Abstract
Objective
To compare myocarditis/pericarditis risk after COVID-19 mRNA vaccination versus SARS-CoV-2 infection, and to assess if myocarditis/pericarditis risk varies by vaccine dosing interval.
Methods
In this retrospective cohort study, we used linked databases in Quebec, Ontario, and British Columbia between January 26, 2020, and September 9, 2021. We included individuals aged 12 or above who received an mRNA vaccine as the second dose or were SARS-CoV-2-positive by RT-PCR. The outcome was hospitalization/emergency department visit for myocarditis/pericarditis within 21 days of exposure. We calculated age- and sex-stratified incidence ratios (IRs) of myocarditis/pericarditis following mRNA vaccination versus SARS-CoV-2 infection. We also calculated myocarditis/pericarditis incidence by vaccine type, homologous/heterologous schedule, and dosing interval. We pooled province-specific estimates using meta-analysis.
Results
Following 18,860,817 mRNA vaccinations and 860,335 SARS-CoV-2 infections, we observed 686 and 160 myocarditis/pericarditis cases, respectively. Myocarditis/pericarditis incidence was lower after vaccination than infection (IR [BNT162b2/SARS-CoV-2], 0.14; 95%CI, 0.07–0.29; IR [mRNA-1273/SARS-CoV-2], 0.28; 95%CI, 0.20–0.39). Within the vaccinated cohort, myocarditis/pericarditis incidence was lower with longer dosing intervals; IR (56 or more days/15–30 days) was 0.28 (95%CI, 0.19–0.41) for BNT162b2 and 0.26 (95%CI, 0.18–0.38) for mRNA-1273.
Conclusion
Myocarditis/pericarditis risk was lower after mRNA vaccination than SARS-CoV-2 infection, and with longer intervals between primary vaccine doses.
Keywords: mRNA-1273, BNT162b2, COVID-19, Myocarditis, Pericarditis, Dosing interval
Highlights
-
•
COVID-19 carries higher risk of myocarditis compared to either mRNA vaccine.
-
•
Longer vaccine dosing interval is associated with lower myocarditis risk.
-
•
Risk of COVID-19 hospitalization is higher than post vaccination myocarditis risk.
1. Introduction
Post-marketing surveillance systems and epidemiological studies have linked myocarditis and, to a lesser extent, pericarditis to mRNA COVID-19 vaccines [[1], [2], [3], [4], [5], [6]]. Given the existing evidence regarding vaccine-associated myocarditis/pericarditis among young adult males, particularly after a second dose of mRNA-1273 [7,8], Canada's National Advisory Committee on Immunization (NACI) recommended preferential use of BNT162b2 (Pfizer-BioNTech Comirnaty) over mRNA-1273 (Moderna Spikevax) as a primary series for individuals aged 6–29 years, and a longer interval between the two primary doses (8 weeks instead of the product monograph-specified 21-day [for BNT162b2] and 28-day [for mRNA-1273] intervals) for all age groups [8,9]. Limited research is available on the difference in post-vaccination myocarditis/pericarditis risk by vaccine schedule and varying dosing intervals [10]. It is now possible to provide insight into these research gaps given ample Canadian data on fully vaccinated people and strategic programmatic changes in Canadian COVID-19 vaccination programs (e.g., extending vaccine eligibility to adolescents, using heterologous mRNA vaccine schedules, and varying the interval between the primary doses).
SARS-CoV-2 infection may also be associated with myocarditis [11,12]. One study found that patients with COVID-19 had, on average, 15.7 times (95%CI, 14.1–17.2) the risk for myocarditis compared with those without COVID-19 (adjusted risk difference = 0.13, 95%CI, 0.11–0.14) [13]. Thus, the objectives of this study were to compare the risk of myocarditis/pericarditis post-vaccination to the risk after confirmed SARS-CoV-2 infection, and to assess whether the risk of myocarditis/pericarditis after the second COVID-19 vaccine dose varies by the dosing interval.
2. Methods
2.1. Data sources
Within each province, population-based COVID-19 vaccination datasets and SARS-CoV-2 laboratory testing datasets were linked with administrative health datasets, using unique encoded identifiers at ICES in Ontario, Institut National de Santé Publique du Québec, and the Provincial Health Services Authority in British Columbia (BC). In our study, we relied on PCR-confirmed COVID-19 cases, which were sourced from comprehensive data sets. For Ontario, we used the COVID19 Integrated Testing Data (C19INTGR), which consolidates diagnostic lab results from multiple sources, including the Ontario Laboratories Information System, distributed testing data within the COVID-19 Provincial Diagnostic Network, and the Public Health Ontario Case and Contact Management System. In Quebec, Nosotech compiled SARS-CoV-2 test data from 100 public and private laboratories. In (BC), we utilized the Integrated Lab Dataset for COVID-19, encompassing results from both private and public laboratories, including real-time polymerase chain reaction (RT-PCR) diagnostic tests, single nucleotide polymorphism (SNP) screening, and whole genome sequencing (details in Supplemental Table 1).
2.2. Study design and population
We applied a cohort study design to population-based data from three Canadian provinces (Ontario, BC, and Quebec) using a common study protocol. Each province provides publicly funded healthcare that includes access to hospital and physician services, SARS-CoV-2 laboratory testing, and COVID-19 vaccination. We created a ‘vaccination cohort’ to assess the risk of myocarditis/pericarditis post-COVID-19 vaccination and determine if the risk varies by dosing interval. This cohort included individuals aged ≥12 years who completed a primary series of COVID-19 vaccination and received an mRNA vaccine as the second dose between December 14, 2020 (i.e., the start date of COVID-19 vaccination in participating provinces) and September 9, 2021. We excluded individuals who: were missing data on the unique encoded identifiers, age, or sex; were not eligible for provincial health insurance on the date of second dose administration (index date); had received either of the two doses outside the province; and/or had a history of myocarditis or pericarditis (depending on outcome assessed) within one year prior to the index date or a history of SARS-CoV-2 infection during 21 days before or after receipt of the second dose.
We created an ‘infection cohort’ to assess the risk of myocarditis/pericarditis following SARS-CoV-2 infection. This cohort included individuals aged ≥12 years who tested positive on a RT-PCR SARS-CoV-2 test between January 26, 2020 (i.e., the first documented SARS-CoV-2 infection in any province) and September 9, 2021. We excluded individuals who: were missing data on the unique encoded identifiers, age, or sex; were not eligible for provincial health insurance on the specimen collection date for the first positive SARS-CoV-2 test (index date); had a history of myocarditis or pericarditis within one year to four days prior to the index date; and/or had received any doses of COVID-19 vaccine during 21 days before or after the index date.
Both cohorts were followed up to September 30, 2021.
2.3. Exposures of interest
For the vaccination cohort, the primary exposure was the receipt of the second mRNA vaccine dose (with varying products and dosing intervals, categorized as 15–30 days, 31–55 days, and ≥56 days). For the infection cohort, the primary exposure was the first laboratory-confirmed SARS-CoV-2 infection ascertained by RT-PCR.
2.4. Outcomes
The primary outcome for both cohorts was a record of an incident hospitalization or emergency department (ED) visit with a diagnosis of myocarditis or pericarditis (with hospitalizations and ED visits treated equally). For the vaccination cohort, an occurrence of myocarditis/pericarditis was considered a vaccine-associated event if it occurred between 1 and 21 days following the receipt of the second vaccine dose. For the infection cohort, the occurrence of myocarditis/pericarditis was deemed as an infection-associated event if it occurred between −3 and 21 days of the specimen collection of the first positive SARS-CoV-2 test, as the test may occur after the ED visit or hospitalization. ICD-10 diagnostic codes I40, I41, and I514, were used to identify cases of myocarditis, whereas I30 and I32 were used to detect cases of pericarditis (Supplemental Table 2) [14].
2.5. Covariate measurements
Covariates included age at the time of exposure (12–17, 18–29, 30–39, and ≥40 years) and sex.
2.6. Statistical analyses
Baseline descriptive statistics (frequencies and percentages) were generated separately for individuals who had received BNT162b2 or mRNA-1273 as the second COVID-19 vaccine dose and for those with a laboratory-confirmed SARS-CoV-2 infection. Additionally, descriptive statistics were computed for those who experienced a hospitalization or ED visit for myocarditis or pericarditis following each of the above exposures, including mean length of stay (LOS) for all cases admitted to hospital, by sex, age group, date of outcome, vaccine brand, dosing interval and vaccine schedule (homologous or heterologous) as appropriate. We calculated overall and age- and sex-stratified incidence of hospitalizations or ED visits for myocarditis or pericarditis per 1,000,000 persons following an exposure, and subsequently estimated incidence ratios (IR) and 95% confidence intervals comparing incidence after mRNA vaccination (by brand) with incidence after SARS-CoV-2 infection. Next, we estimated overall and age- and sex-stratified post-vaccination incidence of hospitalizations or ED visits for myocarditis/pericarditis for each vaccine brand, stratified by varying dosing intervals, and IRs between longer dosing intervals versus the shortest dosing interval. We also estimated crude, and age/sex adjusted (using the random intercept Poisson regression model) incidence of hospitalizations or ED visits for myocarditis/pericarditis for various vaccine schedules by dosing intervals. Finally, to provide context for the prior incidence estimates, the risk of hospitalization for COVID-19 following a laboratory-confirmed SARS-CoV-2 infection was determined. During the initial three months (January to March 2020), hospitalizations related to COVID-19 were identified as any admission taking place within −3 to 21 days of the specimen collection for the first PCR-confirmed SARS-CoV-2 test. However, starting from April 2020 onwards, it was defined as a hospital admission having an ICD-10 diagnostic code of U07.1 occurring within −3 to 21 days of the specimen collection for the first PCR-confirmed SARS-CoV-2 test.
Each province conducted all analyses separately, and the province-specific estimates (incidence and IRs) were pooled. We modeled events directly in a one-stage meta-analysis using the mixed-effects conditional Poisson likelihood within provinces, allowing for random effects on the province-specific effects (the log IRs) [15]. Heterogeneity was estimated using the maximum likelihood estimation method. In instances where the generalized linear mixed model did not converge, the inverse variance random-effects model was used with the restricted maximum likelihood estimator for heterogeneity. Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R version 4.1.2 using the meta package [16].
3. Results
3.1. Descriptive characteristics of the cohorts
A total of 18,860,817 individuals were included in the multi-provincial vaccination cohort. Of these, 13,106,504 (69%) received BNT162b2 and 5,754,313 (31%) received mRNA-1273 as the second COVID-19 vaccine dose. A slightly higher proportion of those administered BNT162b2 were female (53%). Among both vaccine product groups, the majority of individuals (62–71%) had a dosing interval of ≥56 days. Overall, 79% received a homologous vaccine schedule (95% for BNT162b2 and 44% for mRNA-1273). A majority (55%) of individuals received a second dose between July and September 2021. During the enrollment period, a total of 860,335 individuals with a positive SARS-CoV-2 test were identified, with approximately half (51%) aged ≥40 years (Supplemental Table 3).
3.2. Descriptive characteristics of myocarditis/pericarditis cases
In the vaccination cohort, a total of 317 myocarditis and 375 pericarditis events were observed. These individuals were predominantly male (70%) and aged 18–29 years (38%). Higher proportions of cases (54%) received a homologous BNT162b2 primary schedule or had a dosing interval of ≥56 days (56%), consistent with the proportions of participants within the respective categories in the population. In the infection cohort, 84 myocarditis and 77 pericarditis events were observed. Like the vaccination cohort, a higher proportion of events occurred among males, particularly for myocarditis (73%). However, in contrast to the vaccination cohort, the majority (77%) of post-infection myocarditis events occurred among those aged ≥40 years. A total of 56% of post-vaccination and 75% of post-infection myocarditis/pericarditis cases were hospitalized. Mean LOS among hospitalized myocarditis or pericarditis cases was longer for cases following SARS-CoV-2 infection than COVID-19 vaccination for each province (mean LOS with standard deviations = BC: 17.7 (29.7) vs. 2.8 (3.9) days, ON: 17.0 (19.8) vs. 2.9 (3.5) days and QC: 4.2 (3.7) vs. 4.0 (4.5) days respectively; median LOS = BC: 8.0 vs. 1.0, ON: 9.0 vs. 1.0 and QC: 2.0 vs. 3.0) (Supplemental Table 4).
3.3. Incidence of myocarditis/pericarditis per 1,000,000 persons (infections or second doses administered)
Overall (all ages and sexes combined), the incidence of hospitalizations or ED visits per 1,000,000 persons for myocarditis/pericarditis was higher following SARS-CoV-2 infection (193.6; 95%CI, 138.8–270.2) than after receiving a second COVID-19 mRNA vaccine dose (BNT162b2 = 27.5; 95%CI, 17.8–42.4 and mRNA-1273 = 52.5; 95%CI, 46.9–58.7). The incidence of myocarditis/pericarditis was significantly lower after receiving either vaccine than after infection (for BNT162b2, IR = 0.14; 95%CI, 0.07–0.29 and for mRNA-1273, IR = 0.28; 95%CI, 0.20–0.39). Similar findings were observed in the sex-stratified analysis (among all ages) (Table 1). Further stratification by age exhibited significantly lower incidence of myocarditis or pericarditis following BNT162b2 compared to SARS-CoV-2 infection for all age groups (except 12–17 years) among females. However, comparing mRNA-1273 to SARS-CoV-2, significantly lower incidence was observed only for those aged ≥40 years. Among males, those aged ≥18 years had a significantly lower incidence of myocarditis/pericarditis after BNT162b2 vaccination than after SARS-CoV-2 infection. In contrast, the incidence of myocarditis/pericarditis after mRNA-1273 vaccination was significantly lower than after SARS-CoV-2 infection only for those aged ≥40 years (IR = 0.08, 95%CI, 0.03–0.18). For the vaccination cohort, the highest incidence was observed among males aged 18–29 years after receipt of mRNA-1273 as a second dose (264.7; 95%CI, 223.5–313.6). In comparison, in the infection cohort, the incidence among males aged 18–29 years was 158.3 (95%CI, 97.0–258.3) and the IR was 1.68 (95%CI, 1.00–2.82). The highest incidence in the infection cohort was observed among males aged ≥40 years (349.8; 95%CI, 182.5–670.6) (Table 1). The province-specific IRs are presented in Supplemental Table 5.
Table 1.
Incidence of hospitalizations or emergency department visits for myocarditis or pericarditis within 21 days following a laboratory-confirmed SARS-CoV-2 infection or receipt of BNT162b2 (Pfizer-BioNTech Comirnaty) or mRNA-1273 (Moderna SpikeVax) as the second COVID-19 vaccine dose in 3 Canadian provinces, up to September 30, 2021.
| Incidence per 1,000,000 persons (95% CI) |
Incidence ratio, BNT162b2 vs. SARS-CoV-2 infection (95% CI) | Incidence ratio, mRNA-1273 vs. SARS-CoV-2 infection (95% CI) | ||||
|---|---|---|---|---|---|---|
| Sex | Age | SARS-CoV-2 infection | BNT162b2 (Second dose) | mRNA-1273 (Second dose) | ||
| Both sexes | All ages | 193.6 (138.8–270.2) | 27.5 (17.8–42.4) | 52.5 (46.9–58.7) | 0.14 (0.07–0.29) | 0.28 (0.20–0.39) |
| Females | All ages | 132.8 (94.9–185.8) | 14.7 (6.1–34.9) | 28.3 (18.8–42.6) | 0.11 (0.04–0.28) | 0.21 (0.13–0.33) |
| 12–17 yrs | NAa | 22.4 (13.3–37.8) | NAa | NAa | NAa | |
| 18–29 yrs | 98.7 (48.1–203.5) | 19.1 (4.6–79.7) | 58.8 (33.5–103.2) | 0.25 (0.06–0.95) | 0.62 (0.30–1.29) | |
| 30–39 yrs | 139.8 (77.4–252.4) | 13.4 (5.8–31.0) | 41.3 (26.3–64.7) | 0.12 (0.03–0.39) | 0.34 (0.11–1.06) | |
| ≥40 yrs | 174.6 (94.6–322.0) | 12.1 (4.3–34.2) | 16.3 (10.6–25.1) | 0.07 (0.02–0.24) | 0.09 (0.04–0.21) | |
| Males | All ages | 246.4 (177.1–342.9) | 40.6 (31.9–51.8) | 78.1 (68.5–88.9) | 0.16 (0.09–0.29) | 0.32 (0.25–0.40) |
| 12–17 yrs | 90.0 (29.0–279.1) | 132.1 (106.7–163.6) | NAa | 1.16 (0.40–3.41) | NAa | |
| 18–29 yrs | 158.3 (97.0–258.3) | 75.4 (41.9–135.7) | 264.7 (223.5–313.6) | 0.47 (0.27–0.81) | 1.68 (1.00–2.82) | |
| 30–39 yrs | 133.1 (71.6–247.4) | 36.8 (25.9–52.3) | 85.7 (63.3–115.9) | 0.27 (0.13–0.56) | 0.64 (0.32–1.27) | |
| ≥40 yrs | 349.8 (182.5–670.6) | 18.4 (12.7–26.6) | 26.0 (19.7–34.4) | 0.05 (0.02–0.14) | 0.08 (0.03–0.18) | |
The numerator was zero.
3.4. Incidence of myocarditis/pericarditis per 1,000,000 persons by dosing interval
The overall incidence of myocarditis/pericarditis was significantly lower in individuals with longer dosing intervals for both vaccine products. For BNT162b2, the IR was 0.43 (95%CI, 0.30–0.61) when comparing a dosing interval of 31–55 days vs. 15–30 days and 0.28 (95%CI, 0.19–0.41) when comparing a dosing interval of ≥56 days vs. 15–30 days, whereas for mRNA-1273, the corresponding IRs were 0.63 (95%CI, 0.44–0.90) and 0.26 (95%CI, 0.18–0.38). The trend was similar among males and females (Table 2). The age/sex adjusted estimates of the incidence of myocarditis/pericarditis following COVID-19 vaccination by vaccine schedule and dosing interval are presented in Table 3. Within three primary vaccination schedules of mRNA vaccines (two homologous and BNT162b2 followed by mRNA-1273), the adjusted incidence of myocarditis/pericarditis was significantly higher for individuals with shorter dosing intervals (Table 3). Among those who had a dosing interval of 15–30 days, few (<2%) received their second dose before 21 days.
Table 2.
Incidence of hospitalizations or emergency department visits for myocarditis or pericarditis within 21 days following receipt of BNT162b2 (Pfizer-BioNTech Comirnaty) or mRNA-1273 (Moderna SpikeVax) as the second COVID-19 vaccine dose in 3 Canadian provinces, by varying dosing intervals, up to September 30, 2021.
| Vaccine (second dose) | Sex | Age | Incidence per 1,000,000 persons (95% CI) |
Incidence ratio, 31–55 days vs. 15–30 days (95% CI) |
Incidence ratio, ≥56 days vs. 15–30 days (95% CI) |
||
|---|---|---|---|---|---|---|---|
| Dosing interval 15–30 days |
Dosing interval 31–55 days |
Dosing interval ≥56 days |
|||||
| BNT162b2 | Both sexes | All ages | 76.8 (47.6–123.8) | 30.0 (19.9–45.1) | 22.9 (12.7–41.1) | 0.43 (0.30–0.61) | 0.28 (0.19–0.41) |
| Females | All ages | 49.4 (17.8–137.2) | 15.3 (7.4–31.8) | 12.6 (4.5–35.5) | 0.30 (0.15–0.63) | 0.27 (0.15–0.51) | |
| 12–17 yrs | 64.5 (26.8–154.9) | 15.9 (6.6–38.4) | 38.1 (17.1–84.7) | 0.29 (0.07–1.13) | 0.30 (0.02–3.75) | ||
| 18–29 yrs | 129.5 (42.6–393.4) | 17.1 (7.1–40.9) | 22.8 (4.9–105.8) | 0.17 (0.05–0.61) | 0.31 (0.11–0.85) | ||
| 30–39 yrs | 66.3 (19.2–229.1) | 23.4 (8.2–66.6) | 17.3 (9.0–33.3) | 0.26 (0.04–1.66) | 0.35 (0.07–1.79) | ||
| ≥40 yrs | 48.2 (10.4–224.2) | 13.2 (6.3–27.7) | 12.1 (4.2–35.2) | 1.05 (0.07–16.90) | 0.33 (0.10–1.05) | ||
| Males | All ages | 104.1 (73.9–146.4) | 47.1 (34.4–64.3) | 33.9 (23.3–49.2) | 0.45 (0.30–0.69) | 0.28 (0.19–0.41) | |
| 12–17 yrs | 215.8 (135.9–342.5) | 112.5 (82.2–153.9) | 133.6 (91.6–194.8) | 0.57 (0.32–1.00) | 0.71 (0.35–1.44) | ||
| 18–29 yrs | 219.6 (83.9–574.9) | 52.9 (32.9–85.1) | 79.9 (42.8–149.6) | 0.29 (0.12–0.66) | 0.35 (0.16–0.74) | ||
| 30–39 yrs | 111.5 (46.4–267.9) | 22.9 (10.6–49.5) | 43.8 (28.5–67.1) | 0.28 (0.08–1.01) | 0.51 (0.16–1.58) | ||
| ≥40 yrs | 26.1 (8.4–81.0) | 13.3 (6.3–27.9) | 19.4 (13.0–28.9) | 0.78 (0.16–3.89) | 1.11 (0.27–4.59) | ||
| mRNA-1273 | Both sexes | All ages | 117.2 (85.6–160.4) | 70.1 (58.9–83.3) | 39.6 (27.2–57.6) | 0.63 (0.44–0.90) | 0.26 (0.18–0.38) |
| Females | All ages | 60.5 (31.7–115.2) | 25.0 (16.3–38.4) | 26.8 (15.5–46.3) | 0.48 (0.22–1.06) | 0.32 (0.15–0.68) | |
| 12–17 yrs | NA | NA | NA | NA | NA | ||
| 18–29 yrs | 90.1 (26.1–311.2) | 44.8 (24.8–80.9) | 85.4 (50.6–144.2) | 0.87 (0.19–3.93) | 0.92 (0.20–4.33) | ||
| 30–39 yrs | 169.0 (52.7–542.5) | 22.3 (9.3–53.6) | 64.3 (36.5–113.3) | 0.20 (0.02–1.66) | 0.46 (0.12–1.80) | ||
| ≥40 yrs | 50.9 (21.2–122.3) | 17.8 (8.0–39.6) | 15.5 (8.8–27.4) | 0.39 (0.11–1.40) | 0.29 (0.09–0.89) | ||
| Males | All ages | 181.1 (126.6–258.9) | 112.7 (93.2–136.2) | 52.3 (38.4–71.3) | 0.64 (0.42–0.96) | 0.24 (0.16–0.37) | |
| 12–17 yrs | NA | NA | NA | NA | NA | ||
| 18–29 yrs | 447.3 (288.6–693.3) | 257.5 (204.7–323.9) | 239.4 (171.4–334.4) | 0.58 (0.35–0.95) | 0.46 (0.26–0.83) | ||
| 30–39 yrs | 201.1 (98.3–411.3) | 90.6 (59.7–137.6) | 69.9 (40.6–120.5) | 0.44 (0.19–1.04) | 0.39 (0.14–1.04) | ||
| ≥40 yrs | 46.2 (16.2–131.9) | 30.0 (17.0–52.8) | 25.6 (17.5–37.5) | 0.88 (0.25–3.13) | 0.59 (0.18–1.94) | ||
Table 3.
Adjusted incidence of hospitalizations or emergency department visits for myocarditis or pericarditis within 21 days following receipt of BNT162b2 (Pfizer-BioNTech Comirnaty) or mRNA-1273 (Moderna SpikeVax) as the second COVID-19 vaccine dose in 3 Canadian provinces, by varying vaccine schedules, up to September 30, 2021.
| Vaccine schedule (First dose/second dose) | Incidence per 1,000,000 persons (95% CI) |
Incidence ratio, 31–55 days vs. 15–30 days (95% CI) |
Incidence ratio, ≥56 days vs. 15–30 days (95% CI) |
||
|---|---|---|---|---|---|
| Dosing interval 15–30 days |
Dosing interval 31–55 days |
Dosing interval ≥56 days |
|||
| BNT162b2/BNT162b2 | 81.8 (48.8–137.3) | 34.7 (22.9–52.3) | 21.6 (11.6–40.2) | 0.47 (0.33–0.67) | 0.25 (0.17–0.37) |
| mRNA-1273/mRNA-1273 | 100.1 (68.6–145.9) | 59.2 (46.3–75.6) | 49.4 (36.4–66.9) | 0.61 (0.39–0.97) | 0.40 (0.25–0.63) |
| BNT162b2/mRNA-1273 | 173.6 (96.1–313.5) | 70.3 (53.4–92.5) | 35.7 (24.9–51.1) | 0.46 (0.23–0.91) | 0.22 (0.11–0.47) |
3.5. Hospitalization post COVID-19 Vs. myocarditis/pericarditis-related hospitalization or ED visits post mRNA vaccination
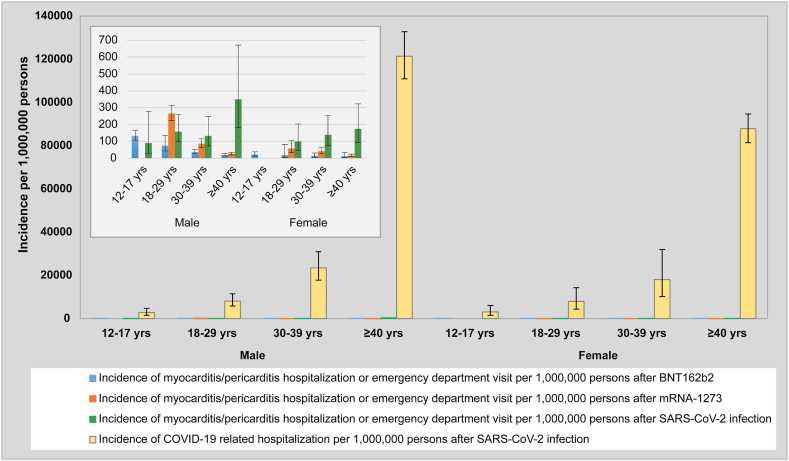
We observed a considerably higher risk of COVID-19-related hospitalization after laboratory-confirmed SARS-CoV-2 infection compared to the risk of myocarditis/pericarditis-related hospitalization or ED visit after either mRNA COVID-19 vaccine (Table 4 and Fig. 1).
Table 4.
Incidence of hospitalizations for COVID-19 within 21 days following a laboratory-confirmed SARS-CoV-2 infection in 3 Canadian provinces, up to September 30, 2021.
| Sex | Age | Incidence per 1,000,000 persons (95% CI) |
|---|---|---|
| Both sexes | All ages | 58,392.5 (54,855.7-62,157.3) |
| Females | All ages | 50,990.1 (47,690.9-54,517.6) |
| 12–17 yrs | 3084.3 (1576.7-6033.4) | |
| 18–29 yrs | 7955.8 (4394.2-14,404.1) | |
| 30–39 yrs | 18,032.9 (10,174.0-31,962.5) | |
| ≥40 yrs | 87,869.3 (81,494.0-94,743.4) | |
| Males | All ages | 66,164.9 (62,537.5-70,002.9) |
| 12–17 yrs | 2785.2 (1610.2-4817.7) | |
| 18–29 yrs | 8176.4 (5835.9-11,455.6) | |
| 30–39 yrs | 23,510.9 (17,804.5-31,046.1) | |
| ≥40 yrs | 121,422.1 (110,949.4-132,883.4) |
Fig. 1.
Title: Incidence of COVID-19 hospitalization vs. incidence of hospitalizations or emergency department visits for mRNA vaccine or SARS-CoV-2 infection associated myocarditis/pericarditis.
Legend: Incidence of hospitalizations for COVID-19 within 21 days following a laboratory-confirmed SARS-CoV-2 infection compared to incidence of hospitalizations or emergency department visits for myocarditis or pericarditis within 21 days following a laboratory confirmed SARS-CoV-2 infection or receipt of BNT162b2 or mRNA-1273 as the second COVID-19 vaccine dose in 3 Canadian provinces.
4. Discussion
In this multi-provincial population-based study, the overall incidence of hospitalizations or ED visits for myocarditis/pericarditis after a second dose of mRNA COVID-19 vaccine was 28 per million doses of BNT162b2, and 53 per million doses of mRNA-1273 vaccine, while the corresponding incidence after SARS-CoV-2 infection was 194 per million laboratory-confirmed infections. Risk of myocarditis/pericarditis after vaccination was higher with a shorter dosing interval and among younger males, while the incidence after infection was higher among older individuals. Importantly, the overall incidence of myocarditis/pericarditis after SARS-CoV-2 infection was 4-fold higher than after receiving mRNA-1273 as a second dose and 7-fold higher than after receiving BNT162b2 as a second dose. This finding was particularly pronounced in individuals aged ≥40 years but was also present in younger males who received BNT162b2. The incidence exhibited a graded negative association with increasing dosing interval for both homologous and heterologous vaccination schedules. To provide additional context, we observed a considerably higher risk of COVID-19-related hospitalization following PCR-confirmed infection (58,393 per million persons) than the risk of myocarditis/pericarditis-related hospitalization or ED visit following receipt of either mRNA vaccine (28–53 events per million persons). These findings suggest a lower risk of myocarditis/pericarditis after vaccination than after infection and highlight that the risk of COVID-19 hospitalization after infection is much higher than the risk of myocarditis/pericarditis after vaccination. Our analysis also provides insight into strategies to reduce risk of vaccine-related myocarditis/pericarditis (e.g., longer dosing intervals, preferential use of BNT162b2 in younger individuals) that were incorporated in provincial COVID-19 vaccination programs in 2021 [9,17].
Our finding of higher incidence of hospitalization or ED visits for myocarditis/pericarditis after SARS-CoV-2 infection than after mRNA vaccines generally aligns with previous studies. Using electronic health record data from 40 healthcare systems in the USA, Block et al. observed a significantly higher risk of myocarditis/pericarditis among males and females aged ≥12 years within 21 days after SARS-CoV-2 infection compared to after a second dose of mRNA vaccines [18]. However, the results were not reported by vaccine product. Similarly, two self-controlled case series studies conducted by Patone et al. reported overall higher numbers of excess myocarditis events per million people after SARS-CoV-2 infection versus the second dose of either mRNA vaccine (risk period of 1–28 days). However, the age-stratified analysis showed a higher risk of myocarditis post-second dose of mRNA-1273 than post SARS-CoV-2 infection among males younger than 40 years [19,20]. In our study, compared to SARS-CoV-2 infection, we observed a non-significant higher incidence of myocarditis/pericarditis after BNT162b2 among males aged 12–17 years and after mRNA-1273 among males aged 18–29 years. Finally, in a systematic review and meta-analysis, Voleti et al. identified a 7-fold higher risk of myocarditis after SARS-CoV-2 infection than after COVID-19 vaccination. While the meta-analysis was limited by varying methods of myocarditis diagnosis and a wide variation in the follow-up times among the studies included, our results corroborate their estimates [12]. Our findings have implications for building vaccine confidence and reducing vaccine hesitancy, considering the higher risk of myocarditis/pericarditis following SARS-CoV-2 infection compared to mRNA vaccination in patients aged ≥40 years as well as in younger patients receiving BNT162b2.
We observed a graded lower incidence of myocarditis or pericarditis with longer dosing intervals compared to the shortest interval (15–30 days), irrespective of the first vaccine product. Overall, the lowest incidence occurred among individuals with dosing intervals of ≥56 days. Our findings corroborate the results of another study conducted in Ontario. Buchan et al. reported significantly higher overall rates of myocarditis/pericarditis identified based on passive safety surveillance reports when the dosing interval was ≤30 days compared with ≥56 days for either mRNA vaccine received as the second dose [10]. In an exploratory analysis presented at the US Advisory Committee on Immunization Practices (ACIP) meeting, for the majority of the myocarditis/pericarditis cases, the mRNA primary series dosing interval was <30 days [21]. However, the US adhered closely to the dosing intervals outlined in the product monographs, with minimal deviations. Although our study was not focused on effectiveness, previous studies have shown that a longer interval between the two primary series doses is associated with a higher antibody response and increased effectiveness against infection [22,23]. Thus, a dosing interval longer than the initial minimum 3–4 weeks recommendation may be optimal, especially for younger age groups among whom we observed a higher risk of post-vaccination myocarditis.
Our study had several strengths. This is the largest Canadian study, including approximately 70% of the country's population, that provides quantitative estimates of the comparative risk of myocarditis/pericarditis after SARS-CoV-2 infection or COVID-19 mRNA vaccination, stratified by mRNA vaccine type, sex, and age. The study also further provides insight into the relatively understudied potential association between dosing interval and myocarditis/pericarditis. The multi-provincial nature of the study enhanced the power to study rare outcomes. Due to the exclusion of any individual with a history of myocarditis/pericarditis within one year preceding the date of exposure, the likelihood of a previous history of myocarditis/pericarditis increasing the risk of postexposure occurrence was mitigated.
Our study did not aim to investigate the molecular basis or pathogenesis of SARS-CoV-2 or vaccine associated myocarditis/pericarditis. Future research, particularly among the younger population, could focus on exploring these critical aspects. Furthermore, we primarily focused on the first occurrence of myocarditis/pericarditis post vaccination or infection. However, given the recent reports of recurrent myocarditis following mRNA COVID-19 vaccine despite a prior episode with full clinical recovery among adolescents [24], follow-up studies to comprehensively examine this phenomenon among both vaccine and infection-associated myocarditis cases are warranted.
5. Limitations
Certain limitations should be considered. First, due to the non-availability of clinical and imaging data, the diagnosis of myocarditis/pericarditis was based on ICD-10 diagnostic codes rather than the standardized case definition criteria from the Brighton Collaboration for myocarditis/pericarditis following COVID-19 vaccination [25,26]. Thus, there is the possibility of outcome misclassification. Second, it is possible that the incidence of post-infection myocarditis/pericarditis has been underestimated using hospitalization data due to coding practices. Specifically, a patient admitted with both COVID-19 and myocarditis may have had COVID-19 recorded as the primary diagnosis, resulting in underreporting of myocarditis cases. Furthermore, based on the definition used in our study, individuals who develop myocarditis during their hospital stay may also be missed in the data. Third, as we relied on hospital and ED visit data, we might have missed less severe cases of myocarditis/pericarditis that did not require medical attention or only required care from primary care providers. Fourth, as we excluded anyone diagnosed with SARS-CoV-2 within 21 days before or after vaccination and those vaccinated within 21 days before or after SARS-CoV-2 infection, we might have underestimated myocarditis/pericarditis events in our two cohorts. However, there is no evident reason to believe that this underestimation would be differential among the groups being compared. Fifth, our study was limited to PCR-confirmed SARS-CoV-2 cases and may have resulted in underestimation of milder myocarditis/pericarditis cases that were probably not linked with preceding PCR testing or in overestimation of SARS-CoV-2-associated myocarditis/pericarditis by considering only PCR-positive infections as a denominator. However, our approach provides enhanced reliability of diagnosis. PCR testing was available without any restriction during the pre-Omicron period. During the Omicron period (starting December 2021) and later, point of care (PoC) tests became widely available and were used by patients at home. At that time, PCR testing was restricted to those presenting at hospitals and people at risk of severe disease. Thus, PoC was not a major issue during the study period (January 2020 to September 2021). Sixth, due to the unavailability of whole genome sequencing data for all PCR-confirmed cases in our study, we were unable to stratify by SARS-CoV-2 variants. Throughout the study period, the Alpha variant was predominant until June 2021, at which point the Delta variant emerged around May 2021 and swiftly became the predominant strain by July 2021. Seventh, due to the rarity of the outcomes, the results of the sub-stratified analysis (by age and sex) should be interpreted cautiously. Finally, the findings of this study are reflective of the SARS-CoV-2 epidemiology and COVID-19 vaccination policy and practices during the study period and may not be generalizable to the present day. Thus, future research is needed to determine if the risk of myocarditis/pericarditis after SARS-CoV-2 infection with Omicron remains higher than the risk of myocarditis/pericarditis after an updated mRNA COVID-19 booster dose.
6. Conclusion
The absolute risk of myocarditis and pericarditis after COVID-19 mRNA vaccination appears to be low. Comparatively, SARS-CoV-2 infection carries a greater risk of myocarditis/pericarditis than receiving a second dose of either of the mRNA vaccines used in Canada, although findings vary by sex and age. For most age and sex groups, the risk of developing myocarditis/pericarditis after vaccination is outweighed by the risk that follows laboratory-confirmed SARS-CoV-2 infection. Furthermore, the risk of a COVID-19 hospitalization after SARS-CoV-2 infection remains much higher than the risk of myocarditis post-vaccination. Dosing intervals that are longer than those recommended by manufacturers may lower the risk of post-vaccination myocarditis/pericarditis. Our study findings support the national guidelines in place at the time of study conduct (i.e., continued use of mRNA COVID-19 vaccines with a longer interval [at least 8 weeks] between the two primary series doses and the preferential use of BNT162b2 for the primary vaccine series in those younger than 30 years).
Ethics approval
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. This study was performed using de-identified data routinely collected as part of public health surveillance and/or routine healthcare encounters. Patient consent was not required in accordance with the Canadian Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans article 5.5B. In British Columbia (BC), the study was reviewed and approved by the Behavioral Research Ethics Board at the University of British Columbia (approval #H20-02097). Use of the Ontario data in this project is authorized under section 45 of Ontario's Personal Health Information Protection Act and does not require review by a Research Ethics Board. ICES is a prescribed entity under PHIPA. Section 45 of PHIPA authorizes ICES to collect personal health information, without consent, for the purpose of analysis or compiling statistical information with respect to the management of, evaluation or monitoring of the allocation of resources to or planning for all or part of the health system.
Funding
This work was supported by the Canadian Immunization Research Network (CIRN) through a grant from the Public Health Agency of Canada and the Canadian Institutes of Health Research (CNF 151944), and also by funding from the Public Health Agency of Canada, through the Vaccine Surveillance Working Party and the COVID-19 Immunity Task Force. This study was supported by Public Health Ontario and by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and Ministry of Long-Term Care (MLTC). This work was also supported by the Ontario Health Data Platform (OHDP), a Province of Ontario initiative to support Ontario's ongoing response to COVID-19 and its related impacts. JCK is supported by Clinician-Scientist Award from the University of Toronto Department of Family and Community Medicine. The study sponsors did not participate in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
Disclaimers
All inferences, opinions, and conclusions drawn in this manuscript are those of the authors, and do not reflect the opinions or policies of the Data Steward(s) in British Columbia. Parts of this material are based on data and/or information compiled and provided by MOH and the Canadian Institute for Health Information (CIHI). The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Data availability
Data associated with this study have not been deposited into a publicly available repository because the authors do not have permission to share data.
In BC the study is based on data contained in various provincial registries and databases. Access to BC data could be requested through the BC Centre for Disease Control Institutional Data.
Access for researchers who meet the criteria for access to confidential data. Requests for the data may be sent to datarequest@bccdc.ca. The dataset for Ontario from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (e.g., healthcare organizations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at https://www.ices.on.ca/DAS (email: das@ices.on.ca). The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification. In QC the study is based on data contained in various provincial registries and databases. Access to data could be requested from the ministère de la Santé et des Services sociaux du Québec for researchers who meet the criteria for access to confidential data. Requests for the data may be sent to: msss_prp@msss.gouv.qc.ca.
CRediT authorship contribution statement
Zaeema Naveed: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation. Cherry Chu: Writing – review & editing, Formal analysis, Data curation. Mina Tadrous: Writing – review & editing, Formal analysis, Data curation. Areti-Angeliki Veroniki: Writing – review & editing, Formal analysis. Julia Li: Writing – review & editing, Formal analysis, Data curation. Isabelle Rouleau: Writing – review & editing, Formal analysis, Data curation. Yossi Febriani: Writing – review & editing, Formal analysis. Andrew Calzavara: Writing – review & editing, Formal analysis, Data curation. Sarah A. Buchan: Writing – review & editing, Methodology, Conceptualization. Sharifa Nasreen: Writing – review & editing, Formal analysis, Data curation. Kevin L. Schwartz: Writing – review & editing, Visualization, Methodology. James Wilton: Writing – review & editing, Methodology. Chi Yon Seo: Writing – review & editing, Methodology. Nisha Thampi: Writing – review & editing, Methodology. Sarah E. Wilson: Writing – review & editing, Methodology, Investigation. Monika Naus: Writing – review & editing, Methodology. Gaston De Serres: Writing – review & editing, Supervision, Methodology, Investigation, Conceptualization. Naveed Z. Janjua: Writing – review & editing, Supervision, Methodology, Investigation, Conceptualization. Jeffrey C. Kwong: Writing – review & editing, Supervision, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Naveed Z. Janjua reports a relationship with AbbVie Inc that includes: consulting or advisory and speaking and lecture fees. Naveed Z. Janjua reports a relationship with Gilead Sciences that includes: consulting or advisory and speaking and lecture fees. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge the assistance of the Provincial Health Services Authority, BC Ministry of Health and Regional Health Authority staff involved in data access, procurement, and management. We would also like to acknowledge Public Health Ontario for access to case-level data from the Public Health Case and Contact Management Solution (CCM) and COVID-19 laboratory data. We also thank the staff of Ontario's public health units who are responsible for COVID-19 case and contact management and data collection within CCM. The authors are grateful to the residents of Ontario, Quebec, and British Columbia, without whom this research would be impossible.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e26551.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Hause A.M., Gee J., Baggs J., Abara W.E., Marquez P., Thompson D., et al. COVID-19 vaccine safety in adolescents aged 12-17 Years - United States, december 14, 2020-july 16, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1053–1058. doi: 10.15585/mmwr.mm7031e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., et al. Safety of the BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N. Engl. J. Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein N.P., Lewis N., Goddard K., Fireman B., Zerbo O., Hanson K.E., et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326:1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ontario Agency for Health Protection and Promotion . Public Health Ontario; Toronto, ON: 2021. Weekly Summary: Adverse Events Following Immunization (AEFIs) for COVID-19 in Ontario: December 13, 2020 to January 9.https://www.publichealthontario.ca/-/media/Documents/nCoV/Archives/AEFI/2022/01/covid-19-aefi-report-2022-01-14.pdf?rev=e5b838b8bcf242019ca58625eca98187&sc_lang=en [Google Scholar]
- 5.Su J.R. COVID-19 vaccine safety updates: primary series in children and adolescents ages 5–11 and 12–15 years, and booster doses in adolescents ages 16–24 years. 2022. https://stacks.cdc.gov/view/cdc/113091
- 6.Goddard K., Lewis N., Fireman B., Weintraub E., Shimabukuro T., Zerbo O., et al. Risk of myocarditis and pericarditis following BNT162b2 and mRNA-1273 COVID-19 vaccination. Vaccine. 2022;40:5153–5159. doi: 10.1016/j.vaccine.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlstad Ø., Hovi P., Husby A., Härkänen T., Selmer R.M., Pihlström N., et al. SARS-CoV-2 vaccination and myocarditis in a nordic cohort study of 23 million residents. JAMA Cardiol. 2022;7:600–612. doi: 10.1001/jamacardio.2022.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naveed Z., Li J., Wilton J., Spencer M., Naus M., Velásquez García H.A., et al. Comparative risk of myocarditis/pericarditis following second doses of BNT162b2 and mRNA-1273 coronavirus vaccines. J. Am. Coll. Cardiol. 2022;80:1900–1908. doi: 10.1016/j.jacc.2022.08.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Advisory Committee on Immunization, Archived 25: NACI rapid response, Updated recommendation on the use of authorized COVID-19 vaccines in individuals aged 12 years and older in the context of myocarditis and pericarditis reported following mRNA COVID-19 vaccines (2021). https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/rapid-response-recommendation-use-covid-19-vaccines-individuals-aged-12-years-older-myocarditis-pericarditis-reported-following-mrna-vaccines.html#a10. (Accessed 19 January 2022).
- 10.Buchan S.A., Seo C.Y., Johnson C., Alley S., Kwong J.C., Nasreen S., et al. Epidemiology of myocarditis and pericarditis following mRNA vaccination by vaccine product, schedule, and interdose interval among adolescents and adults in Ontario, Canada. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.18505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mostafavi A., Tabatabaei S.A.H., Zamani Fard S., Majidi F., Mohagheghi A., Shirani S. The incidence of myopericarditis in patients with COVID-19. J. Cardiovasc. Thorac. Res. 2021;13:203–207. doi: 10.34172/jcvtr.2021.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voleti N., Reddy S.P., Ssentongo P. Myocarditis in SARS-CoV-2 infection vs. COVID-19 vaccination: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.951314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boehmer T.K., Kompaniyets L., Lavery A.M., Hsu J., Ko J.Y., Yusuf H., et al. Association between COVID-19 and myocarditis using hospital-based administrative data - United States, March 2020-january 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1228–1232. doi: 10.15585/mmwr.mm7035e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willame C., Dodd C., Gini R., Durán C., Thomsen R., Wang L., et al. Background rates of adverse events of special interest for monitoring COVID-19 vaccines. Zenodo. 2021 doi: 10.5281/ZENODO.5255869. [DOI] [Google Scholar]
- 15.Stijnen T., Hamza T.H., Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat. Med. 2010;29:3046–3067. doi: 10.1002/sim.4040. [DOI] [PubMed] [Google Scholar]
- 16.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid. Base Ment. Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Advisory Committee on Immunization . 2021. Archive 22: Recommendations on the Use of COVID-19 Vaccines.https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html#a3 [Google Scholar]
- 18.Block J.P., Boehmer T.K., Forrest C.B., Carton T.W., Lee G.M., Ajani U.A., et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination - PCORnet, United States, january 2021-january 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:517–523. doi: 10.15585/mmwr.mm7114e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patone M., Mei X.W., Handunnetthi L., Dixon S., Zaccardi F., Shankar-Hari M., et al. Risk of myocarditis after sequential doses of COVID-19 vaccine and SARS-CoV-2 infection by age and sex. Circulation. 2022;146:743–754. doi: 10.1161/circulationa.122.059970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patone M., Mei X.W., Handunnetthi L., Dixon S., Zaccardi F., Shankar-Hari M., P, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2022;28:410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein Nicola P. 2021. Myocarditis Analyses in the Vaccine Safety Datalink: Rapid Cycle Analyses and “Head-to-head” Product Comparisons.https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-10-20-21/08-COVID-Klein-508.pdf [Google Scholar]
- 22.Amirthalingam G., Bernal J.L., Andrews N.J., Whitaker H., Gower C., Stowe J., et al. Serological responses and vaccine effectiveness for extended COVID-19 vaccine schedules in England. Nat. Commun. 2021;12:7217. doi: 10.1038/s41467-021-27410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grunau B., Asamoah-Boaheng M., Lavoie P.M., Karim M.E., Kirkham T.L., Demers P.A., et al. A higher antibody response is generated with a 6- to 7-week (vs standard) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine dosing interval. Clin. Infect. Dis. 2022;75:e888–e891. doi: 10.1093/cid/ciab938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amodio D., Manno E.C., Cotugno N., Santilli V., Franceschini A., Perrone M.A., Chinali M., Drago F., Cantarutti N., Curione D., Engler R., Secinaro A., Palma P. Relapsing myocarditis following initial recovery of post COVID-19 vaccination in two adolescent males - case reports. Vaccine X. 2023;14 doi: 10.1016/j.jvacx.2023.100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brighton Collaboration Myocarditis/Pericarditis case definition. 2021. https://brightoncollaboration.org/myocarditis/
- 26.Sexson Tejtel S.K., Munoz F.M., Al-Ammouri I., Savorgnan F., Guggilla R.K., Khuri-Bulos N., et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40:1499–1511. doi: 10.1016/j.vaccine.2021.11.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study have not been deposited into a publicly available repository because the authors do not have permission to share data.
In BC the study is based on data contained in various provincial registries and databases. Access to BC data could be requested through the BC Centre for Disease Control Institutional Data.
Access for researchers who meet the criteria for access to confidential data. Requests for the data may be sent to datarequest@bccdc.ca. The dataset for Ontario from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (e.g., healthcare organizations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at https://www.ices.on.ca/DAS (email: das@ices.on.ca). The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification. In QC the study is based on data contained in various provincial registries and databases. Access to data could be requested from the ministère de la Santé et des Services sociaux du Québec for researchers who meet the criteria for access to confidential data. Requests for the data may be sent to: msss_prp@msss.gouv.qc.ca.