Abstract
Dietary egg yolk-derived anti-interleukin (IL)-10 may preserve broiler chicken performance during coccidiosis due to Eimeria spp. infection while effects on secondary Clostridium perfringens (necrotic enteritis) are unknown. Some necrotic enteritis models implement Salmonella Typhimurium to improve repeatability; however, Salmonella upregulation of IL-10 may be a confounder when evaluating anti-IL-10. The study objective was to investigate anti-IL-10 effects on systemic cytokine concentrations and immunometabolism during E. maxima ± C. perfringens challenge in models ± S. Typhimurium. Three 25 d replicate studies using Ross 308 chicks were conducted in wire-floor cages (32 cages/ replicate) with chicks assigned to diets ± 0.03% anti-IL-10. 640 chicks (20/ cage; replicates 1 and 2) were inoculated with sterile saline ± 1×108 colony forming units (CFU) S. Typhimurium while 480 chicks (15/ cage) were placed in replicate 3. In all replicates, blood samples were collected on d 14 (6 chicks/treatment) before administering 15,000 sporulated E. maxima M6 oocysts to S. Typhimurium-inoculated (replicates 1 and 2) or challenge-designated chicks (replicate 3). Half the E. maxima-challenged chicks received 1×108 CFU C. perfringens on d 18 and 19. Blood samples were collected at 1, 3, 7, and 11 d post-inoculation (dpi) with E. maxima and 1, 3, and 7 dpi with secondary C. perfringens. Plasma cytokines were determined by ELISA while immunometabolic assays evaluated peripheral blood mononuclear cell ATP production and glycolytic rate responses. Data were analyzed with diet and challenge fixed effects plus associated interactions (SAS 9.4; P ≤ 0.05). Replicates 1 and 2 showed few immunometabolic responses within 3 dpi with E. maxima, but 25 to 31% increased ATP production and 32% increased compensatory glycolysis at 1 dpi with C. perfringens in challenged vs. unchallenged chicks (P ≤ 0.04). In replicate 3, total ATP production and compensatory glycolysis were increased 25 and 40%, respectively, by the E. maxima main effect at 1dpi (P ≤ 0.05) with unobserved responsiveness to C. perfringens. These outcomes indicate that model type had greater impacts on systemic immunity than anti-IL-10.
Key words: Salmonella Typhimurium, Eimeria maxima, Clostridium perfringens, IgY antibodies, immunometabolism
INTRODUCTION
Understanding immune responses to infectious disease could help identify biological targets for the development of novel mitigation strategies to support poultry health. One approach has been the development of specific immunoglobulin (Ig) Y antibodies from egg yolk to target host interleukin (IL)-10. During infection, Eimeria spp., an intracellular parasite, induces host production of IL-10 to evade the immune system, establish within the intestine, and cause coccidiosis (Hong et al., 2006; Arendt et al., 2019a; López-Osorio et al., 2020). Economic losses related to poor production performance and increased mortality due to coccidiosis have been estimated as high as $14.5 billion USD for the global poultry industry (Blake, et al., 2020). Cellular damage and weakened immune responses due to coccidiosis also create an environment for other pathogens to cause further damage. Increased protein content in the intestinal lumen caused by Eimeria parasites invading and lysing intestinal epithelial cells establishes the ideal environment for pre-existing Clostridium perfringens to expand and cause secondary necrotic enteritis, contributing to an additional estimated economic loss of $3 billion USD (Shojadoost et al., 2012). Existing strategies to address coccidiosis and necrotic enteritis are often ineffective due to conflicts with antibiotic-free (ABF) production practices, increased prevalence of Eimeria resistance to these compounds, and ineffective vaccine coverage across the various strains and species capable of infecting poultry (Dalloul and Lillehoj, 2006; Arabkhazaeli et al., 2013; Noack et al., 2019). Dietary antibodies targeting elements of the host immune response are ideal candidates to mitigate coccidiosis and necrotic enteritis because they do not contribute to drug resistance, align with ABF production standards, can be specifically targeted, and are restricted to the intestinal lumen/mucosa in proximity to Eimeria and C. perfringens (Cook and Trott, 2010; Bobeck, et al., 2016).
Cytokines produced by the host can cause local changes in immune response. For example, IL-10 suppresses macrophage activity and shifts effector helper T cell (TH) responses away from those driven by pro-inflammatory, interferon (IFN)-γ-producing TH1 cells in favor of anti-inflammatory TH2-mediated responses (Couper et al., 2008). In the context of Eimeria spp. infection, increased IL-10 not only allows the parasite to evade innate immune responders but also shifts adaptive lymphocyte responses away from phenotypes considered effective for the clearance of intracellular pathogens (Berger, 2000). Theoretically, dietary anti-IL-10 would counteract Eimeria spp. immune evasion, allowing the host immune system to mobilize favorable TH1 and IFN-γ-mediated responses. This has been supported by preliminary studies where 0.34 g/kg anti-IL-10 preserved bird body weight gain during challenge with high-dose (10X) vaccine-strain Eimeria spp. or direct administration of IFN-γ preserved bird body weight gain during wild-type E. acervulina challenge (Lillehoj and Choi, 1998; Arendt, et al., 2016; Sand, et al., 2016; Arendt, et al., 2019b). Previous work evaluating immune responses to anti-IL-10 have emphasized the direct effect of the antibody on luminal and mucosal IL-10 within the intestine; however, how these changes might translate to systemic responses is undetermined. In research using field-strain E. acervulina and E. tenella, anti-IL-10 did not protect bird performance like in previous studies, but increased circulating T lymphocyte populations, suggesting that anti-IL-10 physiological effects can still be observed in the absence of preserved production performance (Rasheed et al., 2020).
While promising, anti-IL-10 specific mechanisms of action and ramifications for the development of necrotic enteritis have not been evaluated. Likewise, increased systemic T lymphocytes in anti-IL-10-fed birds during Eimeria challenge is intriguing, but immune cell presence does not always equate to increased functionality. One way to approximate immune cell function is through immunometabolic assays. During an immune response, cellular activation, differentiation, and proliferation requires rapid and substantial energy input to mount an adequate response. This can be evaluated as a transition from high yield but relatively slow metabolic profiles dominated by oxidative phosphorylation typically seen in quiescent immune cells towards those favoring rapid, but less efficient ATP production via aerobic glycolysis (Rambold and Pearce, 2018; Kornberg, 2020). Monitoring immunometabolic signatures over the course of a disease challenge can help identify activation timelines and whether diet-induced shifts in these responses are beneficial or detrimental to host health. In chickens, cell culture-based assays using peripheral blood mononuclear cells (PBMC) to provide real-time insight into metabolic behavior during various challenges have been optimized and conducted in the context of vaccine-strain Eimeria challenges but have been minimally evaluated using field-strain E. maxima beyond 1 d post-inoculation (dpi) or during secondary C. perfringens challenge (Meyer et al., 2021; Fries-Craft et al., 2023a; Fries-Craft et al., 2023b).
Modeling necrotic enteritis in research settings is complicated by the ubiquitous nature of C. perfringens, meaning that its presence is not sufficient for inducing a repeatable challenge and model success can be impacted by factors other than predisposing Eimeria (Al-Sheikhly and Al-Saieg, 1980; Kaldhusdal and Hofshagen, 1992; Shojadoost, et al., 2012). To overcome the issue of repeatability, models relying on Salmonella Typhimurium to induce localized immune suppression within the intestine and optimize environments for E. maxima and C. perfringens challenge have been implemented (Shivaramaiah et al., 2011; Hernandez-Patlan et al., 2019; Coles et al., 2021). While it may favorably affect necrotic enteritis model repeatability, S. Typhimurium induces immune tolerance responses in chickens through an IL-10 mediated pathway, potentially saturating the intestinal environment with IL-10 and reducing anti-IL-10 efficacy during disease challenge (Kogut and Arsenault, 2017; Salazar et al., 2017). As such, the study objective was to evaluate the effects of dietary anti-IL-10 on systemic IL-10 and IFN-γ concentrations and PBMC immunometabolic responses during E. maxima and C. perfringens challenge in models with or without early inoculation with S. Typhimurium.
MATERIALS AND METHODS
Animals and Sample Collection
All animal protocols were approved by the Iowa State University Institutional Animal Care and Use Committee. Detailed description of housing, diets, and challenge pathogen preparation can be found in Fries-Craft and Bobeck (2024). Briefly, 3 replicate studies using straight run Ross 308 broilers were completed with the 42 d studies consisting of a challenge period (d 0–25) and grow-out period (d 25–42). Study timelines from d 0 to 25 and each treatment arrangement are presented in Figure 1. Anti-IL-10 antibody was sourced from egg yolks collected from hens vaccinated against IL-10 peptide conjugated to bovine gamma globulin whereas control egg yolk powder was sourced from hens vaccinated against the carrier protein only. Chicks were assigned to diets consisting of a corn-soybean meal basal diet supplemented with 0.03% egg yolk powder ± peptide-specific anti-IL-10 antibody with ad libitum access to mash feed and water. The 0.03% egg powder inclusion rate translated to 150 units of active anti-IL-10 per ton of finished feed using 300 g antibody product per ton. The Chicks in all 3 replicate studies were placed in 2 separate brooders (Petersime Model 2SD20RE; Gettysburg, OH), one designated for unchallenged controls (6 cages/ diet; 12 total) and another for E. maxima or E. maxima + C. perfringens challenged animals (5 cages/ diet; 20 total). Brooders were placed in separate environmentally controlled rooms (initially 84°F stepped down to 79°F with 25-60% recorded humidity) on the same lighting schedule (23 h light, 1 h dark). Daily animal checks started in the unchallenged room with researchers changing clothing and foot coverings between rooms and daily floor washes to prevent cross-contamination. In replicate studies 1 and 2, chicks were inoculated with sterile phosphate buffered saline (PBS) or 1 × 108 colony forming units (CFU) of a Salmonella Typhimurium poultry isolate (Shivaramaiah et al., 2011) before being allowed access to feed while chicks in replicate study 3 were not inoculated with S. Typhimurium. In all 3 replicate studies, blood was collected from 6 chicks/ treatment on d 14 (baseline) by cardiac puncture under CO2 anesthesia before being euthanized by cervical dislocation. Remaining birds received sterile PBS or 15,000 sporulated oocysts of an E. maxima field strain (M6; Martin et al., 1997) by oral gavage. At d 18 and 19, half the E. maxima- challenged birds were orally inoculated with 1 × 108 CFU of C. perfringens TAMU strain (Ausland et al., 2020). Additional blood samples were collected at timepoints corresponding to 1, 3, 7, and 11 dpi for birds challenged with E. maxima with 4 birds/ treatment sampled at 1 and 3dpi and 6 birds/ treatment at 7 and 11 dpi. In birds challenged with E. maxima + C. perfringens, blood from 4 birds/ treatment was collected at 1 dpi and 6 birds/ treatment at 3 and 7 dpi. Challenge timelines for both groups overlapped at 7 dpi with E. maxima and 3 dpi with E. maxima + C. perfringens (study d 21) and 11 dpi with E. maxima and 7 dpi with E. maxima + C. perfringens (study d 25; Fries-Craft and Bobeck, 2024).
Figure 1.
Timelines and treatments for 3 replicate studies evaluating diets ± 0.03% anti-IL-10 in Ross 308 chicks during challenge with Eimeria maxima ± Clostridium perfringens over a 25-d study. Panel A depicts the inoculation and sample collection dates in replicate studies using a model with early Salmonella Typhimurium inoculation (replicate studies 1 and 2) and a follow-up study without S. Typhimurium (replicate study 3). All 3 replicate studies implemented the treatment arrangement displayed in panel B.
Seahorse Immunometabolic Assays
Blood from each chick was collected into heparin-coated needles, syringes, and blood tubes to prevent clotting, diluted 1:1 in sterile PBS, and layered onto a Histopaque 1,119 to 1,077 density gradient (3 mL each, Sigma Aldrich, St. Louis, MO). After centrifugation at 600 × g for 35 min at 18°C with low acceleration and no brakes, 1.5 mL of plasma from each PBMC isolation tube was collected from the top PBS layer and frozen at -80°C for cytokine analyses. Peripheral blood mononuclear cells (PBMC) were harvested from only the PBS-Histopaque 1119 interface as no cells were visually apparent at the Histopaque 1,119- 1,077 interface. Cells were washed twice by centrifugation in sterile PBS (400 × g, 10 min, 18°C), enumerated by hemocytometer, and 200,000 cells/ well were plated in triplicate on a 96-well cell culture plate for Agilent Real-Time ATP and Glycolytic Rate Assays (Agilent, Santa Clara, CA). Four wells per plate were used as assay blanks and did not contain any cells, in accordance with manufacturer recommendations. Therefore, PBMC from 30 chicks could be plated in triplicate on each assay plate. While PBMC were isolated from 6 chicks/ treatment at timepoints corresponding to 7 dpi with E. maxima/ 3 dpi with secondary C. perfringens and 11 dpi with E. maxima/ 7 dpi with secondary C. perfringens due to overlapping sample collection timepoints (36 chicks total), only PBMC from 5 chicks/ treatment could be plated in triplicate on the same plate due to space requirements for assay blanks. In replicate study 1, samples from each treatment were distributed across two 96-well assay plates; however, metabolic readings on the second plate were significantly lower than the first plate (Supplemental Figure 1) and excluded from analysis, resulting in PBMC from 3 chicks/ treatment for immunometabolic assays in replicate study 1. In replicate studies 2 and 3, PBMC from 5 chicks/ treatment were randomly selected for immunometabolic assays.
Assay reagents and cell culture media for all immunometabolic assays were prepared according to manufacturer recommendations and analyzed by Agilent Seahorse XFe96 Analyzer at 40°C (Santa Clara, CA). Each assay implements sequential administration of metabolic inhibitors to determine relative metabolic contributions to ATP production (Real-Time ATP Rate assay) and glycolytic activity (Glycolytic Rate Assay). During the Real-Time ATP rate assay, 15 μM oligomycin followed by 5 μM rotenone + antimycin A (Rot/AA) was injected into the assay media within each well to systematically inhibit ATP production via electron transport chain to calculate ATP production by glycolysis or mitochondrial oxidative respiration. The glycolytic rate assay injects 5 μM Rot/AA into the assay media to inhibit mitochondrial oxidative phosphorylation and evaluate cellular ability to use glycolysis to meet energy needs (compensatory glycolysis) before injecting 500 mM 2-deoxy-D-glucose (2DG), which inhibits the uptake of glucose into glycolytic pathways and assesses residual glycolytic activity (Post-2DG acidification; Supplementary Figure 2). Wave software (Agilent, version 2.6.1) converted measures taken by the real-time ATP and glycolytic rate assays into ATP production rate (pmol ATP/ min) or proton efflux rate (PER; pmol/ min), respectively, and outputs were exported for data analysis. Changes in the slope of the line produced by glycolytic rate assay outputs following Rot/AA (measures 3–4) and 2DG injection (measures 6–7) were calculated to evaluate the magnitude of change in glycolytic rate following in-assay inhibition.
Plasma IL-10 and IFN-γ ELISA
Plasma collected during PBMC isolation at baseline (d 14), E. maxima 7 dpi/ C. perfringens 3 dpi (d 21; peak), and E. maxima 11 dpi/ C. perfringens 7 dpi (d 25; post-peak) was thawed for IL-10 or IFN-γ detection by ELISA (Invitrogen, Waltham, MA). Samples were not diluted further since plasma was harvested from blood diluted 1:1 in PBS for PBMC isolation. Assay reagents and standards were prepared according to the manufacturer's instructions and samples and standards were plated in duplicate. All protocols were conducted according to manufacturer recommendations except for increasing sample incubation to 16 h and secondary antibody incubation to 2 h at room temperature. Developed plates were read at 450 nm on a plate reader (Biotek Software version 3.03.14) and absorbances exported as a Microsoft Excel file (version 16.76). The average blank absorbance was subtracted from all readings and average absorbances for the standards were used to generate a standard curve with the intercept set at 0. The resultant standard curve equation was used to calculate plasma IL-10 (pg/ mL) or IFN-γ (ng/ mL) and multiplied by 2 to account for plasma dilution. The lower limit of detection (LLOD) for IL-10 and IFN-γ assays was 16 pg/ mL and 0.06 ng/ mL, respectively, while average recovery rates in plasma samples were 86% for IL-10 and 103% for IFN-γ.
Statistical Analysis
All data were analyzed within each replicate study and timepoint using the following statistical model:
Wherein yijk denotes the response variable, Di is the main effect of diet at the ith level (i=2; control or anti-IL-10), Cj is the challenge main effect at the jth level (j=3; unchallenged, E. maxima, or E. maxima + C. perfringens), D×Cij is the diet and challenge interaction, and eijk denotes error. The mean cytokine concentration or immunometabolic measure across duplicate or triplicate wells was analyzed with individual chick as the experimental unit. Analysis was conducted using the mixed procedure with Tukey's Honest Significant test to account for multiple comparisons and significant denoted at P ≤ 0.05 (SAS 9.4, SAS Institute, Cary, NC).
RESULTS
ATP Production Rate
Figures depicting the main effect of immunometabolic outcomes for each replicate study are presented in Supplementary Figures 3 to 8. Replicate studies 1 and 2 represent d 14 baseline changes in PBMC ATP production profiles in chicks inoculated with S. Typhimurium at hatch but before challenge with E. maxima and C. perfringens, whereas replicate study 3 challenges purposely did not include early S. Typhimurium exposure. At baseline in replicate study 1, diet and challenge did not alter ATP production profiles in isolated PBMC (Figure 2A). At 1 dpi, the E. maxima challenge main effect increased mitochondrial contributions to ATP production by 27% in challenged birds (P = 0.03) which were resolved by 3 dpi (Figures 2B and 2C; Supplementary Figures 3B and 3C). At 1 dpi with secondary C. perfringens, both main effects of diet and challenge altered ATP production rate. Anti-IL-10 significantly increased oxidative phosphorylation and challenge significantly increased glycolysis along with total ATP production (Supplemental Figure 3D; P ≤ 0.03). When the interaction is taken into account, this culminated in chicks fed anti-IL-10 and challenged with E. maxima + C. perfringens having 26% greater glycolytic contributions to PBMC ATP production, culminating in 25% increased overall ATP production compared to their unchallenged counterparts (Figure 2D; P ≤ 0.04). These differences were resolved by 3 dpi and no recurrences of increased glycolytic or overall ATP production were observed 7 dpi (Figure 2E& F).
Figure 2.
Replicate study 1 ATP production rate in peripheral blood mononuclear cells (PBMC) isolated from Ross 308 chicks fed diets ± 0.03% anti-interleukin-10 before and after challenge with Eimeria maxima ± Clostridium perfringens. Chicks were inoculated with 1×108 colony forming units (CFU) of Salmonella Typhimurium on d0, 15,000 sporulated E. maxima M6 oocysts on d14, and 1×108 CFU C. perfringens on d18 and 19. Data in panels (A–F) represent the interaction effect diet and challenge at timepoints corresponding to 1, 3, 7, and 11d post-inoculation (dpi) with E. maxima or 1, 3, and 7 dpi with E. maxima + C. perfringens. Stacked bars represent the mean ATP production measure measured using the Agilent XFe96 Analyzer and ATP Production rate assay from 4 (E. maxima 1 and 3dpi; E. maxima + C. perfringens 1dpi) or 6 chicks / treatment (baseline; E. maxima 7 dpi/ E. maxima + C. perfringens 3dpi; E. maxima 11dpi/ E. maxima + C. perfringens 7 dpi) ± SEM. Different letter labels within the same-colored bar within a timepoint represent significantly different glycolytic or mitochondrial ATP production rates, whereas different labels above an entire bar represent significantly different total ATP production, P ≤ 0.05.
Figure 3.
Replicate study 2 ATP production rate in peripheral blood mononuclear cells (PBMC) isolated from Ross 308 chicks fed diets ± 0.03% anti-interleukin-10 before and after challenge with Eimeria maxima ± Clostridium perfringens. Chicks were inoculated with 1×108 colony forming units (CFU) of Salmonella Typhimurium on d0, 15,000 sporulated E. maxima M6 oocysts on d14, and 1×108 CFU C. perfringens on d18 and 19. Data in panels (A-F) represent the interaction effect diet and challenge at timepoints corresponding to 1, 3, 7, and 11d post-inoculation (dpi) with E. maxima or 1, 3, and 7dpi with E. maxima + C. perfringens. Stacked bars represent the mean ATP production measure measured using the Agilent XFe96 Analyzer and ATP Production rate assay from 4 (E. maxima 1 and 3dpi; E. maxima + C. perfringens 1dpi) or 6 chicks / treatment (baseline; E. maxima 7dpi/ E. maxima + C. perfringens 3dpi; E. maxima 11dpi/ E. maxima + C. perfringens 7dpi) ± SEM. Different letter labels within the same-colored bar within a timepoint represent significantly different glycolytic or mitochondrial ATP production rates, whereas different labels above an entire bar represent significantly different total ATP production, P ≤ 0.05.
In replicate study 2 at d 14 baseline, the S. Typhimurium challenge main effect increased glycolytic contributions to PBMC ATP production 17% in challenged vs. unchallenged chicks (P = 0.04); however, this did not contribute to subsequent changes in overall ATP production or a significant interaction (Figure 3A; Supplementary Figure 4A). In chicks challenged with E. maxima only, no differences in ATP production were observed in the first 3 dpi (Figures 3B and 3C). At 1 dpi with secondary C. perfringens, the challenge main effect increased glycolytic and mitochondrial contributions to ATP production 26 and 42%, respectively, resulting in 31% greater total ATP production in PBMC isolated from chicks challenged with E. maxima + C. perfringens compared to their unchallenged counterparts (P ≤ 0.01; Supplementary Figure 4D), but no interaction effect (Figure 3D). At 7 dpi E. maxima and 3 dpi with secondary C. perfringens (d 21), a challenge main effect increased glycolytic contributions 21 to 24% and mitochondrial contributions 37%, culminating in 31 to 33% increased total ATP production in challenged vs. unchallenged chicks (P ≤ 0.008) This significant challenge main effect persisted through 11 dpi (Figure 3E and 3F; Supplementary Figure 4E and 4F). A significant interaction occurred where chicks fed anti-IL-10 and challenged with only E. maxima at 11 dpi had 34% greater mitochondrial contributions to ATP production and 30% greater total ATP production compared to their unchallenged counterparts (P ≤ 0.05; Figure 3F), while the remainder were intermediate between challenged or unchallenged.
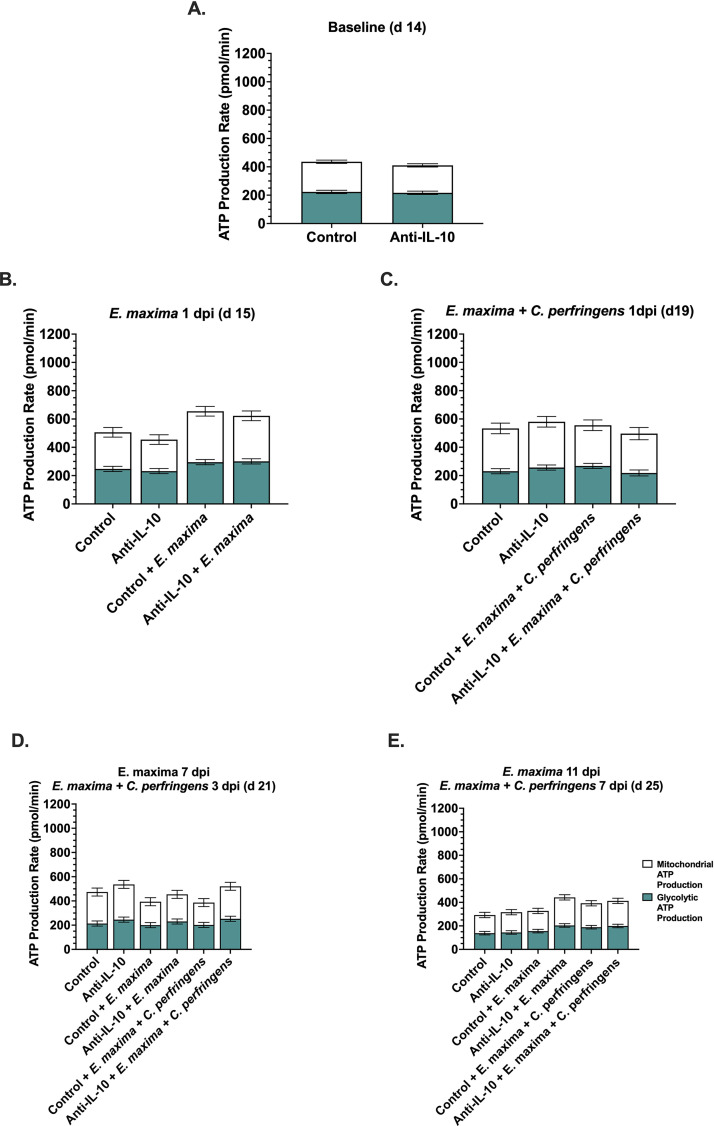
No differences in baseline ATP production rate in replicate study 3 were observed (Figure 4A). At 1 dpi with E. maxima, the challenge main effect increased glycolytic and mitochondrial ATP contributions 20 and 29%, respectively, culminating in 25% greater total ATP production in E. maxima-challenged vs. unchallenged chicks (P ≤ 0.01; Figure 4B; Supplementary Figure 5B). Due to an assay error, ATP production insights could not be obtained at 3 dpi with E. maxima and no differences in ATP production profiles were observed in chicks at 1 dpi with secondary C. perfringens (Figure 4C). A diet main effect increased glycolytic contributions to ATP production and total ATP production by 16 and 17%, respectively, in chicks fed anti-IL-10 vs. the control diet at timepoints corresponding to 7 dpi with E. maxima and 3 dpi with secondary C. perfringens (P ≤ 0.05; Figure 4D; Supplementary Figure 5D). At 11 dpi E. maxima/ 7 dpi C. perfringens, a challenge main effect increased glycolytic ATP production 21 to 27% in chicks challenged with E. maxima ± C. perfringens vs. unchallenged chicks, culminating in 21 to 24% greater total ATP production P = 0.03; Figure 4E; Supplementary Figure 5E).
Figure 4.
Replicate study 3 ATP production rate in peripheral blood mononuclear cells (PBMC) isolated from Ross 308 chicks fed diets ± 0.03% anti-interleukin-10 before and after challenge with Eimeria maxima ± Clostridium perfringens. Chicks were inoculated with 1×108 colony forming units (CFU) on d0, 15,000 sporulated E. maxima M6 oocysts on d14, and 1×108 CFU C. perfringens on d18 and 19. Data in panels A-F represent the interaction effect diet and challenge at timepoints corresponding to 1, 3, 7, and 11 d postinoculation (dpi) with E. maxima or 1, 3, and 7dpi with E. maxima + C. perfringens. Stacked bars represent the mean ATP production measure measured using the Agilent XFe96 Analyzer and ATP Production rate assay from 4 (E. maxima 1 and 3dpi; E. maxima + C. perfringens 1dpi) or 6 chicks / treatment (baseline; E. maxima 7dpi/ E. maxima + C. perfringens 3dpi; E. maxima 11dpi/ E. maxima + C. perfringens 7dpi) ± SEM. Different letter labels within the same-colored bar within a timepoint represent significantly different glycolytic or mitochondrial ATP production rates, whereas different labels above an entire bar represent significantly different total ATP production, P ≤ 0.05.
Glycolytic Rate
Glycolytic rate analysis allows for in-depth evaluation of glycolytic activity as an indicator of inflammation. A detailed breakdown of the glycolytic rate assay output is provided in Supplemental Figure 2. In replicate study 1, the main effect of diet increased baseline total proton efflux rate (PER) 39% in chicks fed anti-IL-10 vs. control (P = 0.03; Figure 5A; Supplementary Figure 6A). At 1 dpi E. maxima, PBMC from chicks fed anti-IL-10, regardless of challenge, had 26% greater ability to mobilize glycolysis to meet metabolic demands (compensatory glycolysis) and 43% greater ability to continue utilizing glycolysis after inhibitor injection (post-2DG acidification) than chicks fed control diets (P ≤ 0.04; Figure 5B; Supplementary Figure 6B). At the same time, the challenge main effect increased compensatory glycolysis 27% and post-2DG acidification 45% in E. maxima-challenged vs. unchallenged birds (P ≤ 0.04; Figure 5B; Supplementary Figure 6G). Diet-induced changes to compensatory glycolysis resolved by 3 dpi, but 38% greater post-2DG acidification was observed in PBMC from E. maxima-challenged vs. unchallenged chicks, regardless of diet (P = 0.02; Figure 5C). An assay error prevented analysis of glycolytic rate changes 1 dpi with secondary C. perfringens and no differences in glycolytic rate were observed beyond 3 dpi with E. maxima in replicate study 1, nor did the interaction between diet and challenge affect glycolytic rate (Figure 5D and 5E; Supplementary Figure 6).
Figure 5.
Replicate study 1 interaction of diet and challenge type on glycolytic rate outputs in peripheral blood mononuclear cells isolated from Ross 308 chicks fed diets ± 0.03% anti-IL-10 before and after challenge with E. maxima ± C. perfringens. Chicks were inoculated with 1×108 colony forming units (CFU) of Salmonella Typhimurium on d0, 15,000 sporulated E. maxima M6 oocysts on d14, and 1×108 CFU C. perfringens on d18 and 19. Samples were collected at (A) baseline (d14) from 6 chicks/ treatment, (B) 1 or (C) 3d postinoculation (dpi) with E. maxima from 4 chicks/treatment, (D), 7dpi with E. maxima or 3dpi with secondary C. perfringens, and (E) 11dpi with E. maxima or 7dpi with C. perfringens from 3 chicks/treatment due to samples being split between 2 plates and outcomes on the second plate being excluded from analysis. Data represent the mean glycolytic rate measure ± SEM.
In replicate study 2, diet did not have a significant main effect on PER output, while challenge did (Figure 6; Supplementary Figure 7A–7F). The S. Typhimurium challenge main effect increased baseline glycolytic rate measures such that chicks challenged with d 0 S. Typhimurium had 17, 18, and 46% greater basal glycolysis, compensatory glycolysis, and post-2DG acidification, respectively, compared to their unchallenged counterparts (P ≤ 0.02; Figure 6A; Supplementary Figure 7G). At E. maxima 1 dpi, the challenge main effect contributed to 30% greater basal glycolysis and 43% greater post-2DG acidification in chicks challenged with E. maxima compared to their unchallenged counterparts (P ≤ 0.04; Figure 6B; Supplemental Figure 7H). While basal glycolytic rate was unaffected at E. maxima 3 dpi, E. maxima-challenged chicks had 22% greater compensatory glycolysis and 39% post-2DG acidification compared to unchallenged chicks, regardless of diet (P ≤ 0.03; Figure 6C; Supplementary Figure 7I). At C. perfringens 1 dpi, the challenge main effect contributed to 27% greater basal glycolytic rate, 32% greater compensatory glycolysis, and 50% greater post-2DG acidification in chicks challenged with E. maxima + C. perfringens vs. unchallenged chicks (P ≤ 0.002; Figure 6D; Supplementary Figure 7J). The continued pattern of increased compensatory glycolysis and post-2DG acidification in replicate study 2 was present at timepoints corresponding to 7 dpi with E. maxima and 3 dpi with secondary C. perfringens. While no differences were observed between PBMC isolated from E. maxima-only and E. maxima + C. perfringens-challenged chicks, the challenge main effect resulted in 26% greater compensatory glycolysis and 42 to 46% greater post-2DG acidification in challenged vs. unchallenged chicks (P ≤ 0.02; Figure 6E; Supplementary Figure 7K). While most interactions were not significant, at the conclusion of the challenge portion of replicate study 2, chicks fed anti-IL-10 and challenged with only E. maxima had 57% greater post-2DG acidification compared to their unchallenged counterparts (P = 0.05; Figure 6F).
Figure 6.
Replicate study 2 interaction of diet and challenge type on glycolytic rate outputs in peripheral blood mononuclear cells isolated from Ross 308 chicks fed diets ± 0.03% anti-IL-10 before and after challenge with E. maxima ± C. perfringens. Chicks were inoculated with 1×108 colony forming units (CFU) of Salmonella Typhimurium on d0, 15,000 sporulated E. maxima M6 oocysts on d14, and 1×108 CFU C. perfringens on d18 and 19. Samples were collected at (A) baseline (d14) from 6 chicks/ treatment, (B) 1 or (C) 3d post-inoculation (dpi) with E. maxima, (D) 1dpi with secondary C. perfringens from 4 chicks/ treatment, (E), 7dpi with E. maxima or 3dpi with secondary C. perfringens, and (F) 11dpi with E. maxima or 7dpi with C. perfringens from 5 chicks/ treatment due to available space on 96-well assay plates. Data represent the mean glycolytic rate measure ± SEM. Different letter assignments in panel (F) denote significant differences between measures in anti-IL-10-fed chicks challenged with E. maxima only vs. all other treatments, P ≤ 0.05.
Diet did not significantly impact PBMC glycolytic rate in replicate study 3 across the sampling timeline (Figure 7), with the exception of E. maxima 7 dpi/ C. perfringens 3 dpi, when birds fed anti-IL-10 had 13% greater basal glycolytic rate compared to their control-fed counterparts (P = 0.05; Supplementary Figure 8E). Challenge significantly affected outcomes at E. maxima 1 dpi, where basal glycolysis was increased 11% in E. maxima-vs. unchallenged chicks (P = 0.04) while also resulting in 40% greater post-2DG acidification (P = 0.0005; Figure 7B; Supplementary Figure 8G). This shift in glycolytic rate due to E. maxima challenge persisted at 3 dpi with 12 and 17% greater basal glycolytic rate and post-2DG-acidification, respectively, observed in challenged vs. unchallenged chicks in replicate study 3 (P ≤ 0.03; Figure 7C; Supplementary Figure 8H). When C. perfringens inoculation was added to the challenge model, significant differences were not found until the final 7 dpi timepoint, when chicks fed anti-IL-10 and challenged with E. maxima ± C. perfringens had 43% greater compensatory glycolysis compared to their unchallenged counterparts, whereas only control-fed chicks challenged with E. maxima + C. perfringens displayed 40% greater compensatory glycolysis compared to their unchallenged counterparts (P = 0.03; Figure 7F).
Figure 7.
Replicate study 3 interaction of diet and challenge type on glycolytic rate outputs in peripheral blood mononuclear cells isolated from Ross 308 chicks fed diets ± 0.03% anti-IL-10 before and after challenge with E. maxima ± C. perfringens. Chicks were inoculated with 15,000 sporulated E. maxima M6 oocysts on d14, and 1×108 CFU C. perfringens on d18 and 19. Samples were collected at (A) baseline (d14) from 6 chicks/ treatment, (B) 1 or (C) 3d postinoculation (dpi) with E. maxima, (D) 1dpi with secondary C. perfringens from 4 chicks/ treatment, (E), 7dpi with E. maxima or 3dpi with secondary C. perfringens, and (F) 11dpi with E. maxima or 7dpi with C. perfringens from 5 chicks/ treatment due to available space on 96-well assay plates. Data represent the mean glycolytic rate measure ± SEM. Different letter assignments in panel (F) denote significant differences between measures in control-fed chicks challenged with E. maxima + C. perfringens and anti-IL-10-fed chicks challenged with E. maxima ± C. perfringens vs. all other treatments, P ≤ 0.05.
Plasma IL-10 and IFN-γ
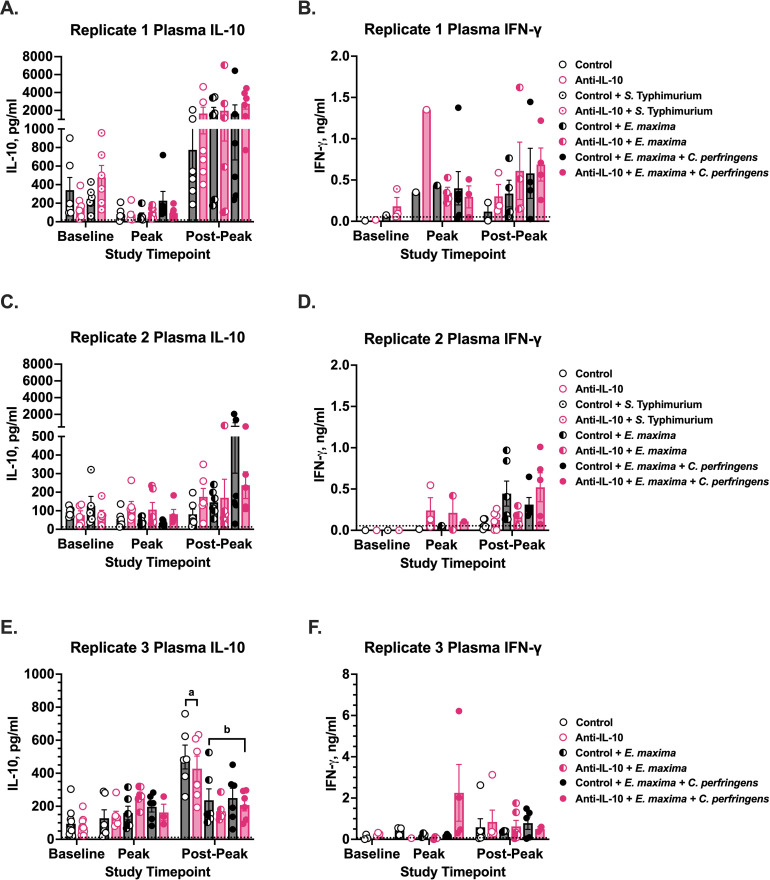
Plasma IL-10 and IFN-γ were measured at key timepoints for all replicate studies (Figure 8). Within each timepoint, neither diet, challenge, nor the interaction between the 2 impacted plasma IL-10 levels in replicate studies 1 or 2. In replicate study 3, at timepoints corresponding to 11 dpi with E. maxima and 7 dpi with secondary C. perfringens (post-peak), unchallenged birds had 53% greater plasma IL-10 compared to chicks challenged with E. maxima ± C. perfringens, regardless of diet (P = 0.0002; Figure 8E). Plasma IFN-γ was detectable in few chicks throughout each study and no differences in plasma IFN-γ were observed at any timepoint in any of the 3 replicate studies (Figure 8B, 8D, and 8F).
Figure 8.
Plasma concentrations of (A, C, E) IL-10 and (B, D, F) IFN-γ in Ross 308 chicks from 3 replicate studies analyzed by ELISA. Chicks in (A–D) replicates 1 and 2 were inoculated with 1×108 colony forming units (CFU) on d0, 15,000 sporulated E. maxima M6 oocysts on d14, and 1×108 CFU C. perfringens on d18 and 19, while chicks in (E&F) replicate 3 had the same challenge timeline without d0 S. Typhimurium inoculation. Cytokines were analyzed from plasma collected at baseline (d14), peak challenge corresponding to 7d post-inoculation (dpi) with E. maxima and 3dpi with secondary C. perfringens, and postpeak corresponding to 11dpi with E. maxima and 7dpi with secondary C. perfringens. Dashed lines represent the lowest limit of detection for each cytokine. Data represent the mean cytokine concentration from 6 chicks/ treatment at each timepoint ± SEM with each point representing individual birds. Brackets with different letter labels denote a significant challenge main effect within a timepoint, P ≤ 0.05.
DISCUSSION
Real-time, primary cell-culture-based assays used in this study allowed for the detection of systemic metabolic shifts over the course of both E. maxima and E. maxima + C. perfringens challenge. Three replicate studies provided the potential identification of consistent immunometabolic responses and the impacts of model type while also identifying variation across different bird hatches raised in similar environments. The 2 assays used provided detailed information about preferred cellular pathways for ATP production during disease challenge while providing greater insight into glycolytic behaviors as indicators of inflammation (Rambold and Pearce, 2018). The first portion of the glycolytic rate assay is very similar to the ATP production rate assay as it measures total metabolic activity of plated PBMC and estimates glycolytic and mitochondrial contributions to PER after injecting Rot/AA, a metabolic inhibitor specifically targeting oxidative phosphorylation. This redundancy simultaneously allows for confirmation of immunometabolic observations while also providing some information at timepoints when one assay failed.
In replicate studies using the S. Typhimurium-E. maxima- C. perfringens co-infection model, the source of baseline immunometabolic changes varied. In replicate study 1, anti-IL-10 generally reduced the baseline PER of PBMC as they were plated in the glycolytic rate assay (Figure 5A). This contrasts with the lack of baseline response to anti-IL-10 observed in replicate study 3 representing a challenge model without d 0 S. Typhimurium inoculation (Figures 4 and 7). While it is possible that early S. Typhimurium may have altered systemic responses to anti-IL-10, this finding needs further investigation. In replicate study 2, baseline glycolytic contributions to ATP production and basal glycolysis in both the ATP production and glycolytic rate assays were increased due to the S. Typhimurium challenge main effect (Figures 3A and 6A). As increased glycolysis is an indicator of immune activation and inflammation, outcomes in replicate study 2 indicate that systemic inflammatory processes were still detectable in chicks in replicate study 2 at d 14, but not replicate study 1.
Salmonella Typhimurium challenge in chicks < 3 d-old results in an acute inflammatory response characterized by increased glycolytic pathways in the cecum that transitions to an immune tolerance phenotype with increased oxidative metabolism by 4 dpi (Kogut and Arsenault, 2017). Systemic immunometabolic responses to S. Typhimurium have not been evaluated extensively, but preliminary work implementing the S. Typhimurium- E. maxima- C. perfringens co-infection model indicated that early S. Typhimurium minimally impacted baseline PBMC ATP production and numerically increased glycolytic profiles in 14 d-old chicks (Fries-Craft et al., 2023b). The findings in this study suggest that chicks in replicate study 2 may have had a prolonged systemic inflammatory response that was not observed in replicate study 1. Chicks in both replicate studies 1 and 2 were given the same S. Typhimurium inoculation dose at d 0, sourced from the same commercial hatchery, and had similar initial body weights (Fries-Craft and Bobeck, 2024). Energy production from glycolysis is comparatively inefficient, resulting in approximately 2 ATP per glucose molecule vs. 36 ATP produced by mitochondrial oxidative phosphorylation (Yetkin-Arik, et al., 2019) and inflammatory glycolytic responses would divert energy from growth to support immune activation (Klasing and Leschinsky, 1999). The S. Typhimurium main effect in both replicate studies 1 and 2 significantly reduced baseline bird performance (Fries-Craft and Bobeck, 2024), indicating that increased glycolytic activity along with factors beyond systemic immune activation, such as localized responses within the intestine, contributed to S. Typhimurium-related production losses in the first 14 d of broiler growth.
Following E. maxima challenge, mitochondrial contributions to ATP production in replicate study 1 were increased by the challenge main effect at 1 dpi but were resolved by 3 dpi; however, increased oxidative phosphorylation is not associated with inflammatory responses (Figures 2 and 5). This, combined with the minimal ATP response outcomes in replicate study 2 indicate that E. maxima likely does not have an inflammatory impact on PBMC ATP production within the first 3 dpi (Figures 2 and 3). While ATP production profiles were unaffected at the early stages of E. maxima challenge, both the main effect of challenge in replicate studies 1 and 2 increased PBMC ability to switch to glycolysis to meet energy demands and/or continue using glycolysis following in-assay inhibition (Supplementary Figures 6G and 6H and 7H and 7I). This suggests that while E. maxima challenge did not affect metabolic activity in PBMC as they were plated, it potentially primed immune cells to respond more glycolytically during additional stressors. Notably, when no S. Typhimurium effect was observed at baseline (replicate study 1), these response patterns were more robust than when a baseline S. Typhimurium effect was observed (replicate study 2) suggesting that S. Typhimurium may impact the magnitude of Eimeria-induced immunometabolic priming and requires further study.
When examining the outputs for each treatment group rather than main effects, PBMC from E. maxima-challenged chicks fed anti-IL-10 had the numerically greatest compensatory glycolysis and post-2DG acidification outcomes compared to other treatment groups at 1 dpi with E. maxima in replicate studies 1 and 2 (Figures 5B and 6B). The proposed immunological effect of anti-IL-10 within the intestine is to counteract anti-inflammatory signaling used by Eimeria spp. to enable inflammatory responses and promote anti-parasitic immune activity (Cyktor and Turner, 2011). In replicate study 1, this anti-IL-10 numerical enhancement in compensatory glycolysis and post-2DG acidification was persistent at E. maxima 3 dpi but was resolved at the same timepoint in replicate study 2 (Figures 5C and 6C). Collectively, this suggests that dietary anti-IL-10 may enhance Eimeria-induced priming of systemic inflammatory responses as detected in the glycolytic rate assay, but the duration of this response may also be impacted by S. Typhimurium.
Consistently in both replicate studies 1 and 2, secondary C. perfringens challenge increased ATP production at 1 dpi. In replicate study 1, chicks fed anti-IL-10 and challenged with E. maxima + C. perfringens had significantly greater ATP production from glycolysis compared to their unchallenged counterparts while ATP production profiles in control-fed birds were unaffected (Figure 2D). This finding was numerically, but not statistically, consistent in replicate study 2 (Figure 3D). In addition to secondary C. perfringens shifting ATP profiles, the challenge main effect universally increased measures within the glycolytic rate assay in replicate study 2 (Supplemental Figure 7J); however, glycolytic rate assay failure at this timepoint in replicate study 1 cannot confirm the consistency of this observation. Collectively, these outcomes indicate secondary C. perfringens challenge caused an early and robust PBMC immunometabolic response in replicate studies 1 and 2 that was not observed with primary E. maxima. During necrotic enteritis, the combined activity of E. maxima and C. perfringens significantly reduces intestinal barrier function, making it more likely for intestinal antigens to enter systemic circulation and cause a systemic immunometabolic response (Latorre, et al., 2018; López-Osorio, et al., 2020). Immunometabolic findings in replicate studies 1 and 2 indicate that responses to E. maxima primary infection were likely restricted to intestinal compartments with some systemic immune priming, but secondary C. perfringens insult caused a systemic inflammatory response that may have been more robust in chicks fed anti-IL-10.
Consistent immunometabolic changes at later timepoints in replicate studies 1 and 2 are difficult to track due to assay limitations identified in replicate study 1. Real-time immunometabolic assays in a 96-well plate require at least 4 blank wells, leaving 92 sample wells and limiting each plate to 30 samples plated in triplicate. As 36 total PBMC were collected at the later challenge timepoints in all replicate studies, samples were initially distributed across 2 plates in order to obtain immunometabolic insights for every sampled chick. During experimentation, cells were plated for the first set of ATP and glycolytic assays while those designated for the second set were incubated in 15 ml tubes with assay media. As cells were metabolically active during this time, it is likely that prolonged incubation exhausted cellular metabolism (Galton and Fain, 1966) as evidenced by the significantly reduced metabolic outcomes between plates 1 and 2 (Supplemental Figure 1). As such, only findings from the first plate in replicate study 1 were analyzed, representing 3 chicks/ treatment, and methods were revised in replicate studies 2 and 3 to maximize observations obtained from a single assay plate. Retrospective analyses demonstrated that plating PBMC from 5 chicks/ treatment resulted in a 0.56-0.73 power, depending on the immunometabolic outcome. Plating samples in duplicate could allow for PBMC from more birds to be analyzed on a single plate and increase power. However, this comes at the cost of technical replicates to account for intra-assay variability when plating PBMC as a heterogenous population of immune and non-immune cell types and should be carefully considered in future research.
In replicate study 2, the challenge main effect at 7 dpi E. maxima/ 3 dpi C. perfringens increased overall ATP production, compensatory glycolysis, and post-2DG acidification but with no apparent anti-IL-10 effects, suggesting a generalized systemic inflammatory effect of E. maxima ± C. perfringens. However, at the end of the study chicks fed anti-IL-10 and challenged with only E. maxima had the greatest mitochondrial contributions to ATP production. Metabolic shifts towards increased oxidative phosphorylation are associated with tissue healing/immunoregulatory responses (Russell, et al., 2019; Shin, et al., 2020) and this could indicate that chicks challenged with only E. maxima exhibited tissue healing responses by 11 dpi that were not observed in those challenged with secondary C. perfringens. The resolution of ATP responses in control-fed chicks challenged with only E. maxima indicates that similar healing responses may have occurred outside of sample collection timepoints and further research is needed to determine the degree of altered healing responses by anti-IL-10.
Replicate study 3 represented a challenge model without early Salmonella inoculation to determine if increased IL-10 by S. Typhimurium was a confounding factor when evaluating anti-IL-10 mechanisms of action during E. maxima ± C. perfringens challenge. At 1 dpi with E. maxima in replicate study 3, ATP production and glycolytic rate profiles indicated that PBMC were immunometabolically activated by the challenge main effect (Figures 4B and 7B). This was unexpected, as previous research has demonstrated that both wild-type and vaccine-strain Eimeria minimally impacts immunometabolism at early post-inoculation timepoints (Fries-Craft et al., 2023a; Fries-Craft et al., 2023b). Due to assay error, prolonged effects on ATP production profiles by E. maxima challenge at 3 dpi could not be determined; however, increased basal glycolysis and post-2DG acidification observed at 3 dpi in the glycolytic rate assay indicates that glycolytic profiles were still generally increased in response to E. maxima (Figure 7C). Unlike outcomes in replicate studies 1 and 2, secondary C. perfringens in replicate study 3 did not cause any observable changes in immunometabolism and it is likely that earlier immunometabolic responses to E. maxima obscured any additional responses to secondary C. perfringens. This is intriguing because replicate study 3 performance outcomes showed that secondary C. perfringens had an additive effect on performance losses not previously observed in replicate studies 1 and 2 (Fries-Craft and Bobeck, 2024). It is possible that early and sustained effects on systemic immunity may have exhausted immune system function (Gigley et al., 2012), causing a less effective immune response to secondary C. perfringens and contributing to greater growth restriction.
Timepoints corresponding to 7 dpi E. maxima and 3 dpi C. perfringens were associated with the greatest challenge-induced reduction in body weight (BW) across all replicate studies (Fries-Craft and Bobeck, 2024). At this time, only PBMC isolated from chicks in replicate study 3 displayed an anti-IL-10-induced increase in glycolytic activity, suggesting that anti-IL-10 promoted more inflammatory phenotypes at peak challenge (Figure 7D). Similarly increased glycolytic activity at the same time in unchallenged chicks fed anti-IL-10 somewhat obscures the meaning of these responses in the context of coccidiosis and necrotic enteritis challenges; however, in the study timeline 7 dpi E. maxima/ 3 dpi C. perfringens sampling represented a timepoint wherein the birds had been repeatedly handled for BW measurement and inoculations. This suggests that birds fed anti-IL-10 had more robust systemic glycolytic responses following behavioral stress, despite stress hormones being associated with immunosuppression (Dhabhar, 2002; Shini, et al., 2008). While the effects of repeated handling stress were not apparent in replicate study 3 unchallenged bird performance outcomes (Fries-Craft and Bobeck, 2024), this introduces an intriguing potential application for anti-IL-10 in preserving broiler immune responses during behavioral stress that requires follow-up study.
By the end of replicate study 3, chicks challenged with E. maxima ± C. perfringens still had increased ATP production from glycolysis and anti-IL-10-fed chicks in either challenge group had increased compensatory glycolytic activity rather than the potential signatures of immune resolution observed in replicate studies 1 and 2 (Figures 4E and 7F). This aligns with expected inflammatory signatures associated with anti-IL-10, but also indicates generally enhanced and sustained inflammatory processes in models without S. Typhimurium that may have been deleterious to performance recovery beyond the 25 d challenge study (Fries-Craft and Bobeck, 2024).
In general, ELISA outcomes indicate that dietary anti-IL-10 does not directly affect systemic IL-10 nor does altered IL-10 signaling within the intestine by anti-IL-10 have an indirect effect on systemic IFN-γ. This confirms that dietary antibody activity is restricted to intestinal compartments, even during challenges that reduce intestinal barrier function. Variability in IFN-γ detectability despite the high recovery rate reported by the ELISA kit manufacturer makes it difficult to associate systemic immunometabolism with cytokine profiles within the same tissues. In contrast, IL-10 was more consistently detected in the frozen and thawed plasma samples (Figure 8), suggesting that cytokines may be differentially susceptible to sample storage conditions and this should be accounted for in future research. Despite this, the generally greater systemic IL-10 concentrations observed at the post-peak timepoint in replicate studies 1 and 2 vs. replicate study 3, corresponding to 11 dpi with E. maxima and 7 dpi with E. maxima + C. perfringens, supports the potential delay in immune resolution timelines observed in models without S. Typhimurium as IL-10 is an important ‘stand down’ signal that promotes tissue healing following an inflammatory response (Couper, et al., 2008). Collectively, outcomes in this study suggest that the timing and duration of a systemic immune response during E. maxima challenge has potentially greater impacts on C. perfringens challenge severity that are altered by S. Typhimurium. Previous research using the S. Typhimurium- E. maxima- C. perfringens coinfection model does not retain an E. maxima-only group to evaluate how early S. Typhimurium may alter immune responses leading up to secondary C. perfringens challenge, nor has it evaluated timepoints beyond 3 dpi with C. perfringens (Shivaramaiah, et al., 2011; Coles, et al., 2021). This creates the perception that S. Typhimurium contributes to a severe challenge at a timepoint when necrotic enteritis challenge is at its peak, regardless of model system. Findings in a model without confounding IL-10 from S. Typhimurium were expected to provide greater insight into anti-IL-10 mechanisms of action during E. maxima and C. perfringens challenges. As findings herein support the lack of a direct effect of dietary anti-IL-10 on systemic IL-10 concentrations and model type likely having a greater impact on systemic immunity, further research at the intestinal level is needed to 1) provide better resolution concerning anti-IL-10 effects where the antibody is present and 2) evaluate how S. Typhimurium may alter the balance of local and systemic immune responses during E. maxima ± C. perfringens challenge to impact disease severity.
ACKNOWLEDGEMENTS
The authors thank Billy Hargis, PhD, and Danielle Graham, PhD, for providing the challenge pathogens used herein. This work was funded by United States Department of Agriculture-National Institute of Food and Agriculture grant number 2021-67015-34533.
DISCLOSURES
The authors declare no conflict of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2024.103551.
Appendix. Supplementary materials
REFERENCES
- Al-Sheikhly F., Al-Saieg A. Role of coccidia in the occurrence of necrotic enteritis of chickens [Clostridium perfringens, Eimeria acervulina, Eimeria necatrix] Avian Dis. 1980;24:324–333. [PubMed] [Google Scholar]
- Arabkhazaeli F., Modrisanei M., Nabian S., Mansoori B., Madani A. Evaluating the resistance of Eimeria spp. field isolates to anticoccidial drugs using three different indices. Iran. J. Parasitol. 2013;8:234–241. [PMC free article] [PubMed] [Google Scholar]
- Arendt M., Elissa J., Schmidt N., Michael E., Potter N., Cook M., Knoll L.J. Investigating the role of interleukin 10 on Eimeria intestinal pathogenesis in broiler chickens. Vet. Immunol. Immunopathol. 2019;218 doi: 10.1016/j.vetimm.2019.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt M.K., Knoll L.J., Cook M.E. Oral antibody to interleukin-10 receptor 2, but not interleukin-10 receptor 1, as an effective Eimeria species immunotherapy in broiler chickens. Poult. Sci. 2019;98:3471–3480. doi: 10.3382/ps/pez064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt M.K., Sand J.M., Marcone T.M., Cook M.E. Interleukin-10 neutralizing antibody for detection of intestinal luminal levels and as a dietary additive in Eimeria challenged broiler chicks. Poult. Sci. 2016;95:430–438. doi: 10.3382/ps/pev365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausland C., Al-Ogaili A.S., Latorre J.D., Tellez-Isaias G., Hargis B.M., Kwon Y.M., Arreguin-Nava M.A., Singh P. Draft genome sequence of Clostridium perfringens strain TAMU, which causes necrotic enteritis in broiler chickens. Microbiol. Resour. Announc. 2020;9 doi: 10.1128/MRA.01357-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A. Th1 and Th2 responses: what are they? BMJ. 2000;321:424. doi: 10.1136/bmj.321.7258.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D.P., Knox J., Dehaeck B., Huntington B., Rathinam T., Ravipati V., Ayoade S., Gilbert W., Adebambo A.O., Jatau I.D., Raman M., Parker D., Rushton J., Tomley F.M. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020;51:115. doi: 10.1186/s13567-020-00837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobeck E.A., Hellestad E.M., Helvig C.F., Petkovich P.M., Cook M.E. Oral antibodies to human intestinal alkaline phosphatase reduce dietary phytate phosphate bioavailability in the presence of dietary 1α-hydroxycholecalciferol. Poult. Sci. 2016;95:570–580. doi: 10.3382/ps/pev341. [DOI] [PubMed] [Google Scholar]
- Coles M.E., Forga A.J., Senas-Cuesta R., Graham B.D., Selby C.M., Uribe A.J., Martinez B.C., Angel-Isaza J.A., Vuong C.N., Hernandez-Velasco X., Hargis B.M., Tellez-Isaias G. Assessment of Lippia origanoides essential oils in a Salmonella typhimurium, Eimeria maxima, and Clostridium perfringens challenge model to induce necrotic enteritis in broiler chickens. Animals (Basel) 2021;11:1111. doi: 10.3390/ani11041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M.E., Trott D.L. IgY – Immune component of eggs as a source of passive immunity for animals and humans. World's Poult. Sci. J. 2010;66:215–226. [Google Scholar]
- Couper K.N., Blount D.G., Riley E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- Cyktor J.C., Turner J. Interleukin-10 and immunity against prokaryotic and eukaryotic intracellular pathogens. Infect. Immun. 2011;79:2964–2973. doi: 10.1128/IAI.00047-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalloul R.A., Lillehoj H.S. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev. Vaccines. 2006;5:143–163. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- Dhabhar F.S. Stress-induced augmentation of immune function—the role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav. Immun. 2002;16:785–798. doi: 10.1016/s0889-1591(02)00036-3. [DOI] [PubMed] [Google Scholar]
- Fries-Craft K., Lamont S.J., Bobeck E.A. Implementing real-time immunometabolic assays and immune cell profiling to evaluate systemic immune response variations to Eimeria challenge in three novel layer genetic lines. Front. Vet. Sci. 2023;10 doi: 10.3389/fvets.2023.1179198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries-Craft K., Graham D., Hargis B.M., Bobeck E.A. Evaluating a Salmonella Typhimurium, Eimeria maxima, and Clostridium perfringens coinfection necrotic enteritis model in broiler chickens: repeatability, dosing, and immune outcomes. Poult Sci. 2023;102 doi: 10.1016/j.psj.2023.103018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries-Craft K., Bobeck E.A. Early Salmonella Typhimurium inoculation may obscure anti-interleukin-10 protective effects on broiler performance during coccidiosis and necrotic enteritis challenge. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2023.103187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton D.J., Fain J.N. Effects of prolonged incubation of isolated fat cells on their response to hormones stimulating lipolysis and glucose metabolism. Biochem. J. 1966;98:557–561. doi: 10.1042/bj0980557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigley J.P., Bhadra R., Moretto M.M., Khan I.A. T cell exhaustion in protozoan disease. Trends Parasitol. 2012;28:377–384. doi: 10.1016/j.pt.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Patlan D., Solis-Cruz B., Pontin K.P., Hernandez-Velasco X., Merino-Guzman R., Adhikari B., López-Arellano R., Kwon Y.M., Hargis B.M., Arreguin-Nava M.A., Tellez-Isaias G., Latorre J.D. Impact of a bacillus direct-fed microbial on growth performance, intestinal barrier integrity, necrotic enteritis lesions, and ileal microbiota in broiler chickens using a laboratory challenge model. Front. Vet. Sci. 2019;6:108. doi: 10.3389/fvets.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y.H., Lillehoj H.S., Lillehoj E.P., Lee S.H. Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Vet. Immunol. Immunopathol. 2006;114:259–272. doi: 10.1016/j.vetimm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Kaldhusdal M., Hofshagen M. Barley inclusion and avoparcin supplementation in broiler diets. 2. Clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis. Poult. Sci. 1992;71:1145–1153. doi: 10.3382/ps.0711145. [DOI] [PubMed] [Google Scholar]
- Klasing K.C., Leschinsky T.V. Functions, costs, and benefits of the immune system during development and growth. Proc. 22nd International Ornithology Congress; Durban, South Africa; 1999. [Google Scholar]
- Kogut M.H., Arsenault R.J. Immunometabolic phenotype alterations associated with the induction of disease tolerance and persistent asymptomatic infection of Salmonella in the chicken intestine. Front. Immunol. 2017;8:372. doi: 10.3389/fimmu.2017.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg M.D. The immunologic Warburg effect: Evidence and therapeutic opportunities in autoimmunity. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020;12:e1486. doi: 10.1002/wsbm.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre J.D., Adhikari B., Park S.H., Teague K.D., Graham L.E., Mahaffey B.D., Baxter M.F.A., Hernandez-Velasco X., Kwon Y.M., Ricke S.C., Bielke L.R., Hargis B.M., Tellez G. Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens. Front. Vet. Sci. 2018;5:199. doi: 10.3389/fvets.2018.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj H.S., Choi K.D. Recombinant chicken interferon-gamma-mediated inhibition of Eimeria tenella development in vitro and reduction of oocyst production and body weight loss following Eimeria acervulina challenge infection. Avian Dis. 1998;42:307–314. [PubMed] [Google Scholar]
- López-Osorio S., Chaparro-Gutiérrez J.J., Gómez-Osorio L.M. Overview of poultry Eimeria life cycle and host-parasite interactions. Front. Vet. Sci. 2020;7:384. doi: 10.3389/fvets.2020.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A.G., Danforth H.D., Barta J.R., Fernando M.A. Analysis of immunological cross-protection and sensitivities to anticoccidial drugs among five geographical and temporal strains of Eimeria maxima. Int. J. Parasitol. 1997;27:527–533. doi: 10.1016/s0020-7519(97)00027-1. [DOI] [PubMed] [Google Scholar]
- Meyer M.M., Lamont S.J., Bobeck E.A. Mitochondrial and glycolytic capacity of peripheral blood mononuclear cells isolated from diverse poultry genetic lines: Optimization and assessment. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.815878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack S., Chapman H.D., Selzer P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019;118:2009–2026. doi: 10.1007/s00436-019-06343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambold A.S., Pearce E.L. Mitochondrial dynamics at the interface of immune cell metabolism and function. Trends Immunol. 2018;39:6–18. doi: 10.1016/j.it.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Rasheed A., M S., Tiwari U.P., Oelschlager M.L., Smith B.N., Jespersen J.C., Escobar J., Olmeda-Geniec N., Dilger R.N. Dietary supplementation with anti–IL-10 antibody during a severe Eimeria challenge in broiler chickens. Poult. Sci. 2020;99:6493–6502. doi: 10.1016/j.psj.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D.G., Huang L., VanderVen B.C. Immunometabolism at the interface between macrophages and pathogens. Nat. Rev. Immunol. 2019;19:291–304. doi: 10.1038/s41577-019-0124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar G.A., Peñaloza H.F., Pardo-Roa C., Schultz B.M., Muñoz-Durango N., Gómez R.S., Salazar F.J., Pizarro D.P., Riedel C.A., González P.A., Alvarez-Lobos M., Kalergis A.M., Bueno S.M. Interleukin-10 production by T and B cells is a key factor to promote systemic Salmonella enterica serovar Typhimurium infection in mice. Front. Immunol. 2017;8:889. doi: 10.3389/fimmu.2017.00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand J.M., Arendt M.K., Repasy A., Deniz G., Cook M.E. Oral antibody to interleukin-10 reduces growth rate depression due to Eimeria spp. infection in broiler chickens. Poult. Sci. 2016;95:439–446. doi: 10.3382/ps/pev352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin B., Benavides G.A., Geng J., Koralov S.B., Hu H., Darley-Usmar V.M., Harrington L.E. Mitochondrial oxidative phosphorylation regulates the fate decision between pathogenic Th17 and regulatory T cells. Cell Rep. 2020;30:1898–1909.e1894. doi: 10.1016/j.celrep.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shini S., Kaiser P., Shini A., Bryden W.L. Biological response of chickens (Gallus gallus domesticus) induced by corticosterone and a bacterial endotoxin. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2008;149:324–333. doi: 10.1016/j.cbpb.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Shivaramaiah S., Wolfenden R.E., Barta J.R., Morgan M.J., Wolfenden A.D., Hargis B.M., Téllez G. The role of an early Salmonella Typhimurium infection as a predisposing factor for necrotic enteritis in a laboratory challenge model. Avian Dis. 2011;55:319–323. doi: 10.1637/9604-112910-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet. Res. 2012;43:74. doi: 10.1186/1297-9716-43-74. -74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetkin-Arik B., Vogels I.M.C., Nowak-Sliwinska P., Weiss A., Houtkooper R.H., van Noorden C.J.F., Klaassen I., Schlingemann R.O. The role of glycolysis and mitochondrial respiration in the formation and functioning of endothelial tip cells during angiogenesis. Sci. Rep. 2019;9:12608–12614. doi: 10.1038/s41598-019-48676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.