Abstract
Background
Data are scarce on respiratory infections during severe acute exacerbation of chronic obstructive pulmonary disease (COPD). This study aimed to investigate respiratory infection patterns in the intensive care unit (ICU) and identify variables associated with infection type and patient outcome.
Methods
A retrospective, single-centre cohort study. All patients admitted (2015–2021) to our ICU for severe acute exacerbation of COPD were included. Logistic multivariable regression analysis was performed to predict factors associated with infection and assess the association between infection and outcome.
Results
We included 473 patients: 288 (60.9%) had respiratory infection and 139 (29.4%) required invasive mechanical ventilation. Eighty-nine (30.9%) had viral, 81 (28.1%) bacterial, 34 (11.8%) mixed, and 84 (29.2%) undocumented infections. Forty-seven (9.9%) patients died in the ICU and 67 (14.2%) in hospital. Factors associated with respiratory infection were temperature (odds ratio [+1°C]=1.43, P=0.008) and blood neutrophils (1.07, P=0.002). Male sex (2.21, P=0.02) and blood neutrophils were associated with bacterial infection (1.06, P=0.04). In a multivariable analysis, pneumonia (cause-specific hazard=1.75, P=0.005), respiratory rate (1.17, P=0.04), arterial partial pressure of carbon-dioxide (1.08, P=0.04), and lactate (1.14, P=0.02) were associated with the need for invasive MV. Age (1.03, P=0.03), immunodeficiency (1.96, P=0.02), and altered performance status (1.78, P=0.002) were associated with hospital mortality.
Conclusions
Respiratory infections, 39.9% of which were bacterial, were the main cause of severe acute exacerbation of COPD. Body temperature and blood neutrophils were single markers of infection. Pneumonia was associated with the need for invasive mechanical ventilation but not with hospital mortality, as opposed to age, immunodeficiency, and altered performance status.
Keywords: pneumonia, infection, COPD, mechanical ventilation, mortality
Plain Language Summary
- This study investigates the prevalence, characteristics, and impact on outcomes of different types of respiratory infections triggering severe acute exacerbations of COPD.
- Our retrospective cohort study of 473 critically ill patients found that respiratory infections, of which 39.9% were bacterial, were the main cause of severe exacerbation.
- The type of infection (viral, bacterial, or mixt) was not associated with the need for invasive mechanical ventilation or mortality.
- Early identification of the infectious agent is crucial for implementing effective therapy; however, the type of infection was not associated with the main outcomes.
Background
Exacerbation of chronic obstructive pulmonary disease (COPD) presents as a transient increase in respiratory symptoms,1 with acute severe exacerbations requiring hospitalization. These events increase short- and mid-term morbidity and mortality, especially in the most severe cases when ventilatory support is mandatory.2 Despite improved management in the intensive care unit (ICU), severe acute exacerbations are associated with a high mortality rate (20%) at hospital discharge.3 Identifying exacerbation triggers in individual patients is a major issue in the ICU.
Respiratory infections are the main cause of COPD exacerbation.4 Recent literature from the ICU provides limited information on the characteristics and impact of the viral or bacterial nature of the infection, even though the therapeutic issue is of primary importance in this context.5,6 Moreover, assessing the association between respiratory infections and the use of invasive mechanical ventilation (MV) and mortality seems relevant and could be an axis for improving management in the acute phase.
The main objectives of this study were to describe, evaluate, and compare the characteristics of respiratory infections and their effect on outcomes in patients admitted to the ICU for a severe acute exacerbation of COPD.
Study Design and Methods
This single-center, observational, retrospective study was granted ethical approval by our national ethical institution, the Ethics Committee of the French Intensive Care Society, under the reference number 21–66 for the “BPCO Réa study” on August 10, 2021. Furthermore, the study was registered with the French National Institute for Health Data under the identifier MR2516271119. Procedures were followed in accordance with institutional ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975. Informed consent was sought from the patients upon recovery, in compliance with French law. The 28-bed ICU is in a university-affiliated tertiary hospital in the Paris area with 800 medical and surgical beds.
Patients
We screened all consecutive COPD patients admitted to our ICU between January 2015 and December 2021. Diagnosis of COPD must be confirmed by spirometry in medical computed records. Only patients over 40 years of age admitted in ICU for a severe acute exacerbation of COPD were included. Definitions for a severe acute exacerbation of COPD, bronchitis, and pneumonia are described in the Data Supplement. Exclusion criteria were admission to the ICU for reasons other than exacerbation of COPD, known asthma according to the Global Initiative for Asthma definition, and patient refusal to participate.
Data Collection
All collected data were obtained retrospectively from medical records and stored in a secured and anonymized standardized electronic file (Excel, Microsoft©, Redmond, WA). Patient data included demographics, comorbidities, immunodeficiency (defined by the presence of a haemopathy, a solid cancer under treatment or in remission for less than 5 years, or in case of active immunosuppressive therapy), triggering factor for the exacerbation, performance status (PS), Simplified Acute Physiology Score II (SAPS II), clinical parameters (including haemodynamic parameters, the Glasgow Coma Scale, temperature and respiratory rate at admission), laboratory tests (such as blood gas analysis, complete blood count, and inflammatory markers) and microbiological samples, type and duration of ventilatory support, length of stay in the ICU and in-hospital, and mortality in the ICU, at day 30, and at day 90. Definitions and description of ICU management, ventilatory support, and standardized microbiological samples are described in the Data Supplement.
Statistical Analysis
Descriptive data are presented as medians with interquartile ranges (IQR) for continuous variables and as absolute values (%) for categorical data.
We first performed a comparison according to the presence of a respiratory infection. We used the χ2 test or Fisher’s exact test for categorical variables and Student’s t-test or the Wilcoxon t-test for continuous variables according to their distribution. We performed a comparison between the identified pathogens; as there were 3 groups of infection, a χ2 test was used for categorical variables, whereas continuous variables were compared via Kruskal–Wallis tests or analysis of variance.
To identify the risk factors associated with the presence of a respiratory infection, a logistic regression model was applied. A set of variables with a P-value <0.20 in the univariate analysis was then used to construct a multivariable model. The first model was defined and then exposed to an automated process of variable selection known as stepwise. Once the final model was obtained during the selection stage, the hypothesis of risk proportionality was evaluated graphically, and the interactions were sought. We assessed the model goodness-of-fit using the Hosmer–Lemeshow test. We used the same strategy to search for risk factors associated with the occurrence of a bacterial infection among patients with a respiratory infection.
We subsequently looked for possible factors associated with the risk of resorting to MV and then with the occurrence of hospital death. As invasive MV is a competing event with discharge from the ICU without intubation (live discharge, death), because death is a competing event with live discharge from the ICU, we applied a Cox cause-specific model. For factors associated with ICU death, the use of invasive MV was considered as a time-dependent explanatory variable. For the construction of the multivariable model, all the covariates were first analyzed in a univariate manner and then a multivariable model was constructed from the covariates presenting a P-value <0.20. The same covariate selection method was used to obtain a final model. In addition to the assumption of proportionality of risk, the log linearity of the continuous variables was assessed. If the linearity assumption of a continuous variable was not verified, it was transformed using splines.
For variables with missing data, the management strategy is as follows: a complete case analysis for variables with less than 5% of missing data or multiple imputation by chained equation for variables with between 5% and 30% missing data. No variable with more than 30% missing data was imputed and was therefore excluded from the multivariable analysis. In the case of multiple imputation, 50 imputed data sets were obtained from an imputation model repeated 10 times. An analysis model was then fitted to each of the 50 separately imputed datasets, and these 50 datasets were pooled according to Rubin’s formulae.
All tests were conducted in a 2-tailed fashion with an alpha risk of 0.05. All analyses were performed with the statistical software R4.2.1© using the packages rms, stepwise, mice, and survival (R foundation© for Statistical Computing Vienna, Austria).
Results
Patient Characteristics
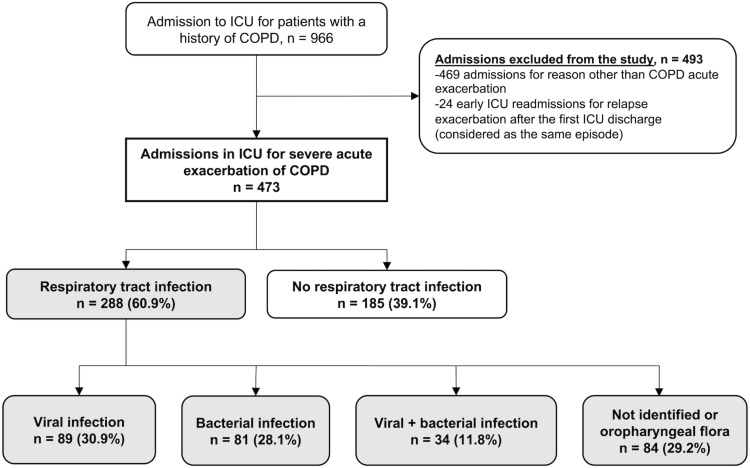
Figure 1 is the patient flowchart and Table S1 reports patient characteristics. A total of 473 patients were included, of whom 304 (64.3%) were men, and the median age was 69 (IQR, 62–77) years. Four hundred and twenty-five (89.9%) patients were hospitalized for acute respiratory distress, 36 (7.6%) for hypercapnic coma, and 12 (2.5%) for acute respiratory distress associated with shock.
Figure 1.
Patient flow chart.
Abbreviations: COPD, chronic obstructive pulmonary disease; ICU, intensive care unit.
Regarding exacerbation triggers, 288 (60.9%) patients presented a respiratory infection, with 182 (63.2%) having pneumonia and 106 (36.8%) acute bronchitis. The origin was viral in 89 (30.9%) patients, bacterial in 81 (28.1%), and mixed (virus and bacteria) in 34 (11.8%). For 84 patients (29.2%), the respiratory infection was either not documented or with oropharyngeal flora.
ICU Management and Outcomes
Table 1 reports ICU management and outcomes. Antibiotic therapies were prescribed for at least 48 hours in 71.5% of patients. Table 2 highlights the distribution of the different types of microbial agents and microbiological diagnosis of respiratory tract infection. Influenza virus was found in 16.0% of patients, followed by undistinguished rhinovirus/enterovirus (12.2%) and respiratory syncytial virus (6.2%). The predominant bacteria were Haemophilus influenzae (12.8%) and Streptococcus pneumoniae (10.8%). Supplementary Figure 1 details the microbiological samples performed in the cohort (Data Supplement).
Table 1.
Intensive Care Unit (ICU) Management and Outcomes in Patients Admitted for Severe Acute Exacerbation of Chronic Obstructive Pulmonary Disease (COPD) According to the Initial Diagnosis of Respiratory Tract Infection as the Triggering Factor
| Variables | All Cases n=473 | Respiratory Tract Infectiona n=288 (60.9) | No Respiratory Tract Infection n=185 (39.1) | N Missing† | |||
|---|---|---|---|---|---|---|---|
| Respiratory Tract Infectiona All Cases n=288 (60.9) | Viral Infection n=89 | Bacterial Infection n=81 | Viral + Bacterial Infection n=34 | ||||
| Ventilatory management during ICU stay | |||||||
| Exclusive spontaneous ventilation | 36 (7.6) | 22 (7.6) | 7 (7.9) | 4 (4.9) | 4 (11.8) | 14 (7.6) | |
| NIV | 420 (88.8) | 252 (87.5) | 81 (91.0) | 69 (85.2) | 27 (79.4) | 168 (90.8) | |
| Duration of NIV, days | 2 [1–4] | 2 [1–5] | 3 (2–5) | 2 [1–5] | 3 [1–5] | 2 [1–4] | |
| NIV failure | 108 (25.7) | 85 (33.7)* | 19 (23.5)** | 32 (46.4) | 12 (44.4) | 23 (13.7) | |
| Invasive MV | 139 (29.4) | 109 (37.8)* | 23 (25.8)** | 43 (53.1) | 18 (52.9) | 30 (16.2) | |
| Duration of invasive MV, days | 6 [3–13] | 7 [3–13]* | 9 [4–15] | 5 [3–11] | 14 [6–18] | 3 [2–11] | |
| Total duration of ventilatory assistance, days | 3 [2–7] | 4 [2–9]* | 4 [2–8]** | 6 [2–10] | 6 [5–16] | 3 [2–5] | |
| Ventilator-associated pneumonia (if intubated) | 27 (19.4) | 20 (6.9) | 5 (5.6) | 6 (7.4) | 5 (14.7) | 7 (3.8) | |
| Tracheostomyb | 7/110 (6.4) | 7/87 (8.0) | 2 (2.2) | 3 (3.7) | 2 (5.9) | 0 (0.0) | |
| NIV after ICU discharge$ | 116 (27.2) | 58 (22.7)* | 15 (18.3) | 17 (25.8) | 7 (22.6) | 58 (34.1) | |
| Adjunctive therapies during ICU stay | |||||||
| Corticosteroids | 242 (51.4) | 153 (53.3) | 50 (56.2) | 38 (46.9) | 20 (60.6) | 89 (48.4) | 8 |
| Antibiotics initiated at ICU admission | 338 (71.5) | 269 (93.4)* | 73 (82.0)** | 81 (100.0) | 34 (100.0) | 69 (37.3) | |
| Antiviral therapy (Oseltamivir) | 87 (18.4) | 81 (28.1)* | 47 (52.8)** | 9 (11.1) | 13 (38.2) | 6 (3.2) | |
| Need for vasoactive drugs in the ICU | 107 (22.6) | 82 (28.5)* | 16 (18.0)** | 35 (43.2) | 14 (41.2) | 25 (13.5) | |
| Outcomes | |||||||
| ICU length of stay, days | 5 [3–9] | 7 [4–11]* | 6 [4–10]** | 7 [4–13] | 9 [5–17] | 4 [3–7] | |
| ICU mortality | 47 (9.9) | 32 (11.1) | 7 (7.9)** | 17 (21.0) | 2 (5.9) | 15 (8.1) | |
| Post-ICU hospital length of stay, days | 10 [6–17] | 10 [6–18] | 9 [6–15] | 9 [4–17] | 12 [6–21] | 10 [5–16] | 2 |
| Total hospital length of stay, days | 17 [11–26] | 18 [12–30]* | 17 [11–26] | 17 [12–28] | 23 [15–39] | 15 [10–23] | 2 |
| Hospital mortality | 67 (14.2) | 46 (16.0) | 11 (12.4)** | 22 (27.1) | 3 (8.8) | 21 (11.4) | 1 |
| Day-30 mortality | 67 (14.2) | 44 (15.5) | 11 (12.4)** | 20 (24.7) | 3 (8.8) | 23 (12.6) | 7 |
| Day-90 mortality | 90 (19.4) | 56 (19.9) | 14 (15.7)** | 24 (29.6) | 5 (14.7) | 34 (18.7) | 10 |
Notes: Data are presented as N (%) or Median [interquartile range]. †Number of missing observations, unless Ø. *p<0.05 between respiratory tract infection and no respiratory tract infection. **p<0.05 between viral, bacterial and [viral + bacterial] respiratory tract infection. a24/288 patients had a suspected respiratory tract infection without microbial identification or oropharyngeal flora. bFor survivors intubated patients. $For ICU survivors.
Abbreviations: SAPS II, Simplified Acute Physiology Score II; ICU, intensive care unit; COPD, chronic obstructive pulmonary disease; NIV, Non-invasive ventilation; MV, Mechanical ventilation.
Table 2.
Antibiotics Used and Microbiological Diagnosis for Patients with Respiratory Tract Infection
| Variables | All Respiratory Tract Infections n=288 | Pneumonia n=182 (63.2) | Bronchitis n=106 (36.8) |
|---|---|---|---|
| Viral diagnosis | 89 (30.9) | 54 (29.7) | 35 (33.0) |
| Bacterial diagnosis | 81 (28.1) | 57 (31.3) | 24 (22.7) |
| Viral and bacterial co-infection | 34 (11.8) | 26 (14.3) | 8 (7.5) |
| Unidentified or oropharyngeal flora | 84 (29.2) | 45 (24.7) | 39 (36.8) |
| Virus type | |||
| Influenzae (type A and B) | 46 (16.0) | 39 (21.4) | 7 (6.6) |
| Respiratory syncytial virus | 18 (6.2) | 8 (4.4) | 10 (9.4) |
| Rhinovirus/Enterovirus | 35 (12.2) | 20 (11.0) | 15 (14.2) |
| Parainfluenzae virus | 11 (3.8) | 4 (2.2) | 7 (6.6) |
| Metapneumovirus | 5 (1.7) | 5 (2.7) | 0 (0.0) |
| Adenovirus | 4 (1.4) | 0 (0.0) | 4 (3.8) |
| Multiples virus coinfection | 4 (1.4) | 2 (1.1) | 2 (1.9) |
| Bacterial type | |||
| Haemophilus influenzae | 37 (12.8) | 27 (14.8) | 10 (9.4) |
| Streptococcus pneumoniae | 31 (10.8) | 24 (13.2) | 7 (6.6) |
| Enterobacteriaceae | 19 (6.6) | 13 (7.1) | 6 (5.7) |
| Others Streptococcus | 12 (4.2) | 9 (4.9) | 3 (2.8) |
| Pseudomonas aeruginosa | 8 (2.8) | 5 (2.7) | 3 (2.8) |
| Staphylococcus aureus oxacilline-sensitive | 6 (2.1) | 5 (2.7) | 1 (0.9) |
| Branhamella catarrhalis | 5 (1.7) | 5 (2.7) | 0 (0.0) |
| Stenotrophomonas maltophilia | 3 (1.0) | 1 (0.5) | 2 (1.9) |
| Legionella pneumophila | 1 (0.3) | 1 (0.5) | 0 (0.0) |
| Mycoplasma pneumoniae | 1 (0.3) | 1 (0.5) | 0 (0.0) |
| Oro-pharyngeal flora | 23 (8.0) | 7 (3.8) | 16 (15.1) |
| Others | 8 (2.8) | 6 (3.3) | 2 (1.9) |
| Multiples bacterial coinfection | 14 (4.9) | 12 (6.6) | 2 (1.9) |
| First line antibiotic therapy at ICU admission | 269 (93.4) | 178 (97.8) | 91 (85.8) |
| One antibiotic | 57 (21.2) | 23 (12.9) | 34 (37.4) |
| Combination therapy (2 antibiotics) | 212 (78.8) | 155 (87.1) | 57 (62.6) |
| Antibiotic type initiated at ICU admission | |||
| Cefotaxime or Ceftriaxone | 123 (45.7) | 100 (56.2) | 23 (25.3) |
| AAC or Amoxicillin | 121 (45.0) | 66 (37.1) | 51 (56.0) |
| Cefepime | 71 (26.4) | 52 (29.2) | 19 (20.9) |
| Piperacillin/tazobactam | 32 (11.9) | 27 (15.2) | 5 (5.5) |
| Imipenem or meropenem | 12 (4.5) | 8 (4.5) | 4 (4.4) |
| Ceftazidime | 5 (1.9) | 3 (1.7) | 2 (2.2) |
| Levofloxacin | 10 (3.7) | 5 (2.8) | 5 (5.5) |
| Spiramycine associated with β-lactamin | 158 (58.7) | 124 (69.7) | 34 (37.4) |
| Antiviral therapy (oseltamivir) | 81 (28.1) | 61 (34.2) | 20 (18.9) |
Note: Data are presented as N (%).
NIV was initiated in 88.8% of patients, with 25.7% NIV failures (Table 1). A total of 139 (29.4%) patients were intubated. Median duration of NIV was 2 days (IQR = 1–4), and invasive MV was 6 days (IQR = 3–13). Median length of stay in the ICU was 5 days (IQR = 3–9). Forty-seven (9.9%) patients died in the ICU and 14.2% died in hospital.
Factors Associated with a Respiratory Infection
Factors independently associated with an infectious exacerbation were initial body temperature +1 °C (odds ratio [OR]=1.43, 95% CI=1.16–1.78, P=0.008) and blood neutrophils (OR=1.07, 95% CI=1.02–1.11, P=0.002) (Table S2). PS score (OR=0.73, 95% CI=0.58–0.93, P=0.01), COPD stage 3 or 4 (OR=0.52, 95% CI=0.34–0.78, P=0.002) and blood eosinophils (OR=0.87, 95% CI=0.79–0.94, P=0.002) were inversely associated with the infectious nature of the exacerbation.
Factors Associated with Bacterial Infection
In multivariable analysis, male sex (OR=2.21, 95% CI=1.14–4.41, P=0.02) and higher blood neutrophils (OR=1.06, 95% CI=1.01–1.13, P=0.04) were independently associated with bacterial infection (Table S3). Higher albumin and lower Glasgow Coma Scale (GCS) were inversely associated with the infectious nature of the exacerbation.
Factors Associated with the Need for Invasive Mechanical Ventilation
In multivariable analysis (Table 3), pneumonia (cause-specific hazard [CSH]=1.75, 95% CI=1.18–2.60, P=0.005), initial respiratory rate (CSH=1.17, 95% CI=1.01–1.36, P=0.04), PaCO2 at ICU admission (CSH=1.08, 95% CI=1.01–1.17, P=0.04), and lactate at ICU admission (CSH=1.14, 95% CI=1.03–1.28, P=0.02) were significantly associated with invasive MV.
Table 3.
Multivariable Analysis of Risk Factors Associated with the Need for Invasive Mechanical Ventilation and Risk Factors Associated with Hospital Mortality
| Variables | Multivariable CSH (95%CI) | P value |
|---|---|---|
| Risk factors associated with the need for invasive mechanical ventilation | ||
| Chronic right heart failure | 0.39 (0.24–0.65) | <0.001 |
| Performance status (+ 1 point) | 0.63 (0.52–0.77) | <0.001 |
| Pneumonia | 1.75 (1.18–2.60) | 0.005 |
| Glasgow coma scale (−1 point) | 0.80 (0.76–0.84) | <0.001 |
| Respiratory rate (+ 5 cycles/min) | 1.17 (1.01–1.36) | 0.04 |
| PaCO2 (+ 10 mmHg) | 1.08 (1.01–1.17) | 0.04 |
| Lactate (+ 1 mmol/L) | 1.14 (1.03–1.28) | 0.02 |
| Serum albumin (+ 1 g/L) | 0.95 (0.93–0.97) | <0.001 |
| Risk factors associated with in-hospital mortality | ||
| Age, years (+1 year) | 1.03 (1.01–1.06) | 0.03 |
| Immunodeficiency | 1.96 (1.08–3.55) | 0.02 |
| Performance status (−1 point) | 1.78 (1.23–2.57) | 0.002 |
| Bacterial infection | 1.71 (0.92–3.18) | 0.08 |
| Corticosteroids use during ICU stay | 0.53 (0.29–0.98) | 0.04 |
Note: Bolded variables are significant in multivariable analysis.
Abbreviations: CSH, Cause-Specific Hazard; 95%CI, 95% Confidence intervals.
Factors inversely associated with the use of invasive MV were chronic right heart failure (OR=0.39, 95% CI=0.24–0.65, P<0.001), increasing PS score (CSH=0.63, 95% CI=0.52–0.77, P<0.001), decreasing GCS score (CSH=0.80, 95% CI=0.76–0.84, P<0.001), and higher albumin levels at ICU admission (CSH=0.95, 95% CI=0.93–0.97, P<0.001). Infection as the trigger of COPD exacerbation was not independently associated with invasive MV in this model.
Factors Associated with Hospital Mortality
Age (CSH=1.03, 95% CI=1.01–1.06, P=0.03), immunodeficiency (CSH=1.96, 95% CI=1.08–3.55, P=0.02), and increasing PS score (CSH=1.78, 95% CI=1.23–2.57, P=0.002) were independently associated with hospital mortality (Table 3). Systemic corticosteroid therapy during the ICU stay was inversely associated with hospital mortality (CSH=0.53, 95% CI=0.29–0.98, P=0.04). Bacterial infection was not independently associated with hospital mortality.
Supplementary Figure 2 shows Kaplan–Meier survival curves at day 90 according to the type of infection (Data Supplement).
Discussion
This is the first study to provide detailed information on respiratory infections in patients admitted to the ICU for severe acute exacerbation of COPD and their impact on invasive MV and on hospital mortality.
Our results show that respiratory infections account for almost two-thirds of the causes of severe acute exacerbations and are consistent with the literature.7–13 In our cohort, 63.2% of infections were pneumonia and 36.8% were acute bronchitis. A French study involving 111 patients with COPD admitted to the ICU described pneumonia in 59% and bronchitis in 20% of cases.14 In our cohort, the origin of infection was viral in 30.9% of cases, bacterial in 28.1%, mixed in 11.8%, and undocumented (or with oropharyngeal flora) in 29.2%. This distribution is consistent with the literature,7,15 although the microbiological description of infectious exacerbations is more frequent in populations hospitalized in the ward than in the ICU. Moreover, microbiological techniques and samples and the seasonality of the recruitment periods could contribute to variations in the distribution of viruses and bacteria between studies. Furthermore, in intubated patients, bronchial samples may also reflect colonization rather than acute infection. One-third of the respiratory infections we describe in this work were not microbiologically documented, and may reflect the high frequency of antibiotic use before admission to the ICU, around 60% in the study by Daubin et al.10
The viruses and bacteria identified in our study appear similar to those described in the literature, with the exception of a lower frequency of Enterobacteria (6.6%) and P. aeruginosa (2.8%).16–18 In a previous study of infectious COPD exacerbations in the ICU, P. aeruginosa was found in 7% of exacerbations.14 Geographic disparity and local ecology may explain these variations. In our study, Haemophilus influenzae and Streptococcus pneumoniae were the most detected bacteria, accounting for 12.8% and 10.8% of respiratory infections, respectively. Furthermore, it is noteworthy that bacterial infections caused by Streptococcus pneumoniae and Haemophilus influenzae can persist in the lower airways even in the absence of exacerbations. Studies have shown an ongoing presence of these pathogenic bacteria, and their burden may escalate during exacerbations, a phenomenon linked to impaired immune cell activity.19,20 This highlights the complex interplay between chronic bacterial colonization and acute exacerbations in COPD patients. This information underscores the need for a more comprehensive understanding of the dynamics of bacterial infections in COPD, as they can have implications for exacerbation risk and severity. While our study primarily focused on acute exacerbations, future research could delve deeper into the mechanisms behind the persistence and escalation of bacterial pathogens in the lower airways, even during stable phases of the disease.
NIV is one of the cornerstones of ICU management of severe COPD exacerbation. In our cohort, the NIV failure rate was 25.7% as previously reported.5,21,22 The heterogeneity of NIV failure between the cohorts could be related to patients demographics, which vary according to the ethical decisions surrounding admission to the ICU, or the definition of NIV failure in case of the decision to withhold care. The use of invasive MV was necessary in 29.4% of patients in our cohort. A recent study from New Zealand described a 34.5% rate of invasive MV.23 The median duration of invasive MV in our study was 6 days, similar to that reported by Gadre et al (4 days) and Abroug et al (6 days).24,25
We observed an ICU mortality rate of 9.9% and a mortality rate of 14.2% at 30 days and 19.4% at 90 days. These rates are consistent with literature.26 In most studies, ICU mortality is in the range of 10–15% and hospital mortality varies between 15% and 30% with similar severity scores or demographic characteristics.23,27,28
In our cohort, factors associated with infection were body temperature and blood neutrophils. Conversely, the increase in performance status score and COPD stage, as well as blood eosinophils, was inversely associated with the infectious origin of the exacerbation. A similar hypothesis can be put forward regarding eosinophils, which could be related to an allergic or environmental trigger, or even to a specific phenotype of patients with COPD. This hypothesis is counterbalanced by the data from Papi et al, who reported a higher eosinophil count in the case of viral infection.7
Anticipating the type of infection would allow for a more accurate antibiotic prescription. In our cohort, male sex and blood neutrophils were associated with a bacterial infection, whereas lower GCS and albumin were inversely correlated with the bacterial nature of the infection. Our results identified an association between male gender and bacterial type of infection, but it could be subject to bias due to the over-representation of males in our population, whereas literature highlights distinct differences between men and women in respiratory chronic diseases,29,30 our results show no difference between gender in terms of type of respiratory infection or patients prognosis (data not shown). Other studies show an increase in blood neutrophils in bacterial exacerbations,17 whereas the link for GCS score is unclear. We would expect a lower score to be the consequence of infection in the context of septic encephalopathy added to hypercapnic encephalopathy or as a severity marker of the infectious exacerbation.
In our study, crude procalcitonin and C-reactive protein values were non-discriminative of the type of infection. A recent meta-analysis reported an area under the curve of 0.77 for procalcitonin to predict a bacterial exacerbation of COPD. Subgroup analysis revealed that the combined sensitivity and specificity of procalcitonin for patients admitted to the ICU was lower than for other patients.31 In a large cohort of COPD patients in the ICU, procalcitonin had poor ability to distinguish between patients with and those without bacterial infection.10 One hypothesis could be that the most chronic severe patients with altered performance status have more exacerbations related to the terminal course of the COPD or to the numerous comorbidities rather than to an infectious trigger. In COPD exacerbation, some markers of exacerbation severity are associated with the need for intubation, such as polypnea or higher PaCO2 and lactate levels.32–35 Pneumonia differs from bronchitis in the more frequent use of MV, which could be explained by more marked pulmonary inflammation.36,37 Our results support these previous findings.
Several studies have shown an association between impairment in daily quality of life, assessed by performance status, and the use of invasive MV.38 Our population included patients subject to withholding a decision on intubation, which could explain why our results do not support this association. Indeed, patients with advanced disease, chronic right heart failure, and higher performance status score could not have been intubated in connection with a “do not intubate” decision. Conversely, patients with higher albumin levels, assumed to be in better nutritional and muscular condition, may have had a lower proportion with intubation thanks to a better ability to support acute respiratory failure.
Invasive MV exposes the patient to an increased risk of complications and a longer length of hospital stay.39 It would therefore seem legitimate to believe that progression to intubation is associated with excess mortality. In our study, invasive MV was not an independent factor associated with hospital mortality. In a recent study, Cao et al described the factors independently associated with hospital mortality, including lymphopenia, leukopenia, chronic heart failure, and invasive MV.40 However, several other studies did not report a relationship between invasive MV and mortality.27,41 Improved management of patients during invasive MV in recent decades has led to a reduction in the mortality rate in COPD exacerbation.5,42
In our study, factors associated with mortality were age, immunodeficiency, and performance status. These findings are in concert with numerous studies of ICU patients regardless of COPD status.27 In view of the numerous comorbidities presented by COPD patients and their frailty, ethical issues and withholding decisions regarding invasive care are frequent during their hospital stay or even from the moment of ICU admission. These reflections and decisions vary according to the ICU and across countries, and contribute to the heterogeneity of mortality risk described in the literature.
Our study has several limitations. First, as a single-center study, the interpretation and generalization of our results is limited. Second, because of its retrospective nature we have missing data. Third, the etiological investigations of the exacerbation were oriented by the clinical condition of the patient; therefore, not all patients had the same microbiological work-up. However, 67.2% of the population had at least one respiratory microbiological sample and 78.2% of patients were investigated for viruses. There is a possible disparity in prevalence between intubated and non-intubated patients in whom distal respiratory sampling is not feasible, leading to an underestimated diagnosis of bacterial infection in non-intubated patients or an overdiagnosis in intubated patients. It is unclear whether a positive result reflects pulmonary infection or colonization. However, despite these limitations, our cohort reflects pragmatic management and shows similar results to the literature. Fourth, we kept the undocumented cases of infection if the clinical picture matched, because of the high frequency of antibiotic use before ICU admission reported in the literature and in real life.10 Fifth, we did not exclude patients who had a withholding decision for intubation, which may have biased our assessment of factors associated with the use of invasive MV and/or mortality.
Conclusions
Respiratory infections seem to be the leading cause of acute severe exacerbation of COPD in critical patients, 39.9% of which were bacterial in our study. Biological factors associated with bacterial infections were higher blood neutrophil and lower albumin levels. Pneumonia was associated with invasive MV but not with hospital mortality as opposed to age, immunodeficiency, and altered performance status. As severe acute exacerbation of COPD is a medical burden, assessing its risk factors to improve treatment is a major challenge in the ICU. A larger prospective study may be necessary to further explore these findings.
Acknowledgment
Sophie Rushton-Smith, PhD (MedLink Healthcare Communications) provided editorial assistance in the final version of the manuscript and was funded by the Délégation à la Recherche Clinique et à l’Innovation (DRCI), Le Chesnay, France. The abstract of this paper was presented at the French Intensive Care Society International Congress’ in June 2023 as a conference talk with interim findings. The poster’s abstract was published in ‘Poster Abstracts’ in Annals of Intensive Care: [https://doi.org/10.1186/s13613-023-01131-y].
Funding Statement
The study was supported by the French public funding agency Délégation à la Recherche Clinique et à l’Innovation (DRCI), Le Chesnay, France.
Data Sharing Statement
The investigators will make the documents and individual data strictly required for monitoring, quality control, and audit of the study available to dedicated persons, in accordance with laws and regulations in force (Articles L.1121-3 and R.5121-13 of the Code de Santé Publique – CSP, French Public Health Code).
The datasets used and/or analyzed during the study will be available from the coordinating investigator (Alexis Ferré) on reasonable request. The procedures carried out under the French data privacy authority (Commission Nationale de l’Informatique et des Libertés) do not permit the transmission of the database, nor do the informed consent documents signed by the patients. Consultation by the editorial board or interested researchers of individual participant data that underlie the results reported in the article after de-identification may nevertheless be considered, subject to prior determination of the terms and conditions of such consultation and in respect of compliance with the applicable regulations.
Ethics Approval and Consent to Participate
This single-center, observational, retrospective study was approved by the ethics committee of the French Intensive Care Society (N° #21-66) and registered at the French National Institute for Health Data (#MR 2516271119). Informed consent was sought from the patients upon recovery, in compliance with French law.
Disclosure
Matthieu Jamme reports honoraria by Sanofi for a lecture during JAMIR Congress 2022, outside the submitted work. Alexis Ferré reports honoraria by Fisher & Paykel for a lecture during SFMU Congress 2022, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Langefeld K 2022 GOLD reports [Internet]. Global Initiative for Chronic Obstructive Lung Disease – GOLD; 2022. Available from: https://goldcopd.org/2022-gold-reports-2/. Accessed February 22, 2024.
- 2.Collaborators GB. GBD 2017 disease and injury incidence and prevalence collaborators: global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dres M, Tran T-C, Aegerter P, et al. Influence of ICU case-volume on the management and hospital outcomes of acute exacerbations of chronic obstructive pulmonary disease*. Crit Care Med. 2013;41:1884–1892. doi: 10.1097/CCM.0b013e31828a2bd8 [DOI] [PubMed] [Google Scholar]
- 4.Roberts CM, Stone RA, Buckingham RJ, et al. Acidosis, non-invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax. 2011;66(1):43–48. doi: 10.1136/thx.2010.153114 [DOI] [PubMed] [Google Scholar]
- 5.Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333(13):817–822. doi: 10.1056/NEJM199509283331301 [DOI] [PubMed] [Google Scholar]
- 6.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351. doi: 10.1016/S0140-6736(11)60968-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114–1121. doi: 10.1164/rccm.200506-859OC [DOI] [PubMed] [Google Scholar]
- 8.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethi S, Sethi R, Eschberger K, et al. Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(4):356–361. doi: 10.1164/rccm.200703-417OC [DOI] [PubMed] [Google Scholar]
- 10.Daubin C, Valette X, Thiollière F, et al. Procalcitonin algorithm to guide initial antibiotic therapy in acute exacerbations of COPD admitted to the ICU: a randomized multicenter study. Intensive Care Med. 2018;44:428–437. doi: 10.1007/s00134-018-5141-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afessa B, Morales IJ, Scanlon PD, et al. Prognostic factors, clinical course, and hospital outcome of patients with chronic obstructive pulmonary disease admitted to an intensive care unit for acute respiratory failure. Crit Care Med. 2002;30(7):1610–1615. doi: 10.1097/00003246-200207000-00035 [DOI] [PubMed] [Google Scholar]
- 12.Fagon JY, Chastre J. Severe exacerbations of COPD patients: the role of pulmonary infections. Semin Respir Infect. 1996;11:109–118. [PubMed] [Google Scholar]
- 13.Mohan A, Chandra S, Agarwal D, et al. Prevalence of viral infection detected by PCR and RT-PCR in patients with acute exacerbation of COPD: a systematic review. Respirology. 2010;15:536–542. doi: 10.1111/j.1440-1843.2010.01722.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Planquette B, Péron J, Dubuisson E, et al. Antibiotics against Pseudomonas aeruginosa for COPD exacerbation in ICU: a 10-year retrospective study. Int J Chron Obstruct Pulmon Dis. 2015;10:379–388. doi: 10.2147/COPD.S71413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cameron RJ, de Wit D, Welsh TN, et al. Virus infection in exacerbations of chronic obstructive pulmonary disease requiring ventilation. Intensive Care Med. 2006;32:1022–1029. doi: 10.1007/s00134-006-0202-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McManus TE, Marley A-M, Baxter N, et al. Respiratory viral infection in exacerbations of COPD. Respir Med. 2008;102(11):1575–1580. doi: 10.1016/j.rmed.2008.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz-González A, Sáez-Huerta E, Martínez-Alonso M, et al. A simple scoring system to differentiate bacterial from viral infections in acute exacerbations of COPD requiring hospitalization. Int J Chron Obstruct Pulmon Dis. 2022;17:773–779. doi: 10.2147/COPD.S356950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta N, Haley R, Gupta A, et al. Chronic obstructive pulmonary disease in the intensive care unit: antibiotic treatment of severe chronic obstructive pulmonary disease exacerbations. Semin Respir Crit Care Med. 2020;41(06):830–841. doi: 10.1055/s-0040-1708837 [DOI] [PubMed] [Google Scholar]
- 19.Ghosh B, Gaike AH, Pyasi K, et al. Bacterial load and defective monocyte-derived macrophage bacterial phagocytosis in biomass smoke-related COPD. Eur Respir J. 2019;53(2):1702273. doi: 10.1183/13993003.02273-2017 [DOI] [PubMed] [Google Scholar]
- 20.Singh R, Mackay AJ, Patel AR, et al. Inflammatory thresholds and the species-specific effects of colonising bacteria in stable chronic obstructive pulmonary disease. Respir Res. 2014;15(1):114. doi: 10.1186/s12931-014-0114-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demoule A, Girou E, Richard J-C, et al. Benefits and risks of success or failure of noninvasive ventilation. Intensive Care Med. 2006;32(11):1756–1765. doi: 10.1007/s00134-006-0324-1 [DOI] [PubMed] [Google Scholar]
- 22.Moretti M, Cilione C, Tampieri A, et al. Incidence and causes of non-invasive mechanical ventilation failure after initial success. Thorax. 2000;55:819–825. doi: 10.1136/thorax.55.10.819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berenyi F, Steinfort DP, Abdelhamid YA, et al. Characteristics and outcomes of critically ill patients with acute exacerbation of chronic obstructive pulmonary disease in Australia and New Zealand. Ann Am Thorac Soc. 2020;17(6):736–745. doi: 10.1513/AnnalsATS.201911-821OC [DOI] [PubMed] [Google Scholar]
- 24.Abroug F, Ouanes-Besbes L, Fkih-Hassen M, et al. Prednisone in COPD exacerbation requiring ventilatory support: an open-label randomised evaluation. Eur Respir J. 2014;43(3):717–724. doi: 10.1183/09031936.00002913 [DOI] [PubMed] [Google Scholar]
- 25.Gadre SK, Duggal A, Mireles-Cabodevila E, et al. Acute respiratory failure requiring mechanical ventilation in severe chronic obstructive pulmonary disease (COPD). Medicine. 2018;97(17):e0487. doi: 10.1097/MD.0000000000010487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galerneau L-M, Bailly S, Terzi N, et al. Management of acute exacerbations of chronic obstructive pulmonary disease in the ICU: an observational study from the OUTCOMEREA database, 1997–2018. Crit Care Med. 2023;51(6):753–764. doi: 10.1097/CCM.0000000000005807 [DOI] [PubMed] [Google Scholar]
- 27.Seneff MG, Wagner DP, Wagner RP, et al. Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA. 1995;274:1852–1857. doi: 10.1001/jama.1995.03530230038027 [DOI] [PubMed] [Google Scholar]
- 28.Chandra D, Stamm JA, Taylor B, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med. 2012;185:152–159. doi: 10.1164/rccm.201106-1094OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh B, Chengala PP, Shah S, et al. Cigarette smoke-induced injury induces distinct sex-specific transcriptional signatures in mice tracheal epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2023;325:L467–L476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy KD, Oliver BGG. Sexual dimorphism in chronic respiratory diseases. Cell Biosci. 2023;13:47. doi: 10.1186/s13578-023-00998-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ni W, Bao J, Yang D, et al. Potential of serum procalcitonin in predicting bacterial exacerbation and guiding antibiotic administration in severe COPD exacerbations: a systematic review and meta-analysis. Infect Dis. 2019;51:639–650. doi: 10.1080/23744235.2019.1644456 [DOI] [PubMed] [Google Scholar]
- 32.Confalonieri M, Garuti G, Cattaruzza MS, et al. A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J. 2005;25:348–355. doi: 10.1183/09031936.05.00085304 [DOI] [PubMed] [Google Scholar]
- 33.Ko BS, Ahn S, Lim KS, et al. Early failure of noninvasive ventilation in chronic obstructive pulmonary disease with acute hypercapnic respiratory failure. Intern Emerg Med. 2015;10(7):855–860. doi: 10.1007/s11739-015-1293-6 [DOI] [PubMed] [Google Scholar]
- 34.Fiorino S, Bacchi-Reggiani L, Detotto E, et al. Efficacy of non-invasive mechanical ventilation in the general ward in patients with chronic obstructive pulmonary disease admitted for hypercapnic acute respiratory failure and pH < 7.35: a feasibility pilot study. Intern Med J. 2015;45(5):527–537. doi: 10.1111/imj.12726 [DOI] [PubMed] [Google Scholar]
- 35.van Gemert JP, Brijker F, Witten MA, et al. Intubation after noninvasive ventilation failure in chronic obstructive pulmonary disease: associated factors at emergency department presentation. Eur J Emerg Med. 2015;22:49–54. doi: 10.1097/MEJ.0000000000000141 [DOI] [PubMed] [Google Scholar]
- 36.Lieberman D, Lieberman D, Gelfer Y, et al. Pneumonic vs nonpneumonic acute exacerbations of COPD. Chest. 2002;122(4):1264–1270. doi: 10.1378/chest.122.4.1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Y, Liu W, Jiang H-L, et al. Pneumonia is associated with increased mortality in hospitalized COPD patients: a systematic review and meta-analysis. Respiration. 2021;100:64–76. [DOI] [PubMed] [Google Scholar]
- 38.Vitacca M, Clini E, Porta R, et al. Acute exacerbations in patients with COPD: predictors of need for mechanical ventilation. Eur Respir J. 1996;9(7):1487–1493. doi: 10.1183/09031936.96.09071487 [DOI] [PubMed] [Google Scholar]
- 39.Brochard L. Mechanical ventilation: invasive versus noninvasive. Eur Respir J Suppl. 2003;22(Supplement 47):31s–37s. doi: 10.1183/09031936.03.00050403 [DOI] [PubMed] [Google Scholar]
- 40.Cao Y, Xing Z, Long H, et al. Predictors of mortality in COPD exacerbation cases presenting to the respiratory intensive care unit. Respir Res. 2021;22(1):77. doi: 10.1186/s12931-021-01657-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ucgun I, Metintas M, Moral H, et al. Predictors of hospital outcome and intubation in COPD patients admitted to the respiratory ICU for acute hypercapnic respiratory failure. Respir Med. 2006;100(1):66–74. doi: 10.1016/j.rmed.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 42.Dumas G, Lemiale V, Rathi N, et al. Survival in immunocompromised patients ultimately requiring invasive mechanical ventilation: a pooled individual patient data analysis. Am J Respir Crit Care Med. 2021;204(2):187–196. doi: 10.1164/rccm.202009-3575OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The investigators will make the documents and individual data strictly required for monitoring, quality control, and audit of the study available to dedicated persons, in accordance with laws and regulations in force (Articles L.1121-3 and R.5121-13 of the Code de Santé Publique – CSP, French Public Health Code).
The datasets used and/or analyzed during the study will be available from the coordinating investigator (Alexis Ferré) on reasonable request. The procedures carried out under the French data privacy authority (Commission Nationale de l’Informatique et des Libertés) do not permit the transmission of the database, nor do the informed consent documents signed by the patients. Consultation by the editorial board or interested researchers of individual participant data that underlie the results reported in the article after de-identification may nevertheless be considered, subject to prior determination of the terms and conditions of such consultation and in respect of compliance with the applicable regulations.