Abstract
Tuberculosis is one of the most common pediatric problems, especially in the developing world. In spite of that, intraocular tuberculosis is a rare disease that can easily be confused with other noninfectious processes, even in regions where tuberculosis is rampant. Diagnosis is difficult, yet it is very important to provide effective antituberculosis treatment and avoid potentially sight-losing interventions. We present a case of a 2-year-old child with a positive contact history of tuberculosis who presented with progressively worsening seizures and constitutional symptoms for 6 months. Brain computed tomography revealed right frontotemporal region conglomerated ring-enhancing lesions with central necrosis consistent with tuberculosis. On the same scan, a calcified right retinal lesion with a contrast-enhancing soft tissue component was identified. A chest radiograph and abdominal sonography showed evidence of disseminated tuberculosis. Subsequently, antituberculosis treatment was initiated, and the right retinal lesion improved, thus leading to the imaging diagnosis of right intraocular tuberculosis.
Early and accurate diagnosis of retinal tuberculosis is of paramount importance in avoiding potentially catastrophic interventions. Neuroimaging is a useful, noninvasive method to consider this difficult diagnosis and also for follow-up.
Keywords: Intraocular tuberculosis, Tuberculosis, Resource-limited setting, Neuroimaging
Introduction
Tuberculosis (TB) is a common diagnosis in the pediatric population and carries a higher chance of dissemination in this age group. Through hematogenous and other means of spread, almost every organ can be involved. Central nervous system (CNS) TB is the most severe form, with high morbidity and mortality [1]. Intraocular TB is rare, with incidence rates as low as 1.4% reported in the literature [2], and presents a diagnostic challenge for clinicians, especially in resource-limited settings where advanced laboratory diagnostic tools such as polymerase chain reaction (PCR), gamma-interferon testing, and culture might not be available. Even in their presence, the diagnosis is difficult [3,4]. The importance of early and accurate recognition lies in the timely institution of effective anti-TB treatment and the avoidance of potentially catastrophic measures such as enucleation and immunosuppressive treatment. In this regard, imaging combined with other clinical clues helps in presumptively diagnosing intraocular TB. The most commonly reported form of intraocular TB is choroidal granuloma [5].
Case history
A 2-year-old male patient was referred to our institution from one of the district health institutions with a history of abnormal body movement, which was characterized by tonic flexion and extension of both upper and lower extremities that lasted for 2 minutes of 3-4 episodes per day of 6 months duration, which progressively worsened over 2 weeks prior to the current presentation. He also had a fever, cough, appetite loss, vomiting, and subjectively significant but unquantified weight loss. His parents did not notice any problems with his vision. There was no history of extremity weakness either. In addition, a right ear discharge of 1-year duration, described as watery initially and later on turning thick, was present. He had a history of contact with a confirmed pulmonary TB patient, his mother, who completed treatment 1 month prior to the current presentation. The child had a complete immunization record but an inadequate duration of exclusive breastfeeding, which was weaned right after the age of 1 month. The parents stated he has fairly comparable growth and development with his peers.
Physical examination at presentation showed a frail child weighing just 8 kg, with anthropometric evidence of severe acute malnutrition. Vital signs were deranged with evidence of tachycardia (160/min), tachypnea (50/min), and fever (38°C). An eye examination did not show redness or swelling. There was a yellowish-thick offensive discharge from the right ear. On the respiratory system, the child had decreased air entry in the upper two-thirds of the right lung with bronchial breath sounds. The other systems, including the central nervous system, were apparently unremarkable, with no evidence of weakness.
Laboratory investigations were done; the complete blood count showed leukocytosis (17,480/uL), and ESR was raised, measuring 30 mm/hour. The GeneXpert examination and the HIV serology test were negative. Given his symptoms and strong contact history, TB was strongly suspected. Concurrent right ear chronic otitis media was determined from ear examination.
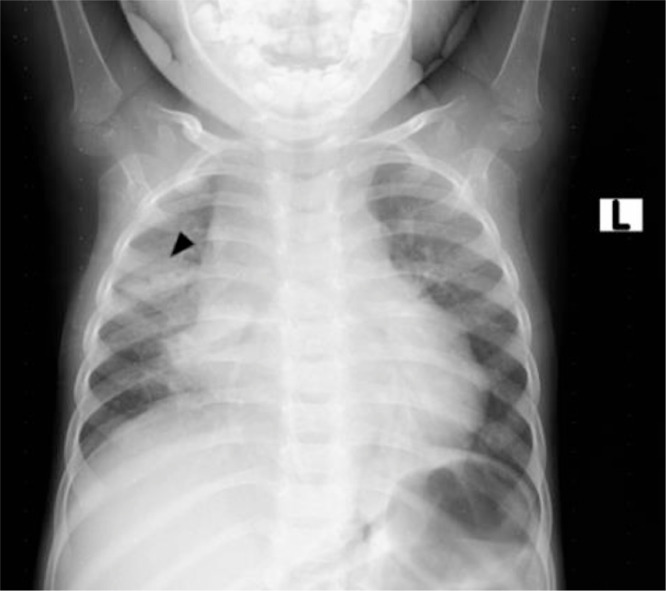
Chest radiography was subsequently performed and showed right middle lung zone air space opacity with ipsilateral hilar density, suggesting enlarged hilar lymph nodes (Fig. 1). Abdominal ultrasound showed multiple round hypoechoic liver lesions and multiple enlarged paraaortic lymph nodes. The spleen was normal in size with no lesions, and the bowel loops were not thickened. No appreciable ascites or peritoneal thickening were seen.
Fig. 1.
AP chest radiograph- right middle lung patchy consolidation (arrowhead) and ipsilateral hilar bulge suggestive of consolidation with hilar lymphadenopathy.
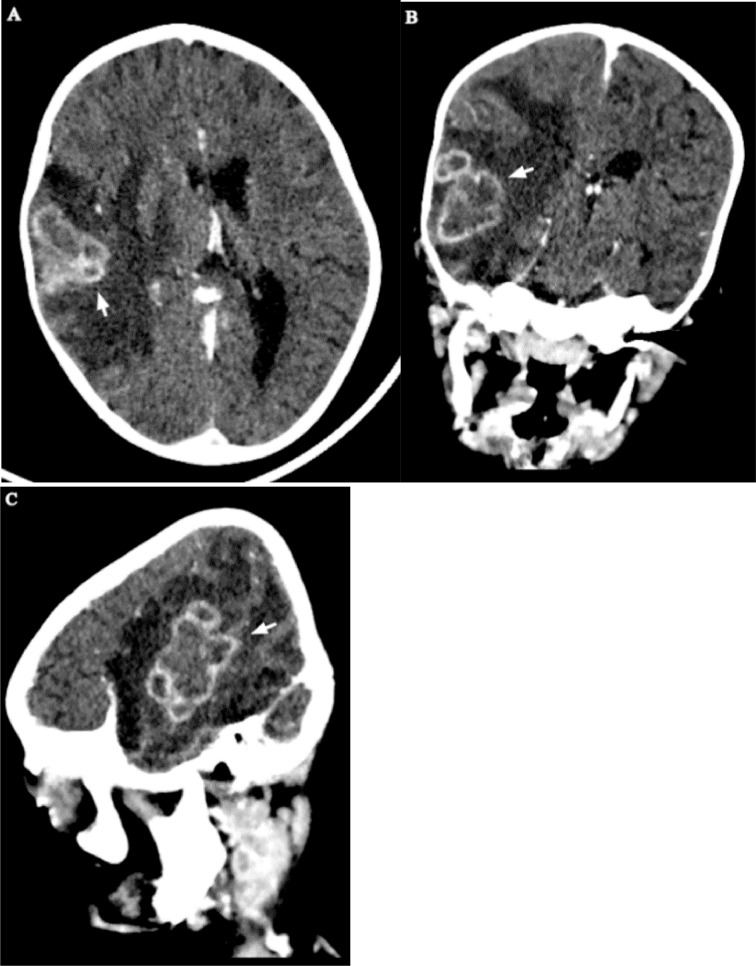
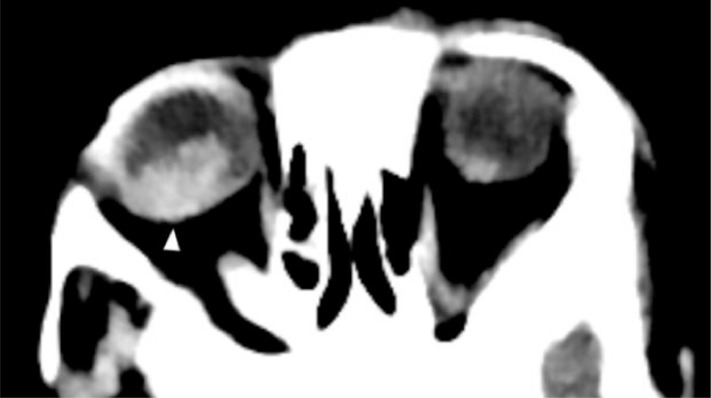
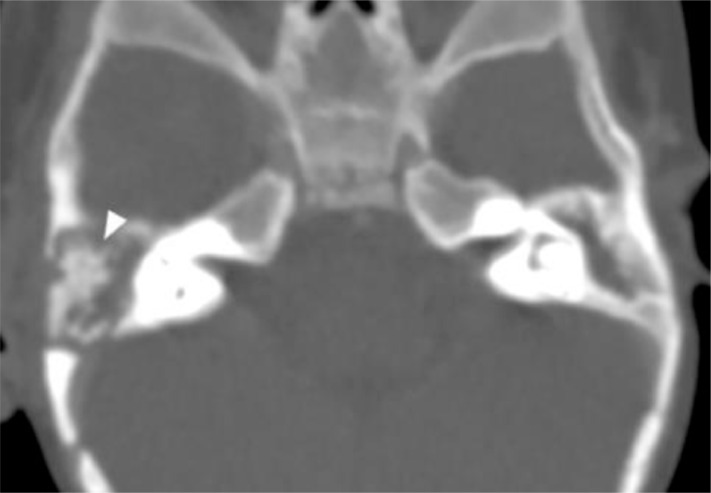
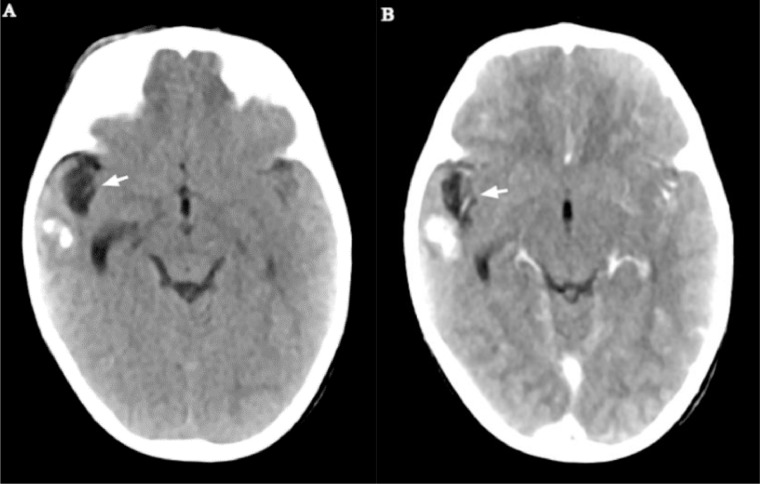
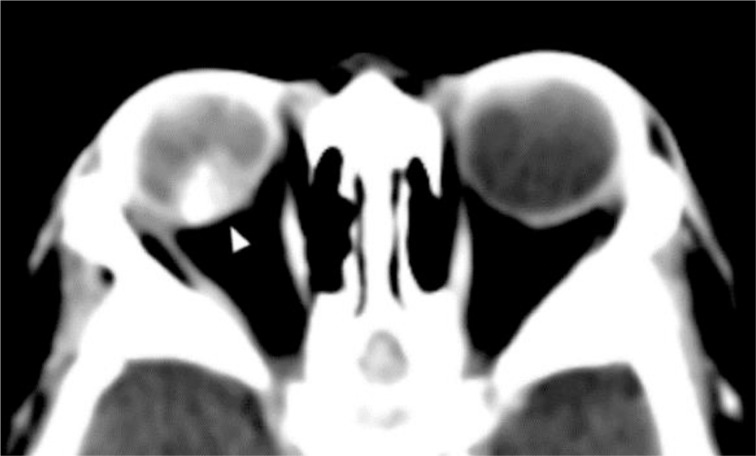
As part of the seizure workup, a contrast-enhanced head CT scan was performed and showed conglomerated and centrally necrotic ring-enhancing lesions in the right frontotemporal region. There was marked perilesional vasogenic edema. There was also a 1.3 × 1.2 cm right ocular lesion with foci of calcifications and heterogeneous enhancement. The lesion occupies slightly more than half of the vitreous space and had a broad relation with the retina. No obvious retrobulbar pathology was seen (Figs. 2 and 3). A soft tissue lesion was seen in the right middle ear, along with lytic ossicular change and mastoid destruction (Fig. 4).
Fig. 2.
Postcontrast head CT - axial (A), coronal (B), and sagittal (C) views show right frontotemporal conglomerated lesions with intense thick peripheral rim enhancement and marked vasogenic edema (arrows).
Fig. 3.
Axial precontrast CT at orbital level- A 1.3 × 1.2 cm orbital globe lesion (arrowhead) with faint calcification and heterogeneous enhancement fills slightly more than half of the vitreous space.
Fig. 4.
Bone window axial CT- Asymmetric soft tissue opacification of the right middle ear with lytic changes of the ossicular chain (arrow). Mastoid destruction also noted.
The brain lesion shown above (Fig. 2) added to the diagnostic confidence of CNS TB. The temporal location and mass effect explained the patient's seizures. The right globe finding (Fig. 3) was completely incidental. Given the faint calcific density, enhancement, location, and commonality, retinoblastoma was the top differential diagnosis. In addition, disseminated TB to the retina was also entertained given the strong contact history, clinical presentation, and imaging findings. As such, caution was exercised from pursuing aggressive surgical treatment of the right ocular lesion. Biopsy from the cerebral or ocular lesion was not attempted due to technical difficulties and poor nutritional status of the patient.
The patient was then started on anti-TB treatment, and follow-up imaging was planned in 3 months. Per Ethiopian national guidelines [6], our patient was put on a four-drug regimen: isoniazid, rifampicin, ethambutol, pyrazinamide, and pyridoxine for 2 months. High-dose prednisolone (2 mg/kg/day) for vasogenic edema and phenytoin (3 mg/kg/day) for seizure were also added. For the right chronic otitis media, regular aural toilet, irrigation with water and hydrogen peroxide, and ciprofloxacin ear drops were prescribed. As described on the follow-up brain CT (Figs. 5 and 6), the patient had interval radiologic improvement of his ring-enhancing right cortical as well as right ocular lesions. Clinically too, improvements in terms of subsiding cough, returning appetite, and weight gain were seen. Seizure control was also optimal. The treatment response of the right retinal lesion, in terms of increased calcification and decreased enhancement proved it was indeed intraocular TB. Anti-TB treatment is planned to continue for a total of 12 months. At the time of writing this report the child is on regular follow-up, adheres to his treatment, and continues to improve.
Fig. 5.
Pre (A) and post (B) contrast axial brain CT after 3 months- Almost complete resolution of the previously illustrated right frontotemporal lesion. Only coarse calcified nodules with small right temporal pole residual encephalomalacia (arrows) remain.
Fig. 6.
Magnified axial CT at orbital level: The right globe lesion has become denser, smaller, and more distinct with decreased enhancement.
Discussion
Tuberculosis is a major communicable disease caused by Mycobacterium tuberculosis, which is the most common causative agent in humans. It is mainly spread when infected individuals expel the bacteria via aerosols [7]. It is a leading infectious cause of morbidity and mortality in children worldwide and a major health concern, particularly in Africa and Southeast Asia, where the highest number of children are affected [8]. Children and young adolescents aged under 15 years represent around 11% of all TB cases globally, with 1.1 million new TB infections every year in this age group [9]. Ethiopia is also one of the 22 high-burden countries with a high pediatric case load [7]. The disease affected 20,000 children under the age of 15, accounting for 11.6% of all TB cases, according to the Global TB Report of 2018 [10].
Thoracic manifestations, including pulmonary disease and lymphadenopathy, are among the most common TB manifestations in children and our case had these features. But, in the background of the coronavirus disease-2019 (COVID-19) pandemic, one needs to be cognizant of its possibility. Unilateral disease occurs more commonly in children and one third can have normal radiological findings [11]. CT scan is a sensitive tool and the preferred modality even in children. The commonest manifestation is multifocal and peripheral ground glass opacities. Consolidation is less common and hilar lymphadenopathy is rare [11,12]. In our case, based on the presented chest radiograph, it can be a reasonable differential diagnosis, but the presence of hilar lymphadenopathy favors TB more than COVID-19.
TB also affects various extra-thoracic sites [4]. Extrapulmonary TB is more common in children than in adults. Our case presented with multiple organ involvement, including the lungs, abdomen, CNS, and eye. Diagnosing tuberculosis at pediatric age has its own difficulties in terms of pathogen detection, specimen collection, and nonspecific imaging findings. Disseminated foci, in the majority, are from a pulmonary focus and are more challenging to diagnose [9].
CNS TB is the most severe extrapulmonary TB manifestation. A history of recent TB contact is common in children, occurring in about 50%-90% of cases [1]. The Mycobacteria reach the CNS via the hematogenous route from an active infectious site or less frequently by local spread from an adjacent focus [13]. CNS TB diagnosis and therapeutic response can be confirmed with clinical and laboratory parameters alone. Neuroimaging provides a noninvasive means to improve diagnostic accuracy and detect complications. Computed tomography and magnetic resonance imaging are the two most commonly utilized imaging tools for the evaluation of CNS TB. Postcontrast CT is an established imaging tool for the evaluation of patients suspected of having CNS TB and has the advantage of fast scanning, which diminishes the need for sedation in children. CNS tuberculomas are one form of CNS TB and are usually small brain parenchymal lesions, uni- or multifocal with central isodensity to the cortex, have ring or nodular enhancement, and usually occur with perilesional edema. Associated basal meningitis can also be seen. However, CT cannot accurately differentiate tuberculoma from other CNS infections or neoplastic conditions, both primary and metastatic [4]. Our patient had multiple ring-enhancing right frontotemporal lesions with isodense centers, which is in accordance with the typical description.
The incidence of ocular tuberculosis has been the subject of controversy due to limitations in ocular sampling for microbiology and inexact diagnostic criteria [14]. As a result, a wide range of incidences ranging from 1.4% to 18% have been reported [2]. Moreover, these studies did not exclusively study children, so the true incidence in the pediatric age group is not certain. Intraocular dissemination of TB is hematogenous, and poor immunity, such as severe malnutrition present in our case, is a key risk factor [5,15,16].
According to Sheu et al. [5], TB infection in the eyes can be both extra- and intraocular. Extraocular disease presents as a lid abscess, chronic blepharitis, atypical chalazions, mucopurulent conjunctivitis, and different forms of corneal lesions. This form is easier to diagnose as it is accessible for sampling. Intraocular TB, on the other hand, can be less symptomatic, and bacteriological diagnosis is almost impossible due to inaccessibility. It can range from uveitis involving the iris and ciliary body to choroiditis and retinitis. If it is severe, extensive endophthalmitis can occur. The commonest among these are probably choroidal lesions, including granulomas, which are significant as they are an early sign of disseminated TB.
Diagnosing intraocular TB is challenging, and the presence of a systemic TB infection strongly suggests but is also not definitive [17]. Sample analyzed by culture and PCR from intraocular fluids, is often negative. PCR is a potentially useful method as it can amplify a small sample size, but it is technically challenging. In many resource-limited settings, access to such technologies is largely limited [2]. The strict criteria for diagnosing intraocular tuberculosis include the existence of giant and epithelioid cell composed tubercles, caseating necrosis, and, uncommonly, the presence of acid-fast bacilli. However, the absence of acid-fast bacilli is not considered evidence against TB [5]. For these reasons, presumptive diagnosis and treatment initiation of intraocular TB need to be considered based on supporting clinical grounds. Response to treatment is also a good retrospective diagnostic mechanism, as demonstrated in our case and stated by Alvarez et al. [3].
Imaging differentiation of intraocular TB from retinoblastoma is difficult as both can present similarly with variably calcified retinal based mass lesion. We did not come across any objective criteria that would enable confident pretreatment imaging diagnosis in such cases. Reported CT findings of tuberculous endophthalmitis include diffuse faint vitreal material and thick conjunctival and uveoscleral thickenings [5,18]. Sen et al. [19] reported a case which illustrates the resemblance between retinoblastoma and intraocular TB. A 4-year-old male child presented with leukocoria of his left eye and swelling over his right eye. The left eye was inflamed with no light perception and fundoscopy showed a retinal lesion. A right inferior palpable orbital lesion was also found. Subsequently, brain CT revealed a non-calcified enhancing left posterior vitreous lesion and left parietal lobe ring-enhancing lesions with mass effect. The initial consideration was retinoblastoma with secondary brain involvement. Fortunately for this child, the accessible right orbital lesion was subjected to fine needle aspiration cytology and revealed epithelioid cell granulomas of TB. As shown in the above case, prudence is required to carefully search for TB when the possibility exists, and should start with a complete history and physical examination, sputum smear, and culture, a tuberculin test, and a chest radiograph [9].
Unfortunately, because of the difficulties in clinching a specific diagnosis of intraocular TB, catastrophic treatment outcomes have occurred, in which enucleations were carried out under a misdiagnosis of retinoblastoma [20]. If retinoblastoma cannot be ruled out with confidence, particularly if the affected eye is painful and/or has severely decreased vision, it is justified to do enucleation and pursue pathological diagnosis [18], [19], [20], [21].
Anti-TB treatment for tuberculoma initially combines isoniazid, rifampicin, ethambutol, and pyrazinamide for 2 months, followed by dual continuation therapy of isoniazid and rifampicin. In treating CNS TB, corticosteroid therapy is usually used in cases of extensive cerebral edema or associated meningeal involvement [1,3,6]. The multidrug combination used for CNS TB is also considered appropriate for treating cases of intraocular TB [22].
In conclusion, it is wise to consider and have high levels of suspicion for intraocular TB, in addition to more familiar and commoner conditions like retinoblastoma, in order to avoid unnecessary and potentially sight-losing interventions in the proper clinical conditions, which include exposure history and fitting physical findings, calcified ocular mass on imaging, and concurrent CNS or extra-CNS evidence of TB. Although it is a tough diagnosis, neuroimaging is a noninvasive gateway to the diagnosis and can be used to follow-up the treatment response.
Patient consent
Written informed consent was obtained from the patient's parents for anonymized patient information to be published in this article.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Synmon B, Das M, Kayal AK, Goswami M, Sarma J, Basumatary L, et al. Clinical and radiological spectrum of intracranial tuberculosis: a hospital based study in Northeast India. Indian J Tuberc. 2017;64(2):109–118. doi: 10.1016/j.ijtb.2016.11.011. Epub December 16, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Yeh S, Sen HN, Colyer M, Zapor M, Wroblewski K. Update on ocular tuberculosis. Curr Opin Ophthalmol. 2012;23(6):551–556. doi: 10.1097/ICU.0b013e328358ba01. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez GG, Roth VR, Hodge W. Ocular tuberculosis: diagnostic and treatment challenges. Int J Infect Dis. 2009;13(4):432–435. doi: 10.1016/j.ijid.2008.09.018. Epub April 22, 2009 Apr 22. [DOI] [PubMed] [Google Scholar]
- 4.Laya BF, Concepcion NDP, Andronikou S, Abdul Manaf Z, Atienza MIM, Sodhi KS. Imaging recommendations and algorithms for pediatric tuberculosis: part 2-extrathoracic tuberculosis. Pediatr Radiol. 2023;53(9):1782–1798. doi: 10.1007/s00247-023-05650-5. Epub April 19, 2023. [DOI] [PubMed] [Google Scholar]
- 5.Sheu SJ, Shyu JS, Chen LM, Chen YY, Chirn SC, Wang JS. Ocular manifestations of tuberculosis. Ophthalmology. 2001;108(9):1580–1585. doi: 10.1016/s0161-6420(01)00693-5. [DOI] [PubMed] [Google Scholar]
- 6.Federal Democratic Republic of Ethiopia Ministry of Health. National guidelines for TB, DR-TB and leprosy in Ethiopia. 2018 [Google Scholar]
- 7.Global Tuberculosis Report 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022.
- 8.Naidoo J., Shelmerdine S.C., Charcape C.F.U., Sodhi A.S. Artificial intelligence in paediatric tuberculosis. Pediatr Radiol. 2023:1–13. doi: 10.1007/s00247-023-05606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain SK, Andronikou S, Goussard P, Antani S, Gomez-Pastrana D, Delacourt C, et al. Advanced imaging tools for childhood tuberculosis: potential applications and research needs. Lancet Infect Dis. 2020;20(11):e289–e297. doi: 10.1016/S1473-3099(20)30177-8. Epub June 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Global tuberculosis report 2018. https://www.who.int/publications-detail-redirect/9789241565646
- 11.Kumar J, Meena J, Yadav A, Yadav J. Radiological findings of COVID-19 in children: a systematic review and meta-analysis. J Trop Pediatr. 2021;67(3):fmaa045. doi: 10.1093/tropej/fmaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chervenkov L, Raycheva R, Rangelova V, Doykova K. Chest CT diagnostic potential as a tool for early detection of suspected COVID-19 cases in pandemic peaks. Folia Med (Plovdiv) 2023;65(1):99–110. doi: 10.3897/folmed.65.e71406. [DOI] [PubMed] [Google Scholar]
- 13.Kaba Ö, Kara M, Odacılar CA, Kamer İ, Sütçü M, Demir SÖ, et al. Evaluation of cases of pediatric extrapulmonary tuberculosis: a single center experience. Turk Arch Pediatr Pediatri Arş. 2019;54(2):86–92. doi: 10.14744/TurkPediatriArs.2019.33239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maheshwari V, Alam K, Jain A, Prasad S, Ahmad S, Amitava AK. Ocular tuberculosis misdiagnosed as retinoblastoma: an interesting case. BMJ Case Rep. 2009:0303. doi: 10.1136/bcr.06.2008.0303. Epub March 20, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalvin L.A., Smith W.M. Intraocular manifestations of mycobacterium tuberculosis: a review of the literature. J Clin Tuberculosis Other Mycobacterial dis. 2017;7:13–21. doi: 10.1016/j.jctube.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanches I, Carvalho A, Duarte R. Who are the patients with extrapulmonary tuberculosis? Rev Port Pneumol. 2006;21(2):90–93. doi: 10.1016/j.rppnen.2014.06.010. Epub February 7, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Thompson MJ, Albert DM. Ocular tuberculosis. Arch Ophthalmol. 2005;123(6):844–849. doi: 10.1001/archopht.123.6.844. [DOI] [PubMed] [Google Scholar]
- 18.Raina UK, Tuli D, Arora R, Mehta DK, Taneja M. Tubercular endophthalmitis simulating retinoblastoma. Am J Ophthalmol. 2000;130(6):843–845. doi: 10.1016/s0002-9394(00)00646-2. [DOI] [PubMed] [Google Scholar]
- 19.Sen S, Kashyap S, Singh UB, NagaSuresh V, Chand M, Garg SP. Intraocular tuberculosis mimicking retinoblastoma: a case report. Diagn Cytopathol. 2003;28(2):107–109. doi: 10.1002/dc.10237. [DOI] [PubMed] [Google Scholar]
- 20.Shields JA, Shields CL, Jr Eagle RC, Barrett J, De Potter P. Endogenous endophthalmitis simulating retinoblastoma. The 1993 David and Mary Seslen Endowment lecture. Retina. 1995;15(3):213–219. [PubMed] [Google Scholar]
- 21.Demirci H, Shields CL, Shields JA, Eagle RC. Ocular tuberculosis masquerading as ocular tumors. Surv Ophthalmol. 2004;49(1):78–89. doi: 10.1016/j.survophthal.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 22.The role of anti-tubercular therapy in patients with presumed ocular tuberculosis - PubMed [Internet]. [cited 2024 Feb 4]. Available from: https://pubmed.ncbi.nlm.nih.gov/25615809/ [DOI] [PubMed]