092. 10.1073/PNAS.0507660103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier, M. , Zaborowska, J. , Caizzi, L. , Mohammad, E. , Velychko, T. , Schwalb, B. , Ferrer‐Vicens, I. , Blears, D. , Nojima, T. , Cramer, P. , & Murphy, S. (2020). CDK12 globally stimulates RNA polymerase II transcription elongation and carboxyl‐terminal domain phosphorylation. Nucleic Acids Research, 48(14), 7712–7727. 10.1093/nar/gkaa514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier, M. , Zaborowska, J. , Neve, J. , Nojima, T. , Hester, S. , Fournier, M. , Furger, A. , & Murphy, S. (2022). CDK9 and PP2A regulate RNA polymerase II transcription termination and coupled RNA maturation. EMBO Reports, 23(10), e54520. 10.15252/EMBR.202154520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo, T. , Kasirzadeh, S. , Albrecht, H. , Sykes, M. J. , Yang, Y. , & Wang, S. (2022). An overview of CDK3 in cancer: Clinical significance and pharmacological implications. Pharmacological Research, 180, 106249. 10.1016/J.PHRS.2022.106249 [DOI] [PubMed] [Google Scholar]
- Teves, S. S. , An, L. , Hansen, A. S. , Xie, L. , Darzacq, X. , & Tjian, R. (2016). A dynamic mode of mitotic bookmarking by transcription factors. eLife, 5, e22280. 10.7554/ELIFE.22280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomázy, V. A. , Luthra, R. , Uthman, M. O. , Davies, P. J. A. , & Medeiros, L. J. (2002). Determination of cyclin D1 and CD20 mRNA levels by real‐time quantitative RT‐PCR from archival tissue sections of mantle cell lymphoma and other non‐Hodgkin's lymphomas. The Journal of Molecular Diagnostics, 4(4), 201–208. 10.1016/S1525-1578(10)60704-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, Y. , Wan, H. , & Tan, G. (2012). Cell cycle‐related kinase in carcinogenesis. Oncology Letters, 4(4), 601. 10.3892/OL.2012.828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietjen, J. R. , Zhang, D. W. , Rodríguez‐Molina, J. B. , White, B. E. , Akhtar, M. S. , Heidemann, M. , Li, X. , Chapman, R. D. , Shokat, K. , Keles, S. , Eick, D. , & Ansari, A. Z. (2010). Chemical‐genomic dissection of the CTD code. Nature Structural & Molecular Biology, 17(9), 1154–1161. 10.1038/nsmb.1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers, H. T. M. , & Verrijzer, C. P. (2017). Mitotic chromosomes: Not so silent after all. Developmental Cell, 43(2), 119–121. 10.1016/j.devcel.2017.10.002 [DOI] [PubMed] [Google Scholar]
- Trembley, J. H. , Hu, D. , Hsu, L. C. , Yeung, C. Y. , Slaughter, C. , Lahti, J. M. , & Kidd, V. J. (2002). PITSLRE p110 protein kinases associate with transcription complexes and affect their activity. Journal of Biological Chemistry, 277(4), 2589–2596. 10.1074/jbc.M109755200 [DOI] [PubMed] [Google Scholar]
- Trembley, J. H. , Hu, D. , Slaughter, C. A. , Lahti, J. M. , & Kidd, V. J. (2003). Casein kinase 2 interacts with cyclin‐dependent kinase 11 (CDK11) in vivo and phosphorylates both the RNA polymerase II carboxyl‐terminal domain and CDK11 in vitro. The Journal of Biological Chemistry, 278(4), 2265–2270. 10.1074/JBC.M207518200 [DOI] [PubMed] [Google Scholar]
- Ubersax, J. A. , Woodbury, E. L. , Quang, P. N. , Paraz, M. , Blethrow, J. D. , Shah, K. , Shokat, K. M. , & Morgan, D. O. (2003). Targets of the cyclin‐dependent kinase Cdk1. Nature, 425(6960), 859–864. 10.1038/nature02062 [DOI] [PubMed] [Google Scholar]
- Urbach, A. , & Witte, O. W. (2019). Divide or commit – Revisiting the role of cell cycle regulators in adult hippocampal neurogenesis. Frontiers in Cell and Developmental Biology, 7, 55. 10.3389/FCELL.2019.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valay, J. G. , Simon, M. , Dubois, M. F. , Bensaude, O. , Facca, C. , & Faye, G. (1995). The KIN28 gene is required both for RNA polymerase II mediated transcription and phosphorylation of the Rpb1p CTD. Journal of Molecular Biology, 249(3), 535–544. 10.1006/JMBI.1995.0316 [DOI] [PubMed] [Google Scholar]
- van den Heuvel, S. , & Harlow, E. (1993). Distinct roles for cyclin‐dependent kinases in cell cycle control. Science, 262(5142), 2050–2054. 10.1126/SCIENCE.8266103 [DOI] [PubMed] [Google Scholar]
- van Driessche, B. , Coddens, S. , van Mullem, V. , & Vandenhaute, J. (2005). Glucose deprivation mediates interaction between CTDK‐I and Snf1 in Saccharomyces cerevisiae. FEBS Letters, 579(24), 5318–5324. 10.1016/J.FEBSLET.2005.08.057 [DOI] [PubMed] [Google Scholar]
- Vanden Bush, T. J. , & Bishop, G. A. (2011). CDK‐mediated regulation of cell functions via c‐Jun phosphorylation and AP‐1 activation. PLoS One, 6(4), e19468. 10.1371/JOURNAL.PONE.0019468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasef, M. A. , Brynes, R. K. , Sturm, M. , Bromley, C. , & Robinson, R. A. (1999). Expression of cyclin D1 in parathyroid carcinomas, adenomas, and hyperplasias: A paraffin immunohistochemical study. Modern Pathology, 12(4), 412–416. [PubMed] [Google Scholar]
- Viladevall, L. , St. Amour, C. V. , Rosebrock, A. , Schneider, S. , Zhang, C. , Allen, J. J. , Shokat, K. M. , Schwer, B. , Leatherwood, J. K. , & Fisher, R. P. (2009). TFIIH and P‐TEFb coordinate transcription with capping enzyme recruitment at specific genes in fission yeast. Molecular Cell, 33(6), 738–751. 10.1016/j.molcel.2009.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent, O. , Kuchin, S. , Hong, S.‐P. , Townley, R. , Vyas, V. K. , & Carlson, M. (2001). Interaction of the Srb10 kinase with Sip4, a transcriptional activator of gluconeogenic genes in Saccharomyces cerevisiae . Molecular and Cellular Biology, 21(17), 5790–5796. 10.1128/MCB.21.17.5790-5796.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahi, M. , & Johnson, A. D. (1995). Identification of genes required for α2 repression in Saccharomyces cerevisiae . Genetics, 140(1), 79. 10.1093/GENETICS/140.1.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenfang, M. R. , & Seydoux, G. (2002). Cdk‐7 is required for mRNA transcription and cell cycle progression in Caenorhabditis elegans embryos. Proceedings of the National Academy of Sciences of the United States of America, 99(8), 5527. 10.1073/PNAS.082618399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Zhang, T. , Kwiatkowski, N. , Abraham, B. J. , Lee, T. I. , Xie, S. , Yuzugullu, H. , Von, T. , Li, H. , Lin, Z. , Stover, D. G. , Lim, E. , Wang, Z. C. , Iglehart, J. D. , Young, R. A. , Gray, N. S. , & Zhao, J. J. (2015). CDK7‐dependent transcriptional addiction in triple‐negative breast cancer. Cell, 163(1), 174–186. 10.1016/j.cell.2015.08.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Zong, H. , Chi, Y. , Hong, Y. , Yang, Y. , Zou, W. , Yun, X. , & Gu, J. (2009). Repression of estrogen receptor alpha by CDK11p58 through promoting its ubiquitin‐proteasome degradation. Journal of Biochemistry, 145(3), 331–343. 10.1093/JB/MVN177 [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Wilson, W. A. , Fujino, M. A. , & Roach, P. J. (2001). Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP‐activated protein kinase, and the cyclin‐dependent kinase Pho85p. Molecular and Cellular Biology, 21(17), 5742–5752. 10.1128/MCB.21.17.5742-5752.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerling, T. , Kuuluvainen, E. , & Maäkelaä, T. P. (2007). Cdk8 is essential for preimplantation mouse development. Molecular and Cellular Biology, 27(17), 6177–6182. 10.1128/MCB.01302-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker, E. W. , van Vugt, M. A. T. M. , Artim, S. A. , Huang, P. H. , Petersen, C. P. , Reinhardt, H. C. , Feng, Y. , Sharp, P. A. , Sonenberg, N. , White, F. M. , & Yaffe, M. B. (2007). 14‐3‐3σ controls mitotic translation to facilitate cytokinesis. Nature, 446(7133), 329–332. 10.1038/nature05584 [DOI] [PubMed] [Google Scholar]
- Wilkinson, S. , Croft, D. R. , O'Prey, J. , Meedendorp, A. , O'Prey, M. , Dufès, C. , & Ryan, K. M. (2011). The cyclin‐dependent kinase PITSLRE/CDK11 is required for successful autophagy. Autophagy, 7(11), 1295–1301. 10.4161/AUTO.7.11.16646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windpassinger, C. , Piard, J. , Bonnard, C. , Alfadhel, M. , Lim, S. , Bisteau, X. , Blouin, S. , Ali, N. B. , Ng, A. Y. J. , Lu, H. , Tohari, S. , Talib, S. Z. A. , van Hul, N. , Caldez, M. J. , Van Maldergem, L. , Yigit, G. , Kayserili, H. , Youssef, S. A. , Coppola, V. , … Kaldis, P. (2017). CDK10 mutations in humans and mice cause severe growth retardation, spine malformations, and developmental delays. American Journal of Human Genetics, 101(3), 391–403. 10.1016/j.ajhg.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters, Z. E. , Hunt, N. C. , Bradburn, M. J. , Royds, J. A. , Turley, H. , Harris, A. L. , & Norbury, C. J. (2001). Subcellular localisation of cyclin B, Cdc2 and p21WAF1/CIP1 in breast cancer: Association with prognosis. European Journal of Cancer, 37, 2405–2412. [DOI] [PubMed] [Google Scholar]
- Winzeler, E. A. , Shoemaker, D. D. , Astromoff, A. , Liang, H. , Anderson, K. , Andre, B. , Bangham, R. , Benito, R. , Boeke, J. D. , Bussey, H. , Chu, A. M. , Connelly, C. , Davis, K. , Dietrich, F. , Dow, S. W. , El Bakkoury, M. , Foury, F. , Friend, S. H. , Gentalen, E. , … Davis, R. W. (1999). Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science, 285(5429), 901–906. 10.1126/SCIENCE.285.5429.901 [DOI] [PubMed] [Google Scholar]
- Wittenberg, C. , & Reed, S. I. (2005). Cell cycle‐dependent transcription in yeast: Promoters, transcription factors, and transcriptomes. Oncogene, 24(17), 2746–2755. 10.1038/sj.onc.1208606 [DOI] [PubMed] [Google Scholar]
- Wohlbold, L. , Larochelle, S. , Liao, J. C. F. , Livshits, G. , Singer, J. , Shokat, K. M. , & Fisher, R. P. (2006). The cyclin‐dependent kinase (CDK) family member PNQALRE/CCRK supports cell proliferation but has no intrinsic CDK‐activating kinase (CAK) activity. Cell Cycle, 5(5), 546–554. 10.4161/CC.5.5.2541 [DOI] [PubMed] [Google Scholar]
- Wong, K. H. , Jin, Y. , & Struhl, K. (2014). TFIIH phosphorylation of the pol II CTD stimulates mediator dissociation from the preinitiation complex and promoter escape. Molecular Cell, 54(4), 601–612. 10.1016/j.molcel.2014.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, A. , & Shilatifard, A. (2006). Bur1/Bur2 and the Ctk complex in yeast: The split personality of mammalian P‐TEFb. Cell Cycle, 5(10), 1066–1068. 10.4161/CC.5.10.2769 [DOI] [PubMed] [Google Scholar]
- Wood, A. , Schneider, J. , Dover, J. , Johnston, M. , & Shilatifard, A. (2005). The Bur1/Bur2 complex is required for histone H2B monoubiquitination by Rad6/Bre1 and histone methylation by COMPASS. Molecular Cell, 20(4), 589–599. 10.1016/j.molcel.2005.09.010 [DOI] [PubMed] [Google Scholar]
- Wood, D. J. , & Endicott, J. A. (2018). Structural insights into the functional diversity of the CDK–cyclin family. Open Biology, 8(9) 180112. 10.1098/rsob.180112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, D. , Zhang, Z. , Chen, X. , Yan, Y. , & Liu, X. (2021). Angel or devil? – CDK8 as the new drug target. European Journal of Medicinal Chemistry, 213, 113043. 10.1016/J.EJMECH.2020.113043 [DOI] [PubMed] [Google Scholar]
- Wu, G. Q. , Xie, D. , Yang, G. F. , Liao, Y. J. , Mai, S. J. , Deng, H. X. , Sze, J. , Guan, X. Y. , Zeng, Y. X. , Lin, M. C. , & Kung, H. F. (2009). Cell cycle‐related kinase supports ovarian carcinoma cell proliferation via regulation of cyclin D1 and is a predictor of outcome in patients with ovarian carcinoma. International Journal of Cancer, 125(11), 2631–2642. 10.1002/IJC.24630 [DOI] [PubMed] [Google Scholar]
- Wu, Z. , Wang, M. , Li, F. , Wang, F. , Jia, J. , Feng, Z. , Huo, X. , Yang, J. , Jin, W. , Sa, R. , Gao, W. , & Yu, L. (2021). CDK13‐mediated cell cycle disorder promotes tumorigenesis of high HMGA2 expression gastric cancer. Frontiers in Molecular Biosciences, 8, 760. 10.3389/FMOLB.2021.707295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, X. , Deng, L. , Zhang, J. , Zhang, X. , Lei, T. , Luan, G. , Yang, C. , Xiao, Z. X. , Li, Q. , & Li, Q. (2014). A distinct expression pattern of cyclin K in mammalian testes suggests a functional role in spermatogenesis. PLoS One, 9(7), e101539. 10.1371/JOURNAL.PONE.0101539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, T. , Hall, H. , Kizer, K. O. , Shibata, Y. , Hall, M. C. , Borchers, C. H. , & Strahl, B. D. (2003). Phosphorylation of RNA polymerase II CTD regulates H3 methylation in yeast. Genes & Development, 17(5), 654–663. 10.1101/GAD.1055503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, B. , Wang, S. , Jiang, N. , & Li, J. J. (2019). Cyclin B1/CDK1‐regulated mitochondrial bioenergetics in cell cycle progression and tumor resistance. Cancer Letters, 443, 56–66. 10.1016/j.canlet.2018.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, J. , Li, Y. , Jiang, K. , Hu, K. , Zhang, S. , Dong, X. , Dai, X. , Liu, L. , Zhang, T. , Yang, K. , Huang, K. , Chen, J. , Shi, S. , Zhang, Y. , Wu, G. , & Xu, S. (2018). CDK16 phosphorylates and degrades p53 to promote radioresistance and predicts prognosis in lung cancer. Theranostics, 8(3), 650–662. 10.7150/THNO.21963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, D. , Li, C. F. , Zhang, X. , Gong, Z. , Chan, C. H. , Lee, S. W. , Jin, G. , Rezaeian, A. H. , Han, F. , Wang, J. , Yang, W. L. , Feng, Z. Z. , Chen, W. , Wu, C. Y. , Wang, Y. J. , Chow, L. P. , Zhu, X. F. , Zeng, Y. X. , & Lin, H. K. (2015). Skp2–MacroH2A1–CDK8 axis orchestrates G2/M transition and tumorigenesis. Nature Communications, 6(1), 1–14. 10.1038/ncomms7641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y. X. , & Manley, J. L. (2004). Pinning down transcription: Regulation of RNA polymerase II activity during the cell cycle. Cell Cycle, 3(4), 430–433. 10.4161/cc.3.4.769 [DOI] [PubMed] [Google Scholar]
- Xu, Y. X. , Hirose, Y. , Zhou, X. Z. , Lu, K. P. , & Manley, J. L. (2003). Pin1 modulates the structure and function of human RNA polymerase II. Genes and Development, 17(22), 2765–2776. 10.1101/gad.1135503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura, M. , Sato, Y. , Takahashi, K. , Sasaki, M. , & Harada, K. (2020). The cyclin‐dependent kinase pathway involving CDK1 is a potential therapeutic target for cholangiocarcinoma. Oncology Reports, 43(1), 306–317. 10.3892/or.2019.7405 [DOI] [PubMed] [Google Scholar]
- Yamochi, T. , Nishimoto, I. , Okuda, T. , & Matsuoka, M. (2001). ik3‐1/Cables is associated with trap and Pctaire2. Biochemical and Biophysical Research Communications, 286(5), 1045–1050. 10.1006/BBRC.2001.5493 [DOI] [PubMed] [Google Scholar]
- Yan, Y. , Tang, Y. D. , & Zheng, C. (2022). When cyclin‐dependent kinases meet viral infections, including SARS‐CoV‐2. Journal of Medical Virology, 94(7), 2962–2968. 10.1002/JMV.27719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi, T. , & Matsuzawa, S. I. (2015). PCTAIRE1/PCTK1/CDK16: A new oncotarget? Cell Cycle, 14(4), 463–464. 10.1080/15384101.2015.1006539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi, T. , Krajewska, M. , Matsuzawa, S. I. , & Reed, J. C. (2014). PCTAIRE1 phosphorylates p27 and regulates mitosis in cancer cells. Cancer Research, 74(20), 5795–5807. 10.1158/0008-5472.CAN-14-0872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Roine, N. , & Mäkelä, T. P. (2013). CCRK depletion inhibits glioblastoma cell proliferation in a cilium‐dependent manner. EMBO Reports, 14(8), 741–747. 10.1038/EMBOR.2013.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. , He, N. , & Zhou, Q. (2008). Brd4 recruits P‐TEFb to chromosomes at late mitosis to promote G 1 gene expression and cell cycle progression. Molecular and Cellular Biology, 28(3), 967–976. 10.1128/MCB.01020-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, S. , & Prelich, G. (2002). Activation of the Bur1‐Bur2 Cyclin‐dependent kinase complex by Cak1. Molecular and Cellular Biology, 22(19), 6750–6758. 10.1128/MCB.22.19.6750-6758.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, S. , Neiman, A. , & Prelich, G. (2000). BUR1 and BUR2 encode a divergent cyclin‐dependent kinase–cyclin complex important for transcription in vivo. Molecular and Cellular Biology, 20(19), 7080–7087. 10.1128/MCB.20.19.7080-7087.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, X. , Zhu, C. , & Harper, J. W. (2001). A premature‐termination mutation in the Mus musculus cyclin‐dependent kinase 3 gene. Proceedings of the National Academy of Sciences of the United States of America, 98(4), 1682–1686. 10.1073/PNAS.98.4.1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama, H. , Gruss, O. J. , Rybina, S. , Caudron, M. , Schelder, M. , Wilm, M. , Mattaj, I. W. , & Karsenti, E. (2008). Cdk11 is a RanGTP‐dependent microtubule stabilization factor that regulates spindle assembly rate. The Journal of Cell Biology, 180(5), 867–875. 10.1083/JCB.200706189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdell, M. L. , Kizer, K. O. , Kisseleva‐Romanova, E. , Fuchs, S. M. , Duro, E. , Strahl, B. D. , & Mellor, J. (2008). Roles for Ctk1 and Spt6 in regulating the different methylation states of histone H3 lysine 36. Molecular and Cellular Biology, 28(16), 4915. 10.1128/MCB.00001-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, D. S. , Zhao, R. , Hsu, E. L. , Cayer, J. , Ye, F. , Guo, Y. , Shyr, Y. , & Cortez, D. (2010). Cyclin‐dependent kinase 9–cyclin K functions in the replication stress response. EMBO Reports, 11(11), 876–882. 10.1038/EMBOR.2010.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Q. , Geng, Y. , & Sicinski, P. (2001). Specific protection against breast cancers by cyclin D1 ablation. Nature, 411(6841), 1017–1021. 10.1038/35082500 [DOI] [PubMed] [Google Scholar]
- Yu, Q. , Sicinska, E. , Geng, Y. , Ahnström, M. , Zagozdzon, A. , Kong, Y. , Gardner, H. , Kiyokawa, H. , Harris, L. N. , Stål, O. , & Sicinski, P. (2006). Requirement for CDK4 kinase function in breast cancer. Cancer Cell, 9(1), 23–32. 10.1016/J.CCR.2005.12.012 [DOI] [PubMed] [Google Scholar]
- Yu, V. P. C. C. , Baskerville, C. , Grünenfelder, B. , & Reed, S. I. (2005). A kinase‐independent function of Cks1 and Cdk1 in regulation of transcription. Molecular Cell, 17(1), 145–151. 10.1016/j.molcel.2004.11.020 [DOI] [PubMed] [Google Scholar]
- Zaborowska, J. , Baumli, S. , Laitem, C. , O'Reilly, D. , Thomas, P. H. , O'Hare, P. , & Murphy, S. (2014). Herpes simplex virus 1 (HSV‐1) ICP22 protein directly interacts with cyclin‐dependent kinase (CDK)9 to inhibit RNA polymerase II transcription elongation. PLoS One, 9(9), e107654. 10.1371/JOURNAL.PONE.0107654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborowska, J. , Egloff, S. , & Murphy, S. (2016). The pol II CTD: New twists in the tail. Nature Structural and Molecular Biology, 23(9), 771–777. 10.1038/nsmb.3285 [DOI] [PubMed] [Google Scholar]
- Zaret, K. S. (2014). Genome reactivation after the silence in mitosis: Recapitulating mechanisms of development? Developmental Cell, 29(2), 132. 10.1016/J.DEVCEL.2014.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Cicero, S. A. , Wang, L. , Romito‐DiGiacomo, R. R. , Yang, Y. , & Herrup, K. (2008). Nuclear localization of Cdk5 is a key determinant in the postmitotic state of neurons. Proceedings of the National Academy of Sciences of the United States of America, 105(25), 8772–8777. 10.1073/PNAS.0711355105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , & Corden, J. L. (1991). Identification of phosphorylation sites in the repetitive carboxyl‐terminal domain of the mouse RNA polymerase II largest subunit. Journal of Biological Chemistry, 266(4), 2290–2296. 10.1016/s0021-9258(18)52242-0 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Krishnamurthy, P. K. , & Johnson, G. V. W. (2002). Cdk5 phosphorylates p53 and regulates its activity. Journal of Neurochemistry, 81(2), 307–313. 10.1046/J.1471-4159.2002.00824.X [DOI] [PubMed] [Google Scholar]
- Zhang, M. , Zhang, L. , Hei, R. , Li, X. , Cai, H. , Wu, X. , Zheng, Q. , & Cai, C. (2021). CDK inhibitors in cancer therapy, an overview of recent development. American Journal of Cancer Research, 11(5), 1913. [PMC free article] [PubMed] [Google Scholar]
- Zhao, L. , Sun, L. H. , Liu, D. M. , He, X. Y. , Tao, B. , Ning, G. , Liu, J. M. , & Zhao, H. Y. (2014). Copy number variation in CCND1 gene is implicated in the pathogenesis of sporadic parathyroid carcinoma. World Journal of Surgery, 38(7), 1730–1737. 10.1007/S00268-014-2455-9 [DOI] [PubMed] [Google Scholar]
- Zheng, D. , Cho, Y. Y. , Lau, A. T. Y. , Zhang, J. , Ma, W. Y. , Bode, A. M. , & Dong, Z. (2008). Cyclin‐dependent kinase 3‐mediated activating transcription factor 1 phosphorylation enhances cell transformation. Cancer Research, 68(18), 7650–7660. 10.1158/0008-5472.CAN-08-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. , Liu, M. , Sun, H. , Feng, Y. , Xu, L. , Chan, A. W. H. , Tong, J. H. , Wong, J. , Chong, C. C. N. , Lai, P. B. S. , Wang, H. K. S. , Tsang, S. W. , Goodwin, T. , Liu, R. , Huang, L. , Chen, Z. , Sung, J. J. Y. , Chow, K. L. , To, K. F. , & Cheng, A. S. L. (2018). Hepatoma‐intrinsic CCRK inhibition diminishes myeloid‐derived suppressor cell immunosuppression and enhances immune‐checkpoint blockade efficacy. Gut, 67(5), 931–944. 10.1136/GUTJNL-2017-314032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J. , Liu, C. , Liu, F. , Wang, Y. , & Zhu, M. (2016). Knockdown of PFTAIRE protein kinase 1 (PFTK1) inhibits proliferation, invasion, and EMT in colon cancer cells. Oncology Research, 24(3), 137. 10.3727/096504016X14611963142218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X. , Wirén, M. , Sinha, I. , Rasmussen, N. N. , Linder, T. , Holmberg, S. , Ekwall, K. , & Gustafsson, C. M. (2006). Genome‐wide occupancy profile of mediator and the Srb8‐11 module reveals interactions with coding regions. Molecular Cell, 22(2), 169–178. 10.1016/j.molcel.2006.03.032 [DOI] [PubMed] [Google Scholar]
- Zuo, L. , Weger, J. , Yang, Q. , Goldstein, A. M. , Tucker, M. A. , Walker, G. J. , Hayward, N. , & Dracopoli, N. C. (1996). Germline mutations in the p16INK4a binding domain of CDK4 in familial melanoma. Nature Genetics, 12(1), 97–99. 10.1038/ng0196-97 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


