Abstract
The activities of HIV‐1 in the central nervous system (CNS) are responsible for a dysregulated neuroinflammatory response and the subsequent development of HIV‐associated neurocognitive disorders (HAND). The use of post‐mortem human brain tissue is pivotal for studying the neuroimmune mechanisms of CNS HIV infection. To date, numerous studies have investigated HIV‐1‐induced neuroinflammation in post‐mortem brain tissue. However, from the commonly investigated studies in this line of research, it is not clear which neuroinflammatory markers are consistently associated with HIV neurocognitive impairment (NCI) and neuropathology (i.e., HIV‐encephalitis, HIVE). Therefore, we conducted a systematic review of the association between neuroinflammation and NCI/HIVE from studies investigating post‐mortem brain tissue. Our aim was to synthesise the published data to date to provide commentary on the most noteworthy markers that are associated with NCI/HIVE. PubMed, Scopus, and Web of Science databases were searched using a search protocol designed specifically for this study. Sixty‐one studies were included that investigated the levels of inflammatory markers based on their gene and protein expression in association with NCI/HIVE. The findings revealed that the (1) transcript expressions of IL‐1β and TNF‐α were consistently associated with NCI/HIVE, whereas CCL2 and IL‐6 were commonly not associated with NCI/HIVE, (2) protein expressions of CD14, CD16, CD68, Iba‐1, IL‐1β and TNF‐α were consistently associated with NCI/HIVE, while CD45, GFAP, HLA‐DR, IL‐1 and IL‐6 were commonly not associated with NCI/HIVE, and (3) gene and protein expressions of CNS IL‐1β and TNF‐α were consistently associated with NCI/HIVE, while IL‐6 was consistently not associated with NCI/HIVE. These markers highlight the commonly investigated markers in this line of research and elucidates the neuroinflammatory mechanisms in the HIV‐1 brain that are involved in the pathophysiology of NCI/HIVE. These markers and related pathways should be investigated for the development of improved diagnostics, prognostics, and therapeutics of HAND.
Keywords: brain tissue, HIV‐associated neurocognitive disorders, markers, neuroinflammation, neuronal damage
Abbreviations
- AAN
American Academy of Neurology
- ACC
anterior cingulate cortex
- AIF‐1
allograft inflammatory factor 1
- AM
amygdala
- ART
antiretroviral therapy
- ARV
antiretroviral
- BG
basal ganglia
- CB
cerebellum
- CCL
C‐C chemokine ligand
- CCR
chemokine receptor
- CD
cluster of differentiation
- CNS
central nervous system
- CSF
cerebrospinal fluid
- CT
computed tomography
- CXCL
chemokine (C‐X‐C motif) ligand
- CXCR
C‐X‐C chemokine receptor type
- DWM
deep white matter
- eGFR
estimated glomerular filtration rate
- ELISA
enzyme‐linked immunosorbent assay
- FC
frontal cortex
- FWM
frontal white matter
- Gal
galectin
- GFAP
glial fibrillary acidic protein
- GP
globus pallidus
- HAART
highly active antiretroviral therapy
- HAD
HIV‐associated dementia
- HAND
HIV‐associated neurocognitive disorders
- HCV
hepatitis C
- HIVE
HIV enecephalitis
- HIVnE
HIV no encephalitis
- HLA‐DR
human leucocyte antigen – DR isotype
- HO
heme oxygenase
- Iba‐1
ionised calcium‐binding adapter molecule
- ICC
immunocytochemistry
- IFN
interferon
- IHC
immunohistochemistry
- IL
interleukin
- JBI
Joanna Briggs Institute
- JNK
c‐Jun N‐terminal kinase
- MAP
microtubule associated protein‐2
- MAPK
mitogen‐activated protein kinase
- MB
midbrain
- MC
motor cortex
- MCs
microglia cells
- MED
medulla
- MFC
midfrontal cortex
- MFG
middle frontal gyrus
- MGCs
multinucleated giant cells
- MHC
major histocompatibility complex
- MIG
monokine induced by gamma interferon
- MIP
macrophage inflammatory protein
- MMP
matrix metalloproteinases
- MMSE
mini‐mental state examination
- MN
microglia nodules
- MND
mild neurocognitive disorder
- MP
mononuclear phagocytes
- MRI
magnetic resonance imaging
- MSK
Memorial Sloan Kettering
- N/A
not available
- NCI
neurocognitive impairement or neurocognitively impaired
- NCN
cognitive/cognitively normal
- ND
neuronal damage
- NE
neuropathological evaluation
- NNTC
National NeuroAIDS Tissue Consortium
- OC
occipital cortex
- OPN
osteopontin
- PAS
periodic acid‐Schiff
- PCC
posterior cingulate cortex
- PCR
polymerase chain reaction
- PET
positron emission tomography
- PI‐3
phosphatidylinositol‐3
- PLWH
people living with HIV
- PN
pons
- PRIMSA
preferred reporting items for systematic reviews and meta‐analyses
- RNA
ribonucleic acid
- S100A8
S100 calcium‐binding protein MRP‐8
- SAPK
stress‐activated protein kinase
- SC
sensory cortex
- SPC
spinal cord
- TC
temporal cortex
- TGF
transforming growth factor
- TIMP
tissue inhibitors of metalloproteinases
- TNFR
tumour necrosis factor receptor
- TRAIL
tumour necrosis factor‐related apoptosis‐inducing ligand
- UK
United Kingdom
- VPR
Viral protein R
- WM
white matter
1. INTRODUCTION
HIV‐1 is well known for its effects on the immune system, but it can also affect the central nervous system (CNS), leading to dysregulated inflammation and induction of neuronal damage and neurocognitive impairment (NCI) in people living with HIV (PLHW), defined as HIV‐associated neurocognitive disorders (HAND). 1 , 2 HIV‐1 is able to invade the CNS by what is known as the trojan horse hypothesis, whereby HIV‐1 is able to cross the blood‐brain barrier through infected mononuclear phagocytes that release viral particles into the brain parenchyma. 3 , 4 , 5 Once within the CNS, the virus primarily infects microglia, the resident macrophages in the CNS. 6 Studies with cell cultures and animals provide compelling evidence that HIV‐1 induces neuronal damage and cell death through direct and indirect mechanisms, leading to the development of HIV‐encephalitis (HIVE) neuropathology and clinical HAND. 7 , 8 , 9
With the introduction of antiretroviral therapy (ART), the most severe form of HAND, HIV‐associated dementia (HAD), has dramatically declined. 2 , 10 , 11 However, mild neurocognitive disorder and asymptomatic neurocognitive impairment have increased and are present in half of the population of PLWH. 2 This persistent development of NCI in the modern ART era has been linked to a chronic inflammatory profile experienced by PLWH, 12 , 13 and neuroinflammation has been proposed as an important element in the pathophysiology of HAND. 14 , 15 Since access to brain tissue in living patients is unobtainable, studies are primarily limited to peripheral blood and cerebrospinal fluid (CSF) to explore the associations between neuroinflammatory markers with HAND in PLWH. In systematic reviews by our group, we have highlighted key immune markers associated with HAND in peripheral blood, that is, soluble cluster of differentiation (sCD14), sCD163, interleukin (IL)‐18 and C‐C motif chemokine ligand (CCL)2 16 and CSF; sCD163, sCD14, Interferon (IFN)‐γ, IL‐1α, IL‐7, IL‐8, soluble tumour necrosis factor‐alpha receptor (sTNFR)‐II and IL‐6. 17 These markers have consistently been associated with neurocognitive impairment in PLWH. Although these studies indicate the potential involvement of neuroinflammatory processes in neurocognitive impairments in PLWH, they do not accurately represent the neuroimmune regulation present in the CNS, particularly the regional expression of inflammatory markers and inflammatory phenotypes of immune cells in the brain (e.g., microglia and astrocytes). More recently, molecular imaging using positron emission tomography (PET) has enabled researchers to perform in vivo imaging of neuroinflammation in cognitively impaired PLWH by evaluating microglia activation with radiotracers specific for increased expression of the translocator protein 18 kDa (TSPO). A review of TSPO PET studies showed that literature on the presence of microglia activation in HIV remains inconclusive. 18 The contradictory results from existing literature may be due to limitations including the limited number of study patients, nonspecific binding of the radiotracers, and the sensitivity of TSPO radiotracers to detect low‐grade neuroinflammation.
Post‐mortem brain tissue is the preferred material for investigating the involvement of CNS inflammation in HIV‐associated NCI and HIVE. HIV‐associated NCI is assessed and categorised through neuropsychological tests, while HIVE is evaluated and defined through post‐mortem brain tissue examination. Numerous studies have investigated inflammation in post‐mortem brain tissue of PLWH to date. 19 , 20 However, it is not clear which are the most commonly investigated markers in this line of research. Furthermore, there is currently no consensus on which of these commonly investigated markers are most consistently associated with NCI or HIVE. Identifying specific neuroinflammatory markers that are associated with NCI/HIVE, as revealed by studies with post‐mortem brain tissue, will enhance our understanding of the neuropathophysiology of HAND. Therefore, this study aims to summarise the current literature on HIV‐1‐induced neuroinflammation, neurocognitive performance, and neuropathology from studies investigating post‐mortem brain tissue.
2. METHODS
2.1. Study design
This is a descriptive and narrative systematic review aimed at summarising the extant literature on the association of neuroinflammatory markers and the presentation of neurocognitive impairment/pathology in studies investigating post‐mortem brain tissue. The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. This study has been approved by the North‐West University Health Research Ethics Committee (NWU‐HREC): NWU‐00090‐23‐S1.
2.2. Eligibility criteria
The eligibility criteria for publications to be included in this study were those that investigated HIV‐positive treatment‐experienced adults (>18 years old, all medication types included and no cut‐off for treatment duration) with neuropsychological/neuropathological and medical assessments. The terms HIVE and HAND are often used interchangeably, however, this remains controversial. 9 For this review, we considered participants to have (1) HIVE when diagnosed using histopathological analysis and (2) clinical NCI when diagnosed using a neuropsychological examination. It is important to note that the pathology of HIV‐associated encephalitis (HIVE) has various forms and classifications 9 , 21 and therefore, these factors were taken into consideration during the inclusion strategy. In order to ensure comparability, studies that included a histopathological diagnosis of HIVE were required to meet the criteria for ‘classic HIVE’, 17 while other types of HIVE such as CD8+ T‐cell encephalitis and HIV leukoencephalopathy were excluded. In this review ‘classic HIVE’ was defined as presence of HIV‐infected cells in the brain, along with the formation of multi‐nucleated giant cells (MNG), microglial nodules (MN), microgliosis, astrogliosis, myelin pallor, and viral proteins such as p. 24. 22 Hence, studies reporting HIVE were expected to report at least one of the criteria associated with ‘classic HIVE’.
For inclusion, all studies had to be investigating neuroinflammation from post‐mortem brain tissue. To ensure comparability across studies, inflammatory protein levels in post‐mortem brain tissue was required to be measured using enzyme‐linked immunosorbent assay (ELISA), immunohistochemistry (IHC), immunocytochemistry (ICC), or western blot. In addition, studies were also included if the gene cytokine expression level was measured using polymerase chain reaction (PCR). Considering that the presence of HIV‐1 in the brain can affect neuroinflammatory processes that are independent of neurocognitive impairment, our objective was to establish a link between dysregulated inflammation and neurocognitive impairment in HIV‐1, rather than solely the presence of the virus. Therefore, studies had to have a control group to be included, which may have been neurocognitively normal (NCN) or HIV non‐encephalitis (HIVnE) serving as negative controls for NCI and HIVE respectively. Exclusionary criteria were fundamental research studies with animals and cell culture models, and literature review studies. Studies investigating only serum and plasma markers were excluded, as these were considered outside the scope of this study. Findings from studies that investigated neuroinflammatory markers but did not report on the association between inflammation and NCI/HIVE were excluded.
2.3. Data sources
We electronically searched for publications in PubMed, Scopus and Web of Science databases based on all studies published until 24/05/2023. Eligible studies included published studies in English only. The search strategy was executed without publication date limitations. The full search criteria for each database are included in Supporting Information S1. The following search terms were applied to PubMed: (HIV [mh] OR HIV [tw] OR Acquired Immunodeficiency Syndrome [mh] OR ‘Acquired Immunodeficiency Syndrome’ [tw] OR AIDS [tw]) AND (HIV associated neurocognitive disorders [mh] OR HAND [tw] OR neurocognitive [tw] OR cogniti* [tw] OR Executive Function [mh] OR executive [tw] OR Memory [mh] OR memory [tw] OR Attention [mh] OR attention [tw] OR Neuropsychological Tests [mh] OR AIDS Dementia Complex [mh]) AND (Cytokines [mh] OR cytokin*[tw] OR Chemokines [mh] OR chemokine [tw] OR Inflammation [mh] OR inflammation [tw] OR Neurogenic Inflammation [mh] OR neuro‐inflammation [tw] OR TNF [tw] OR Interleukins [mh] OR interleukins [tw] OR Microglia [mh] OR microglia [tw] OR Monocytes [mh] OR monocyte* [tw] OR sCD163 [tw] OR sCD14 [tw] OR sCD40 [tw] OR CD68 [tw] OR Neopterin [mh] OR Interferons [mh] OR Ionised calcium binding adaptor molecule 1 [tw] OR IBA1 [tw] OR Glial Fibrillary Acidic Protein [tw] OR S100 Calcium Binding Protein beta Subunit [mh] OR CHI3L1 protein, human [mh]) AND (post‐mortem brain tissue [tw] OR postmortem brain OR Brain [mh] OR Immunohistochemistry [mh])
In addition, we (1) reviewed reference sections of eligible articles and (2) manually searched for relevant publications. This search strategy and the retrieved articles are shown in Figure 1.
FIGURE 1.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only.
2.4. Data selection
All articles were retrieved and loaded onto a single database using a reference manager (EndNote X9, Clarivate). Two authors, MEW and PJWN independently identified studies meeting the inclusion criteria. Where there was a discrepancy in article inclusion/exclusion, this was discussed amongst all authors, and a decision was made regarding its suitability.
2.5. Quality assessment
The quality of the included studies was assessed by MEW. The quality criterion has been adopted from the Joanna Briggs Institute (JBI) critical appraisal tools. Here we have amended the JBI quality questions from the Checklist for Analytical Cross‐Sectional Studies 23 by implementing a Likert‐type scale to provide a quantitative measure of study quality. 16 , 24 , 25 , 26 , 27 For all included studies, we have collated the JBI quality questions which assess those factors, which may significantly affect the findings of the included studies (i.e., level of neuroinflammation and neurocognitive function). These included the questions:
-
(1)
Confounders: Did the study report on potential confounders (e.g., substance misuse, comorbid conditions (e.g., Hepatitis C (HCV)), neurological conditions and psychiatric disorders), a relevant exclusionary criterion and were these controlled for upon statistical analysis?
-
(2)
Study characteristics: Did the study report all key cohort information to contextualise the reported findings (i.e., age of participants at time of death, antemortem CD4+ count/viral load, use and duration of ART use)?
-
(3)
NCI/HIVE diagnosis: Did the study report the antemortem NCI/HAND diagnosis criteria and/or did the study clearly define the criteria for HIVE post‐mortem?
Each question was rated for 0 = no, 1 = partly and 2 = yes. Studies that addressed all of the above questions and had a total rating of 6 were classified as high quality. Studies with a rating between 3 and 5 were considered intermediate‐quality and less than 3 as low quality (Supplementary Table S1).
2.6. Potential confounders
Plasma/CSF viral load, 28 CD4+ count, 29 and the HIV‐1 subtype 30 , 31 can affect immune marker levels and NCI. We, therefore, determined whether these confounders influenced the associations between the neuroinflammatory markers and NCI/HIVE that were reported by studies that were included in this review. First, we stratified studies according to viral load (Supplementary Table S2). We considered viral suppression as <2.6 log (50) copies/ml within blood/CSF as viral suppression 30 , 31 , 32 and >2.6 log (50) copies/ml as non‐viral suppression. All studies reported viral load as a group mean (standard deviation) (Table 1). Second, we also stratified studies according to a mean/median CD4+ count of <200 cells/μL or >200 cells/μL (Supplementary Table S3). Mean values were primarily considered when stratifying studies. However, where the mean values were not available, the median values were used. Last, we wanted to determine if HIV subtype variation may have influenced the association between inflammatory markers and NCI/HIVE in post‐mortem brain tissue of PLWH, considering the fact that the HIV‐1 subtype variation and subtype‐specific viral protein amino acid substitutions can influence the prevalence of HAND, 91 , 92 , 93 , 94 as well as the levels of inflammatory markers. 95 , 96
TABLE 1.
Cohort information of all included studies.
| References | Cohort (n) | Age (years) | CD4 count (cells/μL) | Viral load (Plasma/CSF, log copies/ml) | Treatment status | Neuropsychological evaluation (NE) or neuropathological evaluation (HIVE) | Subtype/geographical location |
|---|---|---|---|---|---|---|---|
| 33 |
People living with HIV (PLWH): 10 Neurocognitive impairment (NCI)/HIV encephalitis (HIVE): 8 Neurocognitive normal (NCN)/HIV non‐encephalitis (HIVnE): 2 HIV‐negative: 0 Female (%): N/A |
PLWH: 40.1 (±6.4) NCI/HIVE: 39.4 (±6.3) NCN/HIVnE: 43 (±6) |
N/A | N/A | N/A | N/A | N/A |
| 34 |
PLWH: 20 NCI/HIVE: 11 NCN/HIVnE: 9 HIV‐negative: 10 Female (%): N/A |
N/A | N/A | N/A | N/A | NE: a history of progressive cognitive and behavioural decline leading to marked impairment of occupational and social functions and usually associated with neurological motor deficit and a serial (at least three) neurological and neuropsychological examinations confirming progressive aggravation over weeks or months | N/A |
| 35 |
PLWH: 36 NCI/HIVE: 8 NCN/HIVnE: 10 HIV‐negative: 5 Female (%): N/A |
N/A | N/A | N/A | N/A | HIVE: Infiltration of macrophages, increase numbers of microglial cells, gliosis, myelin pallor and multinucleated giant cells (MGCs) | Edinburgh, United Kingdom (UK) |
| 36 |
PLWH: 28 NCI/HIVE: 9 NCN/HIVnE: 19 HIV‐negative: 20 Female (%): 15 |
PLWH: 30 (±5) NCI/HIVE: 31.6 (±3.3) NCN/HIVnE: 30 (±5.6) HIV‐negative: 24.8 (±4.6) |
PLWH: 139.4 (±149) NCI/HIVE: 30.65 (±31.32) NCN/HIVnE: 29.74 (±37.5) HIV‐negative: N/A |
All groups >6 | Highly active antiretroviral therapy (HAART) (0%) | N/A | Edinburgh, UK |
| 37 |
PLWH: 45 NCI/HIVE: 27 NCN/HIVnE: 28 HIV‐negative: 9 Female (%): 37 |
PLWH: N/A NCI/HIVE: 33 (22–49) NCN/HIVnE: N/A HIV‐negative: 31 (18–45) |
PLWH: N/A NCI/HIVE: 53 (1–137) NCN/HIVnE: N/A |
All HAART‐treated cases <1.7 | HAART (19%) | HIVE: p24 positivity in any region of the brain | Edinburgh, UK |
| 38 |
PLWH: 27 NCI/HIVE: 15 NCN/HIVnE: 12 HIV‐negative: 10 Female (%): N/A |
N/A | N/A | N/A | Zidovudine therapy, and combination antiretroviral therapy (cART) (14%) | N/A | Edinburgh, UK |
| 39 |
PLWH: 15 NCI/HIVE: 7 NCN/HIVnE: 8 HIV‐negative: 5 Female (%): 16 |
PLWH: 41.2 (±9.3) NCI/HIVE: 45.3 (±9.2) NCN/HIVnE: 36.5 (±6.8) HIV‐negative: 63.4 (±15) |
PLHW: All <300 cells/ul | N/A | ART (0%) |
NE: Memorial Sloan Kettering (MSK) HIVE: p24 positive multinucleated giant cells were observed |
N/A |
| 40 |
PLWH: 17 NCI/HIVE: 15 NCN/HIVnE: 2 HIV‐negative: 0 Female (%): N/A |
PLWH: 45.5 (±7.8) NCI: 44.9 (±8.1) NCN/HIVnE: 49.5 (±0.5) |
N/A | N/A | N/A | NE: MSK | United States of America (USA) |
| 41 |
PLWH: 74 NCI/HIVE: 56 NCN/HIVnE: 18 HIV‐negative: 0 Female (%): 16 |
PLWH: 47 (27–68) NCI: N/A NCN/HIVnE: N/A |
PLWH: 53 (0–577) NCI: N/A NCN/HIVnE: N/A |
PLWH: 4.25 (1.6–5.8) NCI: N/A NCN/HIVnE: N/A |
N/A | NE: Frascati | California, USA |
| 42 |
PLHW: 3 NCI/HIVE: 2 NCN/HIVnE: 1 HIV‐negative: 2 Female (%): N/A |
N/A | N/A | N/A | N/A | NE: American Academy of Neurology (AAN) | N/A |
| 43 |
PLHW: 15 NCI/HIVE: 8 NCN/HIVnE: 7 HIV‐negative: 4 Female (%): 16 |
PLHW: 41.3 (±7.6) NCI/HIVE: 39.87 (±7) NCN/HIVnE: 42.6 (±8) HIV‐negative: 49 (±7.9) |
N/A | N/A | N/A |
NE: Clinical diagnosis based on retrospective chart review at autopsy HIVE: Quantitation of microglial nodules (MN) and MGCs. |
Manhattan, USA |
| 8 |
PLWH: 15 NCI/HIVE: 9 NCN/HIVnE: 6 HIV‐negative: 8 Female (%): N/A |
PLWH: 40.1 (±3.207) NCI/HIVE: 38.5 (±2.5) NCN/HIVnE: 42.5 (±2.7) HIV‐negative: 45.6 (±3.5) |
N/A | N/A | HAART (20%) | HIVE: MSs and/or MGCs | Manhattan, USA |
| 44 |
PLHW: 16 NCI/HIVE: 10 NCN/HIVnE: 6 HIV‐negative: 9 Female (%): 0 |
PLHW: 37.5 (±7.09) NCI/HIVE: 40 (±7) NCN/HIVnE: N/A HIV‐negative: 50 (±12) |
N/A | N/A | N/A | N/A | USA |
| 45 |
PLWH: 32 NCI/HIVE: 10 NCN/HIVnE: 22 HIV‐negative: 0 Female (%): 84 |
PLWH: 45.3 (±3.2 NCI/HIVE: 43 (±2.7) NCN/HIVnE: 46.3 (±2.83) |
PLWH: N/A NCI/HIVE: 24 (18–147) NCN/HIVnE: N/A (4–150) |
PLWH: N/A NCI/HIVE: 6.2 (4.2–6.4) NCN/HIVnE: N/A (1.8–5.8) |
HAART (42%) |
NE: AAN HIVE: The presence of HIV‐p24–positive cells, MNs, astrogliosis, and myelin pallor. |
San Diego, USA |
| 46 |
PLWH: 18 NCI/HIVE: 12 NCN/HIVnE: 6 HIV‐negative: 6 Female (%): N/A |
PLWH: 45.2 (±9.3) NCI/HIVE: 43.4 (±8.6) NCN/HIVnE: 49 (±7.6) HIV‐negative: 50 (±7.6) Female (%): N/A |
N/A | N/A | N/A | N/A | National NeuroAIDS tissue Consortium (NNTC), USA |
| 47 |
PLWH: 11 NCI/HIVE: 7 NCN/HIVnE: 4 HIV‐negative: 8 Female (%): N/A |
N/A | N/A | N/A | N/A | N/A | USA |
| 48 |
PLHW: 13 NCI/HIVE: 8 NCN/HIVnE: 5 HIV‐negative: 0 Female (%): 23 |
PLHW: 44.9 (±4.79) NCI/HIVE: 43 (±5.5) NCN/HIVnE: 40 (±3) |
PLHW: 111.5 (±79.2) NCI/HIVE: 60.5 (±37.1) NCN/HIVnE: 193 (±54) |
PLHW: 4.9 (±0.79) NCI/HIVE: 4.8 (±0.64) NCN/HIVnE: 5.0560 (±1.0534) |
N/A | N/A | San Diego, USA |
| 49 |
PLHW: 83 NCI/HIVE: 25 NCN/HIVnE: 33 Other pathology: 25 HIV‐negative: 0 Female (%): 12 |
PLHW: 45.49 (±9.86) NCI/HIVE: N/A NCN/HIVnE: N/A |
PLHW: 149.3 (±259.89) NCI/HIVE: NCN/HIVnE: 193 |
PLHW: 4.13 (±1.58) NCI/HIVE: NCN/HIVnE: |
Antiretrovirals (ARVs) (36%) |
NE: AAN HIVE: Presence of MNs, astrogliosis, HIV p24‐positive cells, and myelin pallor. |
San Diego, USA |
| 19 |
PLWH: 52 NCI/HIVE: 38 NCN/HIVnE: 14 HIV‐negative: 0 Female (%): 23 |
PLWH: 42.96 (±5.08) NCI: 45.05 (±2.89) NCN/HIVnE: 37.3 (±5.5) |
PLWH: 98.6 (±175.02) NCI: 80.51 (±144.59) NCN/HIVnE: 147.7 (±239.01) |
PLWH: 4.1 (±1.6) NCI: 4.02 (±1.58) NCN/HIVnE: 4.3 (±1.61) |
ART (100%) | NE: AAN | NNTC, USA |
| 50 |
PLWH: 13 NCI/HIVE: 9 NCN/HIVnE: 4 HIV‐negative: 6 Female (%): 20 |
PLWH: 42 (±5.6) NCI/HIVE: 43.7 (±4.9) NCN/HIVnE: 38 (±4.7) HIV‐negative: 48.4 (±15.8) |
N/A | N/A | N/A | N/A | Manhattan, USA |
| 51 |
PLWH: 12 NCI/HIVE: 9 NCN/HIVnE: 3 HIV‐negative: 5 Female (%): 20 |
PLWH: 41.8 (±5.8) NCI/HIVE: 43.8 (±4.9) NCN/HIVnE: 36 (±3.7) HIV‐negative: 47.2 (±14.9) |
N/A | N/A | N/A | N/A | Manhattan, USA |
| 52 |
PLWH: 41 NCI/HIVE: 24 NCN/HIVnE: 17 HIV‐negative: 0 Female (%): 15 |
PLWH: N/A NCI: 41 (N/A) NCN/HIVnE: 41 (N/A) |
N/A | N/A | HAART (0%) | HIVE: Opaque periodic acid‐Schiff (PAS)‐positive HIV macrophages in the brain | N/A |
| 53 |
PLWH: 51 NCI/HIVE: 34 NCN/HIVnE: 16 HIV‐negative: 0 Female (%): 9 |
PLWH: 38.6 (±8.6) HIVE/NCI: 37 (±6.89) NCN/HIVnE: 42 (±11) |
N/A | N/A | N/A | NE: AAN and MSK | N/A |
| 54 | N/A |
PLWH: 38 (±5) NCI: 4 (N/A) NCN/HIVnE: 5 (N/A) HIV‐negative: 42 (±4) |
N/A | N/A | N/A |
NE: MSK HIVE: Identification of macrophage infiltration and/or MGCs |
USA |
| 55 |
PLWH: 30 NCI/HIVE: 20 NCN/HIVnE: 10 HIV‐negative: 10 Female (%): N/A |
N/A | N/A | N/A | N/A | N/A | USA |
| 56 |
PLWH: 12 NCI/HIVE: 6 NCN/HIVnE: 6 HIV‐negative: 6 Female (%): 0 |
PLWH: 41.05 (±21.8) NCI/HIVE: 45.3 (±11.8) NCN/HIVnE: 36.8 (±8.6) HIV‐negative: 32 (±9) |
PLWH: 13.35 (±22.91) NCI/HIVE: 22.5 (±29.4) NCN/HIVnE: 4.2 (±4.6) HIV‐negative: N/A |
N/A | N/A | NE: Mini‐mental state examination (MMSE) | N/A |
| 57 |
PLWH: 44 NCI/HIVE + neuronal damage (ND): 14 NCI/HIVE + no ND:10 No NCI/HIVE + ND:10 No NCI/HIVE + no ND: 10 Female (%): N/A |
PLWH: (28–54) (N/A) NCI/HIVE + neuronal damage (ND): 41.0 (±1.6) NCI/HIVE + no ND: 41.5 (±2.3) No NCI/HIVE + ND: 39.0 (±3) No NCI/HIVE + no ND: 41.0 (±2.5) |
N/A | N/A | N/A | HIVE: The presence of MGCs, HIV‐infected microglial cells and viral load | San Diego, USA |
| 58 |
PLHW: 80 NCI/HIVE: 60 NCN/HIVnE: 20 HIV‐negative: 0 Female (%): 18 |
PLHW: 46.6 (±9) NCI/HIVE: N/A NCN/HIVnE: N/A |
PLHW: 85 (±110.7) NCI/HIVE: N/A NCN/HIVnE: N/A |
PLHW: 3.9 (±1.4) NCI/HIVE: N/A NCN/HIVnE: N/A |
cART (N/A) | NE: Frascati | California, USA |
| 59 |
PLHW: 175 NCI/HIVE: 135 NCN/HIVnE: 50 HIV‐negative: 0 Female (%): 18.6 |
PLHW: 47.4 (±9.2) NCI/HIVE: N/A NCN/HIVnE: N/A |
PLWH: 122 (±168) NCI/HIVE: N/A NCN/HIVnE: N/A |
PLWH: 4.2 (N/A) NCI/HIVE: N/A NCN/HIVnE: N/A |
cART (N/A) | NE: Frascati | USA |
| 60 |
PLWH: 23 NCI/HIVE: 23 HIV‐negative: 7 Female (%): N/A |
All participants: 21–40 (N/A) 21–40 years | N/A | N/A | N/A | HIVE: The presence of MGCs | San Diego, USA |
| 61 |
PLWH: 17 NCI/HIVE: 9 NC: 8 Female (%): N/A |
PLWH: 41.41 (±3.06) NCI/HIVE: 42.5 (±2.3) NCN/HIVnE: 40.2 (±3.5) |
N/A |
PLWH: 6.45 (±6.45) NCI/HIVE: 5.75 (±4.8) NC: 2.4 (±2.1) |
N/A | HIVE: The presence of HIV in the brain, activated microglia, MGCs, astrogliosis and myelin pallor. | San Diego, USA |
| 62 |
PLWH: 20 NCI/HIVE: 8 NCN/HIVnE: 12 HIV‐negative: 0 Female (%): 10 |
PLWH: 38.2 (±3.35) NCI: 39.4 (±4.3) NCN/HIVnE: 37.6 (±2.5) |
PLWH: 71.96 (±44.69) NCI: 47.6 (±27.3) NCN/HIVnE: 88.2 (±47.5) |
N/A | N/A | NE: AAN | N/A |
| 63 |
PLWH: 7 NCI/HIVE: 4 NCN/HIVnE: 3 HIV‐negative: 5 Female (%): N/A |
PLWH: 25.84 (±12.3) NCI: 24.75 (±20.4) NCN/HIVnE: 27.3 (±15.79) HIV‐negative: N/A |
NCI: All <34 NCN/HIVnE: All <30 |
N/A | N/A | N/A | N/A |
| 64 |
PLWH: 10 NCI/HIVE: 6 NCN/HIVnE: 4 HIV‐negative: 5 Female (%): 20% |
PLWH: 39.8 (±10.2) NCI: 63.4 (±15) NCN/HIVnE: 32.3 (±3.8) HIV‐negative: 44 (±10) |
PLWH: All <300 | N/A | Treatment naïve (100%) | NE: MSK | N/A |
| 65 |
PLWH: 6 NCI/HIVE: 4 NCN/HIVnE: 2 HIV‐negative: 3 Female (%): |
PLWH: 38.16 (±15.15) NCI/HIVE: 30.75 (±10.91) NCN/HIVnE: 53 (±11) HIV‐negative: 61 (±8.16) |
All groups <200 | N/A | N/A | HIVE: Presence of MGCs. | N/A |
| 66 |
PLWH; 14 NCI/HIVE: 9 NCN/HIVnE: 5 HIV‐negative: 5 Female (%): N/A |
N/A | All <200 | N/A | N/A | HIVE: Human Leucocyte antigen – DR isotype (HLA‐DR) microglia, HIV‐1 infection of ramified microglia, formation of MN and infiltrating macrophages and abundant microglia cells (MCs) | N/A |
| 67 |
PLWH: 22 NCI/HIVE: 11 NCN/HIVnE: 11 HIV‐negative: 10 Female (%): 40 |
PLWH: 37.2 (±13.9) NCI/HIVE: 35 (±9) NCN/HIVnE: 39 (±18) HIV‐negative: 48 (±19) |
N/A | N/A | ART (22%) | HIVE: Presence of the characteristic MGCs and HIV gp41. | N/A |
| 68 |
PLWH; 21 NCI/HIVE: 10 NCN/HIVnE: 11 HIV‐negative: 7 Female (%): N/A |
PLWH: 37.23 (±13.9) NCI/HIVE: 33.7 (±7.6) NCN/HIVnE: 40.16 (±17) HIV‐negative: N/A |
N/A | N/A | ART (40%) | HIVE: Presence of the characteristic MGCs and HIV gp41. | N/A |
| 69 |
PLHW: 30 NCI/HIVE: 15 NCN/HIVnE: 13 HIV‐negative: 16 Female (%): N/A |
PLHW: 40 (±11.6) NCI/HIVE: 40 (±12) NCN/HIVnE: 40 (±12) HIV‐negative: 42.9 (±14.4) |
PLHW: 271 (±129.7) NCI/HIVE: 37 (±52) NCN/HIVnE: 108 (±160) HIV‐negative: N/A |
N/A | N/A | HIVE: HIV‐1 seropositivity, (2) history of progressive cognitive and behavioural decline, neurological and/or neuropsychological findings consistent with a decline from the premorbid baseline, and (4) opportunistic processes in the NCN/HIVnES excluded by CT or MRI and cerebrospinal fluid (CSF) analysis. | USA |
| 70 a |
PLWH: 18/12 NCI/HIVE: 5/5 NCN/HIVnE: 13/7 HIV‐negative: 6/5 Female (%): N/A/12% |
PLWH: N/A NCI/HIVE: 43 (37–43) NCN/HIVnE: 52 (43–53.5) HIV‐negative: 48 (48‐ 48) |
PLWH: N/A NCI/HIVE: 40 (21–78) NCN/HIVnE: 85 (75–295) |
PLWH: N/A NCI/HIVE: 5.30 (4.7–5.4) NCN/HIVnE: 2.25 (1.7–2.51) |
N/A | N/A | USA |
| 71 |
PLHW: 7 NCI/HIVE: 4 NCN/HIVnE: 3 HIV‐negative: 4 Female (%): 9 |
PLHW: 42.3 (±5.8) NCI/HIVE:38.8 (±4.7) NCN/HIVnE: 47 (±4.3) HIV‐negative: 46.5 (±8.4) |
N/A | PLWH: 6.55 (±6.6)NCI/HIVE: 6.75 (±6.6)NCN/HIVnE: 4.95 (±5.05) | N/A | HIVE: HLA‐DR microglia, HIV‐1 infection of ramified microglia, formation of MN and infiltrating macrophages and abundant MCs. | Washington DC, USA |
| 72 |
PLWH: 17 NCI/HIVE: 13 NCN/HIVnE: 4 HIV‐negative: 2 Female (%): NA |
N/A | N/A | N/A | N/A |
NE: AAN and MSK HIVE: Presences of MGCs and gp41 |
UK |
| 73 |
PLWH: 17 NCI/HIVE: 9 NCN/HIVnE: 8 HIV‐negative: 5 Female (%): NA |
N/A | N/A | N/A | N/A |
NE: AAN and MSK HIVE: Presence of MGCs |
UK |
| 74 |
PLWH: 8 NCI/HIVE: 5 NCN/HIVnE: 3 HIV‐negative: 5 Female (%): 7 |
PLWH: 40.4 (±5) NCI/HIVE: 39.6 (±2.3) NCN/HIVnE: 41.6 (±7.6) HIV‐negative: 41.6 (±9.1) |
N/A | N/A | N/A | N/A | USA |
| 75 |
PLWH: 74 NCI/HIVE: 44 NCN/HIVnE: 30 HIV‐negative: 35 Female (%): 7 |
PLWH: 15–66 years NCI/HIVE: N/A NCN/HIVnE: N/A HIV‐negative: N/A |
N/A | N/A | N/A | N/A | Austria |
| 76 |
PLWH: 12 NCI/HIVE: 3 NCN/HIVnE: 4 HIV‐negative: 6 Female (%): 0 |
PLHW: 41 (±3) years | PLWH: 30 (±11) | N/A | N/A |
NE: MMSE and test battery HIVE: Histology combined with gp41 immunoreactivity. |
France |
| 77 |
PLWH: 12 NCI/HIVE: 3 NCN/HIVnE: 4 HIV‐negative: 6 Female (%): 0 |
PLHW: 41 (±3) years | PLWH: 30 (±11) | N/A | N/A |
NE: MMSE HIVE: Astrogliosis; gp41‐positive cells; the severity of cognitive decline measured by the MMSE |
France |
| 78 |
PLHW: 14 NCI/HIVE: 14 NCI other: 5 NCN/HIVnE: N/A HIV‐negative: 15 Female (%): 34 |
PLWH: 41.57 (±8.9) HIV‐negative: 48.6 (±6.9) |
PLWH: 91.78 (±98.38) | PLWH: 5.2 (±5.45) | Antiretroviral (ARV, 100%) | NE: AAN | USA |
| 79 |
PLWH: 13 NCI/HIVE: 6 NCI other: 3 NCN/HIVnE: 4 HIV‐negative: 4 Female (%): N/A |
PLWH: 24–48 (N/A) NCI/HIVE: N/A NCI other: N/A NCN/HIVnE: N/A HIV‐negative: 33–50 (N/A) |
N/A | N/A | N/A | HIVE: Presence of MGCs and MNs | Los‐Angeles, USA |
| 80 |
PLWH: 28 NCI/HIVE: 16 NCN/HIVnE: 12 HIV‐negative: 5 Female (%): N/A |
N/A | N/A | N/A | N/A | N/A | USA |
| 20 |
PLHW: 47 NCI/HIVE: 39 NCN/HIVnE: 8 HIV‐negative: 0 Female (%): 13 |
PLHW: 45.5 (±9.43) NCI: 47.2 (±9.19) NCN/HIVnE: 37.3 (±5.7) |
PLHW: 154.4 (±173.52) NCI: 155.79 (±160.97) NCN/HIVnE: 147.7 (±239.01) |
PLHW: 5.1 (±1.58) NCI: 3.8 (±1.58) NCN/HIVnE: 4.3 (±1.61) |
ART | NE: AAN | San Diego, USA |
| 81 |
PLHW: 18 NCI/HIVE: 8 NCN/HIVnE: 10 HIV‐negative: 5 Female (%): 50 |
PLHW: 44.6 (±8.59) NCI: 45.5 (±3.5) NCN/HIVnE: 44.75 (±12.1) HIV‐negative: 45.8 (±16.9) |
PLHW: 52.17 (±93.1) NCI: 17.25 (±31.07) NCN/HIVnE: 80.1 (±114.39) |
PLHW: 5.8 (±6.1) NCI: 6 (±6.2) NCN/HIVnE: 5.3 (±5.4) |
cART (44%) | NE: AAN | Manhattan, USA |
| 82 |
PLWH: 8 NCI/HIVE: 4 NCN/HIVnE: 4 HIV‐negative: 4 Female (%): 42 |
PLWH: 33 (±16.69) NCI/HIVE: 33 (±17) NCN/HIVnE: 33 (±19) HIV‐negative: 45 (±13) |
N/A | N/A | N/A | N/A | N/A |
| 83 |
PLWH: 10 NCI/HIVE: 5 NCN/HIVnE: 5 HIV‐negative: 0 Female (%): N/A |
PLWH: N/A NCI/HIVE: 35.8 (±3.9) NCN/HIVnE: N/A |
PLWH: N/A NCI/HIVE: 25.4 (±16.4) NCN/HIVnE: N/A |
PLWH: N/A NCI/HIVE: 5.65 (±5.35) NCN/HIVnE: N/A |
AZT treatment (100%) | HIVE: Perivascular infiltration by mononuclear cells | Buenos Aires, Argentina |
| 84 |
PLWH: 24 NCI/HIVE: 10 NCN/HIVnE: 14 HIV‐negative: 9 Female (%): N/A |
PLWH: 39.6 (±13.4) NCI: 39.8 (±13) NCN/HIVnE: 39.9 (±15) HIV‐negative: 42 (±11) |
PLWH: 56.75 (±37.2) NCI: 55 (±57) NCN/HIVnE: 58 (±14) |
N/A | N/A | NE: MSK | N/A |
| 85 | N/A | N/A | N/A | N/A | N/A | HIVE: HIV‐1 gp41 antigen immunoreactivity in brain sections that showed typical neuropathological alterations | USA |
| 86 |
PLWH: 20 NCI/HIVE: 11 NCN/HIVnE: 9 HIV‐negative: 2 Female (%): N/A |
N/A | N/A | N/A | Treatment naïve (100%) | HIVE: Perivascular infiltration, presence of p24, MGCs, or present along with MNs | Vienna |
| 87 |
PLHW: 12 NCI/HIVE: 6 NCN/HIVnE: 6 HIV‐negative: 6 Female (%): N/A |
N/A | N/A | N/A | N/A | N/A | N/A |
| 88 |
PLWH: 24 NCI/HIVE: 17 NCN/HIVnE: 7 HIV‐negative: 15 Female (%): 31 |
PLWH: 45 (±10) NCI: 45,3 (±8.5) NCN/HIVnE: 47.4 (±9.9) HIV‐negative: 52 (±10) |
PLWH: 112 (±175.2) NCI: 112.1 (±175.2) NCN/HIVnE: 93.7 (±151.8) |
PLWH: 5.4 (±5.5)/4.8 (±5.3) NCI: 5.4 (±5.48)/5 (±5.39) NCN/HIVnE: 5.46 (±5.48)/3.98 (±4.21) |
N/A | NE: Test battery measuring 7 domains | Manhattan, USA |
| 89 |
PLWH: 5 NCI/HIVE: 5 HIV‐negative: 3 Female (%): 29 |
PLWH: 41.4 (±6.7) NCI/HIVE: 41.4 (±6.7) HIV‐negative: 34 (±9.2) |
N/A | N/A | N/A | NE: Retrospective chart review at autopsyHIVE: Quantitation of MN and MGCs | Manhattan, USA |
| 90 |
PLWH: 6 NCI/HIVE: 2 NCN/HIVnE: 4 HIV‐negative: 0 Female (%): 16 |
PLWH: 40.7 (±18.4) NCI/HIVE: 23.5 (±20.5) NCN/HIVnE: 40.7 (±18.4) |
N/A |
PLWH: 5.55 (±5.4) NCI/HIVE: 5.53 (±4.5) NCN/HIVnE: 5.58 (±5.56) |
AZT, DDI (50%) | NE: The American Academy of Neurology | Sydney, Australia |
Abbreviations: AAN, American Academy of Neurology; ARV, Antiretroviral; CSF, cerebrospinal fluid; CT, computed tomography; HAART, Highly active antiretroviral therapy; HIVE, HIV enecephalitis; HIVnE: HIVE no encephalitisHLA‐DR, Human Leucocyte; Antigen – DR isotype, MCs, microglia cells; MGCs, Multinucleated giant cells; MMSE, Mini Mental State Examination; MN, Microglia nodules; MRI, magnetic resonance imaging; MSK, Memorial Sloan Kettering; N/A, Not available; NCI, Neuroongitve impairement or Neurocognitively impaired; NCN: Neurocogntive(ly) normalND: Neuronal damage, NE, Neuropathological evaluation; NNTC, National NeuroAIDS Tissue Consortium; PAS, periodic acid‐Schiff; PLWH, people living with HIV; UK, United Kingdom; USA, United States of America.
Study presents secondary data analysis and primary data analysis and reported as Secondary/primary data within Table 1 (Premeaux et al., 2019).
3. RESULTS
3.1. Study characteristics
Using this criterion and search strategy, 1739 abstracts and titles were screened as indicated in Figure 1. Duplicates (n = 238) were removed, resulting in 1501 studies. Thereafter, abstracts and titles were screened and a total of 1344 studies were removed. A 157 full‐text articles were assessed for eligibility, and 96 articles we excluded. The reasons for exclusion are provided in Figure 1. Using this criterion, a total of 61 studies were included for data extraction. Cohort information was available for n = 59/61 (97%) of studies and across all studies a total sample size of n = 1546 PLWH, n = 949 NCI/HIVE, n = 562 NCN and n = 321 HIV‐negative controls were included. The majority of studies (n = 48, 79%) have reported the age of the study participants, with the mean/median ages ranging from 21 to 56 years old. The majority of the participants were male (Table 1).
3.2. Neuropsychological/neuropathological evaluation
The studies that were included reported the type of antemortem neuropsychological evaluation that was used to classify NCI (n = 17) and/or the post‐mortem neuropathological evaluation used to diagnose HIVE (n = 18). Certain studies included neuropsychological and neuropathological evaluation (n = 9). All studies that included HIVE pathological diagnosis were according the ‘classic HIVE’ criteria. 22 Seventeen studies have not reported the type of neuropsychological evaluation or neuropathological evaluation used to diagnose NCI or HIVE in PLWH (Table 1).
3.3. Quality of assessment of studies
Most of the included studies were rated as intermediate quality (n = 39), with four considered as high quality and 18 as low quality. Only n = 4 studies received a total score of 6 for the overall quality criteria (Supplementary Table S1). The majority of studies did not report all relevant study confounders (n = 49) and key study characteristics (e.g., CD4+ counts and viral loads, n = 54). The majority of studies have reported some type of neuropsychological/neuropathological evaluation for NCI/HIVE diagnosis (n = 43). Based on these findings, recommendations are proposed in the latter part of the review.
3.4. Brain regions investigated
Several brain regions were investigated across all studies which included the adjacent cortex, anterior cingulate cortex (ACC), basal ganglia (BG), brain stem, caudate, caudate nucleus, cerebellum (CB), cerebral white matter (WM), cerebrum, choroid plexus, cingulate gyri, corpus callosum, cortex, dentate nucleus, deep white matter (DWM), entorhinal cortex, frontal cortex (FC), frontal lobe, frontal white matter (FWM), globus pallidus (GP), hippocampus, hypothalamus, insular cortex, internal capsule, medulla (MED), mid frontal cortex (MFC), middle frontal gyrus (MFG), midbrain (MB), neocortex, neostriatum, nucleus basalis of Meynert, occipital cortex (OC), occipital lobe, pallidum, parietal cortex, parietal lobe, pituitary, pons (PN), putamen, right dorsolateral prefrontal cortex, sensory cortex (SC), spinal cord (SPC), subcortical WM, temporal cortex (TC), temporal lobe, thalamus, thoracic and white matter. The majority of studies investigated the FC (n = 24), BG (n = 21), frontal lobe (n = 14), hippocampus (n = 10), CB (n = 5) and PN (n = 5). Three studies have not reported the brain regions that were investigated (Table 2).
TABLE 2.
Studies reporting the association between HIV‐associated neuroinflammation and neurocognitive impairment/HIV encephalitis in PLWH.
| References | Sampling technique | Brain section | Markers | Major findings |
|---|---|---|---|---|
| 33 | Enzyme‐linked immunosorbent assay (ELISA) | Frontal cortex (FC), caudate nucleus, insular cortex, basal ganglia (BG), thalamus, hypothalamus, hippocampus, superior cerebellum (CB), midbrain (MB), pons (PN) and medulla (MED) | Interleukin (IL)‐1, IL‐3, IL‐6, and tumour necrosis factor (TNF)‐α |
|
| 34 | Immunohistochemistry (IHC) | Temporal cortex (TC), BG, and brain stem | Major histocompatibility class II (MHC‐II) antigen, human Leucocyte antigen – DR isotype (HLA‐DR), IL‐1 and TNF‐α |
|
| 35 | (IHC) | Cortex and white matter (WM) | TNF‐α, IL‐1α, IL‐4 and IL‐6 |
|
| 36 | Immunocytochemistry (ICC) | Frontal lobe | Glial fibrillary acidic protein (GFAP) |
|
| 37 | IHC | BG and hippocampus | Cluster of differentiation (CD)68, CD3, CD8, CD20, GFAP and MHC‐II |
|
| 38 | IHC | Frontal, temporal lobes and the thalamus | CD68 |
|
| 39 | IHC and polymerase chain reaction (PCR) | FC | IL‐1β and IL‐10 |
|
| 40 | Western blot | Occipital lobes | Osteopontin (OPN) |
|
| 41 | IHC | Hippocampus, putamen, and internal capsule, FC | HLA‐DR, GFAP, and ionised calcium‐binding adapter molecule (Iba)‐1 |
|
| 42 | In situ hybridisation | FC, hippocampus, and brain stem | C‐C chemokine ligand (CCL)2 |
|
| 43 | Immunostaining, IHC and ICC | Cerebral WM and adjacent cortex and/or BG | CD14, CD45, and CD68 |
|
| 8 | IHC | Frontal lobe | CD45, CD45RA, CRB, CD45RB, CD45RC, CD45RO, CD68, CD3, and CD8 |
|
| 44 | Immunofluorescence | Mid‐frontal cortex (MFC), caudate nucleus, insular cortex, BG, thalamus, hippocampus, CB, MB, PN, MED, and spinal cord (SPC). | CD68 and HLA‐DR |
|
| 45 | PCR and IHC | FC | IL‐1, IL‐6 and TNF‐α |
|
| 46 | IHC | Frontal lobe WM, FC and BG | Iba‐1 |
|
| 47 | PCR and ELISA | FC | Transforming growth factor (TGF)‐β1 and TGF‐β2 |
|
| 48 | PCR microarray | FC | All differential regulated genes due to HIVE |
|
| 49 | IHC | FC | GFAP and Iba‐1 |
|
| 19 | PCR | FC | TNF‐α |
|
| 50 | IHC | N/A | CD14, CD16, and HLA‐DR |
|
| 51 | IHC | N/A | CD68, CD14 and CD16, |
|
| 52 | IHC | Cortex (mostly frontal), BG, and MB or PN | GFAP, and HLA‐DR |
|
| 53 | IHC and PCR | MFG and the BG | HAM‐56 |
|
| 54 | IHC | MFG, parietal cortex and CB | GFAP and HLA‐DR |
|
| 55 | PCR and IHC | FC | IL‐1β and IL‐33 |
|
| 56 | IHC | Frontal lobe and BG | TNF‐α and GFAP |
|
| 57 | ICC | MFC | C‐X‐C motif chemokine ligand (CXCL)12 |
|
| 58 | PCR and IHC | OC | Microtubule‐associated protein‐2 (MAP‐2), HLA‐DR and GFAP. |
|
| 59 | PCR and IHC | Right dorsolateral and MFC. | HLA‐DR, Iba‐1 and GFAP |
|
| 60 | Cytokine labelling | MFC, cortical and subcortical regions | IL‐1β, IL‐2 and TGF‐β1 |
|
| 61 | PCR and IHC | FC | All differentially expressed genes, GFAP and CD45 |
|
| 62 | PCR | Frontal lobe | TNF‐α and IL‐1β |
|
| 63 | PCR | Brain and SPC tissue (cerebrum, CB, and brain stem) | TNF‐α, MIP‐1α and MIP‐1β |
|
| 64 | PCR and IHC | FC | CX3CL1 |
|
| 65 | PCR | Cortex, BG, WM, and CB | TNF‐α, IL‐1β, IL‐6, and CD14 |
|
| 66 | IHC | N/A | MIP‐1α, MIP‐1β, CCL2, and RANTES (CCL5), CD68, HLA‐DR and GFAP |
|
| 67 | IHC | Hippocampus | GFAP and HLA‐DR (LN3) |
|
| 68 | IHC | Hippocampus | CD45RO and CD68 |
|
| 69 | ICC | FC | GFAP, HLA‐DR, CD45 and CD68 |
|
| 70 | PCR and IHC | Frontal lobe, neocortex, WM, and neostriatum | Galectin (Gal)‐9 |
|
| 71 | Immunofluorescence | FC | CD40 and CD68 |
|
| 72 | IHC | WM, BG (GP and the FC | TNF‐α |
|
| 73 | IHC and PCR | (FC) with adjacent deep white matter (DWM) and (BG), including the globus pallidus and frontal region | CXCL12 |
|
| 74 | IHC | FC and BG | Tumour necrosis factor‐related apoptosis‐inducing ligand (TRAIL) |
|
| 75 | PCR | N/A | TNF2 and HLA‐DR3 |
|
| 76 | IHC | MFG, superior temporal and cingulate gyri, hippocampus, occipital cortex, nucleus basalis of Meynert, head of the caudate nucleus, pallidum, WM of the centrum ovale, dentate nucleus, SC at cervical, thoracic and lumbar levels | GFAP |
|
| 77 | IHC |
|
TNF‐α and GFAP |
|
| 78 | ICC | Occipital lobe | OPN, CD68, Iba‐1, and GFAP |
|
| 79 | PCR and IHC | Frontal lobe and subcortical WM | Tumour necrosis factor receptor (TNFR)I, TNFRII and TNF‐α |
|
| 80 | PCR and ELISA | FC and BG (CB, and WM) | IL‐1β, Matrix metalloproteinases (MMP)2 and tissue inhibitors of metalloproteinases (TIMP) |
|
| 20 | PCR | FC | IL‐1β |
|
| 81 | IHC | FWM and BG | CD16, CD163, CD68, HLA‐DR and GFAP |
|
| 82 | IHC | Frontal lobe | GFAP |
|
| 83 | IHC | Entorhinal cortex, hippocampus, subcortical WMFC and BG | GFAP |
|
| 84 | PCR | Cortex, subcortical, and deep WM from the right frontal lobe and GP from the BG | IL‐1β, IL‐6, IFN‐γ, TGF‐ β1, TGF‐ β2, TNF‐α, IL‐2, IL‐4, IL‐10 and monokine induced by gamma interferon (MIG)‐2 |
|
| 85 | ICC | FC | GFAP |
|
| 86 | IHC | Frontal lobe and PN | IL‐1β and TNF‐α |
|
| 87 | PCR | FC and BG | IL‐8 |
|
| 88 | PCR | Caudate and ACC | CCL2, CCR5 and CXCR4 |
|
| 97 | ICC | FC or BG (putamen) | IL‐1 |
|
| 90 | IHC | Frontal lobe, Parietal lobe, temporal lobe occipital lobe, BG, hippocampus, CB, MB, PN, MED, thalamus, corpus callosum, pituitary, Mamlliary bodies, choroid plexus and putamen | CD8, CD68, S100 calcium‐binding protein MRP‐8 (S100A8) |
|
Note: p values were reported in this table for studies that reported the p values.
Abbreviations: ACC, anterior cingulate cortex; AIDS, acquired immunodeficiency syndrome; AM, amygdala; BG, Basal ganglia; CB, cerebellum; CCL, C‐C chemokine ligand; CCR, chemokine receptor; CD, Cluster of differentiation CXCL, Chemokine (C‐X‐C motif) ligand; CXCR, C‐X‐C chemokine receptor type; ELISA, Enzyme‐linked immunosorbent assay; FC, frontal cortex; FWM, frontal white matter; Gal, Galectin; GFAP, Glial fibrillary acidic protein GP, globus pallidus; HAART, Highly active antiretroviral therapy; HAD, HIV‐associated dementia; HIVE, HIVE encephalitis; HIVnE, HIV no encephalitis; HLA‐DR, Human Leucocyte Antigen – DR isotype (HLA‐DR); HO, Heme oxygenase; Iba‐1, ionised calcium‐binding adapter molecule; ICC, Immunocytochemistry; IHC, Immunohistochemistry; IL, Interleukin; MB, midbrain; MC, motor cortex; MED, medulla; MFC, Midfrontal cortex; MHC‐II, Major histocompatibility class II; MIP, macrophage inflammatory protein; MMP, Matrix metalloproteinases; MMSE, mini‐mental state examination; MND, mild neurocognitive disorder; MP, mononuclear phagocytes; mRNA, messenger RNA; NCI, neurocognitive(ly) impaired; NCN, cognitive/cognitively normal; ND, Neuronal damage; OC, occipital cortex; OPN, Osteopontin; PCC, posterior cingulate cortex; PCR, Polymerase chain reaction; PLWH, people living with HIV; PN, pons; RNA, ribonucleic acid; SC, sensory cortex; SPC, spinal cord; TC, temporal cortex; TGF, Transforming growth factor; TIMP, tissue inhibitors of metalloproteinases; TNF, Tumour necrosis factor; TNFR, Tumour necrosis factor receptor; WM, White matter.
3.5. Confounders: Viral load, CD4+ count and HIV‐1 subtype
We explored whether viral load, CD4+ count and HIV‐1 subtype influenced the associations between the level of neuroinflammation and NCI/HIVE as reported by studies investigating post‐mortem brain tissue. First, the majority of the studies (n = 41/61, 67%) have not reported data for antiretroviral therapy (ART) status. Of the n = 20/61 (33%) that have reported ART status, n = 15 studies reported that participants were receiving ART and n = 5 studies reported that participants were treatment naïve. From all selected studies, n = 18/61 (30%) have reported viral loads, with the majority (n = 17/18) reporting a detectable plasma/CSF viral load (>2.6 log copies/ml) in the respective studies. Only one study reported participants with viral suppression (<2.6 log copies/ml). 37 A clear deduction could not be made due to only one study that included participants that were virally suppressed. From the non‐virally suppressed group, the results were not one‐sided as expected. A larger percentage of studies with non‐virally suppressed participants (n = 40/61, 66%) reported that inflammatory markers were significantly higher in NCI/HIVE groups compared to NCN groups (Supplementary Table S2). However, n = 21/61 (34%) of studies reported that certain inflammatory markers levels did not significantly differ between NCI/HIVE versus NCN/HIVnE groups.
Second, n = 26/61 (43%) have reported CD4+ count, and from these studies, all participants had CD4+ counts below 300 cells/μl. Two studies described that their cohorts had <300 cells/μl, however, participants were classified as having AIDS in these studies. Therefore, for this review, we have also stratified these participants as part of the <200 cells/μl (AIDS) group (Supplementary Table S3). When stratifying studies according to CD4+ count (<200 cells/μL (AIDS) or >200 cells/μL (non‐AIDS)), only one study had a mean cohort CD4+ count >200 cells/μL 69 and this study reported that the inflammatory profile was associated with NCI/HIVE. The remaining n = 25/61 (41%) studies investigated cohorts with a mean/median CD4+ count of <200 cells/μL and 88% of these studies reported that the inflammatory profile was associated with HIVE/CI. Similar to the findings of viral load stratification, a clear deduction could not be made for CD4+ count due to only one study including participants that were stratified as having a mean cohort CD4+ count >200 cells/μl.
Last, none of the studies have sequenced HIV from these cohorts and therefore the HIV‐1 subtypes were not reported. However, geographical locations of these samples were provided for n = 47/61 (72%) of the included studies, with the majority of samples sourced from the United States of America (n = 32/61, 52%), followed by the United Kingdom (n = 6/61, 10%), Australia (n = 1/61, 2%), Vienna (n = 1/61, 2%), Argentina (n = 1/61, 2%), France (n = 2/61, 4%), Austria (n = 1/61, 2%). No commentary could be provided regarding the influence of subtype variation on the association of neuroinflammation and HAND/HIVE as reported in post‐mortem brain tissue.
3.6. Markers investigated
Several immune markers were investigated across the studies which included C‐C chemokine ligand (CCL)2, CCL5, chemokine receptor (CCR)5, cluster of differentiation (CD)3, CD8, CD14, CD16, CD163, CD20, CD40, CD45, CD45RA, CD45RB, CD45RC, CD45RO, CD68, CRB, C‐X‐C motif chemokine ligand (CXCL)1, CXCL12, CXCR4, Galectin (Gal)‐9, Glial fibrillary acidic protein (GFAP), HAM‐56, Human Leucocyte Antigen – DR isotype (HLA‐DR), HLA‐DR3, ionised calcium‐binding adapter molecule (Iba)‐1, Interferon (IFN)‐γ, Interleukin (IL)‐1, IL‐10, IL‐1α, IL‐1β, IL‐2, IL‐3, IL‐33, IL‐4, IL‐6, IL‐8, Matrix metalloproteinases (MMP)2, Microtubule associated protein‐2 (MAP‐2), macrophage inflammatory protein (MIP‐1)α, MIP‐1β, Monokine induced by gamma interferon (MIG)‐2, Osteopontin (OPN), S100 calcium‐binding protein MRP‐8 (S100A8), Transforming growth factor (TGF)‐ β1, TGF‐β2, Tissue inhibitors of metalloproteinases (TIMP), Tumour necrosis factor (TNF)2, TNF‐α, Tumour necrosis factor receptor (TNFR)I, TNFRII and Tumour necrosis factor‐related apoptosis‐inducing ligand (TRAIL). From these markers, GFAP (n = 19/61, 31%), TNF‐α (n = 14/61, 23%), CD68 (n = 12/61, 20%), HLA‐DR (n = 12/61, 20%), IL‐1β (n = 9/61, 15%), IL‐6 (n = 5/61, 8%), and Iba‐1 (n = 5/61, 8%) were the most commonly investigated markers. All of these markers highlighted above were either measured by their RNA gene expression (n = 11/61, 18%), protein expression (n = 37/61, 61%) or a combination of these two approaches (n = 13/61, 21%) (Table 2).
3.7. Markers associated with NCI/HIVE in PLWH
Across all studies, certain markers were associated with NCI and/or HIVE in PLWH. Gene‐specific transcript levels were assessed for their association with NCI/HIVE including the markers Bone marrow stromal cell antigen 2, CCL2, CCL3, CCR5, CX3CL1, CXCL12, CXCR1, CXCR4, CD14, Gal‐9, h2‐microglobulin, HLA‐DR3, IgG heavy constant‐g3, IL‐1, IL‐1β, IL‐2, IL‐4, IL‐6, IL‐8, IL‐10, IL‐33, Major histocompatibility complex (MHC) classes 1A, C, F, MIP‐ 1α, MIP‐ 1β, MIG‐2, TGF‐β1, TGF‐β2, TNF2, TNF‐α, TNFRI and TNFRII (Supplementary Table S4). Several gene transcripts were not associated with CI/HIVE (Supplementary Table S4). However, the gene transcripts that were associated with NCI and/or HIVE included higher expression levels of Bone marrow stromal cell antigen 2, CCL2, CX3CL1, CD14, Gal‐9, h2‐microglobulin, IgG heavy constant‐g3, IL‐1β, IL‐8, IL‐10, Major histocompatibility complex (MHC) classes 1A, C, F, MIP‐ 1α, MIP‐ 1β, TGF‐β1, TGF‐β2, TNF‐α and lower transcript expression levels of IL‐1 and IL‐6 (Supplementary Table S4).
The protein levels that were assessed for their associations with NCI/HIVE included inflammatory markers; CCL2, CD8, CD14, CD16, CD40, CD45, CD45RA, CD45RB, CD45RC, CD45R0, CD68, CX3CL1, CXCL12, Galectin‐9, GFAP, HAM‐56, HLA‐DR, Iba‐1, IL‐1, IL‐1α, IL‐1β, IL‐2, IL‐3, IL‐4, IL‐6, IL‐16, IL‐33, MAP2, MHC‐II, MIP‐ 1α, MIP‐1β, MMP‐2, OPN, RANTES, S‐100A8, TGF‐β1, TGF‐β2, TIMP‐1, TNF‐α, TNFRI. TNFRII and TRAIL (Supplementary Table S5). The inflammatory protein markers that were associated with NCI/HIVE included higher levels of CCL2, CD4, CD14, CD16, CD40, CD45, CD45RB, CD45RO, CD68, CXCL1, GFAP, HAM‐56, HLA‐DR, Iba‐1, IL‐1, IL‐1α, IL‐1β, IL‐6, IL‐16, MAP2, MIP‐ 1α, MIP‐1β, MMP‐2, OPN, RANTES, S‐100A8, TGF‐β1, TGF‐β2, and TRAIL and lower levels of IL‐4 and TIMP‐1, as well as higher and lower levels of TNF‐α (Table 2). Several inflammatory markers were also found not to be associated with NCI/HIVE (Supplementary Table S5).
Certain markers were investigated more often and therefore would inherently have more supporting evidence. Therefore, we considered the frequency a marker was investigated when contextualising the findings. For this review, we applied a criterion for identifying ‘noteworthy’ markers as done previously. 17 , 25 For a marker (gene transcript/protein) to be noteworthy it had to be (1) investigated by three or more (>2) independent studies and (2) >50% of the studies investigating the marker had to report a consistent direction in their association with NCI/HIVE. In other words, if >50% of studies that investigated a particular marker found it to have the same direction in association with CI/HIVE, it was considered a noteworthy marker for future investigation. Transcript levels for CCL2, IL‐1β, IL‐6, and TNF‐α were investigated by > 2 independent studies, therefore meeting our first criteria (Figure 2a). Higher transcript expression levels of IL‐1β (n = 5/6, 83%) and TNF‐α (n = 5/7, 71%) were consistently associated with NCI/HIVE as reported in >50% or more of the studies that investigated these gene transcripts, therefore, meeting criteria two as a noteworthy marker (Figure 2a). Interestingly, CCL2 (n = 2/3, 67%) and IL‐6 (n = 2/3, 67%) were more commonly found not to be associated with NCI/HIVE (Figure 2a).
FIGURE 2.
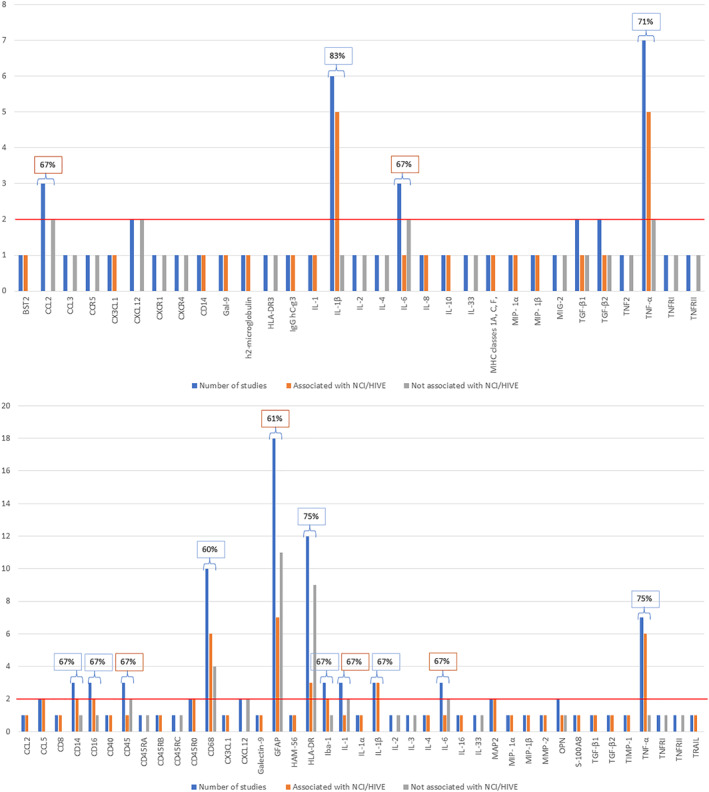
(a and b): The frequency of marker investigation across all studies. The blue bars indicate the number of studies which investigated a particular marker. Markers were considered noteworthy when at >2 independent studies investigated the marker: criteria one (red line cut‐off). The orange bars indicate the number of studies which reported significant associations of a particular marker with NCI/HIVE in PLWH. The grey bars indicate the number of studies which reported no significant associations of a particular marker with NCI/HIVE in PLWH. The percentage indicates the number of studies which report a relationship between a particular marker with NCI/HIVE in PLWH (orange or grey) in relation to the number of studies that investigated the marker (blue). The percentage >50% was indicated in boxes, with blue boxes indicating the association with NCI/HIVE, whereas orange boxes indicate no association with NCI/HIVE (criteria 2). (a) Gene expression markers and (b) Protein expression markers.
From the protein inflammatory markers, CD14, CD16, CD45, CD68, GFAP, HLA‐DR, Iba‐1, IL‐1, IL‐1β, IL‐6 and TNF‐α were investigated by > 2 independent studies, therefore meeting the first criteria for noteworthy (Figure 2b). Higher protein levels of CD14 (n = 2/3, 67%), CD16 (n = 2/3, 67%), CD68 (n = 6/10, 60%), Iba‐1 (n = 2/3, 67%), IL‐1β (n = 3/3, 100%) and TNF‐α (6/7, 85%) were consistently associated with NCI/HIVE as reported in >50% or more of the studies investigating these proteins, therefore, meeting criteria two as a noteworthy marker. From studies investigating TNF‐α, one found lower levels in NCI/HIVE whereas five studies found them to be increased and associated with NCI/HIVE (Supplementary Table S5). Interestingly, CD45 (n = 2/3, 67%), GFAP (n = 11/18, 61%), HLA‐DR (n = 9/12, 75%), IL‐1 (n = 2/3, 67%) and IL‐6 (n = 2/3, 67%) were more commonly found not to be associated with NCI/HIVE (Figure 2b). It is important to note that the inclusion of GFAP and HLA‐DR in a larger number of studies increases the likelihood of reporting non‐significant findings. Conversely, when only three studies focus on a single marker, the probability of discovering significant findings is increased. Therefore, when interpreting the findings presented in this review, it is essential to consider this contextual information.
Collectively, transcripts of IL‐1β and TNF‐α were more consistently associated with NCI/HIVE whereas transcripts of CCL2 and IL‐6 were more commonly not associated with NCI/HIVE. Proteins CD14, CD16, CD68, Iba‐1, IL‐1β and TNF‐α were more consistently associated with NCI/HIVE, whereas proteins CD45, GFAP, HLA‐DR, IL‐1 and IL‐6 were more commonly not associated with NCI/HIVE. Across all studies (gene transcripts and protein), IL‐1β and TNF‐α were associated with NCI/HIVE and IL‐6 was not associated with NCI/HIVE.
4. DISCUSSION
Several findings from studies on human post‐mortem brain tissue are highlighted in this review. These findings include the following: (1) The gene transcripts for IL‐1β and TNF‐α were consistently associated with NCI/HIVE, whereas the gene transcripts for CCL2 and IL‐6 were more consistently not associated with NCI/HIVE. (2) The protein expression levels of CD14, CD16, CD68, Iba‐1, IL‐1β and TNF‐α were more consistently associated with NCI/HIVE, whereas the proteins CD45, GFAP, HLA‐DR, IL‐1 and IL‐6 were more consistently not associated with NCI/HIVE. (3) On both a transcript and protein level, IL‐1β and TNF‐α were consistently associated with NCI/HIVE, whereas IL‐6 was not associated with NCI/HIVE. Based on the findings reported in this review, these markers were considered noteworthy for further investigation into their functions in the pathophysiology of NCI/HIVE, as well as their potential diagnostic, prognostic, and therapeutic potential.
Firstly, the transcript expression of TNF‐α and IL‐1β levels in the brain were consistently associated with the presentation of NCI/HIVE. These findings suggest that transcriptional inflammatory pathways for TNF‐α and IL‐1β may be active in the presence of HIV‐1 within the brain. HIV‐1, particularly its viral proteins, are able to activate several pathways to induce TNF‐α expression. For instance, (1) HIV‐1 gp120‐induced TNF‐α production by primary human macrophages is mediated by phosphatidylinositol‐3 (PI‐3) kinase and mitogen‐activated protein (MAP) kinase pathways, 98 , 99 (2) Viral protein R (Vpr) induced TNF‐α production by astrocytes are produced by Sur1‐Trpm4 channels 100 , 101 and (3) Tat induced mRNA TNF‐α induction is mediated by NF‐κB‐dependent pathways and is also linked to phospholipase C activation in macrophage and astrocytes. 100 Of note, TNF‐α exerts differential effects in the CNS via its TNFR1 and TNFR2. TNFR1 predominantly activates pro‐inflammatory pathways and cell death, whereas activation of TNFR2 leads to neuroprotective signalling pathways. 102 , 103 Thus, future investigations on the balance of TNFR1 and TNFR2 expression levels are needed to elucidate the functional roles of elevated TNF‐α in the pathophysiology of NCI/HIVE.
Similarly, based on the presented findings, it can be suggested that IL‐1β mediated pathways may also be important in the development of HAND. HIV‐1 viral proteins can also activate pathways related to the expression IL‐1β. Examples of these include: (1) HIV‐1 gp120‐induced IL‐1β release by macrophages is mediated through CCR5 and coupling of Giα protein; concomitant activation of Lyn, PI3K and Pyk2; and the co‐ordinately acting of these kinases through the formation of a multi‐kinase signalling complex. 104 (2) Vpr induced IL‐1β production occurs through the activation of p38 and stress‐activated protein kinase (SAPK)/c‐Jun N‐terminal kinase (JNK) in monocyte‐derived macrophages. (3) Tat induced IL‐1β production from human monocytes involves the phospholipase C/protein kinase C signalling cascade. 105 Therefore, targeting signalling pathways linked to the induction of TNF‐α and IL‐1β could potentially reduce the inflammatory burden within the brain and mitigate potential neuronal damage.
Interestingly, we report that the transcript levels of CCL2 and IL‐6 were more consistently found not to be associated with NCI/HIVE. CCL2 is a widely investigated immune chemoattractant, and several studies have indicated that higher peripheral 106 , 107 , 108 and CSF 109 , 110 , 111 , 112 levels were associated with NCI. Studies also reported inconsistent directions for the association of CCL2 with NCI, with higher peripheral and CNS levels 106 , 108 , 109 and lower peripheral and CNS levels 107 , 113 in NCI. Therefore, the exact role of CCL2 in the development of HAND remains elusive. It has been argued the resultant neuronal damage by CCL2 presence is linked to its ability to recruit HIV‐1‐infected monocyte across the blood‐brain barrier. 114 , 115 It has also been suggested that CCL2 may be required for initiation but not the persistence of HIV infection‐mediated neurocognitive disease. A recent preprint study conducted by Kim and Colleagues suggested that CCL2 is required for the development of HAND during systemic EcoHIV infection of mice by promoting monocyte migration during the initial stages of infection, however, once HAND is established CCL2 is dispensable. 115 Findings from this review support the notion that CCL2 levels in the brain may not be directly correlated with the presence of NCI/HIVE, however, may rather function as an initiator of NCI by monocyte recruitment.
Similarly, previous studies investigating peripheral, and CNS IL‐6 have reported inconsistent associations with NCI. 106 , 107 , 116 , 117 In this study, we report that IL‐6 was more consistently not associated with NCI/HIVE. It is important to note that inflammatory factors are influenced by several variables. Specifically, IL‐6 is influenced by older age, nonblack race, higher body mass index, lower serum lipid levels, HIV replication, low nadir CD4⁺ cell count, protease inhibitor use, comorbid conditions, and decreased estimated glomerular filtration rate (eGFR) during HIV‐1 infection. 118 , 119 Considering that participants across all studies were not well matched in terms of clinical variables, this may have contributed to inconsistent associations reported for IL‐6.
Secondly, this review suggests that CD14, CD16, CD68, Iba‐1, IL‐1β and TNF‐α proteins within the brain were consistently associated with NCI/HIVE. Several studies have implicated CD14, CD16, and CD68 as indicators and contributors to the development of HAND. 116 , 120 , 121 , 122 , 123 The findings reported in this review support the notion that monocytes and macrophages play an important function in the mechanistic pathways leading to the development of HAND in the modern ART era. Further, Iba‐1, also known as Allograft inflammatory factor 1 (AIF‐1), is one of the most widely investigated protein markers of microglia activation. Similarly, across all studies reviewed, Iba‐1 was one of the more commonly investigated markers that showed a consistent association with NCI/HIVE. This is in contrast to other commonly investigated markers including GFAP, HLA‐DR, IL‐1 and IL‐6.
GFAP and HLA‐DR were amongst the most commonly investigated markers across all studies reviewed here, however, the majority of studies did not find GFAP and HLA‐DR to be associated with NCI/HIVE. GFAP is the major structural protein of astrocytes, and higher levels of this protein have been implicated in neurodegenerative disorders characterised by astrogliosis including Alzheimer's disease, 124 , 125 multiple sclerosis 126 , 127 and cerebral vasculitis. 128 Considering that one of the neuropathological hallmarks of HIV‐1‐associated dementia (HAD) is the proliferation of astrogliosis, it is expected that a strong association between higher GFAP and NCI/HIVE should exist. However, an earlier study by Sporer and colleagues has shown that CSF GFAP levels and the frequency of increased GFAP levels did not significantly differ between HIV‐infected patients with and without HAD, and, additionally, of HIV‐infected patients with opportunistic CNS diseases. 129 The findings from human post‐mortem brain tissues on GFAP may not reflect the functions of astrocytes in the pathogenesis of NCI/HIVE. Evidence from fundamental research has showed that viral proteins that is, Tat protein, can activate astrocytes, causing glutamate imbalance that causes toxic glutamate signalling in neurons. 130 , 131 The HIV Nef protein on the other hand can downregulate GFAP expression, but still affect glutamate metabolism in astrocytes. 132 It is reasonable to speculate that HIV‐associated proteins influence GFAP expression levels, explaining the non‐significant findings in the reported studies. Together the studies from post‐mortem human brain tissue reviewed here, as well as findings from animal models and cell cultures suggest that GFAP may not be a suitable indicator for the effects of astrocytes in the pathogenesis of NCI/HIVE in PLWH.
Here, we report that HLA‐DR was more commonly not associated with NCI/HIVE. Limited studies have investigated the association between HLA‐DR and NCI/HIVE. Among the limited studies on this topic, one study reported an association between HLA DR‐04 and NCI in participants from China. However, this was performed using HLA typing on blood samples. 133 Another study that evaluated CSF HLA levels did not show an association with NCI. 134 As shown in this review, studies using IHC and/or ICC to investigate brain tissue, more commonly demonstrated no association of HLA‐DR with NCI/HIVE. This difference in findings may be attributed to different techniques and biological specimen types. However, there is a need for further investigation into the role of HLA‐DR in the development of HAND.
Thirdly, we reported that on both a transcript and protein level, IL‐1β and TNF‐α were consistently associated with NCI/HIVE and IL‐6 was not associated with NCI/HIVE. This may suggest that pathways related to the expression of IL‐1β and TNF‐α may be fundamental in the development of NCI/HIVE. To our best knowledge, this is the first study to systematically review neuroinflammation and NCI/HIVE from studies using post‐mortem brain tissue. Therefore, these markers should be investigated to determine their diagnostics, prognostic, and therapeutic potential in addressing the persistent development of HAND in the modern ART era.
5. LIMITATIONS AND RECOMMENDATIONS
Limitations are evident in this review, and accordingly, several recommendations can be made. Firstly, several factors may have influenced the findings that were reported in the studies reviewed here and this made the interpretation of the findings more challenging. These factors included ART status, viral load/CD4+ count, age, gender, neuropsychological and neuropathological assessment, brain region investigated, and the HIV‐1 subtype. First, only n = 15/61 (25%) of the included studies have reported the use of ART, with n = 5/61 (8%) of studies reporting that participants were treatment naïve. The ART status for n = 37/61 (61%) of all included studies was not reported. Therefore, it is not clear what treatment status the included studies and findings present, making it challenging to interpret the findings for the association of neuroinflammation and neurocognitive impairment/HIVE. Furthermore, n = 46/61 (75%) of the studies were conducted prior to the year 2010, and the majority of studies classified as non‐virally suppressed may suggest a greater risk of participants having high levels of viraemia. These factors need to be taken into consideration when contextualising the findings that are reported in this review. Future studies should report the ART treatment status, duration of treatment and the exact treatment regimen, as these may have different CNS penetration effectiveness scores and may influence the viral and neuroinflammatory load and subsequent neuropathology (Letendre et al. 2008).
Secondly, a slightly higher number of studies (n = 26/61, 43%) have reported the mean/median CD4+ count of participants, and the majority of these studies indicating that participants had AIDS (<200 cells/μl). Therefore, this again highlights those participants in the reviewed studies were at an advanced stage of the disease and therefore may inherently have had a greater inflammatory response in the brain. This is supported by previous studies showing associations of higher TNF‐α in blood and CSF 135 , 136 and IL‐1β in CSF 136 with lower CD4+ counts in PLWH.
Thirdly, the participants included in the reviewed studies had an age range of 21‐ to 56‐ years old. However, most studies did not investigate the influence of age on brain inflammation and therefore no conclusions could be drawn. Additionally, the majority of studies investigated male participants, and it is not clear if the reported levels of neuroinflammation in the brain were influenced by sex. It has been established that sex‐related differences can influence inflammatory levels, with men exhibiting higher plasma inflammation compared to women 137 and men showing more associations between CSF inflammatory markers and cognitive performance compared to women with HIV. 119 Consequently, no conclusions could be made regarding age and sex on the reported profiles. Future studies should aim to stratify and analyse neuroinflammatory profiles according to age and gender to fully contextualise the findings. Furthermore, all of the reviewed studies included adult individuals. Future studies should consider investigations using brain tissues of paediatric HIV and HIV‐exposed uninfected children.
Fourthly, we opted to examine the association of inflammatory markers with HIVE and NCI/HAND collectively rather than separately. This decision was influenced by the limited number of studies available per category when stratified. Furthermore, even if studies were stratified per category, it is relevant to note that the different studies employed varied approaches to evaluate neurocognitive impairment and neuropathology. It is known that different criteria for classifying HAND such as MSK, AAN, Frascati criteria, 138 , 139 vary in their strictness. The utilization of different criteria in assessing NCI and HAND across studies may have influenced the reported status of NCI. Recently Nightingale and colleagues highlighted criticisms of the HAND criteria, pointing out that (1) the statistical methods used for cognitive data analysis have the potential for a high false classification rate, (2) cognitive performance is strongly influenced by additional factors (e.g. complex educational, cultural, and socioeconomic factors), which may not necessarily correlate with the pathological state, and (3) cognitive impairment in individuals with HIV is often multifactorial and not solely attributable to the direct effect of HIV on the brain. 140 In this regard, the studies that were included in this review employed different criteria for classifying HIVE neuropathology, ranging from single criterion (the presence of multinucleated giant cells (MGCs)) to multiple criteria (including multinucleated‐cell encephalitis, gliosis, white matter pallor, and vacuolar myelopathy in addition to other pathologies). The reported findings in this review should be interpreted in light of the variations in classification criteria, as these differences may have influenced the outcomes. This further highlights the need that future studies should develop standardized protocols for classifying HAND and HIVE, which would enhance comparability across studies.
Fifthly, the studies included in this review examined different brain regions, all of which may not have been equally vulnerable to HIV‐1‐induced neuroinflammatory effects. Previous investigations of post‐mortem brain tissue have demonstrated distinct patterns of higher type I interferon (IFN)‐stimulated transcript expression in specific brain regions, such as the posterior cingulate cortex, globus pallidus, and cerebellum. 141 Additionally, we observed associations between certain markers and NCI/HIVE in particular brain regions. For example, HLA‐DR and GFAP were found to be increased in the MFG and CB of NCI brains compared to NCN brains. However, no differences were found when comparing these same markers in the PC between the groups. 54
Lastly, it is known that the inflammatory response to HIV‐1 may be influenced by the HIV‐1 subtype, as shown in several fundamental studies. 142 , 143 None of the studies included in this review reported the specific HIV‐1 subtype, although the majority of studies were conducted on participants from the United States of America (n = 32/61, 52%), which is geographically related to subtype B. 144 Therefore, these findings may reflect the inflammatory profile within the brains of participants with subtype B infection, rather than other geographical subtypes. Considering the significant impact that subtype variance has on the underlying mechanisms of HAND, 92 , 145 as well as clinical neurocognitive impairment in PLWH, 94 , 146 , 147 we suggest that viral subtyping should be included as part of routine analysis in human post‐mortem studies of HIV.
6. CONCLUSION
Here, we conducted a systematic review of all studies investigating the relationship between neuroinflammation and NCI/HIVE using post‐mortem brain tissue. The main findings were: (1) In terms of inflammatory transcript expression, IL‐1β and TNF‐α showed a more consisted association with NCI/HIVE, while CCL2 and IL‐6 were more commonly not associated with NCI/HIVE. (2) Protein expression of CD14, CD16, CD68, Iba‐1, IL‐1β and TNF‐α demonstrated a more consistent association with NCI/HIVE, whereas CD45, GFAP, HLA‐DR, IL‐1 and IL‐6 were more commonly not associated with NCI/HIVE. (3) Both at the transcript and protein expression levels within the brain, IL‐1β and TNF‐α were consistently associated with NCI/HIVE, whereas IL‐6 consistently showed no association with NCI/HIVE. These findings highlight the commonly investigated markers in this line of research and emphasise their potential involvement in the development of NCI/HIVE. These markers and associated pathways should be considered for future studies aimed at developing improved therapeutics and diagnostics for NCI/HIVE.
AUTHOR CONTRIBUTIONS
Monray E. Williams and Petrus J.W. Naudé analysed all data and wrote the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS STATEMENT
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
Supporting information
Supporting Information S1
Table S1
Table S2
Table S3
Table S4
Table S5
ACKNOWLEDGEMENTS
MEW was funded by the NRF Thuthuka grant (TTK22031652) and Poliomyelitis Research Foundation (PRF) grant (23/84). PJWN is supported by a Wellcome Trust International Intermediate Fellowship (222020/Z/20/Z).
Williams ME, Naudé PJW. The relationship between HIV‐1 neuroinflammation, neurocognitive impairment and encephalitis pathology: a systematic review of studies investigating post‐mortem brain tissue. Rev Med Virol. 2024;e2519. 10.1002/rmv.2519
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
REFERENCES
- 1. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV‐associated neurocognitive disorders. Neurology. 2007;69(18):1789‐1799. 10.1212/01.WNL.0000287431.88658.8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heaton RK, Clifford DB, Franklin DR, Jr. , et al. HIV‐associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087‐2096. 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. González‐Scarano F, Martín‐García J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69‐81. 10.1038/nri1527 [DOI] [PubMed] [Google Scholar]
- 4. Ivey NS, MacLean AG, Lackner AA. Acquired immunodeficiency syndrome and the blood‐brain barrier. J Neurovirol. 2009;15(2):111‐122. 10.1080/13550280902769764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saylor D, Dickens AM, Sacktor N, et al. HIV‐associated neurocognitive disorder ‐ pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12(5):309. 10.1038/nrneurol.2016.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castellano P, Prevedel L, Eugenin EA. HIV‐infected macrophages and microglia that survive acute infection become viral reservoirs by a mechanism involving Bim. Sci Rep. 2017;7(1):12866. 10.1038/s41598-017-12758-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saylor D, Dickens AM, Sacktor N, et al. HIV‐associated neurocognitive disorder‐‐pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12(4):234‐248. 10.1038/nrneurol.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cosenza‐Nashat MA, Kim MO, Zhao ML, Suh HS, Lee SC. CD45 isoform expression in microglia and inflammatory cells in HIV‐1 encephalitis. Brain Pathol. 2006;16(4):256‐265. 10.1111/j.1750-3639.2006.00027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lucas S Historical and current issues in HIV encephalitis, and the role of neuropathology in HIV disease: a pathological perspective. J Neurol. 2022;270(3):1337‐1345. 10.1007/s00415-022-11503-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Becker JT, Kingsley LA, Molsberry S, et al. Cohort profile: recruitment cohorts in the neuropsychological substudy of the multicenter AIDS cohort study. Int J Epidemiol. 2015;44(5):1506‐1516. 10.1093/ije/dyu092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43(11):2245‐2252. 10.1212/wnl.43.11.2245 [DOI] [PubMed] [Google Scholar]
- 12. Ances BM, Clifford DB. HIV‐associated neurocognitive disorders and the impact of combination antiretroviral therapies. Curr Neurol Neurosci Rep. 2008;8(6):455‐461. 10.1007/s11910-008-0073-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zicari S, Sessa L, Cotugno N, et al. Immune activation, inflammation, and non‐AIDS Co‐morbidities in HIV‐infected patients under long‐term ART. Viruses. 2019;11(3):200. 10.3390/v11030200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hong S, Banks WA. Role of the immune system in HIV‐associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1‐12. 10.1016/j.bbi.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sreeram S, Ye F, Garcia‐Mesa Y, et al. The potential role of HIV‐1 latency in promoting neuroinflammation and HIV‐1‐associated neurocognitive disorder. Trends Immunol. 2022;43(8):630‐639. 10.1016/j.it.2022.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williams ME, Ipser JC, Stein DJ, Joska JA, Naudé PJW. Peripheral immune dysregulation in the ART era of HIV‐associated neurocognitive impairments: a systematic review. Psychoneuroendocrinology. 2020;118:104689. 10.1016/j.psyneuen.2020.104689 [DOI] [PubMed] [Google Scholar]
- 17. Williams ME, Stein DJ, Joska JA, Naudé PJW. Cerebrospinal fluid immune markers and HIV‐associated neurocognitive impairments: a systematic review. J Neuroimmunol. 2021;358:577649. 10.1016/j.jneuroim.2021.577649 [DOI] [PubMed] [Google Scholar]
- 18. Boerwinkle A, Ances BM. Molecular imaging of neuroinflammation in HIV. J Neuroimmune Pharmacol. 2019;14(1):9‐15. 10.1007/s11481-018-9823-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fields JA, Spencer B, Swinton M, et al. Alterations in brain TREM2 and Amyloid‐β levels are associated with neurocognitive impairment in HIV‐infected persons on antiretroviral therapy. J Neurochem. 2018;147(6):784‐802. 10.1111/jnc.14582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Swinton MK, Carson A, Telese F, et al. Mitochondrial biogenesis is altered in HIV+ brains exposed to ART: implications for therapeutic targeting of astroglia. Neurobiol Dis. 2019;130:104502. 10.1016/j.nbd.2019.104502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Everall IP, Hansen LA, Masliah E. The shifting patterns of HIV encephalitis neuropathology. Neurotox Res. 2005;8(1‐2):51‐61. 10.1007/BF03033819 [DOI] [PubMed] [Google Scholar]
- 22. Budka H, Wiley CA, Kleihues P, et al. HIV‐associated disease of the nervous system: review of nomenclature and proposal for neuropathology‐based terminology. Brain Pathol. 1991;1(3):143‐152. 10.1111/j.1750-3639.1991.tb00653.x [DOI] [PubMed] [Google Scholar]
- 23. Moola S, Munn Z, Tufanaru C, et al. Chapter 7: systematic reviews of etiology and risk. Joanna Briggs Inst reviewer’s Man. Joanna Briggs Inst. 2017;5:217‐269. [Google Scholar]
- 24. Williams ME, Naudé PJW, van der Westhuizen FH. Proteomics and metabolomics of HIV‐associated neurocognitive disorders: a systematic review. J Neurochem. 2021;157(3):429‐449. 10.1111/jnc.15295 [DOI] [PubMed] [Google Scholar]
- 25. Asia LK, Jansen Van Vuren E, Williams ME. The influence of viral protein R amino acid substitutions on clinical outcomes in people living with HIV: a systematic review. Eur J Clin Invest;2022:e13943. 10.1111/eci.13943 [DOI] [PubMed] [Google Scholar]
- 26. Joska JA, Gouse H, Paul RH, Stein DJ, Flisher AJ. Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol. 2010;16(2):101‐114. 10.3109/13550281003682513 [DOI] [PubMed] [Google Scholar]
- 27. Likert R. A technique for the measurement of attitudes. Archives Psychol. 1932;22140:55. [Google Scholar]
- 28. Matuzkova A, Pshenichnaya N, Suladze A, Tverdokhlebova T, Dosyagaeva L, Zhuravlev A. Markers of systemic inflammation in HIV‐infected patients with different HIV RNA level. Int J Infect Dis. 2019;79:85. 10.1016/j.ijid.2018.11.214 30579000 [DOI] [Google Scholar]
- 29. Sereti I, Gulick RM, Krishnan S, et al. ART in HIV‐positive persons with low pretreatment viremia: results from the START trial. J Acquir Immune Defic Syndr. 2019;81(4):456‐462. 10.1097/qai.0000000000002052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Biney IJK, Kyei KA, Ganu VJ, et al. Antiretroviral therapy adherence and viral suppression among HIV‐infected adolescents and young adults at a tertiary hospital in Ghana. Afr J AIDS Res. 2021;20(4):270‐276. 10.2989/16085906.2021.1998783 [DOI] [PubMed] [Google Scholar]
- 31. Gabagaya G, Rukundo G, Amone A, et al. Prevalence of undetectable and suppressed viral load in HIV‐infected pregnant women initiating Option B+ in Uganda: an observational study nested within a randomized controlled trial. BMC Infect Dis. 2021;21(1):907. 10.1186/s12879-021-06608-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Euvrard J, Schulz T, Hilderbrand K, et al. How accurately do routinely reported HIV viral load suppression proportions reflect progress towards the 90‐90‐90 target in the population on antiretroviral treatment in Khayelitsha, South Africa? S Afr Med J. 2019;109(3):174‐177. 10.7196/SAMJ.2019.v109i3.13456 [DOI] [PubMed] [Google Scholar]
- 33. Achim CL, Heyes MP, Wiley CA. Quantitation of human immunodeficiency virus, immune activation factors, and quinolinic acid in AIDS brains. J Clin Invest. 1993;91(6):2769‐2775. 10.1172/jci116518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adle‐Biassette H, Chrétien F, Wingertsmann L, et al. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol. 1999;25(2):123‐133. 10.1046/j.1365-2990.1999.00167.x [DOI] [PubMed] [Google Scholar]
- 35. An SF, Ciardi A, Giometto B, Scaravilli T, Gray F, Scaravilli F. Investigation on the expression of major histocompatibility complex class II and cytokines and detection of HIV‐1 DNA within brains of asymptomatic and symptomatic HIV‐1‐positive patients. Acta Neuropathol. 1996;91(5):494‐503. 10.1007/s004010050457 [DOI] [PubMed] [Google Scholar]
- 36. Anderson CE, Tomlinson GS, Pauly B, et al. Relationship of Nef‐positive and GFAP‐reactive astrocytes to drug use in early and late HIV infection. Neuropathol Appl Neurobiol. 2003;29(4):378‐388. 10.1046/j.1365-2990.2003.00475.x [DOI] [PubMed] [Google Scholar]
- 37. Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV‐related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005;64(6):529‐536. 10.1093/jnen/64.6.529 [DOI] [PubMed] [Google Scholar]
- 38. Arango JC, Simmonds P, Brettle RP, Bell JE. Does drug abuse influence the microglial response in AIDS and HIV encephalitis? Aids. 2004;18(Suppl 1):S69‐S74. 10.1097/00002030-200401001-00010 [DOI] [PubMed] [Google Scholar]
- 39. Boven LA, Gomes L, Hery C, et al. Increased peroxynitrite activity in AIDS dementia complex: implications for the neuropathogenesis of HIV‐1 infection. J Immunol. 1999;162(7):4319‐4327. 10.4049/jimmunol.162.7.4319 [DOI] [PubMed] [Google Scholar]
- 40. Brown A, Islam T, Adams R, et al. Osteopontin enhances HIV replication and is increased in the brain and cerebrospinal fluid of HIV‐infected individuals. J Neurovirol. 2011;17(4):382‐392. 10.1007/s13365-011-0035-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bryant AK, Moore DJ, Burdo TH, et al. Plasma soluble CD163 is associated with postmortem brain pathology in human immunodeficiency virus infection. Aids. 2017;31(7):973‐979. 10.1097/qad.0000000000001425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Conant K, Garzino‐Demo A, Nath A, et al. Induction of monocyte chemoattractant protein‐1 in HIV‐1 Tat‐stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A. 1998;95(6):3117‐3121. 10.1073/pnas.95.6.3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cosenza MA, Zhao ML, Si Q, Lee SC. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV‐1 in HIV‐1 encephalitis. Brain Pathol. 2002;12(4):442‐455. 10.1111/j.1750-3639.2002.tb00461.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dallasta LM, Pisarov LA, Esplen JE, et al. Blood‐brain barrier tight junction disruption in human immunodeficiency virus‐1 encephalitis. Am J Pathol. 1999;155(6):1915‐1927. 10.1016/s0002-9440(10)65511-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Desplats P, Dumaop W, Smith D, et al. Molecular and pathologic insights from latent HIV‐1 infection in the human brain. Neurology. 2013;80(15):1415‐1423. 10.1212/WNL.0b013e31828c2e9e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dever SM, Rodriguez M, Lapierre J, Costin BN, El‐Hage N. Differing roles of autophagy in HIV‐associated neurocognitive impairment and encephalitis with implications for morphine co‐exposure. Front Microbiol. 2015;6:653. 10.3389/fmicb.2015.00653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dhar A, Gardner J, Borgmann K, Wu L, Ghorpade A. Novel role of TGF‐beta in differential astrocyte‐TIMP‐1 regulation: implications for HIV‐1‐dementia and neuroinflammation. J Neurosci Res. 2006;83(7):1271‐1280. 10.1002/jnr.20787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Everall I, Salaria S, Roberts E, et al. Methamphetamine stimulates interferon inducible genes in HIV infected brain. J Neuroimmunol. 2005;170(1‐2):158‐171. 10.1016/j.jneuroim.2005.09.009 [DOI] [PubMed] [Google Scholar]
- 49. Fields J, Dumaop W, Rockenstein E, et al. Age‐dependent molecular alterations in the autophagy pathway in HIVE patients and in a gp120 tg mouse model: reversal with beclin‐1 gene transfer. J Neurovirol. 2013;19(1):89‐101. 10.1007/s13365-012-0145-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fischer‐Smith T, Croul S, Sverstiuk AE, et al. CNS invasion by CD14+/CD16+ peripheral blood‐derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7(6):528‐541. 10.1080/135502801753248114 [DOI] [PubMed] [Google Scholar]
- 51. Fischer‐Smith T, Croul S, Adeniyi A, et al. Macrophage/microglial accumulation and proliferating cell nuclear antigen expression in the central nervous system in human immunodeficiency virus encephalopathy. Am J Pathol. 2004;164(6):2089‐2099. 10.1016/s0002-9440(10)63767-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Geiger KD, Stoldt P, Schlote W, Derouiche A. Ezrin immunoreactivity reveals specific astrocyte activation in cerebral HIV. J Neuropathol Exp Neurol. 2006;65(1):87‐96. 10.1097/01.jnen.0000195943.32786.39 [DOI] [PubMed] [Google Scholar]
- 53. Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38(5):755‐762. 10.1002/ana.410380510 [DOI] [PubMed] [Google Scholar]
- 54. Haughey NJ, Cutler RG, Tamara A, et al. Perturbation of sphingolipid metabolism and ceramide production in HIV‐dementia. Ann Neurol. 2004;55(2):257‐267. 10.1002/ana.10828 [DOI] [PubMed] [Google Scholar]
- 55. Kim W, Zekas E, Lodge R, et al. Neuroinflammation‐induced interactions between protease‐activated receptor 1 and proprotein convertases in HIV‐associated neurocognitive disorder. Mol Cell Biol. 2015;35(21):3684‐3700. 10.1128/mcb.00764-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kobayashi K, Seilhean D, Uchihara T, Duyckaerts C, Hauw J.‐J. High densities of tumor necrosis factor‐α in the cerebral cortex and basal ganglia in human immunodeficiency virus‐1‐associated cognitive/motor complex: a quantitative regional analysis study. Neuropathology. 1997;17(3):168‐173. 10.1111/j.1440-1789.1997.tb00033.x [DOI] [Google Scholar]
- 57. Langford D, Sanders VJ, Mallory M, Kaul M, Masliah E. Expression of stromal cell‐derived factor 1alpha protein in HIV encephalitis. J Neuroimmunol. 2002;127(1‐2):115‐126. 10.1016/s0165-5728(02)00068-1 [DOI] [PubMed] [Google Scholar]
- 58. Levine AJ, Soontornniyomkij V, Achim CL, et al. Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J Neurovirol. 2016;22(4):431‐441. 10.1007/s13365-015-0410-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Levine AJ, Soontornniyomkij V, Masliah E, et al. A candidate gene study of intermediate histopathological phenotypes in HIV‐associated neurocognitive disorders. J Neurovirol. 2020;26(4):496‐508. 10.1007/s13365-020-00846-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Masliah E, Ge N, Achim CL, Wiley CA. Cytokine receptor alterations during HIV infection in the human central nervous system. Brain Res. 1994;663:1‐6. 10.1016/0006-8993(94)90456-1 [DOI] [PubMed] [Google Scholar]
- 61. Masliah E, Roberts ES, Langford D, et al. Patterns of gene dysregulation in the frontal cortex of patients with HIV encephalitis. J Neuroimmunol. 2004;157(1‐2):163‐175. 10.1016/j.jneuroim.2004.08.026 [DOI] [PubMed] [Google Scholar]
- 62. Noorbakhsh F, Vergnolle N, McArthur JC, et al. Proteinase‐activated receptor‐2 induction by neuroinflammation prevents neuronal death during HIV infection. J Immunol. 2005;174(11):7320‐7329. 10.4049/jimmunol.174.11.7320 [DOI] [PubMed] [Google Scholar]
- 63. Nuovo GJ, Alfieri ML, Cerami A. AIDS dementia is associated with massive, activated HIV‐1 infection and concomitant expression of several cytokines. Mol Med. 1996;2(3):358‐366. 10.1007/bf03401633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pereira CF, Middel J, Jansen G, Verhoef J, Nottet HS. Enhanced expression of fractalkine in HIV‐1 associated dementia. J Neuroimmunol. 2001;115(1‐2):168‐175. 10.1016/s0165-5728(01)00262-4 [DOI] [PubMed] [Google Scholar]
- 65. Persidsky Y, Buttini M, Limoges J, Bock P, Gendelman HE. An analysis of HIV‐1‐associated inflammatory products in brain tissue of humans and SCID mice with HIV‐1 encephalitis. J Neurovirol. 1997;3(6):401‐416. 10.3109/13550289709031186 [DOI] [PubMed] [Google Scholar]
- 66. Persidsky Y, Ghorpade A, Rasmussen J, et al. Microglial and astrocyte chemokines regulate monocyte migration through the blood‐brain barrier in human immunodeficiency virus‐1 encephalitis. Am J Pathol. 1999;155(5):1599‐1611. 10.1016/s0002-9440(10)65476-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Petito CK, Roberts B, Cantando JD, Rabinstein A, Duncan R. Hippocampal injury and alterations in neuronal chemokine co‐receptor expression in patients with AIDS. J Neuropathol Exp Neurol. 2001;60(4):377‐385. 10.1093/jnen/60.4.377 [DOI] [PubMed] [Google Scholar]
- 68. Petito CK, Adkins B, McCarthy M, Roberts B, Khamis I. CD4+ and CD8+ cells accumulate in the brains of acquired immunodeficiency syndrome patients with human immunodeficiency virus encephalitis. J Neurovirol. 2003;9(1):36‐44. 10.1080/13550280390173391 [DOI] [PubMed] [Google Scholar]
- 69. Power C, Kong PA, Crawford TO, et al. Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood‐brain barrier. Ann Neurol. 1993;34(3):339‐350. 10.1002/ana.410340307 [DOI] [PubMed] [Google Scholar]
- 70. Premeaux TA, D'Antoni ML, Abdel‐Mohsen M, et al. Elevated cerebrospinal fluid Galectin‐9 is associated with central nervous system immune activation and poor cognitive performance in older HIV‐infected individuals. J Neurovirol. 2019;25(2):150‐161. 10.1007/s13365-018-0696-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ramirez SH, Fan S, Dykstra H, et al. Dyad of CD40/CD40 ligand fosters neuroinflammation at the blood‐brain barrier and is regulated via JNK signaling: implications for HIV‐1 encephalitis. J Neurosci. 2010;30(28):9454‐9464. 10.1523/jneurosci.5796-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rostasy K, Monti L, Yiannoutsos C, et al. NFkappaB activation, TNF‐alpha expression, and apoptosis in the AIDS‐Dementia‐Complex. J Neurovirol. 2000;6:537‐543. 10.3109/13550280009091954 [DOI] [PubMed] [Google Scholar]
- 73. Rostasy K, Egles C, Chauhan A, et al. SDF‐1alpha is expressed in astrocytes and neurons in the AIDS dementia complex: an in vivo and in vitro study. J Neuropathol Exp Neurol. 2003;62(6):617‐626. 10.1093/jnen/62.6.617 [DOI] [PubMed] [Google Scholar]
- 74. Ryan LA, Peng H, Erichsen DA, et al. TNF‐related apoptosis‐inducing ligand mediates human neuronal apoptosis: links to HIV‐1‐associated dementia. J Neuroimmunol. 2004;148(1‐2):127‐139. 10.1016/j.jneuroim.2003.11.019 [DOI] [PubMed] [Google Scholar]
- 75. Sato‐Matsumura KC, Berger J, Hainfellner JA, Mazal P, Budka H. Development of HIV encephalitis in AIDS and TNF‐alpha regulatory elements. J Neuroimmunol. 1998;91(1‐2):89‐92. 10.1016/s0165-5728(98)00161-1 [DOI] [PubMed] [Google Scholar]
- 76. Seilhean D, Dzia‐Lepfoundzou A, Sazdovitch V, et al. Astrocytic adhesion molecules are increased in HIV‐1‐associated cognitive/motor complex. Neuropathol Appl Neurobiol. 1997;23(3):83‐92. 10.1046/j.1365-2990.1997.8598085.x [DOI] [PubMed] [Google Scholar]
- 77. Seilhean D, Kobayashi K, He Y, et al. Tumor necrosis factor‐alpha, microglia and astrocytes in AIDS dementia complex. Acta Neuropathol. 1997;93(5):508‐517. 10.1007/s004010050646 [DOI] [PubMed] [Google Scholar]
- 78. Silva K, Hope‐Lucas C, White T, Hairston TK, Rameau T, Brown A. Cortical neurons are a prominent source of the proinflammatory cytokine osteopontin in HIV‐associated neurocognitive disorders. J Neurovirol. 2015;21(2):174‐185. 10.1007/s13365-015-0317-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sippy BD, Hofman FM, Wallach D, Hinton DR. Increased expression of tumor necrosis factor‐alpha receptors in the brains of patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10(5):511‐521. 10.1097/00042560-199510050-00004 [DOI] [PubMed] [Google Scholar]
- 80. Suryadevara R, Holter S, Borgmann K, et al. Regulation of tissue inhibitor of metalloproteinase‐1 by astrocytes: links to HIV‐1 dementia. Glia. 2003;44(1):47‐56. 10.1002/glia.10266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tavazzi E, Morrison D, Sullivan P, Morgello S, Fischer T. Brain inflammation is a common feature of HIV‐infected patients without HIV encephalitis or productive brain infection. Curr HIV Res. 2014;12(2):97‐110. 10.2174/1570162x12666140526114956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Torres‐Muñoz JE, Redondo M, Czeisler C, Roberts B, Tacoronte N, Petito CK. Upregulation of glial clusterin in brains of patients with AIDs. Brain Res. 2001;888(2):297‐301. 10.1016/s0006-8993(00)03052-3 [DOI] [PubMed] [Google Scholar]
- 83. Vanzani MC, Iacono RF, Caccuri RL, Troncoso AR, Berria MI. Regional differences in astrocyte activation in HIV‐associated dementia. Medicina (B Aires). 2006;66:108‐112. [PubMed] [Google Scholar]
- 84. Wesselingh SL, Power C, Glass JD, et al. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol. 1993;33(6):576‐582. 10.1002/ana.410330604 [DOI] [PubMed] [Google Scholar]
- 85. Wyss‐Coray T, Masliah E, Toggas SM, et al. Dysregulation of signal transduction pathways as a potential mechanism of nervous system alterations in HIV‐1 gp120 transgenic mice and humans with HIV‐1 encephalitis. J Clin Invest. 1996;97(3):789‐798. 10.1172/jci118478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xing HQ, Hayakawa H, Gelpi E, Kubota R, Budka H, Izumo S. Reduced expression of excitatory amino acid transporter 2 and diffuse microglial activation in the cerebral cortex in AIDS cases with or without HIV encephalitis. J Neuropathol Exp Neurol. 2009;68:199‐209. 10.1097/NEN.0b013e31819715df [DOI] [PubMed] [Google Scholar]
- 87. Xiong H, Boyle J, Winkelbauer M, et al. Inhibition of long‐term potentiation by interleukin‐8: implications for human immunodeficiency virus‐1‐associated dementia. J Neurosci Res. 2003;71(4):600‐607. 10.1002/jnr.10503 [DOI] [PubMed] [Google Scholar]
- 88. Yuferov V, Ho A, Morgello S, Yang Y, Ott J, Kreek MJ. Expression of ephrin receptors and ligands in postmortem brains of HIV‐infected subjects with and without cognitive impairment. J Neuroimmune Pharmacol. 2013;8(1):333‐344. 10.1007/s11481-012-9429-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhao ML, Si Q, Lee SC. IL‐16 expression in lymphocytes and microglia in HIV‐1 encephalitis. Neuropathol Appl Neurobiol. 2004;30(3):233‐242. 10.1046/j.0305-1846.2003.00527.x [DOI] [PubMed] [Google Scholar]
- 90. Zhou L, Rua R, Ng T, et al. Evidence for predilection of macrophage infiltration patterns in the deeper midline and mesial temporal structures of the brain uniquely in patients with HIV‐associated dementia. BMC Infect Dis. 2009;9(1):192. 10.1186/1471-2334-9-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rao VR, Neogi U, Talboom JS, et al. Clade C HIV‐1 isolates circulating in Southern Africa exhibit a greater frequency of dicysteine motif‐containing Tat variants than those in Southeast Asia and cause increased neurovirulence. Retrovirology. 2013;10(1):61. 10.1186/1742-4690-10-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Santerre M, Wang Y, Arjona S, Allen C, Sawaya BE. Differential contribution of HIV‐1 subtypes B and C to neurological disorders: mechanisms and possible treatments. AIDS Rev. 2019;21(2):76‐83. 10.24875/AIDSRev.19000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Boivin MJ, Ruel TD, Boal HE, et al. HIV‐subtype A is associated with poorer neuropsychological performance compared with subtype D in antiretroviral therapy‐naive Ugandan children. Aids. 2010;24(8):1163‐1170. 10.1097/qad.0b013e3283389dcc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sacktor N, Nakasujja N, Skolasky RL, et al. HIV subtype D is associated with dementia, compared with subtype A, in immunosuppressed individuals at risk of cognitive impairment in Kampala, Uganda. Clin Infect Dis. 2009;49(5):780‐786. 10.1086/605284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ruiz AP, Ajasin DO, Ramasamy S, DesMarais V, Eugenin EA, Prasad VR. A naturally occurring polymorphism in the HIV‐1 Tat basic domain inhibits uptake by bystander cells and leads to reduced neuroinflammation. Sci Rep. 2019;9(1):3308. 10.1038/s41598-019-39531-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Williams ME, Ruhanya V, Paul RH, et al. An investigation of the HIV Tat C31S and R57S mutation on peripheral immune marker levels in South African participants: a pilot study. J Med Virol. 2022;94(7):2936‐2938. 10.1002/jmv.27720 [DOI] [PubMed] [Google Scholar]
- 97. Zhao ML, Kim MO, Morgello S, Lee SC. Expression of inducible nitric oxide synthase, interleukin‐1 and caspase‐1 in HIV‐1 encephalitis. J Neuroimmunol. 2001;115(1‐2):182‐191. 10.1016/s0165-5728(00)00463-x [DOI] [PubMed] [Google Scholar]
- 98. Lee C, Tomkowicz B, Freedman BD, Collman RG. HIV‐1 gp120‐induced TNF‐{alpha} production by primary human macrophages is mediated by phosphatidylinositol‐3 (PI‐3) kinase and mitogen‐activated protein (MAP) kinase pathways. J Leukoc Biol. 2005;78(4):1016‐1023. 10.1189/jlb.0105056 [DOI] [PubMed] [Google Scholar]
- 99. Furler RL, Uittenbogaart CH. Signaling through the P38 and ERK pathways: a common link between HIV replication and the immune response. Immunol Res. 2010;48(1‐3):99‐109. 10.1007/s12026-010-8170-1 [DOI] [PubMed] [Google Scholar]
- 100. Li G, Makar T, Gerzanich V, et al. HIV‐1 Vpr‐induced proinflammatory response and apoptosis are mediated through the Sur1‐Trpm4 channel in astrocytes. mBio. 2020;11(6). 10.1128/mBio.02939-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Williams ME, Williams AA, Naudé PJ. Viral protein R (Vpr)‐induced neuroinflammation and its potential contribution to neuronal dysfunction: a scoping review. BMC Infect Dis. 2023;23(1):512. 10.1186/s12879-023-08495-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Naudé PJ, den Boer JA, Luiten PG, Eisel UL. Tumor necrosis factor receptor cross‐talk. FEBS J. 2011;278(6):888‐898. 10.1111/j.1742-4658.2011.08017.x [DOI] [PubMed] [Google Scholar]
- 103. Yang S, Wang J, Brand DD, Zheng SG. Role of TNF‐TNF receptor 2 signal in regulatory T cells and its therapeutic implications. Front Immunol. 2018;9:784. 10.3389/fimmu.2018.00784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cheung R, Ravyn V, Wang L, Ptasznik A, Collman RG. Signaling mechanism of HIV‐1 gp120 and virion‐induced IL‐1beta release in primary human macrophages. J Immunol. 2008;180(10):6675‐6684. 10.4049/jimmunol.180.10.6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yang Y, Wu J, Lu Y. Mechanism of HIV‐1‐TAT induction of interleukin‐1beta from human monocytes: involvement of the phospholipase C/protein kinase C signaling cascade. J Med Virol. 2010;82(5):735‐746. 10.1002/jmv.21720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3(6):e2516. 10.1371/journal.pone.0002516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cohen RA, de la Monte S, Gongvatana A, et al. Plasma cytokine concentrations associated with HIV/hepatitis C coinfection are related to attention, executive and psychomotor functioning. J Neuroimmunol. 2011;233(1‐2):204‐210. 10.1016/j.jneuroim.2010.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Woods SP, Morgan EE, Marquie‐Beck J, Carey CL, Grant I, Letendre SL. Markers of macrophage activation and axonal injury are associated with prospective memory in HIV‐1 disease. Cogn Behav Neurol. 2006;19(4):217‐221. 10.1097/01.wnn.0000213916.10514.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yuan L, Qiao L, Wei F, et al. Cytokines in CSF correlate with HIV‐associated neurocognitive disorders in the post‐HAART era in China. J Neurovirol. 2013;19(2):144‐149. 10.1007/s13365-013-0150-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gougeon ML, Poirier‐Beaudouin B, Durant J, et al. HMGB1/anti‐HMGB1 antibodies define a molecular signature of early stages of HIV‐Associated Neurocognitive Isorders (HAND). Heliyon. 2017;3(2):e00245. 10.1016/j.heliyon.2017.e00245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Meeker RB, Poulton W, Markovic‐Plese S, Hall C, Robertson K. Protein changes in CSF of HIV‐infected patients: evidence for loss of neuroprotection. J Neurovirol. 2011;17(3):258‐273. 10.1007/s13365-011-0034-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Xing Y, Shepherd N, Lan J, et al. MMPs/TIMPs imbalances in the peripheral blood and cerebrospinal fluid are associated with the pathogenesis of HIV‐1‐associated neurocognitive disorders. Brain Behav Immun. 2017;65:161‐172. 10.1016/j.bbi.2017.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Schrier RD, Hong S, Crescini M, et al. Cerebrospinal fluid (CSF) CD8+ T‐cells that express interferon‐gamma contribute to HIV associated neurocognitive disorders (HAND). PLoS One. 2015;10(2):e0116526. 10.1371/journal.pone.0116526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein‐1 mediates enhanced transmigration of human immunodeficiency virus (HIV)‐infected leukocytes across the blood‐brain barrier: a potential mechanism of HIV‐CNS invasion and NeuroAIDS. J Neurosci. 2006;26(4):1098‐1106. 10.1523/jneurosci.3863-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kim B.‐H, Hadas E, Kelschenbach J, et al. CCL2 is required for initiation but not persistence of HIV infection mediated neurocognitive disease in mice. Research Square. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kamat A, Lyons JL, Misra V, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60(3):234‐243. 10.1097/QAI.0b013e318256f3bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rubin LH, Benning L, Keating SM, et al. Variability in C‐reactive protein is associated with cognitive impairment in women living with and without HIV: a longitudinal study. J Neurovirol. 2018;24(1):41‐51. 10.1007/s13365-017-0590-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Borges Á H, O'Connor JL, Phillips AN, et al. Factors associated with plasma IL‐6 levels during HIV infection. J Infect Dis. 2015;212(4):585‐595. 10.1093/infdis/jiv123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Vecchio AC, Williams DW, Xu Y, et al. Sex‐specific associations between cerebrospinal fluid inflammatory marker levels and cognitive function in antiretroviral treated people living with HIV in rural Uganda. Brain Behav Immun. 2021;93:111‐118. 10.1016/j.bbi.2020.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Williams DW, Byrd D, Rubin LH, Anastos K, Morgello S, Berman JW. CCR2 on CD14+CD16+ monocytes is a biomarker of HIV‐associated neurocognitive disorders. Neurology ‐ Neuroimmunology Neuroinflammation. 2014;1(3):e36. 10.1212/nxi.0000000000000036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Veenstra M, León‐Rivera R, Li M, Gama L, Clements JE, Berman JW. Mechanisms of CNS viral seeding by HIV(+) CD14(+) CD16(+) monocytes: establishment and reseeding of viral reservoirs contributing to HIV‐associated neurocognitive disorders. mBio. 2017;8(5). 10.1128/mBio.01280-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Veenhuis RT, Williams DW, Shirk EN, et al. Higher circulating intermediate monocytes are associated with cognitive function in women with HIV. JCI Insight. 2021:6. 10.1172/jci.insight.146215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Montoya JL, Campbell LM, Paolillo EW, et al. Inflammation relates to poorer complex motor performance among adults living with HIV on suppressive antiretroviral therapy. JAIDS J Acquir Immune Defic Syndromes. 2019;80(1):15‐23. 10.1097/qai.0000000000001881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Crols R, Saerens J, Noppe M, Lowenthal A. Increased GFAp levels in CSF as a marker of organicity in patients with Alzheimer's disease and other types of irreversible chronic organic brain syndrome. J Neurol. 1986;233(3):157‐160. 10.1007/bf00314423 [DOI] [PubMed] [Google Scholar]
- 125. Shir D, Graff‐Radford J, Hofrenning EI, et al. Association of plasma glial fibrillary acidic protein (GFAP) with neuroimaging of Alzheimer's disease and vascular pathology. Alzheimers Dement (Amst). 2022;14(1):e12291. 10.1002/dad2.12291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Petzold A, Eikelenboom MJ, Gveric D, et al. Markers for different glial cell responses in multiple sclerosis: clinical and pathological correlations. Brain. 2002;125(7):1462‐1473. 10.1093/brain/awf165 [DOI] [PubMed] [Google Scholar]
- 127. Ayrignac X, Le Bars E, Duflos C, et al. Serum GFAP in multiple sclerosis: correlation with disease type and MRI markers of disease severity. Sci Rep. 2020;10(1):10923. 10.1038/s41598-020-67934-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nylén K, Karlsson JE, Blomstrand C, Tarkowski A, Trysberg E, Rosengren LE. Cerebrospinal fluid neurofilament and glial fibrillary acidic protein in patients with cerebral vasculitis. J Neurosci Res. 2002;67(6):844‐851. 10.1002/jnr.10180 [DOI] [PubMed] [Google Scholar]
- 129. Sporer B, Missler U, Magerkurth O, Koedel U, Wiesmann M, Pfister HW. Evaluation of CSF glial fibrillary acidic protein (GFAP) as a putative marker for HIV‐associated dementia. Infection. 2004;32(1):20‐23. 10.1007/s15010-004-3048-6 [DOI] [PubMed] [Google Scholar]
- 130. Rao VR, Ruiz AP, Prasad VR. Viral and cellular factors underlying neuropathogenesis in HIV associated neurocognitive disorders (HAND). AIDS Res Ther. 2014;11(1):13. 10.1186/1742-6405-11-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Bozzelli PL, Yin T, Avdoshina V, Mocchetti I, Conant KE, Maguire‐Zeiss KA. HIV‐1 Tat promotes astrocytic release of CCL2 through MMP/PAR‐1 signaling. Glia. 2019;67(9):1719‐1729. 10.1002/glia.23642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wilson KM, He JJ. HIV Nef expression down‐modulated GFAP expression and altered glutamate uptake and release and proliferation in astrocytes. Aging and Disease. 2023;14(1):152‐169. 10.14336/AD.2022.0712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Schrier RD, Gupta S, Riggs P, et al. The influence of HLA on HIV‐associated neurocognitive impairment in Anhui, China. PLoS One. 2012;7(5):e32303. 10.1371/journal.pone.0032303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Neuenburg JK, Cho TA, Nilsson A, et al. T‐cell activation and memory phenotypes in cerebrospinal fluid during HIV infection. JAIDS J Acquir Immune Defic Syndromes. 2005;39(1):16‐22. 10.1097/01.qai.0000155036.03004.a0 [DOI] [PubMed] [Google Scholar]
- 135. Vaidya SA, Korner C, Sirignano MN, et al. Tumor necrosis factor α is associated with viral control and early disease progression in patients with HIV type 1 infection. J Infect Dis. 2014;210(7):1042‐1046. 10.1093/infdis/jiu206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Abassi M, Morawski BM, Nakigozi G, et al. Cerebrospinal fluid biomarkers and HIV‐associated neurocognitive disorders in HIV‐infected individuals in Rakai, Uganda. J Neurovirol. 2017;23(3):369‐375. 10.1007/s13365-016-0505-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Mathad JS, Gupte N, Balagopal A, et al. Sex‐related differences in inflammatory and immune activation markers before and after combined antiretroviral therapy initiation. J Acquir Immune Defic Syndr. 2016;73(2):123‐129. 10.1097/qai.0000000000001095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. De Francesco D, Underwood J, Post FA, et al. Defining cognitive impairment in people‐living‐with‐HIV: the POPPY study. BMC Infect Dis. 2016;16(1):617. 10.1186/s12879-016-1970-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gisslén M, Price RW, Nilsson S. The definition of HIV‐associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect Dis. 2011;11(1):356. 10.1186/1471-2334-11-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Nightingale S, Cinque P, Joska JA, et al. A new approach to cognitive impairment in people with HIV. Lancet HIV. 2022;9(12):e815‐e817. 10.1016/s2352-3018(22)00267-3 [DOI] [PubMed] [Google Scholar]
- 141. Gruenewald AL, Garcia‐Mesa Y, Gill AJ, Garza R, Gelman BB, Kolson DL. Neuroinflammation associates with antioxidant heme oxygenase‐1 response throughout the brain in persons living with HIV. J Neurovirol. 2020;26(6):846‐862. 10.1007/s13365-020-00902-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Gandhi N, Saiyed Z, Thangavel S, Rodriguez J, Rao K, Nair MP. Differential effects of HIV type 1 clade B and clade C Tat protein on expression of proinflammatory and antiinflammatory cytokines by primary monocytes. AIDS Res Hum retroviruses. 2009;25(7):691‐699. 10.1089/aid.2008.0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yndart A, Kaushik A, Agudelo M, et al. Investigation of neuropathogenesis in HIV‐1 clade B and C infection associated with IL‐33 and ST2 regulation. ACS Chem Neurosci. 2015;6(9):1600‐1612. 10.1021/acschemneuro.5b00156 [DOI] [PubMed] [Google Scholar]
- 144. Taylor BS, Hammer SM. The challenge of HIV‐1 subtype diversity. N Engl J Med. 2008;359(18):1965‐1966. 10.1056/NEJMc086373 [DOI] [PubMed] [Google Scholar]
- 145. Williams ME, Zulu SS, Stein DJ, Joska JA, Naudé PJW. Signatures of HIV‐1 subtype B and C Tat proteins and their effects in the neuropathogenesis of HIV‐associated neurocognitive impairments. Neurobiol Dis. 2020;136:104701. 10.1016/j.nbd.2019.104701 [DOI] [PubMed] [Google Scholar]
- 146. Rao VR, Sas AR, Eugenin EA, et al. HIV‐1 clade‐specific differences in the induction of neuropathogenesis. J Neurosci. 2008;28(40):10010‐10016. 10.1523/jneurosci.2955-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Yepthomi T, Paul R, Vallabhaneni S, et al. Neurocognitive consequences of HIV in southern India: a preliminary study of clade C virus. J Int Neuropsychol Soc. 2006;12(03):424‐430. 10.1017/s1355617706060516 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Table S1
Table S2
Table S3
Table S4
Table S5
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].


