1. Introduction
Traumatic brain injuries or large-vessel occlusion strokes can lead to the development of medically refractory intracranial hypertension and malignant cerebral edema. Contained within the bony cranium, an engorging brain can become compressed, leading to catastrophic outcomes. Decompressive craniectomy can therefore be a life-saving intervention during states of high intracranial pressure.1,2 Once the causal pathology has been sufficiently treated and brain swelling has subsided, a cranioplasty is performed to provide renewed physical protection of neurological structures and to restore aesthetic cranial morphology.3 Traditionally, an autologous cranioplasty is fashioned using the patient's own cranial bone, which was stored in freezers or subcutaneously within the patient's abdominal wall. The bone is prepped and cleaned before being reapproximated and secured to its original place.4,5
Complications such as infection, bone resorption, and skin flap breakdown are commonplace after autologous cranioplasties, with failure rates as high as 40%.6, 7, 8 To address these shortcomings, synthetic cranioplasty materials such as polymethylmethacrylate, hydroxyapatite implants, titanium mesh, and polyetheretherketone (PEEK) have been developed. Many of these synthetic implants can be intricately fashioned with computer-aided design techniques that allow for a patient-specific custom fit.9,10 Although they are more expensive than autologous bone grafts, custom implants ideally offer multiple advantages, including faster operating times (because they are prefabricated and require no bone retrieval operations or bone preparation), alleviation of bone flap storage complexities, no potential for bone flap resorption, reduced postoperative infection rates, and a more precise fit, which reduces the likelihood of imbalanced skin pressures with resultant skin breakdown.6,11, 12, 13, 14, 15, 16, 17, 18 These benefits suggest that custom implants could supersede autologous implants as the new gold standard. To this end, a limited number of studies have examined whether the increased initial costs of a custom cranioplasty can be economically justified by reducing the long-term complications, and thus total costs, more commonly observed among autologous grafts.11,19,20 Considering the many nuances such as synthetic material type, surface area required, and length of follow-up, it has been difficult to fully address this question. We examined our experience managing patients who underwent autologous or custom cranioplasty and assessed the comparative short- and aggregated long-term costs of the two techniques, using a proprietary institutional cost database. To enhance the robustness of our analysis and better isolate the effects of each implant modality, we employed propensity score-matching controlling for known risk factors for cranioplasty failure, an approach that has yet to be utilized in this arena.
2. Methods
2.1. Study cohort
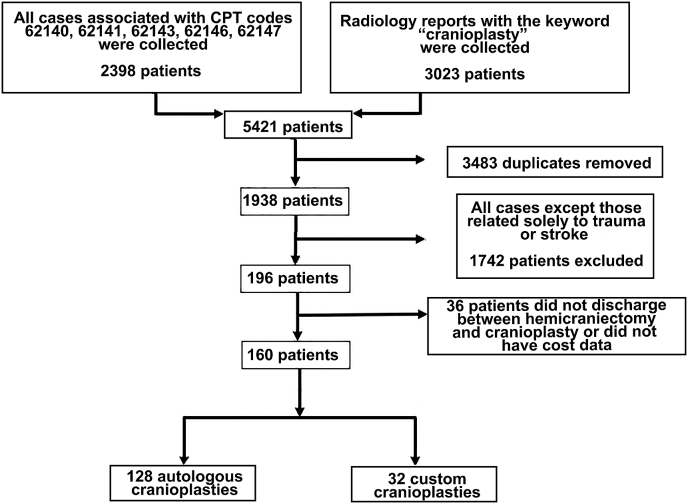
This retrospective study was approved by the institutional review board with a waiver of informed consent. We queried the University of Utah billing records to identify all patient encounters between January 1, 2011, and June 1, 2022, that were coded with Common Procedural Terminology codes corresponding to cranioplasty (62,140, 62,141, 62,143, 62,146, 62,147). All autologous and custom implant types were included. To ensure that no patients were missed, we also reviewed the charts of all patients who had imaging reports with the keyword “cranioplasty.” Duplicate patients and pediatric cases (patients <18 years old) were excluded (Fig. 1). Likewise, all cranioplasties undertaken for reasons other than trauma or stroke were excluded. This was done to control for confounding pathologies like brain tumors, which might impart additional costs and long-term complication events that could bias our results. Lastly, patients were excluded if they were not discharged from the hospital between the hemicraniectomy and cranioplasty or if there were no available cost data.
Fig. 1.
Patient inclusion diagram.
2.2. Assessed variables
Baseline demographics of the patients and clinical characteristics of the hemicraniectomy and cranioplasty procedures were collected. These variables included age, sex, race, American Society of Anesthesiologists (ASA) class, and body mass index (BMI); history of diabetes mellitus (DM), hyperlipidemia (HLD), hypertension (HTN), smoking, and alcohol use; and procedures before hemicraniectomy, shunts placed before hemicraniectomy, ASA class at hemicraniectomy, Glasgow Coma Scale (GCS) score at hemicraniectomy, interval from hemicraniectomy to cranioplasty, cranioplasty operation duration, length of hospital stay after cranioplasty, disposition after cranioplasty, and unplanned reoperation <30 days after cranioplasty. Additional data regarding cranioplasty size and materials and long-term outcomes were collected. These included rates of failure after initial cranioplasties but also after second and third revision operations, if applicable. Rates of implant resorption and infection were captured, and each patient's length of clinical follow-up was noted.
2.3. Value driven outcomes cost data
The Value Driven Outcomes (VDO) database is an innovative resource that calculates healthcare-related costs based on the prices of instruments, drugs, and medical procedures, instead of patient or insurer charges. Its methodology has been described in previous studies.21,22 We employed the VDO tool to capture the relevant cranioplasty costs for the patients identified through the mechanisms described. Recorded cost data included initial cranioplasty total hospitalization costs as well as subsequent cranioplasty-related hospitalization costs. For each hospitalization, subcategory costs of pharmacy, imaging, supplies, laboratory, facility, and other were captured. All cost data are expressed as a percentage of the initial custom cranioplasty hospitalization total cost. Actual dollar amounts are not disclosed because of an agreement with University of Utah Health.
2.4. Statistical analysis
SPSS version 20 (IBM Corp.) was used for statistical analysis. Data normality was assessed using the Wilk–Shapiro test. Student's T-test or the Mann–Whitney U test was used in assessment of continuous variables per distribution. The Pearson Chi-square test was used for comparison of categorical variables. Linear regression was used to perform multivariate analysis. The multivariate models were adjusted for clinically notable implant failure risk factors.23, 24, 25, 26, 27 Statistical significance was determined at α = 0.05.
A 1:1 “nearest-neighbor” propensity-score matching scheme was completed using the MatchIt R package. Matches were based on predictors of cranioplasty failure and included age, interval from hemicraniectomy to cranioplasty (days), smoking status, procedures performed before hemicraniectomy, implant surface area, and postoperative length of stay.23, 24, 25, 26, 27 A caliper weight of 0.1 was used. Statistical significance remained at α = 0.05.
3. Results
3.1. Unmatched demographic comparisons
A total of 32 patients with custom implants were compared with 128 patients with autologous grafts (Table 1). The custom cohort was more predominantly male (81.3% vs. 60.9%, p = 0.03) and had less hypertension (15.6% vs. 36.7%, p = 0.02) than the autologous cohort. There were no significant differences in other assessed demographic variables, including age (37.7 vs. 43.1 years), race, median BMI (25.8 vs. 28.4), DM (9.4% vs. 15.6%), HLD (9.4% vs. 20.3%), active smoker (25.0% vs. 28.9%), and alcohol abuse (9.4% vs. 11.7%) (all p ≥ 0.05).
Table 1.
Unmatched and matched comparisons of demographic and clinical variables for initial cranioplasty.
| Variable | Unmatched |
Matched |
||||
|---|---|---|---|---|---|---|
| Autologous (n = 128) | Custom (n = 32) | P value | Autologous (n = 29) | Custom (n = 29) | P value | |
| Age at HC | 43.1 ± 15.1 | 37.7 ± 16.2 | 0.07 | 30 (23–50) | 39 (24–53) | 0.76 |
| Male sex | 78 (60.9) | 26 (81.3) | 0.03 | 16 (55.2) | 24 (82.8) | 0.02 |
| Race | 0.47 | 0.23 | ||||
| White | 100 (78.1) | 27 (84.4) | 22 (75.9) | 25 (86.2) | ||
| Black | 2 (1.6) | 1 (3.1) | 0 | 1 (3.4) | ||
| Asian or Pacific Islander | 8 (6.3) | 0 | 3 (10.3) | 0 | ||
| Other | 18 (14.1) | 4 (12.5) | 4 (13.8) | 3 (10.3) | ||
| BMIa | 28.4 (23.5–31.5) | 25.8 (23.0–30.8) | 0.54 | 26.1 (23.3–30.2) | 25.9 (23.4–30.7) | 0.70 |
| DM | 20 (15.6) | 3 (9.4) | 0.37 | 2 (6.9) | 3 (10.3) | 0.64 |
| HLD | 26 (20.3) | 3 (9.4) | 0.15 | 4 (13.8) | 3 (10.3) | 0.69 |
| HTN | 47 (36.7) | 5 (15.6) | 0.02 | 9 (31.0) | 5 (17.2) | 0.22 |
| Active smoker | 37 (28.9) | 8 (25.0) | 0.66 | 6 (20.7) | 7 (24.1) | 0.75 |
| Alcohol abuse | 15 (11.7) | 3 (9.4) | 0.71 | 3 (10.3) | 2 (6.9) | 0.64 |
| Procedures prior to HC | 10 (7.9) | 6 (18.8) | 0.07 | 4 (13.8) | 6 (20.7) | 0.49 |
| Perioperative shunt for HC | 26 (20.3) | 6 (18.8) | 0.84 | 7 (24.1) | 5 (17.2) | 0.52 |
| ASA class at HC | 0.18 | 0.36 | ||||
| Class I | 3 (2.3) | 0 | 0 | 0 | ||
| Class II | 5 (3.9) | 4 (12.5) | 1 (3.4) | 2 (6.9) | ||
| Class III | 24 (18.8) | 9 (28.1) | 4 (13.8) | 9 (31.0) | ||
| Class IV | 56 (43.8) | 12 (37.5) | 15 (51.7) | 12 (41.4) | ||
| Class V | 40 (31.3) | 7 (21.9) | 9 (31.0) | 6 (20.7) | ||
| GCS score at HC | 9.3 ± 3.7 | 9.3 ± 4.3 | 0.99 | 7 (6–10) | 9 (5–13) | 0.34 |
| Time from HC to CP (days)a | 61 (44–81) | 80 (61–131) | 0.04 | 49 (41–80) | 80 (57–123) | <0.01 |
| Reason for HC | 0.08 | 0.75 | ||||
| Trauma | 83 (64.8) | 26 (81.3) | 22 (75.9) | 23 (79.3) | ||
| Stroke | 45 (35.2) | 6 (18.8) | 7 (24.1) | 6 (20.7) | ||
| Interval complication between HC and CP | 37 (28.9) | 9 (28.1) | 0.93 | 6 (20.7) | 8 (27.6) | 0.54 |
| CP operation durationa | 127 (96–142) | 125 (90–162) | 0.87 | 124 (100–138) | 128 (97–160) | 0.45 |
| Length of stay (days)a | 3 (2–5) | 3 (2–4) | 0.60 | 3.0 (2.0–5.0) | 3 (1.8–4.5) | 0.74 |
| Disposition following CP | 0.66 | 0.29 | ||||
| Home | 70 (54.7) | 19 (59.4) | 18 (62.1) | 18 (62.1) | ||
| Rehab | 24 (18.8) | 4 (12.5) | 2 (6.9) | 4 (13.8) | ||
| SNF | 23 (18.0) | 8 (25.0) | 5 (17.2) | 1 (3.4) | ||
| Long-term acute care | 10 (7.8) | 1 (3.1) | 4 (13.8) | 6 (20.7) | ||
| Prison | 1 (0.8) | 0 | 2 (6.9) | 3 (10.3) | 0.64 | |
| Unplanned reoperation <30 days after CP | 11 (8.6) | 3 (9.4) | 0.89 | |||
Values reported as number (%), mean ± standard deviation.
Boldface font indicates statistical significance.
ASA, American Association of Anesthesiologists'; BMI, body mass index; CP, cranioplasty; DM, diabetes mellitus; GCS, Glasgow coma scale; HC, hemicraniectomy; HLD, hyperlipidemia; HTN, hypertension; SNF, skilled nursing facility.
Non-parametric continuous data reported as median (interquartile range).
3.2. Unmatched hemicraniectomy and initial cranioplasty management
Patients who underwent custom cranioplasties had a significantly longer median interval (80 days) between hemicraniectomy and cranioplasty than autologous patients (61 days, p = 0.04) (Table 1). However, other variables were not significantly different between the custom and autologous groups, including procedures before hemicraniectomy (18.8% vs. 7.9%), ASA class, mean GCS at hemicraniectomy (9.3 vs 9.3), reason for cranioplasty (81.3%/18.8% vs. 64.8%/35.2% trauma/stroke), complication rate between hemicraniectomy and cranioplasty (28.1% vs. 28.9%), median cranioplasty operation duration (125 vs. 127 min), disposition, and unplanned reoperation within 30 days of cranioplasty (9.4% vs. 8.6%) (all p > 0.05).
3.3. Unmatched long-term outcomes
The rate of initial graft failure was not significantly different between the custom (12.5%) and autologous (23.4%) graft groups (p = 0.18) (Table 2). Understandably, however, the rates of implant failure resulting from bone resorption were significantly higher in the autologous cohort (14.1%) than in the custom group (0%) (p = 0.02). Differences in other long-term outcomes between custom and autologous groups also failed to reach levels of significance, including median days from cranioplasty to failure (256 vs. 170 days), failure due to infection (12.5% vs. 12.5%), mean implant surface area (144 vs. 153 cm2), second cranioplasty (12.5% vs. 22.7%), second cranioplasty failure (6.3% vs. 7.8%), third cranioplasty (6.3% vs. 7.0%), and median clinical follow-up (5.0 vs. 5.2 months) (all p > 0.05).
Table 2.
Unmatched and matched comparisons of initial cranioplasty outcomes.
| Unmatched |
Matched |
|||||
|---|---|---|---|---|---|---|
| Autologous (n = 128) | Custom (n = 32) | P value | Autologous (n = 29) | Custom (n = 29) | P value | |
| Initial CP failure | 30 (23.4) | 4 (12.5) | 0.18 | 5 (17.2) | 4 (13.8) | 0.72 |
| Median time from CP to failure (days)a | 170 (56–475) | 256 (60–523) | 0.60 | 163 (28–448) | 256 (60–523) | 0.87 |
| Reason for CP failure | ||||||
| Infection | 16 (12.5) | 4 (12.5) | 1.00 | 2 (6.9) | 4 (13.8) | 0.39 |
| Bone resorption | 18 (14.1) | 0 | 0.02 | 3 (10.3) | 0 | 0.08 |
| CP surface area (cm2) | 152.7 ± 27.3 | 143.7 ± 40.6 | 0.14 | 155 (135–172) | 150 (132–164) | 0.62 |
| Second CP | 29 (22.7) | 4 (12.5) | 0.20 | 5 (17.2) | 4 (13.8) | 0.72 |
| Second CP failure | 10 (7.8) | 2 (6.3) | 0.76 | 3 (10.3) | 2 (6.9) | 0.64 |
| Third CP | 9 (7.0) | 2 (6.3) | 0.88 | 3 (10.3) | 2 (6.9) | 0.64 |
| Time to last clinical follow-up (months)a | 5.2 (1.4–19.3) | 5.0 (1.1–10.7) | 0.26 | 3.9 (1.1–11.7) | 4.9 (1.1–11.6) | 0.87 |
Values reported as number (%) or mean ± standard deviation.
Boldface font indicates statistical significance.
CP, cranioplasty.
Non-parametric continuous data reported as median (interquartile range).
3.4. Unmatched custom graft types
For the initial cranioplasty, 32 patients received custom grafts. In these cases, PEEK was used most often for initial cranioplasty (34.4%), with fewer patients receiving a calcium phosphate graft reinforced with 3D printed titanium (21.9%), titanium mesh (18.8%), porous polyethylene sheet (15.6%), and calcium phosphate bone cement (9.4%) (Supplemental Table 1). In the 33 patients (including both originally autologous and custom grafts) who required a second cranioplasty, titanium mesh was used most frequently (48.5%), followed by calcium phosphate graft reinforced with 3D printed titanium (27.3%), porous polyethylene sheet (12.1%), PEEK (9.1%), and methyl methacrylate (3.0%). In the 11 patients who required a third cranioplasty, titanium mesh was used most often (72.7%), followed by equal numbers of calcium phosphate graft reinforced with 3D printed titanium (9.1%), porous polyethylene sheet (9.1%), and methyl methacrylate (9.1%).
3.5. Unmatched cost analysis of initial cranioplasty implants
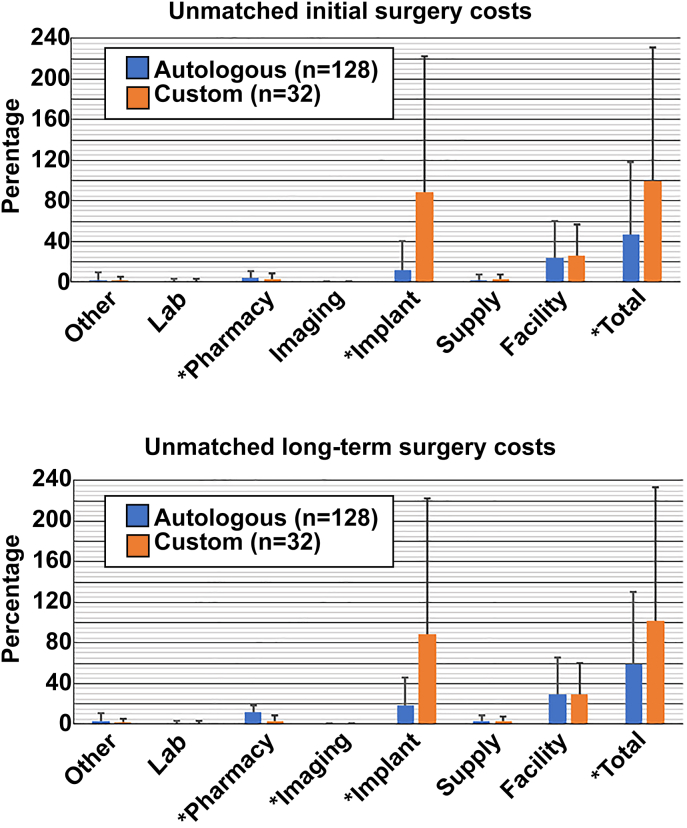
Assessment of initial cranioplasty costs found that custom cranioplasties (100%) were significantly more expensive than autologous grafts (46.8%, p < 0.01) (Table 3, Fig. 2, top). Cost subcategories with statistically significant differences between groups included implant costs (89.2%vs. 12.2%, p < 0.01) and pharmacy costs (3.1% vs. 4.4%, p = 0.03). Other subcategories had nonsignificant costs differences, including other costs (1.6% vs. 2.1%, p = 0.42), laboratory costs (1.0% vs. 1.0%, p = 0.55), imaging costs (0.2% vs. 0.2%, p = 0.18), supply costs (2.7% vs. 2.4%, p = 0.88), and facility costs (26.0% vs. 24.0%, p = 0.80).
Table 3.
Unmatched and matched comparisons of initial and long-term costs reported as median percent of the custom approach (interquartile range).
| Costs type | Unmatched |
Matched |
||||
|---|---|---|---|---|---|---|
| Autologous (n = 128) | Custom (n = 32) | P value | Autologous (n = 29) | Custom (n = 29) | P value | |
| Initial surgery costs | ||||||
| Other | 2.1 (1.0–7.5) | 1.6 (1.1–3.8) | 0.42 | 1.6 (0.9–4.5) | 1.6 (1.0–4.2) | 0.97 |
| Lab | 1.0 (0.5–2.0) | 1.0 (0.6–2.2) | 0.55 | 1.2 (0.6–3.0) | 0.9 (0.6–1.9) | 0.37 |
| Pharmacy | 4.4 (3.2–6.7) | 3.1 (2.2–5.3) | 0.03 | 3.6 (2.2–4.8) | 3.3 (2.0–6.1) | 0.99 |
| Imaging | 0.2 (0.2–0.6) | 0.2 (0.1–0.4) | 0.18 | 0.2 (0.1–0.6) | 0.2 (0.1–0.4) | 0.78 |
| Implant | 12.2 (4.9–27.7) | 89.2 (24.2–133.6) | <0.01 | 6.5 (4.2–26.8) | 88.5 (30.7–132.5) | <0.01 |
| Supply | 2.4 (1.3–5.3) | 2.7 (1.4–5.0) | 0.88 | 2.9 (1.4–4.8) | 2.9 (1.6–5.4) | 0.62 |
| Facility | 24.0 (18.8–36.6) | 26.0 (17.1–31.2) | 0.80 | 21.2 (19.1–33.9) | 26.1 (17.4–31.6) | 0.70 |
| Total direct | 46.8 (36.0–71.1) | 100.0 (76.3–131.5) | <0.01 | 58.3 (31.8–68.9) | 100.0 (76.3–126.6) | <0.01 |
| Initial plus long-term costs | ||||||
| Other | 2.9 (1.2–10.2) | 1.7 (1.1–4.1) | 0.07 | 1.7 (1.0–4.9) | 1.7 (1.0–4.3) | 0.73 |
| Lab | 1.4 (0.6–4.1) | 1.0 (0.7–2.8) | 0.74 | 1.3 (0.7–3.0) | 1.0 (0.7–2.5) | 0.32 |
| Pharmacy | 12.2 (3.4–9.8) | 3.2 (2.3–6.4) | <0.01 | 3.8 (2.3–7.0) | 3.7 (2.2–6.8) | 0.41 |
| Imaging | 0.3 (0.2–0.7) | 0.2 (0.1–0.4) | 0.04 | 0.3 (0.2–0.6) | 0.3 (0.1–0.4) | 0.41 |
| Implant | 18.5 (6.0–46.6) | 89.2 (46.0–133.6) | <0.01 | 44.7 (5.4–112.4) | 96.3 (49.6–132.1) | <0.01 |
| Supply | 3.3 (1.4–7.2) | 2.8 (1.4–7.2) | 0.47 | 2.9 (1.5–5.9) | 2.9 (2.1–6.0) | 0.76 |
| Facility | 29.5 (19.7–65.4) | 29.4 (18.7–33.21) | 0.23 | 26.6 (19.3–38.4) | 29.9 (21.0–33.2) | 0.77 |
| Total direct | 58.7 (35.5–136.8) | 101.8 (78.2–133.2) | 0.03 | 86.3 (36.5–134.8) | 101.9 (78.9–125.5) | 0.03 |
Boldface font indicates statistical significance.
Data reported as median percent (interquartile range).
Fig. 2.
Unmatched initial (top) and long-term (bottom) hospitalization costs related to cranioplasty surgery are illustrated. For the unmatched initial surgery costs, custom cranioplasty was significantly cheaper in pharmacy costs, but more expensive in imaging and total direct costs. Upon long-term analysis, custom cranioplasty was less costly in pharmacy and imaging costs, but increased in implant and total direct costs (all p < 0.05). All costs (initial and long-term) are represented as a percentage of unmatched initial total custom cranioplasty costs (Statistical significance indicated with asterisk.)
3.6. Longitudinal cost analysis of custom vs. autologous implants
After univariate aggregation of all initial and long-term cranioplasty hospital costs, using initial custom cranioplasty hospitalization costs as a baseline, custom cranioplasties remained significantly more expensive than autologous grafts (101.8% custom vs. 58.7% autologous, p = 0.03) (Table 3, Fig. 2, bottom). Cost subcategories with significant differences included implant costs (89.2% vs. 18.5%, p < 0.01), pharmacy costs (3.2% vs. 12.2%, p < 0.01), and imaging costs (0.2% vs. 0.3%, p = 0.04). Subcategories that were not signficantly different between the two groups included other costs (1.7% vs. 2.9%, p = 0.07), laboratory costs (1.0% vs. 1.4%, p = 0.74), supply costs (2.8% vs. 3.3%, p = 0.47), and facility costs (29.4% vs. 29.5%, p = 0.23).
A subanalysis was performed to assess whether differences in initial and long-term costs exist depending upon injury cause (i.e., trauma vs. stroke) (Supplemental Table 2). No differences were detected among the custom or autologous cohorts regardless of timeframe (all p > 0.05).
3.7. Multivariate analysis of longitudinal total direct costs for autologous and nonautologous implants
Multivariate linear regression was performed to isolate the independent effects of cranioplasty implant type on initial and long-term costs. The model was adjusted for age, sex, smoking status, HTN, GCS, trauma vs. stroke cause, graft surface area, interval (days) from hemicraniectomy to cranioplasty, cranioplasty operation duration, and length of stay. For initial costs, custom cranioplasty implants were significantly associated with higher costs (standardized β = 0.20, p < 0.01) (Table 4). The other significant contributer to initial cost was length of stay (standardized β = 0.92, p < 0.01). A similar multivariate linear regression was performed for long-term costs (Table 4). Notably, custom cranioplasty was not an independently significant contributer to the aggregated long-term total costs (standardized β = 0.10, p = 0.13), whereas initial craniplasty operation time (standardized β = 0.13, p = 0.03) and initial cranioplasty length of stay (standardized β = 0.63, p < 0.01) were.
Table 4.
Unmatched multivariate linear regression for initial cranioplasty and long-term total direct costs ($).
| Variable | Standardized Coefficients | 95% confidence intervals |
P value | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Initial surgery total direct costs | ||||
| Custom CP | 0.20 | 0.13 | 0.26 | <0.01 |
| Smoker | −0.02 | −0.08 | 0.04 | 0.52 |
| HTN | −0.03 | −0.09 | 0.04 | 0.44 |
| Age | 0.04 | −0.03 | 0.11 | 0.23 |
| Male sex | 0.00 | −0.06 | 0.06 | 0.93 |
| GCS score | −0.01 | −0.08 | 0.05 | 0.68 |
| Reason for HC (stroke) | −0.01 | −0.07 | 0.05 | 0.80 |
| CP surface area (cm2) | 0.02 | −0.04 | 0.08 | 0.51 |
| Time from HC to CP (days) | 0.02 | −0.04 | 0.08 | 0.48 |
| CP operative duration (min) | 0.03 | −0.03 | 0.03 | 0.30 |
| Length of stay (days) | 0.92 | 0.86 | 0.98 | <0.01 |
| Initial plus long-term total direct costs | ||||
| Custom CP | 0.10 | −0.03 | 0.22 | 0.13 |
| Smoker | −0.02 | −0.14 | 0.10 | 0.75 |
| Hypertension | 0.02 | −0.11 | 0.16 | 0.76 |
| Age | 0.13 | 0.00 | 0.27 | 0.04 |
| Male sex | −0.10 | −0.22 | 0.03 | 0.12 |
| GCS score | −0.02 | −0.14 | 0.11 | 0.80 |
| Reason for HC (stroke) | 0.00 | −0.12 | 0.13 | 0.97 |
| CP surface area (cm2) | 0.07 | −0.05 | 0.20 | 0.25 |
| Time from HC to CP (days) | −0.03 | −0.16 | 0.09 | 0.59 |
| CP operative duration (min) | 0.13 | 0.01 | 0.25 | 0.03 |
| Length of stay (days) | 0.63 | 0.51 | 0.75 | <0.01 |
For initial cranioplasty costs, R2 = 0.87, p < 0.01. For long-term cranioplasty costs, R2 = 0.50, p < 0.01.
CP, cranioplasty; GCS, Glasgow Coma Scale; HC, hemicraniectomy; HTN, hypertension.
Boldface font indicates statistical significance.
3.8. Matched analysis of demographic and clinical variables
After a 1:1 propensity-score matching based on implant type, 29 patient pairs were available for analysis. Comparisons for baseline demographics and clinical course were performed as previously described (Table 1, Table 2). Statistically significant differences included custom implants being used more often in male patients (82.8% vs. 55.2%, p = 0.02) and a longer interval from hemicraniectomy to cranioplasty in patients undergoing custom cranioplasty (80 vs. 49 days, p < 0.01). There were no significant differences in outcomes between the two groups (all p > 0.05).
3.9. Matched initial cranioplasty and long-term cost analyses
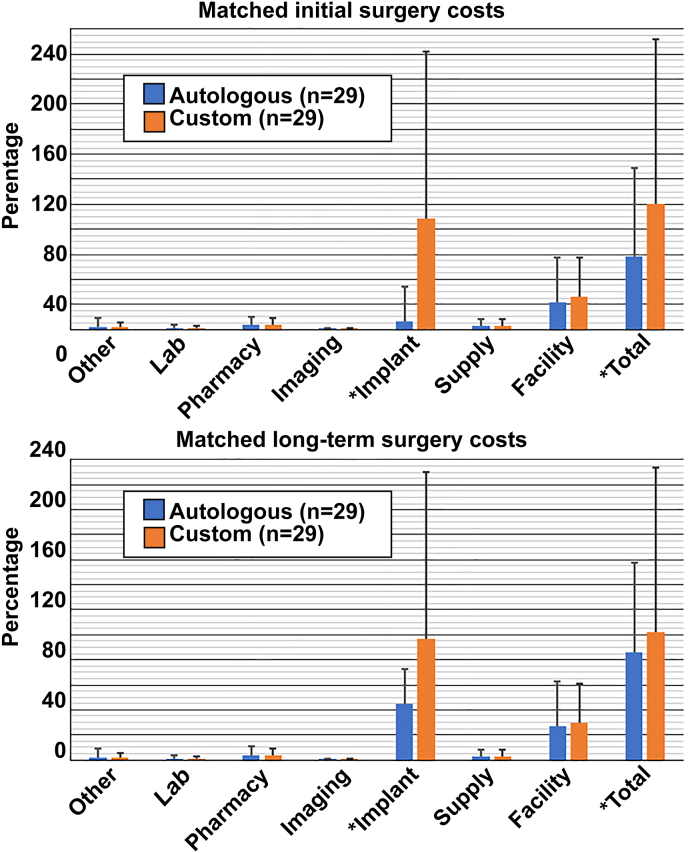
Univariate analysis of the matched cohorts likewise found custom grafts were more expensive in the initial surgery (100.0% custom vs. 58.3% autologous, p < 0.01) and in the long term (101.9% vs. 86.3%, p = 0.03) (Table 3, Fig. 3, top). In the matched cohorts, multivariate linear regression also demonstrated that costs for initial surgeries were greater in the custom group (standardized β = 0.56, p < 0.01) but long-term costs were not (standardized β = 0.22, p = 0.11) (Table 5, Fig. 3, bottom).
Fig. 3.
Matched initial (top) and long-term (bottom) hospitalization costs related to cranioplasty surgery and are shown. For the matched initial surgery costs, custom craniplpasty was statistically more costly for implant and total direct costs. Upon long-term analysis, implant and total direct costs remained statistically more expensive for custom grafts (all p < 0.05). All costs (initial and long-term) are represented as a percentage of matched initial total custom cranioplasty costs (Statistical significance indicated with asterisk.)
Table 5.
Matched multivariate linear regression for initial cranioplasty and long-term total direct costs.
| Variable | Unstandardized Coefficients | 95% confidence intervals |
P value | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Initial surgery total direct costs | ||||
| Custom CP | 0.56 | 0.39 | 0.74 | <0.01 |
| Smoker | −0.02 | −0.21 | 0.16 | 0.80 |
| HTN | −0.02 | −0.21 | 0.17 | 0.84 |
| Age | 0.02 | −0.18 | 0.23 | 0.83 |
| Sex | −0.11 | −0.29 | 0.07 | 0.24 |
| GCS | −0.18 | −0.36 | 0.01 | 0.06 |
| Reason for HC (stroke) | 0.01 | −0.22 | 0.25 | 0.92 |
| CP surface area (cm2) | 0.01 | −0.17 | 0.19 | 0.91 |
| Time from HC to CP (days) | 0.12 | −0.08 | 0.32 | 0.24 |
| CP operative duration (min) | 0.04 | −0.13 | 0.21 | 0.63 |
| Length of stay (days) | 0.66 | 0.47 | 0.85 | <0.01 |
| Initial plus long-term total direct costsa | ||||
| Custom CP | 0.22 | −0.05 | 0.49 | 0.11 |
| HTN | 0.18 | −0.10 | −0.10 | 0.46 |
| Age | 0.16 | −0.15 | 0.48 | 0.30 |
| Sex | −0.27 | −0.53 | −0.01 | 0.04 |
| GCS | −0.15 | −0.44 | 0.13 | 0.28 |
| Reason for HC (stroke) | 0.09 | −0.25 | 0.43 | 0.60 |
| Cranioplasty surface area (cm2) | 0.04 | −0.23 | 0.32 | 0.75 |
| Time from HC to CP (days) | −0.01 | −0.31 | 0.30 | 0.97 |
| CP operative duration (min) | 0.21 | −0.06 | 0.47 | 0.12 |
| Length of stay (days) | 0.21 | −0.08 | 0.49 | 0.16 |
For initial cranioplasty costs, R2 = 0.72, p < 0.01. For long-term cranioplasty costs, R2 = 0.32, p = 0.03.
CP, cranioplasty; GCS, Glasgow coma score, HC, hemicraniectomy; HTN, hypertension.
Boldface font indicates statistical significance.
Smoking status removed to achieve adequate goodness of fit.
4. Discussion
Decompressive hemicraniectomy and subsequent cranioplasty surgery are fundamental, life-saving procedures in the care of neurosurgical patients, but rates of implant failure remain quite high. To improve implant longevity, enhance cosmesis, and mitigate complication profile, multiple custom implant materials have been developed. However, the cost implications of these synthetic implants remain insufficiently described, and questions remain as to whether custom grafts are economically justified as a primary treatment. In this study, we compared cranioplasty patients with custom or autologous implants to evaluate for cost differences in the short- or long-term using a well-validated tool that tracks costs at our institution.
4.1. Cost comparison of custom vs. autologous implants
Our unmatched multivariate cost analysis demonstrated significant differences in favor of autologous grafts in the initial surgery period (custom implants: standardized β = 0.20, p < 0.01) while no statistical difference was observed in the long term (custom implants: standardized β = 0.10, p = 0.13). These findings were replicated upon matched analysis (standardized β = 0.56, p < 0.01; standardized β = 0.22, p = 0.11; respectively). It is difficult to contextualize and directly compare these findings with those of other reports given the limited number of available studies and the heterogeneity in custom implant materials and follow-up protocols. Typically, however, although cost analysis results have been mixed, autologous grafts have often been favored in the short term and custom implants in the long term.
For example, in a randomized controlled trial assessing the cost disparity between titanium implants (n = 32) and autologous grafts (n = 32), Honeybul et al28 demonstrated that healthcare costs were statistically equivalent in the initial cranioplasty encounter regardless of graft type ($3281 mean difference in favor of titanium cranioplasty; p = 0.327). However, upon 24-month follow-up, this same trial demonstrated a significant cost advantage favoring titanium implants, with average healthcare savings of $9999 (p = 0.015) compared with autologous grafts.29 In an analysis of 33 patients (17 custom and 16 autolous), Lethaus et al30 found that patient-specific implants (10 PEEK, 7 titanium) were more costly than autologous grafts in the initial surgery (€15,532 vs. €10,850); however, once long-term subsequent costs were factored in, custom implants became the cheaper option (€15,532 vs. €26,086). Conversely, other analyses such as that by Binhammer et al19 have reported mixed results. In their analysis, titanium mesh grafts were more cost-effective than autologous grafts in the setting of smaller cranial defects; however, autologous materials were more financially favorable for larger defects (>5 cm).
In our analysis, 6 different synthetic material types were used and follow-up was variable.
As reflected in the previously discussed studies,19,28,29 the most significant contributer to custom cranioplasty costs in the present analysis were the implants themselves. In the unmatched analysis, custom implants accounted for 89.2% of the total initial custom cranioplasty hospitalization costs. This drastically contrasted with the median percentage cost of autologous grafts, which was only 12.2% of the total initial custom cranioplasty hospitalization costs (p < 0.01). Although median initial implant costs were significantly higher in custom grafts, other hospitalization subcategory costs that were statistically equivalent to that of autologous grafts included other costs, laboratory costs, supply costs, and facility costs. Moreover, custom grafts were comparatively significantly cheaper in initial pharmacy costs and imaging costs (although these were still much smaller than the cost of the implants).
4.2. Failure rates
A key element in evaluating cost differences is ensuring that the outcomes in the comparison group are noninferior to those in the control group. Complicating variables related to patient age, cause of the bony defect, condition of the flap or implant, size and location of the cranial defect, or other concurrently existing pathologies can impede proper cranioplasty formation or otherwise predispose to ultimate implant failure.31 Partly because of their biological nature and capacity for resorption, autologous implants have demonstrated greater risk of implant failure compared with custom materials.29 In our study, 14.1% of initial autologous cranioplasties failed because of bone resorption (Table 2). Likewise, some custom cranioplasty materials are lauded for their nonbiologic nature and other properties that reduce infection risk.32 For example, PEEK is built with a porous design that facilitates tissue integration, thereby reducing residual empty space that can act as a nidus for infection.33 In our study, autologous implants generally demonstrated elevated, though statistically insignificantly, rates of initial cranioplasty failure (12.5% custom vs. 23.4% autologous; p = 0.18). The lack of significant differences between failure rates is likely a limitation of our study's sample size. However, our autologous failure rate falls within the range of other published reports, which describe autologous cranioplasty failure rates of 7–40%.6, 7, 8,17,34, 35, 36 Significant efforts are being made within both autologous and custom cranioplasty protocols/materials to lessen rates of postimplantation infection, necrosis, and resorption.37,38
4.3. Future directions
Growing clinical evidence strongly suggests that currently available custom materials demonstrate notable clinical advantages over autologous implants.17 A major concern impeding a more widespread use of custom implants has been related to cost.13 Although the present analysis suggests that in select contexts, presently available materials may be economically noninferior to traditional autologous implants, streamlined manufacturing pipelines, on-site 3-D printing, and continued optimization of low-cost graft materials should further reduce custom implant costs and allow for increased popularity as a primary cranioplasty approach.39,40
In all, the mixed results reported in the literature demonstrate the high complexity of cranioplasty cost analysis, which is complicated by highly diverse clinical presentations, varying surgical indications, numerous custom implant materials, and heterogeneity in follow-up duration. Future, high-quality retrospective meta-analyses or prospective randomized clinical trials with consistent surgical techniques and periprocedural protocols from surgeon to surgeon will be required to adequately characterize the cost profile associated with each graft material and to define the clinical scenarios in which custom or autologous implants yield greater economic advantage. At present, and in the absence of convincing cost data for one approach over the other, the decision to use autologous or custom grafts is left to surgeon and patient discretion, factoring in elements such as bone flap integrity, cosmesis projections, local resources, insurance requirements, and surgeon experience.
5. Limitations
Among this study's limitations, our analysis only included patients who underwent reconstructive cranioplasty after either stroke or trauma. In cases of trauma, our study did not capture fracture severity or type. For this reason, our analysis may conflict with other published reports that may have included patients who required cranioplasty after tumor removal or aneurysm occclusion. Additionally, our study—and particularly our matched comparison—contains a limited sample size, which inhibited our ability to robustly assess wide-ranging cost data or potentially detect differences in failure rates or operative times, increasing the possibility of a type 2 error. Likewise, because of the sample size limitations, we were unable to thoroughly assess the various custom implant materials—e.g., titanium mesh, PEEK—in comparison with one another. Finally, our assessments are largely dependent on the charges for the custom implant. Our analysis may or may not apply to systems where because of competition or other factors different implant cost negotiations have decreased custom graft costs.
6. Conclusion
Our results suggest that during the initial cranioplasty surgery, autologous implants are significantly cheaper; however, upon unmatched and matched analyses, multivariate assessment of long-term costs data suggested that custom cranioplasty may be economically noninferior to autologous grafts. Similarly, patient outcomes were not significantly different in the two groups. From a financial perspective, it may be reasonable to consider custom cranioplasty implants as a primary approach for individuals requiring surgical correction of cranial defects, although more study is required.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Matthew Findlay: Writing – original draft, Formal analysis, Data curation. Sawyer Z. Bauer: Data curation. Diwas Gautam: Data curation. Matthew Holdaway: Data curation. Robert B. Kim: Writing – review & editing, Conceptualization. Walid K. Salah: Formal analysis. Spencer Twitchell: Data curation. Sarah T. Menacho: Writing – review & editing. Gurpreet S. Gandhoke: Writing – review & editing, Formal analysis. Ramesh Grandhi: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
This study did not receive any funding or financial support. RG: consults for Balt Neurovascular, Cerenovus, Medtronic Neurovascular, Integra, Rapid Medical, and Stryker Neurovascular.
Acknowledgments
We thank Kristin Kraus, MSc, for her expert editing.
Abbreviations
- ASA
American Society of Anesthesiologists
- BMI
body mass index
- DM
diabetes mellitus
- GCS
Glasgow Coma Scale
- HLD
hyperlipidemia
- HTN
hypertension
- PEEK
polyeteretherketone
- VDO
Value Driven Outcomes
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.wnsx.2024.100358.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hutchinson P.J., Kolias A.G., Timofeev I.S., et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016;375:1119–1130. doi: 10.1056/NEJMoa1605215. [DOI] [PubMed] [Google Scholar]
- 2.Vahedi K., Hofmeijer J., Juettler E., et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–222. doi: 10.1016/s1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- 3.Han S.E., Lim S.Y., Pyon J.K., Mun G.H., Bang S.I., Oh K.S. Aesthetic refinement of secondary cranioplasty using methyl methacrylate bone cements. Aesthetic Plast Surg. 2013;37:592–600. doi: 10.1007/s00266-013-0110-8. [DOI] [PubMed] [Google Scholar]
- 4.Morina A., Kelmendi F., Morina Q., et al. Cranioplasty with subcutaneously preserved autologous bone grafts in abdominal wall—experience with 75 cases in a post-war country Kosova. Surg Neurol Int. 2011;2:72. doi: 10.4103/2152-7806.81735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osawa M., Hara H., Ichinose Y., Koyama T., Kobayashi S., Sugita Y. Cranioplasty with a frozen and autoclaved bone flap. Acta Neurochir. 1990;102:38–41. doi: 10.1007/bf01402184. [DOI] [PubMed] [Google Scholar]
- 6.Bobinski L., Koskinen L.O., Lindvall P. Complications following cranioplasty using autologous bone or polymethylmethacrylate---retrospective experience from a single center. Clin Neurol Neurosurg. 2013;115:1788–1791. doi: 10.1016/j.clineuro.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Lee C.-H., Chung Y.S., Lee S.H., Yang H.-J., Son Y.-J. Analysis of the factors influencing bone graft infection after cranioplasty. J Trauma Acute Care Surg. 2012;73:255–260. doi: 10.1097/TA.0b013e318256a150. [DOI] [PubMed] [Google Scholar]
- 8.Coulter I.C., Pesic-Smith J.D., Cato-Addison W.B., et al. Routine but risky: a multi-centre analysis of the outcomes of cranioplasty in the Northeast of England. Acta Neurochir. 2014;156:1361–1368. doi: 10.1007/s00701-014-2081-1. [DOI] [PubMed] [Google Scholar]
- 9.Rotaru H., Stan H., Florian I.S., et al. Cranioplasty with custom-made implants: analyzing the cases of 10 patients. J Oral Maxillofac Surg. 2012;70:e169–e176. doi: 10.1016/j.joms.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 10.Metwali H., Hassanin M., Ibrahim T. A customized technique of cranioplasty for patients with large skull defects: a technical note. World Neurosurg. 2021;148:110–114. doi: 10.1016/j.wneu.2020.12.157. [DOI] [PubMed] [Google Scholar]
- 11.Gilardino M.S., Karunanayake M., Al-Humsi T., et al. A comparison and cost analysis of cranioplasty techniques: autologous bone versus custom computer-generated implants. J Craniofac Surg. 2015;26:113–117. doi: 10.1097/scs.0000000000001305. [DOI] [PubMed] [Google Scholar]
- 12.Matsuno A., Tanaka H., Iwamuro H., et al. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir. 2006;148:535–540. doi: 10.1007/s00701-006-0740-6. [DOI] [PubMed] [Google Scholar]
- 13.Shah A.M., Jung H., Skirboll S. Materials used in cranioplasty: a history and analysis. Neurosurg Focus. 2014;36(4):E19. doi: 10.3171/2014.2.Focus13561. [DOI] [PubMed] [Google Scholar]
- 14.Henderson D., Sinha S. OssDsign cranioplasty in children: a single-centre experience. Childs Nerv Syst. 2020;36:1773–1776. doi: 10.1007/s00381-020-04584-9. [DOI] [PubMed] [Google Scholar]
- 15.Pöppe J.P., Spendel M., Schwartz C., Winkler P.A., Wittig J. The “springform” technique in cranioplasty: custom made 3D-printed templates for intraoperative modelling of polymethylmethacrylate cranial implants. Acta Neurochir. 2022;164:679–688. doi: 10.1007/s00701-021-05077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J.-K., Lee S.-B., Yang S.-Y. Cranioplasty using autologous bone versus porous polyethylene versus custom-made titanium mesh: a retrospective review of 108 patients. J Korean Neurosurg Soc. 2018;61:737–746. doi: 10.3340/jkns.2018.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van de Vijfeijken S.E.C.M., Münker T.J.A.G., Spijker R., et al. Autologous bone is inferior to alloplastic cranioplasties: safety of autograft and allograft materials for cranioplasties, a systematic review. World Neurosurg. 2018;117:443–452.e448. doi: 10.1016/j.wneu.2018.05.193. [DOI] [PubMed] [Google Scholar]
- 18.Lilly G.L., Santucci N.M., Petrisor D., Wax M.K. Soft tissue coverage of cranial defects: an update. Plastic Aesthet Res. 2021;8:24. doi: 10.20517/2347-9264.2021.21. [DOI] [Google Scholar]
- 19.Binhammer A., Jakubowski J., Antonyshyn O., Binhammer P. Comparative cost-effectiveness of cranioplasty implants. Plast Surg (Oakv). 2020;28:29–39. doi: 10.1177/2292550319880922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mrad M., Murrad K., Antonyshyn O. Analyzing the cost of autogenous cranioplasty versus custom-made patient-specific alloplastic cranioplasty. J Craniofac Surg. 2017;28:1260–1263. doi: 10.1097/SCS.0000000000003708. [DOI] [PubMed] [Google Scholar]
- 21.Karsy M., Brock A.A., Guan J., Bisson E.F., Couldwell W.T. Assessment of cost drivers in transsphenoidal approaches for resection of pituitary tumors using the Value Driven Outcome database. World Neurosurg. 2017;105:818–823. doi: 10.1016/j.wneu.2017.05.148. [DOI] [PubMed] [Google Scholar]
- 22.Kawamoto K., Martin C.J., Williams K., et al. Value Driven Outcomes (VDO): a pragmatic, modular, and extensible software framework for understanding and improving health care costs and outcomes. J Am Med Inf Assoc. 2015;22:223–235. doi: 10.1136/amiajnl-2013-002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanaty M., Chalouhi N., Starke R.M., et al. Complications following cranioplasty: incidence and predictors in 348 cases. J Neurosurg. 2015;123:182–188. doi: 10.3171/2014.9.Jns14405. [DOI] [PubMed] [Google Scholar]
- 24.Johnson W., Ravindra V., Fielder T., et al. Surface area of decompressive craniectomy predicts bone flap failure after autologous cranioplasty: a radiographic cohort study. Neurotrauma Rep. 2021;2:391–398. doi: 10.1089/neur.2021.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park S.P., Kim J.H., Kang H.I., Kim D.R., Moon B.G., Kim J.S. Bone flap resorption following cranioplasty with autologous bone : quantitative measurement of bone flap resorption and predictive factors. J Korean Neurosurg Soc. 2017;60:749–754. doi: 10.3340/jkns.2017.0203.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrabi S.M., Sarmast A.H., Kirmani A.R., Bhat A.R. Cranioplasty: indications, procedures, and outcome - an institutional experience. Surg Neurol Int. 2017;8:91. doi: 10.4103/sni.sni_45_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walcott B.P., Kwon C.-S., Sheth S.A., et al. Predictors of cranioplasty complications in stroke and trauma patients: clinical article. J Neurosurg. 2013;118:757–762. doi: 10.3171/2013.1.Jns121626. [DOI] [PubMed] [Google Scholar]
- 28.Honeybul S., Morrison D.A., Ho K.M., Lind C.R.P., Geelhoed E. A randomized controlled trial comparing autologous cranioplasty with custom-made titanium cranioplasty. J Neurosurg. 2017;126:81–90. doi: 10.3171/2015.12.Jns152004. [DOI] [PubMed] [Google Scholar]
- 29.Honeybul S., Morrison D.A., Ho K.M., Lind C.R.P., Geelhoed E. A randomised controlled trial comparing autologous cranioplasty with custom-made titanium cranioplasty: long-term follow-up. Acta Neurochir. 2018;160:885–891. doi: 10.1007/s00701-018-3514-z. [DOI] [PubMed] [Google Scholar]
- 30.Lethaus B., Bloebaum M., Koper D., Poort-Ter Laak M., Kessler P. Interval cranioplasty with patient-specific implants and autogenous bone grafts--success and cost analysis. J Cranio-Maxillo-Fac Surg. 2014;42:1948–1951. doi: 10.1016/j.jcms.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Sahoo N.K., Tomar K., Thakral A., Kumar S. Failures in cranioplasty - a clinical audit & review. J Oral Biol Craniofac Res. 2021;11:66–70. doi: 10.1016/j.jobcr.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alkhaibary A., Alharbi A., Alnefaie N., Oqalaa Almubarak A., Aloraidi A., Khairy S. Cranioplasty: a comprehensive review of the history, materials, surgical aspects, and complications. World Neurosurg. 2020;139:445–452. doi: 10.1016/j.wneu.2020.04.211. [DOI] [PubMed] [Google Scholar]
- 33.Soto E., Restrepo R.D., Grant J.H.I., Myers R.P. Outcomes of cranioplasty strategies for high-risk complex cranial defects: a 10-year experience. Ann Plast Surg. 2022;88:S449–S454. doi: 10.1097/sap.0000000000003019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee L., Ker J., Quah B.L., Chou N., Choy D., Yeo T.T. A retrospective analysis and review of an institution's experience with the complications of cranioplasty. Br J Neurosurg. 2013;27:629–635. doi: 10.3109/02688697.2013.815313. [DOI] [PubMed] [Google Scholar]
- 35.Cheah P.P., Rosman A.K., Cheang C.K., Idris B. Autologous cranioplasty post-operative surgical site infection: does it matter if the bone flaps were stored and handled differently? Malays J Med Sci. 2017;24:68–74. doi: 10.21315/mjms2017.24.6.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koller M., Rafter D., Shok G., Murphy S., Kiaei S., Samadani U. A retrospective descriptive study of cranioplasty failure rates and contributing factors in novel 3D printed calcium phosphate implants compared to traditional materials. 3D Print Med. 2020;6:14. doi: 10.1186/s41205-020-00066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolff A., Santiago G.F., Belzberg M., et al. Adult cranioplasty reconstruction with customized cranial implants: preferred technique, timing, and biomaterials. J Craniofac Surg. 2018;29:887–894. doi: 10.1097/scs.0000000000004385. [DOI] [PubMed] [Google Scholar]
- 38.Arnaoutakis D., Bahrami A., Cohn J.E., Smith J.E. Cranioplasty using a mixture of biologic and nonbiologic agents. JAMA Facial Plast Surg. 2018;20:9–13. doi: 10.1001/jamafacial.2017.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morales-Gómez J.A., Garcia-Estrada E., Leos-Bortoni J.E., et al. Cranioplasty with a low-cost customized polymethylmethacrylate implant using a desktop 3D printer. J Neurosurg. 2019;130:1721–1727. doi: 10.3171/2017.12.Jns172574. [DOI] [PubMed] [Google Scholar]
- 40.De La Peña A., De La Peña-Brambila J., Pérez-De La Torre J., Ochoa M., Gallardo G.J. Low-cost customized cranioplasty using a 3D digital printing model: a case report. 3D Print Med. 2018;4:4. doi: 10.1186/s41205-018-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.