Abstract
OBJECTIVE
Diabetes presenting at a younger age has a more aggressive nature. We aimed to explore the association of age at type 2 diabetes mellitus (T2DM) diagnosis with subsequent cancer incidence in a large Chinese population.
RESEARCH DESIGN AND METHODS
The prospective population-based longitudinal cohort included 428,568 newly diagnosed T2DM patients from 2011 to 2018. Participants were divided into six groups according to their age at diagnosis: 20–54, 55–59, 60–64, 65–69, 70–74, and ≥75 years. The incidence of overall and 14 site-specific cancers was compared with the Shanghai general population including 100,649,346 person-years.
RESULTS
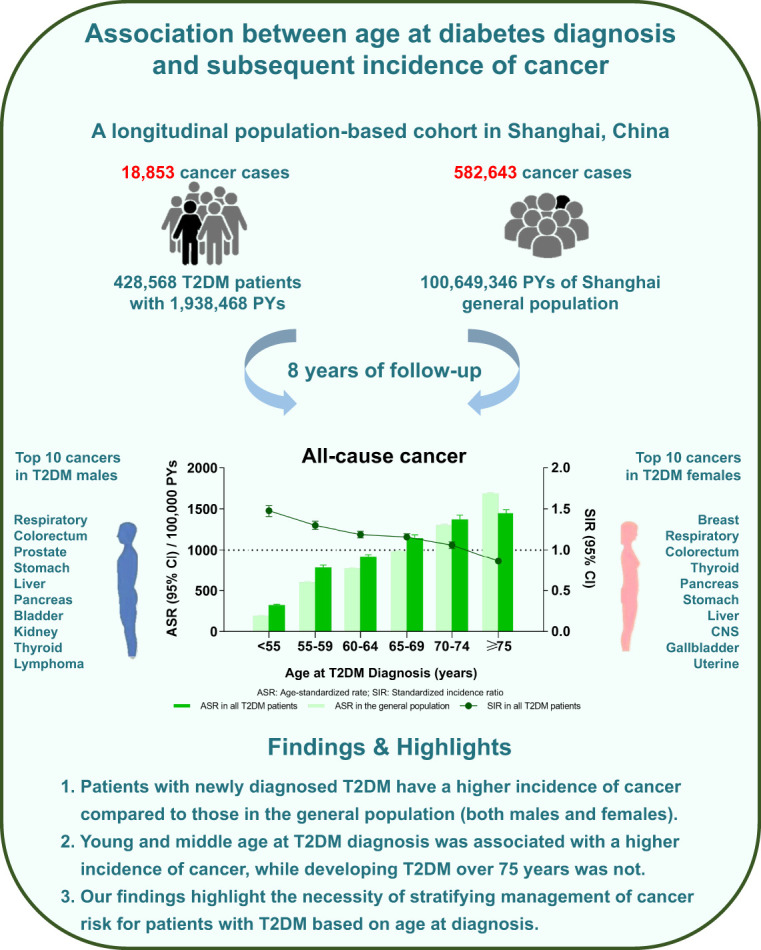
A total of 18,853 and 582,643 overall cancer cases were recorded in the T2DM cohort and the general population. The age-standardized rate of overall cancer in T2DM patients was 501 (95% CI: 491, 511) per 100,000 person-years, and the standardized incidence ratio (SIR) was 1.10 (1.09, 1.12). Younger age at T2DM diagnosis was associated with higher incidence of overall and site-specific cancers. SIRs for overall cancer with T2DM diagnosis at ages 20–54, 55–59, 60–64, 65–69, 70–74, and ≥75 years were 1.48 (1.41, 1.54), 1.30 (1.25, 1.35), 1.19 (1.15, 1.23), 1.16 (1.12, 1.20), 1.06 (1.02, 1.10), and 0.86 (0.84, 0.89), respectively. Similar trends were observed for site-specific cancers, including respiratory, colorectum, stomach, liver, pancreatic, bladder, central nervous system, kidney, and gallbladder cancer and lymphoma among both males and females.
CONCLUSIONS
Our findings highlight the necessity of stratifying management for T2DM according to age of diagnosis. As with a range of vascular outcomes, age-standardized cancer risks are greater in earlier compared with later onset T2DM.
Graphical Abstract
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disorder characterized by hyperglycemia associated with obesity, insulin resistance, and subsequent complications (1,2). The prevalence of T2DM has increased worldwide over the past 30 years, and, of note, age of T2DM onset between 45 and 65 years has increased disproportionately rapidly (3,4). Compared with diabetes developing at older ages, that presenting at a young age has a more aggressive nature and becomes a challenging clinical entity (5–11). There are increasing reports that young and middle age T2DM diagnosis is associated with greater risk of several complications and multimorbidity, including comorbidities such as cardiovascular diseases, retinopathy, dementia, and nephropathy. The earlier the age of diabetes diagnosis, the higher the risk of complications (5,12–14). However, few studies have explored the association between diabetes diagnosis age and overall cancer incidence across a large population. Moreover, previous studies had insufficient numbers of specific cancer types to explore the relationship between age at diabetes diagnosis and incidence of specific malignancies (15).
The current study aimed to explore the association of age at T2DM diagnosis with the subsequent incidence of both overall and site-specific cancer incidence based on a longitudinal population-based cohort including 428,568 newly diagnosed T2DM patients during 8 years of follow-up in Shanghai, China. Results from a large population-based longitudinal cohort will provide more authoritative evidence for clinics to care for patients with T2DM according to their age of diagnosis.
Research Design and Methods
Study Design and T2DM Population
We assessed the epidemiological association between T2DM diagnosis age and the risk of cancer incidence based on 428,568 newly diagnosed T2DM patients in a longitudinal population-based cohort and the general population of Shanghai aged 20 years or older including 100,649,346 person-years (PYs) from 2011 to 2018.
The longitudinal cohort was established based on the Shanghai Standardized Diabetes Management System (SSDMS) and the malignant tumor registry system operated by Shanghai Municipal Center for Disease Control and Prevention (SCDC). All diagnosed cases of T2DM in Shanghai were registered in the system, including newly diagnosed cases in community-based screenings and previously diagnosed cases reported by routine outpatient visits (16). T2DM cases were diagnosed based on fasting plasma glucose of ≥7.0 mmol/L and/or a 2-h glucose (after 75 g oral glucose tolerance test) level of ≥11.1 mmol/L or use of glucose-lowering medication according to the 1998 World Health Organization criteria. Patients with type 1 diabetes were not included in the system. Baseline information for each patient in the SSDMS, including height, weight, blood pressure, and fasting glucose, was collected during an initial assessment at registration. Information on smoking history, alcohol consumption, and physical activity was available from about 60% of patients in the current system.
The malignant tumor registry was initiated in 1963 and well established by the SCDC in 2002. All cancer cases must be registered in the system by general hospitals and specialized hospitals with cancer diagnosis qualification. The relevant technical standards issued by the International Agency for Research on Cancer and the relevant technical requirements issued by the National Office of Cancer Prevention and Treatment were used for tumor registration. The classification of tumor location was coded according to the ICD-10 codes (Supplementary Material).
The geographic distribution of both registries includes all 16 districts of Shanghai. From 1 January 2011 to 31 December 2018, a total of 428,568 newly diagnosed patients with T2DM had been registered in the SSDMS (17). The median time from the diagnosis of T2DM to registration was 8.6 months. New cases of cancer occurrence were identified through the malignant tumor registry system during an 8-year period. Incident cancers were defined as the occurrence of any type of cancer from the diagnosis of T2DM to the study end point of 31 December 2018. This study was approved by the Ethical Review Committee of SCDC in Shanghai, China.
Statistical Analysis
Baseline characteristics were summarized as the number and percentage for categorical data and mean ± SD and median (5th, 95th) percentiles for continuous variables. Patients with T2DM were classified into six age of diagnosis groups: 20–54, 55–59, 60–64, 65–69, 70–74, and ≥75 years. We used one-way ANOVA for continuous variables to test the significant difference.
The overall cancer incidence rate was calculated for the occurrence of any type of cancer, and the site-specific cancer incidence rates were calculated for the occurrence of specific cancer types. The crude incidence rates were the number of cancer cases that occurred during the study period divided by the total PYs. PYs of follow-up were calculated from the date of T2DM diagnosis to the date of cancer diagnosis, loss to follow-up, or study end point, 31 December 2018. The age-standardized rates (ASRs; per 100,000 PYs) were calculated using the direct method, based on the China sixth nationwide population census by 5-year age groups and age-specific incidence.
The standardized incidence ratio (SIR) with a 95% CI was calculated as the ratio of the observed to the expected number of cancers in each year. The expected numbers of cancer were computed by a weighted sum of stratified incidence rates by sex and age from the reference population if the population has the same cancer experience as a larger comparison population designated as “normal” or average (18). The expected numbers were defined by multiplying the accumulated PYs of follow-up in each year by the 5-year age group cancer incidence rate in the Shanghai cancer registry system. The annual 5-year age group incidence of cancer for the reference population was calculated by dividing the number of incident cancers by the number of adults aged 20 years or older in the Shanghai general population for every 5-year age group from 2011 to 2018. The 95% CIs for the SIR were calculated based on the Poisson distribution. Analyses were undertaken in participants overall and by sex. Cox proportional hazards models were used to compute the adjusted hazard ratios (HRs) with 95% CIs of BMI, sex, fasting plasma glucose (FPG), systolic blood pressure (SBP), diastolic blood pressure (DBP), and age at T2DM diagnosis in all T2DM patients and male and female T2DM patients. Sensitivity analysis was conducted in 60% of patients, for whom smoking history, alcohol consumption, and physical activity information was available. The proportionality assumption was verified by Schoenfeld residuals. The sensitivity analysis was conducted to assess the effect of death as a competing risk using the Fine and Gray subdistribution hazard competing risk model. Statistical analyses were performed using SAS software (version 9.4; SAS Institute, Inc., Cary, NC). P values of <0.05 were considered statistically significant.
Data and Resource Availability
Patient-level data cannot be shared without approval from data custodians, owing to local information governance and data protection regulations. Additional requests for materials may be addressed to Y.L.
Results
Baseline Characteristics Across Different Ages in T2DM Diagnosis Groups
From January 2011 to December 2018, a total of 428,568 (222,766 females, 51.98%) patients with T2DM were enrolled in the cohort, with a median (5th, 95th) follow-up time of 4.58 (0.99, 8.00) years (Table 1 and Supplementary Table 1). The mean T2DM diagnosis age was 63.81 ± 11.09 years overall, 62.95 ± 11.27 years in males, and 64.61 ± 10.87 years in females (P < 0.001). Compared with T2DM patients with age of diagnosis older than 75 years, those with age of diagnosis less than 55 years had higher BMI (24.66 ± 3.23 vs. 24.00 ± 3.14 kg/m2, P < 0.001) and higher FBG (7.42 ± 1.90 vs. 7.17 ± 1.60 mmol/L, P < 0.001).
Table 1.
Baseline characteristics in the newly diagnosed T2DM cohort
| Total | Males | Females | |
|---|---|---|---|
| Total N | 428,568 | 205,802 | 222,766 |
| Age at diagnosis of diabetes, N (%) | |||
| 20–54 years | 83,823 (19.56) | 45,170 (21.95) | 38,653 (17.35) |
| 55–59 years | 73,124 (17.06) | 34,466 (16.75) | 38,658 (17.35) |
| 60–64 years | 86,698 (20.23) | 41,622 (20.22) | 45,076 (20.23) |
| 65–69 years | 68,606 (16.01) | 32,791 (15.93) | 35,815 (16.08) |
| 70–74 years | 44,218 (10.32) | 21,083 (10.24) | 23,135 (10.39) |
| ≥75 years | 72,099 (16.82) | 30,670 (14.90) | 41,429 (18.60) |
| Follow-up time, years | 4.58 (0.99, 8.00) | 4.52 (0.93, 8.00) | 4.65 (1.03, 8.00) |
| BMI, kg/m2, average | 24.43 ± 3.11 | 24.41 ± 2.92 | 24.45 ± 3.28 |
| BMI, N (%) | |||
| <23 kg/m2 | 5,370 (1.35) | 2,205 (1.16) | 3,165 (1.54) |
| 23–24.9 kg/m2 | 247,445 (62.34) | 120,245 (63.01) | 127,200 (61.72) |
| 25.0–29.9 kg/m2 | 125,433 (31.60) | 60,931 (31.93) | 64,502 (31.30) |
| ≥30.0 kg/m2 | 18,671 (4.70) | 7,458 (3.91) | 11,213 (5.44) |
| FPG, mmol/L, average | 7.26 ± 1.71 | 7.34 ± 1.81 | 7.18 ± 1.61 |
| FPG, N (%) | |||
| <6.0 mmol/L | 43,523 (11.00) | 20,006 (10.54) | 23,517 (11.43) |
| 6.0–7.9 mmol/L | 270,279 (68.32) | 127,355 (67.07) | 142,924 (69.48) |
| 8.0–9.9 mmol/L | 58,057 (14.68) | 29,344 (15.45) | 28,713 (13.96) |
| ≥10.0 mmol/L | 23,723 (6.00) | 13,166 (6.93) | 10,557 (5.13) |
| SBP, mmHg | 130.36 ± 10.18 | 130.13 ± 10.24 | 130.62 ± 10.09 |
| DBP, mmHg | 79.44 ± 6.51 | 79.78 ± 6.59 | 79.13 ± 6.43 |
| Smoking, N (%) | 38,846 (13.62) | 37,851 (29.61) | 995 (0.63) |
| Drinking, N (%) | 62,211 (21.11) | 49,286 (36.09) | 12,925 (8.17) |
| Physical exercise, N (%) | |||
| Every day | 38,206 (12.96) | 18,127 (13.27) | 20,079 (12.69) |
| ≥1 time per week | 27,337 (9.27) | 12,864 (9.42) | 14,473 (9.15) |
| Occasionally | 135,967 (46.13) | 63,781 (46.70) | 72,186 (45.64) |
| Never | 93,229 (31.63) | 41,799 (30.61) | 51,430 (32.52) |
Incidence Rates of Overall Cancer and Top 10 Site-Specific Cancers
A total of 18,853 cancer cases occurred in the T2DM cohort during the 8 years of follow-up. Among adults aged 20 years or older in the Shanghai general population from 2011 to 2018, there were 582,643 cancer cases. Table 2 showed the crude incidence rate, ASR, and SIR of overall cancer in the total, male, and female general populations and among T2DM patients.
Table 2.
Cancer incidence in Shanghai general population and the newly diagnosed T2DM cohort across different age groups
| General population | T2DM patients | SIR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PYs | Number of cancer cases | Annual incidence per 100,000 PYs | ASR (95% CI) | Population at risk | Number of cancer cases | Annual incidence per 100,000 PYs | ASR (95% CI) | ||
| Total | 100,649,346 | 582,643 | 579 | 394 (393, 395) | 428,568 | 18,853 | 973 | 501 (491, 511) | 1.10 (1.09, 1.12) |
| 20–54 years | 55,180,008 | 121,605 | 220 | 193 (191, 194) | 83,823 | 2,215 | 540 | 324 (307, 342) | 1.48 (1.41, 1.54) |
| 55–59 years | 12,285,775 | 74,057 | 603 | 603 (599, 607) | 7,3124 | 2,728 | 783 | 784 (754, 813) | 1.30 (1.25, 1.35) |
| 60–64 years | 11,328,656 | 87,306 | 771 | 772 (767, 777) | 86,698 | 3,471 | 910 | 915 (884, 945) | 1.19 (1.15, 1.23) |
| 65–69 years | 7,531,302 | 74,448 | 989 | 988 (981, 995) | 68,606 | 3,266 | 1,134 | 1,146 (1,108, 1,185) | 1.16 (1.12, 1.20) |
| 70–74 | 4,594,238 | 59,903 | 1,304 | 1,305 (1,295, 1,316) | 44,218 | 2,601 | 1,360 | 1,374 (1,322, 1,427) | 1.06 (1.02, 1.10) |
| ≥75 years | 9,729,367 | 165,324 | 1,699 | 1,690 (1,682, 1,698) | 72,099 | 4,572 | 1,434 | 1,449 (1,408, 1,491) | 0.83 (0.84, 0.89) |
| Males | 49,780,959 | 305,030 | 613 | 390 (388, 392) | 205,802 | 9,996 | 1,088 | 488 (474, 503) | 1.10 (1.07, 1.12) |
| 20–54 years | 27,796,554 | 45,917 | 165 | 140 (139, 142) | 45,170 | 1,004 | 463 | 264 (242, 285) | 1.63 (1.53, 1.73) |
| 55–59 years | 6,137,418 | 37,339 | 608 | 608 (602, 615) | 34,466 | 1,292 | 799 | 799 (756, 843) | 1.31 (1.24, 1.39) |
| 60–64 years | 5,640,934 | 47,878 | 849 | 849 (841, 856) | 41,622 | 1,811 | 1,002 | 1,002 (956, 1,048) | 1.18 (1.13, 1.24) |
| 65–69 years | 3,812,396 | 44,268 | 1,161 | 1,161 (1,150, 1,172) | 32,791 | 1,892 | 1,384 | 1,384 (1,322, 1,446) | 1.19 (1.14, 1.25) |
| 70–74 years | 2,275,814 | 36,922 | 1,622 | 1,622 (1,606, 1,639) | 21,083 | 1,549 | 1,731 | 1,731 (1,646, 1,817) | 1.07 (1.01, 1.12) |
| ≥75 years | 4,117,843 | 92,706 | 2,251 | 2,210 (2,196, 2,224) | 30,670 | 2,448 | 1,841 | 1,841 (1,769, 1,913) | 0.83 (0.80, 0.86) |
| Females | 50,868,387 | 277,613 | 546 | 399 (397, 400) | 222,766 | 8,857 | 868 | 513 (499, 527) | 1.11 (1.09, 1.13) |
| 20–54 years | 27,383,454 | 75,688 | 276 | 247 (245, 248) | 38,653 | 1,211 | 627 | 385 (358, 413) | 1.37 (1.29, 1.45) |
| 55–59 years | 6,148,357 | 36,718 | 597 | 597 (591, 603) | 38,658 | 1,436 | 768 | 768 (729, 808) | 1.29 (1.22, 1.36) |
| 60–64 years | 5,687,722 | 39,428 | 693 | 693 (686, 700) | 45,076 | 1,660 | 826 | 826 (787, 866) | 1.19 (1.14, 1.25) |
| 65–69 years | 3,718,906 | 30,180 | 812 | 812 (802, 821) | 35,815 | 1,374 | 908 | 908 (860, 955) | 1.12 (1.06, 1.18) |
| 70–74 years | 2,318,424 | 22,981 | 991 | 991 (978, 1,004) | 23,135 | 1,052 | 1,033 | 1,033 (971, 1,096) | 1.04 (0.98, 1.11) |
| ≥75 years | 5,611,524 | 72,618 | 1,294 | 1,269 (1,260, 1,279) | 41,429 | 2,124 | 1,143 | 1,142 (1,093, 1,190) | 0.90 (0.86, 0.94) |
The crude incidence rate and ASR of overall cancer were 973 of 100,000 PYs and 501 of 100,000 PYs, respectively, in all T2DM patients and 579 of 100,000 PYs and 394 of 100,000 PYs, respectively, in the general population. Compared with the general population, the relative risks (SIRs) of overall cancer were 1.10 (1.09, 1.12) in all T2DM patients, 1.10 (1.07, 1.12) in males, and 1.11 (1.09, 1.13) in females.
Findings for the most frequent 10 site-specific cancers among total, male, and female T2DM patients are presented in Supplementary Table 2. Respiratory, colorectum, stomach, breast, thyroid, liver, pancreas, bladder, central nervous system (CNS), and kidney cancers were the most frequent 10 among all T2DM patients, while respiratory, colorectum, prostate, stomach, liver, pancreas, bladder, kidney, thyroid, and lymphoma cancers were the most frequent cancers among males, and breast, respiratory, colorectum, thyroid, pancreas, stomach, liver, CNS, gallbladder, and uterine cancers were the most frequent cancers among females.
Notably, T2DM patients exhibited a higher risk of pancreas, bladder, thyroid, kidney, breast, colorectum, and liver cancers compared with the general population, with respective SIR values of 1.45, 1.31, 1.21, 1.18, 1.17, 1.17, and 1.12. Among male T2DM patients, the SIR values for pancreas, prostate, thyroid, bladder, lymphoma, colorectum, kidney, and liver cancers were 1.42, 1.30, 1.28, 1.27, 1.26, 1.23, 1.16, and 1.09, respectively. The risk of pancreas, uterine, thyroid, liver, breast, and colorectum cancers were higher among female T2DM patients, with SIRs of 1.49, 1.41, 1.19, 1.17, 1.17, and 1.09, respectively. There was no observed increased incidence of respiratory and stomach cancers among male T2DM patients and respiratory, stomach, CNS, and gallbladder cancers in female T2DM patients relative to the general population.
Association Between Age at T2DM Diagnosis and the Relative Risks of All-Cause Cancer
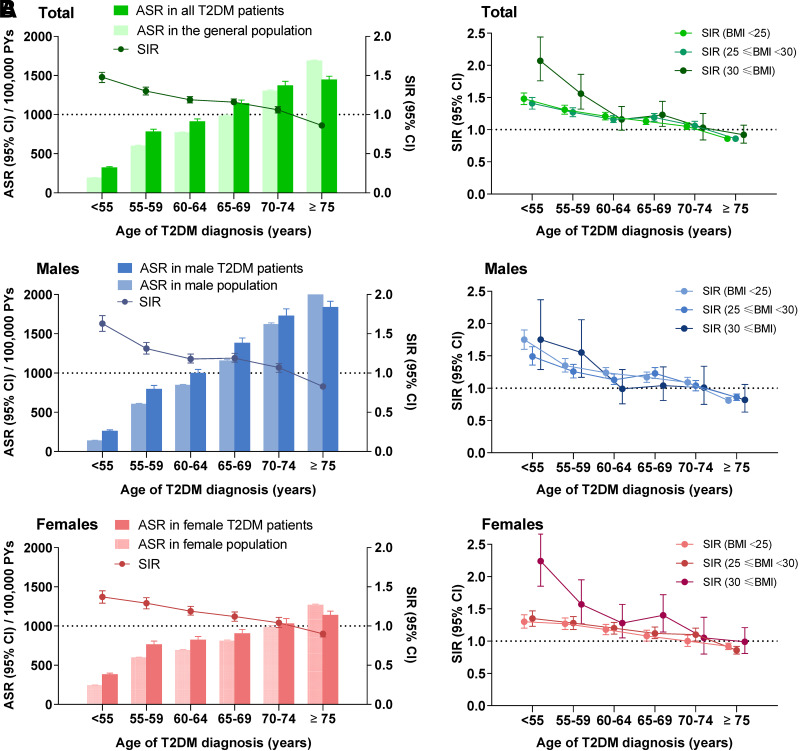
The incidence of cancers across different age of diagnosis groups is shown in Table 2 and Fig. 1A. The incidence of overall cancer increased from 540 of 100,000 PYs in patients with age at T2DM diagnosis of 20–54 years to 783 in those with age at diagnosis of 55–59 years, 910 with age at diagnosis of 60–64 years, 1,134 with age at diagnosis of 65–69 years, 1,360 with age at diagnosis of 70–74 years, and 1,434 with age at diagnosis of over 75 years.
Figure 1.
All-cause cancer incidences and relative risks across different age at T2DM diagnosis groups and the relative risks of all-cause cancer among patients with BMI <25, 25 ≤ BMI <30, and BMI ≥30. A: ASR. B: SIR.
Meanwhile, we have conducted the Cox regression analysis to investigate the factors influencing the risk of all-cause cancer among diabetes patients, in Supplementary Table 3, with BMI, FPG, SBP, and DPP as continuous variables. Male, age, FPG, smoking, and physical exercise were the impact factors of all caused cancer in patients with type 2 diabetes. The HRs of overall cancer were 0.34 (0.32, 0.35) in those with age at diagnosis of T2DM 20–54 years, 0.50 (0.47, 0.53) in those with age at diagnosis 55–59 years, 0.63 (0.60, 0.66) in those with age at diagnosis 60–64 years, 0.81 (0.77, 0.85) in those with age at diagnosis 65–69 years, and 0.93 (0.88, 0.98) in those with age at diagnosis 70–74 years compared with the patients with age at diagnosis of T2DM ≥75 years old. There were similar patterns of overall cancer in males and females when analyzed separately.
The relative risk of cancer was present as SIR, and a significantly higher SIR of 1.48 (1.41, 1.54) was observed in those with age at diagnosis of T2DM 20–54 years, while SIRs were 1.30 (1.25, 1.35) in patients with age at diagnosis 55–59 years, 1.19 (1.15, 1.23) in those with age at diagnosis 60–64 years, 1.16 (1.12, 1.20) in those with age at diagnosis 65–69 years, 1.06 (1.02, 1.10) in those with age at diagnosis 70–74 years, and 0.86 (0.84, 0.89) in those with T2DM diagnosis at age ≥75 years old. There were similar patterns of overall cancer in males and females when analyzed separately (Table 2 and Fig. 1A).
Association Between Age at T2DM Diagnosis and the Relative Risks of Site-Specific Cancers
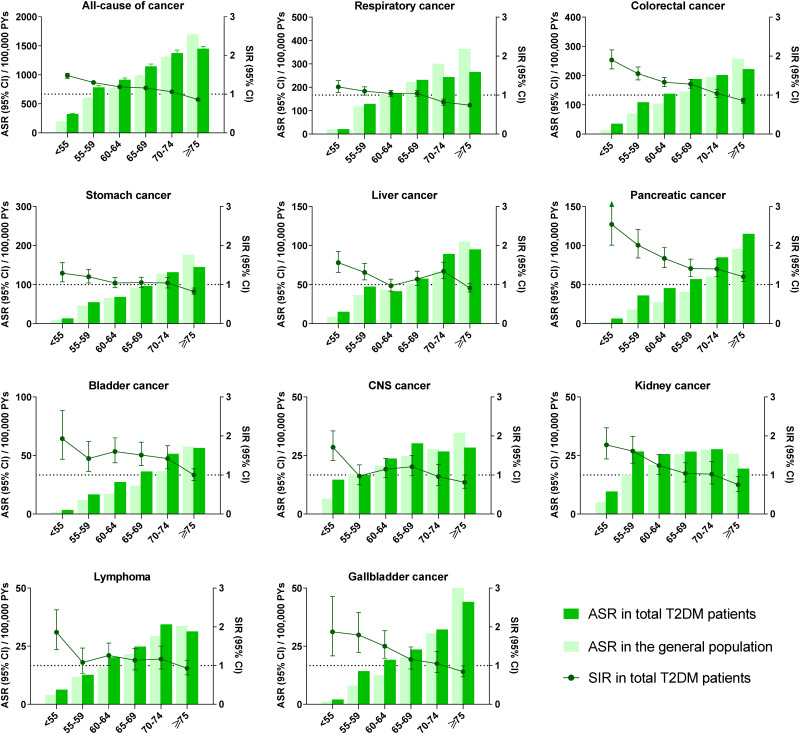
As with overall cancer rates, most site-specific cancers reported in the top 10 incident cancers had an increased ASR and decreased SIR with increasing age of diagnosis, including respiratory, colorectal, stomach, liver, pancreatic, bladder, CNS, kidney, and gallbladder cancer and lymphoma. The sex stratification of cancer incidence among most site-specific cancers had a trend consistent with that seen for overall cancer incidence. We found increased incidences and decreased relative risks with increasing T2DM diagnosis age among both male and female patients in this cohort (Fig. 2, Supplementary Table 4, and Supplementary Fig. 1).
Figure 2.
Site-specific cancer incidence and relative risks across different age at T2DM diagnosis groups.
As far as hormone-related cancers, prostate cancer in males had the same trend seen with overall cancer. The incidence of breast cancer was higher among female patients with age of diabetes diagnosis 55–74 years, while no significant trend was found in male patients, because of limited cases. The SIR of uterine cancer in females increased in T2DM diagnosis age 20–54 years, while the incidence of uterine cancer was higher among female patients with age of diabetes diagnosis 55–74 years. However, The ASR and SIR of thyroid cancer decreased with increasing age of T2DM diagnosis in both male and female patients (Supplementary Table 4 and Supplementary Fig. 2).
Compared with the patients with age at diagnosis of T2DM ≥75 years old, the patients with lower age at diagnosis of T2DM have lower HRs of most site-specific cancers, including respiratory, colorectal, stomach, liver, pancreatic, bladder, and gallbladder cancer and lymphoma. The HR of thyroid cancer increased with decreasing age of T2DM diagnosis in both male and female patients (Supplementary Table 3).
Effect of BMI on the Risks of Overall and Site-Specific Cancers
The associations of age at diabetes diagnosis and relative cancer risk among patients with different BMI groups were similar to the overall results. Compared with the general population, young and middle age at diagnosis of T2DM were associated with higher incidence of overall cancer, and relative risks were decreased with increasing age at diagnosis among T2DM patients with BMI <25, 25 ≤ BMI <30, and BMI ≥30 (Fig. 1B).
Compared with patients with BMI <25, the HRs of overall cancer of patients with 25 ≤ BMI <30 and BMI ≥30 were 0.99 (0.96, 1.03) and 1.06 (0.98, 1.14), respectively, in all patients, 0.98 (0.93, 1.02) and 0.91 (0.80, 1.02), respectively, in male patients, and 1.19 (1.08, 1.30) and 1.02 (0.97, 1.07), respectively, in female patients. The incidence of thyroid cancer increased in all T2DM patients with 25 ≤ BMI <30 and BMI ≥30. The incidence of breast and uterine cancer increased in female patients with 25 ≤ BMI <30 and BMI ≥30, while, in male patients with 25 ≤ BMI <30, the incidence of prostate cancer increased (Supplementary Fig. 3). Sensitivity analysis using data from about 60% patients with smoking history, alcohol consumption, and physical activity showed similar patterns of overall cancer and most site-specific cancers, except there was no significant trend in thyroid cancer among male T2DM patients. (Supplementary Fig. 4).
Sensitivity Analysis of the First Occurrence of Cancer and All Cancers After Diagnosis of T2DM
In the study, some participants had multiple cancer cases after the diagnosis of T2DM. However, all cancers included in the calculation occurred independently, instead of being metastatic cancers. A total of 18,149 patients in this study developed 18,853 cancers after the diagnosis of diabetes. Among them, 17,532 patients had one cancer, 533 patients suffered two cancers, 81 patients suffered three cancers, and three patients each had four cancers.
We further conducted a sensitivity analysis using the results of the first occurrence of cancer and all cancers definitions in Supplementary Table 5; the association between age at diagnosis of diabetes and incidence of all-cause and site-specific cancer subtypes is similar in both methods of cancer calculation.
Conclusions
To the best of our knowledge, this study is the first to examine the association between age at T2DM diagnosis and the incidence of multiple types of cancer. We found that overall cancer incidence was higher in T2DM patients than in the general Chinese population. Compared with the general population, young and middle age at diagnosis of T2DM was significantly associated with a higher incidence of overall cancer, while patients developing T2DM at an age over 75 years did not show increased risk. The BMI stratification among the overall cancer population showed a consistent trend. Taken together, these findings highlight the importance of treatment target recommendations on stratified management for T2DM patients according to their age at diagnosis.
The association between T2DM and cancer incidence has been widely discussed (19–21). Participants with T2DM in the China Kadoorie Biobank study had increased risks of total and certain site-specific cancers; HRs were 1.13 for total cancer, 1.51 for liver cancer, 1.86 for pancreatic cancer, and 1.21 for female breast cancer (22). Our study found that T2DM was associated with a 10% increased risk of all-cause cancers, which was consistent with other observational studies (22,23). Specifically, T2DM patients had a higher incidence of pancreas, bladder, thyroid, kidney, breast, colorectum, and liver cancers compared with the general population. Previous studies have investigated the association between age at diabetes diagnosis and a series of subsequent complications, including CVD, retinopathy, dementia, nephropathy, and mortality (12–14,24–26). However, few studies explicitly considered the association between cancer incidence and age at diabetes diagnosis. One prospective study with 125 all-cause cancer cases illustrated a mildly elevated risk of cancer in women with diabetes onset earlier in childhood (15). Studies of specific types of cancer suggested that early-onset diabetes confers a higher relative incidence of pancreatic cancer and colon cancer than late-onset diabetes (27,28). A national study found slightly lower cancer mortality in patients with T2DM diagnosed before 40 years of age, but a significantly higher CVD mortality (29). However, given their heterogeneity of study design and populations, whether T2DM diagnosis age affected all-cancer risk remained unsubstantiated. We found that the incidence of overall and most site-specific cancers increased with age at diagnosis of T2DM patients. Even more interesting is that our results suggest that the relative risk of overall cancer was highest in the patients whose T2DM was diagnosed at a younger age, and these risks decreased with increasing age at diagnosis, so that, for patients with T2DM diagnosed at over 75 years of age, overall cancer incidence risk was even lower than that in the general population.
Although observational results suggest that individuals with young-onset T2DM seem to have a higher risk of complications, the potential reasons for this excess risk are unclear. There are several possible explanations for younger age at diagnosis of diabetes being an important predictor for subsequent adverse outcomes. Younger age of diagnosis of T2DM is associated with worse metabolic status (1). Accumulating data suggest that young-onset T2DM has a more rapid deterioration of β-cell function than that in later-onset T2DM (9,11,30,31). We found, in our study, that those with age of diagnosis less than 55 years had higher FBG compared with T2DM patients with age of diagnosis older than 75 years. It is well known that high blood glucose is a risk factor for future CVD and cancers (19,32,33). Furthermore, earlier studies show that young people with T2DM had higher fat content in liver and muscle, in association with higher BMI and Homeostatic Model Assessment for Insulin Resistance, than that in older adults with T2DM (11). After following 228,073 eligible participants in the Nurses’ Health Studies, Zhang et al. (34) found that incident early-onset type 2 diabetes was associated with increased risk of early-onset total cancer and diabetes- and obesity-related cancer in females, especially in those with higher BMI at 18 years of age. We also found that those with age of T2DM diagnosis less than 55 years had higher BMI in our cohort. Moreover, BMI ≥ 25 had more effect on certain cancers, such as thyroid cancer in all patients, breast and uterine cancer in female patients, and prostate cancer in male patients. Thus, given the oncogenicity of obesity and insulin resistance, more severe hyperinsulinemia in the young appears to promote cancer development, especially in the patients with earlier-onset T2DM (1). Therefore, these findings from the observational data set highlight the importance of combating obesity and insulin resistance in young people, in order to postpone T2DM onset and prevent subsequent certain cancers.
The sex stratification among most site-specific cancers had a trend consistent with the overall cancer, including respiratory, colorectum, stomach, liver, pancreatic, bladder, CNS, kidney, and gallbladder cancer and lymphoma. As for hormone-related cancers, prostate cancer is the most frequently diagnosed cancer and the second leading cause of death among American men (35). In our study, prostate cancer was the third most frequently diagnosed cancer and had the same trend as overall cancer. Breast cancer is the most common cancer, and uterine cancer is the tenth most common cancer among Chinese female T2DM patients in this cohort. Although there were very different trends among breast and uterine cancer in females, the incidence of breast and uterine cancer was higher among patients with diagnosis of T2DM at 55–74 years old. BMI remains the most crucial factor for these hormone-related cancers, and T2DM patients with BMI ≥ 25 had more incidences of thyroid, prostate, breast, and uterine cancers.
Our study has several strengths. Firstly, leveraging a large, newly diagnosed T2DM cohort of 428,568 patients with 8 years of follow-up and the comparison Shanghai general population aged 20 years or older with more than 100 million PYs designated as “normal” or average, we were able to detect even small differences in cancer incidence and to evaluate the relative risks in an extensive range of subtypes. Secondly, in order to ensure the integrity of the reported cancer information, the SCDC regularly organized 16 district centers including 241 community health centers to carry out missing report investigations at relevant medical institutions every year. The prevalence of missing reports was found to be 0.5%. Thirdly, we conducted a sensitivity analysis and found the associations are similar in either including only the first occurrence of cancer (sensitivity analysis) or all cancers (primary analysis) after the diagnosis of T2DM.
Our findings should be interpreted in the light of the limitations. Information on smoking history, alcohol consumption, and physical activity was available for approximately 60% of patients in the current system. We conducted a sensitivity analysis and found the BMI stratification effect on the risk of all-cause cancer was similar among T2DM patients for whom smoking history, alcohol consumption, and physical activity information was available and among all T2DM patients. Because of the exclusion from the registry of people with prevalent diabetes, the results may only be applicable to those surviving longer than average for the T2DM population, and thus potentially of less relevance to the general population with diabetes. It is not possible to incorporate the competing risk of death in this study, as we lack such information for the general population. However, SIR is a standard methodology and allows for comparison between other studies. This is observational evidence, and it would take further evaluation to translate findings to clinical practice. Despite the 8 years of follow-up, patients with young-onset T2DM had not yet reached an age when cancers are more prevalent. Continuing follow-up will allow further examination of the association between age at T2DM onset and cancer incidence.
In conclusion, we found a negative association of age at T2DM diagnosis with subsequent relative risk of overall cancer and most site-specific cancers. Our findings suggest that the carcinogenicity of T2DM differs markedly by age at diagnosis and highlight the necessity of stratifying patients according to diagnosis age in management, screening, and preventative strategies. More aggressive cancer risk screening in patients developing diabetes mellitus at young and middle age and delaying the age of T2DM appearance by combating obesity or insulin resistance would be effective methods for reducing the subsequent cancer incidence. As for many elderly patients with newly diagnosed T2DM, reassessment of screening and treatment goals might be helpful.
This article contains supplementary material online at https://doi.org/10.2337/figshare.24773148.
Article Information
Acknowledgments. The authors want to acknowledge all participants and investigators of Shanghai Cohort Study. We give our supreme appreciation to Dr. Linjie Yu and Mingqing Yuan at Zhejiang University for their kindly assistance and suggestion.
Funding. This work was supported by the Foundation of National Facility for Translational Medicine (Shanghai) (TMSK-2021-506), National Natural Science Foundation of China (82088102, 82370902, and 81270935), Shanghai Municipal Health Commission (no. GWVI-11.1-22), and Three-Year Action Plan of Shanghai Public Health (GWV-7).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.L., J.T., T.H., and G.N. conceptualized and designed the study. Y.L., K.G., and Q.Y. were responsible for statistical analysis. Y.L., J.T., T.H., K.H., Z.T.B., J.C., and G.N. engaged in the interpretation of data. Y.L., J.T., and T.H. wrote the draft of the manuscript. K.H., Z.T.B., J.C., and G.N. reviewed and edited the manuscript. All authors contributed to discussion and approved the final version of the manuscript. C.F. and G.N. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Statement
This work was supported by the Foundation of National Facility for Translational Medicine (Shanghai) (TMSK-2021-506), National Natural Science Foundation of China (82088102, 82370902, and 81270935), Shanghai Municipal Health Commission (no. GWVI-11.1-22), and Three-Year Action Plan of Shanghai Public Health (GWV-7).
Footnotes
Y.L., J.T., T.H., and K.G. contributed equally to this work.
References
- 1. Magliano DJ, Sacre JW, Harding JL, Gregg EW, Zimmet PZ, Shaw JE. Young-onset type 2 diabetes mellitus - implications for morbidity and mortality. Nat Rev Endocrinol 2020;16:321–331 [DOI] [PubMed] [Google Scholar]
- 2. Dev R, Bruera E, Dalal S. Insulin resistance and body composition in cancer patients. Ann Oncol 2018;29:ii18–ii26 [DOI] [PubMed] [Google Scholar]
- 3. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 2022;183:109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lawrence JM, Divers J, Isom S, et al.; SEARCH for Diabetes in Youth Study Group . Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001-2017. JAMA 2021;326:717–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bjornstad P, Drews KL, Caprio S, et al.; TODAY Study Group . Long-term complications in youth-onset type 2 diabetes. N Engl J Med 2021;385:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ke C, Lau E, Shah BR, et al. Excess burden of mental illness and hospitalization in young-onset type 2 diabetes: a population-based cohort study. Ann Intern Med 2019;170:145–154 [DOI] [PubMed] [Google Scholar]
- 7. Yuan C, Kim J, Wang QL, et al.; PanScan/PanC4 I-III Consortium . The age-dependent association of risk factors with pancreatic cancer. Ann Oncol 2022;33:693–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and management of youth-onset type 2 diabetes: a position statement by the American Diabetes Association. Diabetes Care 2018;41:2648–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Viner R, White B, Christie D. Type 2 diabetes in adolescents: a severe phenotype posing major clinical challenges and public health burden. Lancet 2017;389:2252–2260 [DOI] [PubMed] [Google Scholar]
- 10. Nadeau KJ, Anderson BJ, Berg EG, et al. Youth-onset type 2 diabetes consensus report: current status, challenges, and priorities. Diabetes Care 2016;39:1635–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol 2018;6:69–80 [DOI] [PubMed] [Google Scholar]
- 12. Barbiellini Amidei C, Fayosse A, Dumurgier J, et al. Association between age at diabetes onset and subsequent risk of dementia. JAMA 2021;325:1640–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao M, Song L, Sun L, et al. Associations of type 2 diabetes onset age with cardiovascular disease and mortality: The Kailuan Study. Diabetes Care 2021;44:1426–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morton JI, Liew D, McDonald SP, Shaw JE, Magliano DJ. The association between age of onset of type 2 diabetes and the long-term risk of end-stage kidney disease: a National Registry study. Diabetes Care 2020;43:1788–1795 [DOI] [PubMed] [Google Scholar]
- 15. Fredriksson M, Persson E, Dahlquist G, Möllsten A, Lind T. Risk of cancer in young and middle-aged adults with childhood-onset type 1 diabetes in Sweden–a prospective cohort study. Diabet Med 2022;39:e14771. [DOI] [PubMed] [Google Scholar]
- 16. Li X, Lu J, Hu S, et al. The primary health-care system in China. Lancet 2017;390:2584–2594 [DOI] [PubMed] [Google Scholar]
- 17. Shen B, Li Y, Sheng CS, et al. Association between age at diabetes onset or diabetes duration and subsequent risk of pancreatic cancer: results from a longitudinal cohort and mendelian randomization study. Lancet Reg Health West Pac 2022;30:100596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sung H, Hyun N, Leach CR, Yabroff KR, Jemal A. Association of first primary cancer with risk of subsequent primary cancer among survivors of adult-onset cancers in the United States. JAMA 2020;324:2521–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yuan S, Kar S, Carter P, et al. Is type 2 diabetes causally associated with cancer risk? Evidence from a two-sample Mendelian randomization study. Diabetes 2020;69:1588–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ballotari P, Vicentini M, Manicardi V, et al. Diabetes and risk of cancer incidence: results from a population-based cohort study in northern Italy. BMC Cancer 2017;17:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol 2009;10:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pan XF, He M, Yu C, et al.; China Kadoorie Biobank Collaborative Group . Type 2 diabetes and risk of incident cancer in China: a prospective study among 0.5 million Chinese adults. Am J Epidemiol 2018;187:1380–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mayor S. High glucose and diabetes increase cancer risk. Lancet Oncol 2005;6:71. [DOI] [PubMed] [Google Scholar]
- 24. Al-Saeed AH, Constantino MI, Molyneaux L, et al. An inverse relationship between age of type 2 diabetes onset and complication risk and mortality: the impact of youth-onset type 2 diabetes. Diabetes Care 2016;39:823–829 [DOI] [PubMed] [Google Scholar]
- 25. Sattar N, Rawshani A, Franzén S, et al. Age at diagnosis of type 2 diabetes mellitus and associations withcCardiovascular and mortality risks. Circulation 2019;139:2228–2237 [DOI] [PubMed] [Google Scholar]
- 26. Wong J, Molyneaux L, Constantino M, Twigg SM, Yue DK. Timing is everything: age of onset influences long-term retinopathy risk in type 2 diabetes, independent of traditional risk factors. Diabetes Care 2008;31:1985–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gupta S, Vittinghoff E, Bertenthal D, et al. New-onset diabetes and pancreatic cancer. Clin Gastroenterol Hepatol 2006;4:1366–1372; quiz 1301 [DOI] [PubMed] [Google Scholar]
- 28. Ali Khan U, Fallah M, Sundquist K, Sundquist J, Brenner H, Kharazmi E. Risk of colorectal cancer in patients with diabetes mellitus: A Swedish nationwide cohort study. PLoS Med 2020;17:ce1003431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huo L, Magliano DJ, Rancière F, et al. Impact of age at diagnosis and duration of type 2 diabetes on mortality in Australia 1997-2011. Diabetologia 2018;61:1055–1063 [DOI] [PubMed] [Google Scholar]
- 30. Wang T, Lu J, Shi L, et al.; China Car diometabolic Disease and Cancer Cohort Study Group . Association of insulin resistance and β-cell dysfunction with incident diabetes among adults in China: a nationwide, population-based, prospective cohort study. Lancet Diabetes Endocrinol 2020;8:115–124 [DOI] [PubMed] [Google Scholar]
- 31. Fan B, Wu H, Shi M, et al. Associations of the HOMA2-%B and HOMA2-IR with progression to diabetes and glycaemic deterioration in young and middle-aged Chinese. Diabetes Metab Res Rev 2022;38:e3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tian J, Sheng CS, Sun W, et al. Effects of high blood pressure on cardiovascular disease events among Chinese adults with different glucose metabolism. Diabetes Care 2018;41:1895–1900 [DOI] [PubMed] [Google Scholar]
- 33. Qiu M, Shen W, Song X, et al. Effects of prediabetes mellitus alone or plus hypertension on subsequent occurrence of cardiovascular disease and diabetes mellitus: longitudinal study. Hypertension 2015;65:525–530 [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Song M, Cao Y, et al. Incident early- and later-onset type 2 diabetes and risk of early- and later-onset cancer: prospective cohort study. Diabetes Care 2023;46:120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sandhu S, Moore CM, Chiong E, Beltran H, Bristow RG, Williams SG. Prostate cancer. Lancet 2021;398:1075–1090 [DOI] [PubMed] [Google Scholar]