Summary
Background
Dry eye disease has a high prevalence and exerts a significant negative effect on quality of life. In China, there are currently no available nasal sprays to promote natural tear production in patients with dry eye disease. We therefore evaluated the efficacy and safety of OC-01 (varenicline solution) nasal spray versus vehicle in Chinese patients with dry eye disease.
Methods
This was a randomized, multicenter, double-masked, vehicle-controlled, phase 3 clinical trial conducted at ophthalmology departments in 20 hospitals across China (NCT05378945). Eligible patients had a diagnosis of dry eye disease based on patient symptoms, Eye Dryness Score (EDS), Schirmer's Test (with topical anesthesia) Score (STS), and corneal fluorescein staining (CFS) score. Participants were randomly assigned 1:1 using an Interactive Web Response System (IWRS) to receive OC-01 0.6 mg/mL twice daily (BID) or vehicle nasal spray. Participants, investigators, and sponsor were all masked to treatment assignment. The primary endpoint was the percentage of subjects in the intention-to-treat population achieving ≥10 mm improvement in STS from baseline at week 4.
Findings
In total, 340 patients were randomized from 21 July 2022 to 04 April 2023, 78.8% were female. Patients in the OC-01 group (n = 176) had significantly higher achievement of ≥10 mm improvement in STS (35.8% [n = 63] versus 17.7% [n = 29], stratified odds ratio: 2.67, 95% CI: 1.570–4.533, p = 0.0002) and a significantly greater increase from baseline STS (least-squares mean difference [SE]: 3.87 [0.794], p < 0.0001) at week 4 versus the vehicle group (n = 164). In addition, OC-01 led to a numerically greater reduction in mean EDS from baseline at week 4 compared to the vehicle group (LS mean [SE] difference: −1.3 [2.20]; 95% CI: −5.64 to 2.99, p = 0.5467). The most common adverse event was mild, transient sneezing (78% of OC-01 administrations). No serious adverse events related to nasal administration occurred.
Interpretation
OC-01 (varenicline solution) nasal spray BID has clinically meaningful efficacy for reducing the signs (as measured by STS) and may improve the symptoms (as measured by EDS) of dry eye disease, with an excellent safety and tolerability profile, in the Chinese population.
Funding
Jixing Pharmaceutical Co. Ltd.
Keywords: Dry eye disease, Nicotinic acetylcholine receptor agonist, Varenicline nasal spray, Randomized clinical trial, Schirmer's test score, Eye dryness score
Research in context.
Evidence before this study
In China, it is estimated that 21–30% of people over 40 years of age have dry eye disease, a higher prevalence than reported in the USA or Europe. Dry eye disease is associated with a significant negative effect on quality of life. Multiple studies have demonstrated that the nicotinic acetylcholine receptor (nAChR) can mediate activation of the trigeminal parasympathetic pathway (TPP), which accounts for approximately one-third of basal tear film production. The OC-01 nasal spray contains varenicline, which is a nAChR agonist. We searched PubMed using the terms “OC-01” AND “varenicline” AND “dry eye” and restricted the results to clinical trials (no language restrictions). Our search identified three clinical trials conducted in North America that evaluated the safety and efficacy of OC-01 for the treatment of dry eye disease. The Phase 2 ONSET-1 trial established the safety and efficacy of three different doses of OC-01 relative to vehicle given for 4 weeks to patients with dry eye. The Phase 2 MYSTIC trial evaluated the safety and efficacy of two doses of OC-01 nasal spray at Day 84 and showed a continuous response over this time period. Finally, the Phase 3 ONSET-2 trial confirmed the safety and efficacy of OC-01 relative to vehicle in a larger population of patients with dry eye disease. However, the clinical benefits and risks of OC-01 still require evaluation in a Chinese population due to potential ethnic differences in dry eye disease characteristics, causative factors, and medical practice compared to the USA or Europe.
Added value of this study
This is the first Phase 3 study of the OC-01 nasal spray conducted in patients with dry eye disease in China. The results show that OC-01 is well-tolerated and efficacious for the treatment of the signs and symptoms of dry eye disease and is superior to vehicle/placebo in this patient population. The trial found that OC-01 nasal spray both increases natural tear secretion and improves dry eye symptoms. The most-frequently reported non-ocular treatment-emergent adverse event in patients receiving OC-01 or vehicle was sneezing, which was all mild and did not require medical intervention.
Implications of all the available evidence
The patient population enrolled in this study represents a broad and representative sample of the patients with dry eye who present to eye care practitioners in China. Therefore, the findings of this study have high generalizability to the real-world population of patients with dry eye in China and support the use of OC-01 in this patient population. In addition to significantly increasing tear production via the novel mechanism of action of agonizing nAChRs, the route of administration of OC-01 offers many advantages over traditional topical therapies including avoidance of the ocular surface in patients who have undergone ocular surgery or with eye disorders, and a potentially easier delivery method for patients with reduced physical activity or with other eye conditions for which eye drops are not convenient or feasible to use.
Introduction
Dry eye disease is a multifactorial, age-related disorder of the ocular surface resulting in severe pain, visual impairment, tear film hyperosmolarity and instability, inflammation, and corneal wound formation.1,2 A persistently unstable and/or deficient tear film is one of the critical causes of dry eye disease.1,2 Female sex, advanced age, Asian ethnicity, meibomian gland dysfunction, connective tissue disease, and Sjogren's syndrome are the main and most apparent risk factors for this condition.3 In China, it is estimated that 21–30% of people over 40 years of age have dry eye, and this prevalence is higher than reported in the USA or Europe.4,5 There is also a trend for early onset of dry eye in China, which may be related to risk factors such as myopia, use of mobile phones and computers, and prolonged video-based teaching time.6 Generally, the therapeutic options for dry eye disease in China, including tear substitutes, anti-inflammatory medications, and physiotherapies are similar to the treatments recommended in the guidelines published by the Tear Film & Ocular Surface Society.7,8 However, due to some differences in disease diagnosis and classification, as well as drug accessibility compared to the USA or Europe, the treatment of patients may differ in clinical practice.1,9 Furthermore, the currently approved therapies for dry eye disease in China are typically administered as eye-drop formulations, which are commonly associated with ocular burning sensation, blurred vision, and dysgeusia, causing challenges for some patients and reducing compliance.7,8 At the time the present study was completed, no nasal spray-delivered therapy to restore tear film homeostasis with natural tear production was available in China.
The OC-01 nasal spray contains varenicline, a small-molecule nicotinic acetylcholine receptor (nAChR) agonist, which binds with high selectivity and affinity to human α4β2, α4α6β2, α3β4, α3α5β4 and α7 nAChRs.10,11 Tear film production is mediated by the lacrimal functional unit, which comprises the ocular surface, the main lacrimal gland, and the interconnecting innervation.12 Tear secretion is regulated by neural reflex arcs, including those that are triggered by activation of trigeminal afferent nerves in the nasal cavity, which lead to stimulation of trigeminal efferent parasympathetic nerves innervating the lacrimal functional unit.13 This pathway is known as the trigeminal parasympathetic pathway (TPP) and accounts for approximately one-third of basal tear film production.14 Multiple studies have demonstrated that nAChR can mediate afferent signals within the trigeminal nerve in response to nasal stimuli.15,16 Therefore, when delivered as a multi-dose, preservative-free nasal spray, the nAChR agonist varenicline may promote activation of the TPP, providing a novel method to improve the signs and symptoms of dry eye disease. Furthermore, the nasal route of administration of OC-01 offers advantages over traditional topical therapies including avoidance of the ocular surface burden present in patients who have undergone ocular surgery or with eye disorders, and a potentially easier delivery method for patients with reduced physical activity or mobility.9, 10, 11
Three pivotal clinical trials were conducted in North America to evaluate the safety and efficacy of OC-01 for the treatment of the signs and symptoms of dry eye disease.17, 18, 19 The Phase 2 ONSET-1 trial established the safety and efficacy of three different doses of OC-01 (0.12 mg/mL, 0.6 mg/mL, and 1.2 mg/mL) relative to vehicle given for 4 weeks in subjects with dry eye disease.18 The Phase 2 MYSTIC trial evaluated the safety and efficacy of two doses of OC-01 nasal spray (0.6 mg/mL and 1.2 mg/mL) at Day 84 and showed a continuous response.18 Finally, the Phase 3 ONSET-2 trial confirmed the safety and efficacy of two doses of OC-01 (0.6 mg/mL and 1.2 mg/mL) relative to vehicle in a larger population of patients with dry eye disease.17 As discussed above, due to differences in dry eye disease characteristics, classification, and treatment in China compared to the USA and Europe, OC-01 requires further evaluation in a Chinese population. Here, we report a clinical trial (JX03002) conducted to evaluate the efficacy and safety of OC-01 in Chinese patients with dry eye disease.
Methods
Study design
This was a randomized, double-masked, vehicle-controlled, multicenter, phase 3 clinical trial conducted at ophthalmology departments at 20 hospitals in China. The study consisted of a screening phase followed by four study visits at randomization (Day 1), Weeks 1, 2, and 4. The protocol and statistical analysis plan are available in the Supplementary Materials. This study was performed in accordance with the principles of the Declaration of Helsinki and good clinical practice. The study protocol was approved by the ethics committees of all 20 sites (eTable 1). This study report adheres to the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Participants
Eligible participants were aged 18 years or older with a physician diagnosis of dry eye disease and had used or desired to use an artificial tear substitute for dry eye symptoms within 6 months prior to the screening visit. Participants were also required to have an Eye Dryness Score (EDS) ≥40, and a corneal fluorescein staining (CFS) score of ≥2 in at least 1 corneal region or a sum of ≥4 in all regions, a baseline Schirmer's Test (with topical anesthesia) Score (STS) of ≤10 mm/5 min and increased at least 7 mm in the same eye with a cotton swab nasal stimulation in the study eye at the Screening Visit, as well as a <20 mm difference between the STS of the study eye and the non-study eye at baseline, and a physician's diagnosis of dry eye disease. Other inclusion criteria were a best corrected visual acuity (BCVA) of 0.7 logarithm of the minimum angle of resolution (logMAR) or better (logMAR ≤0.7) in each eye at the screening visit. In patients for whom both eyes were eligible for treatment, the study eye was defined as the eye with the greatest increase in tear production with stimulation by a cotton swab at the Screening Visit or, if there was no difference in stimulated tear production, the eye with the lower basal Schirmer's score at screening. If there was no difference for either measure, the right eye was used as the study eye. Participants provided written, informed consent before enrollment. Data on patient sex were self-reported.
Key exclusion criteria were: (1) clinically significant corneal epithelial defects (such as neurotrophic keratitis) at the screening visit prior to performing Schirmer's Test; (2) a history of chronic or recurrent epistaxis, coagulation disorders or other conditions that, in the opinion of the Investigator, may have led to a clinically significant risk of increased bleeding; (3) prior nasal or sinus surgery (including history of application of nasal cautery) or significant trauma to these areas that had impacted the function of the trigeminal nerve; (4) a vascularized polyp, severely deviated septum, chronic recurrent nosebleeds, or severe nasal airway obstruction as confirmed by intranasal examination performed prior to the screening visit; (5) patients who were not able to stop wearing contact lenses. Eligible patients with autoimmune system disease were not excluded unless deemed appropriate by the investigators.
Randomization and masking
Eligible participants were assigned 1:1 to either OC-01 nasal spray or vehicle using a central web-based randomization system and utilizing permuted blocks. The randomization table was generated by an independent and unblind statistician using the PLAN process in SAS software, version 9.4 (SAS Institute Inc). Randomization was stratified by baseline EDS (<60 versus ≥60) and baseline STS (≤5 versus >5). Bottles of OC-01 and the vehicle were identical in appearance to facilitate masking. Participants, investigators, and sponsor were all masked to treatment assignment.
Procedures
Participants received OC-01 0.6 mg/mL twice daily (BID) or vehicle BID, delivered as a 50-μL intranasal spray in each nostril. The 0.6 mg/mL BID dose was selected based on a Phase 3 study conducted in North America which showed no difference between 0.6 mg/mL and 1.2 mg/mL doses of OC-01.17 Patients were trained on the self-administration of the nasal spray. During the study, the utilization of artificial tears was allowed for temporary relief of dry eye disease symptoms in both study arms.
The Schirmer's test with topical anesthetic was conducted in both eyes. At the screening visit, one basal Schirmer's test was performed followed by a Schirmer's test with cotton swab nasal stimulation. Schirmer's test with topical anesthetic was conducted again at randomization (Day 1), Week 2 and Week 4 (eFig. 1). Corneal fluorescein staining was performed after assessment of STS with anesthesia at screening and Week 4. EDS was assessed at screening and Weeks 1, 2 and 4 in the clinic environment using a visual analog scale, which is a validated assessment in which the patient rates their ocular symptoms (both eyes simultaneously) related to eye dryness by placing a vertical mark on a 100 mm horizontal assessment line to indicate the level of discomfort, with 0 corresponding to “no discomfort” and 100 corresponding to “maximum discomfort”. Signs of dry eye disease were also assessed by CFS score at screening and Week 4 using the National Eye Institute scale (range, 0–3, with higher scores representing a worse condition). Five regions of the cornea were assessed for corneal staining (central, superior, inferior, nasal, and temporal), and a total score was calculated that included all regions.
Outcomes
The primary study endpoint was the percentage of patients who achieved a ≥10 mm improvement of STS in the study eye from baseline to Week 4 (Day 28). Secondary efficacy endpoints were change from baseline in STS at Week 4, and change from baseline in EDS at Weeks 1, 2, and 4. Other efficacy endpoints were: change from baseline at Week 4 in Total CFS, Inferior CFS, Nasal CFS, Temporal CFS, Central CFS, and Superior CFS.
Safety assessments included evaluation of treatment-emergent adverse events (TEAEs) which were defined as adverse events (AEs) that were new or had worsened in severity since the first study drug use, sneezing (collected by diary), slit-lamp biomicroscopy, best-corrected visual acuity assessment and intranasal examination. Medical history, ocular history, and AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) (version 25.0) and concomitant medications using the World Health Organization (WHO) Drug Global Dictionary, Format B3 (Version March 2022), as appropriate. Safety was evaluated at baseline, Weeks 1, 2 and 4, 7 days after treatment completion and 28 days post-study. Treatment compliance was defined as the number of doses administered (in both nostrils) that were actually performed relative to the number specified per the protocol for the duration of actual treatment exposure.
A pre-specified subgroup analysis was conducted to evaluate the percentage of patients who achieved ≥10 mm improvement of STS in the study eye, change in STS, and change in EDS from baseline to Week 4 by randomization stratification factors.
The percentage of subjects who achieved an absolute value of STS above 10 mm at Week 4 was investigated as a post hoc analysis.
Statistical analysis
In the ONSET-2 study, 38.5% of patients treated with OC-01 0.6 mg/mL and 22.2% in the vehicle group achieved a ≥10 mm improvement in STS from baseline at Week 4.16 Assuming a similar treatment effect in Chinese subjects, and a 2-sided type I error rate of 0.05, a total of 340 patients randomized 1:1 (170 in each treatment arm) was estimated to provide over 90% power to detect superiority of OC-01 over vehicle.
Efficacy was analyzed in the Intention-To-Treat (ITT) population (all randomized patients), the modified ITT (mITT) population (excluding randomized patients infected with COVID-19) and Per-Protocol (PP) populations. Two PP populations were defined: PP based on the ITT population, including all patients who received ≥1 dose of investigational product with ≥1 non-missing baseline and one post-baseline data point and excluding patients with protocol deviations, and PP based on the mITT population, excluding randomized patients infected with COVID-19 as well as those with important protocol deviations that may have affected the efficacy analysis. All analyses in the PP population of the mITT were post hoc. The safety population included all randomized patients who received at least one dose of the investigational product.
The primary efficacy endpoint was analyzed using a Cochran Mantel Haenszel test, controlling for the study site and randomization strata. The analysis of change in STS from baseline to Visit 4 compared the least squares (LS) mean difference between the treatment groups using a mixed model for repeated measures (MMRM) including visit, treatment group and treatment–visit interaction as fixed effects, and study site, baseline STS, and baseline EDS as covariates (continuous value, linear effect). Change from baseline in EDS was analysed using the same method as the STS analysis. A sensitivity analysis was conducted using analysis of covariance (ANCOVA) models for each of the secondary endpoints. The change from baseline in CFS was analyzed for the total CFS score as well as the CFS scores for each area (inferior, superior, central, temporal, and nasal) with an ANCOVA model including study site, baseline STS, baseline EDS and the baseline corresponding location CFS score as covariates. No interim analyses were planned.
Patients were considered non-responders if they discontinued treatment due to an AE related to study drug, lack of efficacy, or using a prohibited concomitant drug/device that may have significantly affected the study outcome, or patients with missing endpoint values in the study eye at Week 4 for any reason. Therefore, no imputation method was used for patients with missing data. All analyses were conducted using SAS software, version 9.4. The study protocol was registered on ClinicalTrials.gov (NCT05378945).
Role of the funding source
The study sponsor participated in the design of the study, conduct of the study, data collection, data management, data analysis, interpretation of the data, and review of the manuscript.
Results
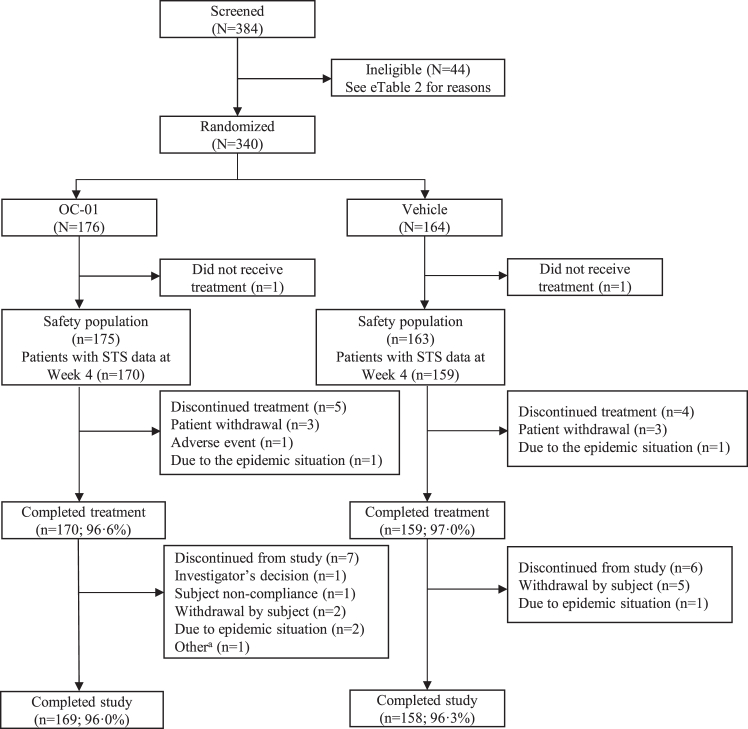
A total of 340 patients with dry eye disease were randomized to the study from 21 July 2022 to 04 April 2023. Reasons for exclusion at screening are summarized in eTable 2. The trial ended when all enrolled patients had completed follow-up. Overall, 327 (96.2%) patients completed the study, and 13 (3.8%) withdrew from the study for non-safety reasons (Fig. 1, Table 1 & eFig. 1). All patients (100%) were Asian, and most (78.8%) were female. In general, patient demographics and baseline characteristics were well balanced across the two treatment groups, including use of topical eye drops (Table 1). A total of six patients in the OC-01 group and five in the placebo group were considered non-responders (eTable 3).
Fig. 1.
Patient flow chart. aThe patient developed a foreign body sensation without the use of the study drug STS, Schirmer's Test Score.
Table 1.
Patient demographic and baseline clinical characteristics.
| Characteristic statistic | OC-01 0.6 mg/mL (n = 176) | Placebo (n = 164) | Total (N = 340) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 45.0 (15.27) | 41.8 (14.82) | 43.5 (15.12) |
| Age Category (years), n (%) | |||
| ≤65 | 159 (90.3) | 154 (93.9) | 313 (92.1) |
| >65– ≤75 | 16 (9.1) | 10 (6.1) | 26 (7.6) |
| >75 | 1 (0.6) | 0 | 1 (0.3) |
| Race, n (%) | |||
| Asian | 176 (100.0) | 164 (100.0) | 340 (100.0) |
| Sex, n (%) | |||
| Male | 41 (23.3) | 31 (18.9) | 72 (21.2) |
| Female | 135 (76.7) | 133 (81.1) | 268 (78.8) |
| Childbearing potential, n (%)a | |||
| Yes | 82 (60.7) | 99 (74.4) | 181 (67.5) |
| No | 53 (39.3) | 34 (25.6) | 87 (32.5) |
| STS (mm)–Study eye | |||
| Mean (SD) | 5.09 (2.351) | 5.05 (2.414) | 5.07 (2.378) |
| STS with cotton swab stimulation (mm)–Study eye | |||
| Mean (SD) | 21.22 (6.091) | 21.42 (5.997) | 21.31 (6.038) |
| EDS (mm) | |||
| Mean (SD) | 66.3 (13.44) | 65.6 (13.60) | 66.0 (13.50) |
| Total CFS–Study eye | |||
| Mean (SD) | 5.3 (2.49) | 5.3 (2.43) | 5.3 (2.46) |
| BCVA (logMAR)–Study eye | |||
| Mean (SD) | 0.092 (0.1615) | 0.102 (0.1465) | 0.097 (0.1543) |
| Intraocular pressure (mmHg)–Study eye | |||
| Mean (SD) | 14.01 (2.675) | 14.38 (2.676) | 14.19 (2.678) |
| Pupil size (mm)–Study eye | |||
| Mean (SD) | 3.09 (0.416) | 3.11 (0.473) | 3.10 (0.444) |
| Irrigation of lacrimal passage–Study Eye, n (%) | |||
| Normal | 174 (98.9) | 160 (97.6) | 334 (98.2) |
| Abnormal, not clinical significance | 2 (1.1) | 4 (2.4) | 6 (1.8) |
| Use of allowed topical eye drops, n (%) | |||
| Glycerol eye drops | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| Sodium hyaluronate eye drops | 52 (29.5) | 38 (23.2) | 90 (26.5) |
| Polyvinyl alcohol eye drops | 6 (3.4) | 5 (3.0) | 11 (3.2) |
Abbreviations: %, percentages are calculated based on group total as the denominator; BCVA, best corrected visual acuity; CFS, corneal fluorescein staining; EDS, Eye Dryness Score; LogMAR, logarithm of the minimum angle of resolution; SD, Standard deviation; STS, Schirmer's Test Score.
This percentage was calculated using the number of female subjects as the denominator.
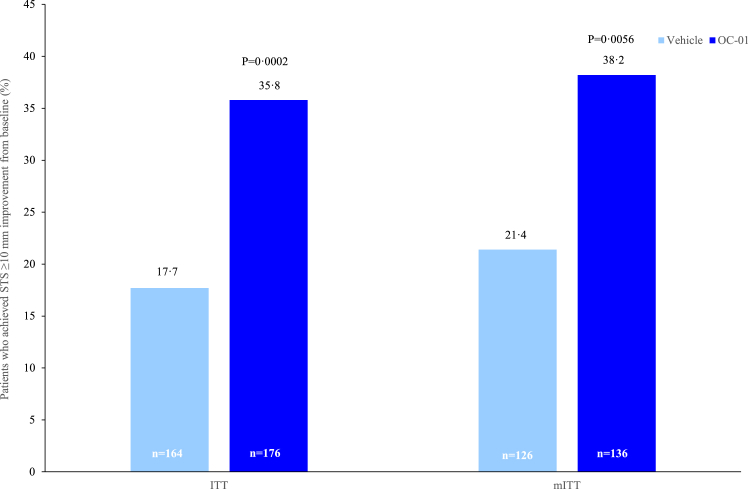
The primary efficacy endpoint was met and a significantly higher proportion of patients in the OC-01 group achieved an STS response (≥10 mm improvement in STS from baseline to Week 4) compared with the vehicle group (35.8% [n = 63] versus 17.7% [n = 29], stratified odds ratio: 2.67 [95% CI: 1.570–4.533], p = 0.0002) (Fig. 2). These results in the ITT population were supported by a sensitivity analysis in the PP population (eTable 4). In addition, outcomes in the mITT population were similar to those observed in the ITT population; a significantly higher proportion of patients receiving OC-01 achieved an STS response at Week 4 compared with the vehicle group (38.2% [n = 52] versus 21.4% [n = 27], stratified odds ratio: 2.25 [95% CI: 1.257–4.022], p = 0.0056) (Fig. 2).
Fig. 2.
The percentage of patients who achieved a ≥10 mm improvement in STS at Week 4 (ITT & mITT Population). STS, Schirmer's Test Score.
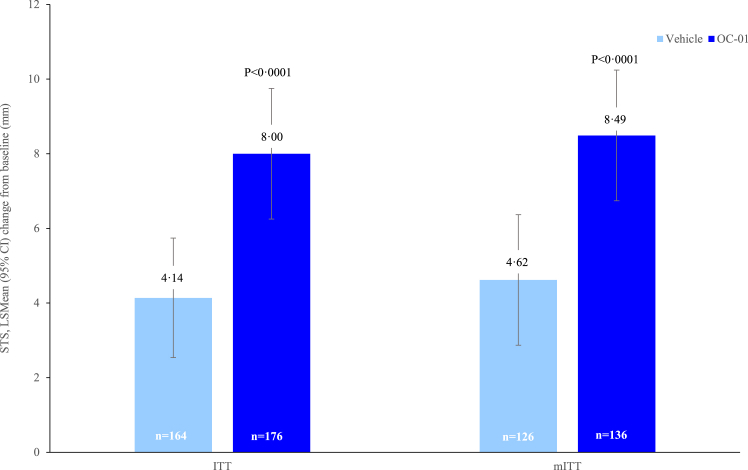
Patients receiving OC-01 achieved a significantly greater increase in mean STS from baseline at Week 4 versus vehicle (LS mean difference [standard error, SE]: 3.79 [0.790], 95% CI: 2.234–5.344, p < 0.0001) (Fig. 3). These findings were supported by a sensitivity analysis performed in the ITT population using an ANCOVA model, which showed greater increases in mean STS from baseline to Week 4 in the OC-01 group versus the vehicle group (eTable 5). In addition, consistent results were observed in the mITT population; OC-01 resulted in a significantly greater increase in mean STS from baseline at Week 4 versus vehicle (LS mean difference [SE]: 3.87 [0.794], 95% CI: 2.087–5.653, p < 0.0001) (Fig. 3).
Fig. 3.
Mean change of STS at Week 4 from baseline (ITT & mITT Population). STS, Schirmer's Test Score.
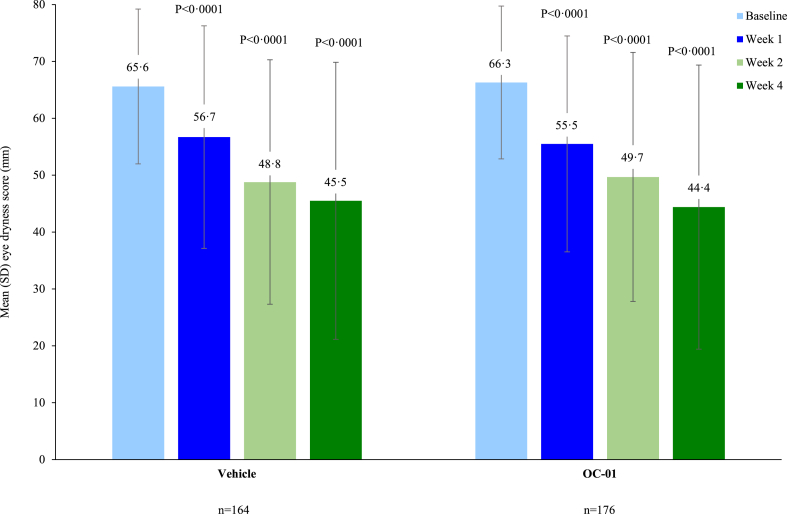
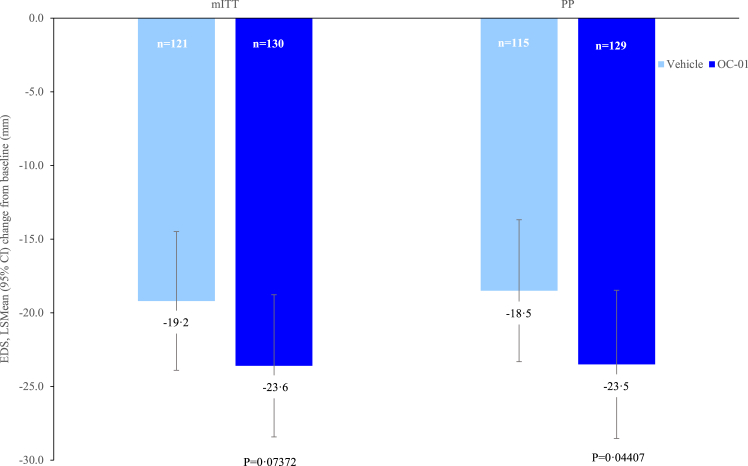
Patients in the OC-01 group achieved a numerically greater reduction in mean EDS from baseline at Week 4 compared to the vehicle group (LS mean [SE] difference: −1.3 [2.20]; 95% CI: −5.64 to 2.99, p = 0.5467) (Fig. 4). The numerically greater reductions in EDS with OC-01 versus placebo were apparent from as early as Week 1 (LS mean [SE] difference: −1.4 [1.60]; 95% CI: −4.57 to 1.72, p = 0.3725) (Fig. 4). A sensitivity analysis using an ANCOVA model was consistent with the primary analysis (eTable 6). Similar results were also observed in the mITT population, with a trend towards a greater reduction from baseline in mean EDS at Week 4 for OC-01 versus vehicle (LS mean [SE] difference: −4.35 [2.429]; 95% CI: −9.119 to 0.419, p = 0.07372) (Fig. 5). In addition, in a post hoc analysis, patients receiving OC-01 in the PP population of the mITT showed a nominally greater reduction in mean EDS from baseline at Week 4 versus vehicle (LS mean [SE] difference: −5.00 [2.480]; 95% CI: −9.870 to −0.133, p = 0.04407) (Fig. 5).
Fig. 4.
Mean eye dryness score (mm).
Fig. 5.
Changes in dry eye disease signs (mITT Population and mITT per protocol).
The change from baseline in CFS at Week 4 indicated directional improvements in nasal, temporal and central, as well as a total, score that included all regions, for OC-01 versus vehicle but the differences did not reach statistical significance (eFig. 2).
Subgroup analysis of the primary study endpoint in the ITT population showed significantly higher achievement of STS response with OC-01 versus vehicle across the randomization stratification factors (EDS <60 versus ≥60, and STS ≤5 versus >5), consistent with the primary analysis (eFig. 3). Subgroup analysis also revealed a favorable trend in change from baseline STS at Week 4 with OC-01 versus vehicle across patient subgroups by randomization stratification factors at baseline (eFig. 4). A subgroup analysis of change from baseline in EDS using MMRM by randomization stratification factors at baseline showed a numerically larger reduction from baseline in mean EDS at Week 4 in the OC-01 group versus the vehicle group in patients with baseline EDS ≥60 versus <60 and inferior CFS ≥1.5 versus <1.5 (eFig. 5).
Post hoc analysis showed an absolute value of STS above 10 mm at Week 4 was achieved by a significantly higher proportion of patients receiving OC-01 versus the vehicle group for the study eye: 54.0% (n = 95) versus 35.4% (n = 58), respectively; stratified odds ratio: 2.37 (95% CI: 1.415–3.964, p = 0.0007) (eTable 7). Furthermore, improvements in EDS were observed at Week 4 compared with baseline in both treatment groups. In the OC-01 group, a significant difference was observed between the mean EDS (observed value) at Week 4 compared to baseline (44.4 versus 66.3, p < 0.0001). The result was supported by a sensitivity analysis using the last observation carried forward (LOCF) rule for missing data at Week 4 (44.8 versus 66.3, p < 0.0001) (Fig. 4).
The overall mean treatment compliance in the safety population was 99.3% (range: 51.0%–104.8%) and was comparable in the OC-01 group (98.8% [range: 51.0%–102.9%]) and vehicle group (99.8% [range: 80.2%–104.8%]). Most subjects (99.1%) had treatment compliance ≥80% and ≤120%.
At least one TEAE was reported by 87.4% of patients in the OC-01 group and 47.2% in the vehicle group, the majority of which occurred during the treatment period. Most TEAEs were mild or moderate in severity, with only 1.2% of patients reporting a severe TEAE during the study (1.7% of patients in the OC-01 group reported the severe TEAEs pyrexia, and COVID-19, respectively, and 0.6% of patients in the vehicle group reported the severe TEAE pain); no severe TEAEs were classified as ocular TEAEs, and none were considered related to the study drug. There were no deaths reported during the study. Overall, 0.9% of subjects reported serious adverse events (SAEs): 1.1% of patients in the OC-01 group had arteriosclerosis coronary artery and pneumonia and 0.6% in the vehicle group had melanocytic naevus. All SAEs were moderate in severity, and none were considered related to the study drug.
The incidence of ocular TEAEs was similar across the treatment groups; reported by 4.6% of patients in the OC-01 group and 5.5% in the vehicle group. The most common ocular TEAE in both groups was corneal epithelium punctuate defect (OC-01 group: 1.1%, vehicle group: 2.5%). All other ocular TEAEs were reported by <1% of total subjects (eTable 8).
Non-ocular TEAEs occurred in a higher proportion of patients in the OC-01 group compared to the vehicle group (86.9% versus 43.6%). The most common non-ocular TEAE in both treatment groups was sneezing (78.3% in the OC 01 group and 22.1% in the vehicle group). The other most commonly reported non-ocular TEAEs had a similar incidence in the OC-01 and vehicle groups: COVID-19 (17.1% and 15.3%, respectively), COVID-19 pneumonia (4.6% and 4.9%, respectively), upper respiratory tract infection (1.7% and 3.1%, respectively), and pyrexia (0.6% and 2.5%, respectively) (Table 2). All other non-ocular TEAEs were reported in <1% of total subjects.
Table 2.
Non-ocular treatment-emergent adverse events occurring in at least 1% of patients.
| n (%) | OC-01 0.6 mg/mL (n = 175) | Placebo (n = 163) |
|---|---|---|
| Non-ocular TEAE | 152 (86.9) | 71 (43.6) |
| Pyrexia | 1 (0.6) | 4 (2.5) |
| COVID-19 | 30 (17.1) | 25 (15.3) |
| COVID-19 pneumonia | 8 (4.6) | 8 (4.9) |
| Upper respiratory tract infection | 3 (1.7) | 5 (3.1) |
| Dizziness | 2 (1.1) | 1 (0.6) |
| Insomnia | 1 (0.6) | 2 (1.2) |
| Sneezing | 137 (78.3) | 36 (22.1) |
| Cough | 1 (0.6) | 2 (1.2) |
| Epistaxis | 2 (1.1) | 0 |
| Rhinorrhoea | 2 (1.1) | 0 |
| Throat irritation | 2 (1.1) | 0 |
Sneezing data collected from patient diaries showed that all sneezing events were mild among both treatment groups (no intervention was needed). In the OC-01 group, 17.2% of administrations caused 1–2 sneezes, 9.1% of administrations caused 3–5 sneezes, and 0.5% of administrations caused ≥6 sneezes. Among subjects who sneezed after administration, most sneezing started and stopped within the first minute.
Visual acuity and pupil size did not indicate any clinically significant change at any visit. Clinically relevant changes in slit lamp biomicroscopy and intranasal examination from baseline at the end of treatment were reported for around 1% of patients in each group. Slit-lamp biomicroscopy was performed at a post-treatment follow-up visit and clinically relevant changes were observed for <1% of patients in each group.
Discussion
To the authors' knowledge, this is the first phase 3 study of OC-01 (varenicline solution) nasal spray conducted in patients with dry eye disease in China. The study results revealed a clinically meaningful improvement in the signs of dry eye disease (the proportion of patients achieving ≥10 mm increase in STS) after 4 weeks of treatment compared with vehicle. An increase of 10 mm in STS indicates a significant improvement in the patient's tear production with apparent clinical meaningfulness and is recommended by the US FDA and NMPA as an endpoint in clinical trials in dry eye disease.20,21 These findings were consistent in a sensitivity analysis conducted in the per protocol population and in a subgroup analysis regardless of the baseline severity of compromised tear production or eye dryness symptoms. Furthermore, OC-01 led to significantly greater improvements in mean changes from baseline in STS at Week 4 and over 50% of patients receiving OC-01 achieved an STS of 10 mm after 4 weeks of treatment, which is entering the normal range for tear production. Taken together, these results show OC-01 leads to a robust and clinically meaningful promotion of tear production in a Chinese patient population. The results are consistent with findings in US populations from the ONSET-1 and ONSET-2 studies.18,19
It is generally accepted that dry eye disease signs and symptoms have high heterogeneity. Therefore, our study also included change in EDS measured in the clinic environment as a secondary endpoint. A post hoc analysis showed that patients receiving OC-01 achieved statistically significant reductions in mean EDS from baseline at Weeks, 1, 3 and 4, which reached around 30% reduction at Week 4. Although this study was not powered to demonstrate a difference in change in EDS between OC-01 and vehicle, OC-01 provided a directional benefit in EDS improvement compared with vehicle. Some in vitro studies have found that the S protein of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) inhibits certain nAChRs subtypes, such as α4β2 or α4α6β2.22,23 In our study, after excluding subjects infected with COVID-19 (the mITT population), numerically greater improvements in EDS and STS at Week 4 were observed in both treatment groups compared with the ITT population. Additionally, in the mITT population analyzed per protocol, patients receiving OC-01 achieved a nominally greater reduction from baseline in EDS at Week 4 versus vehicle. Therefore, infection with COVID-19 may have impacted the sensitivity of the ITT analysis to detect differences between the treatment groups. However, it should be noted that post hoc analyses of EDS in the mITT and PP populations were not adjusted for multiplicity and therefore the probability of type I error may be inflated.
The effect of OC-01 on the signs of dry eye disease was also supported by improvements in CFS. Although both treatment groups showed a gradual decrease in mean CFS scores from baseline, there was a directional benefit favoring OC-01 compared to vehicle in nasal, temporal, and central, as well as total score (including all regions). However, it should be noted that this study was not designed or powered to show small treatment differences in CFS at Week 4. In addition, a moderate baseline corneal lesion and a treatment period of only 4 weeks further reduced the probability of observing statistical differences in CFS. Furthermore, the results from this study should be interpreted with caution as there is evidence that topical anesthesia may enhance corneal staining.24 Despite this, our findings indicate that OC-01 may have a beneficial effect on the improvement in transparency of the central and nasal areas of the cornea, which would have an important clinical impact on patient vision.
Our results show that OC-01 is safe and well tolerated, with no unexpected safety signals observed in the Chinese patient population. Treatment compliance was high (99.27%), which may have been influenced by the good safety profile as well as correct drug administration following effective patient training. The most commonly-reported drug-related TEAE by PT was sneezing (73.1% of subjects in the OC-01 group and 19.0% of subjects in the vehicle group). All sneezing instances were mild among both treatment groups, and no intervention was needed. Among subjects who sneezed after administration, most sneezing started and stopped within the first minute. Sneezing is likely caused by stimulation of the trigeminal nerve and to some extent due to the mechanical administration of the nasal spray, as demonstrated by the sneezing in the vehicle group.25 The safety profile of OC-01 in the present Chinese population was similar to that observed in US populations in the ONSET studies, in which the most common adverse reaction (82% of patients) was sneezing and events reported in 5–16% of patients were cough, throat irritation, and instillation-site (nose) irritation.18,19
We believe the patient population of this study is representative of the broader dry eye disease population in China and the results are generalizable to this population. For example, the mean age of patients was 43.5 years and the majority were female, consistent with dry eye disease epidemiology and the trend of early onset of dry eye disease in China.4, 5, 6 In addition, the concomitant use of artificial tears was allowed, in-line with real clinical practice and the study included patients with minimal signs (i.e., anesthetized STS and CFS) and moderate to severe symptoms (EDS ≥40), which represents the group willing to seek medical treatment with ocular symptoms.
This study had several limitations. Firstly, the study had a relatively short follow-up period. However, despite this limitation, results from the MYSTIC trial have shown a durable effect of OC-01 over a 12-week treatment duration. In addition, long-term safety data for OC-01 from the ONSET-2 and ONSET-1 extension study have shown a consistent safety profile to the present study. Secondly, this study excluded patients who were not able to stop wearing contact lenses or had undergone refractive surgery within 12 months of the screening visit. Therefore, the efficacy and safety of OC-01 in these patient populations remains uncertain. On-going studies are investigating OC-01 in these patient populations, including the CONtaCt (NCT05161208) and RANK (NCT05082974) studies.
In conclusion, OC-01 (varenicline solution) nasal spray BID demonstrated robust, clinically meaningful efficacy for the treatment of the signs of dry eye disease (as measured by STS) and may lead to improvements in symptoms (as measured by EDS), with an excellent safety and tolerability profile, in a Chinese patient population. The novel delivery method of OC-01, via a multi-dose preservative-free nasal spray, spares the ocular surface and represents an effective and convenient treatment option for patients with dry eye disease.
Contributors
J-Y, L-ZP, and D-FM conceived and designed the study.
J-Y, T-L, W-LN, Z-SZ, Z-H, Z-L, F-Y, L-CW, J-XM, X-YG, W-JB, L-XH, Z-Q, T-LM, D-H, Z-MC, Z-QY, G-H, M-LX, R-Q and L-SX enrolled patients and collected the data.
T-L, D-FM and L-ZP drafted the manuscript.
Z-SL conducted the statistical analyses.
All authors contributed to the interpretation of data and critically reviewed and revised the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
J-Y, L-ZP, and D-FM accessed and verified the data.
Data sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted after review.
Declaration of interests
No conflicts of interest to declare.
Acknowledgements
This study was supported by Jixing Pharmaceutical Co Ltd. L-ZP, D-FM, Z-SL and Z-SP reported being employed by and receiving a salary from Jixing Pharmaceutical Co Ltd. Marian Macsai and Kristen Striffler (Oyster Point Pharma, Inc., Princeton, New Jersey) participated in the design of the study, interpretation of the data, and review of the manuscript. Medical writing support, funded by Jixing Pharmaceutical Co Ltd, was provided by Jake Burrell PhD (Rude Health Consulting Ltd, Shanghai, China), in accordance with the Good Publication Practice 3 guidelines.
We are grateful to all patients and their families, the investigators, research nurses, study coordinators, and operations staff who participated in this trial. We thank the following investigators for participating in this study: Lin Lin (The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China); Pengfei Zhang (Weifang Eye Hospital, Shandong, China); Dan Li (Bethune First Hospital of Jilin University, Changchun, China); Liu Yang (Tianjin Medical University Eye Hospital, Tianjin, China); Yunli Niu (Tongji Hospital of Tongji University, Shanghai, China); Zheng Wang (Beijing Hospital, Beijing, China); Liying Zhang (Affiliated Hospital of Guizhou Medical University, Guiyang, China); Huatao Xie (Union Hospital Tongji Medical College Huazhong University of Science and Technology, Hubei, China); Chunsheng Chen (Nanjing First Hospital, Nanjing, China); Yanyan Zhong (Zhujiang Hospital of Southern Medical University, Guangdong, China); Rui Gao (Hebei Eye Hospital, Xing Tai, China); Zhanrong Li (Henan Eye Hospital, Henan Provincial People's Hospital, Zhengzhou, China); Chen Qiao (Wuhan Aier Eye Hospital Hankou Hospital, Hubei, China); Xuedong Chen (The First Affiliated Hospital of Harbin Medical University, Harbin, China); Yang Lu (Shanghai Ninth People's Hospital, Shanghai Jiao tong University School of Medicine, Shanghai, China); Guangwei Li (Eye Hospital of Shandong First Medical University, Shandong, China); Qing Zhang (The Second Hospital of Anhui Medical University, Anhui, China).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2024.101032.
Appendix A. Supplementary data
References
- 1.Dry eye association China chapter, ocular surface and tear disease group of the ophthalmology professional committee of the cross-strait medical and health exchange association, ocular surface and dry eye group of the ophthalmologist branch of the Chinese medical doctor association. Chinese dry eye expert consensus: definition and classification (2020) Chin J Ophthalmol. 2020;56(6):418–422. [Google Scholar]
- 2.Craig J.P., Nichols K.K., Akpek E.K., et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z., Zhang X. Interpret the definition and classification of dry eye in the 2017 dry eye expert consensus of the international tear film and ocular surface association. Chin J Ophthalmol. 2018;54(4):246–248. [Google Scholar]
- 4.Expert Consensus Expert Group on the Standardized Construction of Dry Eye Diagnosis and Treatment Centres. Dry Eye Rehabilitation Professional Group of Visual Rehabilitation Special Committee of Chinese Rehabilitation Medical Association Expert consensus on the standardization of dry eye diagnosis and treatment centres in China (2021) Chin J Exp Ophthalmol. 2021;39(6):473–476. [Google Scholar]
- 5.Corneal Disease Group, Ophthalmology Branch of Chinese Medical Association Expert consensus on clinical diagnosis and treatment of dry eye (2013) Chin J Ophthalmol. 2013;49(1):73–75. [Google Scholar]
- 6.Wei Z.Y., Li H.R., Liang Q.F. Advances on the epidemiology of the dry eye Chinese Journal of Ophthalmology (electronic version) 2020;10(1):46–50. [Google Scholar]
- 7.Dry eye association of Asia, China chapter, ocular surface and tear disease group of the ophthalmology professional committee of the cross-strait medical and health exchange association, ocular surface and dry eye group of the ophthalmologist branch of the Chinese medical doctor association. Chinese dry eye expert consensus: treatment (2020) Chin J Ophthalmol. 2020;56(12):907–913. [Google Scholar]
- 8.Jones L., Downie L.E., Korb D., et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. doi: 10.1016/j.jtos.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Tsubota K., Yokoi N., Watanabe H., et al. A new perspective on dry eye classification: proposal by the Asia dry eye society. Eye Contact Lens. 2020;46 Suppl 1(1):2–13. doi: 10.1097/ICL.0000000000000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bordia T., Hrachova M., Chin M., McIntosh J.M., Quik M. Varenicline is a potent partial agonist at α6β2∗ nicotinic acetylcholine receptors in rat and monkey striatum. J Pharmacol Exp Ther. 2012;342(2):327–334. doi: 10.1124/jpet.112.194852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mihalak K.B., Carroll F.I., Luetje C.W. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha 7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70(3):801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- 12.Dartt D.A. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28(3):155–177. doi: 10.1016/j.preteyeres.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu M.D., Park J.K., Kossler A.L. Stimulating tear production: spotlight on neurostimulation. Clin Ophthalmol. 2021;15:4219–4226. doi: 10.2147/OPTH.S284622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta A., Heigle T., Pflugfelder S.C. Nasolacrimal stimulation of aqueous tear production. Cornea. 1997;16(6):645–648. [PubMed] [Google Scholar]
- 15.Alimohammadi H., Silver W.L. Evidence for nicotinic acetylcholine receptors on nasal trigeminal nerve endings of the rat. Chem Senses. 2000;25(1):61–66. doi: 10.1093/chemse/25.1.61. [DOI] [PubMed] [Google Scholar]
- 16.Albrecht J., Kopietz R., Linn J., et al. Activation of olfactory and trigeminal cortical areas following stimulation of the nasal mucosa with low concentrations of S(-)-nicotine vapor--an fMRI study on chemosensory perception. Hum Brain Mapp. 2009;30(3):699–710. doi: 10.1002/hbm.20535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wirta D., Vollmer P., Paauw J., et al. Efficacy and safety of OC-01 (Varenicline Solution) nasal spray on signs and symptoms of dry eye disease: the ONSET-2 phase 3 randomized trial. Ophthalmology. 2022;129(4):379–387. doi: 10.1016/j.ophtha.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Wirta D., Torkildsen G.L., Boehmer B., et al. ONSET-1 phase 2b randomized trial to evaluate the safety and efficacy of OC-01 (Varenicline Solution) nasal spray on signs and symptoms of dry eye disease. Cornea. 2022;41(10):1207–1216. doi: 10.1097/ICO.0000000000002941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quiroz-Mercado H., Hernandez-Quintela E., Chiu K.H., Henry E., Nau J.A. A phase II randomized trial to evaluate the long-term (12-week) efficacy and safety of OC-01 (Varenicline Solution) nasal spray for dry eye disease: the MYSTIC study. Ocul Surf. 2022;24:15–21. doi: 10.1016/j.jtos.2021.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Food and Drug Administration, Center for Drug Evaluation and Research . 2020. Dry eye: developing drugs for treatment guidance for industry: draft guidance.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/dry-eye-developing-drugs-treatment-guidance-industry Available from: [Google Scholar]
- 21.National Medical Products Administration (NMPA) Technical guidelines for clinical trials of dry eye therapy drugs. 2022. https://www.cde.org.cn/main/news/viewInfoCommon/4da74ab70a9f4e8f799db9d8b9c91982 Available from:
- 22.Carlson E.C., Macsai M., Bertrand S., Bertrand D., Nau J. The SARS-CoV-2 virus and the cholinergic system: spike protein interaction with human nicotinic acetylcholine receptors and the nicotinic agonist varenicline. Int J Mol Sci. 2023;24(6):5597. doi: 10.3390/ijms24065597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadwa E.H., Al-Kuraishy H.M., Al-Gareeb A.I., et al. Cholinergic dysfunction in COVID-19: frantic search and hoping for the best. Naunyn Schmiedebergs Arch Pharmacol. 2023;396(3):453–468. doi: 10.1007/s00210-022-02346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Josephson J.E., Caffery B.E. Corneal staining after instillation of topical anesthetic (SSII) Invest Ophthalmol Vis Sci. 1988;29(7):1096–1099. [PubMed] [Google Scholar]
- 25.Önerci T.M. 2013. Nasal physiology and pathophysiology of nasal disorders; pp. 1–616. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.