Abstract
The results of several studies aiming to tailor early breast cancer treatment to individual risk were released in 2023. Axillary lymph node dissections and radiotherapy may be safely omitted in carefully selected patients. Sustained benefit from adjuvant CDK4/6 inhibitors was observed in high-risk hormone receptor-positive disease and the addition of immunotherapy to neoadjuvant chemotherapy improved pathological response. Continued benefit from perioperative pembrolizumab was reported in patients with triple negative breast cancer, while atezolizumab did not improve the risk of recurrence either pre- or postoperatively. The chance of pregnancy was higher in younger patients attempting to conceive after breast cancer.
Keywords: Early breast cancer, Adjuvant therapy, Immunotherapy, Radiation therapy, Survivorship
Highlights
-
•
Risk-adapted management of early breast cancer (BC) is becoming increasingly relevant.
-
•
Axillary dissections and radiotherapy may be safely omitted in carefully selected patients.
-
•
Adjuvant CDK4/6 inhibitors improved outcomes in high-risk hormone receptor-positive (HR+) BC.
-
•
Chemo-immunotherapy regimens increased the pathological response in HR + BC.
-
•
Pregnancy after breast cancer appears to be safe in patients with HR + disease and BRCA1/2 carriers.
1. Introduction
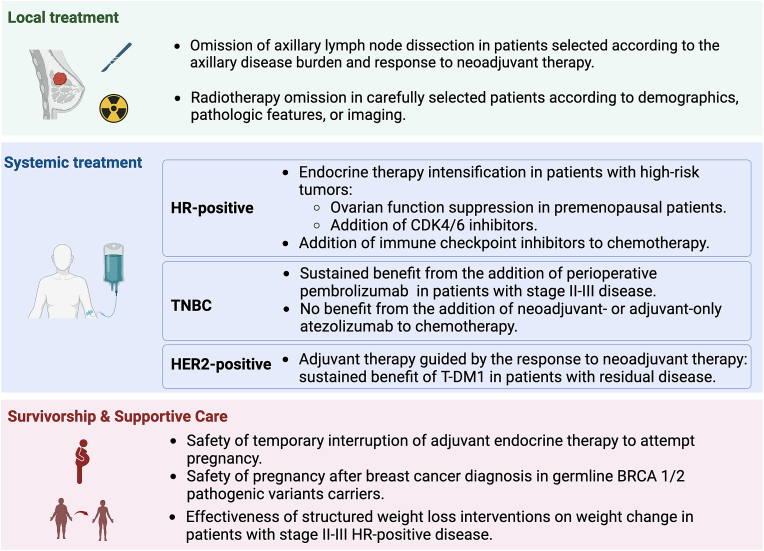
2023 was marked by long-awaited research results that directly influence early breast cancer management. Several studies shared a common objective of tailoring treatment intensity according to individual risk. In this article, we highlight recent studies addressing dominant topics in this field that will inform local and systemic breast cancer treatment and survivorship (Fig. 1).
Fig. 1.
Dominant topics in early breast cancer research in 2023
Abbreviations: HR: hormone receptor, TNBC: triple negative breast cancer. Created with BioRender.com.
2. Breast cancer treatment
2.1. Local therapy
Routine use of sentinel lymph node biopsy (SLN) has allowed many patients to safely avoid axillary lymph node dissection (ALND) and its associated morbidity [[1], [2], [3]]. Recently presented results have contributed to filling some knowledge gaps in this field, particularly in patients with a low volume of nodal disease. The ICARO study (OPBC05/EUBREAST-14R) was a large (N = 583), international, real-world data (RWD) study evaluating the role of ALND in patients with isolated tumor cells (ITCs) after neoadjuvant chemotherapy (NACT) [4]. In this study, among patients with ITCs who underwent ALND, 29.5% were found to have additional positive nodes, most of which with low metastatic burden (6.2% micrometastases, and 15.8% ITCs), with only 7.5% of cases having macrometasis [4]. After a median follow-up of 3.2 years, there were no significant differences in axillary recurrence, locoregional recurrence, and any invasive recurrence between patients treated with or without ALND, suggesting little value from routine ALND in patients with ITCs after NACT [4]. The SENOMAC trial compared ALND with omission of ALND in patients with cT1-3, cN0 primary breast cancer and 1–2 SLN macrometastases who were treated with upfront surgery, and included a more broad population than prior studies assessing this issue including more patients who had undergone mastectomy, with extranodal extension and men [5]. After a median follow-up of 37.1 months, no differences in recurrence-free survival were observed between patients treated with or without ALND [5]. Considering that the majority (93.6%) of the patients included in this study had ER-positive disease, long-term follow-up is essential though these results confirm and extend prior research regarding safety of forgoing ALND in this setting [5].
The NRG Oncology/NSABP B-51/RTOG 1304 phase III trial (NCT01872975) sought to further reduce local therapy in select patients, randomizing those with cT1-3, cN1 breast cancer with a complete nodal pathological response (ypN0) after NACT to receive adjuvant regional nodal irradiation (RNI) or not [6]. In a predominantly ER-negative population (78%), no statistically significant differences were observed in invasive breast cancer recurrence-free interval (HR = 0.88, 95%CI 0.60–1.29) or distant recurrence-free interval (HR 1.0, 95%CI 0.67–1.51) between the groups [6]. These findings add to the growing number of studies demonstrating the safety of radiotherapy omission in carefully selected patients whether using demographics, pathologic features, or imaging, and several studies are ongoing to refine further the identification of low-risk groups who can safely avoid radiotherapy [[6], [7], [8], [9], [10]].
2.2. Systemic therapy
2.2.1. Hormone receptor-positive breast cancer
Ovarian function suppression (OFS) has been used as part of the adjuvant treatment of premenopausal patients with high-risk ER-positive breast cancer [11]. The magnitude of the benefit derived from this strategy was confirmed in a large patient-level meta-analysis carried out by the Early Breast Cancer Trialists' Collaborative Group, including data from 14,993 women [12]. Among premenopausal women, the risk of recurrence at 15 years was reduced by 12.1% (28.9% vs. 41.0%) and all-cause mortality was improved by 7.2% (26.0% vs. 33.1%; RR = 0.73, 0.64–0.82) with the addition of ovarian ablation or suppression [12]. Importantly, OFS was not associated with increased risk of deaths without recurrence (RR = 0.88, 0.67–1.14) [12].
Another strategy to optimize the adjuvant treatment of patients with ER-positive breast cancer is the addition of CDK4/6 inhibitors to ET. Updated results from the monarchE trial demonstrated that, with a median follow-up of 54 months, the addition of abemaciclib to ET was associated with improvements in invasive disease-free survival (IDFS) (HR 0.68, 0.60–0.77) and distant relapse-free survival (DRFS) (HR 0.67, 95% CI 0.59–0.77) in patients with high-risk ER-positive disease, corresponding to 5-year absolute improvements of 7.6% in IDFS and 6.7% in DRFS [13]. Biomarker analysis from this trial showed the benefit associated with abemaciclib was fairly consistent across all intrinsic breast cancer subtypes, Recurrence Scores, and the most common molecular alterations, except for MYC amplification, which was associated with less benefit from abemaciclib [14]. The NATALEE trial presented initial results in 2023 from the addition of three years of ribociclib to adjuvant ET in patients with stage II-III ER-positive breast cancer [15]. With a median follow-up of 34 months, improvements in IDFS (HR 0.75, 95% CI 0.63–0.89) and DDFS (HR 0.75, 95% 0.62–0.90) favoring the ribociclib-containing arm were observed [15]. Importantly, the impact of adjuvant CDK4/6 blockade on OS remains to be determined with long-term follow-up.
Although immune checkpoint inhibitors have shown limited activity as monotherapy in patients with ER-positive breast cancer [16], early signs of efficacy were demonstrated by their combination with cytotoxic chemotherapy in higher risk ER-positive disease in two randomized trials. KEYNOTE-756 is a phase III placebo-controlled trial testing the addition of pembrolizumab to neoadjuvant chemotherapy followed by adjuvant pembrolizumab in combination with ET, having pathologic complete response (pCR) and event-free survival (EFS) as co-primary endpoints [17]. Similarly, CheckMate 7FL is a phase III study comparing neoadjuvant chemotherapy with or without nivolumab followed by adjuvant ET monotherapy or in combination with the anti-PD-1 agent, with pCR as primary endpoint (EFS as secondary endpoint) [18]. In both studies, patients treated with the immunotherapy-containing regimens had higher pCR rates, with absolute improvements of 8.5% (95%CI 4.2–12.8) in KEYNOTE-756, and 10.5% (95%CI 4.0–16.9) in CheckMate-7FL [17,18]. Biomarker analyses of both trials showed greater magnitudes of benefit in patients with higher PD-L1 expression, lower ER expression and, in CheckMate-7FL, in higher stromal tumor infiltrating lymphocytes [19,20]. The impact of immune checkpoint blockade on EFS will have a critical influence on the interpretation of these results and on the decision to incorporate or not this strategy into clinical practice.
2.2.2. Triple negative breast cancer
Updated results from KEYNOTE-522 which randomized patients with stage II-III TNBC to receive pembrolizumab or placebo starting in the preoperative period with chemotherapy revealed continued benefit from the addition of pembrolizumab in EFS (HR 0.63, 95% CI 0.49–0.81), and in distant recurrence-free survival (HR 0.64, 95%CI 0.49–0.84) at median follow-up of 63 months [21]. Key subgroup analyses confirmed the EFS benefit of adding pembrolizumab including for those with stage II (HR: 0.59, 95% CI, 0.43–0.82), and node-negative disease (HR: 0.56, 95% CI, 0.38–0.84) [21]. Overall survival data has not been presented yet.
In contrast, in the phase III ALEXANDRA/IMpassion030 study, which randomized 2300 patients treated with upfront surgery for stage II-III I TNBC to all adjuvant chemotherapy ± atezolizumab, there was no significant difference in invasive disease-free survival (IDFS) between treatment arms in the overall population (HR 1.12, 95%CI 0.87–1.45) or in the PD-L1-positive subgroup (HR 1.03, 95%CI 0.75–1.42) [22]. Similarly, the addition of atezolizumab to neoadjuvant chemotherapy did not improve EFS (HR 1.07, 95%CI 0.67–1.73) in the NeoTRIP trial [23]. It remains unclear whether the negative results of these studies stem from different populations, study designs (purely adjuvant in the case of the ALEXANDRA trial), different immunotherapy mechanisms (PD-L1 inhibitor), differences in the chemotherapy backbone or other factors.
2.2.3. HER2-positive breast cancer
The final DFS analysis from the KATHERINE study, assessing adjuvant T-DM1 in patients with HER2-positive residual disease after neoadjuvant anti-HER2 based chemotherapy, confirmed the benefit of T-DM1 compared to trastuzumab (HR 0.54, 95% CI 0.44–0.66), representing a 13.7% absolute improvement in 7-year IDFS rate [24]. Post-neoadjuvant T-DM1 was also associated with a 34% reduction in the risk of death (HR 0.66, 95%CI 0.51–0.87), translating into a 4.7% absolute benefit in OS at 7 years, confirming the tremendous value of this strategy for patients [24].
2.2.4. Survivorship and supportive care
A growing number of studies have been interested in addressing important questions related to quality of life, lifestyle, and supportive care for breast cancer patients. Building on knowledge of the negative prognostic impact of obesity in patients with breast cancer [25], the BWEL trial evaluated the impact of a structured telephone-based weight loss intervention (WLI) on the risk of recurrence and on weight change in patients with ER-positive stage II-III breast cancer [26]. In this trial, the WLI was associated with significant weight loss in patients with overweight and obesity compared to regular health education [26]. Although the impact of this intervention on cancer-related outcomes remains unknown, this study provides proof of concept that telephone interventions can be effective in promoting healthy habits and generating positive impacts on general health after breast cancer treatment.
The safety of pregnancy after breast cancer diagnosis has been an area of active research. The POSITIVE trial demonstrated that a temporary interruption of ET to attempt pregnancy is not associated with a significant increase in the short-term risk of breast cancer events [27]. This trial further demonstrated that younger age (<35 years old) was the only factor associated with time-to-pregnancy, while both younger age and the use of assisted reproductive technology (ART) were predictors of the chance of pregnancy [28]. The use of ovarian stimulation was also not associated with an excess of breast cancer events, either as part of embryo/oocyte cryopreservation (at breast cancer diagnosis) or as part of ART, although the latter analysis should be interpreted with caution due to the low number of patients and events [28]. Further, new data confirmed the safety of pregnancy in young survivors with germline BRCA 1 and/or BRCA2 pathogenic variants carriers [29].
3. Conclusions
The management of early breast cancer is consistently evolving towards personalized risk-adapted therapies, which have been facilitated by improvements in the accuracy of prognostic and predictive determinants. While randomized studies remain essential, studies with unconventional designs and real-world data help us to answer important clinical questions in a timely manner. Moving forward, as our treatments evolve and the chances of a cure increase, maintaining quality of life becomes a central priority to be considered throughout patients’ trajectories.
CRediT authorship contribution statement
Guilherme Nader-Marta: Writing – original draft. Ann H. Partridge: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
Wolters-Kluwer-royalties (AHP); Novartis-research support (AHP). AstraZeneca - travel grants for meetings (GNM).
References
- 1.Giuliano A.E., Ballman K.V., McCall L., et al. Effect of axillary dissection vs No axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318(10):918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartels S.A.L., Donker M., Poncet C., et al. Radiotherapy or surgery of the Axilla after a positive sentinel node in breast cancer: 10-year results of the randomized controlled EORTC 10981-22023 AMAROS trial. J Clin Oncol. 2023;41(12):2159–2165. doi: 10.1200/JCO.22.01565. [DOI] [PubMed] [Google Scholar]
- 3.Sakorafas G.H., Peros G., Cataliotti L., Vlastos G. Lymphedema following axillary lymph node dissection for breast cancer. Surg Oncol. 2006;15(3):153–165. doi: 10.1016/j.suronc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Montagna G., Laws A., Ferrucci M. San Antonio Breast Cancer Symposium. 2023. GS02-02 Are nodal ITCs after neoadjuvant chemotherapy an indication for axillary dissection? The OPBC05/EUBREAST-14R/ICARO study. [Google Scholar]
- 5.de Boniface J., Tvedskov T., Leif B. San Antonio Breast Cancer Symposium. 2023. GS02-06 Recurrence-free survival following sentinel node-positive breast cancer without completion axillary lymph node dissection – first results from the international randomized SENOMAC trial. [Google Scholar]
- 6.Mamounas E., Bando H., White J. San Antonio breast cancer Symposium. 2023. GS02-07 Loco-regional irradiation in patients with biopsy-proven axillary node involvement at presentation who become pathologically node-negative after neoadjuvant chemotherapy: primary outcomes of NRG Oncology/NSABP B-51/RTOG 1304. [Google Scholar]
- 7.Chadha M., White J., Swain S.M., et al. Optimal adjuvant therapy in older (≥70 years of age) women with low-risk early-stage breast cancer. Npj Breast Cancer. 2023;9(1):1–7. doi: 10.1038/s41523-023-00591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunkler I.H., Williams L.J., Jack W.J.L., Cameron D.A., Dixon J.M. Breast-Conserving surgery with or without irradiation in early breast cancer. N Engl J Med. 2023;388(7):585–594. doi: 10.1056/NEJMoa2207586. [DOI] [PubMed] [Google Scholar]
- 9.Jagsi R., Griffith K., Harris E. San Antonio Breast Cancer Symposium. 2023. GS02-08) Five-year outcomes of the IDEA trial of endocrine therapy without radiotherapy after breast-conserving surgery for postmenopausal patients age 50-69 with genomically-selected favorable Stage I breast cancer. [Google Scholar]
- 10.Riaz N., Jeen T., Whelan T.J., Nielsen T.O. Recent advances in optimizing radiation therapy decisions in early invasive breast cancer. Cancers. 2023;15(4):1260. doi: 10.3390/cancers15041260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curigliano G., Burstein H.J., Gnant M., et al. Understanding breast cancer complexity to improve patient outcomes: the st. Gallen international consensus conference for the primary therapy of individuals with early breast cancer 2023. Ann Oncol. 2023;0(0) doi: 10.1016/j.annonc.2023.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Gray R.G., Bradley R., Braybrooke J., et al. Effects of ovarian ablation or suppression on breast cancer recurrence and survival: patient-level meta-analysis of 14,993 pre-menopausal women in 25 randomized trials. J Clin Oncol. 2023;41(16_suppl) doi: 10.1200/JCO.2023.41.16_suppl.503. 503-503. [DOI] [Google Scholar]
- 13.Rastogi P., O'Shaughnessy J., Martin M., et al. Adjuvant abemaciclib plus endocrine therapy for hormone receptor–positive, Human epidermal growth factor receptor 2–negative, high-risk early breast cancer: results from a preplanned monarchE overall survival interim analysis, including 5-year efficacy outcomes. J Clin Oncol. Published online January. 2024;9 doi: 10.1200/JCO.23.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner N., Reis-Filho J.S., Goetz M. 2023. (GS03-06) Genomic and transcriptomic profiling of primary tumors from patients with HR+, HER2-, node-positive, high-risk early breast cancer in the monarchE trial. [Google Scholar]
- 15.Slamon D.J., Stroyakovskiy D., Yardley D.A., et al. Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2- early breast cancer: primary results from the phase III NATALEE trial. J Clin Oncol. 2023;41(17_suppl) doi: 10.1200/JCO.2023.41.17_suppl.LBA500. LBA500-LBA500. [DOI] [Google Scholar]
- 16.Rugo H.S., Delord J.P., Im S.A., et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2018;24(12):2804–2811. doi: 10.1158/1078-0432.CCR-17-3452. [DOI] [PubMed] [Google Scholar]
- 17.Cardoso F., McArthur H.L., Schmid P., et al. LBA21 KEYNOTE-756: phase III study of neoadjuvant pembrolizumab (pembro) or placebo (pbo) + chemotherapy (chemo), followed by adjuvant pembro or pbo + endocrine therapy (ET) for early-stage high-risk ER+/HER2– breast cancer. Ann Oncol. 2023;34:S1260–S1261. doi: 10.1016/j.annonc.2023.10.011. [DOI] [Google Scholar]
- 18.Loi S., Curigliano G., Salgado R.F., et al. LBA20 A randomized, double-blind trial of nivolumab (NIVO) vs placebo (PBO) with neoadjuvant chemotherapy (NACT) followed by adjuvant endocrine therapy (ET) ± NIVO in patients (pts) with high-risk, ER+ HER2− primary breast cancer (BC) Ann Oncol. 2023;34:S1259–S1260. doi: 10.1016/j.annonc.2023.10.010. [DOI] [Google Scholar]
- 19.Cardoso F., O'Shaughnessy J., McArthur H. San antonio breast cancer symposium. 2023. GS01-02) Phase 3 study of neoadjuvant pembrolizumab or placebo plus chemotherapy, followed by adjuvant pembrolizumab or placebo plus endocrine therapy for early-stage high-risk ER+/HER2− breast cancer: KEYNOTE-756. [Google Scholar]
- 20.Loi S., Curigliano G., Salgado R. San Antonio Breast Cancer Symposium. 2023. GS01-01) biomarker results in high-risk Estrogen receptor positive, Human Epidermal Growth factor receptor 2 negative primary breast cancer following neoadjuvant chemotherapy ± nivolumab: an exploratory analysis of CheckMate 7FL. [Google Scholar]
- 21.Schmid P., Cortes J., Dent R. San Antonio Breast Cancer Symposium. 2023. (LBO1-01) Neoadjuvant pembrolizumab or placebo plus chemotherapy followed by adjuvant pembrolizumab or placebo for early-stage triple-negative breast cancer: updated event-free survival results from the phase 3 KEYNOTE-522 study. [Google Scholar]
- 22.Ignatiadis M., Bailey A., McArthur H. San Antonio Breast Cancer Symposium. 2023. GS01-03) Adding atezolizumab to adjuvant chemotherapy for stage II and III triple-negative breast cancer is unlikely to improve efficacy: interim analysis of the ALEXANDRA/IMpassion030 phase 3 trial. [Google Scholar]
- 23.Gianni L., Huang C., Egle D., et al. LBA19 Event-free survival (EFS) analysis of neoadjuvant taxane/carboplatin with or without atezolizumab followed by an adjuvant anthracycline regimen in high-risk triple negative breast cancer (TNBC): NeoTRIP Michelangelo randomized study. Ann Oncol. 2023;34:S1258–S1259. doi: 10.1016/j.annonc.2023.10.009. [DOI] [Google Scholar]
- 24.Loibl S., Mano M.S., Untch M. San Antonio Breast Cancer Symposium. 2023. GS03-12) Phase III study of adjuvant ado-trastuzumab emtansine vs trastuzumab for residual invasive HER2-positive early breast cancer after neoadjuvant chemotherapy and HER2-targeted therapy: KATHERINE final IDFS and updated OS analysis. [Google Scholar]
- 25.Chan D.S.M., Vieira R., Abar L., et al. Postdiagnosis body fatness, weight change and breast cancer prognosis: global Cancer Update Program (CUP global) systematic literature review and meta-analysis. Int J Cancer. 2023;152(4):572–599. doi: 10.1002/ijc.34322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ligibel J.A., Ballman K.V., McCall L.M., et al. Effect of a telephone-based weight loss intervention (WLI) on weight at 12-months in women with early breast cancer: results from the Breast Cancer Weight Loss (BWEL) trial. J Clin Oncol. 2023;41(16_suppl) doi: 10.1200/JCO.2023.41.16_suppl.12001. 12001-12001. [DOI] [Google Scholar]
- 27.Partridge A.H., Niman S.M., Ruggeri M., et al. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med. 2023;388(18):1645–1656. doi: 10.1056/NEJMoa2212856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azim H.A., Niman S.M., Partridge A.H. San Antonio Breast Cancer Symposium. 2023. GS02-11) Fertility preservation and assisted reproductive technologies (ART) in breast cancer (BC) patients (pts) interrupting endocrine therapy (ET) to attempt pregnancy. [Google Scholar]
- 29.Lambertini M., Blondeaux E., Agostinetto E., et al. Pregnancy after breast cancer in young BRCA carriers: an international hospital-based cohort study. JAMA. 2024;331(1):49–59. doi: 10.1001/jama.2023.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]