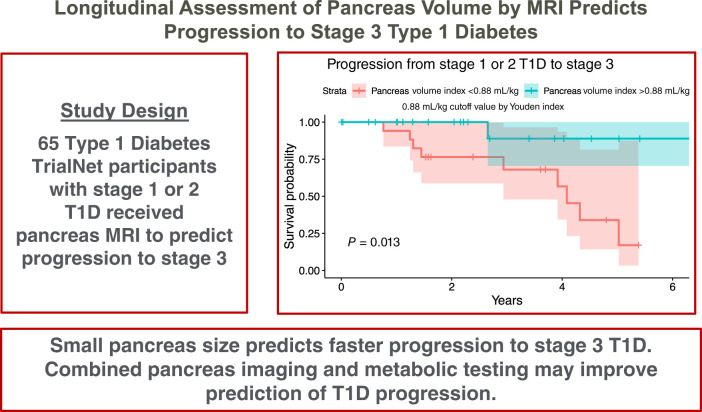
Abstract
OBJECTIVE
This multicenter prospective cohort study compared pancreas volume as assessed by MRI, metabolic scores derived from oral glucose tolerance testing (OGTT), and a combination of pancreas volume and metabolic scores for predicting progression to stage 3 type 1 diabetes (T1D) in individuals with multiple diabetes-related autoantibodies.
RESEARCH DESIGN AND METHODS
Pancreas MRI was performed in 65 multiple autoantibody-positive participants enrolled in the Type 1 Diabetes TrialNet Pathway to Prevention study. Prediction of progression to stage 3 T1D was assessed using pancreas volume index (PVI), OGTT-derived Index60 score and Diabetes Prevention Trial–Type 1 Risk Score (DPTRS), and a combination of PVI and DPTRS.
RESULTS
PVI, Index60, and DPTRS were all significantly different at study entry in 11 individuals who subsequently experienced progression to stage 3 T1D compared with 54 participants who did not experience progression (P < 0.005). PVI did not correlate with metabolic testing across individual study participants. PVI declined longitudinally in the 11 individuals diagnosed with stage 3 T1D, whereas Index60 and DPTRS increased. The area under the receiver operating characteristic curve for predicting progression to stage 3 from measurements at study entry was 0.76 for PVI, 0.79 for Index60, 0.79 for DPTRS, and 0.91 for PVI plus DPTRS.
CONCLUSIONS
These findings suggest that measures of pancreas volume and metabolism reflect distinct components of risk for developing stage 3 type 1 diabetes and that a combination of these measures may provide superior prediction than either alone.
Graphical Abstract
Introduction
In type 1 diabetes (T1D), a long prodromal period of β-cell loss occurs before diagnosis (1,2). The recognition that T1D pathogenesis follows a temporal progression resulted in staging guidelines, marking the progression from stage 1 (autoimmunity defined as two or more β-cell–related antibodies with euglycemia) to stage 2 (autoimmunity with dysglycemia) and finally stage 3 T1D (clinical disease according to current diagnostic criteria) (3). Better prediction of the timing of disease progression is needed to guide patient monitoring as well as to recruit and stratify patients in prevention trials (4). Recent success in immune therapy for T1D prevention (5) has further highlighted the need for accurate prediction of diabetes progression in order to identify therapeutic windows when interventions may be most successful.
Risk for developing T1D is known to be related to immunologic, genetic, and metabolic factors. Islet-cell autoantibodies have proven useful for identifying individuals at risk of T1D (6), but the expression of these biomarkers can be transient (7), and predicting time to progression remains difficult. A number of genetic markers have also been associated with T1D risk (8). Metabolic dysregulation is an early marker of T1D progression, usually showing an increase in risk ∼18 months before stage 3 develops (9). A number of indices based on oral glucose tolerance testing (OGTT) for classification of T1D risk and prediction of progression have been proposed. Among these, Index60 (10) and the Diabetes Prevention Trial–Type 1 Risk Score (DPTRS) (11) have shown utility for predicting progression to stage 3. Prediction accuracy may be increased by incorporating different aspects of disease pathogenesis. For example, models combining both metabolic and immunologic measures may better predict the timing of T1D progression (12) than either assay alone.
Current methods for evaluating progression to stage 3 are primarily serologic, but there is growing evidence that monitoring the size of the pancreas may also inform our prediction of disease progression. MRI studies have detected smaller pancreas size at the onset of T1D (13,14) as well as in autoantibody-positive individuals at risk of stage 3 T1D (15,16). However, prior studies did not correlate pancreas size with progression of T1D to assess the predictive capacity of pancreas imaging. In this study, we assessed pancreas volume longitudinally in autoantibody-positive relatives of patients with T1D and compared the ability of MRI of the pancreas to predict progression to stage 3 T1D with the OGTT-based predictors Index60 and DPTRS.
Research Design and Methods
Participants
Study participants (N = 65) were enrolled at one of three Type 1 Diabetes TrialNet International Clinical Centers as part of the Multicenter Assessment of the Pancreas in Type 1 Diabetes (MAP-T1D) consortium (www.map-t1d.com): Vanderbilt University Medical Center, Barbara Davis Center, or University of Chicago. Participant demographics are listed in Table 1. Participants were relatives of patients with T1D identified as having multiple diabetes-associated autoantibodies through the Type 1 Diabetes TrialNet Pathway to Prevention study (17). TrialNet screens relatives of probands with T1D, including first-degree relatives age 2.5–45 years as well as second- or third-degree relatives age 2.5–20 years for antibodies to insulin, GAD, and islet antigen 2. Islet cell and Zn transporter autoantibodies were measured if a participant tested positive for at least one other antibody. Participants were monitored with autoantibody testing, HbA1c measurement, and OGTT at 6- or 12-month intervals depending on diabetes risk. T1D staging was performed according to TrialNet guidelines: stage 1 was defined by the presence of two or more islet autoantibodies with euglycemia, stage 2 as the presence of β-cell autoimmunity with dysglycemia, and stage 3 as onset of symptomatic disease or meeting of specified criteria on OGTT (3). Individuals with stage 1 or 2 T1D age ≥8 years at each study center who had no contraindications for MRI and were not pregnant were offered enrollment in this study. All protocols were approved by the institutional review board of the enrolling institution.
Table 1.
Study participant demographics and measurements at study entry
| Stage 1 or 2 during study (n = 54) | Progression to stage 3 (n = 11) | P | |
|---|---|---|---|
| Sex | 0.79 | ||
| Male | 25 (46.3) | 4 (36.4) | |
| Female | 29 (53.7) | 7 (63.6) | |
| Self-reported race | 0.67 | ||
| Asian | 1 (1.9) | 0 (0) | |
| Black or African American | 3 (5.6) | 1 (9.1) | |
| White | 50 (92.6) | 10 (90.9) | |
| Self-reported ethnicity | 1 | ||
| Hispanic or Latino | 1 (1.9) | 0 (0) | |
| Not Hispanic or Latino | 53 (98.1) | 11 (100) | |
| Mean (SD) age, years | 21.2 (11.9) | 15.0 (8.42) | 0.054 |
| Mean (SD) weight, kg | 66.0 (23.8) | 52.6 (22.8) | 0.097 |
| Mean (SD) BMI, kg/m2 | 23.5 (6.3) | 21.5 (6.2) | 0.35 |
| Mean (SD) body surface area, m2 | 1.72 (0.34) | 1.47 (0.38) | 0.07 |
| Type 1 diabetes stage | 0.86 | ||
| 1 | 31 (57.4) | 7 (63.6) | |
| 2 | 20 (37.0) | 4 (36.4) | |
| Unknown (no recent OGTT) | 3 (5.6) | 0 (0) | |
| Mean (SD) n of autoantibodies | 2.72 (1.5) | 4.27 (0.9) | <0.001 |
| Follow-up time, months | 7 (0–27) | 18 (15–43) | 0.14 |
| N of MRIs | 2 (1–3) | 3 (2–4.5) | 0.09 |
Data are given as n (%) or median (IQR) unless otherwise indicated.
MRI
Pancreas MRI was performed using a Philips 3T Ingenia scanner (Vanderbilt and Chicago) or Siemens 3T Skyra scanner (Barbara Davis Center) according to a standardized protocol validated for quantitative pancreas evaluation across imaging centers and hardware (18). Imaging was performed at ∼6- or 12-month intervals, customarily aligning with OGTT, with additional MRI performed proximal to progression events. The second MRI was performed a median of 7 months after the first MRI, and the third MRI was performed a median of 9 months after the second MRI. A total of 181 MRIs were performed. One participant received 10 MRIs, one received nine MRIs, one received eight MRIs, two received seven MRIs, three received six MRIs, five received five MRIs, six received four MRIs, seven received three MRIs, 13 received two MRIs, and 26 received a single MRI. Imaging was performed in the axial plane during breath hold. Acquisitions included a fat-suppressed T2-weighted fast-spin echo sequence with 1.5 × 1.5 × 4 mm spatial resolution as well as a T1-weighted ultrafast gradient echo sequence with spatial resolution of 1.5 × 1.5 × 4 mm. Both images were acquired during breath hold to minimize respiratory motion.
Image Analysis
The pancreas was outlined on each T2-weighted slice by an experienced radiologist (M.A.H.) blinded to the status of each participant. The T1-weighted image was consulted to help guide delineation of the pancreas border. Regions of interest delineating the pancreas on each slice were created using MIPAV (National Institutes of Health; https://mipav.cit.nih.gov). Areas within the region of interest were multiplied by slice thickness and summed to yield total pancreas volume. Pancreas volume index (PVI) was calculated by dividing the pancreas volume by the participant’s body weight (14) to account for interindividual differences in body habitus.
Metabolic Testing
Participants in the Type 1 Diabetes TrialNet Pathway to Prevention study underwent OGTT annually or biannually (17). OGTT consisted of blood sampling of glucose, insulin, and C-peptide levels −10, 0, 30, 60, 90, and 120 min after administration of an oral glucose challenge of 1.75 g/kg (maximum 75 g). Plasma glucose levels were measured by standard glucose oxidase testing, and C-peptide levels were measured by a two-sided immunoenzymometric assay performed on a Tosoh 600 II analyzer at a TrialNet central laboratory, as previously described (19). Glucose and C-peptide measurements were used to calculate Index60 (20) and DPTRS (21). Most (71%) of the corresponding OGTT and MRI measurements were acquired on the same day during a single study visit as part of TrialNet monitoring. When measurements from the same day were not available, the closest measurements in time were used. The average time between OGTT and MRI data acquisition was 17 days.
Statistical Analysis
Statistical analysis was performed using R software (version 4.1.1). Longitudinal data were analyzed using a linear mixed-effects model with each participant set as a random factor (i.e., participant-specific random intercept). Associations between independent variables were assessed using Pearson correlation. Pairwise comparisons of independent groups were assessed using a Student t test. Comparisons of more than two groups were performed using ANOVA. Cox proportional hazards models were used to assess the predictive capacity for progression to stage 3 T1D and quantified using the C-index (22). The area under the receiver operating characteristic (ROC) curve (AUC) was calculated using the pROC package in R, and significant differences were assessed using a one-sided DeLong test. Time-dependent ROC curves were generated using Cox regression and the R package survivalROC (23). Kaplan-Meier analyses and log-rank tests were performed using the Youden index, the value that maximizes the sensitivity and specificity of dichotomization for each predictor. Continuous data are presented as mean ± SD or median with interquartile range (IQR), as noted. P values were adjusted for multiple comparisons using the Holm method. An adjusted P value of 0.05 was considered significant for all statistical analyses.
Results
Demographics of the participants at study entry are listed in Table 1. Study participants exhibited a wide range of pancreas volumes at the initial MRI, ranging from 11 to 110 mL. When normalizing for body weight, PVI also varied at the initial MRI, ranging from a high of 1.69 mL/kg, similar to that in healthy individuals without T1D, to a low of 0.39 mL/kg, similar to that in individuals with T1D (16) or individuals with diabetes and insulin deficiency from a mutation in the insulin gene (24). All study participants were in stage 1 or 2 when entering the study. Over the course of the study, 11 of the 65 individuals experienced progression to stage 3 T1D (i.e., progressors). As expected, progressors tended to be younger (age 15 ± 8 years) than nonprogressors (age 21 ± 12 years; P = 0.054). Median time between study entry and diabetes diagnosis for individuals who experienced progression to stage 3 T1D was 18 months (IQR 15–43 weeks), whereas nonprogressors were observed for a median time of 7 months (IQR 0–27 months). Pancreas volume did not correlate with the number of autoantibodies present at study entry (Supplementary Fig. 1A), but it was significantly lower in individuals positive for islet cell antibody (Supplementary Fig. 1B–E). Progressors were positive for more autoantibodies at study entry (Table 1).
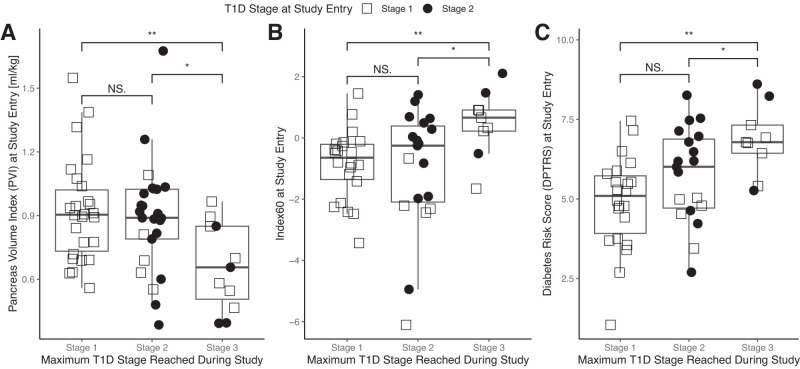
Individuals who experienced progression to stage 3 T1D had a smaller pancreas volume at study entry (32 ± 14 vs. 57 ± 21 mL for nonprogressors; P < 0.001). To account for any difference in pancreas volume as a function of age (Supplementary Fig. 2A), we normalized pancreas volume by body weight to yield the PVI, which was age insensitive (Supplementary Fig. 2B). Linear regression indicated that pancreas volume was dependent on age (P < 0.0001), but PVI was not (P = 0.98). PVI was smaller at study entry in individuals who experienced progression to stage 3 T1D compared with that in nonprogressors (P < 0.005) (Fig. 1A). OGTT-derived scores were also different between progressors and nonprogressors at study entry, with progressors having a higher Index60 (P < 0.001) (Fig. 1B) and DPTRS (P < 0.005) (Fig. 1C) than nonprogressors. Cox proportional hazards modeling using PVI as a predictor yielded a C-index of 0.88 (95% CI 0.78–0.98), whereas DPTRS had a C-index of 0.81 (95% CI 0.64–0.97), and Index60 had a C-index of 0.77 (95% CI 0.59–0.96).
Figure 1.
A: PVI at study entry is smaller in individuals who ultimately experienced progression to stage 3 T1D than in individuals who remained in stage 1 or 2. B and C: At study entry, Index60 (B) and DPTRS (C) were higher in individuals who ultimately experienced progression to stage 3 T1D than in individuals who remained in stage 1 or 2. T1D stage at study entry is indicated by shape. *P < 0.05, **P < 0.01. NS, not significant.
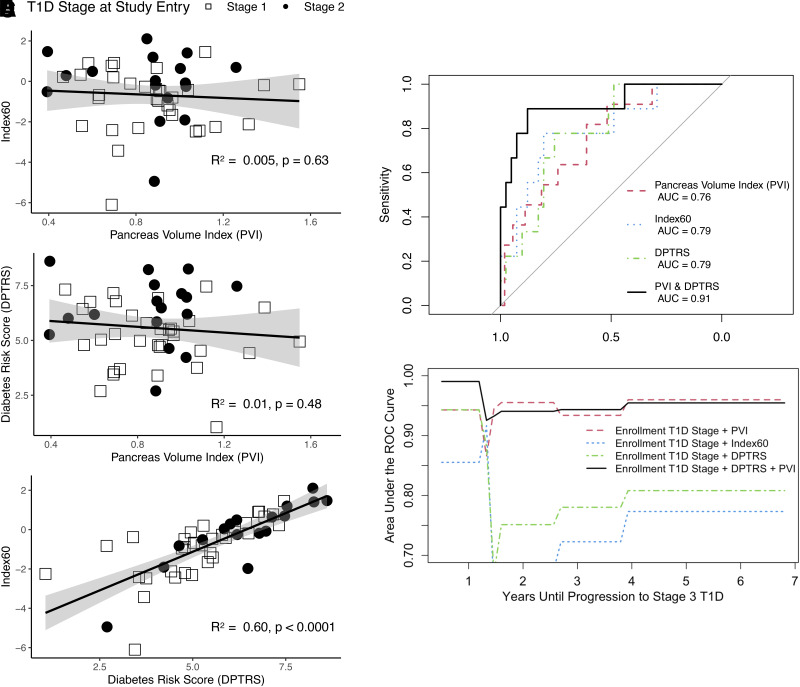
We next sought to assess the relationship between PVI and metabolic measures. We found that PVI had no significant correlation with either Index60 or DPTRS at the initial MRI (Fig. 2A and B). However, the two metabolic measures, Index60 and DPTRS, were significantly correlated, as expected (R2 = 0.60; P < 0.0001) (Fig. 2C). To determine whether imaging and metabolic measures could provide complementary information regarding T1D progression risk, we performed Cox modeling using PVI and DPTRS as predictors, which yielded a C-index of 0.94 (95% CI 0.87–1.00). A Cox model incorporating PVI and Index60 yielded a similar C-index of 0.93 (95% CI 0.85–1.00).
Figure 2.
A and B: Scatterplots of PVI (A) versus Index60 and PVI versus DPTRS (B) demonstrate no correlation between PVI and either of the metabolic measures. C: In contrast, Index60 and DPTRS, which are both derived from metabolic testing, are significantly correlated. Shaded area displays 95% CI for each linear fit. D: ROC curves for stratifying individuals who experienced progression to stage 3 T1D versus nonprogressors based on measurement at study entry. ROC AUC for logistic regression incorporating PVI and DPTRS exceeds any single measure alone (P < 0.05). E: Time-dependent ROC analysis demonstrates that PVI or combination of T1D stage, DPTRS, and PVI predicts individuals who will develop stage 3 T1D years before progression.
We assessed the predictive capacity of MRI and OGTT performed at study entry for classifying progressors versus nonprogressors. PVI had an ROC AUC of 0.76 (95% CI 0.62–0.91), Index60 had an ROC AUC of 0.79 (95% CI 0.62–0.97), and DPTRS had an ROC AUC of 0.79 (95% CI 0.64–0.93), none of which were significantly different from one another (Fig. 3A). Logistic regression using both PVI and DPTRS as predictors achieved an ROC AUC of 0.91 (95% CI 0.78–1.00), which was significantly higher than that seen with DPTRS or Index 60 (P < 0.05) but not with PVI (P = 0.07) (Fig. 2D). The Akaike information criterion of the model combining PVI and DPTRS was 32.9, which demonstrated an improved model compared to values for PVI (54.3), DPTRS (42.1), and Index60 (42.7). Time-dependent ROC analysis from Cox modeling (Fig. 2E) suggested that PVI and combined PVI plus DPTRS predicted stage 3 T1D progression earlier than either Index60 or DPTRS alone, although this study was not powered to assess significance. We also performed Kaplan-Meier analysis and log-rank tests to compare progression to stage 3 T1D using the Youden index of each predictor. The Youden indices for dichotomous discrimination were calculated to be 0.88 mL/kg for PVI, 0.22 mL/kg for Index60, and 6.44 mL/kg for DPTRS. These Youden indices for PVI, Index60, and DPTRS separated cohorts with different progression to stage 3 T1D (P < 0.05) (Supplementary Fig. 3).
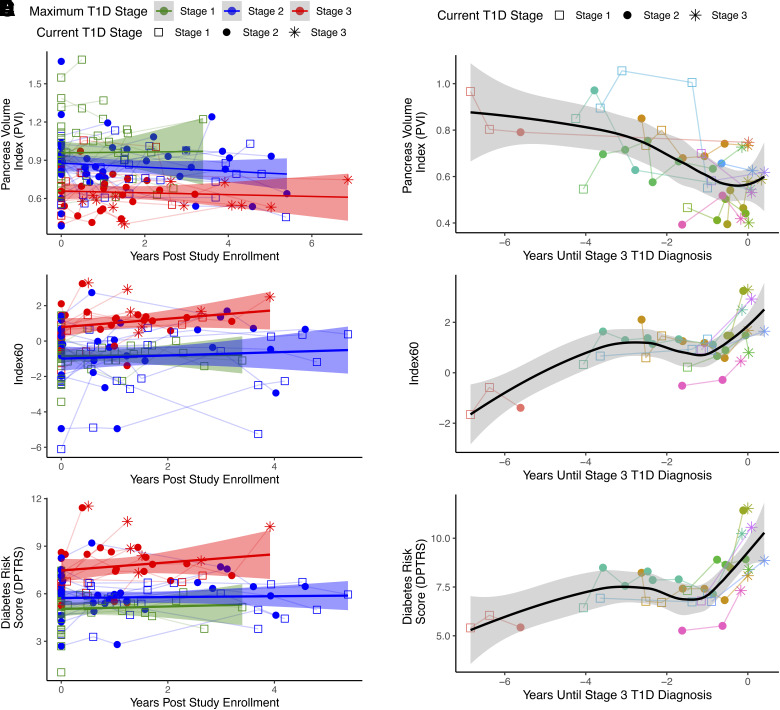
Figure 3.
A, C, and E: Longitudinal plots of PVI (A), Index60 (C), and DPTRS (E) for all study participants over the time course of study enrollment. Current T1D stage at MRI is indicated by marker shape. Highest T1D stage reached by study participant is indicated by color. Some individuals did not receive MRI or OGTT after experiencing progression to stage 3 T1D. Red indicates participants who experienced progression to stage 3 T1D, whereas green (stage 1) and blue (stage 2) indicate participants who did not experience progression to symptomatic disease. Weighted line displays cohort average, and shaded area displays 95% CI. B, D, and F: Longitudinal plots of PVI (B), Index60 (D), and DPTRS (F) versus time to diagnosis for the 11 study participants who experienced progression to stage 3 T1D. Individual participants are color coded, and T1D stage at MRI is indicated by marker shape. PVI declined over time, whereas Index60 and DPTRS increased proximally to diagnosis in individuals diagnosed with stage 3 T1D.
To assess changes in the pancreas as individuals experienced progression to stage 3 T1D, we performed MRI studies longitudinally up to 6.9 years after the initial MRI. We did not detect significant changes in PVI in individuals who did not experience progression to stage 3 (Fig. 3A). However, PVI declined by >10% in seven of the 11 individuals who experienced progression to stage 3 T1D (P < 0.005) (Fig. 3B). Similarly, Index60 (Fig. 3C) and DPTRS (Fig. 3E) were stable in individuals who did not experience progression to stage 3. However, each of these measures increased proximally to diagnosis with stage 3 T1D (P < 0.005) (Fig. 3D and F).
Conclusions
In this study, we investigated whether MRI measurement of pancreas volume in individuals identified as being at risk of T1D could predict progression to stage 3 disease and how these imaging measures correlated with metabolic testing. Our results suggest that small pancreas volume can predict progression to stage 3 T1D, with discrimination similar to that of the OGTT-derived measurements Index60 and DPTRS. Of note, pancreas volume and metabolic measures were not correlated, suggesting that they reflect different aspects of the disease process underlying T1D and provide different information regarding disease risk. We therefore created a prediction model with both pancreas volume and metabolic measures, and this model outperformed imaging or metabolic testing alone for predicting progression to stage 3 T1D.
This study used MRI performed longitudinally to assess changes in pancreas volume during progression of T1D. Pancreas volume increased in study participants who either did or did not experience progression to stage 3 T1D. This observation likely reflects the known increase in pancreas volume up to age 20 years (25). When pancreas volume was normalized by body weight to account for adolescent growth, we detected longitudinal declines in PVI in the cohort who experienced progression to stage 3 T1D but not in those who remained in stage 1 or 2. We previously demonstrated a decline in pancreas volume in both individuals with recent-onset T1D and those with longstanding T1D (26). In this study, three individuals who experienced progression to stage 3 displayed a >30% decline in PVI over a year before diagnosis, four individuals displayed a 14–24% decline in PVI, and four individuals displayed low but stable PVI before diagnosis with stage 3 T1D. Individuals may have entered the study at different points in their progression (e.g., the individuals who did not exhibit a decline in pancreas volume may have enrolled at a later stage of disease progression). This finding suggests interindividual variability in disease progression and its impact on pancreas volume, which may be related to different pathways or risk factors contributing to disease progression. The OGTT-derived measures Index60 and DPTRS increased in the cohort who experienced progression to stage 3 T1D within ∼6 months of diagnosis, consistent with prior reports (27). Time-dependent ROC analysis indicated that PVI may predict progression to stage 3 earlier than metabolic testing. Although limited by a relatively short time to progression in this study, this finding suggests a divergence in the temporal dynamics of pancreas volume and dysglycemia, with pancreas volume representing the earliest identifiable risk factor. Importantly, OGTT seems to be the best-established predictor at present, surpassing continuous glucose monitoring (28). Additional studies are required to define the capacity of pancreas imaging to predict progression early in the disease process.
The recent approval of the first immunomodulatory agent to delay T1D onset highlights the importance of accurate prediction of disease progression. Clinically, improved accuracy of prediction may help to identify those patients most likely to benefit from preventive therapies. In clinical trials, identifying individuals at the highest risk of imminent disease progression could enable more rapid determination of the effect of an intervention. In addition, understanding the risk profile of study enrollees is important for interpreting the outcome of therapeutic studies. Monitoring of the pancreas volume in response to immune therapy and in relation to metabolic recovery is another important opportunity for future studies.
This study and its results have limitations, namely, a relatively small sample size and limited progression events. Given this small sample size, we were not able to validate our model using a separate training and validation cohort. A larger data set of pancreas MRI is needed to test the generalizability of combined pancreas volume and OGTT measures. Study participants were overwhelmingly non-Hispanic White. Future studies are needed to see if this study is reproducible in diverse populations. Individuals who experienced progression to stage 3 tended to be younger and weigh less than individuals who remained in stage 1 or 2. Pancreas volume measurements were normalized by body weight to account for known relationships between pancreas volume and age (25). Study participants who experienced progression to stage 3 had longer median follow-up than individuals who did not, although follow-up time was not significantly different between groups. We used measurements at study entry to mitigate bias from differences in follow-up time. Furthermore, study participants continue to be monitored to detect future diagnoses of stage 3 T1D. Given that all members of the cohort presented with two or more autoantibodies, it is anticipated that nearly all will eventually experience progression to stage 3 T1D; the focus of the current study was defining the degree to which MRI can refine prediction of the timing of the stage 3 diagnosis.
The current cost and convenience of MRI could limit its use as a screening tool in the general population. However, MRI does have advantages over glucose tolerance testing, namely, preclusion of intravenous sampling and fasting, which may improve participant retention. Moreover, 2-h OGTT requires a significant time commitment by both participants and staff, which also leads to high costs. On the other hand, a noncontrast pancreas MRI sufficient for quantifying pancreas volume can be acquired in 15 s. Thus, MRI screening can be performed at multiple sites and MRI machines if a standardized imaging protocol, such as the one we have developed, is used (18). Importantly, the movement toward shorter and less expensive MRI screening protocols in other organs such as the breast (29) demonstrates a potential pathway for reducing MRI cost and improving screening throughput through shorter scan times. Furthermore, the development of machine learning techniques that automatically segment the pancreas and calculate pancreas volume (30) provides a viable path for clinical implementation.
In summary, this study introduces the potential for pancreas imaging in individuals at risk of T1D to predict which individuals will experience the most rapid progression to symptomatic disease. Pancreas size may reflect different aspects of T1D progression than metabolic measures, because imaging does not correlate with two common OGTT-based indices, and the longitudinal dynamics of imaging and metabolism diverge. The orthogonal relationship between pancreas imaging and metabolic assays suggests a role for incorporating imaging into T1D staging. Ultimately, pancreas imaging may be useful for informing clinical trials aimed at T1D prevention.
This article contains supplementary material online at https://doi.org/10.2337/figshare.24713238.
Article Information
Funding. Research support was received from the Leona M. and Harry B. Helmsley Charitable Trust (2207-05374), JDRF International (3-SRA-2015-102-M-B and 3-SRA-2019-759-M-B), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; R03 DK129979), the JP Fletcher Foundation, and the Thomas J. Beatson Jr. Foundation (2021-003). Funding was provided by grant U24 DK097771 via the NIDDK Information Network New Investigator Pilot Program in Bioinformatics. This work used REDCap and VCTRS resources, which are supported by grant UL1 TR000445 from National Center for Advancing Translational Sciences, National Institutes of Health (NIH). Support was also received from the Vanderbilt Diabetes Research and Training Center, Division of Diabetes, Endocrinology, and Metabolic Diseases (DK 020593), and the Vanderbilt University Institute of Imaging Science Center for Human Imaging (1 S10OD021771 01). This study was performed under the auspices of the Multicenter Assessment of the Pancreas in Type 1 Diabetes international consortium (https://www.map-t1d.com). The Type 1 Diabetes TrialNet Study Group is a clinical trials network funded through a cooperative agreement by the NIH, through the NIDDK, the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and JDRF.
The study sponsors were not involved in the design of the study; the collection, analysis, or interpretation of data; or the writing of the report and did not impose any restrictions regarding the publication of the report.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.V., J.J.W., J.M.W., W.E.R., L.H.P., T.W.H.K., H.E.T., S.A.W.G., A.K.S., A.C.P., and D.J.M. were responsible for study conceptualization. J.V., J.J.W., and D.J.M. wrote the original manuscript draft. J.V., L.D., and H.K. curated data. J.V., A.C.P., and D.J.M. acquired funding. J.M.W., T.M.T., H.B., S.N., L.G.V., D.R., B.H., and M.W. performed investigations. M.A.H., L.D., and H.K. performed formal analyses. All authors edited, reviewed, and approved the final version of the manuscript. J.V. and D.J.M. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Statement
Research support was received from the Leona M. and Harry B. Helmsley Charitable Trust (2207-05374), JDRF International (3-SRA-2015-102-M-B and 3-SRA-2019-759-M-B), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; R03 DK129979), the JP Fletcher Foundation, and the Thomas J. Beatson Jr. Foundation (2021-003). Funding was provided by grant U24 DK097771 via the NIDDK Information Network New Investigator Pilot Program in Bioinformatics. This work used REDCap and VCTRS resources, which are supported by grant UL1 TR000445 from National Center for Advancing Translational Sciences, National Institutes of Health (NIH). Support was also received from the Vanderbilt Diabetes Research and Training Center, Division of Diabetes, Endocrinology, and Metabolic Diseases (DK 020593), and the Vanderbilt University Institute of Imaging Science Center for Human Imaging (1 S10OD021771 01). This study was performed under the auspices of the Multicenter Assessment of the Pancreas in Type 1 Diabetes international consortium (https://www.map-t1d.com). The Type 1 Diabetes TrialNet Study Group is a clinical trials network funded through a cooperative agreement by the NIH, through the NIDDK, the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and JDRF.
Footnotes
Clinical trial reg. no. NCT03585153, clinicaltrials.gov
References
- 1. Gorsuch AN, Spencer KM, Lister J, et al. Evidence for a long prediabetic period in type I (insulin-dependent) diabetes mellitus. Lancet 1981;2:1363–1365 [DOI] [PubMed] [Google Scholar]
- 2. Sosenko JM, Palmer JP, Greenbaum CJ, et al. Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial–Type 1. Diabetes Care 2006;29:643–649 [DOI] [PubMed] [Google Scholar]
- 3. Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015;38:1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Warshauer JT, Bluestone JA, Anderson MS. New frontiers in the treatment of type 1 diabetes. Cell Metab 2020;31:46–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sims EK, Bundy BN, Stier K, et al.; Type 1 Diabetes TrialNet Study Group . Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci Transl Med 2021;13:eabc8980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonifacio E, Bingley PJ, Shattock M, et al. Quantification of islet-cell antibodies and prediction of insulin-dependent diabetes. Lancet 1990;335:147–149 [DOI] [PubMed] [Google Scholar]
- 7. Barker JM, Barriga KJ, Yu L, et al.; Diabetes Autoimmunity Study in the Young . Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab 2004;89:3896–3902 [DOI] [PubMed] [Google Scholar]
- 8. Graham J, Hagopian WA, Kockum I, et al.; Diabetes Incidence in Sweden Study Group; Swedish Childhood Diabetes Study Group . Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes 2002;51:1346–1355 [DOI] [PubMed] [Google Scholar]
- 9. Sosenko JM, Palmer JP, Rafkin-Mervis L, et al.; Diabetes Prevention Trial-Type 1 Study Group . Incident dysglycemia and progression to type 1 diabetes among participants in the Diabetes Prevention Trial–Type 1. Diabetes Care 2009;32:1603–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nathan BM, Redondo MJ, Ismail H, et al. Index60 identifies individuals at appreciable risk for stage 3 among an autoantibody-positive population with normal 2-hour glucose levels: implications for current staging criteria of type 1 diabetes. Diabetes Care 2022;45:311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sosenko JM, Skyler JS, Mahon J, et al.; Type 1 Diabetes TrialNet and Diabetes Prevention Trial-Type 1 Study Groups . Use of the Diabetes Prevention Trial–Type 1 Risk Score (DPTRS) for improving the accuracy of the risk classification of type 1 diabetes. Diabetes Care 2014;37:979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morales JF, Muse R, Podichetty JT, et al. Disease progression joint model predicts time to type 1 diabetes onset: Optimizing future type 1 diabetes prevention studies. CPT Pharmacometrics Syst Pharmacol 2023;12:1016–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gaglia JL, Guimaraes AR, Harisinghani M, et al. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest 2011;121:442–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williams AJ, Thrower SL, Sequeiros IM, et al. Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J Clin Endocrinol Metab 2012;97:E2109–E2113 [DOI] [PubMed] [Google Scholar]
- 15. Campbell-Thompson ML, Filipp SL, Grajo JR, et al. Relative pancreas volume is reduced in first-degree relatives of patients with type 1 diabetes. Diabetes Care 2019;42:281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Virostko J, Williams J, Hilmes M, et al. Pancreas volume declines during the first year after diagnosis of type 1 diabetes and exhibits altered diffusion at disease onset. Diabetes Care 2019;42:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mahon JL, Sosenko JM, Rafkin-Mervis L, et al.; TrialNet Natural History Committee; Type 1 Diabetes TrialNet Study Group . The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97–104 [DOI] [PubMed] [Google Scholar]
- 18. Virostko J, Craddock RC, Williams JM, et al. Development of a standardized MRI protocol for pancreas assessment in humans. PLoS One 2021;16:e0256029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Voss MG, Cuthbertson DD, Cleves MM, et al.; DPT-1 and TrialNet Study Groups . Time to peak glucose and peak C-peptide during the progression to type 1 diabetes in the Diabetes Prevention Trial and TrialNet cohorts. Diabetes Care 2021;44:2329–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sosenko JM, Skyler JS, DiMeglio LA, et al.; Type 1 Diabetes TrialNet Study Group; Diabetes Prevention Trial-Type 1 Study Group . A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care 2015;38:271–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sosenko JM, Skyler JS; Diabetes Type 1 TrialNet and Diabetes Prevention Trial-Type 1 Study Groups . The development, validation, and utility of the Diabetes Prevention Trial–Type 1 Risk Score (DPTRS). Curr Diab Rep 2015;15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA 1982;247:2543–2546 [PubMed] [Google Scholar]
- 23. Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000;56:337–344 [DOI] [PubMed] [Google Scholar]
- 24. Wright JJ, Williams JM, Letourneau-Freiberg LR, et al. Insulin deficiency from insulin gene mutation leads to smaller pancreas. Diabetes Care 2023;46:773–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saisho Y, Butler AE, Meier JJ, et al. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat 2007;20:933–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wright JJ, Dulaney A, Williams JM, et al. Longitudinal MRI shows progressive decline in pancreas size and altered pancreas shape in type 1 diabetes. J Clin Endocrinol Metab 2023;108:2699–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bogun MM, Bundy BN, Goland RS, Greenbaum CJ. C-peptide levels in subjects followed longitudinally before and after type 1 diabetes diagnosis in TrialNet. Diabetes Care 2020;43:1836–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ylescupidez A, Speake C, Pietropaolo SL, et al. OGTT metrics surpass continuous glucose monitoring data for T1D prediction in multiple-autoantibody-positive individuals [published correction appears in J Clin Endocrinol Metab 2023;109:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang J, Greuter MJW, Vermeulen KM, et al. Cost-effectiveness of abbreviated-protocol MRI screening for women with mammographically dense breasts in a national breast cancer screening program. Breast 2022;61:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roger R, Hilmes MA, Williams JM, et al. Deep learning-based pancreas volume assessment in individuals with type 1 diabetes. BMC Med Imaging 2022;22:5. [DOI] [PMC free article] [PubMed] [Google Scholar]