Abstract
Objectives
Current diameter-based guidelines for ascending thoracic aortic aneurysms (aTAA) do not consistently predict risk of dissection/rupture. ATAA wall stresses may enhance risk stratification independent of diameter. The relation of wall stresses and diameter indexed to height and body surface area (BSA) is unknown. Our objective was to compare aTAA wall stresses with indexed diameters in relation to all-cause mortality at 3.75 years follow-up.
Methods
Finite element analyses were performed in a veteran population with aortas ≥ 4.0 cm. Three-dimensional geometries were reconstructed from computed tomography with models accounting for pre-stress geometries. A fiber-embedded hyperelastic material model was applied to obtain wall stress distributions under systolic pressure. Peak wall stresses were compared across guideline thresholds for diameter/BSA and diameter/height. Hazard ratios for all-cause mortality and surgical aneurysm repair were estimated using cause-specific Cox proportional hazards models.
Results
Of 253 veterans, 54 (21 %) had aneurysm repair at 3.75 years. Indexed diameter alone would have prompted repair at baseline in 17/253 (6.7 %) patients, including only 4/230 (1.7 %) with diameter < 5.5 cm. Peak wall stresses did not significantly differ across guideline thresholds for diameter/BSA (circumferential: p = 0.15; longitudinal: p = 0.18), but did differ for diameter/height (circumferential: p = 0.003; longitudinal: p = 0.048). All-cause mortality was independently associated with peak longitudinal stresses (p = 0.04). Peak longitudinal stresses were best predicted by diameter (c-statistic = 0.66), followed by diameter/height (c-statistic = 0.59), and diameter/BSA (c-statistic = 0.55).
Conclusions
Diameter/height improved stratification of peak wall stresses compared to diameter/BSA. Peak longitudinal stresses predicted all-cause mortality independent of age and indexed diameter and may aid risk stratification for aTAA adverse events.
Keywords: Ascending aortic aneurysm, Biomechanics, Computed tomography, Finite element analysis, Outcomes, Risk factors
1. Introduction
The question of when to intervene in ascending thoracic aortic aneurysms (aTAA) is motivated by the potentially fatal complications of aortic dissection, a major cause of mortality that has seen little improvement in the past two decades [1], [2]. Current international guidelines based on maximal aortic diameter fall short of robust prediction of adverse events [3]. The newest American guidelines incorporate indexed diameter as a potentially reasonable metric for prophylactic repair [4]. In an observational study of 780 patients, c-statistics for indexed diameter ranged from 0.617 for diameter/[body surface area (BSA)] to 0.645 for diameter/height [5]. Thus, 36–38 % of patients who experienced an adverse event were assigned an event probability at or below those assigned for non-event patients. In a more recent observational study of over 6000 patients at a large integrated health care system, there was no meaningful difference in risk stratification of adverse aortic events between aortic diameter and diameter indexed to body surface area [6]. Notably, there was no evaluation of diameter indexed to height alone.
Biomechanically, aortic dissection may be initiated when wall stress exceeds wall strength. Wall stress, the force per unit area applied to the aortic wall, peaks at systole and can be expressed as directional components. We have shown that normal aortic wall strength is weaker in the longitudinal direction while others have reported similar findings in aortic aneurysms [7], [8], [9]. We previously showed that wall stresses largely overlap across diameter-based criteria using aTAA finite element analyses (FEA), a rigorous computational method for determining wall stresses in vivo [10], [11], [12]. Another study of 7 patients with pre-dissection imaging suggested that peak longitudinal stresses were elevated prior to dissection, compared to non-dissected, non-aneurysmal controls [13]. We similarly demonstrated that peak longitudinal stresses were associated with 3-year all-cause mortality independent of aTAA diameter and cross-sectional area/height [14], [15]. FEA-derived wall stresses allow us to independently evaluate risk metrics derived from clinical outcomes to incorporate new biomechanical predictors into risk calculations.
This study examines the relationship of diameter/BSA and diameter/height with peak circumferential (CIRC) and longitudinal (LONG) wall stresses. All-cause mortality and indicated and observed rates of surgical intervention were also determined at a median follow-up of 3.75 years.
2. Patients and methods
2.1. Ethical statement
This study was approved by Institutional Review Board at University of California San Francisco Medical Center and San Francisco Veterans Affairs Health Care System (SFVAHCS; CHR 13-10932, Nov. 23, 2020; CHR 10-03594, July 2, 2020; CHR 18-25716, Aug. 18, 2020). Retrospective review of imaging and clinical data was exempt from obtaining informed consent. SFVAHCS veterans presenting to our aortic clinic between June 2017 and June 2020 with ascending aortas ≥ 4.0 cm measured on chest computed tomography (CT) were evaluated for study inclusion. Exclusion criteria were a history of surgical aneurysm repair or aortic valve replacement, type A aortic dissection or aneurysm rupture, poor image quality, or motion artifact on imaging. We also excluded patients with monogenic aortopathies (e.g., Marfan, Ehlers-Danlos, or Loeys-Dietz syndromes), as suggested by a compelling clinical syndrome and/or confirmed by genetic testing.
Maximum aTAA diameter was assessed from clinical radiologic reports. Height and weight were captured from date closest to the imaging scan used for FEA. BSA was estimated with Du Bois–Du Bois formula: . Diameter indices were calculated as: diameter/BSA (cm/m2) and diameter/height (cm/m). Patients were grouped according to literature-proposed clinical risk groups: diameter/BSA ≤ 2.05, 2.08–2.95, 3.00–3.95, ≥4.00 cm/m2 and diameter/height ≤ 2.43, 2.44–3.17, 3.21–4.06, ≥4.10 cm/m5. Values falling into discontinuities within these definitions were assigned to the nearest group (e.g. diameter/height of 3.18 cm/m was assigned to 2.44–3.17 cm/m). These groups were previously found to have average yearly complication rates of 4 %, 7 %, 12 %, and 18 %, respectively, with the authors suggesting surveillance for the first two groups and surgical repair for the latter two [5].
2.2. Clinical outcomes
Patient records were reviewed to assess age, sex, valve type, valvular disease by echocardiography, and comorbidities of hypertension, hyperlipidemia, smoking, and diabetes mellitus. Patients were followed up to 3.75 years beyond the FEA scan to determine incidence of aTAA repair and death as competing risks. ATAA repair or death from any cause were documented outcomes. All-cause mortality rather than aorta-related mortality was the primary outcome to avoid bias in the absence of autopsy-confirmed causes of death. Patients were censored at latest confirmation of vital status, such as documented clinical visit.
2.3. Biomechanical outcomes
Finite element analysis was performed as previously described and validated [16]. Patient-specific geometries were derived from CT, including left ventricular outflow tract (LVOT), aortic annulus, sinuses, ascending aorta, arch, and a portion of the descending thoracic aorta (DTA). Models were developed as follows: 1) aortic lumen was segmented with MeVisLab (MeVis Medical Solutions AG and Fraunhofer MEVIS, Bremen, Germany); 2) 3D surface was reconstructed with Geomagic (Morrisville, NC) using image slices orthogonal to aortic long axis; 3) finite element (FE) mesh was generated with TrueGrid (XYZ Scientific Applications, Inc., Pleasant Hill, CA) and convergence studies were performed to optimize mesh density; and 4) FEA was performed with LS-DYNA (LSTC Inc., Livermore, CA). Meshes comprised ∼ 11,202 hexahedral elements with 1.80 mm thickness and three layers reflecting aortic intima, media, and adventitia.
Initial contours had geometries at systemic pressure. A modified update-Lagrangian method was applied to account for prestress and obtain zero-pressure geometries suitable for FEA [17]. The aortic wall was modeled as incompressible hyperelastic material with collagen-embedded material model [18]. Average aTAA material properties were separately specified for bicuspid and tricuspid valves based upon previous stretch testing [19]. To allow for aortic movement during the cardiac cycle while considering restraint from ligamentum arteriosum, we fixed translational motion at LVOT (20 mm proximal to aortic annulus) and distally at DTA. No constraints to rotational motion were placed. FEA was performed by applying physiologic arterial pressure to aTAA lumen. Simulations started with initial lumen pressurization to 80 mmHg. Cardiac cycle was subsequently simulated by gradual increase in pressure from 80 to 120 mmHg over 300 ms, followed by decrease to 80 mmHg over an additional 500 ms. Peak wall stresses were calculated at systole as 99th percentile stress to avoid artifacts from inhomogeneities in FE mesh.
2.4. Statistical analysis
All statistical analyses were performed in R version 4.1.1 (R Foundation for Statistical Computing). Characteristics of the patient population were summarized as count (percentages) for categorical variables and median [interquartile range (IQR)] for continuous variables.
For clinical outcomes, surgical aTAA repair and all-cause mortality were considered competing risks. Cumulative incidence functions were estimated and cause-specific Cox proportional hazards models were fit using R package riskRegression [20]. To address multiplicity of testing, the following predetermined model-building strategy was carried out. First, univariate associations of age, diameter/BSA, diameter/height, CIRC, and LONG were calculated. The p-values for these associations were not adjusted. Then, four adjusted models combining age, indexed diameter, and peak wall stresses were fit: (1) age, diameter/BSA, and CIRC; (2) age, diameter/height, and CIRC; (3) age, diameter/BSA, and LONG; and (4) age, diameter/height, and LONG. P-values for these adjusted models were corrected for multiple testing by controlling the false discovery rate using the Benjamini-Hochberg procedure. As a sensitivity analysis, these models were refit after excluding two patients whose deaths were attributed to non-cardiac causes. The proportional hazards assumption was assessed by plotting time-dependent beta coefficients. Median follow-up was estimated by applying Kaplan-Meier method to censored times. Cohort-specific binary thresholds were found for peak wall stresses as follows: each threshold from the 10th to 90th percentile values in the study population was tested in the respective adjusted model by 1-unit increments for peak wall stress. The threshold that minimized the p-value for the covariate of interest was chosen.
For biomechanical outcomes, distributions of peak wall stresses across proposed diameter index risk groups were compared using the Kruskal-Wallis test. For cohort-specific analyses, associations of CIRC/LONG with diameter indices as continuous variables were calculated as the Pearson correlation. Nonlinear trends were assessed by locally estimated scatterplot smoothing (LOESS). Lastly, the concordance(c-)-statistic, sensitivity, and specificity of diameter and diameter indices were computed for classification of high- and low-stress groups. P < 0.05 was considered significant.
3. Results
3.1. Patient population
The study population comprised 253 veterans (Table 1). Median age was 69 [IQR, 64–73] years, and 98 % (249/253) were male. Median diameter was 4.6 [4.3–4.9] cm, diameter/BSA was 2.21 [1.99–2.45] cm/m2, and diameter/height was 2.57 [2.43–2.77] cm/m. At baseline, 76 % (192/253) of patients had aTAA diameter 4.0–4.9 cm, 14 % (36/253) had 5.0–5.4 cm, and 10 % (25/253) had ≥ 5.5 cm.
Table 1.
Characteristics of the study population at baseline.
| Characteristic | Overall |
|---|---|
| N | 253 |
| Age (median [IQR]) | 69 [64–73] |
| Male sex(%) | 249 (98) |
| Bicuspid aortic valve (%) | 48 (19) |
| Aortic valve disease (%) | 150 (71) |
| Regurgitation (%) | |
| None–Mild | 174 (83) |
| Moderate–Severe | 35 (17) |
| Stenosis (%) | |
| None–Mild | 181 (87) |
| Moderate–Severe | 28 (13) |
| Hypertension (%) | 200 (79) |
| Hyperlipidemia (%) | 188 (74) |
| Smoking history (%) | 174 (69) |
| Diabetes mellitus (%) | 50 (20) |
| Aortic diameter (median [IQR])—cm | 4.6 [4.3–4.9] |
| Diameter group (%) | |
| <5.0 | 192 (76) |
| 5.0–5.4 | 36 (14) |
| ≥5.5 | 25 (10) |
| Diameter/BSA (median [IQR])—cm/m2 | 2.21 [1.99–2.45] |
| Diameter/BSA group (%) | |
| ≤2.05 | 81 (32) |
| 2.08–2.95 | 163 (64) |
| 3.00–3.95 | 8 (3.2) |
| ≥4.00 | 1 (0.4) |
| Diameter/height (median [IQR])—cm/m | 2.57 [2.43–2.77] |
| Diameter/height group (%) | |
| ≤2.43 | 70 (28) |
| 2.44–3.17 | 168 (66) |
| 3.21–4.06 | 14 (5.5) |
| ≥4.10 | 1 (0.4) |
| CIRC (median [IQR])—kPa | 500 [440–594] |
| CIRC ≥ 607 kPa (%) | 53 (21) |
| LONG (median [IQR])—kPa | 305 [275–349] |
| LONG ≥ 354 kPa (%) | 55 (22) |
Counts and percentages for aortic valve disease, regurgitation, and stenosis are out of 209/253 (83%) patients with echocardiography. BSA, body surface area; CIRC, peak circumferential stress; LONG, peak longitudinal stress; IQR, interquartile range.
3.2. Distribution of peak wall stresses by diameter/BSA and diameter/height
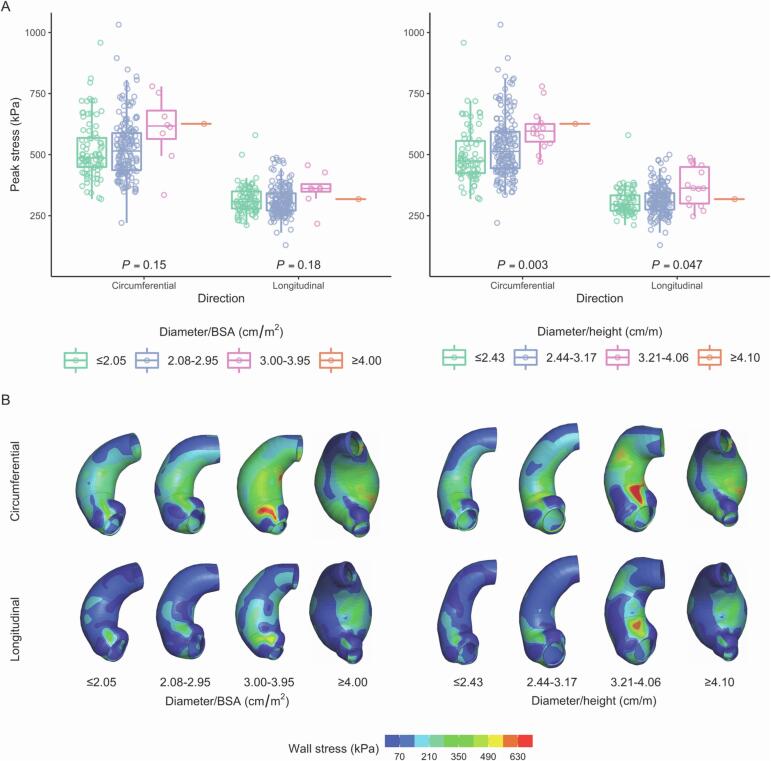
Across proposed diameter/BSA groups ≤ 2.05 (N = 81), 2.08–2.95 (N = 163), 3.00–3.95 (N = 8), and ≥ 4.00 (N = 1) cm/m2, CIRC/LONG were not significantly different (Fig. 1). Across proposed diameter/height groups ≤ 2.43 (N = 70), 2.44–3.17 (N = 168), 3.21–4.06 (N = 14), and ≥ 4.10 (N = 1) cm/m, CIRC were significantly different (p = 0.003), as were LONG (p = 0.047) (Fig. 1).
Fig. 1.
(A) Distributions of peak wall stresses across proposed risk groups based on diameter/[body surface area (BSA)] and diameter/height. (B) Circumferential and longitudinal stress profiles for one representative patient in each risk group. Same patient is shown for diameter/BSA ≥ 4.00 and diameter/height ≥ 4.10.
3.3. Association of peak wall stresses with diameter, diameter/BSA, and diameter/height
CIRC had the strongest linear relationship with diameter (r = 0.36), followed by diameter/height (r = 0.29) and diameter/BSA (r = 0.13) (Supplemental Fig. 2A). Similarly, LONG had the strongest linear relationship with diameter (r = 0.30), followed by diameter/height (r = 0.22) and diameter/BSA (r = 0.10) (Supplemental Fig. 2B). For both stress directions, smoothed trends with diameter, diameter/BSA, and diameter/height were largely linear.
3.4. Classification of high peak wall stresses
We examined the ability of diameter, diameter/BSA, and diameter/height to predict high peak stresses. High peak stresses were defined according to thresholds that maximized all-cause mortality hazard ratio: CIRC ≥ 607 kPa and LONG ≥ 354 kPa. Diameter was most predictive of high peak stresses (CIRC c-statistic: 0.65, 95 % CI, 0.57–0.74; LONG c-statistic: 0.62, 95 % CI, 0.55–0.69), followed by diameter/height (CIRC c-statistic: 0.61, 95 % CI, 0.51–0.70; LONG c-statistic: 0.57, 95 % CI, 0.50–0.64), and diameter/BSA (CIRC c-statistic: 0.52, 95 % CI, 0.42–0.62; LONG c-statistic: 0.49, 95 % CI, 0.42–0.57). Diameter 5.5 cm was most sensitive for high CIRC and LONG at 21 % and 13 %, respectively. Diameter/height 3.21 cm/m had lower sensitivity for high CIRC and LONG at 13 % and 8.4 %, respectively. Diameter/BSA 3.00 cm/m2 had the lowest sensitivity for high CIRC and LONG at 11 % and 6.1 %, respectively. Conversely, diameter/BSA had the highest specificity for high CIRC and LONG at 99 % for both stress directions, followed by diameter/height at 96–97 % specificity for high CIRC and LONG, and diameter at 93 % specificity for high CIRC and LONG.
3.5. Surgical aTAA repair operative results
ATAA diameter ≥ 5.5 cm was the repair indication for tricuspid aortic valves (TAV-aTAA). For bicuspid aortic valves (BAV-aTAA), diameter ≥ 5 cm was the primary indication prior to 2018, and diameter ≥ 5.5 cm thereafter following updated guidelines [21]. Concomitant procedures generally decreased the corresponding diameter threshold to 4.5 cm. Overall, 21 % (54/253) of patients underwent aTAA repair. Repairs included 52 % (28/54) TAV-aTAA and 48 % (26/54) BAV-aTAA. No urgent/emergent repairs were performed for type A dissection, rupture, or intramural hematoma. There were no intraoperative or 30-day deaths.
3.6. Indications for surgical repair based upon indexed diameter thresholds
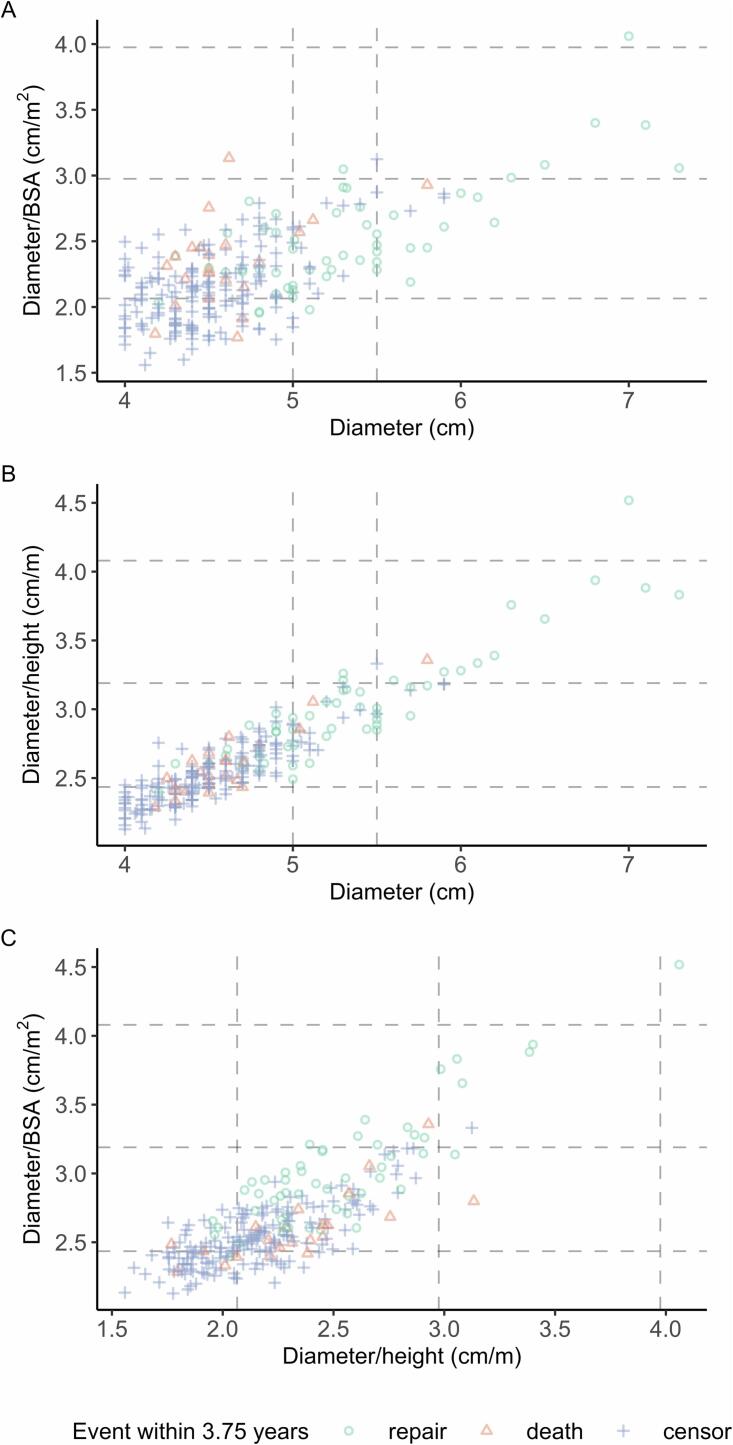
Indications for surgical aTAA repair at baseline are summarized in Fig. 2/Supplemental Table 1. Among 90 % (228/253) of patients with baseline diameter < 5.5 cm, 2 (0.9 %) had diameter/BSA ≥ 3.00 cm/m2 and 2 (0.9 %) had diameter/height ≥ 3.21 cm/m, which may be used to indicate prophylactic repair. Among 25 (10 %) patients with baseline diameter ≥ 5.5 cm, 28 % (7/25) had diameter/BSA ≥ 3.00 cm/m2 and 52 % (13/25) had diameter/height ≥ 3.21 cm/m.
Fig. 2.
Joint distributions of diameter and diameter indices. Dashed lines delineate current guideline thresholds for diameter, diameter/BSA, and diameter/height.
3.7. All-cause mortality
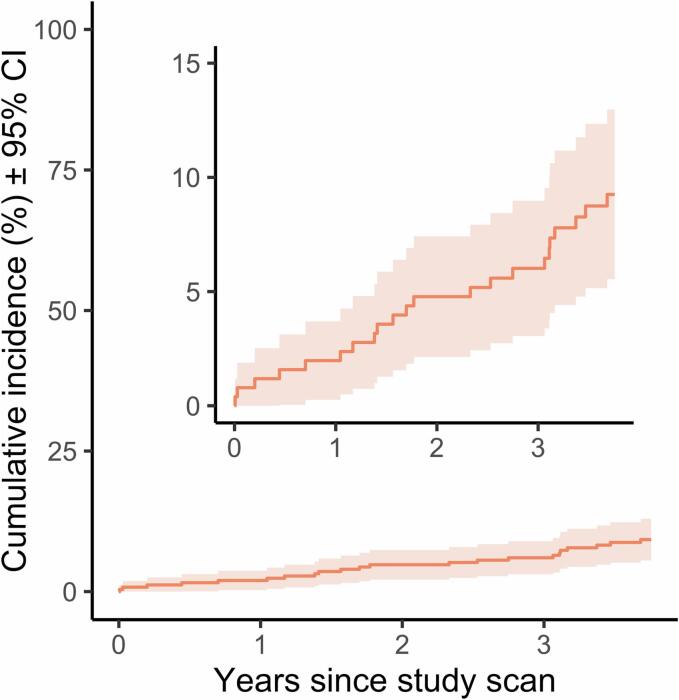
Median follow-up was 3.75 [IQR, 3.75–3.75] years. Cumulative incidence of all-cause mortality was 1.6 % (95 % CI, 0–3.1 %), 2.0 % (0.3–3.7 %), 3.6 % (1.3–5.9 %), 4.8 % (2.1–7.4 %), 5.2 % (2.4–7.9 %), 6.0 % (3.1–9.0 %), and 8.7 % (5.2–12.3 %) at 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, and 3.5 years, respectively, with 22/253 (8.7 %) total patients deceased within 3.75 years (Fig. 3). Death was due to dissection in 2 patients, and non-cardiac causes in 2 patients. In general, it was not possible to ascertain precise causes of death for the patients who died during the study period retrospectively without autopsy studies.
Fig. 3.
Cumulative incidence of all-cause mortality. Shading represents 95% confidence interval. Inset is identical plot with smaller y-axis limits.
Patients with diameter ≥ 5.5 cm at baseline typically received surgical repair and were considered not at risk for mortality thereafter. Thus, among the 8.7 % (22/253) patients who died within 3.75 years, only 1 (4.5 %) had baseline diameter ≥ 5.5 cm. Among the remaining 21 deceased patients, 19 (90 %) had baseline diameter < 5.0 cm, 2 (9.5 %) had diameter 5.0–5.4 cm, 20 (95 %) had diameter/BSA < 3.00 cm/m2, and 21 (100 %) had diameter/height < 3.21 cm/m. Of the 19 patients with baseline diameter < 5.0 cm who were deceased within 3.75 years, peak longitudinal stress ≥ 354 kPa threshold would have added indication for repair in 6 (32 %).
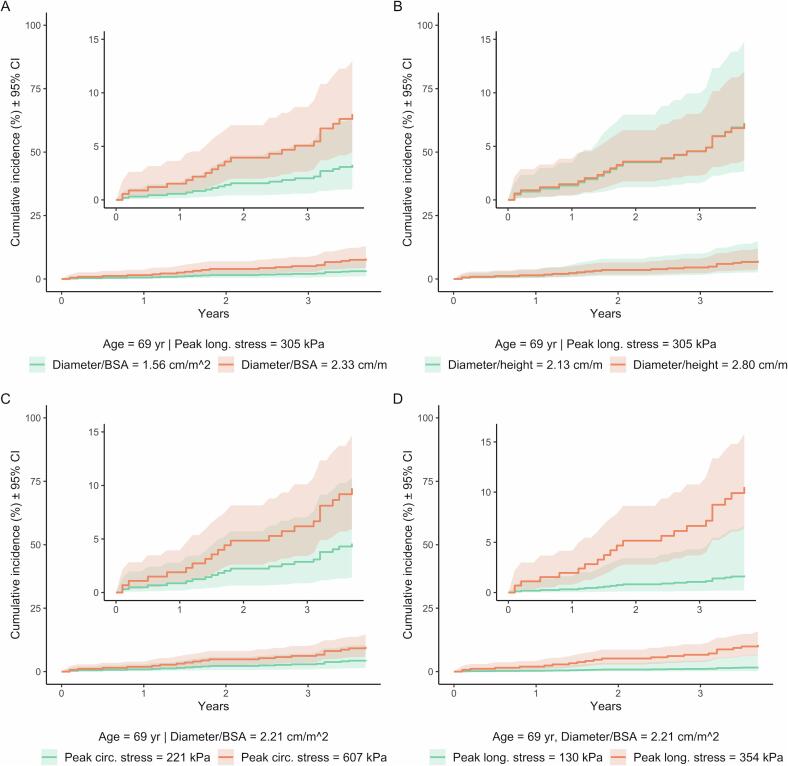
Four models were evaluated that adjusted for age, indexed diameter, and peak stresses (Table 2). CIRC was not associated with an increased hazard of all-cause mortality when accounting for age and diameter/BSA or diameter/height. However, adjusted hazard ratios for LONG were significant when accounting for age and diameter/BSA (hazard ratio 1.81 [95 % CI, 1.09–3.00]; p = 0.04) and for age and diameter/height (1.78 [1.08–2.92]; p = 0.04). Diameter/BSA was significantly associated with an increased hazard all-cause mortality when adjusting for age and CIRC (1.95 [1.06–3.60]; p = 0.04) but not age and LONG. Diameter/height was not significantly associated with an increased hazard of all-cause mortality when accounting for age and CIRC/LONG. The exclusion of two patients with non-cardiac deaths did not meaningfully alter these results (Supplemental Table 2). Fig. 4 shows cumulative incidence curves based on the above models.
Table 2.
All-Cause Mortality with Aneurysm Repair as a Competing Risk.
| Unadjusted | Hazard ratio (95 % CI) | P |
|---|---|---|
| Age – 9-year increase | 2.02 (1.14–3.56) | 0.016 |
| Diameter/BSA – 0.46-cm/m2 increase | 2.29 (1.26–4.17) | 0.007 |
| Diameter/height – 0.35-cm/m increase | 1.31 (0.75–2.28) | 0.35 |
| Peak circumferential stress – 154-kPa increase | 1.46 (0.92–2.33) | 0.11 |
| Peak longitudinal stress – 75-kPa increase | 1.67 (1.02–2.73) | 0.04 |
| Adjusted model 1 | ||
| Age | 1.76 (1.02–3.02) | 0.06 |
| Diameter/BSA | 1.95 (1.06–3.60) | 0.051 |
| Peak circumferential stress | 1.36 (0.89–2.08) | 0.18 |
| Adjusted model 2 | ||
| Age | 1.98 (1.12–3.48) | 0.04 |
| Diameter/height | 1.09 (0.61–1.97) | 0.76 |
| Peak circumferential stress | 1.41 (0.91–2.19) | 0.17 |
| Adjusted model 3 | ||
| Age | 1.91 (1.09–3.35) | 0.04 |
| Diameter/BSA | 2.03 (1.10–3.74) | 0.04 |
| Peak longitudinal stress | 1.81 (1.09–3.00) | 0.04 |
| Adjusted model 4 | ||
| Age | 2.13 (1.20–3.81) | 0.04 |
| Diameter/height | 1.12 (0.63–1.99) | 0.76 |
| Peak longitudinal stress | 1.78 (1.08–2.92) | 0.04 |
CI, confidence interval. P-values in the four adjusted models were corrected for multiple testing by controlling the false discovery rate using the Benjamini-Hochberg procedure.
Fig. 4.
Cumulative incidences according to all-cause mortality Cox proportional hazards models. One covariate is plotted at minimum value in study population and an optimal binary threshold, while the other covariates are constant at their respective medians. Shading represents 95% confidence intervals. Insets are identical respective plots with smaller y-axis limits.
4. Discussion
An intervention strategy led solely by diameter/BSA or diameter/height would fail to capture 48 % of patients meeting the diameter threshold, but as adjunct, diameter/BSA or diameter/height added a novel repair indication in < 2 % of patients not indicated by diameter. Notably, LONG ≥ 354 kPa would have added a novel repair indication in 35 % of non-repaired patients who were deceased within 3.75 years after having no baseline size-based indication.
No metric reliably predicted high CIRC/LONG. For the newest diameter guidelines and indexed diameter thresholds, specificity (93–99 %) far outweighed sensitivity (6.1 %–21 %). Therefore, size-based thresholds may be more helpful for ruling in rather than ruling out high peak stresses—patients who meet size-based metrics likely have elevated CIRC/LONG, whereas those who do not meet size-based metrics may have low or high CIRC/LONG. Largely linear smoothed trends of CIRC/LONG with diameter, diameter/BSA, and diameter/height suggest a lack of a critical value beyond which peak stresses rapidly escalate, although our data lack many > 6 cm aneurysms.
We did not find an association between all-cause mortality and diameter/height, and the association that we did observe with diameter/BSA was negated when accounting from LONG. This finding contrasts with the largest study of indexed diameter from the Yale Aortic Institute [5]. However, demographics in a veteran population differ due to the paucity of women compared to the civilian population from which these metrics were originally derived. Size-based metrics would add relatively more women to indications for surgical repair than men, as diameter indices increase as BSA or height decreases. Given findings of worse aneurysm outcomes in women [22], [23], [24], [25], [26], women may require surgical intervention earlier than indicated by diameter alone. Other indices have been proposed including area/height ratio ≥ 10 cm2/m as a threshold related to all-cause mortality and aortic length in relation to adverse aortic events [27], [28], [29], [30], [31]. In prior work, we found an association of LONG and all-cause mortality independent of area/height ratio at 3 years follow-up [15]. Investigating the association of aortic length, peak wall stresses, and mortality is a future area of work.
With diameter-based guidelines in effect, there was a 6.0 % cumulative incidence of all-cause death at 3 years, which was consistent with the adjusted yearly mortality rate of 2 % among 70-year-old male veterans 2010–2017 [32]. When adjusting for age and indexed diameter, LONG was associated with an increased hazard of death. We previously showed that the normal aortic wall is weaker in the longitudinal than the circumferential direction, and others reported similar findings in aTAA [7], [8], [9]. Moreover, we and others have reported elevated LONG preceding dissection and death [13], [14], [15]. Mechanistically, relatively larger LONG compared to longitudinal strength may initiate type A dissection as a transverse tear, which then propagates distally both circumferentially and longitudinally. A prospective evaluation of biomechanical and higher-order geometric predictors of adverse aTAA outcomes such as dissection, rupture, or intramural hematoma is needed to confirm this hypothesis.
5. Limitations
This study has several limitations. The study population comprised mostly male veterans, who may have different characteristics than other populations. FEA methods were also optimized to process large numbers of patients. We did not use patient-specific material properties, which are challenging to obtain in vivo; however, our previous work has shown that average material properties yield similar results [33]. While we did not consider aortic stenosis or regurgitation in FEA, hydrostatic pressures dominate shear stresses due to blood flow patterns by several orders of magnitude [34]. Finally, it was not possible to ascertain aortic causes of death without autopsy reports in this retrospective study. However, only two deaths were attributed to non-cardiac causes, and acute aortic dissection is an underappreciated cause of out-of-hospital cardiac arrest [35].
6. Conclusion
In this veteran study population, indexed diameter added few patients to the indications for surgical aTAA repair, and if used solely, would have decreased the number of patients undergoing repair. CIRC/LONG significantly differed across proposed thresholds for diameter/height but not diameter/BSA, albeit with largely overlapping distributions. Indexed diameter did not improve classification of high CIRC/LONG than diameter alone. LONG was an independent predictor of mortality at 3.75 years follow-up when accounting for age and indexed diameter. Investigating longitudinal stresses and adverse aortic events prospectively may yield an additional prognostic indicator for aortic dissection and improve patient-specific risk stratification for prophylactic surgical repair.
CRediT authorship contribution statement
Siavash Zamirpour: Writing – review & editing, Writing – original draft, Visualization, Software, Methodology, Investigation, Formal analysis, Data curation. Yue Xuan: Writing – review & editing, Software, Methodology, Investigation, Data curation. Zhongjie Wang: Writing – review & editing, Software, Methodology, Investigation, Data curation. Axel Gomez: Writing – review & editing, Methodology, Investigation, Data curation. Joseph R. Leach: Writing – review & editing, Conceptualization. Dimitrios Mitsouras: Writing – review & editing, Conceptualization. David A. Saloner: Writing – review & editing, Resources, Methodology, Conceptualization. Julius M. Guccione: Writing – review & editing, Resources, Methodology, Conceptualization. Liang Ge: Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Funding acquisition, Formal analysis, Conceptualization. Elaine E. Tseng: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the UCSF School of Medicine Summer Explore Fellowship, AATS Summer Intern Scholarship, and AHA/CVSA Student Scholarship in Cardiovascular Surgery to S.Z.; National Institutes of Health (NIH) K25HL150408 to Y.X.; American Heart Association 20POST35211107 to Z.W.; NIH R01HL119857, Veterans Affairs (VA) Merit I01CX002365-01A1, and Marfan Foundation A131894 to E.E.T./L.G.; and VA Merit I01-CX002071 to D.M./D.A.S.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101375.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Saliba E., Sia Y., Dore A., El Hamamsy I. The ascending aortic aneurysm: when to intervene? IJC Heart Vasc. 2015;6:91–100. doi: 10.1016/j.ijcha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bossone E., Eagle K.A. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol. 2021;18:331–348. doi: 10.1038/s41569-020-00472-6. [DOI] [PubMed] [Google Scholar]
- 3.Hiratzka L.F., Bakris G.L., Beckman J.A., et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and Management of Patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, American Association for Thoracic Surgery, american College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121(13) doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 4.Isselbacher E.M., Preventza O., Hamilton Black J., et al. 2022 ACC/AHA guideline for the diagnosis and Management of Aortic Disease. J Am Coll Cardiol. 2022;80(24):e223–e393. doi: 10.1016/j.jacc.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zafar M.A., Li Y., Rizzo J.A., et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J Thorac Cardiovasc Surg. 2018;155(5):1938–1950. doi: 10.1016/j.jtcvs.2017.10.140. [DOI] [PubMed] [Google Scholar]
- 6.Solomon M.D., Leong T., Sung S.H., et al. Association of Thoracic Aortic Aneurysm Size with Long-term Patient Outcomes: the KP-TAA study. JAMA Cardiol. 2022;7(11):1160–1169. doi: 10.1001/jamacardio.2022.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xuan Y., Wisneski A.D., Wang Z., et al. Regional biomechanical and failure properties of healthy human ascending aorta and root. J Mech Behav Biomed Mater. 2021;123 doi: 10.1016/j.jmbbm.2021.104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasta S., Phillippi J.A., Gleason T.G., Vorp D.A. Effect of aneurysm on the mechanical dissection properties of the human ascending thoracic aorta. J Thorac Cardiovasc Surg. 2012;143(2):460–467. doi: 10.1016/j.jtcvs.2011.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iliopoulos D.C., Deveja R.P., Kritharis E.P., et al. Regional and directional variations in the mechanical properties of ascending thoracic aortic aneurysms. Med Eng Phys. 2009;31(1):1–9. doi: 10.1016/j.medengphy.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Gomez A., Wang Z., Xuan Y., et al. Wall stress distribution in bicuspid aortic valve-associated ascending thoracic aortic aneurysms. Ann Thorac Surg. 2020;110(3):807–814. doi: 10.1016/j.athoracsur.2019.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez A, Wang Z, Xuan Y, et al. Association of diameter and wall stresses of tricuspid aortic valve ascending thoracic aortic aneurysms. J Thorac Cardiovasc Surg. Published online June 2021:S0022522321009090. doi:10.1016/j.jtcvs.2021.05.049. [DOI] [PMC free article] [PubMed]
- 12.Wang Z, Flores N, Lum M, et al. Wall stress analyses in patients with ≥5 cm versus <5 cm ascending thoracic aortic aneurysm. J Thorac Cardiovasc Surg. Published online February 2020:S0022522320304566. doi:10.1016/j.jtcvs.2020.02.046. [DOI] [PMC free article] [PubMed]
- 13.Emerel L., Thunes J., Kickliter T., et al. Predissection-derived geometric and distensibility indices reveal increased peak longitudinal stress and stiffness in patients sustaining acute type a aortic dissection: implications for predicting dissection. J Thorac Cardiovasc Surg. 2019;158(2):355–363. doi: 10.1016/j.jtcvs.2018.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamirpour S, Xuan Y, Wang Z, et al. Association of 3-Year All-Cause Mortality and Peak Wall Stresses of Ascending Thoracic Aortic Aneurysms in Veterans. Semin Thorac Cardiovasc Surg. Published online June 2022:S1043067922001332. doi:10.1053/j.semtcvs.2022.06.002. [DOI] [PubMed]
- 15.Zamirpour S, Xuan Y, Wang Z, et al. Aortic Area/Height Ratio, Peak Wall Stresses, and Outcomes in Veterans with Tricuspid vs Bicuspid Aortic Valve Associated Ascending Thoracic Aortic Aneurysms. J Thorac Cardiovasc Surg. Published online June 2023:S0022522323004567. doi:10.1016/j.jtcvs.2023.05.031. [DOI] [PubMed]
- 16.Krishnan K., Ge L., Haraldsson H., et al. Ascending thoracic aortic aneurysm wall stress analysis using patient-specific finite element modeling of in vivo magnetic resonance imaging. Interact Cardiovasc Thorac Surg. 2015;21(4):471–480. doi: 10.1093/icvts/ivv186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gee M.W., Förster C.h., Wall W.A. A computational strategy for prestressing patient-specific biomechanical problems under finite deformation. Int J Numer Methods Biomed Eng. 2010;26(1):52–72. doi: 10.1002/cnm.1236. [DOI] [Google Scholar]
- 18.Gasser T.C., Ogden R.W., Holzapfel G.A. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J R Soc Interface. 2006;3(6):15–35. doi: 10.1098/rsif.2005.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azadani A.N., Chitsaz S., Mannion A., et al. Biomechanical properties of human ascending thoracic aortic aneurysms. Ann Thorac Surg. 2013;96(1):50–58. doi: 10.1016/j.athoracsur.2013.03.094. [DOI] [PubMed] [Google Scholar]
- 20.Ozenne B., Lyngholm S.A., Scheike T., Torp-Pedersen C., Alexander G.T. riskRegression: predicting the risk of an event using cox regression models. R J. 2017;9(2):440. doi: 10.32614/RJ-2017-062. [DOI] [Google Scholar]
- 21.Borger M.A., Fedak P.W.M., Stephens E.H., et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve–related aortopathy: full online-only version. J Thorac Cardiovasc Surg. 2018;156(2):e41–e74. doi: 10.1016/j.jtcvs.2018.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung J., Coutinho T., Chu M.W.A., Ouzounian M. Sex differences in thoracic aortic disease: a review of the literature and a call to action. J Thorac Cardiovasc Surg. 2020;160(3):656–660. doi: 10.1016/j.jtcvs.2019.09.194. [DOI] [PubMed] [Google Scholar]
- 23.Harris K.M., Strauss C.E., Eagle K.A., et al. Correlates of delayed recognition and treatment of acute type a aortic dissection: the international registry of acute aortic dissection (IRAD) Circulation. 2011;124(18):1911–1918. doi: 10.1161/CIRCULATIONAHA.110.006320. [DOI] [PubMed] [Google Scholar]
- 24.Nienaber C.A., Fattori R., Mehta R.H., et al. Gender-related differences in acute aortic dissection. Circulation. 2004;109(24):3014–3021. doi: 10.1161/01.CIR.0000130644.78677.2C. [DOI] [PubMed] [Google Scholar]
- 25.Huckaby L.V., Sultan I., Trimarchi S., et al. Sex-based aortic dissection outcomes from the international registry of acute aortic dissection. Ann Thorac Surg. 2022;113(2):498–505. doi: 10.1016/j.athoracsur.2021.03.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beller CJ, Farag M, Wannaku S, et al. Gender-Specific Differences in Outcome of Ascending Aortic Aneurysm Surgery. Nikitovic-Tzanakaki D, ed. PLOS ONE. 2015;10(4):e0124461. doi:10.1371/journal.pone.0124461. [DOI] [PMC free article] [PubMed]
- 27.Masri A., Kalahasti V., Svensson L.G., et al. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation. 2016;134(22):1724–1737. doi: 10.1161/CIRCULATIONAHA.116.022995. [DOI] [PubMed] [Google Scholar]
- 28.Masri A., Kalahasti V., Svensson L.G., et al. Aortic cross-sectional area/height ratio and outcomes in patients with bicuspid aortic valve and a dilated ascending aorta. Circ Cardiovasc Imaging. 2017;10(6) doi: 10.1161/CIRCIMAGING.116.006249. [DOI] [PubMed] [Google Scholar]
- 29.Krüger T., Oikonomou A., Schibilsky D., et al. Aortic elongation and the risk for dissection: the Tübingen aortic pathoanatomy (TAIPAN) project†. Eur J Cardiothorac Surg. 2017;51(6):1119–1126. doi: 10.1093/ejcts/ezx005. [DOI] [PubMed] [Google Scholar]
- 30.Krüger T., Sandoval Boburg R., Lescan M., et al. Aortic elongation in aortic aneurysm and dissection: the Tübingen aortic pathoanatomy (TAIPAN) project†. Eur J Cardiothorac Surg. 2018;54(1):26–33. doi: 10.1093/ejcts/ezx503. [DOI] [PubMed] [Google Scholar]
- 31.Wu J., Zafar M.A., Li Y., et al. Ascending aortic length and risk of aortic adverse events. J Am Coll Cardiol. 2019;74(15):1883–1894. doi: 10.1016/j.jacc.2019.07.078. [DOI] [PubMed] [Google Scholar]
- 32.Mortality Rates and Life Expectancy of Veterans from 1980 to 2017, and by Education, Income, and Period of Service. National Center for Veterans Analysis and Statistics, Department of Veterans Affairs; 2021.
- 33.Wang Z., Xuan Y., Guccione J.M., Tseng E.E., Ge L. Impact of patient-specific material properties on Aneurysm Wall stress: a finite element study. J Heart Valve Dis. 2018;27(5):275–284. [PMC free article] [PubMed] [Google Scholar]
- 34.Pasta S., Rinaudo A., Luca A., et al. Difference in hemodynamic and wall stress of ascending thoracic aortic aneurysms with bicuspid and tricuspid aortic valve. J Biomech. 2013;46(10):1729–1738. doi: 10.1016/j.jbiomech.2013.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gouveia e Melo R, Machado C, Caldeira D, et al. Incidence of acute aortic dissections in patients with out of hospital cardiac arrest: a systematic review and meta-analysis of observational studies. IJC Heart Vasc. 2022;38 doi: 10.1016/j.ijcha.2021.100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.