Highlights
-
•
This study explores the sociodemographic factors of Kyasanur forest disease (KFD) in humans.
-
•
Our results show that most KFD cases and deaths occur in the 15-64 years age group.
-
•
More deaths due to KFD have been reported in men than women.
-
•
We found statistically significant differences in KFD prevalence by state.
-
•
The underreporting of sociodemographic data limits understanding of KFD impact.
Keywords: Kyasanur forest disease, Sociodemographic factors, KFD cases, KFD deaths, Prevalence
Abstract
Objectives
Kyasanur forest disease (KFD) is a tick-borne disease in India affecting humans and two local non-human primate species. A critical knowledge gap in the scientific literature is the lack of information on how people's sociodemographic factors influence KFD occurrence.
Methods
We analyzed available data on KFD from three data sources: (a) 104 peer-reviewed articles using keyword searches on PubMed Central and Google Scholar, (b) 116 Program for Monitoring Emerging Diseases reports, and (c) an acute febrile illness surveillance data set on KFD from a report by the government of India. We performed statistical analyses to calculate the prevalence of KFD by state and differences in KFD cases by sex and age group.
Results
All three data sets used indicate that KFD cases and deaths have occurred predominantly in the 15-64 years age group (literature: 87% cases and 95% deaths, Program for Monitoring Emerging Diseases: 78% cases and 78% deaths, acute febrile illness: 96% cases [no breakdown for acute febrile illness death data]). Data reporting varies across states and is non-standardized.
Conclusions
The inconsistent reporting of sociodemographic data on KFD in India has created a gap in our understanding of its impact on different social groups. Collecting and reporting data on sociodemographic factors is critical to understanding the epidemiology of KFD and designing effective public health interventions.
Introduction
Kyasanur forest disease (KFD) is a tick-borne disease that is prevalent along the western coast of India since its discovery in 1957. Although it has been expanding into new areas [1,2], it continues to be a neglected disease [1], [2], [3]. KFD virus (KFDV) is transmitted by Haemaphysalis spinigera and Haemaphysalis turturis tick species, which serve as the primary vectors to humans [3]. The disease is caused by the KFDV, a member of the Flaviviridae group of viruses [4]. Although KFD has only occurred in India, KFDV is part of the Russian spring-summer encephalitis group of viruses that include the Alkhumra virus and Omsk hemorrhagic fever virus [1,4].
KFD affects humans by causing symptoms such as fever, myalgia, hemorrhage in the mouth and gastrointestinal tract, and encephalitis, along with neurologic complications, bleeding manifestations, and persistent shock, which can lead to death [4,5]. In addition, a history of tick bites and travel to forested areas are considered part of the case definition for KFD. The methods used to diagnose KFD cases have changed over time, such as virus isolation, hemagglutination inhibition, complement fixation and neutralization tests, real-time polymerase chain reaction (PCR), real-time reverse transcriptase PCR, and immunoglobulin M enzyme-linked immunosorbent assay [5,6]. The combination of these physical symptoms and the use of sensitive reverse transcription–PCR tests have recently been used to confirm KFD cases. However, across the sources used in this study and over the 60+ year period, the estimates have not always been reported as being “laboratory confirmed” cases, impacting the epidemiological analyses of KFD. From 1957 until 2017, an estimated 9594 KFD cases have been reported within several districts in India along the western coast [1]. KFD also affects two local non-human primates (i.e. Macaca radiata and Semnopithecus entellus). Between 1957 and 2020, at least 3314 monkey deaths have been attributed to KFD [7]. The KFD season typically occurs every year between December and May, after the end of the monsoons [1].
Social determinants of health are conditions that affect a wide range of health and quality-of-life risks and outcomes where people live, learn, work, and play [8]. Sociodemographic data are a combination of social and demographic data that define people in a specific group or population, such as age, sex, occupation, education level, and income [8]. Sociodemographic data provide crucial information to help researchers determine how infectious diseases can spread to different population groups and design appropriate public health interventions accordingly [9,10]. Sociodemographic factors play critical roles in the transmission of vector-borne diseases (VBDs) [9] and can influence treatment access and health outcomes [10]. Social determinants and sociodemographic factors are critical to investigate in the context of VBDs. For instance, in the United States, the incidence of Lyme disease was found to be highest in counties with relatively higher proportions of White and educated persons and lower poverty and crime rates [11]. The authors also found that the incidence of human monocytic ehrlichiosis was highest in counties with relatively higher proportions of White and less educated persons, higher unemployment rates, and lower crime rates [11].
Previous research on KFD has significantly advanced our knowledge of the genetic structure of the virus [12], the clinical characteristics of both the virus and the disease [5], and information related to vaccines [13]. However, despite these contributions, since the disease's discovery, much of the research on KFD has predominantly concentrated on its virology and epidemiology, with limited attention given to investigating the socioeconomic determinants and risk factors associated with the disease in India [14]. Exploring publicly available databases that contain patients’ social and demographic information offers a valuable avenue for assessing the influence of these factors on the epidemiology of KFD. However, the absence of comprehensive data examination has left an important gap in understanding the disease impact across social and demographic groups, hindering the development of effective public health interventions. This study seeks to address some of these limitations by providing insight into the sociodemographic information on cases and deaths associated with KFD in India using a retrospective review approach.
Materials and methods
Data
We conducted a retrospective analysis of openly available data on KFD to determine the sociodemographic factors of this disease. Information for this study was collected primarily from the Program for Monitoring Emerging Diseases (Pro-MED) and the scientific literature. Pro‐MED is a publicly available, integrated, global disease surveillance reporting system that reports emerging diseases and outbreaks worldwide [15]. We reviewed 116 reports of KFD on Pro-MED. We also used the search term “Kyasanur forest disease” in the Pro-MED database and collected information on reported cases and deaths due to KFD. Case data on Pro-MED for KFD were available for 1983-1984, 2000, and 2011 to July 2022. Our period of analysis for this study is 1957-2022. We used the search terms “Kyasanur forest disease” and “KFD” to identify articles in PubMed Central and Google Scholar databases. We reviewed 104 peer-reviewed articles that were accessed from the University of Illinois Library and WorldCat database. Data from published literature were available for the periods 1957, 1959-1966, 1975, 1982-1988, 1990-1992, and 2000 to May 2020. We also obtained KFD data from 2014-2019 as part of an acute febrile illness (AFI) surveillance data set [16]. A technical report published by the Manipal Center of Viral Research [17] reported that human cases of KFD occurred in Tamil Nadu (TN) in 2017. Because we only used peer-reviewed literature sources and Pro-MED for our analysis, we did not use the results of this report. Our study examined the demographic data for KFD from 1957 until 2022. The methodology used in this study to collect and organize the KFD data is comparable to that in previously published studies [1,7]. Because we dealt with secondary data sources for this study, institutional review board approval for this study was not required.
The scientific literature and Pro-MED reported human cases and deaths due to KFD in the four-state region of Karnataka (KN), Kerala (KL), Maharashtra (MH), and Goa (GA) along the western coast of India. There have been reports of tick and monkey positivity for KFDV from TN [18]; however, we found no reported figures for human KFD cases in this state from the literature or Pro-MED. The AFI surveillance data set, which reported 32 KFD cases in TN from 2014 to 2019, is the sole source for human cases in the region [16] and has been cited by Naren Babu et al. in a table [19]. The Manipal Center of Viral Research technical report mentions KFD cases in TN; however, these were excluded from our analysis. We conducted separate analyses for the scientific literature, Pro-MED, and AFI surveillance data sets because they cover different periods. Furthermore, the way data are reported does not allow us to determine any case overlap. To avoid duplication, we analyzed each of the three data sources independently.
We also explored the population data from the official Indian census website for 2011 [20] and from the Registrar General and Census Commissioner of India [21] for 1951 to generate the population age and sex distributions.
Variables
Our study adopted an inclusive approach in which we comprehensively collected data from articles that contained sociodemographic information about affected patients, including age, sex, occupation, case and death details, and location. We prioritized data from studies reporting confirmed cases or deaths due to KFD over suspected cases or deaths. The final analysis was based on confirmed case and death data, as presented in Table 1. Unfortunately, the scientific literature, Pro-MED, and AFI data sets lacked other sociodemographic details, such as income, education level, and health care access.
Table 1.
Demographic information reported from Pro-MED, literature, and AFI surveillance data sources.
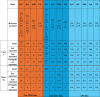 |
AFI: acute febrile illness; GA: Goa; KL: Kerala; KN: Karnataka; MH: Maharashtra; NA: not available; Pro-MED: Program for Monitoring Emerging Diseases; TN: Tamil Nadu.
We classified the population into three distinct groups: 0-14 (children), 15-64 (working-age adults), and 65 years and older (older adults), following a well-established practice in demographics literature [22]. This approach proved necessary because the data sources lacked consistent and detailed age breakdowns for KFD cases and deaths. Assumptions had to be made to input data when data were missing. (i) If data were given as an aggregate number and overlapped between different age groups, the total number of cases/deaths was divided equally between the age groups to estimate cases/deaths for each age group. For example, Kiran et al. [23] reported an aggregate number of cases among the >60 years age group from December 2013 to April 2014. For these final case estimates, the number of cases was divided by 2, half allocated for the 15-64 years group and the remaining for the 65 years and older age category [1]. (ii) In some cases, data on the sex or occupation of the KFD cases and deaths were missing. However, we input demographic data, such as sex or occupation, based on relevant descriptive text provided in the source when available. For example, Mourya et al. [18] reported, “six humans from Mole Hole village and Madhur colony in the Bandipur Tiger Reserve who handled and incinerated the sick monkeys were reported to have clinical signs and symptoms typical of KFD.” In this case, we assumed the sex of the affected persons to be male because men are most likely to have these jobs. We had to perform data imputations to perform the analyses when only partial data were available in the sources. From the literature data, sex was assumed for 0.3% of cases, and age was assumed for 8.7% of cases. No assumptions were made for death data from the literature. For Pro-MED data, sex was assumed for 5.6% of cases, and no assumptions were made for age or death data. Most sources report KFD cases and deaths occurring in villages and areas near forest fringes without any other information on the patient's occupation; thus, we mostly used the term villager under the job/occupation field in our database.
Because the 2011 census data are the latest available census information for India and there are no census data available for 2023, we used the population data in 2011 for the four-state KFD region to calculate the per capita case and death rates and used these data for statistical analyses. We used absolute values for cases and deaths from the data reported in the literature and Pro-MED to generate the graphs.
Analysis
We examined the distribution of KFD cases and deaths by age and sex by data sources separately. We calculated rates using the total cases or deaths with available demographic data as the denominator and excluded data without demographic information. Statistical analyses were conducted separately for the scientific literature and Pro-MED data using Microsoft Excel and R Studio [24]. Owing to its limited reporting period, we did not perform statistical analyses for the AFI surveillance data set. We used the Pearson chi-square test to determine if there were differences in KFD prevalence by state. The P-value for the significance tests was set as P <0.05. We also analyzed whether KFD cases differed by sex and age group for each state and the pooled four-state KFD region. When the expected cases were less than five, we used Fischer's exact test instead of the Pearson chi-square. We calculated the prevalence of KFD over the entire period that KFD case data are available for the respective states from the literature and Pro-MED data. We used the 2011 census data to calculate the expected prevalence values in performing chi-square or Fischer's exact tests. Because neither data source reported any cases in TN, we omitted this state from our analyses. We created data visualizations using Microsoft Excel to represent the data and the significant gaps in the available information.
Results
All KFD demographic data reported in Pro-MED, published literature, and the AFI surveillance data set are summarized in Table 1. Pro-MED had more data for cases and deaths in recent years, whereas we found more data from the published literature in the early years of KFD reporting. Neither of the three sources reported demographic data clearly and consistently. For example, in the scientific literature, demographic data are often grouped, making it difficult to parse out demographic information of individual patients for analyses. Pro-MED mainly reports cases and deaths; however, there are fewer instances when demographic information of individuals affected with KFD are reported. The AFI surveillance data set provides demographic information on most cases but no information on deaths.
Data from the literature suggested approximately 6971 human cases of KFD, whereas Pro-MED reported an estimated 1437 cases within the four-state KFD region. The AFI surveillance data set indicated 910 KFD cases occurring between 2014 and 2019 across five states. However, it is important to note that not all cases and deaths in these sources had available demographic data, potentially leading to underreporting and lower estimates. Aggregated numbers in peer-reviewed papers often cover large time frames, resulting in case estimates varying from one article to another. We also observed that the breakdown of data by demographics is better provided for cases than for deaths across the three data sources. Data gaps across all data sources have been evident for several years, exacerbating the scarcity of information on KFD. The impact of the COVID-19 pandemic from 2020 to 2022 led to even more substantial underreporting of KFD cases, deaths, and demographic details.
Distribution of cases and deaths by sex and age groups
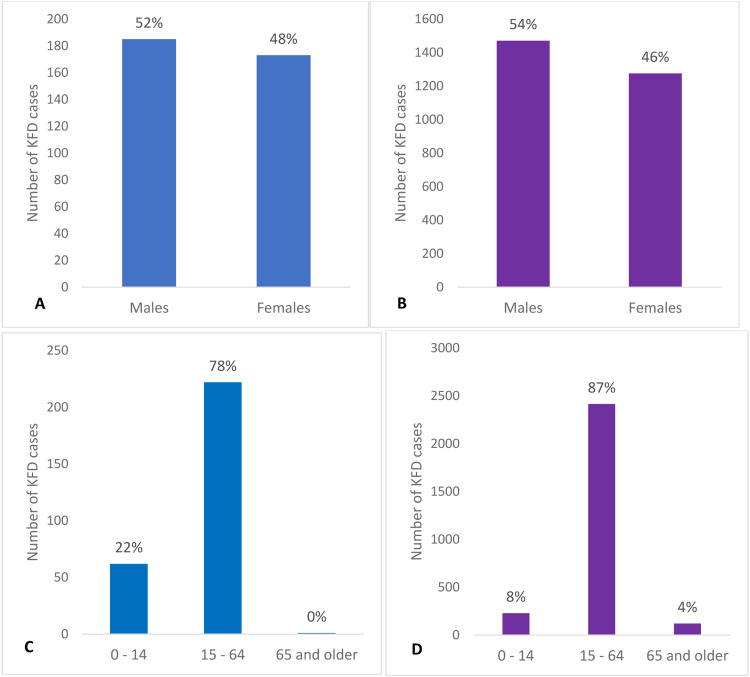
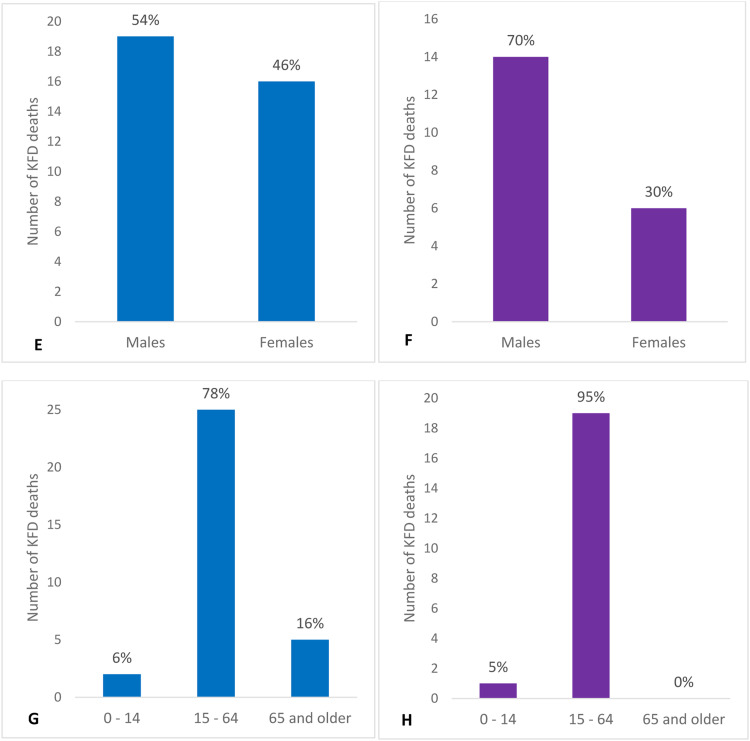
In Figure 1 (Figures 1a-h), we present the number and percentage of cases and deaths by sex and age groups from Pro-MED and literature, respectively.
Figure 1.
Number and percentage of KFD cases and deaths by sex and age group as determined from Pro-MED and literature data. KFD: Kyasanur forest disease; Pro-MED: Program for Monitoring Emerging Diseases.
In the cases for which age was reported in the KFD region, literature data indicate that approximately 87% of cases occurred in the 15-64 years age group, 8% in the 0-14 years age group, and 4% in the 65 years and older age group. Pro-MED data indicate that 78% of cases occurred in the 15-64 age group, 22% in the 0-14 age group, and less than 1% in the 65 and older age group. The AFI surveillance data set indicates that between 2014 and 2019, 96% of the cases occurred in the 15-64 years age group and 4% in the 0-14 years age group.
Data from Pro-MED and the scientific literature reveal that most deaths due to KFD have occurred among individuals aged 15 to 64 years. The 65 years and older age category provides an interesting comparison of differences in coverage across two sources: Pro-MED reports one case and five deaths. In contrast, the literature reports 121 cases with zero deaths, indicating a severe gap in data reporting. The AFI data set only reports 10 deaths between 2014 and 2019 but does not provide any age information for deaths. The limited data for the 65 years and older age group might be because of underreporting and lower prevalence of KFD within this age group.
In the KFD region, cases seem to be more common in men than in women. The literature data suggest that 54% of the cases occurred in males and 46% in females, whereas Pro-MED data report that 52% occurred in males and 48% in females. The AFI data, however, showed that 46% of cases in all five states were in males and 54% in females. We also found more deaths in males than in females in the literature and Pro-MED data (literature data: 70%, Pro-MED data: 54%, and AFI data set: no sex information for deaths).
We performed statistical analyses separately for literature and Pro-MED data sources for KN, KL, GA, and MH. On the prevalence of KFD in the four-state region, we found a statistical difference among states from both literature data (P <0.001, df = 3, χ2 = 13,887.219) and Pro-MED data (P <0.001, df = 3, χ2 = 4555.702). In both data sources, the prevalence of KFD is highest in GA, followed by KN and KL, and the least in MH.
For both literature data (P <0.001, df = 2, χ2 = 523.239) and Pro-MED data (P <0.001, df = 2, χ2 = 24.199), we found that persons in the 15-64 years age group are at a higher risk of contracting KFD than the other age groups. However, the literature indicates that the next at-risk group is the 65 years and older age group, and the least at risk is the 0-14 years group, whereas Pro-MED reports the reverse for these two age groups. We calculated the relative risk of contracting KFD by age (Table 2a) and sex (Table 2b) for cases and deaths for each of the three data sources. Literature data for the entire four-state KFD region indicate that males are statistically more likely to contract KFD than females (P = 0.0049, df = 1, χ2 = 7.899); however, Pro-MED data indicate no statistical difference (P = 0.76, df = 1, χ2 = 0.093). The results disaggregated by state indicate that from the data reported in the literature, there is a statistical difference in cases by sex only in KN (P <0.001, df = 1, χ2 = 33.099). For data reported in Pro-MED, there is a statistical difference in the cases by sex only in GA (P = 0.0013, df = 1, χ2 = 10.239). Similarly, for the data reported in literature and Pro-MED, there is a statistical difference in the cases by age in KN (literature: P <0.001, df = 2, χ2 = 375.677; Pro-MED: P <0.001, df = 2, χ2 = 67.981), KL (literature: P <0.001, df = 2, χ2 = 27.253; Pro-MED: two-tailed P = 0.004 [performed Fischer's exact test here]), and GA (literature: P <0.001, df = 2, χ2 = 89.829; Pro-MED: P <0.001, df = 2, χ2 = 63.718). However, the order of cases from the most to least affected age group varies by data source and state. For instance, the literature data indicate that most cases in KN occurred in the 15-64 years age group, followed by the 65 years and older group, then the under-15 years age group. Whereas for Pro-MED, most cases were in the under-15 years age group, followed by the 15-64 years group, and least in the 65 years and older group. Similar differences have been noted for KL and GA when comparing literature and Pro-MED data.
Table 2a.
Relative risk calculations for KFD cases and deaths by age group.
| Relative risk (cases) | 0-14 years | 15-64 years | 65 and older |
|---|---|---|---|
| Pro-MED | 0.84 [95% CI: 0.67-1.03] | 1.16 [95% CI: 1.08-1.23] | 0.05 [95% CI: 0.01-0.29] |
| Literature | 0.32 [95% CI: 0.28-0.36] | 1.30 [95% CI: 1.28-1.32] | 0.65 [95% CI: 0.54-0.77] |
| AFI | 0.16 [95% CI: 0.12-0.22] | 1.42 [95% CI: 1.40-1.44] | 0.00 [95% CI: 0.00-0.07] |
| Relative risk (deaths) | 0-14 years | 15-64 years | 65 and older |
| Pro-MED | 0.24 [95% CI: 0.07-0.77] | 1.16 [95% CI: 0.91-1.32] | 2.31 [95% CI: 1.01-4.69] |
| Literature | 0.19 [95% CI: 0.03-0.91] | 1.41 [95% CI: 1.14-1.47] | 0.00 [95% CI: 0.00-2.38] |
| AFI (no age data for deaths available) | NA | NA | NA |
Table 2b.
Relative risk calculations for KFD cases and deaths by sex.
| Relative risk (cases) | Males | Females |
|---|---|---|
| Pro-MED | 1.02 [95% CI: 0.91-1.12] | 0.98 [95% CI: 0.88-1.09] |
| Literature | 1.05 [95% CI: 1.02-1.09] | 0.95 [95% CI: 0.91-0.98] |
| AFI | 0.90 [95% CI: 0.83-0.96] | 1.11 [95% CI: 1.04-1.17] |
| Relative risk (deaths) | Males | Females |
| Pro-MED | 1.07 [95% CI: 0.75-1.37] | 0.93 [95% CI: 0.62-1.26] |
| Literature | 1.38 [95% CI: 0.95-1.68] | 0.61 [95% CI: 0.30-1.06] |
| AFI (no sex data for deaths available) | NA | NA |
AFI: acute febrile illness; CI, confidence interval; KFD: Kyasanur forest disease; NA: not available; Pro-MED: Program for Monitoring Emerging Diseases.
Significant variation exists in how the demographic data are reported from state to state, limiting the possibility of making geographic comparisons. For instance, the reporting of sex in literature data for KFD cases is only about 32% in KN, 96% in KL, 42% in MH, and 100% in GA. Similarly, data from Pro-MED indicate that sex was reported for 27% of cases in KN, 7% in KL, 0% in MH, and 15% in GA. The AFI surveillance data set reported sex for 100% of cases in KN, 100% in KL, 76% in MH, 100% in GA, and 97% in TN. Similarly, when we look at age data reporting for the cases, literature data points to age being reported in 38% of cases in KN, 37% in KL, less than 1% in MH, and 100% in GA. The age reporting for Pro-MED data is 8% of cases in KN, 82% in KL, 0% in MH, and 100% in GA. In the AFI surveillance data set, age is reported for 100% of cases in KN, 100% in KL, 76% in MH, 100% in GA, and 97% in TN. This illustrates the lack of standardized case reporting methods by different states and institutions.
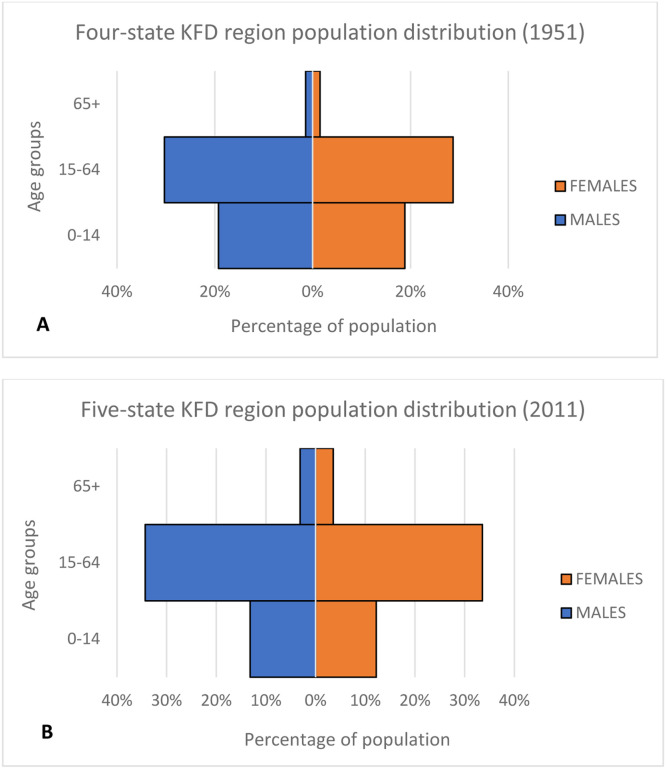
We examined the trends in population change in India for the KFD region based on census data from 1951 and 2011. The population in India has increased, but the age distribution remains relatively young, with most of its population in the 15-64 years age group. In 1951, 38% of the population was in the 0-14 years age group, 59% in the 15-64 years age group, and only 3% were 65 years or older. Note that GA was still under Portuguese territory and was not yet annexed by India in 1951 so we could not find population data available for GA in 1951. By 2011, the percentage of individuals aged 0-14 years had been reduced to 25%, the proportion in the 15-64 years age group increased to 68%, and the percentage in the 65 years and older group more than doubled, reaching 7% of the population. In 1951, the overall population of males and females was almost the same, with slightly more males in the 15-64 years age group and slightly more females in the 65 years and older age group. The population in 2011 also follows a similar trend. There are slightly more males in the 0-14 years age group and slightly more females in the 65 years and older age group. In Figure 2 (Figures 2a-b), we demonstrate the percentage of the total population by sex and age group for 1951 and 2011.
Figure 2.
Percentage of the total population in India in the KFD region by sex and age group for 1951 and 2011. A: Population distribution by age and sex in 1951 in the KFD region. (Females: 0 – 14 years – 19%, 15 – 64 years – 29% and 65 years and older – 2%. Males: 0 – 14 years – 19%, 15 – 64 years – 30% and 65 years and older – 1%.) B: Population distribution by age and sex in 2011 in the KFD region. (Females: 0 – 14 years – 12%, 15 – 64 years – 34% and 65 years and older – 4%. Males: 0 – 14 years – 13%, 15 – 64 years – 34% and 65 years and older – 3%.)
Distribution of cases and deaths by occupational groups
The occupations most frequently associated with KFD cases, as reported in the Pro-MED, literature, and AFI surveillance data set, are farming activities, plantation farming (e.g. cashews, areca nut, and coffee), cattle grazers, individuals working for the forest department, and migrant workers/laborers who travel for work to other states. For the districts where these KFD cases were reported, the main occupational categories defined by the 2011 District Census Handbook of India [20] are cultivators, agricultural laborers, household industry, and other workers. Because occupational information on KFD cases and deaths has been poorly reported across the three data sources, we could not quantify the risk of contracting KFD by occupation.
Discussion
Sociodemographic factors can impact the transmission of several VBDs. In a study by Donnelly et al. [25], the authors found that Aedes aegypti had increased in abundance in lower-income communities in Los Angeles, and certain factors, such as the use of potted plants and the use of air-conditioning impacted the abundance of this vector. Kikuti et al. [26] found a significant association between income and dengue cases in Brazil. Ruiz et al. [27] found that West Nile virus cases occurred in the urban class associated with the inner suburbs in Chicago, Illinois, and Detroit, Michigan. Factors such as housing structures, vegetation cover, and population density also affected where West Nile virus cases occurred. Thus, investigating sociodemographic factors is critical in infectious disease epidemiology and important for public health efforts.
Prevalence
KFDV has been reported in both the districts of GA (North GA and South GA), in at least 10 of 31 districts in KN (Shimoga, Udupi, Chikmagalur, Dakshin Kannada, Uttar Kannada, Belgaum, Chamarajanagar, Mysore, Gulbarga, and Hassan), in four of 14 districts in KL (Wayanad, Mallappuram, Alappuzha, and Palakkad), in two of 36 districts in MH (Sindhudurg and Pune [one report of laboratory exposure]), and one of 38 districts in TN (Nilgiris) [1,6,7,16]. KFD continues to expand to new geographic areas over time. We found a statistical difference between the states in terms of KFD prevalence in literature and Pro-MED data. In both data sources, the prevalence of KFD appears to be highest in GA, followed by KN and KL, and the least in MH. Given that the population and size of these states greatly vary, standardized reporting of cases and deaths is essential to determine the disease burden and help inform prevention measures. Although KFD has historically been endemic along the western coast of India [1,4], there are indications that it may be silently spreading to other regions within the country. This is suggested by reports of hemagglutination and neutralizing antibodies detected in individuals from different states [4]. Given favorable environmental, ecological, behavioral, and climatic conditions, KFD cases could potentially emerge in various states and regions across India [4].
Age
We found that for data reported in the scientific literature and Pro-MED for KFD cases in the four-state region of KN, KL, MH, and GA, KFD cases are significantly more frequent in the 15-64 years age group. However, the order of cases from the most to least affected age group varies by data source. Kiran et al. [23] briefly discussed that most cases tend to occur in the 15-64 years age group without providing further details. This also aligns with the population distribution in the KFD region, as demonstrated by the census data. When we examined states individually, we report a statistical association of KFD cases by age in KN, KL, and GA. However, the most affected age group tends to vary by state and data source. Issues such as underreporting, bias in data reporting, non-standardized data collection and reporting methods, and insufficient demographic data make it challenging to analyze the epidemiological characteristics of KFD. Deaths due to KFD have also been frequently reported in the 15-64 years age group, followed by those in the 0-14 years age group.
Sex
More cases were reported among males than females for the cases that reported demographic information. Demographic data reported in the literature indicates that in the four-state region, KFD cases occur more significantly in men than in women. However, the data for Pro-MED do not show any significant difference by sex. For individual states, data based on the literature show an association of KFD cases by sex only in KN, whereas data in Pro-MED report significance for cases by sex in KL and in GA (more male persons affected than female persons). Comparable to the issue with age, biases in data reporting, poor reporting of cases, and paucity of demographic information may have led to these different results.
At the time of disease discovery, Work and Trapido [28] stated that KFD cases occurred in males and females at approximately equivalent rates across the data sources. In addition, reports also indicate that there have been periods when more males have been affected in certain districts [23] and others have reported more females being affected by KFD [29]. Fatality due to KFD is observed numerically more in males than in females.
Occupation
Several reservoirs of KFDV in the environment, such as monkeys, small mammals, and bats, can maintain the virus and transmit it to ticks during blood meals, which can then be passed on to monkeys or humans [1,4]. Humans must either be bitten by an infected tick or acquire the infection after handling dead monkeys. Human-to-human transmission of KFDV has not yet been reported. The aerosolization of the KFDV has been hypothesized as a potential transmission route; however, there is no scientific evidence to prove this. The scientific literature does not provide information on other transmission pathways through which humans may be at risk for KFD. Thus, occupations or behaviors requiring humans to venture into forests predispose them to be exposed to ticks or dead monkeys infected with KFDV. Affected individuals might encounter ticks on farmlands, in plantations, or when they enter the forests. Some villagers in the KFD-affected districts may own free-ranging cattle that graze in the forests and potentially bring in ticks from the forests, which can bite and infect people.
Our review indicates that occupational data reporting is highly patchy. Often, terms like villager or persons dependent on the forest for survival are used in the literature and Pro-MED to describe individuals with KFD. Predominantly, cases have been associated with persons involved in agriculture, cattle grazing, forestry work, and plantation farming. In addition, indigenous tribal groups that might capture monkeys for game meat or forage for food in forests or are involved in fire line work, migrant workers who travel from state to state in search of agricultural/plantation work, children of individuals who go into forests, and monkey handlers are at high risk of contracting KFD [1,4,29]. There have been reports in Pro-MED, the scientific literature, and AFI surveillance data set about cases occurring in children, students, and unemployed men and women. It was not possible to conduct statistical analysis to determine if a specific occupation is associated with KFD cases as the available information is insufficient to conduct further analyses. The AFI surveillance data set also noted that for KFD cases after December 2015 in the five states for which socioeconomic status data were available, 56% belonged to low-income, 44% middle-income, and less than 1% to high-income backgrounds.
Differences across sources
Regarding demographic data reported in the data sources used in this study, there are gaps in how data are reported. Pro-MED usually provides information on the age, sex, location, and occupation of patients when the number of reported cases is small (usually five or fewer). However, when the number of reported cases/deaths increases, demographic data in the Pro-MED reports are often incomplete. Our analysis did not use newspaper data as an independent source. However, it is worth noting that some of the information contained in Pro-MED alerts is compiled from diverse media sources, including newspapers, websites, and other outlets. Although data from the literature are often aggregated, making it difficult to parse out individual demographic data from all cases. In this study, we focused on KFD-confirmed cases and deaths, specifically, those for which demographic data were accessible from the three data sources. It is important to note that there were occasions when aggregate KFD-suspected cases were reported but no demographic data were provided, adding complexity to our analysis. We also observed that mortality data have little to no associated demographic data across the literature, Pro-MED, and AFI surveillance data. In the literature, we also observed that studies sometimes report different case estimates than others for the same years. Some papers might report suspected cases and others might report confirmed cases, whereas some report data for only a handful of cases in a certain period. Several papers provide aggregate data for multiple years, which makes it challenging to get individual-level sociodemographic data.
Differences across states
There is also variation in how cases and demographic data are reported from state to state. Although no case estimates are provided for human cases in TN, neither in Pro-MED nor in the literature, we know cases have occurred in this state owing to the AFI surveillance data. We found that cases reported in KL and GA provide more demographic coverage than cases in KN and MH. This could be because of the different reporting measures used in different states and a lack of systematic and standardized data reporting protocol. KFD has been reported in KN for a long period, and the state government has taken several steps to raise awareness and contain the spread of the disease. These efforts include establishing the Virus Diagnostic Laboratory in Shimoga (KFD endemic district in KN) to test, diagnose, and provide care whenever KFD cases are found. However, there is still a need to strengthen surveillance activities, implement unified data reporting protocols and tools in all states, and adopt transparent data sharing practices. This issue has also been raised by Bhat et al. [30] who found that human case reporting measures are not standardized across local public health centers; there were delays in testing, lack of systematic data sharing practices, outdated surveillance guidelines, limited vaccine production capacity, inconsistent training and attitudes among surveyed personnel, and inadequate operational research.
Implications
In a study by Assaga et al. [14], the authors found that factors such as poor access to land, being at or below the poverty line, households headed by an older person, distance to private hospitals and main roads, and study location were important determinants of vulnerability to KFD. They also found that concerns over acquiring KFD varied and not enough people were taking the necessary tick-prevention measures [14]. Pattnaik et al. [31] also discussed the unavailability of national-level statistics and epidemiological data on KFD, the absence of community screening and sentinel surveillance systems, a low-efficacy vaccine, lack of health awareness, deforestation, and insufficient vector control measures as being responsible for continued occurrence of KFD cases. The culmination of all these factors, where there is a lack of knowledge and lack of tick and disease prevention measures in the local population who may be at risk for KFD and among personnel who handle cases and deaths in humans and monkeys, indicate that KFD is a widely neglected disease that tends to be associated with low-income groups.
We recommend that public health agencies standardize their reporting format for KFD for human cases, deaths, and monkey deaths systematically, which is maintained in the same format for all the KFD states [7,[30], [31], [32]. Investing in open and transparent data reporting practices and effectively training staff and personnel in disease reduction and prevention measures is crucial. Regularly screening for and reporting clinical, demographic, and diagnostic data from humans, monkeys, and ticks will significantly help further our understanding of the epidemiology of the disease. In addition, using a One Health approach to ensure consistent monitoring, communication between stakeholders, and implementation of timely and effective public health prevention measures can help reduce the burden of KFD.
Limitations
This study analyzed openly available data from the literature, Pro-MED reports, and an AFI surveillance data set. We excluded sources such as newspapers and technical publications because of the limited availability of sociodemographic data. Our statistical analyses revealed varying state and data source results, indicating data reporting limitations. We were unable to statistically analyze the relationship between occupation and KFD cases nor calculate the KFD risk associated with occupation owing to the descriptive nature of the data. For the calculations and visualizations, we made some assumptions about sex, age, and occupation based on available descriptions in the data sources. Data reporting inconsistency and periods of low to no reporting challenge precise case and demographic estimates. KFD cases have likely been historically underreported, including during the COVID-19 pandemic. Although KFD typically occurs from December to May, inconsistent information on the months of KFD cases and deaths in the three sources hindered our analysis of temporal patterns.
Another challenge with performing an epidemiological analysis of KFD has been the lack of case definitions across the 60+-year period. For this study, we prioritized use of confirmed cases when reported in the sources; when confirmed cases were not available, we adopted the case definition provided by the source. Because of the unavailability of consistently reported case and death information over time, there may be limitations in our ability to understand the full range of risk factors for KFD.
Conclusion
KFD is a neglected tick-borne disease that is expanding into new areas, impacting humans and non-human primates. We reviewed various data sources and found that the current data reporting lacks critical sociodemographic information. There are also variations across data sources on the completeness of the data. The absence of these data leads to incomplete knowledge regarding the disease, virus, and risk factors for KFD. Future steps should include setting up standardized case and death reporting forms across all affected states and publicly sharing the data to allow epidemiological analysis and the development of effective prevention measures.
Declarations of competing interest
The authors have no competing interest to declare.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval and consent to participate
Not applicable.
Acknowledgments
This study was possible thanks to the University of Illinois at Urbana-Champaign library and digital access to various peer-reviewed journal articles and resources used in the study.
Author contributions
SC and FCDA conceived the study, surveyed the literature and data sources, extracted information from published articles, and drafted the first version of the manuscript. SC, WS, BFA, and FCDA contributed to the data analysis and manuscript writing. All authors read and approved the final manuscript.
References
- 1.Chakraborty S, Andrade FCD, Ghosh S, Uelmen J, Ruiz MO. Historical expansion of Kyasanur Forest Disease in India from 1957 to 2017: A retrospective analysis. GeoHealth. 2019;3:44–55. doi: 10.1029/2018GH000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purse BV, Darshan N, Kasabi GS, Gerard F, Samrat A, George C, et al. Predicting disease risk areas through co-production of spatial models: the example of Kyasanur Forest Disease in India's forest landscapes. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holbrook MR. Kyasanur forest disease. Antiviral Res. 2012;96:353–362. doi: 10.1016/j.antiviral.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pattnaik P. Kyasanur forest disease: an epidemiological view in India. Rev Med Virol. 2006;16:151–165. doi: 10.1002/rmv.495. [DOI] [PubMed] [Google Scholar]
- 5.Gladson V, Moosan H, Mathew S, Dineesh P. Clinical and laboratory diagnostic features of Kyasanur Forest disease: a study from Wayanad, South India. Cureus. 2021;13:e20194. doi: 10.7759/cureus.20194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munivenkatappa A, Sahay RR, Yadav PD, Viswanathan R, Mourya DT. Clinical & epidemiological significance of Kyasanur forest disease. Indian J Med Res. 2018;148:145–150. doi: 10.4103/ijmr.IJMR_688_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakraborty S, Sander WE, Allan BF, Andrade FCD. Retrospective study of Kyasanur Forest disease and deaths among nonhuman primates, India, 1957–2020. Emerg Infect Dis. 2021;27:1969–1973. doi: 10.3201/eid2707.210463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Social determinants of health: know what affects health, https://www.cdc.gov/socialdeterminants/index.htm; 2022 [accessed 07 December 2022].
- 9.Hacker K, Houry D. Social needs and social determinants: the role of the Centers for Disease Control and Prevention and Public Health. Public Health Rep. 2022;137:1049–1052. doi: 10.1177/00333549221120244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riley WJ. Health disparities: gaps in access, quality and affordability of medical care. Trans Am Clin Climatol Assoc. 2012;123:167–172. discussion 172. [PMC free article] [PubMed] [Google Scholar]
- 11.Springer YP, Johnson PTJ. Large-scale health disparities associated with Lyme disease and human monocytic ehrlichiosis in the United States, 2007–2013. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadav PD, Patil S, Jadhav SM, Nyayanit DA, Kumar V, Jain S, et al. Phylogeography of Kyasanur Forest Disease virus in India (1957–2017) reveals evolution and spread in the Western Ghats region. Sci Rep. 2020;10:1966. doi: 10.1038/s41598-020-58242-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatia B, Meade-White K, Haddock E, Feldmann F, Marzi A, Feldmann H. A live-attenuated viral vector vaccine protects mice against lethal challenge with Kyasanur Forest disease virus. npj Vaccines. 2021;6:152. doi: 10.1038/s41541-021-00416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asaaga FA, Purse BV, Rahman M, Srinivas PN, Kalegowda SD, Seshadri T, et al. The role of social vulnerability in improving interventions for neglected zoonotic diseases: the example of Kyasanur Forest Disease in India. PLoS Glob Pub Health. 2023;3 doi: 10.1371/journal.pgph.0000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Program for Monitoring Emerging Diseases (ProMED-mail). Kyasanur Forest Disease reports, https://promedmail.org/promed-posts/; n.d. [accessed 18 February 2022].
- 16.Department of Health & Family Welfare Services, National Center for Disease Control. Kyasanur Forest Disease Compendium of the scientific literature, https://monkeyfeverrisk.ceh.ac.uk/sites/default/files/2022-05/NCDC-Consultation-book-3rd-update.pdf; n.d. [accessed 10 February 2022].
- 17.Manipal Center for Virus Research. Annual Report of Hospital-based surveillance of acute febrile illness in India, https://careernext.manipal.edu/content/dam/manipal/mu/dovr/Documents/AFI/MCVR_AFI%20Brochure%20final.pdf; 2017 [accessed 05 January 2024].
- 18.Mourya DT, Yadav PD, Sandhya VK, Reddy S. Spread of Kyasanur forest disease, Bandipur tiger reserve, India, 2012–2013. Emerg Infect Dis. 2013;19:1540–1541. doi: 10.3201/eid1909.121884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naren Babu N, Jayaram A, Hemanth Kumar H, Pareet P, Pattanaik S, Auti AM, et al. Spatial distribution of Haemaphysalis species ticks and human Kyasanur Forest Disease cases along the Western Ghats of India, 2017–2018. Exp Appl Acarol. 2019;77:435–447. doi: 10.1007/s10493-019-00345-9. [DOI] [PubMed] [Google Scholar]
- 20.Office of the Registrar General and Census Commissioner . 2011. District census handbook. [Google Scholar]; https://censusindia.gov.in/2011census/dchb/DCHB.html; [accessed 10 February 2022].
- 21.Office of the Registrar General and Census Commissioner of India, Ministry of Home Affairs, Government of India, Census Digital Library. Census of India 1951, https://censusindia.gov.in/nada/index.php/catalog/28409; n.d. [accessed 18 January 2022].
- 22.Ritchie H, Roser M. Age structure. OurWorldInData.org, https://ourworldindata.org/age-structure; 2019 [accessed 19 January 2023].
- 23.Kiran SK, Pasi A, Kumar S, Kasabi GS, Gujjarappa P, Shrivastava A, et al. Kyasanur Forest disease outbreak and vaccination strategy, Shimoga District, India, 2013–2014. Emerg Infect Dis. 2015;21:146–149. doi: 10.3201/eid2101.141227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.RStudio Team . PBC, Rstudio; Boston: 2020. RStudio: integrated development for R. [Google Scholar]; http://www.rstudio.com/; [accessed 10 April 2023].
- 25.Donnelly MAP, Kluh S, Snyder RE, Barker CM. Quantifying sociodemographic heterogeneities in the distribution of Aedes aegypti among California households. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kikuti M, Cunha GM, Paploski IAD, Kasper AM, Silva MMO, Tavares AS, et al. Spatial distribution of dengue in a Brazilian urban slum setting: role of socioeconomic gradient in disease risk. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruiz MO, Walker ED, Foster ES, Haramis LD, Kitron UD. Association of West Nile virus illness and urban landscapes in Chicago and Detroit. Int J Health Geogr. 2007;6:1–11. doi: 10.1186/1476-072X-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Work TH, Trapido H. Summary of preliminary report of investigations of the virus research centre on an epidemic disease affecting forest villagers and wild monkeys in Shimoga district, Mysore. Indian J Med Sci. 1957;11:341–342. [PubMed] [Google Scholar]
- 29.Sadanandane C, Elango A, Marja N, Sasidharan PV, Raju KHK, Jambulingam P. An outbreak of Kyasanur Forest disease in the Wayanad and Malappuram districts of Kerala, India. Ticks Tick Borne Dis. 2017;8:25–30. doi: 10.1016/j.ttbdis.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Bhat P, Jagadeesha HS, Raju MK, Sooda S, Premanand K, Kumar R. Kyasanur Forest Disease, is our surveillance system healthy to prevent a larger outbreak? A mixed-method study, Shivamogga, Karnataka, India: 2019. Int J Infect Dis. 2021;110:S50–S61. doi: 10.1016/j.ijid.2021.07.076. [DOI] [PubMed] [Google Scholar]
- 31.Pattnaik S, Agrawal R, Murmu J, Kanungo S, Pati S. Does the rise in cases of Kyasanur forest disease call for implementation one health in India? IJID Reg. 2023;7:18–21. doi: 10.1016/j.ijregi.2023.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keshavamurthy R, Charles LE. Predicting Kyasanur forest disease in resource-limited settings using event-based surveillance and transfer learning. Sci Rep. 2023;13:11067. doi: 10.1038/s41598-023-38074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]