Abstract
Background
In patients with mild type 1 von Willebrand disease (VWD), treatment guidelines suggest individualization of surgical management. However, these conditional recommendations are based on very low–certainty evidence due to limited data on surgical outcomes in this population.
Objectives
To characterize procedural bleeding prophylaxis strategies and outcomes in children with mild type 1 VWD.
Methods
This is a retrospective cohort study that included patients aged between 0 and 21 years with mild type 1 VWD (defined as von Willebrand factor antigen and/or an activity of 30-50 IU/dL) who underwent a procedure from July 1, 2017, to July 1, 2022. Demographic, surgical, medication, and bleeding data were collected by manual chart review.
Results
A total of 161 procedures were performed in 108 patients. The population was primarily female (75%), White (77.8%), and non-Hispanic (79.6%). Median age was 15.8 years (IQR, 8.2-17.6). Fifty-nine surgeries were classified as major, 66 as minor, and 36 as dental. For most procedures, patients received only antifibrinolytics for bleeding prophylaxis (n = 128, 79.5%); desmopressin was used in 17 (10.6%) procedures, and von Willebrand factor concentrate was used in 12 (7.5%) procedures. Bleeding complications occurred in 8 (5.0%) procedures: these included 1 major, 4 clinically relevant nonmajor, and 3 minor bleeding events. No patient required blood transfusion or an additional procedure to achieve hemostasis. Most bleeding complications were seen following intrauterine device (IUD) placement (5/8). Nearly 30% of patients who underwent IUD placement reported bleeding.
Conclusion
Pediatric patients with mild type 1 VWD can safely undergo procedures using a tailored approach. Bleeding complications were uncommon, with the majority following IUD placement.
Keywords: antifibrinolytic agents, bleeding, child, postoperative hemorrhage, surgery, type 1 von Willebrand disease
Graphical abstract
Essentials
-
•
There is limited data to guide the procedural management of mild von Willebrand disease.
-
•
Children with mild von Willebrand disease undergo procedures using various approaches to prevent bleeding.
-
•
Many children safely had procedures with antifibrinolytic medication only to prevent bleeding.
-
•
Most bleeding occurred with intrauterine device insertion, a known cause of irregular bleeding.
1. Introduction
von Willebrand disease (VWD) is the most common inherited bleeding disorder and is characterized by a quantitative (types 1 and 3) or qualitative (type 2) defect in the von Willebrand factor (VWF) protein. Bleeding symptoms in patients with VWD are typically mucocutaneous in nature, often consisting of epistaxis, oral bleeding, heavy menstrual bleeding, and bruising. Individuals with VWD have an increased risk of periprocedural bleeding. Among patients with VWD, the bleeding phenotype is quite heterogeneous, varying between individuals with the same subtype. Fortunately, an individual’s bleeding phenotype can be characterized by validated bleeding assessment tools (BATs) [1]. BATs provide bleeding scores (BS), which have been shown to help predict periprocedural bleeding risk within the adult population [2,3]. While normal BS has been established in the pediatric population, it is less clear how well BS predict procedural bleeding in this population, as its predictive value has not yet been studied in children [4]. Since children have fewer hemostatic challenges, their BS may not be indicative of their true bleeding risk.
The most common VWD subtype, type 1, is characterized by a partial quantitative VWF deficiency [5]. The definition of type 1 VWD has changed throughout the years. In 2021, a multidisciplinary group of international stakeholders developed evidence-based guidelines to standardize the diagnosis. These guidelines placed a high priority on uniformity of diagnosis and access to care [6]. In these guidelines, individuals with VWF levels less than 30 IU/dL, regardless of bleeding, and individuals with VWF levels 30 to 50 IU/dL and a bleeding phenotype are considered to have type 1 VWD. While these individuals were labeled as type 1 VWD at some time points, they were classified as “low VWF” at other times. Low VWF was considered more a risk factor for bleeding than a bleeding disorder [7]. Pathologic genetic variants in VWF are often not found in individuals with moderate reductions in VWF as opposed to those with VWF levels <30 IU/dL [8]. Despite lacking an identifiable genetic variant in VWF, the majority of those followed in hematology centers have abnormal bleeding that is not explained by concurrent hemostatic defects [8,9]. For the purposes of this study, children with VWF antigen (VWF:Ag) and/or activity levels 30 to 50 IU/dL were considered to have “mild VWD,” the population of interest. This population will include all children regardless of BS, given the limitations of BS in the pediatric population.
The mainstays of prevention and treatment of bleeding in VWD are antifibrinolytic medications, desmopressin (DDAVP) acetate, and VWF concentrate. These medications are used alone and in combination to prevent periprocedural bleeding. The periprocedural bleeding prophylaxis plans for individuals with VWD should be tailored to both the inherent risk of the procedure and the bleeding phenotype of the patient [10]. The VWD guidelines provide conditional recommendations based on low certainty of evidence [11].
The guideline recommends targeting factor (F)VIII and VWF activity levels ≥50 IU/dL prior to and for 3 days after major surgical procedures. It also recommends increasing VWF activity levels to ≥50 IU/dL prior to minor surgical or mucosal procedures with the addition of antifibrinolytic therapy over increasing levels without antifibrinolytic therapy. Additionally, the guideline suggests giving antifibrinolytic therapy alone over increasing factor levels for individuals with type 1 VWD, levels >30 IU/dL, and a mild bleeding phenotype undergoing mild mucosal procedures [11]. The guideline highlights the importance of individualized therapy plans based on the procedure and bleeding phenotype.
With new diagnostic guidelines that increase the number of children who qualify for a type 1 VWD diagnosis, there needs to be a focus on optimal periprocedural management. There is a paucity of data evaluating the periprocedural management of children with mild type 1 VWD and associated bleeding complications. We aimed to characterize medications used for procedural bleeding prophylaxis in children with mild VWD and characterize the prevalence of bleeding complications based on a prophylactic medication plan. We hypothesized that these patients would tolerate minor surgeries using only fibrinolytics without adverse bleeding events.
2. Methods
2.1. Study design and participants
This single-center retrospective chart review evaluated the procedural outcomes of pediatric patients with mild type 1 VWD at the Aflac Cancer and Blood Disorders Center at the Children’s Healthcare of Atlanta (CHOA). Approval was obtained from the CHOA Institutional Review Board with a waiver of informed consent and assent. Subjects were identified using the International Classification of Diseases, Ninth and Tenth Revision codes for disorders related to VWD (D68.0, D68.00, D68.01, D68.09, and R79.1). Charts of patients between the ages of 0 and 21, with one of the diagnosis codes for VWD in their problem list, were compiled and reviewed for inclusion in the study.
2.2. Inclusion and exclusion criteria
For inclusion, subjects required a diagnosis of mild type 1 VWD; this was defined as a VWF:Ag, VWF ristocetin cofactor assay (VWF:RCo), and/or VWF:glycoprotein IbM (GPIbM) between 30 and 50 IU/dL and a documented bleeding phenotype. Patients were included even if their only low value was an isolated VWF:RCo. Patients were included only if they underwent a minor, major, or dental procedure between July 1, 2017, and July 1, 2022. Patients were excluded if the most recent VWF laboratory results were drawn more than 3.5 years before the surgery and if the patient had a history of any VWF level less than 30 IU/dL, a history of abnormal VWF multimer distribution, or a concurrent diagnosis of hemophilia. A cutoff of 3.5 years was chosen as the institutional practice is to repeat VWD laboratory tests every 3 years; this study spans the COVID-19 pandemic; thus, this was extended to 3.5 years to allow for brief delays in follow-up given the pandemic.
2.3. Data collection
For each subject, age of diagnosis, sex assigned at birth, self-identified race and ethnicity, and International Society on Thrombosis and Haemostasis (ISTH) BS were collected. BS were calculated by the investigators based on all bleeding documented in the medical record prior to the first procedure. Throughout the manuscript, when the terms male or female are used, they refer to sex assigned at birth, independent of gender identity. The study population includes individuals who identify as non-binary and transgender. VWD laboratory data were collected for each subject: VWF:Ag, VWF:RCo, VWF:GPIbM, and VWF:collagen binding (CB) results from January 1, 2014, to July 1, 2022. To identify those with isolated low VWF:RCo, study team members extracting data from the electronic medical record were asked if VWF:RCo <50 was the only abnormal value. Individuals with isolated low VWF:RCo were analyzed both in the full group and separately, given the limitations of the VWF:RCo assay. Those classified as isolated low VWF:RCo had no other values (VWF:Ag, VWF:GPIbM, and VWF:CB) <50 IU/dL at any point in their laboratory evaluation. This includes laboratory data that fell outside of the time frame of analysis for the study.
For each procedure, the procedure date, procedure type, procedure name, prophylactic medication plan (medications, dosing, and frequency), bleeding complications, thrombotic complications, and follow-up care were reviewed and documented. For individuals with multiple procedures, these data were collected for each procedure. Surgery type (major, minor, and dental) was classified based on previous literature and is defined in the Supplementary Table [12]. Generally, procedures during which a body cavity was entered, a mesenchymal barrier was opened, a fascial plane was entered, normal anatomy was operatively altered, joint space was entered, or a third molar was extracted were considered major. Minor procedures consisted of invasive procedures only involving skin, mucous membranes, or superficial connective tissue. Dental procedures included nonmolar extractions and dental fillings.
Surgical outcomes were defined by the ISTH bleeding definitions. The definition of major bleeding includes fatal bleeding, symptomatic bleeding in a critical area or organ (intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, or pericardial), intramuscular bleeding with compartment syndrome, bleeding that leads to a drop in hemoglobin of 2 g/dL, or leading to a transfusion of 2 units (or 2 10-mL/kg aliquots) of packed red blood cells. Major bleeding also includes surgical site bleeding requiring a second surgical intervention and hemarthrosis that interferes with rehabilitation. Nonmajor clinically relevant bleeding includes surgical site bleeding that is unexpected and prolonged and/or sufficiently large to cause hemodynamic instability. It also includes hospital admission for bleeding, medical or surgical treatment for bleeding (that does not meet criteria for major bleeding), intraoperative bleeding, return visit to the emergency department for bleeding, need for blood product support, and need for unplanned medication. Nonclinical relevant minor bleeding is defined as patient-relevant bleeding, which leads to a call to physicians or nurses postoperatively for bleeding but does not lead to any intervention or any complications. Charts were reviewed for any bleeding events in the 30 days following the procedure.
Data were gathered in Research Electronic Data Capture, a secure Health Insurance Portability and Accountability Act–compliant database hosted through CHOA. Data was collected from the electronic medical record by manual extraction. All members of the research team followed a uniform protocol for data extraction to limit bias or subjective influence.
2.4. Statistical analysis
Descriptive statistics were performed for all demographic and clinical variables. A median with IQR was identified for each continuous variable. Comparisons between groups were performed using Fisher’s exact test and Mann–Whitney U-test. Analysis was performed on the group in its entirety, with subgroup analysis for individuals with isolated low VWF:RCo assay and individuals with normal BS.
3. Results
3.1. Study participants
Initial screening with International Classification of Diseases, 10th Revision codes and the presence of a periprocedural note from the bleeding disorder team provided a list of 379 unique subjects. A manual chart review for inclusion and exclusion criteria identified 108 unique patients with mild type 1 VWD and a surgical or dental procedure within the last 5 years. A total of 161 procedures in the 108 patients were analyzed for bleeding complications.
Subject demographics are outlined in Table 1. The patient population was primarily female (75%), White (77.8%), and non-Hispanic (79.6%). The median age at the time of surgery was 15.8 years (IQR, 8.2-17.6). One-half of the subjects had type O blood (50.5%). About 30% (33 subjects) had isolated low VWF:RCo; of these, 26 had normal VWF:GPIbM or VWF:CB, and 7 had no other VWF:activity testing. The median number of times VWF levels were drawn during the study period was 2 (IQR, 2-3).
Table 1.
Study population demographics.
| Characteristic | Values (N = 108) |
|---|---|
| Age at the time of procedure (y), median (IQR) | 15.8 (8.3-17.6) |
| Sex, n (%) | |
| Female | 81 (75.6) |
| Race, n (%) | |
| Asian | 1 (0.9) |
| Black or African American | 15 (14.0) |
| White or Caucasian | 83 (77.6) |
| Multiracial | 1 (0.9) |
| Not known, unlisted | 7 (6.5) |
| Ethnicity, n (%) | |
| Hispanic/Latino | 20 (18.7) |
| Age at diagnosis, median (IQR) | 14.0 (7.7-16.3) |
| Blood type, n (%) | |
| A | 18 (16.8) |
| B | 4 (3.7) |
| AB | 0 (0) |
| O | 54 (50.5) |
| Unknown | 31 (29.0) |
| Diagnostic laboratory values,a median (IQR) | |
| FVIII (n = 108) | 90 (78-108.5) |
| VWF:Ag (n = 108) | 56 (48-62) |
| VWF:RCo (n = 108) | 41 (37.5-45) |
| VWF:GPIbM (n = 80) | 48 (43-54) |
| VWF:CB (n = 8) | 46 (41.75-52) |
| Isolated low VWF:RCo, n (%) | 33 (30.8) |
| Isolated low VWF:RCo, other functional level drawn, and normal, n (%) | 26 (24.3) |
| Number of times VWD levels drawn in the timeframe, median (IQR) | 2 (2-3) |
| Bleeding score, median (IQR) | 4 (3-5) |
Ag, antigen; CB, collagen binding; FVIII, factor VIII; GP, glycoprotein; RCo, ristocetin cofactor; VWD, von Willebrand disease; VWF, von Willebrand factor.
Earliest full set of diagnostic levels.
Subjects had a variable phenotype with ISTH BAT BSs ranging from 0 to 14 (median, 4; IQR, 3-5). Ten patients had a normal BS (range, 0-2.) Of those with normal BS, 5 had a family history of VWD. Two patients experienced isolated heavy menstrual bleeding (BS, 2), 1 had severe unexplained anemia as a toddler, 1 experienced a prolonged, severe bleeding episode after a minor procedure requiring an emergency room visit at the age of 2 years, and 1 patient had concurrent hereditary hemorrhagic telangiectasia.
3.2. Procedures and periprocedural prophylaxis
Of the 161 procedures, 59 were classified as major surgeries, 66 were minor surgeries, and 36 were dental (Table 2).
Table 2.
Prophylaxis details by surgery risk status.
| Type of surgery | Procedural characteristics | Antifibrinolytic therapy only | Antifibrinolytic therapy + DDAVP | Antifibrinolytic therapy + VWF concentrate | DDAVP only | None |
|---|---|---|---|---|---|---|
Major
|
Number of events | 44 | 12 | 3 | 0 | 0 |
| Antifibrinolytic frequency | Twice daily (1); 3 times daily (41); 4 times daily (1) | Three times daily | Three times daily | NA | NA | |
| Antifibrinolytic duration (d) | 8 (5-10) | 7 (7-10) | 10 (7.5-10) | NA | NA | |
| DDAVP or VWF concentrate dose (n), route of administration,and dosage | NA | Dose (n): 1 Route: IN (10), i.v. (2) Dose: weight-based (IN) 0.3 mcg/kg i.v. | Dose (n): median 1, range 1-3 Route: i.v. Dose: 40-50 units/kg | NA | NA | |
| DDAVP or VWF concentrate timing | NA | Preprocedural (n = 12) | Preprocedural (n = 2) Preprocedural, days 1 and 3 (n = 1) |
NA | NA | |
| Bleeding events | 0 | 1 major: hemoglobin drop >2 g, hospital admission | 0 | 0 | 0 | |
| Bleeding event treatment | NA | Antifibrinolytics, DDAVP | NA | NA | NA | |
| Minor Invasive operative procedure in which only skin, mucous membranes, or superficial connective tissue was manipulated. Includes scopes, IUD insertion, therapeutic intramuscular injections, tympanostomy, and bone marrow biopsy. |
Number of events | 56 | 2 | 4 | 1 | 3 |
| Antifibrinolytic frequency | Daily (6); twice daily (6); 3 times daily (40); 4 times daily (2); unknown (2) | Three times daily (1); 4 times daily (1) | Three times daily (4) | NA | NA | |
| Antifibrinolytic duration (d) | 2 (2-3) | 3.5 (2.75-4.25) | 4 (2.75-5) | NA | NA | |
| DDAVP or VWF concentrate dose (n), route of administration, and dosage | NA | Dose: 1 Route: IN (2) Dose: weight-based (IN) |
Dose: 1 Route: i.v. (4) Dose: 40 (37.1-42.5) units/kg | Dose: 1 Route: IN Dose: weight-based | NA | |
| DDAVP or VWF concentrate timing | NA | Preprocedural (n = 2) | Preprocedural (n = 4) | Preprocedural | NA | |
| Bleeding events | 4 - 2 CRNM - 2 calls to the nursing team |
1 1 CRNM: ER visit for surgical site bleeding |
1 1 CRNM: surgical site bleeding requiring medication |
0 | 1 1 CRNM: surgical site bleeding, call to nurses, ER visit for bleeding |
|
| Bleeding event treatment | Antifibrinolytics (2) | Antifibrinolytics | VWF concentrate | NA | Antifibrinolytics | |
| Dental Nonthird molar extractions, crowns, and fillings |
Number of events | 28 | 3 | 5 | 0 | 0 |
| Antifibrinolytic frequency | Daily (2); twice daily (3); 3 times daily (23) | Three times daily (3) | Three times daily (5) | |||
| Antifibrinolytic duration (d) | 5 (3-10) | 10 (8.5-10) | 3 (2-7) | |||
| DDAVP or VWF concentrate dose (n), route of administration, and dosage | NA | Dose (n): 1 Route: IN (2), i.v. (1) Dose: weight-based (IN) 0.3 mcg/kg (i.v.) |
Dose (n): 1 Route: i.v. (5) Dose: 50 units/kg (range, 30-50) |
|||
| DDAVP or VWF concentrate timing | NA | Preoperative (3) | Preoperative (5) | |||
| Bleeding events | 0 | 0 | 0 | |||
| Bleeding event treatment | NA | NA | NA |
Data are reported as median with IQR, unless otherwise stated.
CRNM, clinically relevant nonmajor bleeding; DDAVP, desmopressin; Dose (n), number of doses; ER, emergency room; IN, intranasal; IUD, intrauterine device; i.v., intravenous; NA, not applicable; VWF, von Willebrand factor.
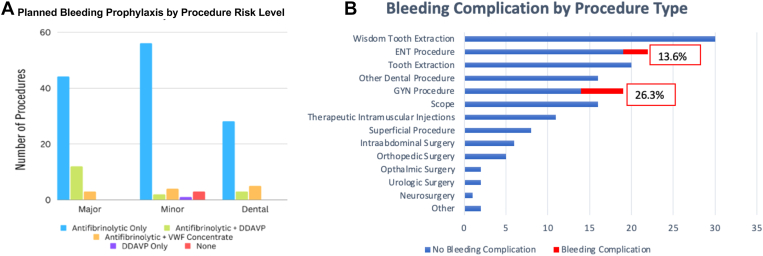
Bleeding management plans varied in both duration and medications given. Bleeding prevention plans included antifibrinolytic therapy only (79.5%), antifibrinolytic therapy with DDAVP (10.6%), antifibrinolytic therapy with VWF concentrate (7.5%), DDAVP alone (0.6%), and no medications (1.9%). Various prophylactic regimens were used for each procedure type (Figure). The first dose of antifibrinolytic therapy was given prior to the procedure in all instances. In all cases, antifibrinolytics were continued postprocedurally for a duration ranging from 3 to 10 days. VWF concentrate, and DDAVP doses were given once preprocedurally. One patient also received VWF concentrate on postprocedural days 1 and 3. All individuals given DDAVP for procedural prophylaxis had undergone a DDAVP trial to ensure adequate response to DDAVP.
Figure.
(A) Planned bleeding prophylaxis by procedure risk level and (B) bleeding complication incidence by procedure type. DDAVP, desmopressin; ENT, ear, nose, and throat; GYN, gynecologic; VWF, von Willebrand factor.
3.3. Bleeding outcomes in the full cohort
Of the 161 procedures, 8 bleeding complications occurred (5%; 95% CI, 2.2%-9.6%). One was classified as a major bleeding event, 4 were clinically relevant nonmajor bleeding events, and 3 were minor (nonclinically relevant) bleeding events. Bleeding events occurred with a variety of different treatment plans, as outlined in Table 2. Details about each bleeding event are outlined in Table 3.
Table 3.
Bleeding events with surgery risk status, prophylaxis plan, and bleeding treatment.
| Surgical procedure | Risk level for the procedure | Medication plan for the procedure | Documented noncompliance | Bleeding events | ISTH severity | Medications needed |
|---|---|---|---|---|---|---|
| IUD insertion | Minor | Tranexamic acid 1300 mg (24.6 mg/kg) 3 times daily for 2 d | Yes | Call to nurses for vaginal bleeding | Minor | Tranexamic acid |
| IUD insertion | Minor | Tranexamic acid 1300 mg (27 mg/kg) 3 times daily for 3 d | No | Call to nurses for vaginal bleeding. Need for unplanned medication | Clinically relevant nonmajor bleeding | Tranexamic acid |
| IUD insertion | Minor | Tranexamic acid 1300 mg (18.5 mg/kg) 3 times daily for 2 d and VWF concentrate 28 units/kg before surgery | No | Call to nurses for vaginal bleeding. Unplanned medication | Clinically relevant nonmajor bleeding | VWF concentrate |
| IUD insertion | Minor | None | No | Call to nurses for vaginal bleeding. Need for unplanned medication | Clinically relevant nonmajor bleeding | Tranexamic acid |
| IUD insertion | Minor | Tranexamic acid 1300 mg (18 mg/kg) 3 times daily for 2 d | No | Call to nurses for vaginal bleeding | Minor | None |
| Tympanostomy tube replacement and dental debridement | Minor | Tranexamic acid 325 mg (17.6 mg/kg) twice daily for 1 d | No | Call to nurses | Minor | None |
| Nasal endoscopy with inferior turbinate reduction | Minor | Aminocaproic acid 30 mg/kg 4 times daily × 2 d DDAVP IN × 1 before the procedure | Yes | Surgical site bleeding. ER visit for bleeding. Need for unplanned medication | Clinically relevant nonmajor bleeding | Aminocaproic acid |
| Tonsillectomy and adenoidectomy | Major | Tranexamic acid 650 mg (15.5 mg/kg) 3 times daily × 10 d DDAVP IN × 1 before the procedure | No | ER visit and unplanned hospitalization for bleeding. Need for unplanned medication. Drop in hemoglobin >2 g/dL | Major | DDAVP, tranexamic acid |
BAT, bleeding assessment tool; BS, bleeding score; DDAVP, desmopressin; ISTH, International Society on Thrombosis and Haemostasis; VWF, von Willebrand factor; RCo, ristocetin cofactor.
DDAVP, desmopressin; ER, emergency room; IN, intranasal; ISTH, International Society of Thrombosis and Haemostasis; IUD, intrauterine device; VWF, von Willebrand factor.
The only major bleeding event occurred after a tonsillectomy and adenoidectomy. Despite DDAVP and tranexamic acid with reported compliance, the patient experienced postoperative bleeding, leading to a drop in hemoglobin of greater than 2 g/dL, hospitalization, and additional DDAVP and tranexamic acid. This bleeding event occurred approximately 48 hours postoperatively, which is considered secondary hemorrhage.
One clinically relevant nonmajor bleeding episode occurred after inferior nasal turbinate reduction. The patient required an extension of antifibrinolytic therapy past the 48 hours originally planned. The 3 additional clinically relevant nonmajor bleeding events occurred after intrauterine device (IUD) insertion for heavy menstrual bleeding. In total, 5 of the 8 bleeding events (62.5%) occurred after IUD insertions. Bleeding complications were reported in 26.3% of all IUD gynecologic procedures and 13.6% of all ear, nose, and throat (ENT)–related procedures. Bleeding event prevalence by surgical type is outlined in the Figure.
The population who experienced a bleeding complication had no significant difference in age, sex, or proportion of individuals with isolated low VWF:RCo (Table 4). The median preprocedural ISTH BAT BS was 5 (IQR, 4-7) in the group experiencing a bleeding complication vs 4 (IQR, 3-5) in those without a bleeding complication (P = .055).
Table 4.
Population comparisons between groups who experienced a bleeding complication vs those who did not experience a bleeding complication.
| Characteristic | Bleeding complication | No bleeding complication | P value |
|---|---|---|---|
| Demographics | n = 7 | n = 101 | |
| Age | 14.5 (11.5-16.8) | 15.8 (8.1-17.6) | .719a |
| Female sex | 7 (100%) | 74 (73.3%) | .19b |
| ISTH BAT BS | 5 (4-7) | 4 (3-5) | .055a |
| Isolated low VWF:RCo | 2 (28.6) | 31 (30.7) | .837b |
| Procedure information | |||
| No. of procedures | 8 | 153 | |
| Surgical type | |||
| Major | 1 (12.5) | 58 (38.2) | |
| Minor | 7 (87.5) | 59 (38.8) | |
| Dental | 0 | 36 (23.5) | .031b |
| Prophylaxis plan | |||
| Antifibrinolytic only | 4 (50) | 124 (81.7) | |
| Antifibrinolytic + DDAVP | 2 (25) | 15 (9.8) | |
| Antifibrinolytic + VWF concentrate | 1 (12.5) | 11 (7.2) | |
| DDAVP only | 0 | 1 (0.7) | |
| None | 1 (12.5) | 2 (1.3) | .048b |
BAT, bleeding assessment tool; BS, bleeding score; DDAVP, desmopressin; ISTH, International Society on Thrombosis and Haemostasis; VWF, von Willebrand factor; RCo, ristocetin cofactor.
DDAVP, desmopressin; ER, emergency room; IN, intranasal; ISTH, International Society of Thrombosis and Haemostasis; IUD, intrauterine device; VWF, von Willebrand factor.
Mann–Whitney U-test.
Fisher’s exact test.
There was no significant difference in bleeding rates between the first procedure performed on any one individual (5.6%; 95% CI, 2.1%-11.7%) vs a subsequent procedure performed on an individual (3.8%; 95% CI, 0.4%-13.0%). One major bleeding event occurred in a subsequent procedure.
The hemostatic evaluation of all individuals who experienced bleeding events includes prothrombin time, activated partial thromboplastin time, and fibrinogen. Three individuals had platelet aggregation testing, 2 prior to the bleeding event and 1 following the bleeding event. Two individuals had testing of FIX and FXIII levels. All of the testing for additional bleeding disorders was normal.
3.4. Analysis removing the isolated VWF:RCo cohort
The definition of mild type 1 VWD in this population included any VWF:Ag or activity assay of 30 to 50 IU/dL; therefore, individuals with isolated low VWF:RCo were included in the analysis. Thirty-three subjects had an isolated low VWF:RCo. Given the concerns for the accuracy of VWF:RCo in measuring VWF activity, particularly in populations with a high frequency of the benign variant c.4414G>C; p.D1472DH, recent guidelines give preference for nonristocetin-based VWF functional assays [13]. As this group may not truly qualify for a diagnosis of mild VWD, we performed an analysis that excluded individuals with isolated low VWF:RCo. There were 41 procedures in 33 patients with isolated low VWF:RCo.
After removing the 33 individuals with isolated low VWF:RCo, there were 120 procedures in 75 individuals. Overall, there were 6 bleeding events (5%); 3 were IUD placement, and the remaining 3 were related to procedures involving the ears, nose, tonsils, and adenoids (Table 5). When comparing the isolated low VWF:RCo cohort to the VWD cohort, there was no difference in bleeding frequency or surgical types (Table 5). There was some variation in treatment plans; no subjects with isolated low VWF:RCo received VWF concentrates.
Table 5.
Comparison of the population with isolated low von Willebrand factor ristocetin cofactor vs type 1 von Willebrand disease.
| Characteristic | Isolated low VWF:RCo n = 33 | Type 1 VWD n = 75 | P value |
|---|---|---|---|
| Demographics | |||
| Age | 15.8 (11.0-17.9) | 15.8 (7.7-17.4) | .90a |
| Female sex | 25 (61.0%) | 56 (74.7%) | .12b |
| ISTH BAT BS | 4 (3-5) | 4 (3-5) | .26a |
| Procedure information | |||
| No. of procedures | 41 | 120 | |
| Bleeding events | 2 | 6 | |
| Bleeding frequency | 4.9% | 5.0% | .84b |
| Surgical type | |||
| Major | 16 (39.0%) | 43 (35.8%) | |
| Minor | 17 (41.5%) | 49 (40.8%) | |
| Dental | 8 (19.5%) | 28 (23.3%) | .86b |
| Prophylaxis plan | |||
| Antifibrinolytic only | 35 (85.4) | 93 (77.5) | |
| Antifibrinolytic + DDAVP | 4 (9.8) | 13 (10.8) | |
| Antifibrinolytic + VWF concentrate | 0 | 12 (10.0) | |
| DDAVP only | 0 | 1 (0.8) | |
| None | 2 (4.9) | 1 (0.8) | .07b |
BAT, bleeding assessment tool; BS, bleeding score; DDAVP, desmopressin; ISTH, International Society on Thrombosis and Haemostasis; VWD, von Willebrand disease; VWF, von Willebrand factor; RCo, ristocetin cofactor.
Mann–Whitney U-test.
Fisher’s exact test.
3.5. Stratification of bleeding in individuals with normal BSs
There were 9 procedures performed in 9 children with normal BSs. Four were classified as major, 4 minor, and 1 dental. Antifibrinolytic therapy was used in all cases, and 1 subject received a VWF concentrate infusion. No bleeding events occurred in this cohort. When this cohort was removed from the overall population, the bleeding incidence in individuals with abnormal BSs was 5.3%, similar to the overall incidence of bleeding in the full cohort (5%).
3.6. Bleeding related to specific procedure types
3.6.1. Wisdom tooth extraction
Extraction of the third molars, commonly known as wisdom teeth, is one of the most frequently performed surgeries in the United States each year. In this cohort, 30 cases of third molar extraction were performed without any bleeding complications. Each of the 30 cases involved oral antifibrinolytic therapy, with an average duration of 8.5 days. In addition, 23% of patients undergoing third molar extraction received a single preoperative dose of DDAVP.
3.6.2. Simple tooth extractions and simple dental procedures
Roughly 22% (35) of the recorded procedures were simple tooth extractions or simple dental procedures. No bleeding complications were reported in any of these procedures. Each of the 35 cases received periprocedural antifibrinolytic therapy. The duration of antifibrinolytic therapy ranged from 1 to 10 days, with a median of 7 days (IQR, 3-10). Three patients received 1 preprocedural dose of DDAVP, and 5 patients received 1 preprocedural dose of VWF concentrate for bleeding prevention.
3.6.3. ENT procedures: general ENT tonsillectomy and adenoidectomy
Twenty-two of the 161 procedures were ENT procedures; 8 of these were tonsillectomy and adenoidectomy. Tonsillectomy and adenoidectomy procedures were primarily managed with high-dose antifibrinolytic therapy, with 2 patients receiving antifibrinolytic therapy and intranasal DDAVP. Antifibrinolytic therapy was typically continued for 10 days. Antifibrinolytic therapy was provided in each of the 16 general ENT procedures for a median duration of 2 days (IQR, 1-2). In the general ENT cohort, 1 participant received a single dose of DDAVP, and 1 participant received a single dose of a VWF concentrate product.
In the ENT procedures cohort, there were 3 reported bleeding complications (13%; 95% CI, 2.9%-34.9%). One bleeding complication was classified as a major event (hemoglobin drop of >2 g/dL) in a patient undergoing tonsillectomy and adenoidectomy; the other 2 were clinically relevant nonmajor bleeding events.
3.6.4. IUD insertion
There were 17 IUD placements during the study period. All but 1 individual received antifibrinolytic therapy prior to IUD insertion. Two patients received a single VWF concentrate infusion (28-40 units/kg) prior to the procedure. One patient received no prophylactic medication. There were 5 bleeding events (Table 4). The prevalence of bleeding events in this cohort was 29.4% (95% CI, 10.3%-56.0%). Three events were classified as clinically relevant nonmajor bleeding events: 2 patients received additional doses of tranexamic acid, 1 patient received a progesterone-only hormonal therapy, and 1 patient received an additional dose of VWF concentrate. Two bleeding events were classified as minor bleeding events; in these instances, the subjects had contact with the medical team about bleeding concerns, but no clinical actions were initiated. The duration of bleeding ranged from 3 to 7 days following insertion, with 1 subject reporting ongoing bleeding for 5 weeks. There was 1 IUD expulsion, which occurred in an individual who reported heavy bleeding and received both additional antifibrinolytic therapy and hormonal therapy after insertion.
4. Discussion
This study demonstrates a low overall incidence of bleeding complications in children and adolescents with mild VWD undergoing procedures using various medication strategies when compared to known bleeding rates of these procedures. With an appropriate bleeding plan in place, children and adolescents with mild VWD can safely undergo a wide variety of surgeries and procedures, often without the need for VWF concentrate infusion. Most subjects underwent procedures with antifibrinolytic therapy alone; this strategy aligns with recommendations in the American Society of Hematology 2021 VWD Treatment Guidelines, which suggest giving antifibrinolytic therapy alone for mild mucosal procedures performed in individuals with type 1 VWD, levels >30 IU/dL, and a mild bleeding phenotype [11]. Our study provides real-world data supporting this recommendation and will help inform future prospective studies evaluating this strategy. Few patients received DDAVP despite the high prevalence of DDAVP responsiveness in patients with mild type 1 VWD. This is related to the lack of commercial availability of intranasal DDAVP coinciding with the study time period. The use of DDAVP carries a risk of hyponatremia and requires fluid restriction; the need for intravenous fluids during procedures may also limit the use of DDAVP, especially in the pediatric population, which is more susceptible to hyponatremia.
This study reports the procedural outcomes of patients with VWF levels of 30% to 50%, regardless of BS. While the new diagnostic guidelines require abnormal bleeding in addition to abnormal VWF levels, BS poses a particular challenge in the pediatric population. As young children often have not experienced a bleeding challenge, their bleeding phenotype has not been established. Several children in our cohort had a normal personal BS but a family history of VWD with a bleeding phenotype. In these circumstances, our standard was to give periprocedural medications, primarily with antifibrinolytic agents, to prevent bleeding events.
IUD insertions accounted for most bleeding complications (62.5%), and gynecological procedures had the highest prevalence of bleeding complications (26.3%). It is unclear given the available literature if this rate of bleeding is atypical for individuals undergoing IUD insertion. One historical paper estimated that up to 94% of users reported bleeding in the days following IUD insertion [14]. Given the retrospective nature of this project, we were unable to quantify the amount of bleeding to determine if the bleeding experienced by adolescents with VWD was more extensive than the general population. The rate of bleeding after IUD placement in patients with bleeding disorders has not been previously described; the prevalence of IUD-associated bleeding complications leading to interaction with the medical team in our cohort was 30% (5 of 17 subjects). This is a small cohort, and larger prospective data can help to provide a more accurate estimation of the rate of periprocedural bleeding for women and girls with VWD.
Given the known and frequent irregular bleeding associated with IUD placement, it is unclear if using antifibrinolytic therapy around the time of insertion impacted the bleeding rates. While 3 subjects received medications for postprocedural bleeding, 2 subjects contacted their medical team and had no recommended changes to their treatment plan. In our analysis, we considered this minor bleeding. The ISTH Subcommittee on Pediatric and Neonatal Thrombosis and Hemostasis published a practice guideline defining these outcomes as “patient important bleeding, no intervention” [15] and specifically as “bleeding for which a patient seeks care but there is no change in management.” This type of bleeding event is important to consider in clinical studies; although these events do not require a change in management, they are bothersome to families and patients and impact the quality of life. As such, this bleeding classification should be included in prospective studies in this population.
Tonsillectomy and adenoidectomy procedures, and ENT procedures more generally, are associated with higher bleeding risks and complications in the nonbleeding disorder population. Hemorrhagic complications after tonsillectomy with or without adenoidectomy are well documented in the literature. Studies have shown rates of primary hemorrhage (within 24 hours) to be between 0.2% and 2.2% and rates of secondary hemorrhage (>24 hours after surgery) to be between 0.1% and 3% [[16], [17], [18], [19], [20]]. A 2011 multicenter cohort study that defined hemorrhage more broadly reported hemorrhage complication rates of up to 15% for tonsillectomy with or without adenoidectomy [21]. A study in children with VWD or hemophilia showed a primary hemorrhage rate of 1.6% and a secondary hemorrhage rate of 15% [22]. In our cohort, 1 out of 8 (12.5%) of the tonsillectomy and adenoidectomy procedures ended with a bleeding event. Given the small sample size, the focus of our prospective work should center on the optimal strategy to prevent postoperative hemorrhage in tonsillectomy and adenoidectomy in children with VWD.
Overall, bleeding rates for tonsillectomy appear to align with previously documented literature, and most overall bleeding events in our cohort occurred after IUD placement. Further prospective studies will help determine the optimal treatment of children with mild type 1 VWD undergoing procedures.
4.1. Limitations
The major limitations of this study are the retrospective nature and single-center setting. All data was reported from 1 urban academic center; treatment plans were created by a variety of providers, some hemostasis experts, and other general pediatric hematology oncology physicians. Given the single institution, the treatment decisions are biased to the treating patterns of that institution and likely are not representative of other centers. Further research could prospectively enroll at academic and nonacademic locations with geographic diversity.
Given the paucity of data on bleeding events following IUD placement in the adolescent population, we are unable to compare the rate of bleeding events in the study to the general population.
As mentioned in the discussion, the study is also limited by the inclusion of patients with normal BS and those with isolated low VWF:RCo. Individuals with normal BS were included due to the challenges associated with using BS for risk stratification in young patients without bleeding challenges and a known family history of bleeding; however, their individual bleeding phenotype had not been determined. Secondly, the inclusion of individuals with isolated low VWF:RCo may lead to the inclusion of individuals who do not have VWD but rather have the benign variant c.4414G>C; p.D1472DH, which leads to low VWF:RCo despite normal VWF activity. Given the lack of data for alternative VWF activity assays in many patients, these patients were included in the overall analysis.
A final limitation of the retrospective chart review study is the self-reported nature of some bleeding complications. Bleeding events were identified via chart review and are subject to reporting bias. Since most patients have antifibrinolytic medications at home for as-needed use, it is possible that patients received additional medication for bleeding without contacting the medical team. Prospective studies with follow-up phone calls postprocedurally would provide complete data. We characterized any phone call to the clinical team for bleeding concerns as a minor complication; given that some bleeding after IUD insertion is often considered normal, we could be overcounting bleeding episodes.
4.2. Generalizability
The study used data from the Aflac Cancer and Blood Disorders Center at CHOA, a pediatric specialty clinic at an academic institution in the United States. The single-center nature of this study limits the overall generalizability, and a prospective multicenter study would help to determine if these findings are generalizable to children and adolescents with mild VWD across a variety of settings.
Acknowledgments
Funding
S.F.M. was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under award numbers TL1TR002382 and UL1TR002378.
Ethics statement
The study was approved by the Children’s Healthcare of Atlanta Institutional Review Board with a waiver of informed consent and assent.
Author contributions
M.C.B. created the concept and design of the study, analyzed the data, and wrote the manuscript. R.F.S. assisted in creating and designing the study. S.H. assisted in designing the study, analyzed the data, and wrote the manuscript. K.Z., S.F.M., M.H.W., S.D., and C.T. collected data and contributed to writing and editing the manuscript. All authors edited and approved the manuscript.
Relationship Disclosure
R.F.S. participated in advisory boards for Takeda, Novo Nordisk, Bayer, Pfizer, Octapharma, Vega Therapeutics, Roche/Genentech, Hema Biologics, and LFB. R.F.S. has investigator initiated support from Takeda, Genentech, Octapharma, Hema Biologics, and LFB. R.F.S. has received honoraria from Sanofi/Sobi, Octapharma, Takeda, Bayer, Pfizer, Guardian Therapeutics, Vega, LFB, Hema Biologics, Novo Nordisk and Genentech. R.F.S. participates on Data and Safety Monitoring Board for Uniqure. R.F.S. has a leadership role on American Thrombosis and Hemostasis Network Board, Hemophilia Federation of American medical advisor, Medical and Scientific Advisory Council, and International Society of Thrombosis and Haemostasis Chair of Women’s Health Issues in Haemostasis and Thrombosis. M.C.B. has investigator-initiated support from Star Therapeutics and Sanguina. M.C.B. has served on an advisory board for Genentech and Hema Biologics. M.C.B. serves on the advisory board of Partners Physician Academy; and is a cochair of ISTH Von Willebrand Factor group. K.Z. has research funding from Pfizer, CSL Behring (through Hemostasis and Thrombosis Research Society), and Takeda (through National Hemophilia Foundation). K.S. has received travel support from Octapharma and Hemophilia of Georgia and honoraria from Hemophilia of Georgia. K.S. has participated on an advisory board for Hema Biologics. M.H.W. is the Hemostasis and Thrombosis Research Society Trainee Workshop Committee Chair.
Footnotes
Handling Editor: Dr Bethany Samuelson Bannow
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2024.102334.
Supporting Information
References
- 1.Rydz N., James P.D. The evolution and value of bleeding assessment tools. J Thromb Haemost. 2012;10:2223–2229. doi: 10.1111/j.1538-7836.2012.04923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Federici A.B., Bucciarelli P., Castaman G., Mazzucconi M.G., Morfini M., Rocino A., et al. The bleeding score predicts clinical outcomes and replacement therapy in adults with von Willebrand disease. Blood. 2014;123:4037–4044. doi: 10.1182/blood-2014-02-557264. [DOI] [PubMed] [Google Scholar]
- 3.Tosetto A., Rodeghiero F., Castaman G., Goodeve A., Federici A.B., Batlle J., et al. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM-1 VWD) J Thromb Haemost. 2006;4:766–773. doi: 10.1111/j.1538-7836.2006.01847.x. [DOI] [PubMed] [Google Scholar]
- 4.Elbatarny M., Mollah S., Grabell J., Bae S., Deforest M., Tuttle A., et al. Normal range of bleeding scores for the ISTH-BAT: adult and pediatric data from the merging project. Haemophilia. 2014;20:831–835. doi: 10.1111/hae.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabih A., Babiker H.M. StatPearls; 2022. Von Willebrand disease. Treasure Island (FL) [PubMed] [Google Scholar]
- 6.James P.D., Connell N.T., Ameer B., Di Paola J., Eikenboom J., Giraud N., et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021;5:280–300. doi: 10.1182/bloodadvances.2020003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadler J.E. Von Willebrand disease type 1: a diagnosis in search of a disease. Blood. 2003;101:2089–2093. doi: 10.1182/blood-2002-09-2892. [DOI] [PubMed] [Google Scholar]
- 8.Lavin M., Aguila S., Schneppenheim S., Dalton N., Jones K.L., O'Sullivan J.M., et al. Novel insights into the clinical phenotype and pathophysiology underlying low VWF levels. Blood. 2017;130:2344–2353. doi: 10.1182/blood-2017-05-786699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivaths L., Minard C.G., O’Brien S.H., Wheeler A.P., Mullins E., Sharma M., et al. The spectrum and severity of bleeding in adolescents with low von Willebrand factor-associated heavy menstrual bleeding. Blood Adv. 2020;4:3209–3216. doi: 10.1182/bloodadvances.2020002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Donnell J.S., Lavin M. Perioperative management of patients with von Willebrand disease. Hematology Am Soc Hematol Educ Program. 2019;2019:604–609. doi: 10.1182/hematology.2019000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connell N.T., Flood V.H., Brignardello-Petersen R., Abdul-Kadir R., Arapshian A., Couper S., et al. ASH ISTH NHF WFH 2021 guidelines on the management of von Willebrand disease. Blood Adv. 2021;5:301–325. doi: 10.1182/bloodadvances.2020003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orsini S., Noris P., Bury L., Heller P.G., Santoro C., Kadir R.A., et al. Bleeding risk of surgery and its prevention in patients with inherited platelet disorders. Haematologica. 2017;102:1192–1203. doi: 10.3324/haematol.2016.160754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flood V.H., Gill J.C., Morateck P.A., Christopherson P.A., Friedman K.D., Haberichter S.L., et al. Common VWF exon 28 polymorphisms in African Americans affecting the VWF activity assay by ristocetin cofactor. Blood. 2010;116:280–286. doi: 10.1182/blood-2009-10-249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ylikorkala O., Kauppila A., Siljander M. Anti-prostglandin therapy in prevention of side-effects of intrauterine contraceptive devices. Lancet. 1978;2:393–395. doi: 10.1016/s0140-6736(78)91864-0. [DOI] [PubMed] [Google Scholar]
- 15.Whitworth H., Amankwah E.K., Betensky M., Castellucci L.A., Cuker A., Goldenberg N.A., et al. Updated guidance for efficacy and safety outcomes for clinical trials in venous thromboembolism in children: communication from the ISTH SSC Subcommittee on Pediatric and Neonatal Thrombosis and Hemostasis. J Thromb Haemost. 2023;21:1666–1673. doi: 10.1016/j.jtha.2023.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Luca Canto G., Pachêco-Pereira C., Aydinoz S., Bhattacharjee R., Tan H.L., Kheirandish-Gozal L., et al. Adenotonsillectomy complications: a meta-analysis. Pediatrics. 2015;136:702–718. doi: 10.1542/peds.2015-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramanyam R., Varughese A., Willging J.P., Sadhasivam S. Future of pediatric tonsillectomy and perioperative outcomes. Int J Pediatr Otorhinolaryngol. 2013;77:194–199. doi: 10.1016/j.ijporl.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Lowe D., van der Meulen J., Cromwell D., Lewsey J., Copley L., Browne J., et al. Key messages from the National Prospective Tonsillectomy Audit. Laryngoscope. 2007;117:717–724. doi: 10.1097/mlg.0b013e318031f0b0. [DOI] [PubMed] [Google Scholar]
- 19.Østvoll E., Sunnergren O., Stalfors J. Increasing readmission rates for hemorrhage after tonsil surgery: a longitudinal (26 years) national study. Otolaryngol Head Neck Surg. 2018;158:167–176. doi: 10.1177/0194599817725680. [DOI] [PubMed] [Google Scholar]
- 20.Odhagen E., Stalfors J., Sunnergren O. Morbidity after pediatric tonsillotomy versus tonsillectomy: a population-based cohort study. Laryngoscope. 2019;129:2619–2626. doi: 10.1002/lary.27665. [DOI] [PubMed] [Google Scholar]
- 21.Sarny S., Ossimitz G., Habermann W., Stammberger H. Hemorrhage following tonsil surgery: a multicenter prospective study. Laryngoscope. 2011;121:2553–2560. doi: 10.1002/lary.22347. [DOI] [PubMed] [Google Scholar]
- 22.Sun G.H., Auger K.A., Aliu O., Patrick S.W., DeMonner S., Davis M.M. Posttonsillectomy hemorrhage in children with von Willebrand disease or hemophilia. JAMA Otolaryngol Head Neck Surg. 2013;139:245–249. doi: 10.1001/jamaoto.2013.1821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.