Abstract
We analyzed here the in silico biological activities of caffeine, (+)-catechin, and theobromine. For this, the PubChem database of the NIH (National Institutes of Health) was used to obtain the SMILE canonical form of the bioactive molecules, and the free software PASS Online (Prediction of Activity Spectra for Substances) from the Way2Drug portal. Also, we conducted an in vitro experiment using a chronic myeloid leukemia (CML) cell line (K562) to confirm some results found in in silico investigation. These cells were exposed to different concentrations of caffeine, (+)-catechin, and theobromine for 72 h. The results found in this in silico study suggested that caffeine, (+)-catechin, and theobromine showed excellent biological properties, such as antioxidant, anti-inflammatory, and anticarcinogenic, as well as protection against cardiovascular, diabetes, neurological, allergic, respiratory, and other therapeutic activities. These findings can be elucidated through the modulation exerted by these bioactive molecules in many biochemical pathways involved in organism homeostasis, such as free radical scavenger action, oxidoreductase inhibitor, membrane permeability inhibitor, and lipid peroxidase inhibitor. In addition, we have found here that caffeine, (+)-catechin, and theobromine have a remarkable anti-inflammatory activity which plays an important role in the therapeutic approach of COVID-19. Moreover, our in vitro findings confirmed the in silico results regarding anticancer activity since these molecules reduce cell proliferation at all tested concentrations. Therefore, since these molecules exhibit important medicinal activities, further investigations should be conducted to reveal new therapies to improve the treatments and prevention of numerous disorders and, consequently, promote human health.
Keywords: Computational platform, Bioactive molecules, Medicinal properties, Screening
Introduction
The use of medicinal plants is an ancient practice that has been carried out throughout the development of civilizations to achieve a better quality of life and health. One of the oldest reports is from Egyptians, between 3000 and 6000 years ago. They compiled a pharmacological compendium describing numerous medicinal substances extracted from natural resources (Halberstein 2005).
Currently, the importance of medicinal plants remains emphasize, due to their easy access, the existence of ethnopharmacological consumption, whose knowledge and tradition of use is transmitted through generations (Dutra et al. 2016; Ladio and Acosta 2019). Another factor that emphasizes the relevance of medicinal plants is their contribution to the discovery of new drugs (Newman and Cragg 2016). It is estimated that around 30% of all medicines come from natural sources. In some therapeutic areas, such as oncology, the amount of medicines derived from plants exceeds 50% (Dutra et al. 2016; Mazumder et al. 2018).
Throughout evolution, plants have survived, thanks to their secondary metabolism, protecting themselves from pests and UV rays, attracting pollinators, and adapting to different environmental conditions. As a result, they developed potent natural products which in turn are sources of pharmacologically relevant molecules. Phenolic acids, flavonoids, alkaloids, methylxanthines, anthraquinones, tannins, saponins, and terpenoids are important natural products (Langeder et al. 2020).
Some investigations (Cadoná et al. 2017; Martínez-Pinilla et al. 2015) reported that natural extracts such as guarana (Paullinia cupana) and cocoa (Theobroma cacao) present a matrix rich in bioactive molecules, such as flavonoids and methylxanthines, such as, for example, caffeine, (+)-catechin, and theobromine. These bioactive molecules confer important biological activities to these extracts, such as anticarcinogenic, antioxidant, and anti-inflammatory effects.
(+)-Catechin is a representative of flavonoids. A review conducted by Isemura (2019) emphasized the beneficial effects of (+)-catechin on human health. This study reports that this bioactive molecule has a remarkable antioxidant activity that can act against several chronic-degenerative disorders, such as cancer, cardiovascular, and neurodegenerative diseases.
(+)-Catechin is a renowned bioactive molecule, belonging to the group of phenols, found in many natural products, such as guarana. The phenolic substances present in guarana impart an astringent flavor and some studies demonstrate its antioxidant, antiviral, bactericidal, molluscicidal, and enzymatic inhibition activity (Bae et al. 2020). In addition, catechin has also been identified in different types of Camellia sinensis teas, conferring a remarkable antioxidant activity to these natural products (Filippini et al. 2020).
In addition, caffeine and theobromine are considered important methylxanthines. There are historical and anthropological references that methylxanthines were included in the human diet a long time ago, being regularly consumed by the general population (Monteiro et al. 2019).
Methylxanthines also have renowned biological activities, which have several benefits for human health. They are effective against respiratory and cardiovascular diseases, cancer, obesity and diabetes, human infertility, neurological and neurodegenerative diseases (Monteiro et al. 2019).
Given the scenario involving the identification of biological properties of bioactive molecules, such as caffeine, (+)-catechin and theobromine (Fig. 1), baseline studies that provide results that make it possible to elucidate biological actions, which leverage investigations for the development of new therapeutic agents with null side effects or reduced, aimed at the treatment and prevention of various human diseases, are extremely necessary.
Fig. 1.
Chemical structures of caffeine and theobromine (methylxanthines) and (+)-catechin (flavonoids)
Therefore, pre-clinical studies provide subsidies for the evaluation of the safety and biological activities of natural products and bioactive molecules for the development of new drugs. Taking this into account, in silico screening studies are increasingly being used to foster studies in this area. Therefore, we analyzed here in silico the biological activities of caffeine, (+)-catechin, and theobromine.
Materials and methods
Screening analyses that evaluate the biological activities of compounds are remarkable to reveal new therapies. There are several tools to predict the profile of compounds based on their medicinal activity. In this scenario, we conduct an in silico study using the software PASS Online.
This in silico study was carried out through the PubChem database of the NIH (National Institutes of Health) (https://pubchem.ncbi.nlm.nih.gov/). The SMILE canonical form of these bioactive molecules was assessed in this database and used in the free software PASS Online (Prediction of Activity Spectra for Substances) from the Way2Drug portal (http://www.way2drug.comPassOnline/community) to analyze the potential biological activities of these molecules.
In the PASS platform, a screening of biological action was possible by estimating the probability of the chemical molecule being active (Pa) and inactive (Pi), shown as a percentage.
The platform represents the “intrinsic” property of a substance, depending only on its structure and physicochemical characteristics. The bioactive molecules were evaluated according to their antioxidant, anti-inflammatory, and anticarcinogenic activities, as well as protection against cardiovascular, diabetes, neurological, allergic, and respiratory diseases, and other pharmacological properties and protection against other disorders. To confirm the in silico experiment, an in vitro study was conducted.
In vitro experimental design
Cell culture
Chronic myeloid leukemia (CML) cell line (K562) was commercially purchased from the Rio de Janeiro Cell Bank and cultivated under ideal cell culture conditions, using Roswell Park Memorial Institute (RPMI), supplemented with 10% fetal bovine serum (FBS) and 1% penicillin (100 U/mL)/streptomycin (100 mg/mL) antibiotics.
Treatments
K562 cells, at a concentration of 5 × 104 cells per well, were exposed to different concentrations of caffeine, (+)-catechin, and theobromine. Caffeine and (+)-catechin concentrations were determined at 0.1, 0.3, 1, 3, and 10 µg/mL, following a pharmacological curve screening. However, theobromine concentrations were lower than caffeine and (+)-catechin (0.2 and 0.6 µg/mL), based on previous experiments (data not yet published) since this molecule was diluted in DMSO solution and the concentration of this solvent should achieve low percentual per well to not interfere in the experiment (0.02 and 0.06% for 0.2 and 0.6 µg/mL concentration of theobromine, respectively). After theobromine DMSO dilution, it was followed by magnetic stirring for 10 min, and subsequently dilution in culture medium, in a ratio of 1:3, reaching a concentration of 0.25 mg/mL at pH 7.2.
Cell proliferation measurement
Cell viability and proliferation were measured by the MTT assay. The supernatant of the treatments was discarded, and the cells were washed and resuspended in a phosphate buffer (PBS, 0.01 M; pH 7.4), to avoid the interference of the polyphenols present in cocoa. The sample treatments were disposed in a 96-well plate. Then MTT reagent (Sigma-Aldrich, St. Louis, MO, USA) was added (1:10) (dissolved in 5 mg/mL PBS) and incubated for 1 h, at 37 °C. The formazan crystals generated were released from the cells through DMSO added. The quantification was performed colorimetrically by spectrophotometry at a wavelength of 560 nm, the absorbance value being proportional to the number of viable cells (Kang et al. 2010).
Results
Therapeutic properties of caffeine, (+)-catechin, and theobromine
The results found through in silico analysis of the therapeutic properties of caffeine, theobromine, and (+)-catechin indicated that these bioactive molecules showed excellent biological activities. In all analyses carried out, the probability of being active (Pa) was higher than the probability of being inactive (Pi).
The results of antioxidant, anti-inflammatory, and anticarcinogenic activities can be seen in Tables 1, 2, and 3, respectively. Regarding the findings obtained for protection against cardiovascular, diabetes, neurological, allergic and respiratory diseases, respectively, it is possible to observe these data in Table 4, 5, 6, 7, and 8, respectively. In addition, other pharmacological properties and protection against other disorders can be found in Table 9.
Table 1.
Antioxidant activity of caffeine, (+)-catechin, and theobromine
| Antioxidant properties | Caffeine | (+)- Catechin | Theobromine | |
|---|---|---|---|---|
| Free radical scavenger | P.a | 0.468 | 0.270 | 0.616 |
| P.i | 0.062 | 0.219 | 0.019 | |
| Oxidoreductase inhibitor | P.a | – | 0.571 | 0.261 |
| P.i | – | 0.047 | 0.202 | |
| Membrane permeability inhibitor | P.a | 0.526 | 0.790 | 0.382 |
| P.i | 0.526 | 0.011 | 0.212 | |
| Lipid peroxidase inhibitor | P.a | 0.189 | 0.888 | 0.261 |
| P.i | 0.143 | 0.003 | 0.202 | |
Table 2.
Anti-inflammatory activity of caffeine, theobromine, and (+)-catechin
| Anti-inflammatory activity | Caffeine | (+)- Catechin | Theobromine | |
|---|---|---|---|---|
| Immunomodulator | P.a | 0.561 | – | 0.539 |
| P.i | 0.007 | – | 0.009 | |
| Immunosuppressant | P.a | 0.450 | 0.348 | 0.558 |
| P.i | 0.053 | 0.086 | 0.034 | |
| Interleukin 8 antagonist | P.a | 0.213 | – | 0.359 |
| P.i | 0.009 | – | 0.005 | |
| Tumor necrosis factor antagonist | P.a | 0.309 | – | 0.241 |
| P.i | 0.015 | – | 0.027 | |
| Interleukin 5 antagonist | P.a | – | – | 0.106 |
| P.i | – | – | 0.036 | |
| Interleukin 1 antagonist | P.a | 0.168 | – | 0.126 |
| P.i | 0.050 | – | 0.083 | |
| Mucositis treatment | P.a | – | 0.375 | 0.246 |
| P.i | – | 0.123 | 0.243 | |
| TNF expression inhibitor | P.a | 0.488 | 0.517 | 0.415 |
| P.i | 0.032 | 0.026 | 0.052 | |
| Cyclic AMP phosphodiesterase inhibitor | P.a | 0.943 | 0.266 | 0.858 |
| P.i | 0.003 | 0.134 | 0.003 | |
Table 3.
Anticarcinogenic activity of caffeine, theobromine, and (+)-catechin
| Anticarcinogenic activity | Caffeine | (+)- Catechin | Theobromine | |
|---|---|---|---|---|
| Regulator of nucleotide metabolism | P.a | 0.745 | 0.329 | 0.782 |
| P.i | 0.009 | 0.111 | 0.007 | |
| Leukopoiesis stimulant | P.a | 0.684 | 0.272 | 0.762 |
| P.i | 0.008 | 0.217 | 0.004 | |
| Testosterone 17-beta dehydrogenase (NADP +) inhibitor | P.a | 0.608 | 0.716 | 0.695 |
| P.i | 0.087 | 0.050 | 0.057 | |
| Radiosensitizer | P.a | 0.722 | 0.253 | 0.634 |
| P.i | 0.004 | 0.177 | 0.009 | |
| Chemoprotective | P.a | 0.438 | 0.546 | 0.391 |
| P.i | 0.018 | 0.008 | 0.024 | |
| Antineoplastic antimetabolite | P.a | 0.233 | – | 0.374 |
| P.i | 0.028 | – | 0.019 | |
| Antineoplastic potentiator | P.a | – | – | 0.276 |
| P.i | – | – | 0.037 | |
| Anticarcinogenic | P.a | 0.204 | 0.795 | 0.245 |
| P.i | 0.115 | 0.005 | 0.086 | |
| Antineoplastic (solid tumors) | P.a | 0.224 | – | 0.263 |
| P.i | 0.176 | – | 0.124 | |
| MAP kinase 1 inhibitor | P.a | – | – | 0.180 |
| P.i | – | – | 0.059 | |
| Treatment of proliferative diseases | P.a | – | 0.681 | 0.199 |
| P.i | – | 0.007 | 0.096 | |
| Alpha agonist interferon | P.a | 0.229 | – | 0.223 |
| P.i | 0.118 | – | 0.124 | |
| Antimetastatic | P.a | – | 0.290 | 0.225 |
| P.i | – | 0.086 | 0.130 | |
| BCR-ABL kinase inhibitor | P.a | 0.139 | – | 0.140 |
| P.i | 0.047 | – | 0.046 | |
| MAP kinase 4 inhibitor | P.a | 0.214 | 0.377 | 0.228 |
| P.i | 0.163 | 0.008 | 0.137 | |
| VEGFR2 expression inhibitor | P.a | 0.009 | – | 0.008 |
| P.i | 0.005 | – | 0.007 | |
Table 4.
Protection against cardiovascular disease of caffeine, theobromine, and (+)-catechin
| Protection against cardiovascular disease | Caffeine | (+)- Catechin | Theobromine | |
|---|---|---|---|---|
| Peripheral vasodilator | P.a | 0.806 | 0.357 | 0.695 |
| P.i | 0.004 | 0.106 | 0.010 | |
| Antiischemic cerebral | P.a | 0.535 | – | 0.461 |
| P.i | 0.088 | – | 0.133 | |
| Antithrombotic | P.a | 0.428 | 0.234 | 0.319 |
| P.i | 0.044 | 0.162 | 0.090 | |
| Vasoprotector | P.a | 0.391 | 0.652 | 0.309 |
| P.i | 0.082 | 0.014 | 0.147 | |
| Adenosine A1 receptor antagonist | P.a | 0.422 | – | 0.359 |
| P.i | 0.004 | – | 0.005 | |
| Cardiotonic | P.a | 0.551 | – | 0.369 |
| P.i | 0.013 | – | 0.047 | |
| Antihypotensive | P.a | – | 0.217 | 0.277 |
| P.i | – | 0.038 | 0.012 | |
| Thromboxane B2 antagonist | P.a | 0.395 | 0.459 | 0.378 |
| P.i | 0.106 | 0.054 | 0.122 | |
| Antithrombotic | P.a | 0.428 | 0.234 | 0.319 |
| P.i | 0.044 | 0.162 | 0.090 | |
| Platelet adhesion inhibitor | P.a | 0.310 | 0.514 | 0.322 |
| P.i | 0.251 | 0.054 | 0.240 | |
| Treatment of vascular (peripheral) diseases | P.a | 0.225 | 0.440 | 0.190 |
| P.i | 0.091 | 0.010 | 0.137 | |
| Heart failure treatment | P.a | 0.239 | – | 0.129 |
| P.i | 0.025 | – | 0.079 | |
| Anti-hypoxic | P.a | 0.252 | 0.432 | 0.250 |
| P.i | 0.203 | 0.062 | 0.206 | |
| Cardioprotectant | P.a | 0.227 | 0.647 | 0.197 |
| P.i | 0.152 | 0.005 | 0.194 | |
| Nitric oxide synthase stimulant | P.a | – | – | 0.143 |
| P.i | – | – | 0.120 | |
| Cyclic phosphodiesterase amp inhibitor | P.a | 0.943 | 0.266 | 0.858 |
| P.i | 0.003 | 0.134 | 0.003 | |
Table 5.
Benefits against diabetes of caffeine, theobromine, and (+)-catechin
| Diabetes protection | Caffeine | (+)- Catechin | Theobromine | |
|---|---|---|---|---|
| Symptomatic antidiabetic | P.a | 0.264 | – | 0.362 |
| P.i | 0.064 | – | 0.018 | |
| Antidiabetic (type 1) | P.a | 0.227 | – | 0.168 |
| P.i | 0.010 | – | 0.034 | |
| Insulin promoter | P.a | 0.254 | – | 0.253 |
| P.i | 0.213 | – | 0.216 | |
| Diabetic nephropathy treatment | P.a | 0.357 | – | 0.323 |
| P.i | 0.004 | – | 0.005 | |
Table 6.
Protection against neurological diseases of caffeine, theobromine, and (+)-catechin
| Protection against neurological diseases | Caffeine | (+)- Catechin | Theobromine | |
|---|---|---|---|---|
| Antiparkinsonian | P.a | 0.522 | – | 0.376 |
| P.i | 0.011 | – | 0.035 | |
| Cerebral anti-ischemic | P.a | 0.535 | – | 0.461 |
| P.i | 0.088 | – | 0.133 | |
| Anticonvulsant | P.a | 0.298 | – | 0.333 |
| P.i | 0.122 | – | 0.100 | |
| Treatment of obsessive–compulsive disorder | P.a | 0.122 | – | 0.176 |
| P.i | 0.103 | – | 0.016 | |
| Treatment of neurodegenerative diseases | P.a | 0.427 | – | 0.268 |
| P.i | 0.055 | – | 0.169 | |
| Antiepileptic | P.a | 0.218 | – | 0.193 |
| P.i | 0.077 | – | 0.097 | |
| Treatment of Cerebrovascular Disorders | P.a | 0.192 | 0.170 | 0.172 |
| P.i | 0.069 | 0.099 | 0.096 | |
| Dementia treatment | P.a | 0.267 | 0.426 | 0.250 |
| P.i | 0.192 | 0.038 | 0.223 | |
| Antiparkinsonian, relieving stiffness | P.a | – | – | 0.172 |
| P.i | – | – | 0.171 | |
Table 7.
Benefits against allergies of caffeine, theobromine, and (+)-catechin
| Allergy protection | Caffeine | (+)- Catechin | Theobromine | |
|---|---|---|---|---|
| Antieczematic | P.a | 0.198 | – | 0.608 |
| P.i | 0.132 | – | 0.083 | |
| Urticaria treatment | P.a | 0.176 | – | 0.216 |
| P.i | 0.086 | – | 0.046 | |
| Histamine release inhibitor | P.a | 0.301 | 0.791 | 0.305 |
| P.i | 0.148 | 0.003 | 0.146 | |
Table 8.
Protection against respiratory diseases of caffeine, theobromine, and (+)-catechin
| Protection against respiratory diseases | Caffeine | (+)- Catechin | Theobromine | |
|---|---|---|---|---|
| Respiratory analeptic | P.a | 0.906 | 0.403 | 0,700 |
| P.i | 0.004 | 0.067 | 0.015 | |
| Rhinitis treatment | P.a | 0.475 | – | 0.433 |
| P.i | 0.024 | – | 0.039 | |
| Antiasthmatic | P.a | 0.597 | – | 0.393 |
| P.i | 0.016 | – | 0.050 | |
| Bronchodilator | P.a | 0.379 | 0.143 | 0.286 |
| P.i | 0.006 | 0.045 | 0.011 | |
| Treatment of respiratory distress syndrome | P.a | 0.299 | – | 0.198 |
| P.i | 0.029 | – | 0.096 | |
Table 9.
Additional pharmacological properties of caffeine, theobromine, and (+)-catechin
| Additional pharmacological properties | Caffeine | (+)- Catechin | Theobromine | |
|---|---|---|---|---|
| Kidney function stimulant | P.a | 0.858 | 0.610 | 0.821 |
| P.i | 0.002 | 0.036 | 0.003 | |
| Fibroblast growth factor agonist | P.a | 0.484 | – | 0.623 |
| P.i | 0.037 | – | 0.014 | |
| Diuretic | P.a | 0.720 | 0.231 | 0.537 |
| P.i | 0.003 | 0.064 | 0.005 | |
| Treatment of biliary tract disorders | P.a | 0.647 | – | 0.514 |
| P.i | 0.004 | – | 0.007 | |
| Analgesic stimulant | P.a | – | – | 0.407 |
| P.i | – | – | 0.005 | |
| Antidiarrheal | P.a | 0.546 | – | 0,400 |
| P.i | 0.004 | – | 0.005 | |
| Muscle relaxant | P.a | 0.702 | 0.185 | 0.420 |
| P.i | 0.004 | 0.127 | 0.026 | |
| Regulator of lipid metabolism | P.a | 0.627 | 0.334 | 0.446 |
| P.i | 0.018 | 0.122 | 0.070 | |
| Spasmolytic | P.a | 0.743 | – | 0.382 |
| P.i | 0.004 | – | 0.030 | |
| Septic shock treatment | P.a | 0.383 | – | 0.351 |
| P.i | 0.012 | – | 0.036 | |
| Treatment of male reproductive dysfunction | P.a | 0.268 | – | 0.331 |
| P.i | 0.033 | – | 0.020 | |
| Antiprotozoan (Leishmania) | P.a | 0.415 | 0.398 | 0.355 |
| P.i | 0.043 | 0.049 | 0.069 | |
| Treatment for erectile dysfunction | P.a | 0.273 | – | 0.293 |
| P.i | 0.024 | – | 0.018 | |
| Antiviral (Hepatitis B) | P.a | 0.216 | 0.352 | 0.301 |
| P.i | 0.072 | 0.022 | 0.033 | |
| Anti-infective | P.a | – | 0.245 | 0.289 |
| P.i | – | 0.158 | 0.112 | |
| Antianemic | P.a | 0.137 | – | 0.174 |
| P.i | 0.066 | – | 0.023 | |
| Cytochrome P450 Stimulant | P.a | 0.233 | 0.507 | 0.279 |
| P.i | 0.233 | 0.026 | 0.146 | |
| Antiprotozoan (Amoeba) | P.a | – | 0.221 | 0.243 |
| P.i | – | 0.187 | 0.142 | |
| Treatment of kidney disease | P.a | 0.263 | – | 0.156 |
| P.i | 0.022 | – | 0.156 | |
| Treatment of liver disorders | P.a | 0.183 | 0.495 | 0.206 |
| P.i | 0.146 | 0.012 | 0.206 | |
| Antiuremic | P.a | 0.402 | 0.186 | 0.177 |
| P.i | 0.015 | 0.087 | 0.093 | |
| Cystic fibrosis treatment | P.a | 0.239 | – | 0.224 |
| P.i | 0.121 | – | 0.152 | |
| Treatment of gastrointestinal disorders | P.a | 0.271 | – | 0.116 |
| P.i | 0.006 | – | 0.068 | |
| Anti-migraine | P.a | – | – | 0.130 |
| P.i | – | – | 0.097 | |
| Treatment of movement disorders | P.a | – | – | 0.105 |
| P.i | – | – | 0.103 | |
| Antinociceptive | P.a | 0.238 | 0.447 | 0.237 |
| P.i | 0.235 | 0.073 | 0.237 | |
| Treatment of liver cirrhosis | P.a | 0.116 | – | 0.152 |
| P.i | 0.046 | – | 0.049 | |
Antioxidant activity of caffeine, (+)-catechin, and theobromine
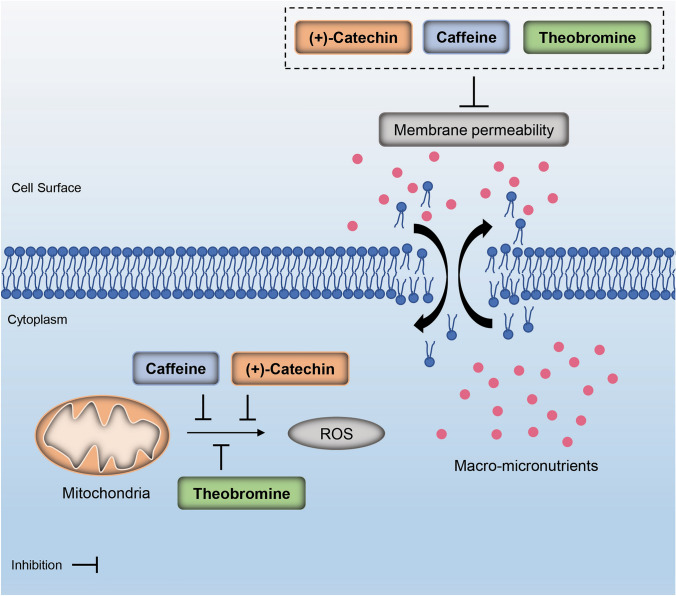
We found that all the bioactive molecules tested exhibited excellent antioxidant activities (Table 1). Theobromine can be emphasized in terms of free radical scavenger action since presented more activity than (+)-catechin and caffeine (Pa = 0.616).
On the other hand, (+)-catechin showed better action regarding oxidoreductase inhibitor (Pa = 0.571), membrane permeability inhibitor (Pa = 0.790), and lipid peroxidase inhibitor (Pa = 0.888).
Anti-inflammatory activity of caffeine, theobromine, and (+)-catechin
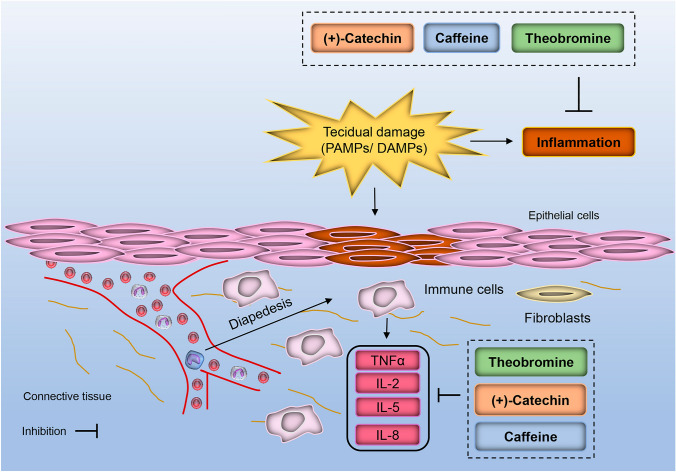
The results reported remarkable anti-inflammatory capacity of caffeine, theobromine, and (+)-catechin (Table 2). All tested molecules exhibited excellent immunosuppressant, TNF expression inhibitor, and cyclic AMP phosphodiesterase inhibitor capacity.
Caffeine and theobromine also showed a significant immunomodulator, interleukin 8 antagonist, tumor necrosis factor antagonist, and interleukin-1 antagonist action.
Moreover, (+)-catechin and theobromine indicated a potential effect to mucositis treatment (Pa = 0.375 and Pa = 0.246, respectively).
Further, only theobromine showed interleukin 5 antagonist capacity (Pa = 0.106).
Anticarcinogenic activity of caffeine, theobromine, and (+)-catechin
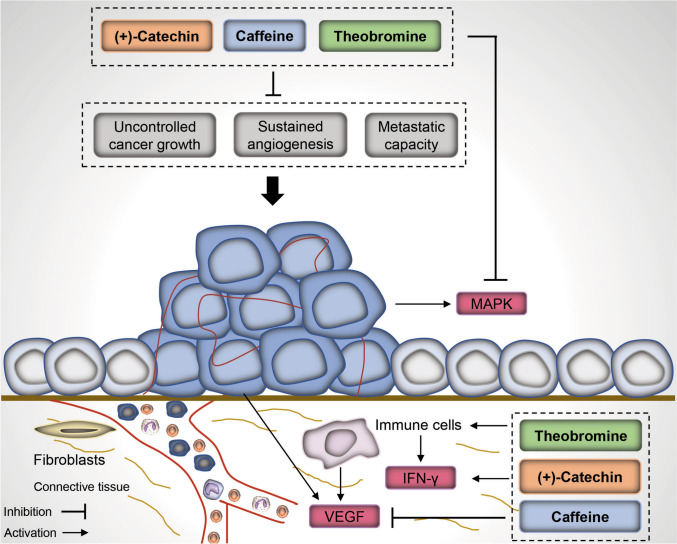
We found a significant anticarcinogenic activity of caffeine, theobromine and (+)-catechin (Table 3), showing important actions, such as radiosensitizer, chemoprotective, and anticarcinogenic effects.
All these molecules presented potential action in some important pathways to cancer proliferation and survival control, such as regulator of nucleotide metabolism, leukopoiesis stimulant, testosterone 17-beta dehydrogenase (NADP+) inhibitor, and MAP kinase 4 inhibitor.
The results emphasize theobromine and caffeine in this field since these bioactive molecules exhibited activities of antineoplastic antimetabolite, antineoplastic (solid tumors), alpha agonist interferon, BCR-ABL kinase inhibitor, and VEGFR2 expression inhibitor.
Also, theobromine presented action of antineoplastic potentiator (Pa = 0.276) and MAP kinase 1 inhibitor (Pa = 0.180) capacity.
Protection against cardiovascular disease of caffeine, theobromine, and (+)-catechin
Our results indicated that these bioactive molecules presented a great therapeutic capacity regarding protection against cardiovascular disease (Table 4). We found that caffeine, theobromine, and (+)-catechin exhibited peripheral vasodilator, antithrombotic, vasoprotector, thromboxane B2 antagonist, antithrombotic, platelet adhesion inhibitor, treatment of vascular (peripheral) diseases, anti-hypoxic, cardioprotective, and cyclic phosphodiesterase amp inhibitor.
Moreover, our findings indicated that caffeine and theobromine showed anti-ischemic cerebral, adenosine A1 receptor antagonist, cardiotonic, and heart failure treatment. Further, theobromine showed nitric oxide synthase stimulant action (Pa = 0.143).
Benefits against diabetes of caffeine, theobromine, and (+)-catechin
We found that caffeine and theobromine have a protective effect against diabetes (Table 5). However, (+)-catechin did not show action in this area.
In general, caffeine and theobromine exhibited similar effects in some aspects of diabetes, such as symptomatic antidiabetic, antidiabetic (type 1), insulin promoter, and diabetic nephropathy treatment.
Protection against neurological diseases of caffeine, theobromine, and (+)-catechin
Our findings indicated that caffeine, theobromine, and ( +)-catechin showed therapeutic activities against neurological diseases (Table 6). However, caffeine and theobromine showed better action than (+)-catechin, since this molecule only acted in the treatment of cerebrovascular disorders and treatment of cerebrovascular disorders.
While caffeine and theobromine showed activity in the treatment of neurodegenerative disorders, anti-parkinsonism, cerebral anti-ischemic disorders, anticonvulsants, treatment of obsessive–compulsive disorder, antiepileptic drugs, and relief of rigidity.
Benefits against allergies of caffeine, theobromine, and (+)-catechin
Our results reported that all the tested molecules offer protection against allergies (Table 7). The molecule (+)-catechin was less effective as it only showed the ability to inhibit histamine release (Pa = 0.791). Caffeine and theobromine, on the other hand, have anti-eczematous effects, treat urticaria, and inhibit histamine release.
Protection against respiratory diseases of caffeine, theobromine, and (+)-catechin
We found that caffeine, theobromine, and (+)-catechin provide protection against respiratory diseases (Table 8). Nevertheless, caffeine and theobromine showed a better effect than (+)-catechin, as this molecule only acted on respiratory analeptic (Pa = 0.403) and bronchodilator (Pa = 0.143).
While, caffeine and theobromine presented respiratory analeptic, rhinitis treatment, anti-asthmatic, bronchodilator, and treatment of respiratory distress syndrome effects.
Additional pharmacological properties of caffeine, theobromine, and (+)-catechin
Further, we found additional pharmacological properties of caffeine theobromine, and (+)-catechin (Table 9). Caffeine, theobromine, and (+)-catechin presented kidney function stimulant, diuretic, muscle relaxant, regulator of lipid metabolism, anti-protozoan (Leishmania), antiviral (Hepatitis B), cytochrome P450 stimulant, treatment of liver disorders, anti-uremic, and anti-nociceptive effects.
Theobromine and (+)-catechin showed several therapeutic effects such as fibroblast growth factor agonist, treatment of biliary tract disorders, antidiarrheal, spasmolytic, septic shock treatment, treatment of male reproductive dysfunction, treatment for erectile dysfunction, anti-anemic, treatment of kidney disease, cystic fibrosis treatment, treatment of gastrointestinal disorders, and treatment of liver cirrhosis.
Theobromine can be emphasized in this field due to additional medicinal effects than the other molecules, for example, analgesic stimulant (Pa = 0.407), anti-migraine (Pa = 0.130), and treatment of movement disorders (Pa = 0.105).
Caffeine, (+)-catechin, and theobromine: therapeutic perspectives in COVID-19
We found here that caffeine, (+)-catechin, and theobromine present remarkable anti-inflammatory activity. We found that all the molecules are immunosuppressant and can inhibit tumor necrosis factor expression, and cyclic AMP phosphodiesterase, which are associated with COVID-19 development (Table 2).
Moreover, we revealed that caffeine and theobromine can inhibit interleukin 8, tumor necrosis factor, and interleukin-1, important molecules involved in COVID-19 physiopathology. Also, theobromine presents interleukin 5 inhibition. Given the reason that these molecules present a crucial role in modulating pathways that are involved in COVID-19 development, we analyzed here the therapeutic perspectives of these bioactive molecules in COVID-19 (Table 10).
Table 10.
Caffeine (+)-catechin and theobromine: therapeutic perspectives in COVID-19
| Caffeine | (+)-Catechin | Theobromine | ||
|---|---|---|---|---|
| Cytokines storm inhibition | ||||
| Interleukin 8 antagonist | P.a | 0.213 | – | 0.359 |
| P.i | 0.009 | – | 0.005 | |
| Interleukin 1 antagonist | P.a | 0.168 | – | 0.126 |
| P.i | 0.050 | – | 0.083 | |
| Interleukin 4 antagonist | P.a | – | 0.222 | – |
| P.i | – | 0.016 | – | |
| Interleukin 10 antagonist | P.a | – | 0.161 | – |
| P.i | – | 0.013 | – | |
| Interleukin 6 antagonist | P.a | – | 0.208 | – |
| P.i | – | 0.072 | – | |
| Interferon gamma antagonist | P.a | – | 0.138 | – |
| P.i | – | 0.056 | – | |
| Interleukin 5 antagonist | P.a | – | – | 0.106 |
| P.i | – | – | 0.036 | |
| Tumour necrosis factor antagonist | P.a | 0.309 | – | 0.241 |
| P.i | 0.015 | – | 0.027 | |
| Tumour necrosis factor alpha antagonist | P.a | 0.227 | – | 0.181 |
| P.i | 0.019 | – | 0.032 | |
| Respiratory analeptic | P.a | 0.906 | 0.403 | 0.700 |
| P.i | 0.004 | 0.067 | 0.015 | |
| Antitussive | P.a | 0.453 | 0.524 | 0.347 |
| P.i | 0.014 | 0.009 | 0.027 | |
| Bronchodilator | P.a | 0.379 | 0.143 | 0.286 |
| P.i | 0.006 | 0.045 | 0.011 | |
| Vasoprotector | P.a | 0.391 | 0.652 | 0.309 |
| P.i | 0.082 | 0.014 | 0.147 | |
| Thromboxane B2 antagonist | P.a | 0.395 | 0.459 | 0.378 |
| P.i | 0.106 | 0.054 | 0.122 | |
| Gastrointestinal disorders treatment | P.a | 0.271 | – | 0.166 |
| P.i | 0.006 | – | 0.068 | |
| Prostaglandin-A1 DELTA-isomerase inhibitor | P.a | 0.274 | 0.217 | 0.209 |
| P.i | 0.109 | 0.166 | 0.177 | |
| Antihistaminic | P.a | 0.153 | – | – |
| P.i | 0.065 | – | – | |
| Heat shock protein 90 antagonist | P.a | 0.101 | 0.107 | – |
| P.i | 0.042 | 0.038 | – | |
| MAP kinase kinase 4 inhibitor | P.a | 0.214 | 0.377 | 0.228 |
| P.i | 0.163 | 0.008 | 0.137 | |
| Antinociceptive | P.a | 0.238 | 0.447 | 0.237 |
| P.i | 0.235 | 0.073 | 0.236 | |
We found that caffeine can modulate the cytokines storm via the inhibition of interleukin-1 and -8, tumor necrosis factor-alpha, as well as present a remarkable therapeutical potential against COVID-19 since some activities can be highlighted such as respiratory analeptic, antitussive, bronchodilator, vasoprotector, thromboxane B2 antagonist, gastrointestinal disorders treatment, prostaglandin-A1 DELTA-isomerase inhibitor, gastrointestinal disorders treatment, antihistaminic, heat shock protein 90 antagonist, MAP kinase kinase 4 inhibitor, anti-nociceptive and kidney function stimulant.
Also, (+)-catechin showed great control in cytokines storm via interleukin 4, 6, 10, and interferon-gamma inhibition. This molecule also presents great medicinal properties important to improve COVID-19 treatment, such as respiratory analeptic, antitussive, bronchodilator, vasoprotector, thromboxane B2 antagonist, prostaglandin-A1 DELTA-isomerase inhibitor, gastrointestinal disorders treatment, heat shock protein 90 antagonist, MAP kinase 4 inhibitor, anti-nociceptive, and kidney function stimulant.
Theobromine also exhibited potential action against cytokines storm by interleukin-1, -5, -8 inhibition and tumor necrosis factor-alpha inhibition. Besides, this molecule showed therapeutic activities such as respiratory analeptic, antitussive, bronchodilator, vasoprotector, thromboxane B2 antagonist, gastrointestinal disorders treatment, prostaglandin-A1 DELTA-isomerase inhibitor, gastrointestinal disorders treatment, MAP kinase kinase 4 inhibitor, anti-nociceptive, and kidney function stimulant.
Caffeine, (+)-catechin, and theobromine presented in vitro anticarcinogenic activity against chronic myeloid leukemia (CML) cell line (K562)
We developed here an additional in vitro experiment regarding the anticancer action of caffeine, (+)-catechin, and theobromine, one of the most significant biological activities found in our in silico investigation.
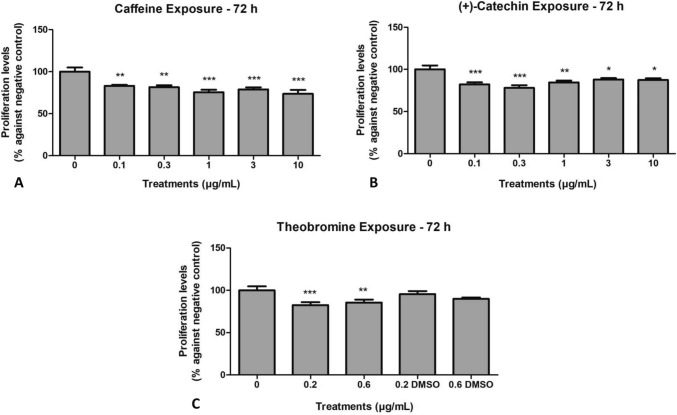
Our results revealed that all the molecules at all the concentrations tested exhibited anti-proliferative activity in the chronic myeloid leukemia (CML) cell line (K562), caffeine (0.1, 0.3 µg/mL (p < 0.001); 1, 3, 10 µg/mL (p < 0.0001), (+)-catechin (0.1, 0.3 µg/mL (p < 0.0001); 1 µg/mL (p < 0.001); 3, 10 µg/mL (p < 0.005), theobromine (0.2 µg/mL (p < 0.0001), 0.6 µg/mL (p < 0.001). Our findings showed a significant cell proliferation reduction when compared to the negative control (only cells and culture media). DMSO theobromine control dilution did not interfere in the experiment since the cell proliferation levels were the same as the negative control (Fig. 2).
Fig. 2.
K562 cells were exposed to different caffeine (A) and (+)-catechin (B) concentrations (0.1, 0.3, 1, 3, and 10 µg/mL) for 72 h. Theobromine concentrations were determined at 0.2 and 0.6 µg/mL (C). Cells were also exposed to DMSO (theobromine dilution vehicle control, at a concentration of 0.02 and 0.06% for 0.2 and 0.6 µg/mL concentration of theobromine, respectively), for 72 h. Cell proliferation was determined by the MTT assay. The results were compared with the percentage of the negative control (cells and medium only). N = 3, *(p < 0.005), **(p < 0.001), ***(p < 0.0001)
Discussion
The results found in this study suggested that caffeine, (+)-catechin, and theobromine showed excellent biological properties, such as antioxidant, anti-inflammatory, and anticarcinogenic, as well as protection against cardiovascular, diabetes, neurological, allergic, respiratory, and other therapeutic activities. These findings can be elucidated through the modulation exerted by these bioactive molecules in many biochemical pathways involved in body homeostasis.
The results found in the present study suggested that the bioactive molecules studied here showed excellent antioxidant properties, modulating several pathways involved in this issue (Fig. 3).
Fig. 3.
Antioxidant properties of caffeine, (+)-catechin, and theobromine. These molecules exhibited excellent antioxidant activities, such as free radical scavenger action, oxidoreductase inhibitor, membrane permeability inhibitor, lipid peroxidase inhibitor
These findings are in line with what has been reported by previous studies that indicated the antioxidant action of these bioactive molecules (Demir et al. 2016; Ulvia et al. 2020; Vieira et al. 2020). In this scenario, Vieira et al. (2020) suggested that caffeine showed remarkable protection against the oxidative degradation of adenine exposed to the hydroxyl radical generated by gamma irradiation or by photolysis reaction by ultraviolet radiation.
In addition, another study reported that caffeine intake decreased oxidative stress and inflammatory biomarkers in thioacetamide (TAA)-induced liver disease in male rats. TAA-induced liver toxicity in the liver elevated enzymes and histological changes, fatty changes, apoptosis and fibrosis evidenced by increased reaction to matrix metalloproteinase-9 (MMP-9) and collagen type IV in hepatocytes. In addition, serum levels of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) were significantly elevated. Co-treatment with caffeine and TAA restored normal liver structure and function. Caffeine provided an anti-fibrogenic, anti-inflammatory, and antioxidant effect that were associated with recovery from hepatic histological and functional changes from TAA-induced hepatotoxicity (Amer et al. 2017).
Furthermore, Ulvia et al. (2020) reported remarkable antioxidant activity of the main molecules found in Indonesian cocoa. Among the components found in the chemical matrix of this extract, theobromine, (+)-catechin, and caffeine stood out for their excellent antioxidant properties.
Corroborating these studies, Demir et al. (2016) showed the short-term effects of caffeine on renal antioxidant activity in rats. The results found in this study indicated that the administration of caffeine generated a dose-dependent decrease in lipid peroxidation and advanced oxidation of protein products in the kidney.
Regarding the findings involving the anti-inflammatory action of bioactive molecules, it is possible to suggest that caffeine, (+)-catechin, and theobromine showed remarkable modulatory activities involving inflammatory pathways (Fig. 4).
Fig. 4.
Anti-inflammatory activity of caffeine, theobromine, and (+)-catechin. These molecules presented remarkable anti-inflammatory as well as immunosuppressant, interleukins expression inhibitor, and cyclic AMP phosphodiesterase inhibitor capacity
Corroborating these findings, Paiva et al. (2019) showed, through a systematic review regarding coffee and caffeine, a positive correlation between coffee or caffeine consumption and the modulation of inflammatory markers in serum. The study reported that caffeine generated an increase in the anti-inflammatory cytokine, interleukin-10.
In addition, another study showed an inhibitory effect of caffeine on TNF-α secretion in LPS-activated human monocytes, as well as in two subpopulations of monocyte-derived macrophages differentiated in the presence of M-CSF or GM-CSF (Kovács et al. 2021).
The anti-inflammatory effects of (+)-catechin were also observed in the study conducted by Tang et al. (2007). The investigation evaluated the adjuvant effects of catechin on arthritis in rats and its possible mechanisms of action. Arthritis was induced by metatarsal pad injection with complete Freund’s adjuvant in male rats. Intragastric administration of (+)-catechin (60 and 120 mg/kg) significantly suppressed secondary inflammatory swelling of the paw, the pain response index of polyarthritis. It also inhibited the production of interleukin-1, necrosis factor α, PGE2, and increased levels of cyclic adenosine monophosphate in rats with arthritis. Furthermore, catechin regulated EP2 expression in synoviocytes of rats with this dysfunction.
Also, a previous study reported the anti-inflammatory action of theobromine acting against obesogenesis (Jang et al. 2015). Theobromine inhibited the accumulation of lipid droplets, the expression of PPARγ and C/EBPα, and the expression of aP2 and leptin mRNA. Inhibition of adipogenic differentiation by theobromine occurred mainly in the early stages of differentiation. Furthermore, theobromine interrupted the cell cycle in the G0/G1 phase and regulated the expressions of CDK2, p27, and p21. Theobromine reduced the phosphorylation of ERK and JNK. In addition, the secretion and mRNA level of TNF-α and IL-6 were inhibited by theobromine treatment.
We investigated here the therapeutic potential of these bioactive molecules in COVID-19 (Table 10). COVID-19 can cause severe symptoms such as respiratory tract infections, pneumonia, acute respiratory distress syndrome (ARDS), multiple organ failure, and death. This can be explained due to the fact that the virus triggers severe host immune responses (Montazersaheb et al. 2022).
In agreement with our results, Yang and Shen (2020) reported that green tea polyphenol catechins inhibit coronavirus replication by decreasing cytokines storm, such as interleukin 5 and TNF-α. The study was conducted in Vero E6 cells infected with SARS-CoV, and in vivo model investigating the protective effect of catechins on CD25/CD69/CD94/CD8 + cytotoxic T lymphocytes-mediated adaptive immunity and the preventive action on lipopolysaccharide-induced acute lung injury (ALI) in mice. Also, a previous study reported that catechins from green tea promote in vitro SARS-CoV-2 inactivation (Nishimura et al. 2021 Sep 22).
Caffeine also was studied to prevent or improve COVID-19 recovery. Romero-Martínez et al. (2021) highlighted the potential action molecule against coronavirus possible by blocking the viral entrance into host cells via inhibition of receptor-binding domain connection with the angiotensin-converting enzyme complex, and also by reducing viral replication by blocking 3-chymotrypsin-like proteases. Besides, the study reported the effective anti-inflammatory and immunomodulator action of this molecule. Our results also suggested that caffeine presents great potential against COVID-19 since this molecule can inhibit cytokine storm and present protection in tissues that are deeply affected by coronavirus.
Moreover, Rolta et al. (2022) carried out a docking study that suggested that methylxanthines present potential COVID-19 inhibition. This investigation reported that caffeine/thiene, methylxanthine, theobromine, theophylline, and xanthine prevented the SARS-CoV-2 spike protein and S1 receptor-binding domain (S1RBD) avoiding the cell infection via ACE2 receptor.
Moreover, since we found that these molecules exhibit also neurological action, as shown in Table 6, caffeine, (+)-catechin, and theobromine could be used against neurological symptoms associated with COVID-19, such as long COVID. In agreement with this scenario, Almasi et al. (2022) reported that (+)-catechin presented benefits to recovery of neurological disorders caused by COVID-19. This molecule showed neuroprotection via modulation of monoamine oxidase (MAO). MAO is responsible for catalyzing monoamines oxidative deamination reaction. MOA inhibitors exhibit neuroprotection via monoamine substances. In this scenario, (+)-catechin was able to reduce MOA-B activity promoting neurological benefits on rats.
Besides, a previous study reported that caffeine showed protective benefits against disease. The neuroprotective effects of this methylxanthine could be associated with anti-inflammatory and antioxidant activity (Monji et al. 2020). Another investigation suggested that caffeine showed neuroprotective effects against Alzheimer’s, Parkinson disease. Also, caffeine exhibits A2AR antagonism action and consequently can protect against amyotrophic lateral sclerosis (ALS) (Kolahdouzan and Hamadeh 2017).
Also, the neuroprotective action of theobromine was reported in a previous study carried out by Bhat and Kumar (2022). This investigation suggested that rats with permanent bilateral common carotid artery occlusion (BCCAO) treated with theobromine presented an improvement in some neurological aspects, such as sensorimotor functions and memory. Besides, theobromine reduced the oxidative stress in the brain, inflammatory molecules (tumor necrosis factor-α, interleukin-1β and -6, nuclear factor-κB), biomarkers of cellular injury (lactate dehydrogenase, caspase-3), acetylcholinesterase activity, and improvement in γ-aminobutyric acid quantity.
When the in silico analyses were intended for antitumor investigation, the results indicated that the bioactive molecules showed excellent anticarcinogenic activities, since they modulate altered pathways in cancer cells, such as, for example, accelerated growth and cell survival signals, angiogenesis activation and ability to metastasis (Fig. 5).
Fig. 5.
Anticarcinogenic activity of caffeine, theobromine, and (+)-catechin. All these molecules presented potential action on some important pathways to cancer proliferation and survival control, sustained angiogenesis, and metastatic capacity, such as regulator of nucleotide metabolism, leukopoiesis stimulant, and MAP kinase 4 inhibitor, alpha agonist interferon, BCR-ABL kinase inhibitor, and VEGFR2 expression inhibitor
Since one of the most important effects of these molecules is anticancer, we performed an in vitro activity with a chronic myeloid leukemia (CML) cell line (K562) to confirm these results. These cells were exposed to different concentrations of caffeine, (+)-catechin, and theobromine for 72 h. Our in vitro findings confirmed the in silico results as these molecules reduce cell proliferation even at lower concentrations.
Our in silico study showed that caffeine, (+)-catechin, and theobromine can inhibit uncontrolled cell proliferation via inhibition of some anticancer biomarkers, such as nucleotide metabolism, MAPK1 and 4, BCR-ABL kinase, and VEGRF2. These findings were confirmed in in vitro experiments, since these molecules control cell proliferation, exhibiting anticancer activity.
Consolidating these findings, a systematic review pointed to numerous studies already reported in the literature that showed the anticarcinogenic activities of caffeine, (+)-catechin, and theobromine, highlighting the hallmarks of cancer, showing the numerous ways in which these molecules act to reduce proliferation and survival of different types of cancer cells (Cadoná et al. 2021). In general, in this study, it is possible to verify that caffeine showed inhibition of PD-1, colony formation, VEGF, ATM and ATR, mTOR, cyclins, and MAPKs, and activation of p53, of cytokines to stimulate the immune system (TNF- α and IFN-γ), caspase 3 and PTEN. Regarding theobromine, it was possible to identify activation of BAX/BLC2 and inhibition of A2aR, mTOR, MAPKs, and NF-kB. While (+)-catechin showed anti-proliferative activity activating p53, IL-2, p21, caspase 3, 8, TIMP-1, generating DNA fragmentation and inhibiting IL-1B, IL-6, NO, VEGF, mTOR, and cyclins.
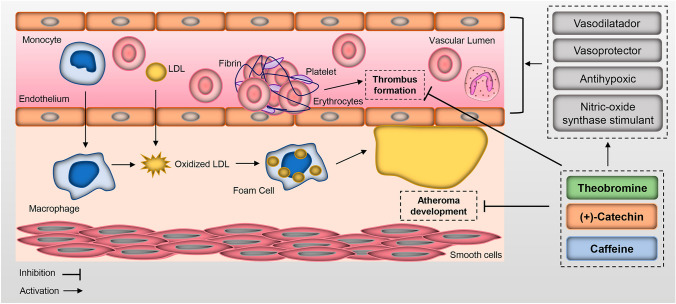
Furthermore, in silico analyses aimed at investigating the protective action against cardiovascular diseases indicated that caffeine, (+)-catechin, and theobromine had excellent cardioprotective activities (Fig. 6).
Fig. 6.
Protection against cardiovascular disease of caffeine, theobromine, and (+)-catechin. These bioactive molecules have a great therapeutic capacity regarding peripheral vasodilator, antithrombotic, vasoprotector, thromboxane B2 antagonist, antithrombotic, platelet adhesion inhibitor, treatment of vascular (peripheral) diseases, anti-hypoxic, cardioprotective, and cyclic phosphodiesterase amp inhibitor, and nitric oxide synthase stimulant action
The study conducted by Martínez-Pinilla et al. (2015) indicated that theobromine showed cardiovascular protection by increasing plasma levels of HDL cholesterol and decreasing LDL. Furthermore, the study by Dam and Hu (2022) demonstrated that moderate coffee consumption (2–5 cups per day) has been consistently associated with a lower risk of cardiovascular disease in epidemiological studies. For most individuals, a caffeine intake of up to 400 mg per day is safe and moderate coffee consumption can be included as part of a healthy lifestyle. The cardioprotective property of (+)-catechin has also been recognized in the literature. The review study conducted by Chen et al. (2016) reported the remarkable action of (+)-catechin against cardiovascular disease via regulation of lipid metabolism, control of blood lipid metabolism, vascular endothelial protection, and blood pressure reduction.
Furthermore, the results found in the present study suggested numerous therapeutic properties against various disorders, such as diabetes, neurological diseases, asthma, allergies, and infertility-related problems (Fig. 7).
Fig. 7.
Pharmacological properties of caffeine, theobromine, and (+)-catechin. These bioactive molecules exhibited important therapeutic actions, such as, anti-allergic, antidiabetic, erythropoiesis stimulant, hepatic disorders treatment, antiprotozoal, kidney function stimulant, gastrointestinal disorders treatment, respiratory system protection, anti-nociceptive, and neuroprotection
Corroborating these findings, previous studies reported that caffeine was associated with a likely decreased risk of Parkinson’s disease and type 2 diabetes (Grosso et al. 2017). Caffeine and theobromine also act against respiratory and cardiovascular diseases, cancer, obesity and diabetes, human infertility, neurological diseases and neurodegenerative diseases (Monteiro et al. 2019).
In addition, (+)-catechin has also been associated with numerous therapeutic properties. A review by Isemura (2019) emphasized the beneficial effects of (+)-catechin on human health. This study reported that this bioactive molecule has a remarkable antioxidant activity that can act against several chronic-degenerative disorders, such as cancer, cardiovascular, and neurodegenerative diseases.
A previous docking study reported that caffeine could be considered MAO-B inhibitor/AA2AR antagonist for Parkinson’s disease. Also, other docking study suggested that caffeine could interact with human hair protein keratin (4ZRY) via van der Waals attraction by ARG432, GLU397, GLY433, LEU404, TYR400 whereas by alkyl/Pi-alkyl side chains with ALA429, CYS401 residues. These findings suggested that caffeine could improve hair growth and skin care (Gajalakshmi and Kavitha 2022).
Docking studies were also carried out using (+)-catechin. In this scenario, the interaction of this molecule and its derivatives was investigated on Glucosamine-6-Phosphate Synthase (GlmS). GlmS inhibition is considered to lead to promote an important antibacterial action. This investigation revealed that catechin derivatives, such as epicatechin, gallocatechin, and epigallocatechin showed a significant binding on GlmS (Fikrika et al. 2016). Moreover, a previous docking investigation study analyzed (+)-catechin and its different synthetic derivatives to find new potential inhibitors of Coronavirus. This study revealed that these molecules showed significant therapeutic potential to inhibit COVID-19 main protease (Arif 2020).
Theobromine was also investigated in docking studies. The study of Eissa et al. (2023) investigated new semisynthetic derivative theobromine, (m-tolyl)acetamide theobromine derivative (T-1-MTA), to exhibit anticancer activity via epidermal growth factor receptors (EGFR) protein inhibition. The results revealed significant potential for T-1-MTA to bind to EGFR.
Moreover, Rolta et al. (2022) carried out a docking study that suggested that methylxanthines present potential COVID-19 inhibition. This investigation reported that caffeine/thiene, methylxanthine, theobromine, theophylline, and xanthine prevented the SARS-CoV-2 spike protein and S1 receptor-binding domain (S1RBD) avoiding the cell infection via ACE2 receptor.
Conclusion
To find new therapeutic agents with little or no side effects, this in silico study proved to be very relevant as it aimed to measure and identify numerous biological properties of biological molecules contained in various natural products. The computational software used to conduct this study identified various biological properties, which are often restricted to just a few in vivo and in vitro studies.
In this scenario, the results of the present study suggested that caffeine, (+)-catechin, and theobromine showed excellent biological properties, as well as protection against cardiovascular, diabetes, neurological, allergic, respiratory diseases, human infertility, and obesity. These findings can be explained by the remarkable antioxidant and anti-inflammatory activities present in these molecules, which in this way are able to modulate numerous important biological pathways to ensure body homeostasis. Our in vitro findings confirmed the in silico results regarding anticancer activity since these molecules reduce cell proliferation at all tested concentrations.
In this way, the benefit that natural antioxidants bring to the human organism is remarkable, so it is possible to observe through literary research and on PASS online how their bioactive effects help in the prevention and treatment of numerous diseases and thus leverage more studies in the area to conduct further investigations for better treatments and prevention of numerous disorders and thus ensure the promotion of human health.
Acknowledgements
The authors would like to express their gratitude to the Franciscan University research team for all their help and support.
Author contributions
Data curation: NRD, CMSA, TCS, TOM, ICL, ACZB, FKS, FCC. Formal analysis: NRD, CMSA, TCS, FCC. Investigation: NRD, CMSA, TCS, TOM, ICL, ACZB, FKS, FCC. Methodology: FCC. Project administration: FCC. Supervision: FCC. Roles/writing—original draft: NRD, CMSA, TCS, TOM, ICL, ACZB, FKS, FCC. Writing—review and editing: NRD, CMSA, TCS, TOM, ICL, ACZB, FKS, FCC.
Data availability
Data will be made available on request.
Declarations
Conflict of interest
The authors have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
References
- Almasi F, Dang W, Mohammadipanah F, Li N. Neurological disorders of COVID-19: insights to applications of natural products from plants and microorganisms. Arch Pharmacal Res. 2022 doi: 10.1007/s12272-022-01420-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer MG, Mazen NF, Mohamed AM. Caffeine intake decreases oxidative stress and inflammatory biomarkers in experimental liver diseases induced by thioacetamide: biochemical and histological study. Int J Immunopathol Pharmacol. 2017;30(1):13–24. doi: 10.1177/0394632017694898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif MN. Catechin derivatives as inhibitor of COVID-19 main protease (Mpro): molecular docking studies unveil an opportunity against CORONA. Comb Chem High Throughput Screening. 2020;25(1):197–203. doi: 10.2174/1871520620666201123101002. [DOI] [PubMed] [Google Scholar]
- Bae J, Kim N, Shin Y, Kim S-Y, Kim Y-J. Activity of catechins and their applications. Biomed Dermatol. 2020 doi: 10.1186/s41702-020-0057-8. [DOI] [Google Scholar]
- Bhat JA, Kumar M. Neuroprotective Effects of Theobromine in permanent bilateral common carotid artery occlusion rat model of cerebral hypoperfusion. Metab Brain Dis. 2022;37(6):1787–1801. doi: 10.1007/s11011-022-00995-6. [DOI] [PubMed] [Google Scholar]
- Cadoná FC, Rosa JL, Schneider T, Cubillos-Rojas M, Sánchez-Tena S, Azzolin VF, da Cruz IBM. Guaraná, a highly caffeinated food, presents in vitro antitumor activity in colorectal and breast cancer cell lines by inhibiting AKT/mTOR/S6K and MAPKs pathways. Nutr Cancer. 2017;69(5):800–810. doi: 10.1080/01635581.2017.1324994. [DOI] [PubMed] [Google Scholar]
- Cadoná FC, Dantas RF, de Mello GH, Silva-Jr FP. Natural products targeting into cancer hallmarks: an update on caffeine, theobromine, and (+)-catechin. Crit Rev Food Sci Nutr. 2021 doi: 10.1080/10408398.2021.1913091. [DOI] [PubMed] [Google Scholar]
- Chen XQ, Hu T, Han Y, Huang W, Yuan HB, Zhang YT, Jiang YW. Preventive effects of catechins on cardiovascular disease. Molecules. 2016 doi: 10.3390/molecules21121759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir EO, Demirtaş CY, Paşaoğlu ÖT. Ratlarda böbrek antioksidan aktivitesi üzerine kafeinin etkileri. Turk J Biochem. 2016;41(3):216–222. doi: 10.1515/tjb-2016-0032. [DOI] [Google Scholar]
- Dutra RC, Campos MM, Santos ARS, Calixto JB. Medicinal plants in Brazil: pharmacological studies, drug discovery, challenges and perspectives. Pharmacol Res. 2016 doi: 10.1016/j.phrs.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Eissa IH, Yousef RG, Elkaeed EB, Alsfouk AA, Husein DZ, Ibrahim IM, Metwaly AM. Anticancer derivative of the natural alkaloid, theobromine, inhibiting EGFR protein: computer-aided drug discovery approach. PLoS ONE. 2023 doi: 10.1371/journal.pone.0282586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrika H, Ambarsari L, Sumaryada T. Molecular docking studies of catechin and its derivatives as anti-bacterial inhibitor for glucosamine-6-phosphate synthase. IOP Conf Ser: Earth Environ Sci. 2016 doi: 10.1088/1755-1315/31/1/012009. [DOI] [Google Scholar]
- Filippini T, Malavolti M, Borrelli F, Izzo AA, Fairweather-Tait SJ, Horneber M, Vinceti M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst Rev. 2020 doi: 10.1002/14651858.CD005004.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajalakshmi D, Kavitha E. Caffeine docking studies with keratin: implications for its cosmetic applications. Mater Today: Proc. 2022;69:1408–1412. doi: 10.1016/j.matpr.2022.09.206. [DOI] [Google Scholar]
- Grosso G, Godos J, Galvano F, Giovannucci EL. Coffee, caffeine, and health outcomes: an umbrella. Review. 2017 doi: 10.1146/annurev-nutr-071816. [DOI] [PubMed] [Google Scholar]
- Halberstein RA. Medicinal plants: historical and cross-cultural usage patterns. Ann Epidemiol. 2005;15(9):686–699. doi: 10.1016/j.annepidem.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Isemura M. Catechin in human health and disease. Molecules. 2019 doi: 10.3390/molecules24030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YJ, Koo HJ, Sohn EH, Kang SC, Rhee DK, Pyo S. Theobromine inhibits differentiation of 3T3-L1 cells during the early stage of adipogenesis via AMPK and MAPK signaling pathways. Food Funct. 2015;6(7):2365–2374. doi: 10.1039/c5fo00397k. [DOI] [PubMed] [Google Scholar]
- Kang P, Liao M, Wester MR, Leeder JS, Pearce RE. NIH public access. Ratio. 2010;36(3):490–499. doi: 10.1124/dmd.107.016501.CYP3A4-Mediated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolahdouzan M, Hamadeh MJ. The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci Therap. 2017 doi: 10.1111/cns.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács EG, Alatshan A, Budai MM, Czimmerer Z, Bíró E, Benkő S. Caffeine has different immunomodulatory effect on the cytokine expression and nlrp3 inflammasome function in various human macrophage subpopulations. Nutrients. 2021 doi: 10.3390/nu13072409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladio AH, Acosta M. Urban medicinal plant use: do migrant and non-migrant populations have similar hybridisation processes? J Ethnopharmacol. 2019 doi: 10.1016/j.jep.2019.01.013. [DOI] [PubMed] [Google Scholar]
- Langeder J, Grienke U, Chen Y, Kirchmair J, Schmidtke M, Rollinger JM. Natural products against acute respiratory infections: strategies and lessons learned. J Ethnopharmacol. 2020 doi: 10.1016/j.jep.2019.112298. [DOI] [PubMed] [Google Scholar]
- Martínez-Pinilla E, Oñatibia-Astibia A, Franco R. The relevance of theobromine for the beneficial effects of cocoa consumption. Front Pharmacol. 2015 doi: 10.3389/fphar.2015.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder A, Cerella C, Diederich M. Natural scaffolds in anticancer therapy and precision medicine. Biotechnol Adv. 2018 doi: 10.1016/j.biotechadv.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Monji F, Siddiquee A, Hashemian F. Potential role of methylxanthines as an adjuvant to COVID-19 treatment: a review of pentoxifylline and caffeine as the case of any port in the storm. Authorea. 2020 doi: 10.22541/au.159015270.02586731. [DOI] [Google Scholar]
- Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, Tarhriz V, Farjami A, Ghasemian Sorbeni F, Ghasemnejad T. COVID-19 infection: an overview on cytokine storm and related interventions. Virol J. 2022 doi: 10.1186/s12985-022-01814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro J, Alves MG, Oliveira PF, Silva BM. Pharmacological potential of methylxanthines: Retrospective analysis and future expectations. Crit Rev Food Sci Nutr. 2019 doi: 10.1080/10408398.2018.1461607. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016 doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Okamoto M, Dapat I, Katsumi M, Oshitani H. Inactivation of SARS-CoV-2 by catechins from green tea. Jpn J Infect Dis. 2021;74(5):421–423. doi: 10.7883/yoken.JJID.2020.902. [DOI] [PubMed] [Google Scholar]
- Paiva CLRS, Beserra BTS, Reis CEG, Dorea JG, da Costa THM, Amato AA. Consumption of coffee or caffeine and serum concentration of inflammatory markers: a systematic review. Crit Rev Food Sci Nutr. 2019 doi: 10.1080/10408398.2017.1386159. [DOI] [PubMed] [Google Scholar]
- Rolta R, Salaria D, Sharma B, Awofisayo O, Fadare OA, Sharma S, Dev K. Methylxanthines as potential inhibitor of SARS-CoV-2: an in silico approach. Curr Pharmacol Rep. 2022 doi: 10.1007/s40495-021-00276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Martínez BS, Montaño LM, Solís-Chagoyán H, Sommer B, Ramírez-Salinas GL, Pérez-Figueroa GE, Flores-Soto E. Possible beneficial actions of caffeine in sars-cov-2. Int J Mol Sci. 2021 doi: 10.3390/ijms22115460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TangL-Q, Wei W, Wang X-Y (2017) Effects and mechanisms of catechin for adjuvant arthritis in rats [DOI] [PubMed]
- Ulvia B, Andarwulan N, Hunaefi D. Profile of bioactive compounds and antioxidant capacity of Indonesian cocoa powder: a case of food processing authentication. Scitepress; 2020. pp. 97–105. [Google Scholar]
- van Dam RM, Hu FB. Caffeine consumption and cardiovascular health. Nat Rev Cardiol. 2022 doi: 10.1038/s41569-022-00719-4. [DOI] [PubMed] [Google Scholar]
- Vieira AJSC, Gaspar EM, Santos PMP. Mechanisms of potential antioxidant activity of caffeine. Radiat Phys Chem. 2020 doi: 10.1016/j.radphyschem.2020.108968. [DOI] [Google Scholar]
- Yang N, Shen H-M. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int J Biol Sci. 2020;16(10):1724–1731. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.