Abstract
Tendon-bone interface injuries pose a significant challenge in tissue regeneration, necessitating innovative approaches. Hydrogels with integrated supportive features and controlled release of therapeutic agents have emerged as promising candidates for the treatment of such injuries. In this study, we aimed to develop a temperature-sensitive composite hydrogel capable of providing sustained release of magnesium ions (Mg2+). We synthesized magnesium-Procyanidin coordinated metal polyphenol nanoparticles (Mg-PC) through a self-assembly process and integrated them into a two-component hydrogel. The hydrogel was composed of dopamine-modified hyaluronic acid (Dop-HA) and F127. To ensure controlled release and mitigate the “burst release” effect of Mg2+, we covalently crosslinked the Mg-PC nanoparticles through coordination bonds with the catechol moiety within the hydrogel. This crosslinking strategy extended the release window of Mg2+ concentrations for up to 56 days. The resulting hydrogel (Mg-PC@Dop-HA/F127) exhibited favorable properties, including injectability, thermosensitivity and shape adaptability, making it suitable for injection and adaptation to irregularly shaped supraspinatus implantation sites. Furthermore, the hydrogel sustained the release of Mg2+ and Procyanidins, which attracted mesenchymal stem and progenitor cells, alleviated inflammation, and promoted macrophage polarization towards the M2 phenotype. Additionally, it enhanced collagen synthesis and mineralization, facilitating the repair of the tendon-bone interface. By incorporating multilevel metal phenolic networks (MPN) to control ion release, these hybridized hydrogels can be customized for various biomedical applications.
Keywords: Magnesium, Metal–phenolic networks, Self-assembly process, Controlled release, Immunomodulation, Tendon-bone interface
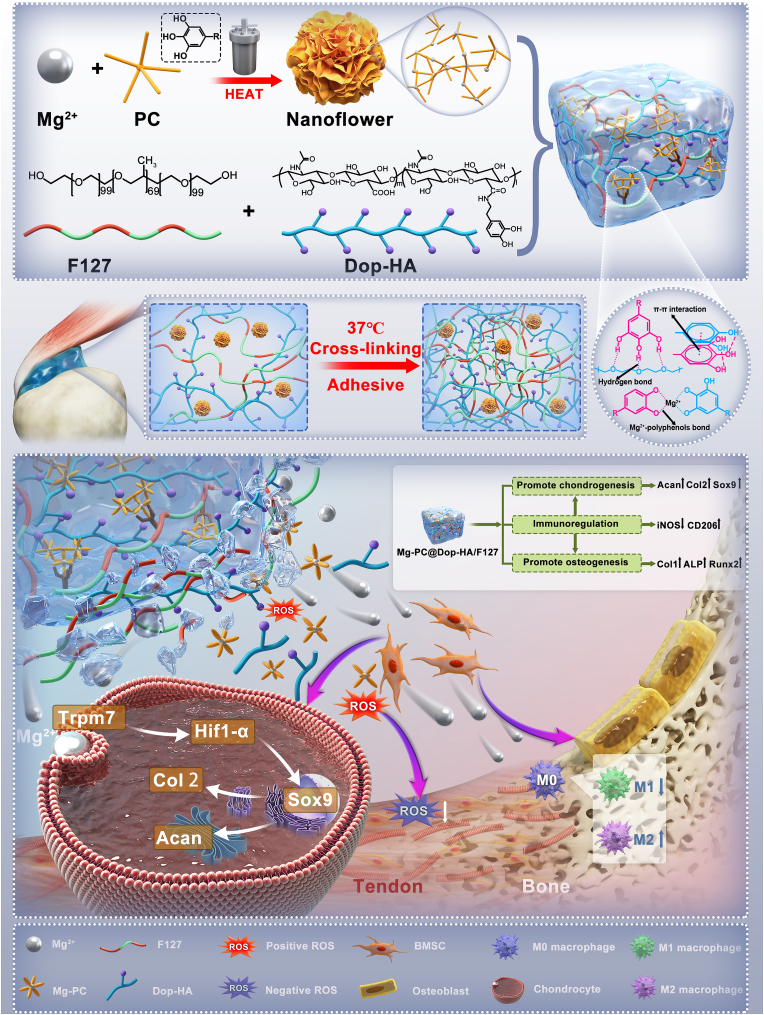
Graphical abstract
Through the incorporation of a polyphenol block-modified pathway, the composite hydrogel served as a multifunctional platform. It acted as a metal ion donor, releasing proanthocyanidin (PC) with potent antioxidant activity. This dual functionality effectively mitigated oxidative stress damage during the repair process. Moreover, incorporating polyphenol molecules facilitated the integration of organic and inorganic phases, enhancing the anchoring of nanosized structures within the hydrogel network and further modulating the release rate of ions. Consequently, the resulting composite hydrogel exhibited improved antioxidant properties, enhanced immunomodulatory activity, and superior tendon-bone repair capabilities.

Highlights
-
•
Self-assembling nature of the nanoflower particles, dispersed in a hydrogel system forming a composite hydrogel.
-
•
Excellent injectability, shape adaptability, controlled and sustained release of magnesium ions and proanthocyanidins.
-
•
Improved antioxidant property, enhanced immunomodulatory activity, and superior tendon-bone repair capability.
-
•
The ion-releasing design strategy inspires the development of innovative regenerative drugs and biomaterials.
1. Introduction
The tendon-bone interface, such as the connection between the rotator cuff and the humeral head, or the connection between the cruciate ligament and the tibia or femur, is an important connector between muscles and bones, promoting complex movements and functions in the body [1,2]. Injuries to tendon-bone interface are often observed in individuals engaged in high-energy physical activities [3,4]. They can be broadly categorized as avulsion injuries at the tendon insertion points on bones and tears at the tendon-bone interface [5]. The latter category, characterized by intricate tissue interactions, presents a particular challenge, additionally, due to the dynamic nature of the tendon-bone interface, which features a gradient modulus composition, long-term repair processes need to account for motion [6,7]. Treatment options for tendon-bone injuries encompass surgical interventions, closure therapy, shockwave therapy, non-steroidal medications, and platelet-rich plasma (PRP) injections [8,9]. While orthopedic anchoring techniques represent the conventional clinical approach for physically stabilizing tendon-bone interface injuries, achieving functional regeneration of the natural interface remains formidable. This is primarily due to factors such as the postoperative inflammatory environment, limitations in stem cell differentiation, and the slow pace of repair and remodeling within the tendon-bone interface [[10], [11], [12]].
Tissue engineering is a promising avenue for regenerating the tendon-bone interface, often leveraging hydrogel materials as scaffolds, drug carriers, and protective barriers [13,14]. However, the critical forces experienced during sliding between tendons and bone tissues must be addressed. Hydrogels, though versatile, typically exhibit weak mechanical properties and susceptibility to integrity compromise [15]. Therefore, it becomes imperative to imbue hydrogels with deformability and self-healing capabilities. Adhesive and self-healing hydrogels, acting as scaffolding systems, hold the potential to enhance tendon-bone interface healing by offering physical support and facilitating precise drug delivery [16,17]. These characteristics ensure sustained contact between the hydrogel and tendon-bone interface, even during movement, thereby minimizing the risk of displacement at the treatment site [18].
Hyaluronic acid (HA) is a natural polymer found in the human body and a constituent of the extracellular matrix (ECM) [19,20]. It boasts excellent biocompatibility and fosters cell migration, proliferation, and collagen deposition [21]. Nevertheless, its limited mechanical strength and low tissue adhesion restrict its utility. Modifying HA by adjusting its molecular weight or creating chemical derivatives can yield more adaptable hydrogels [22]. On the other hand, some products or devices prepared by Pluronic F127(F127) have been approved by the FDA. F127 are composed of hydrophilic polyethylene oxide (PEO) and hydrophobic polypropylene oxide (PPO) blocks arranged in a basic a-b-a triblock structure. They are widely used in tissue engineering, such as drug delivery, bioactive cargo transportation, and cell encapsulation [23,24]. In recent years, strategies inspired by mussel and plant polyphenols have found application in tissue engineering [25]. In the present study, we combined dopamine-modified hyaluronic acid (Dop-HA) with F127 to develop a composite hydrogel characterized by improved bio-interfacial adhesion and mechanical properties, tailored for the repair of irregularly shaped tendons.
Procyanidins (PC), potent free radical scavengers abundant in grape skins, are renowned for their anti-inflammatory, anti-aging, antioxidant, and antimicrobial effects [26]. However, their development has been hampered by low bioavailability and catechin release [27]. Metallophenolic complexes, formed through interactions between various metal ions and phenolic molecules, have emerged as a strategy for constructing drug delivery platforms [25]. In addition, magnesium ions have garnered attention in research and development of orthopedic biomaterials due to their pro-repair bioactivities on bone, cartilage, tendon, and periosteum [28,29]. Magnesium ions are metal ligands for Procyanidins, giving rise to flower-shaped nanoparticles featuring a high specific surface area [30]. The chelation of magnesium ions with PC retains the biological effects of PC while enabling the slow release of both magnesium ions and Procyanidins synergistically, thus promoting healing throughout the physiological cycle of tendon-bone interface repair.
In this study, we developed a composite hydrogel with metal-polyphenol nanoflower particles that precisely controlled the release of PC and magnesium ions and demonstrated the synergistic potential of this hydrogel in enhancing the healing of rotator cuff tears in vivo. Initially, metal polyphenol nanoparticles (Mg-PC) were synthesized through a straightforward one-pot method at room temperature, employing a one-step ion/molecule assembly process. Simultaneously, dopamine-modified hyaluronic acid (Dop-HA) with F127 was prepared to formulate a two-component hydrogel as a nanoparticle carrier. This hydrogel exhibited favorable injectability, temperature sensitivity, and plasticity, enabling easy injection and subsequent adaptation to the irregularly shaped implantation sites within the supraspinatus muscle. Notably, the metal polyphenol nanoflower particles (Mg-PC) not only acted as loading components but also chemically cross-linked with the two-component hydrogel, thereby enhancing the composite hydrogel's mechanical strength, adhesion, and the sustained release of therapeutic factors. We have thoroughly studied and evaluated hydrogels' stability, swelling, compression, and rheological properties. Furthermore, the intervention effect of the composite hydrogel on cells relevant to tendon-bone repair, including macrophages, osteoblasts, and chondrocytes, was assessed. The hydrogel demonstrated anti-inflammatory and antioxidant properties through the release of PC, creating a protective environment for stem cells during the healing of rotator cuffs. It also modulated the immune microenvironment and promoted the polarization of macrophages toward the M2 phenotype, thus expediting tendon-bone repair. Additionally, the composite hydrogel released magnesium ions, facilitating stem cell migration, adhesion, proliferation, and differentiation at the injury site. This dual-action approach held promise for promoting tendon-bone inter-healing. Moreover, it was found that magnesium ions facilitated Mg2+ entry into cells via TRPM7 channel proteins during cartilage differentiation, leading to the upregulation of hypoxia-inducible factor-1α (Hif-1α), which, in turn, promoted cartilage matrix production. Thus, this composite hydrogel is envisioned as a novel delivery system that seamlessly integrates material properties with biotherapeutics, making valuable contributions to the development of future drug delivery strategies.
2. Material and methods
2.1. Materials
Procyanidin (PC, Mw = 468.42 Da), magnesium chloride (MgCl2), Pluronic F-127 (F127, Mw = 304.293 Da), and 2, 2-diphenyl-1-picrylhydrazyl (DPPH, 96%) were purchased from Macklin Reagent. Dopamine, 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide (EDC), N-Hydroxy succinimide (NHS) and hyaluronic acid (HA, 40–100 K Da) were purchased from Sigma-Aldrich. All chemicals were used without further purification.
2.2. Synthesis of Mg-PC
Procyanidins (PC) were used to modify MgCl2 (Mg-PC) through a hydrothermal reaction. A hydrothermal reactor with a capacity of 100 mL was charged with 2.5 g of magnesium chloride and 40 mL of ethanol for the synthesis of Mg-PC. Additionally, 0.125 g of PC was added to the reactor. Gradually adjust the pH to alkaline (9–12) after mixing the solution well under acidic or neutral conditions. The reactor was sealed and heated at 180 °C for 24 h. After the hydrothermal reaction, the crude product was collected by centrifugation at 8000 rpm for 5 min. Further purification was achieved by washing the product with ethanol and water for at least three cycles. Finally, the purified Mg-PC was obtained through freeze-drying.
2.3. Preparation of Dop-HA/F127
A cold method was employed to prepare the hydrogel. The F127 concentration of 30% was selected based on its established gelatinizing properties in the literature [31]. To elaborate, a mixture of 3 g F127 and 7 mL deionized water was stirred at 4 °C for 12 h, resulting in a colorless and transparent solution. 0.2 g of hyaluronic acid (HA) with a molecular weight of 40–100 kDa (Macklin) was dissolved in 20 mL of deionized water for the amidation reaction. Subsequently, 0.02 g of dopamine (DA, Macklin), 0.04 g of EDC (Macklin), and 0.04 g of NHS (Macklin) were added to the solution. This process led to the formation of dopamine-modified hyaluronic acid hydrogel (DOP-HA) [32]. As a control group, 0.2 g of HA was dissolved in 20 mL of deionized water. The ratio of F127 to DOP-HA was 1:1 to form the double network hydrogel system. To create composite hydrogel systems (Mg-PC@Dop-HA/F127), nanoparticles of Mg-PC were incorporated at different ratios (weight/hydrogel volume of 0%, 5%, 10%, 20%) into the double network hydrogels. These different ratios of the composite hydrogel systems were then utilized in the subsequent experiments.
2.4. Characterization of Mg-PC
The size of Mg-PC was determined using Dynamic Light Scanning (DLS) on a Nano-ZS 90 instrument manufactured by Malvern Instruments, UK. Scanning electron microscopy (SEM) images and energy dispersive (EDS) data of Mg-PC were obtained using a ZEISS Sigma 300 microscope from Germany. Fourier transform infrared spectra (FTIR) were recorded using a Thermo Scientific Nicolet-iS10 FTIR spectrometer. X-ray diffraction (XRD) measurements of Mg-PC were conducted using a Bruker D8 X-ray powder diffractometer with Cu Kα radiation, operating at 45 kV and 40 mA, with a detection range from 5 to 80°. Thermogravimetric analysis (TGA) was performed using a thermogravimetric analyzer (NETZSCH STA 449F3) from Nai, Germany, under an N2 atmosphere with a heating rate of 10 °C per minute.
The antioxidant activity of Mg-PC was evaluated using the DPPH radical scavenging method [33]. 3 mg sample was added to a 3.0 mL methanol solution of DPPH (100 μmol/L). The mixture was incubated in darkness for 3 min. Subsequently, a wavelength scan was performed using a UV–vis spectrophotometer (Shimadzu 2600i, Japan). The degradation rate of DPPH was determined using Equation (1), where AB represents the absorbance of the blank (DPPH + ethanol), and AS represents the absorbance of the sample (DPPH + methanol + sample).
| (1) |
2.5. Characterization of hydrogels
2.5.1 To obtain scanning electron microscopy (SEM) images and energy dispersive (EDS) data of Mg-PC@Dop-HA/F127, a ZEISS Sigma 300 microscope from Germany was utilized. Initially, the hydrogels were completely lyophilized by subjecting them to a lyophilizer. Subsequently, the lyophilized hydrogels were subjected to morphology scanning using a scanning electron microscope. Fourier transform infrared spectra (FTIR) were measured using a Thermo Scientific Nicolet-iS10 FTIR spectrometer. 1H NMR analysis was measured using a fourier 80 spectrometer (AVANCE NEO 400, Bruker).
2.5.2. To determine the minimum sol/gel transfer temperature for the test formulation, we performed a tilt test in a water bath. A glass tube containing 1 mL of the mixed solution was placed in the water bath. The water bath temperature was gradually increased from 15 to 40 °C, with each temperature being maintained for at least 2 min. At every one °C interval, the tube was tilted to observe the flowability of the solution. Gelation formation was indicated if the solution at the top of the testing tube remained immobile for more than 30 s. The temperature at which gelation occurred was recorded as the gelation temperature. This tilting test in the water bath was repeated at least three times for each sample, and the results were averaged to obtain the minimum sol/gel transfer temperature.
2.5.3 The rapid shape adaptability of Mg-PC@Dop-HA/F127, with Mg-PC@Dop- HA/F127-10% as a representative, was investigated. The Mg-PC@Dop-HA/F127-10% hydrogel was first loaded into a syringe at room temperature. Then, the hydrogel was injected into an irregular foam mold, maintained at a temperature of 37 °C. A syringe was used for the injection process. Additionally, the hydrogel was also injected into water at a temperature of 37 °C to observe its morphology. Data was collected throughout the experiment by capturing photographs and recording videos. The adhesive strengths of hydrogels to different substrates including muscle, tendon and bone. At the same time, we also verified the self-healing ability of the hydrogel.
2.5.4 Catechol content was confirmed by measuring the absorbance at 280 nm with a UV-visible spectrophotometer and quantitatively determined with a dopamine standard. And the swelling ratio (SR) of the hydrogels was determined by the swelling test. The hydrogels were put into 20 mL PBS in sealed vials at 37 °C. At the pre-set time interval, the hydrogels were taken out from the solution and weighted. SR was calculated using the following equation:
| (2) |
Where the W0 and Wt were initial weight of hydrogels and the weight after swelling at a pre-set time, respectively. The test was repeated three times.
2.5.5 The mechanical properties of the hydrogels were measured using an Instron 34TM-10 testing machine equipped with a 5 N load cell, following the guidelines outlined in the American Society for Testing and Materials (ASTM) standard D412A. To prepare the samples, composite hydrogels with different formulations were cast in a Teflon mold and dried for three days. Rectangular samples measuring 15 mm in length, 3 mm in width, and 1 mm in thickness were obtained. These samples were then subjected to tensile testing at a 100 mm/min strain rate until failure.
2.5.6 The rheological properties of the hydrogels were evaluated using a rheometer equipped with a vertebral plate measurement system (MARS60, Thermo Scientific). The following parameters were used for the measurements: Strain amplitude: 1%; Frequency: 10 rad/s; Frequency range: 1–100%; Scanning temperature: 37 °C.During the test, both the storage modulus (G′) and the loss modulus (G″) were recorded separately. The setting parameters for the rheological behavior of the hydrogel at different temperatures are as follows. The test temperature is 10 °C–40 °C, the frequency is 1 Hz, and the strain is 1%. The changes in storage modulus (G′) and loss modulus (G″) of the hydrogel at different temperatures are tested. An alternate step strain sweep test was performed at a fixed angular frequency of 10 rad/s and a temperature of 37 °C. The amplitude of oscillatory strains was changed from a slight strain (1.0%) to subsequent significant strains (100%) with a duration of 60 s for each strain interval. This allowed for the characterization of the rheological behavior of the hydrogels under different strain conditions.
2.5.7 To study the release behavior of Mg2+ and PC from the composite hydrogel, we loaded 0.1 g of dried sample into a dialysis bag with a molecular weight cut-off (MWCO) of 500 Da.The dialysis bag was then placed in a capped glass vial containing 50 mL of PBS solution with a pH of 7.4. The glass vial was incubated at 37 °C in a shaking incubator. At each predetermined time interval, 1.0 mL of the solution was extracted from the glass vial, and an equal volume of fresh PBS was added back to maintain a constant volume. The released Mg2+ was measured using an inductively coupled plasma mass spectrometer (ICP-MS, icpms-2030, Shimadzu, Japan). The concentration of PC in the release medium was determined using an ultraviolet/visible spectrophotometer (Shimadzu 2600i, Japan) at a wavelength of 280 nm. Three parallel measurements were conducted for each sample, and the results were averaged to obtain more accurate data. This experimental setup allowed for monitoring the release profiles of both Mg2+ and PC from the composite hydrogels over time.
2.5.8 To study the degradation of the hydrogels, we placed a lyophilized hydrogel sample weighing 0.1 g into a plastic tube containing 10 ml of phosphate-buffered saline (PBS) at pH 7.4. The tubes were then incubated at a temperature of 37 °C. The PBS solution was changed once a week to ensure a fresh environment for degradation. After the designated incubation period, the samples were thoroughly washed at least three times with water to remove residual salts. Subsequently, the samples were freeze-dried to remove any remaining moisture. The mass loss of the hydrogels was calculated using equation (3), where W0 represents the initial weight of the hydrogel, and W1 represents the weight of the hydrogel after degradation. At least three samples were tested for each hydrogel formulation, and the average results were recorded. This experimental procedure allows us to determine the mass loss of the hydrogel during degradation, thus providing insight into the degradation behavior of the hydrogel over time.
| (3) |
2.5.9 A ROS assay was performed to evaluate the intracellular reactive oxygen species (ROS) scavenging ability of Mg-PC@Dop-HA/F127.BMSCs were seeded in the wells of a 24-well plate and incubated at 37 °C for 12 h to allow cell attachment and growth. The material suspension of Mg-PC@Dop-HA/F127 was added to the cell culture medium at a 100 μg/mL concentration. The cells were then incubated with the material suspension for 2–4 h. After incubation, the Rosup reagent (from the reactive oxygen species assay kit, Beyotime, S0033S, diluted at 1:1000) was added to the cell culture medium containing the cells and material suspension. The mixture was allowed to react for 30 min. The cell culture medium was discarded, and the cells were gently washed twice with α-MEM to remove any residual reagents. The cells were then incubated with the DCFH-DA assay reagent (diluted at a ratio of 1:1000) for 20–30 min, enabling the visualization of intracellular ROS using an inverted fluorescence microscope (Leica DMI4000 B; Leica et al.) for the 24-well plates. For 96-well plates, a quantitative assay using a fluorescent enzyme marker was conducted. It is essential to avoid exposure to light during this step. Untreated cells served as the negative control, while ROS UP-treated cells (without the sample) were used as the positive control for comparison. By performing this ROS assay, the intracellular ROS scavenging ability of Mg-PC@Dop-HA/F127 could be evaluated and observed under a fluorescence microscope, providing insights into its potential antioxidative properties.
2.6. Isolation and culture of BMSCs, identification of stem cell characteristics
Male Sprague-Dawley rats (4 weeks old) were sacrificed by cervical dislocation. rBMSCs were isolated from the bilateral femurs and tibias of the rats. Under sterile conditions, the ends of the bones were cut off, and the bone marrow was flushed out using 1 mL of Alpha-modified Eagle's medium (αMEM). The bone marrow was then centrifuged to remove the supernatant, and the resulting cell pellet was re-suspended in Alpha-modified Eagle's medium (αMEM) supplemented with 10% FBS and 1 × penicillin-streptomycin. After 48 h, non-adherent cells were removed. When the cells reached 100% confluence, they were sub-cultured, and the culture passage was increased to Passage 1 (P1). To confirm the characteristics of the rat BMSCs (bone marrow-derived mesenchymal stem cells), mesenchymal stem cell-related surface markers such as CD34, CD45, CD29, CD90, and CD44 were used. Flow cytometric analysis was performed according to the manufacturer's instructions. The cells were passed to Passage 3 (P3) for subsequent experiments. For osteogenic differentiation, P3 BMSCs were seeded at a density of 1 × 105 cells in a six-well plate and cultured until they reached 70–80% confluence. Then, 2 mL/well of osteogenic medium was added. For chondrogenic differentiation, 2 mL/well of the chondrogenic medium was added, and 2 mL/well of the adipogenic medium was added for adipogenic differentiation. The culture media were changed every three days, and the induction was performed at 37 °C with 5% CO2 for 14 days. Finally, the cells were fixed in 4% formalin and stained with Alizarin Red S, Alcian Blue, and Oil Red O to visualize their osteogenesis, chondrogenesis, and adipogenesis abilities, respectively.
This experimental procedure allowed for the characterization of rat BMSCs and the evaluation of their differentiation potential into osteogenic, chondrogenic, and adipogenic lineages.
2.7. Biocompatibility of hydrogels in vitro
To test the in vitro compatibility of the hydrogels, we performed a cell counting kit-8 (CCK-8) assay and live/dead cell staining. Rat BMSCs at passages 3–7 were cultured in complete Alpha-modified Eagle's medium (αMEM) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic solution. The cells were incubated at 37 °C, 5% CO2, and 95% relative humidity. The hydrogel degradation products were used to study the cytocompatibility of the hydrogels. A 1.0 g hydrogel sample was wholly degraded in a 1.0 mol/L NaOH solution to obtain a 1 × degradation product solution (10 mL). This solution was then neutralized to pH 7.4 and sterilized by passing it through a 0.22 μm Teflon filter. The sterilized 1 × degradation product solution was diluted 10 or 100 times with sterile PBS to create 10 × or 100 × degradation product dilutions. In a 96-well plate, each well was seeded with 100 μL of rat BMSCs suspension in complete αMEM at 3 × 104 cells/mL concentration. The cells were incubated overnight, and then 10 μL of the degradation product in different dilutions was added to each well. The cells were cultured for an additional day before being quantified using the CCK-8 assay. At least six parallel wells were set up for each group, and the results were averaged. For cells treated with the 100 × (1 mg/mL) degradation products, a Live/Dead Viability/Cytotoxicity Kit was used to stain the cells. The morphology of the cells was observed using an inverted fluorescence microscope (Leica DMI4000 B; Leica Microsystems GmbH, Wetzlar, Germany).
This experimental setup allowed for the assessment of cell viability and morphology following exposure to different dilutions of the hydrogel degradation products, providing information on the cytocompatibility of the hydrogels.
2.8. Cell migration experiments
The scratch and transwell assays allow for the assessment of cell migration in response to the hydrogel degradation products. The scratch assay involves creating scratches in a cell monolayer and monitoring cell migration into the scratched area over time. The transwell assay typically involves placing cells on a porous membrane, allowing cell migration through the membrane, and quantifying the extent of migration. We applied interventions using 100 × degradation products to assess the effect of hydrogels on cell migration. Rat BMSCs were seeded in 6-well plates at a cell density of 3 × 104 cells/well. The cells were cultured in complete αMEM for 24 h to form a cell monolayer. Three parallel scratches were created in the cell monolayer using a 200 μL pipette tip. The floating cells were washed with sterile PBS (pH 7.4) three times to remove non-adherent cells. The remaining cells were incubated in complete αMEM containing 100 × degradation products (1/10 to complete αMEM, v/v) of different hydrogel formulations for an additional day. After 6, 12, and 24 h, the cells were observed and photographed using an inverted microscope (Olympus CKX41, Tokyo, Japan). The cell migration rate was quantitatively calculated using ImageJ or analysis software based on Equation (4).
| (4) |
Here, Wi denotes the initial scratch width at 0 h, and Wt represents the scratch width after being cultured for t hours (t = 6,12 or 24).
The transwell assay was conducted using transwell chambers (Corning, 353,097) placed within 24-well plates containing αMEM wells. The chambers were seeded with BMSCs at 3 × 104 cells/mL density in 100 × degradation products (500 μL/chamber) and incubated for 24 h. After incubation, the cells on the upper surface of the chamber were entirely removed by gently wiping them with a cotton swab. Subsequently, the cells on the lower surface were fixed using a 5% glutaraldehyde solution for 30 min and stained with crystal violet for 15 min. The chambers were washed with sterile PBS before being observed and photographed using a microscope (Olympus CKX41, Tokyo, Japan).
2.9. Validation of hydrogel-regulated macrophage polarization
RAW264.7 cells were utilized in a co-culture experiment with 100 × degradation products to evaluate the ability of the hydrogel to induce macrophage polarization. The ratio of M1 and M2 macrophages was determined using flow cytometry, employing flow antibodies procured from eBioscience. RAW264.7 cells were seeded onto 12-well plates and treated with 100 μL of the hydrogel's 100 × degradation products in the presence of 100 ng/mL lipopolysaccharide (LPS) or 40 ng/mL Interleukin 4 (IL-4) to stimulate cell polarization. Following a 24-h incubation period, the cells were collected and subjected to flow cytometry analysis using a BD Canto II instrument (USA). The collected cells were treated with FcR Blocking Reagent (BioLegend) and incubated with PE F4/80BioLegend, 111,703), PE/Cy7 CD11b (BioLegend), and FITC CD86 (BioLegend) antibodies. Next, cells were fixed with a Fixation Buffer (Biolegend) and permeated with an IntracellularStaining Permeabilization Wash Buffer (BioLegend). APC CD206 (BioLegend) antibody was then used to incubate cells for flow cytometry assay. Macrophages were firstly screened based on the expression of F4/80 and CD11b, and CD86 (M1 marker) and CD206 (M2 marker) expression were then determined. Data were analyzed by Flowjo Analysis Software. Immunofluorescence staining was conducted using the F4/80 primary antibody, iNOS primary antibody (Abcam, USA), and CD206 primary antibody (Abcam, USA). In brief, the culture medium was removed from each well, and the cells were fixed with 4% paraformaldehyde for 30 min. Following three rinses with PBS, the cells were treated with 0.5% Triton for 30 min. After three additional rinses, a rapid blocking solution (beyotime, China) was applied for a 1-h incubation period. Subsequently, the corresponding primary antibodies were added and incubated overnight at 4 °C. The following day, secondary antibodies (Abcam, USA) were added following three rinses and incubated at 37 °C for 2 h. The secondary antibodies were washed away, and the cell nuclei were stained with DAPI (beyotime, China) for 5 min. The immunofluorescent cells were observed and imaged using an inverted fluorescence microscope.
2.10. Validation of hydrogel promoted osteogenesis
To summarize, the protocol involved seeding rBMSCs in the wells of 24-well plates and culturing them in complete αMEM supplemented with 100 × degradation products (1/10 to αMEM) from different hydrogel formulations. The medium was changed every two days. For ALP staining, after seven days of culture in osteogenic media, the cells were fixed with 4% PFA and stained using the BCIP/NBT Alkaline Phosphatase Color Development Kit (beyotime) for 30 min, followed by washing with PBS. For Alizarin red S staining, after 14 days of osteogenic culture, the cells were fixed with 4% PFA, stained with 0.2% Alizarin red S solution for 30 min, and washed with PBS three times. The cells were visualized and quantified using an inverted fluorescence microscope (Leica DMI4000 B; Leica Microsystems GmbH, Wetzlar, Germany) and appropriate staining kits.
Immunofluorescence staining was also employed to confirm the hydrogels’ ability to promote osteogenic differentiation. Following the protocol above, after seven days of co-culture, BMSCs in different groups were subjected to immunofluorescence staining using OCN and Runx2 primary antibodies (SAB, USA). The immunofluorescent cells were then observed and photographed under an inverted fluorescence microscope.
2.11. Pellets of BMSCs and the examination of hydrogel's ability to induce chondrogenesis
To assess the pro-chondrogenic effects of hydrogels, pellets of BMSCs (n = 3, passage ≤5) were formed by centrifuging a cell suspension (2.5 × 105 cells) at 500×g for 10 min. The pellets were then cultured in αMEM supplemented with 1% ITS (Gibco), 0.1 μM dexamethasone (Sigma-Aldrich), 50 μM ascorbate-2-phosphate (Sigma-Aldrich), 50 μM l-proline (Sigma-Aldrich), and 1 mM sodium pyruvate (Gibco). The pellets were treated with 5 ng/mL TGF-β3 (positive control) with or without 100 × degradation products (1/10 to complete culture medium, v/v) by changing the medium every three days. After 21 days of culture, the pellets were fixed in a 4% paraformaldehyde solution and embedded in paraffin. Slices of the embedded pellets were then used for toluidine blue and immunofluorescence staining [34]. Following the previously described protocol, immunofluorescence staining was performed using Col II (Bioss) and Aggrecan (Bioss) primary antibodies on sections from different groups. Furthermore, to evaluate the hydrogels’ ability to promote chondrogenic differentiation, BMSCs with a density of 3 × 104 cells/ml were cultured in a chondrogenic induction medium containing 100 × degradation products (10% v/v). After 7 days, immunofluorescence staining using Sox9 (ABclonal) and Mag-Fluo-4 AM (Maokangbio) was carried out following the procedure. To verify whether Mg2+ enters the cells to promote chondrogenesis, we labeled Mg2+ using a Mag-Fluo-4 AM flow cytometer. The experiments for flow cytometry were performed according to the previously described protocol.
2.12. Gene expression analysis
Quantitative PCR was applied to detect the expression of specific genes. Total RNA was isolated using a Trizol reagent (Invitrogen, USA). 1000 ng RNA was reverse transcripted using the First Strand cDNA Synthesis Kit (Thermofisher, USA). SYBR green PCR Master Mix (Thermofisher, USA) was used in qPCR with CFX96 Connect Real-Time PCR Detection System (BIO-RAD, USA). ΔΔCt method was applied to calculate the results. The primers used in this study were designed by Primer 3.0 (Table S2).
2.13. Protein expression analysis
The expressions of related proteins were examined using WB. Briefly, total protein was extracted using RIPA lysis buffer (Beyotime), and protein lysis products were quantified using a bicinchoninic acid (BCA) kit (Solarbio). Subsequently, 20 μg of protein was loaded onto SDS PAGE gels, followed by electrophoresis, membrane transfer, and closure. Then, the membranes were incubated with correspondent primary antibodies (ABclonal). The membranes were incubated with the secondary antibody solution labeled by horseradish peroxidase (HRP) (Beyotime), and the protein bands were detected using enhanced chemiluminescence (ECL) kit (Fdbio science) and scanned by gel imaging system. Using the Image J software, the blots (n = 3 per group) were quantified by a densitometric method. The results were expressed as mean ± SD.
2.14. Animal studies
2.14.1. Surgical procedures
The surgical procedures and perioperative handling followed the protocols approved by the Animal Experimental Committee of the Daoke Pharmaceutical Technology (Guangdong) Co., Ltd (Approval IACUC-DK-2022-08-12). Sixty-four male Sprague Dawley (SD) rats at 8 weeks of age were obtained from the Medical Animal Experiment Center of Southern Medical University. They were randomly divided into four groups: the standard group, the control group, the Dop-HA/F127 group, and the Mg-PC@Dop-HA/F127 group, with 16 rats in each group. The rotator cuff tear model was created using an established method [35]. After administering anesthesia, the rats were placed in the lateral decubitus position. A longitudinal incision was made in the anterolateral portion of the shoulder to expose the supraspinatus tendon. The deltoid muscle was also split to visualize the tendon better. The tendon was then detached at its insertion site on the greater tuberosity of the humerus. A tunnel was drilled using a 7# angle needle at the critical prominence of the humerus. After removing any remnants on the footprint, hydrogels (50 μL) were injected into the bone groove. No hydrogel injection was performed in the control group, and the retracted tendon was pulled over the bone groove. The supraspinatus muscle was then sutured back to the greater tuberosity using a 5# suture. Finally, the wound was closed, and the rats had free access to water and food in their cages.
2.14.2. Micro-CT Analysis, Finite element analysis and biomechanical Testing
At weeks 4 and 8 post-operation, SD rats were euthanized, and the bone-tendon interface specimens (4 for micro-CT and histology study, 4 for biomechanical testing) were fixed in 4% paraformaldehyde and stored at 4 °C.
Micro-CT (Viva CT40; Scanco et al. dorf, Switzerland) was conducted in an isolated bone mode to evaluate the bone regeneration in the defect area with the following settings: pixel size, 480 μm; slice thickness, 15 μm; rotation angle, 360°; X-ray voltage, low; artifact removal, lean; sync. Scan, no; metal artifact reduction, no. The coronal image of the bone-tendon interface sections was reconstructed using Mimics software (version 20.0). A cylindrical space representing the volume of interest (VOI) was designated to evaluate new bone formation by calculating BMD, bone volume fraction (BV/TV), number of bone trabeculae (Tb. N), thickness of bone trabeculae (Tb. Th), separation of bone trabeculae (Tb. Sp) using Latheta software (Scanco Medical AG, Bassersdorf, Switzerland).
The finite element model was constructed using Ansys 19.0 software, on which the material properties of each part of the structure of the 3D model were defined [36]. The material properties of the humeral head cortical bone were 1.5 × 104 MPa, and the material properties of the supraspinatus tendon were 10 MPa. The tensile force of the supraspinatus tendon was simulated (200 N), and the stress-strain data were outputted simultaneously.
Supraspinatus tendon-humerus complexes of each group (n = 4) were harvested 4 and 8 weeks after RCR surgery. All the specimens were tested using an electronic universal materials testing system (Instron 5569). In brief, the specimens were preconditioned with 0.1 N and then were loaded to failure under uniaxial tension at an elongation rate of 10 mm/min. The failure loads were noted, and stiffness was calculated from the load-deformation curve.
2.14.3. Histological analysis
The supraspinatus tendon humerus complex was harvested 4 and 8 weeks after the operation, fixed with 4% paraformaldehyde after micro-CT scanning, and decalcified with 0.5 M EDTA at 37 °C for 4 weeks. After dehydration and embedding in paraffin, the samples were sectioned with a thickness of 4 mm. These samples were stained with hematoxylin and eosin (H&E), toluidine blue (TB), and picrosirius red. A light microscope (IX71SBF-2; Olympus) examined the H&E and TB-stained slides. The metachromasia area in the TB-stained slides was measured using the Image J software (National Institutes of Health). A polarized light microscope (Eclipse E800; Nikon) was used to detect the collagenous tissue of the tendon near the greater tuberosity in the picrosirius red slides. Higher values indicate higher levels of collagen maturation. A histological scoring system was used to assess the interface between the tendon and bone semi-quantitatively. Higher histological scores suggest better tendon-to-bone healing. Two independent observers performed the histological evaluation, blinded to group allocation.
2.14.4. Immunohistochemical staining and immunofluorescence staining
Immunohistochemical staining of Trpm7, Hif-1α, Sox9, and Col II, as well as immunofluorescence staining of BMSCs (CD44 as the BMSCs surface marker), were performed and observed with an upright fluorescence microscope (Leica DMI4000 B; Leica et al.). Semi-quantitative analysis of Trpm7, Hif-1α, Sox9, and Col II positively stained cells was also conducted using Image J.
2.15. Statistical analysis
Statistical significance was estimated with one-way ANOVA with Tukey's multiple comparisons test or Student's test within groups GraphPad Prism 9 (GraphPad Software, USA). A value of P < 0.05 was considered a statistically significant difference. *, ** and *** represent p < 0.05,p < 0.01 and p < 0.001, respectively.
3. Results
3.1. Synthesis and characterizations of Mg-PC
As depicted in Fig. 1A, heating conditions within a reactor expedited the coordination process, facilitating the formation of Mg-PC. Fig. 1B illustrates the XRD curve of Mg-PC, displaying the characteristic peak of Mg2+ and affirming the successful integration of Mg2+ into Mg-PC. Additionally, the successful incorporation of PC into Mg-PC is substantiated by the presence of characteristic peaks at 1479 cm−1 and 1285 cm−1 in the FTIR spectra of Mg-PC [37] (Fig. 1C). These peaks, related to the absorbance of aromatic rings and phenolic hydroxyl groups in PC molecules, are absent in the FTIR spectrum of MgCl2. Thermogravimetric analysis (TGA) indicates that Mg-PC undergoes a mass loss of approximately 80% when subjected to temperatures of up to 1000 °C under controlled heating conditions (Fig. 1D). These results confirm the successful introduction of organic components, with an organic-to-inorganic mass ratio of approximately 1:4. The morphology of Mg-PC nanoparticles is unveiled through SEM analysis (Fig. 1E), revealing a “nanoflower” structure reminiscent of a rose, with dimensions spanning from 300 nm to 500 nm. Dynamic light scattering further shows that the particle size of Mg-PC nanoparticles ranges from 200 nm to 600 nm (Fig. 1F). Moreover, the element mapping images of Mg-PC (Fig. 1G) further confirm the successful inclusion of PC in Mg-PC and showcase the uniform distribution of Mg2+ within the Mg-PC structure.
Fig. 1.
Schematic synthesis and characterization of Mg-PC. A) Schematic synthesis of Mg-PC by hydrothermal method; B) XRD patterns of PC and Mg-PC; C) FTIR spectra of MgCl2, PC, and Mg-PC; D) TGA curves of Mg-PC; E) Scanning electron microscope images and magnified images of Mg-PC; F) Particle size distribution of Mg-PC; G) Elemental mapping of Mg-PC.
3.2. Synthesis and characterizations of Mg-PC@Dop-HA/F127
We link DA to HA molecular chains via EDC/NHS chemistry. Under the catalysis of NHS and EDC, Dopamine functional groups can be grafted onto long chains of hyaluronic acid via an amidation reaction. The successful grafting of DA was first verified by FTIR and nuclear magnetic spectrum analysis. In the FTIR spectrum of Dop-HA, it can be observed that two peaks at 1680 cm−1 and 1516 cm−1 respectively correspond to the two characteristic peaks of the amide bond, confirmed that dopamine was successfully grafted onto the long chain of HA (Fig. S1A). We then further confirmed the successful synthesis of Dop-HA through 1H NMR analysis. As shown in Fig. S1B, the new peaks appear at 6.7 ppm, indicating the presence of benzene rings in dopamine, and the proton peak at 2.76 ppm is because the –CH2- group is close to the catechol ring. Then we used the UV–visible method to determine the grafting rate of DA. As shown in Fig. S2, according to the dopamine standard curve, the degree of substitution of Dop-HA by dopamine is about 6.88%.
To further examine the impact of Mg-PC introduction on hydrogel properties, various hydrogel groups were obtained by adjusting the proportions of Mg-PC (0%, 5%, 10%, 20%): Dop-HA/F127, 5% Mg-PC@Dop-HA/F127, 10% Mg-PC@Dop-HA/F127, and 20% Mg-PC@Dop-HA/F127. As shown in Scheme 1, Dop-HA itself is connected through π-π bonds, and Dop-HA is connected with F127 through hydrogen bonds. In aqueous solution, F127 forms micelles. In addition, the polyphenol PC in our synthesized Mg-PC can form a network with F127 through hydrogen bonds. Gelation time and temperature were recorded for these prepared gel-forming systems (Fig. 2B). Both parameters were dependent on Mg-PC concentration and overall composition. As Mg-PC content increased, both gel time and temperature gradually decreased, possibly due to increased cross-linking sites with higher Mg-PC content. The 10% Mg-PC@Dop-HA/F127 group exhibited superior temperature responsiveness and nanoparticle loading, making it the representative choice for synthesis validation and hydrogel performance demonstration. The successful synthesis of Mg-PC@Dop-HA/F127 was confirmed through Fourier-transform infrared spectroscopy (FTIR) (Fig. 2C). Temperature-sensitive properties of the hydrogel system (10% Mg-PC@Dop-HA/F127) were evident (Fig. 2D). At 4 °C or 25 °C, the hydrogel remained fluid, while at 37 °C (body temperature), it solidified. Recognizing the importance of injectability in biomedical applications of hydrogels, we confirmed the hydrogels' excellent injectability, thermosensitivity, and shape adaptability in vitro models, facilitating their application to irregular positions such as the supraspinatus muscle insertion (Fig. 2E, Video S1–S3). SEM images of various hydrogel groups are displayed in Fig. 2F. Additionally, Fig. S3 presents the mapping image of the hydrogel scanning electron microscope and the element proportions (Table S1). Meanwhile, the swelling rates of Dop-HA and 10% Mg-PC@Dop-HA/F127 hydrogels are shown in Fig. S4. After adding Mg-PC and F127, the swelling rate of the composite hydrogel decreased compared with that of Dop-HA. Rheological measurements, including frequency and temperature sweeps, were conducted to examine the mechanical properties (Fig. 2G and Fig. S5). The curves of elastic modulus (G′) and viscosity modulus (G″) of different hydrogel groups showed stability within the frequency range of 1–100 rad/s. F127 had the lowest G′ and G″ compared to other groups. The addition of Mg PC increased both G′ and G″ as the nanoparticle amount increased.
Scheme 1.
Schematic representation of the design of an injectable composite hydrogel. This hydrogel could control the release of Mg-PC for bone-tendon interface repair by modulating the inflammatory environment and promoting cartilage regeneration.
Fig. 2.
Mechanism and characterization of injectable composite hydrogels. A) Schematic diagram of the mechanism of injectable composite hydrogels; B) Gel characteristics (temperature and time) of composite hydrogels with different contents of Mg-PC (0%, 5%, 10%, and 20%); C) FTIR spectra of Mg-PC, Dop-HA, F127, and 10% Mg– PC@Dop-HA/F127; D) Macroscopic photos of hydrogels at different temperatures (Scale bars indicate 1 cm); E) Injectability, thermosensitivity and shape adaptability; F) Scanning electron microscopy images of composite hydrogels with different contents of Mg-PC (0%, 5%, 10%, and 20%); G) The elastic modulus (G′) and viscosity modulus (G″), and the rheological recovery behavior (H) of composite hydrogels with different contents of Mg-PC (0%, 5%, 10% and 20%); I) Mechanical properties of composite hydrogels with different contents of Mg-PC (0%, 5%, 10% and 20%); J) Weight loss of composite hydrogels with different contents of Mg-PC (0%, 5%, 10% and 20%); and release characteristics of Mg2+ (K) and PC (L) with different contents of Mg-PC (5%, 10% and 20%).
Subsequently, rheological recovery behavior was determined using a continuous step strain test (Fig. 2H). When applying a 1000% strain exceeding the critical limit, the G′ value immediately dropped from 2600 to 470 Pa, lower than G". Upon strain recovery to 1%, both G′ and G″ values returned to or exceeded their initial values. This demonstrated the hydrogel's rapid and highly efficient self-healing capacity, with the collapse and recovery behaviors of the hydrogel network alternately repeated several times. We verified the adhesion properties to tendon and bone and the excellent self-healing capacity of the composite hydrogel (Fig. S6). The tensile strength of the hydrogel as a tendon-filling material after solidification was verified (Fig. 2I). Dop-HA exhibited a weak tensile strength of approximately 0.1 N as a substrate material. The addition of Mg-PC nanoparticles increased the tensile strength of the hydrogel system. After adding 10% w/v Mg-PC, the tensile strength substantially increased, indicating that introducing inorganic components is crucial for enhancing hydrogel mechanical properties. However, when 20% w/v Mg-PC was added, the hydrogel stretched and broke quickly, with lower strength than the 10% w/v group. This phenomenon was attributed to the failure of the hydrogel network's energy dissipation mechanism due to excessive inorganic component content. The degradation and release properties of composite hydrogels in PBS were also investigated. The degradation curve of the composite hydrogel is shown in Fig. 2J, with Dop-HA/F127 serving as the control. Overall, the composite hydrogel exhibited a moderate degradation rate in PBS (pH 7.4), with a mass loss of approximately 50–60% after 35 days. Adding Mg-PC slowed the hydrogel's mass loss rate, possibly due to the introduction of the metal-polyphenol structure that enhanced hydrogel cohesion. However, there was almost no difference in degradation rates between the two groups, with 20% Mg-PC and 10% Mg-PC, as Dop-HA/F127 reached the upper loading limit for the inorganic component. This observation was confirmed by the cumulative release curves (Fig. 2K&J), wherein the Mg2+ and PC release rates exhibited an increasing trend in the 20% Mg-PC@Dop-HA/F127 group.
The other two groups entered the stable release interval at 15 days. The experiment also confirmed that the strategy of combining Mg-PC with Dop-HA/F127 could effectively and consistently release magnesium ions and Procyanidins. In injury repair, this composite hydrogel can deliver therapeutic components in situ over an extended period, thereby aiding the body in achieving a more comprehensive and effective repair process.
3.3. Cellular viability, proliferation and migration In vitro
Initial stemness testing was conducted on primary bone marrow mesenchymal stem cells (BMSCs). Flow cytometric analysis of rat BMSC-related markers revealed that more than 90% of BMSCs expressed positive markers (CD29 and CD90). In comparison, less than 5% of cells expressed negative markers (CD34 and CD45), confirming their identity as stem cells (Fig. S7A&B).
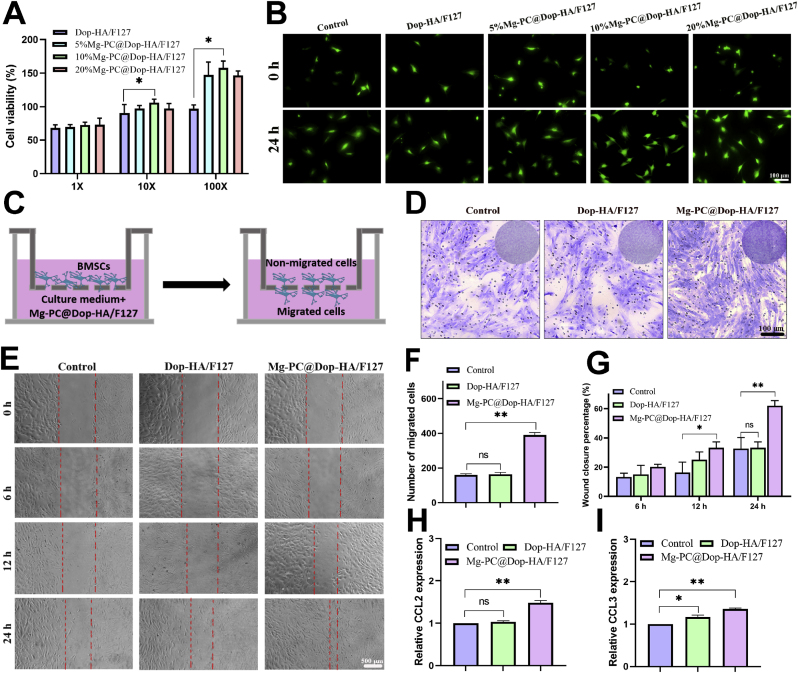
BMSCs exhibited a spindle-shaped morphology (Fig. S7C), and following differentiation induction, they demonstrated the capacity to differentiate into osteocytes, adipocytes, and chondrocytes (Figs. S7D–F). These results confirmed the successful isolation and utilization of BMSCs in subsequent cell experiments. The cytocompatibility of the hydrogels was evaluated using the Live/Dead staining kit and cell counting kit 8 (CCK-8) assay for BMSCs. Fig. 3A illustrates that in the presence of complete degradation products of hydrogels diluted 100 times, cell viability was notably higher in groups with the addition of Mg-PC, especially in the 10% Mg-PC@Dop-HA/F127 group. The actual cytocompatibility performance of various hydrogel groups containing different proportions of Mg-PC (0%, 5%, 10%, 20%, w/v) is presented in Fig. 3A. While cell viability in the four hydrogel groups was low at 1-fold degradation product dilution (approximately 50–60%), it significantly increased at 10-fold and 100-fold dilutions, particularly at 100-fold dilutions (with the 10% Mg-PC group reaching up to 150% cell viability). Live/dead staining images in Fig. 3B support these findings, confirming the strong cytocompatibility of Mg-PC@Dop-HA/F127, making it a promising candidate for in vivo applications. Due to its exceptional cytocompatibility and in vitro mechanical properties, the 10% Mg-PC@Dop-HA/F127 group was selected for subsequent cell and animal experiments. Furthermore, the effect of Mg-PC@Dop-HA/F127 on BMSC migration was assessed using transwell and cell scratch assays. Fig. 3C outlines the Transwell experiment, while Fig. 3D&F demonstrate longitudinal cell migration, with the Mg-PC@Dop-HA/F127 group exhibiting significantly higher migration rates compared to the control group. Fig. 3E&G, illustrating the cell scratch test assessing lateral cell migration, further confirms the increased migration rate in the Mg-PC@Dop-HA/F127 group (approximately 60% after 24 h) compared to the control group (approximately 30% after 24 h). This indicates that Mg and PC enhance cell migration and proliferation. Additionally, qPCR results (CCL2 and CCL3, Fig. 3H&I) further support the hydrogel's ability to promote migration. These findings underscore the favorable cytocompatibility of Mg-PC@Dop-HA/F127, as well as its significant potential in promoting cell proliferation and migration, which is advantageous for its application in tendon-bone healing.
Fig. 3.
In vitro cytocompatibility and cell migration properties of composite hydrogels. A) Cytotoxicity of degradation products of composite hydrogels with different contents of Mg-PC (0%, 5%, 10% and 20%) on BMSCs; B) Live/dead images of BMSCs treated with 100-fold degradation products of different composite hydrogels for 24 h; C) Schematic diagram of the Transwell assay; D) Transwell images and migration rates of different composite hydrogels quantification (F); E) Cell migration images and related quantitative data obtained from scratch experiments (G); H) and I) are qRT-PCR results of CCL2 and CCL3 mRNA expression of BMSCs 3 days after treatment with degradation products of different hydrogels. *p < 0.05, **p < 0.01, “ns” represents no significant difference.
3.4. In vitro antioxidant activity performance
Rotator cuff injuries often trigger an inflammatory response, leading to the generation of ROS (Reactive Oxygen Species) in the local environment, which can have adverse effects and impede the regeneration of the bone-tendon interface [38]. Procyanidin (PC), known for its abundance of phenolic groups, is recognized for its superior antioxidant capabilities. Therefore, we thoroughly investigated the antioxidant capacity of Mg-PC and Mg-PC@Dop-HA/F127 using the 2,2-diphenyl-1-trinitrophenylhydrazine (DPPH) assay and intracellular ROS assay. With its numerous phenolic hydroxyl groups, PC can supply ample electrons to reduce DPPH, as observed in Fig. 4A. The DPPH radical solution starts purple, and, as shown in Fig. 4B, its color changes based on the duration of Mg-PC addition. Fig. 4C&D reveal that, after 10 min, the absorption intensity of DPPH at 517 nm in the Mg-PC group significantly decreased by approximately 38% compared to the control group. When the treatment duration was extended to 60 min, the DPPH clearance rate reached approximately 60% (Fig. 4E&F). Intracellularly, the ROS level in the Dop-HA/F127 group closely resembled that of the control group, while the ROS level in the Rosup group notably increased. Notably, the Mg-PC@Dop-HA/F127 group exhibited a significant decrease in intracellular ROS levels (Fig. 4H). This observation was further supported by quantitative fluorescence intensity results presented in Fig. 4G, with the addition of Mg-PC@Dop-HA/F127 reducing fluorescence intensity by approximately 40%. These findings confirm the excellent antioxidant efficacy of Mg-PC, which can effectively scavenge ROS in the environment, creating a conducive microenvironment for tendon-bone interface regeneration.
Fig. 4.
Antioxidant activity of Mg-PC and Mg-PC@Dop-HA/F127. A) Schematic diagram of the DPPH radical reduction reaction; B) Photographs of DPPH solution, MgCl2-treated DPPH solution, and Mg-PC-treated DPPH solution at different times; C) UV–visible spectra of the different modes of treatment and D) DPPH scavenging rate (n = 3); E) UV–visible spectral changes of DPPH solutions treated by Mg-PC for different times; F) UV–vis curves of Mg-PC; H) Fluorescence images and G) relative fluorescence intensities of oxidation inhibition obtained by ROS test kit on BMSCs (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001,"ns” represents no significant difference.
3.5. In vitro immunomodulatory activity performance
The state and function of local immune cells play a crucial role in tissue repair processes. Among these cells, the differentiation of macrophages holds particular significance, as an immune microenvironment dominated by M2-type macrophages promotes optimal healing of the tendon-bone interface [39]. After labeling macrophage populations with F4/80, we employed iNOS and CD206 as markers for M1 and M2-type macrophages, respectively. Cellular immunofluorescence staining and quantitative results revealed that the fluorescence associated with iNOS (M1) in the Mg-PC@Dop-HA/F127 group was significantly weaker compared to the other groups (Fig. 5B&D). In contrast, the fluorescence of CD206 (M2) was notably stronger than in the other groups (Fig. S8). This data suggests that the presence of magnesium ions induces a more significant differentiation toward M2-type macrophages (Fig. 5C&E). Western blot (WB) testing (Fig. 5F) and the corresponding relative protein expression levels (Fig. S9) further confirmed that Mg-PC@Dop-HA/F127 significantly reduced the expression of iNOS while promoting the expression of CD206, underscoring the hydrogel's efficacy in inducing macrophage polarization from M0 to M2. Flow cytometry analysis of the expression of CD86 and CD206 cell surface markers in RAW264.7 cells showed a significant decrease in the percentage of CD86-positive cells in the Mg-PC@Dop-HA/F127 group (from 42.1% in the LPS control group and 40.2% in the Dop-HA/F127 group to 19.1% in the Mg-PC@Dop-HA/F127 group) (Fig. 5G). Furthermore, qPCR results (Fig. 5H) confirmed that, compared to the control group, the expression of iNOS and TNF-α(M1) genes significantly decreased in the Mg-PC@Dop-HA/F127 group, while the expression of CD206 and Arg (M2) genes substantially increased. These results demonstrate that Mg-PC@Dop-HA/F127 effectively modulates macrophage polarization, shifting them from M0 to M2, which holds significant promise for tissue healing [40].
Fig. 5.
Immunomodulatory activity of composite hydrogels. A) Schematic diagram of the experiment to study the polarizing effect of hydrogel on M0 macrophages; B) and C) Immunofluorescence staining of iNOS and CD206 after induction of M0 macrophages by hydrogel degradation products; D) and E) Quantification of immunofluorescence staining of iNOS and CD206; F) Western blotting showing expression of iNOS,CD206 and GAPDH. G) Representative images of RAW264.7 surface markers (CD86 and CD206) analyzed by flow cytometry. H) RT-qPCR measurements of gene expression of iNOS, TNF-α, CD206, and Arg after induction of M0 macrophages by hydrogel degradation products for 3 days. *p < 0.05, **p < 0.01, ***p < 0.001.
3.6. In vitro osteogenic differentiation performance
Throughout the reparative process of the tendon-bone interface, regenerating tissue often transitions from chondrogenesis to osteogenesis. Consequently, it is postulated that the establishment of the tendon-bone interface relies on the chondrogenic differentiation of bone marrow stem cells, with the ultimate goal being osteogenic differentiation. These two processes are intricately interconnected within the physiological milieu and temporal progression [41].
Building upon prior research, we know that Mg2+ can induce the osteogenic differentiation of bone marrow mesenchymal stem cells and stimulate the secretion of osteogenic marker proteins, such as Runx2 and ALP. In this context, we utilized Mg-PC@Dop-HA/F127 to investigate its potential to promote the osteogenic differentiation of BMSCs. The control group consisted solely of fresh medium culture. To assess the osteogenic differentiation-promoting properties and bone mineralization of Mg-PC@Dop-HA/F127, we conducted alkaline phosphatase (ALP) and alizarin red staining (ARS). As illustrated in Fig. 6A&B, 14 days after introducing 100x diluted degradation products of Mg-PC@Dop-HA/F127 into the osteogenic medium, there was a significant increase in ALP expression. Furthermore, as evident in the alizarin red-stained image (Fig. 6A) and the quantitative red area analysis at day 21 (Fig. 6C), the presence of Mg2+ significantly enhanced the mineralization of osteogenically differentiated BMSCs. After 7 days of co-cultivation with BMSCs, the fluorescence intensity of OCN (Osteocalcin) and Runx2 in the Mg-PC@Dop-HA/F127 group was notably higher, reaching four-fold and six-fold, respectively, when compared to the Control group and Dop-HA/F127 group (Fig. 4C–E). Subsequently, we subjected 100-fold diluted degradation products of various hydrogels to an osteogenic medium for 7 days, and we further examined the expression levels of Col I, ALP, and Runx2 in osteogenically differentiated BMSCs using Western blotting. Observing the Western blot bands (Fig. 6D) and the corresponding relative protein expression levels (Fig. S10A), it becomes evident that Mg-PC@Dop-HA/F127 significantly promoted the expression of Col I, ALP, and Runx2. Additionally, qPCR results about Osx, ALP, and Runx2 (Fig. S10B) reinforced the notion that the Mg-PC@Dop-HA/F127 group possessed the capability to induce the expression of genes associated with osteogenesis. This further validates the osteogenic differentiation-promoting effect of magnesium-based biomaterials.
Fig. 6.
Osteogenic differentiation-promoting activity of composite hydrogels and mechanism of promoting chondrogenic differentiation. A) Alkaline phosphatase (ALP) staining images and alizarin red staining images; B) Quantification of ALP activity (at day 14); C) Alizarin red staining of the corresponding quantified red areas (at day 21); D) Western blotting bands of Col I, ALP and Runx2 after hydrogel degradation products in osteogenic medium treated BMSCs; (E) Toluidine blue staining images of hydrogel degradation products after treatment of BMSC pellets in osteogenic medium; (F) Western blotting bands of Acan, Col II and Sox9 after hydrogel degradation products in chondrogenic medium treated BMSCs; G) and H) RT-qPCR measurements of Sox9 and Col II gene expression by hydrogel degradation products after treating BMSCs in chondrogenic medium for 7 days; I) Toluidine blue staining images of hydrogel degradation products and Mg2+ channel inhibitor (2APB) after chondrogenic medium treatment of BMSC pellets; J) Images of immunofluorescence staining (Col II and Aggrecan) and corresponding fluorescence intensity quantification (N) of hydrogel degradation products and Mg2+ channel inhibitor (2APB) in chondrogenic medium after treatment of BMSC pellets; K) Representative images of Mg2+ analyzed by flow cytometry; L) Immunofluorescence photos of Mg2+ labeling and associated quantification (M); O) Western blot bands of TRPM7, Hif-1α, Acan, Col II, and Sox9 by hydrogel degradation products and Mg2+ channel inhibitor (2APB) after treating BMSCs in chondrogenic medium for 7 days.(P) RT-qPCR measurements of Sox9, Col II and Aggrecan gene expression by hydrogel degradation products and Mg2+ channel inhibitor (2APB) in chondrogenic medium after treating BMSCs for 7 days.*p < 0.05, **p < 0.01, ***p < 0.001,"ns” represents no significant difference.
3.7. In vitro chondrogenic differentiation performance
We subjected BMSC pellets to co-treatment with hydrogel degradation products and a chondrogenic induction medium to assess their chondrogenic properties. Our conclusions are supported by toluidine blue-stained images obtained on day 21 (Fig. 6E) and the quantification of chondrogenic marker proteins (Acan, Col II, and Sox9) on the 14th day (Fig. 6F). Compared to the Control group and the Dop-HA/F127 group, the Mg-PC@Dop-HA/F127 group demonstrated superior capabilities in inducing chondrogenic differentiation of BMSCs.
Concurrently, the results for related gene expression (Acan, Col II, and Sox9) on the 14th day echoed the same trend (Fig. 6G&H). Previous research has indicated that Mg2+ has the potential to induce chondrogenic differentiation of mesenchymal stem cells [42]. The Trpm7 channel plays a crucial role in facilitating Mg2+ entry into cells as a divalent metal ion channel [43]. Additionally, studies have shown that Mg2+ can promote cartilage differentiation by activating Hif-1α [44]. To delve deeper into the involvement of Mg2+ in the chondrogenesis process and its activation of Hif-1α, we conducted an experiment using the Trpm7 channel blocker 2-Aminoethyl diphenylborinate (2APB) to inhibit Mg2+ entry into the cell. We aimed to determine whether the hydrogel could induce cartilage differentiation even with a reduced intracellular Mg2+ concentration. We established Control, Dop-HA/F127, Mg-PC@Dop-HA/F127, and 2APB + Mg-PC@Dop-HA/F127 groups for this research.
Toluidine blue staining results in Fig. 6I corroborated our earlier findings. The Mg-PC@Dop-HA/F127 group exhibited a higher number of chondrocytes, underscoring the hydrogel's excellent chondrogenic properties. However, the 2APB + Mg-PC@Dop-HA/F127 group displayed a similar trend to the Control group. We employed flow cytometry to investigate the entry of Mg2+ into BMSCs through the Trpm7 channel (Fig. 6K). It was observed that 2APB + Mg-PC@Dop-HA/F127 prevented the entry of Mg2+ into cells and inhibited its ability to promote chondrogenesis. Compared to the Control group and the Dop-HA/F127 group, the positive cell rate in the Mg-PC@Dop-HA/F127 group was significantly increased (32.9%), while it decreased to 14.6% in the 2APB + Mg-PC@Dop-HA/F127 group. Immunofluorescence staining supported these findings (Fig. 6L&M), as Mg2+ fluorescence intensity was significantly higher in the Mg-PC@Dop-HA/F127 group, while it was notably lower in the 2APB + Mg-PC@Dop-HA/F127 group. BMSC pellets were embedded in wax and sectioned for immunofluorescence staining (Col II, Acan) to study the chondrogenic properties of the hydrogels. 2APB + Mg-PC@Dop-HA/F127 served as the inhibition control group. As demonstrated in Fig. 6J&N, both Col II and Acan fluorescent staining yielded higher fluorescence intensity in the Mg-PC@Dop-HA/F127 group compared to the Control and Dop-HA/F127 groups. However, upon the addition of 2APB, the Trpm7 channel blocker, both fluorescence intensities were significantly reduced. Corresponding fluorescence quantification further substantiated these results. To delve further into the chondrogenic differentiation of BMSCs, we analyzed the expression of chondrogenic markers Sox9, Acan, and Col II using qRT-PCR and WB. The Mg-PC@Dop-HA/F127 group exhibited significantly higher expression of chondrogenic genes compared to other groups. As depicted in Fig. 6P, in comparison to the Control group and the Dop-HA/F127 group, the expression of chondrogenic genes in the Mg-PC@Dop-HA/F127 hydrogel group increased significantly after 7 days of co-culture. In comparison to the Control group, the gene expression levels of chondrogenic genes, including Sox9, Acan, and Col II in the Mg-PC@Dop-HA/F127 group, increased by 2.24-fold, 1.93-fold, and 1.49-fold, respectively, after 7 days of culture. Notably, gene expression in the 2APB + Mg-PC@Dop-HA/F127 group decreased significantly, even falling below the levels in the Control group. These results confirm that the hydrogel can gradually release Mg2+ into BMSCs through Trpm7 channels, promoting chondrogenic differentiation. In alignment with the qRT-PCR results, the Mg-PC@Dop-HA/F127 group exhibited increased expression of Acan, Sox9, and Col II proteins. Additionally, compared to other groups, the Mg-PC@Dop-HA/F127 group demonstrated significantly elevated expressions of Trpm7 and Hif-1α proteins (Fig. 6O and Fig. S11).
3.8. In vivo study of rat rotator cuff tears (RCT) model
After establishing the rat RCT model, we administered injections of Dop-HA/F127 and Mg-PC@Dop-HA/F127 hydrogels into the bone groove. In addition, we included a normal group and a suture group as controls. We assessed the efficacy of these composite hydrogels in repairing rotator cuff injuries in rats through bone volume analysis, mechanical analysis, and histological analysis (Fig. 7A).
Fig. 7.
Micro-CT analysis results and Data for biomechanical tests of in vivo animal experiments. A) Schematic of the animal experiments; B) Three-dimensional reconstruction images and coronal images of the proximal humerus micro CT of rats at 4 and 8 weeks after surgery; C) Images of a finite element model of the proximal humerus in rats at 4 and 8 weeks after surgery; D) Images of biomechanical tests; E) BMD,BV/TV and Tb. Sp values of the tendon-to-bone interface; F) Data for biomechanical tests, including maximum load, stiffness, and distance at maximum load.*p < 0.05, **p < 0.01, “ns” represents no significant difference.
3.8.1. Micro-CT analysis, finite element analysis and biomechanical Testing
At 4 and 8 weeks post-operation, we harvested the rats' shoulders for Micro-CT analysis. As depicted in Fig. 7B (including 3D visualization and coronal slice images), the Mg-PC@Dop-HA/F127 group exhibited superior morphology at the healing interface compared to the other groups at both postoperative weeks 4 and 8. Particularly at 8 weeks, the healing interface's morphology in the Mg-PC@Dop-HA/F127 group closely resembled that of the Normal group. Statistical analysis results for subchondral bone morphological parameters are presented in Fig. 7E and Fig. S12. The Mg-PC@Dop-HA/F127 group displayed higher values for bone mineral density, bone volume fraction, number of trabecular bone, and trabecular bone thickness compared to the other groups. Conversely, the Suture group exhibited the lowest values for bone mineral density, bone volume fraction, and trabecular thickness at both 4 and 8 weeks. Analysis of the trabecular separation rate among the groups revealed the Mg-PC@Dop-HA/F127 group had the lowest rate. These results establish that the injection of Mg-PC@Dop-HA/F127 significantly improved the osteogenic properties of subchondral bone in rats with rotator cuff injuries. By 8 weeks post-surgery, recovery was excellent and approached that of the normal group.
A finite element model of the shoulder joint was reconstructed using CT scan data from the rat shoulder joint. Based on this model, we applied a 200 N tension load to simulate tendon behavior [36] (Fig. 7C). Under this 200 N load, the highest stress levels at the junction of the supraspinatus tendon and humeral head were as follows: normal group - 969.4 MPa (8 weeks), suture group - 2608.8 MPa (8 weeks), Dop-HA/F127 group - 1625 MPa (8 weeks), and Mg-PC@Dop-HA/F127 group - 1002 MPa (8 weeks). These results indicate that the suture group and the Dop-HA/F127 group had substantially higher localized stress values compared to the normal group and the Mg-PC@Dop-HA/F127 group at both 4 and 8 weeks post-surgery. Injection of Mg-PC@Dop-HA/F127 resulted in maximum stress values approaching those of the normal group, suggesting a more even distribution of stress at the tendon-bone interface, reducing the risk of tendon re-rupture due to excessive localized stress.
Moreover, as illustrated in Fig. 7D&F, the Mg-PC@Dop-HA/F127 group exhibited higher maximum load and stiffness values compared to the other groups at both 4 and 8 weeks. No significant differences were observed in maximum load and stiffness values between the Dop-HA/F127 and pure suture groups. Moreover, at the point of maximum load, there were no significant differences in performance between the Mg-PC@Dop-HA/F127 and Normal groups. These findings indicate superior tendon tissue healing in the Mg-PC@Dop-HA/F127 group.
3.8.2. Histological analysis
To observe the microstructure of the repaired tissues, we stained and analyzed local tissues (Fig. 8). By week 4, all groups displayed incomplete tendon healing, with immature granulation tissue containing limited collagen present at the tendon-bone interface. However, compared to the pure suture group and the Dop-HA/F127 group, the Mg-PC@Dop-HA/F127 group exhibited reduced inflammatory cell infiltration and more regular tendon tissue morphology and arrangement. By week 8, the repair process had substantially progressed, and the Mg-PC@Dop-HA/F127 group demonstrated more organized collagen fibers compared to the other groups (Fig. 8A). According to maturation scoring criteria, the Mg-PC@Dop-HA/F127 group exhibited significantly increased tendon maturity at week 8, compared to the pure suture group and the Dop-HA/F127 group (P < 0.05) (Fig. 8D) [35]. Toluidine blue staining was employed to assess fibrocartilage in tendon-to-bone sections, as shown in Fig. 8B. The fibrocartilage area at the tendon-bone interface increased over time. At 4 weeks post-operation, the fibrocartilage displayed irregular shapes and immature structure. However, by 8 weeks, the tissue structure had matured and become complete, with standard cell shapes and tissue structure. The fibrocartilage area in the Mg-PC@Dop-HA/F127 group was significantly larger than that in the suture repair group and the Dop-HA/F127 group at 8 weeks (Fig. 8D). These results highlight the superior ability of the Mg-PC@Dop-HA/F127 group to repair RCT. Magnesium played a crucial role in recruiting stem cells and inducing cartilage regeneration, effectively promoting the reconstruction of the tendon-bone interface. Furthermore, the analysis of histological sections stained with picrosirius red and viewed under polarized light revealed increased deposition of fibrous tissue (bright areas) in the Mg-PC@Dop-HA/F127 group compared to the control and Dop-HA/F127 groups (Fig. 8C). After 8 weeks, the collagen tissue in the Mg-PC@Dop-HA/F127 group exhibited significant improvement and closely resembled that of the normal group.
Fig. 8.
Morphological analysis of newly formed tendon-bone interface tissues after treatment. A) Representative images of hematoxylin and eosin staining (H&E) and toluidine blue/fast green staining (B) of slices in rats treated with normal, suture, Dop-HA/F127, and Mg-PC@Dop-HA/F127 groups, respectively. T: tendon; I: Interface; B: Bone. C) Sirius red stained photos of different treatment groups; D) Bone-tendon interface maturing score of different treatment groups (n = 3) and E) the area of newly formed fibrocartilage in the different treatment groups (n = 3). Scale bars indicate 200 μm for 40 × and 100 μm for 100 × .*p < 0.05, **p < 0.01.
To elucidate the mechanism behind the effects of the Mg-PC@Dop-HA/F127 hydrogel on tendon-bone interface repair in vivo, we conducted immunohistochemical staining (Fig. 9A). At the designated time points (4 and 8 weeks), the expression levels in the Mg-PC@Dop-HA/F127 group exceeded those in both the control (suture only) and Dop-HA/F127 groups. Immunohistochemical staining of Col II and Sox9, along with their quantitative analysis, confirmed the upregulation of chondrocyte matrix synthesis (4w and 8w, Fig. 9D–F). As shown in Fig. 9A–C, at 4 weeks, the expression of Trpm7 and Hif-1α closely approximated that of the normal group in the suture group (97.8 ± 5.5% and 81.6 ± 6.3%) and the Dop-HA/F127 group (86.9 ± 4.5% and 94.9 ± 4.2%). In contrast, the Trpm7 and Hif-1α expression in the Mg-PC@Dop-HA/F127 group was significantly higher than in the other three groups (168.6 ± 8.2% and 174.5 ± 8.8%). At 8 weeks, the expression of Trpm7 and Hif-1α across the groups displayed a similar trend to that observed at 4 weeks. In Fig. 9D–F, it can be observed that at 4 weeks, the expression of Col II (195.1 ± 8.0%) and Sox9 (198.3 ± 9.8%) in the Mg-PC@Dop-HA/F127 group significantly exceeded that in the other three groups. However, at 8 weeks, the rates of Col II and Sox9 positive cells in the Mg-PC@Dop-HA/F127 group (140.0 ± 10.7% and 124.4 ± 8.4%) decreased compared to those at 4 weeks. This suggests that the hydrogel substantially increased the expression of cartilage indicators (Col II and Sox9) during the early stages of implantation. Over time, while the expression of these indicators remained elevated in the Mg-PC@Dop-HA/F127 group relative to the suture-only group, it decreased compared to the early stage (4 weeks). This phenomenon may be attributed to further repair remodeling of the tendon-bone interface, resulting in chondrocyte gradient mineralization. These results collectively indicate that the Mg-PC@Dop-HA/F127 hydrogel possesses the capability to stabilize and continuously release Mg2+ in vivo. It maintains a high magnesium-ion environment within cells, induces high intracellular Trpm7 expression, subsequently promotes Hif-1α expression, accelerates cartilage production, and enhances tendon-bone interface repair.
Fig. 9.
Immunohistochemical staining of newly formed tendon bone interface tissues after treatment. A) Immunohistochemical staining images and associated expression levels of Trpm7 and Hif-1α at the tendon bone interface in different treatment groups (n = 3) at 4 and 8 weeks after treatment (B&C). D) Immunohistochemical staining images and associated expression levels of Col II and Sox9 at the tendon bone interface in different treatment groups (n = 3) at 4 and 8 weeks after treatment (E&F). Scale bars indicate 200 μm for 40 × and 100 μm for 100 × .*p < 0.05, **p < 0.01.
For a more comprehensive understanding of Mg-PC@Dop-HA/F127's efficacy in promoting stem cell migration and creating a conducive microenvironment, we conducted immunofluorescence staining to assess its migration-promoting capacity (CD44, a representative stem cell marker) and in vivo immunomodulatory properties (F4/80, a representative surface marker for macrophages, and CD206, a surface marker for anti-inflammatory M2 macrophages, co-staining). As depicted in Fig. 10A and B, the number of CD44 positive cells in the Mg-PC@Dop-HA/F127 group (299.8 ± 20.4% and 326.6 ± 24.6%) significantly exceeded that in the remaining three groups at 4 and 8 weeks. This suggests that the Mg-PC@Dop-HA/F127 hydrogel maintains an optimal Mg2+ concentration through controlled release, providing enhanced cellular support for differentiation into chondrocytes and consequently facilitating stem cell migration to the tendon-bone interface [45]. For the analysis of inflammatory immune responses, we selected the early repair stage (4 weeks) as the observation window, and we co-stained macrophages with F4/80 and CD206 (Fig. 10B). At week 4, the number of CD206-positive cells in the Mg-PC@Dop-HA/F127 group (188.3 ± 7.1%) was significantly higher than that in the other groups, suggesting that the combined release of procyanidins and Mg2+ more effectively induced the differentiation of M2-type macrophages within the immune microenvironment. This reduction in inflammation accelerated tissue regeneration.
Fig. 10.
Immunofluorescence (CD44 and CD206) staining results. A) Immunofluorescence and relative intensity quantification(C) of CD44 at the tendon bone interface at 4 and 8 weeks after treatment in different groups; B) Immunofluorescence and relative intensity quantification(D) of CD206 at the tendon bone interface at 4 weeks after treatment in different groups. **p < 0.01, ***p < 0.001,"ns” represents no significant difference.
4. Discussion
The normal tendon-bone insertion is a series of highly specifically ordered tissues whose main function is to transmit complex mechanical stress from soft tissue to bone. It contains 4 different types of soft tissue: tendon/ligament, non-calcified fibrocartilage, calcified fibrocartilage, and bone tissue [2]. At the same time, tendon-bone interface injury is accompanied by local inflammatory reactions. For tendon injuries such as rotator cuff tears and anterior cruciate ligament rupture, surgical reconstruction is a regular method. Although reconstructive surgery restores the position of the tendon anatomically, it can only provide effective mechanical strength after firm tendon-bone healing is achieved. Despite advances in repair technology, secure tendon bone healing after reconstruction remains a challenge. This is one of the main reasons why we designed a composite hydrogel for tendon-bone interface reconstruction in this study.
In this study, Mg-PC was synthesized through a straightforward one-pot hydrothermal reaction. The protonation of polyphenolic groups within Procyanidins initially obstructed their coordination with Mg in an acidic liquid environment, allowing for thorough solution mixing. Subsequently, the solution's pH was adjusted from acidic to alkaline, resulting in the deprotonation of hydroxides. Consequently, free Mg2+ ions and polyphenolic groups formed more extensive and structurally intricate aggregates through continuous combination and coordination. Given the excellent performance of magnesium ions in inhibiting cartilage and tendon calcification and inducing chondrogenic differentiation of stem cells, we used PC to control release the of magnesium ions in the construction of the material [46]. In the composite hydrogel system, incorporating a block copolymer (F127) in the Dop-HA/F127 design imparts temperature-sensitive properties. At lower temperatures, Mg-PC enhances the hydrogel's cross-linking through coordination bonding. Subsequently, after the phase transition at elevated temperatures, the entanglement of block molecular chains reinforces the hydrogel's strength and the encapsulation of Mg-PC nanoparticles. This assembly strategy significantly enhances the performance of the Mg-PC@Dop-HA/F127 hydrogel, rendering the degradation and release of Mg-PC smoother and more stable (Fig. 2A). At the same time, the addition of Mg-PC nanoparticles increased the tensile strength of the hydrogel system ((Fig. 2I). After adding 10% w/v Mg-PC, the tensile strength substantially increased, indicating that introducing inorganic components is crucial for enhancing hydrogel mechanical properties. However, when 20% w/v Mg-PC was added, the hydrogel stretched and broke quickly, with lower strength than the 10% w/v group. This phenomenon was attributed to the failure of the hydrogel network's energy dissipation mechanism due to excessive inorganic component content.
Rotator cuff injuries often trigger an inflammatory response, leading to the generation of ROS (Reactive Oxygen Species) in the local environment, which can have adverse effects and impede the regeneration of the bone-tendon interface [38]. Procyanidin (PC) is one of the strongest and most effective free radical scavengers discovered so far, with anti-inflammatory, anti-aging, antioxidant, and antibacterial effects. It is introduced into the hydrogel system to scavenge locally generated reactive oxygen species, thus providing a suitable microenvironment for tendon-bone interface healing. Besides. the state and function of local immune cells play a crucial role in tissue repair processes [39]. Compared to other groups, the presence of magnesium ions induces a more significant differentiation toward M2-type macrophages in this study. In our findings, Mg-PC@Dop-HA/F127 extract effectively promoted CD206 and Arg genes in cell culture. These findings undoubtedly indicate that Mg2+ dissolved in the extract is responsible for the improved immune environment.
We evaluated the chondrogenic differentiation-promoting properties of the hydrogel by co-treating BMSC microspheres with hydrogel degradation products and chondrogenic induction medium. Previous studies have shown that the Trpm7 channel, as a divalent metal ion channel, plays a crucial role in promoting the entry of Mg2+ into cells. At the same time, Mg2+ can promote cartilage differentiation by activating Hif-1α. To further study the role of Mg2+ in the chondrogenesis process and its activation of Hif-1α, we participated in the study using the Trpm7 channel blocker 2-aminoethyl diphenylborate (2APB). Through toluidine blue staining, Western blot, qPCR experiments, and immunofluorescence staining experiments, it was verified that Mg-PC@Dop-HA/F127 can promote the differentiation of BMSCs into chondrocytes. These findings confirmed our previous conjecture: Mg-PC@Dop-HA/F127 hydrogel stably releases Mg2+, enters bone marrow mesenchymal stem cells through the Trpm7 channel, activates Hif-1α, and thereby promotes the secretion of Sox9 in the nucleus. The enhanced expression of Sox9, in turn, further enhanced the expression of Acan and Col Ⅱproteins. This supports the idea that hydrogels can promote the differentiation of BMSCs into chondrocytes and promote tendon-bone interface remodeling in vivo.
Based on the results of these in vitro studies, we expect that Mg-PC@Dop-HA/F127 can be evaluated in vivo to obtain the best effect in situ tendon-bone healing. At 4 and 8 weeks post-operation, we harvested the rats' shoulders for micro-CT analysis. The mg- PC@Dop-HA/F127 group exhibited superior morphology at the healing interface compared to the other groups at both postoperative weeks 4 and 8. We verified that the maximum stress value was close to the normal group after 4 and 8 weeks of injection of Mg-PC@Dop-HA/F127, and the stress was more even distribution of stress at the tendon-bone interface, reducing the risk of tendon re-rupture due to excessive localized stress. From the newly formed bone tissue sections, we can conclude that Mg-PC@Dop-HA/F127 showed the strongest ability to manipulate these key events related to chondrogenesis and immune regulation. In general, the composite hydrogel we designed promoted tendon-bone interface healing in vivo experiments and showed consistent results in in vitro experiments.
In summary, as an endogenous bioavailable therapeutic ion, Mg2+ has multiple functions in regulating cell proliferation and differentiation. The Mg-PC nanoflower-shaped particles we synthesized are matched with Dop-HA and F127 hydrogels to form a hydrogel system that can sustainably release Mg2+. We have used it in vivo and in vitro experiments to verify its effect on repairing the tendon-bone interface. This provides a novel design for bioactive hydrogels in tendon-bone therapy.
5. Conclusions
In this study, we demonstrated the exceptional qualities of Mg-PC@Dop-HA/F127 hydrogels, which consisted of Dop-HA and F127 two-component hydrogels infused with Mg-PC. These hydrogels exhibited impressive injectability, rapid self-healing capabilities, and strong adhesion properties. These characteristics facilitated the injection process and enabled the hydrogel to adapt seamlessly to irregularly shaped supraspinatus muscle implantation sites. Mg-PC@Dop-HA/F127 stood out for its ability to deliver a sustained, controlled release of both Mg and PC. This controlled release exerted anti-inflammatory effects and supported cartilage regeneration precisely at the tendon-bone interface. With its anti-inflammatory and antioxidant properties, PC creates a protective environment for stem cells during the healing of rotator cuff injuries. It achieved this by modulating the immune microenvironment, polarizing macrophages toward the M2 phenotype, and accelerating the repair of the tendon-bone interface. Furthermore, introducing the polyphenol system enabled the controlled release of magnesium ions. These ions played a pivotal role in inducing the early migration of peripheral MSCs to the bone-tendon interface, thereby promoting their differentiation. Guided by magnesium ions and microenvironmental cues, MSCs at the tendon-bone interface underwent chondrogenic differentiation, significantly enhancing the healing process of the bone-tendon interface. This chondrogenic differentiation was mediated by magnesium ions, which facilitated Mg2+ entry into cells through the TRPM7 channel protein, subsequently upregulating hypoxia-inducible factor-1α (Hif-1α) and promoting the synthesis of the cartilage matrix.
In summary, we successfully engineered a hydrogel system with nano-bioactive properties to enhance the healing of the tendon-bone interface following surgical reconstruction of rotator cuff injuries. This innovative composite hydrogel introduces a novel approach to expedite recovery after reconstruction surgery while mitigating local inflammatory responses.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers: 82302639,81974327,81974328 and 82372358], National Students’ Platform for Innovation and Entrepreneurship Training Program of China [grant number: No.202212121004], Natural Science Funds for Distinguished Young Scholar of Guangdong province [grant number:2022B1515020044] and the Natural Science Foundation of Guangdong Province [grant number: 2022A1515011101].
CRediT authorship contribution statement
Jintao Li: Writing – original draft, Formal analysis, Data curation, Conceptualization. Haolin Ke: Software, Formal analysis, Data curation, Conceptualization. Xiangcheng Lei: Software, Resources, Formal analysis, Data curation. Jiexin Zhang: Validation, Data curation, Conceptualization. Zhicheng Wen: Software, Methodology, Investigation, Formal analysis. Zhisheng Xiao: Resources, Project administration, Methodology. Huabin Chen: Software, Resources, Investigation. Juncheng Yao: Software, Methodology. Xuan Wang: Validation, Resources. Zhengnong Wei: Software, Methodology. Hongrui Zhang: Validation, Investigation. Weilun Pan: Visualization, Validation, Methodology. Yan Shao: Visualization, Supervision, Project administration. Yitao Zhao: Writing – original draft, Visualization, Supervision, Project administration. Denghui Xie: Writing – review & editing, Visualization, Supervision, Funding acquisition. Chun Zeng: Writing – review & editing, Supervision, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2024.02.024.
Contributor Information
Yan Shao, Email: shaoyan_n@163.com.
Yitao Zhao, Email: zyt36634@163.com.
Denghui Xie, Email: smuspine@163.com.
Chun Zeng, Email: zengdavid@126.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Font Tellado S., Balmayor E.R., Van Griensven M. Strategies to engineer tendon/ligament-to-bone interface: biomaterials, cells and growth factors. Adv. Drug Deliv. Rev. 2015;94:126–140. doi: 10.1016/j.addr.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Shaw H.M., Benjamin M. Structure-function relationships of entheses in relation to mechanical load and exercise. Scand. J. Med. Sci. Sports. 2007;17(4):303–315. doi: 10.1111/j.1600-0838.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 3.Atesok K., Doral M.N., Karlsson J., Egol K.A., Jazrawi L.M., Coelho P.G., Martinez A., Matsumoto T., Owens B.D., Ochi M., Hurwitz S.R., Atala A., Fu F.H., Lu H.H., Rodeo S.A. Multilayer scaffolds in orthopaedic tissue engineering. Knee Surg. Sports Traumatol. Arthrosc. J. ESSKA. 2016;24(7):2365–2373. doi: 10.1007/s00167-014-3453-z. [DOI] [PubMed] [Google Scholar]
- 4.Lim W.L., Liau L.L., Ng M.H., Chowdhury S.R., Law J.X. Current progress in tendon and ligament tissue engineering. Tissue Eng. Regen. Med. 2019;16(6):549–571. doi: 10.1007/s13770-019-00196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Zhang X., Huang S., Liu Y., Fu B.S., Mak K.K., Blocki A.M., Yung P.S., Tuan R.S., Ker D.F.E. Engineering multi-tissue units for regenerative Medicine: bone-tendon-muscle units of the rotator cuff. Biomaterials. 2021;272 doi: 10.1016/j.biomaterials.2021.120789. [DOI] [PubMed] [Google Scholar]
- 6.Rossetti L., Kuntz L.A., Kunold E., Schock J., Müller K.W., Grabmayr H., Stolberg-Stolberg J., Pfeiffer F., Sieber S.A., Burgkart R., Bausch A.R. The microstructure and micromechanics of the tendon-bone insertion. Nat. Mater. 2017;16(6):664–670. doi: 10.1038/nmat4863. [DOI] [PubMed] [Google Scholar]
- 7.Jiang X., Wu S., Kuss M., Kong Y., Shi W., Streubel P.N., Li T., Duan B. 3D printing of multilayered scaffolds for rotator cuff tendon regeneration. Bioact. Mater. 2020;5(3):636–643. doi: 10.1016/j.bioactmat.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang C., Teng Y., Geng B., Xiao H., Chen C., Chen R., Yang F., Xia Y. Strategies for promoting tendon-bone healing: Current status and prospects. Front. Bioeng. Biotechnol. 2023;11 doi: 10.3389/fbioe.2023.1118468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warth R.J., Dornan G.J., James E.W., Horan M.P., Millett P.J. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthrosc. J. Arthrosc. Relat. Surg. official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2015;31(2):306–320. doi: 10.1016/j.arthro.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Rashid M.S., Cooper C., Cook J., Cooper D., Dakin S.G., Snelling S., Carr A.J. Increasing age and tear size reduce rotator cuff repair healing rate at 1 year. Acta Orthop. 2017;88(6):606–611. doi: 10.1080/17453674.2017.1370844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei T., Zhang T., Ju W., Chen X., Heng B.C., Shen W., Yin Z. Biomimetic strategies for tendon/ligament-to-bone interface regeneration. Bioact. Mater. 2021;6(8):2491–2510. doi: 10.1016/j.bioactmat.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang P., Liu X., Guo P., Li X., He Z., Li Z., Stoddart M.J., Grad S., Tian W., Chen D., Zou X., Zhou Z., Liu S. Effect of cyclic mechanical loading on immunoinflammatory microenvironment in biofabricating hydroxyapatite scaffold for bone regeneration. Bioact. Mater. 2021;6(10):3097–3108. doi: 10.1016/j.bioactmat.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou J., Yang W., Cui W., Li C., Ma C., Ji X., Hong J., Qu Z., Chen J., Liu A., Wu H. Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon-bone healing. J. Nanobiotechnol. 2023;21(1):14. doi: 10.1186/s12951-023-01778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu C., Qiu J., Thomopoulos S., Xia Y. Augmenting tendon-to-bone repair with functionally graded scaffolds. Adv. Healthcare Mater. 2021;10(9) doi: 10.1002/adhm.202002269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chimene D., Kaunas R., Gaharwar A.K. Hydrogel Bioink Reinforcement for additive manufacturing: a Focused review of emerging strategies. Advanced materials (Deerfield Beach, Fla.) 2020;32(1) doi: 10.1002/adma.201902026. [DOI] [PubMed] [Google Scholar]
- 16.Yuan X., Zhao Y., Li J., Chen X., Lu Z., Li L., Guo J. Citrate-based mussel-inspired magnesium whitlockite composite adhesives augmented bone-to-tendon healing. J. Mater. Chem. B. 2021;9(39):8202–8210. doi: 10.1039/d1tb01710a. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Q., Wang L., Liu Z., Su J., Tang Y., Tan P., Zhu X., Zhang K., Ma X., Jiang J., Zhao J., Lin H., Zhang X. Canine ACL reconstruction with an injectable hydroxyapatite/collagen paste for accelerated healing of tendon-bone interface. Bioact. Mater. 2023;20:1–15. doi: 10.1016/j.bioactmat.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lv B., Lu L., Hu L., Cheng P., Hu Y., Xie X., Dai G., Mi B., Liu X., Liu G. Recent advances in GelMA hydrogel transplantation for musculoskeletal disorders and related disease treatment. Theranostics. 2023;13(6):2015–2039. doi: 10.7150/thno.80615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toole B.P. Hyaluronan: from extracellular glue to pericellular cue, Nature reviews. Cancer. 2004;4(7):528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 20.Fraser J.R., Laurent T.C., Laurent U.B. Hyaluronan: its nature, distribution, functions and turnover. J.inter med. 1997;242(1):27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 21.Toole B.P. Hyaluronan in morphogenesis. Semin. Cell Dev. Biol. 2001;12(2):79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 22.Luo Y., Tan J., Zhou Y., Guo Y., Liao X., He L., Li D., Li X., Liu Y. From crosslinking strategies to biomedical applications of hyaluronic acid-based hydrogels: a review. Int. J. Biol. Macromol. 2023;231 doi: 10.1016/j.ijbiomac.2023.123308. [DOI] [PubMed] [Google Scholar]
- 23.Shamma R.N., Sayed R.H., Madry H., El Sayed N.S., Cucchiarini M. Triblock copolymer Bioinks in hydrogel three-dimensional printing for regenerative medicine: a Focus on Pluronic F127, tissue engineering. Part B, Reviews. 2022;28(2):451–463. doi: 10.1089/ten.TEB.2021.0026. [DOI] [PubMed] [Google Scholar]
- 24.Li S., Yang C., Li J., Zhang C., Zhu L., Song Y., Guo Y., Wang R., Gan D., Shi J., Ma P., Gao F., Su H. Progress in Pluronic F127 derivatives for application in wound healing and repair. Int. J. Nanomed. 2023;18:4485–4505. doi: 10.2147/ijn.S418534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y., Li J., Liu L., Wang Y., Ju Y., Zeng C., Lu Z., Xie D., Guo J. Zinc-based Tannin-modified composite Microparticulate scaffolds with Balanced antimicrobial activity and osteogenesis for Infected bone defect repair. Adv. Healthcare Mater. 2023;12(20) doi: 10.1002/adhm.202300303. [DOI] [PubMed] [Google Scholar]
- 26.Huang X., Ye Y., Zhang J., Zhang X., Ma H., Zhang Y., Fu X., Tang J., Jiang N., Han Y., Liu H., Chen H. Reactive oxygen species scavenging functional hydrogel delivers procyanidins for the treatment of Traumatic Brain injury in Mice. ACS Appl. Mater. Interfaces. 2022 doi: 10.1021/acsami.2c04930. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L., Wang Y., Li D., Ho C.T., Li J., Wan X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016;7(3):1273–1281. doi: 10.1039/c5fo01244a. [DOI] [PubMed] [Google Scholar]
- 28.Li X., Dai B., Guo J., Zhu Y., Xu J., Xu S., Yao Z., Chang L., Li Y., He X., Chow D.H.K., Zhang S., Yao H., Tong W., Ngai T., Qin L. Biosynthesized Bandages Carrying magnesium oxide nanoparticles induce cortical bone formation by modulating endogenous periosteal cells. ACS Nano. 2022;16(11):18071–18089. doi: 10.1021/acsnano.2c04747. [DOI] [PubMed] [Google Scholar]
- 29.Luo Y., Zhang C., Wang J., Liu F., Chau K.W., Qin L., Wang J. Clinical translation and challenges of biodegradable magnesium-based interference screws in ACL reconstruction. Bioact. Mater. 2021;6(10):3231–3243. doi: 10.1016/j.bioactmat.2021.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S., Chang Y.Y., Lee J., Madhurakkat Perikamana S.K., Kim E.M., Jung Y.H., Yun J.H., Shin H. Surface engineering of titanium alloy using metal-polyphenol network coating with magnesium ions for improved osseointegration. Biomater. Sci. 2020;8(12):3404–3417. doi: 10.1039/d0bm00566e. [DOI] [PubMed] [Google Scholar]
- 31.Chen S., Yao W., Wang H., Wang T., Xiao X., Sun G., Yang J., Guan Y., Zhang Z., Xia Z., Li M., Tao Y., Hei Z. Injectable electrospun fiber-hydrogel composite sequentially releasing clonidine and ropivacaine for prolonged and walking regional analgesia. Theranostics. 2022;12(11):4904–4921. doi: 10.7150/thno.74845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arjama M., Mehnath S., Jeyaraj M. Self-assembled hydrogel nanocube for stimuli responsive drug delivery and tumor ablation by phototherapy against breast cancer. Int. J. Biol. Macromol. 2022;213:435–446. doi: 10.1016/j.ijbiomac.2022.05.190. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Z., Song X., Jiang Y., Yao J., Jiang Y., Li Z., Dai F. Chemical structure and antioxidant activity of a neutral polysaccharide from Asteris Radix et Rhizoma. Carbohydrate Polym. 2022;286 doi: 10.1016/j.carbpol.2022.119309. [DOI] [PubMed] [Google Scholar]
- 34.Kim H.S., Shin Y.M., Chung S., Kim D., Park D.B., Baek S., Park J., Kim S.Y., Kim D.H., Yi S.W., Lee S., Lee J.B., Ko J.Y., Im G.I., Kang M.L., Sung H.J. Cell-membrane-derived nanoparticles with Notch-1 Suppressor delivery promote hypoxic cell-cell Packing and inhibit angiogenesis acting as a two-Edged Sword. Adv. Mater. (Deerfield Beach, Fla.) 2021;33(40) doi: 10.1002/adma.202101558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang R., Li G., Zhuang C., Yu P., Ye T., Zhang Y., Shang P., Huang J., Cai M., Wang L., Cui W., Deng L. Gradient bimetallic ion-based hydrogels for tissue microstructure reconstruction of tendon-to-bone insertion. Sci. Adv. 2021;7(26) doi: 10.1126/sciadv.abg3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Büchler P., Ramaniraka N.A., Rakotomanana L.R., Iannotti J.P., Farron A. A finite element model of the shoulder: application to the comparison of normal and osteoarthritic joints. Clin. Biomech. 2002;17(9–10):630–639. doi: 10.1016/s0268-0033(02)00106-7. [DOI] [PubMed] [Google Scholar]
- 37.Hu Y., Pan Z.J., Liao W., Li J., Gruget P., Kitts D.D., Lu X. Determination of antioxidant capacity and phenolic content of chocolate by attenuated total reflectance-Fourier transformed-infrared spectroscopy. Food Chem. 2016;202:254–261. doi: 10.1016/j.foodchem.2016.01.130. [DOI] [PubMed] [Google Scholar]
- 38.Khan K.M., Cook J.L., Bonar F., Harcourt P., Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management, Sports medicine (Auckland. N.Z.) 1999;27(6):393–408. doi: 10.2165/00007256-199927060-00004. [DOI] [PubMed] [Google Scholar]
- 39.Ye J., Xie C., Wang C., Huang J., Yin Z., Heng B.C., Chen X., Shen W. Promoting musculoskeletal system soft tissue regeneration by biomaterial-mediated modulation of macrophage polarization. Bioact. Mater. 2021;6(11):4096–4109. doi: 10.1016/j.bioactmat.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu H., Zhu Y., Hsiao A.W., Xu J., Tong W., Chang L., Zhang X., Chen Y.F., Li J., Chen W., Zhang Y., Chan H.F., Lee C.W. Bioactive glass-elicited stem cell-derived extracellular vesicles regulate M2 macrophage polarization and angiogenesis to improve tendon regeneration and functional recovery. Biomaterials. 2023;294 doi: 10.1016/j.biomaterials.2023.121998. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J., Wang J.H. Kartogenin induces cartilage-like tissue formation in tendon-bone junction. Bone Res. 2014;2 doi: 10.1038/boneres.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martínez Sánchez A.H., Omidi M., Wurlitzer M., Fuh M.M., Feyerabend F., Schlüter H., Willumeit-Römer R., Luthringer B.J.C. Proteome analysis of human mesenchymal stem cells undergoing chondrogenesis when exposed to the products of various magnesium-based materials degradation. Bioact. Mater. 2019;4:168–188. doi: 10.1016/j.bioactmat.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu D., Qu J., Sun L., Li Q., Ling H., Yang N., Ma T., Wang Q., Li M., Zhang K., Li Z. Ca2+/Mg2+ homeostasis-related TRPM7 channel mediates chondrocyte hypertrophy via regulation of the PI3K-Akt signaling pathway. Mol. Med. Rep. 2017;16(4):5699–5705. doi: 10.3892/mmr.2017.7300. [DOI] [PubMed] [Google Scholar]
- 44.Cao B., Li Z., Peng R., Ding J. Effects of cell-cell contact and oxygen tension on chondrogenic differentiation of stem cells. Biomaterials. 2015;64:21–32. doi: 10.1016/j.biomaterials.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 45.Shen J., Chen B., Zhai X., Qiao W., Wu S., Liu X., Zhao Y., Ruan C., Pan H., Chu P.K., Cheung K.M.C., Yeung K.W.K. Stepwise 3D-spatio-temporal magnesium cationic niche: Nanocomposite scaffold mediated microenvironment for modulating intramembranous ossification. Bioact. Mater. 2021;6(2):503–519. doi: 10.1016/j.bioactmat.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.No Y.J., Castilho M., Ramaswamy Y., Zreiqat H. Role of biomaterials and controlled Architecture on tendon/ligament repair and regeneration. Adv. Mater. 2020;32(18) doi: 10.1002/adma.201904511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.