Abstract
Eggshell quality is among the most important factors affecting hatchability in broiler breeders, and therefore several methods for its assessment are available in the poultry industry. Among them, eggshell translucency has received special attention in recent years due to its connection with ultrastructural disorganization of the shell layers. However, there is very limited data on the impact of translucency on hatching eggs and on the possible links between this trait and specific gravity (SG) or shell color. Thus, our study investigated associations and interactions between eggshell translucency, SG, and color on incubation parameters of eggs from the same breeding flock (Ross 308AP, 51 wk of age). To this end, light and dark eggs within 5 different SG categories (≥1.065, 1.070, 1.075, 1.080, and ≤1.085) were selected from 15,976 eggs, graded into 3 translucency scores, and later incubated to evaluate egg weight loss, hatchability and embryonic mortalities. In general, translucency scores were evenly distributed within SG categories (χ2 [8, N = 1,138] = 13.67, P = 0.090) and color (χ2 [2, N = 1,138] = 4.93, P = 0.084). No interactions between eggshell translucency and SG or between translucency and color were found for the analyzed variables. An interaction was observed between SG and eggshell color for the variable egg weight loss, where the light-shelled eggs, in most SG categories lost more weight throughout incubation than dark eggs. Eggshell translucency affected egg weight loss, hatchability, and embryonic mortality on 11 to 18 d of incubation, with highly translucent eggs showing the worst results. At the same time, eggs with SG lower than 1.070 displayed the greatest weight loss, lowest hatchability, and highest contamination. We found no influence of eggshell color on weight loss or hatchability, but light-shelled eggs exhibited higher late embryonic mortality. Together, these data suggest that despite its effects on certain hatching parameters, shell translucency bears no relationship to SG or color.
Key words: Chicken, broiler breeder, egg characteristic, shell quality, eggshell mottling
INTRODUCTION
Modern fast-growing lines of chickens have advanced so far genetically that today more than a third of broiler's life is spent inside the egg (Ismail et al., 2016; Tallentire et al., 2018). This circumstance brings out the importance of hatcheries in commercial operations, and how the monitoring of egg and incubation parameters is critical for the maintenance of the broiler production chain (Kroetz Neto et al., 2023). In light of this, fertility and hatchability (i.e., the percentage of fertile eggs that hatch) are 2 key elements in the hatchery routine given their impact on the supply of day-old chicks (Wolc et al., 2009; Wolc et al., 2010; King'ori, 2011). However, while fertility is a trait influenced by genetic and nongenetic factors linked to both the hen and the rooster, hatchability is a trait more related to the embryo and the hen due to the quality of the environment provided by the egg (Wolc et al., 2009; Wolc et al., 2010). Therefore, breeding companies carefully watch several egg parameters that are known to affect hatchability and chick quality such as egg weight, shape, shell features, and consistency of the contents (Peebles and Brake, 1987; Narushin and Romanov, 2002). Among these, eggshell quality stands out as one of the most important components because of the shell's role in embryo protection, respiration, and nourishment (Roque and Soares, 1994; Ketta and Tůmová, 2016). Although there are several methods for assessing eggshell quality, nondestructive ones are often preferred for their applicability in hatchery operations and genetic selection programs (Narushin, 1997; De Ketelaere et al., 2004; Kibala et al., 2018).
Egg specific gravity (SG), an indirect, nondestructive method, is probably the most employed approach for measuring shell quality in the field, due to its low cost, practicality, and significant correlation with shell thickness (Roque and Soares, 1994; Narushin, 1997; Kibala et al., 2018). Nevertheless, as useful as it is, SG fails to detect some eggshell abnormalities that can be observed during candling such as hairline cracks and translucent spots (also referred to as eggshell mottling). Translucent spots on eggshells are areas where the mamillary and palisade layers are disorganized and the shell structure becomes weaker (Tyler and Geake, 1964; Talbot and Tyler, 1974; Bain, 1992; Wong et al., 2020; Cheng and Ning, 2023). This structural failure allows moisture from the egg's contents that pass through the shell membranes to accumulate in the eggshell, leading to increased light transmission (Wang et al., 2017; Wang et al., 2019; Wong et al., 2020). Eggshell translucency can vary from numerous tiny spots to almost the entire shell, and its occurrence is associated with potential risks of bacterial penetration and crack development (Bain, 1992; Bain et al., 2006; Chousalkar et al., 2010; Wong et al., 2020). Despite the amount of research on translucent eggs in laying hens and broiler breeders, most of it deals with structural analysis, egg grading systems or the influence of dietary factors on the incidence of this type of shell abnormality (Tyler and Geake, 1964; Talbot and Tyler, 1974; Bain, 1992; Bain et al., 2006; Chousalkar et al., 2010; Wang et al., 2017; Wang et al., 2019; Wong et al., 2020; van den Brand et al., 2023; Cheng and Ning, 2023). To our knowledge, only one presented study has addressed the effect of eggshell translucency on hatchability of broiler eggs and its interaction with eggshell color (Galindo et al., 2022). These authors concluded that dark eggs exhibiting low translucency had the best hatchability and chick weight. Thus, an interest was generated in investigating possible interactions among eggshell translucency, specific gravity, and color over egg weight loss, hatchability, and embryonic mortalities.
MATERIALS AND METHODS
All procedures complied with the current regulations established by the Ethics Committee of the College of Veterinary Medicine and Animal Sciences at the São Paulo University (CEUAVET No 6556290520).
Experimental Design
A total of 15,976 eggs from the same breeding flock (Ross 308 AP, 51 wk of age) housed by AD'ORO S/A (São Carlos, São Paulo, Brazil) had their shells evaluated for specific gravity (SG: ≥1.065, 1.070, 1.075, 1.080, and ≤1.085) and color (light, intermediate and dark). Subsequently, only light and dark eggs within each SG category were separated, individually numbered, weighed, and analyzed for shell translucency (scores 1, 2, and 3, 1 being the least mottled and 3 the heavily mottled). Eggs from the same SG and eggshell color (e.g., 1.065 light, 1.065 dark, 1.070 light, and so on) were incubated and hatched in the same tray to enable the monitoring of hatching parameters. Every time the number of eggs with the same SG and color did not complete a hatcher tray, screen dividers were placed to allow the separation of chicks and unhatched eggs belonging to different categories. Eggs were incubated using a single-stage incubator (Coopermaq 1290 for 129,024 eggs with trays of 84 eggs) and transferred to a hatcher after 19 d of incubation (Coopermaq 430 for 43,008 eggs with trays of 84 eggs). Egg weight loss was individually measured when transferring, whereas hatchability (i.e., the percentage of fertile eggs that hatch), embryonic mortalities were determined immediately after removal from the hatcher.
Egg Storage, Processing, and Incubation
Eggs used in this study were stored for 3 d at the breeders’ farm at a temperature of 20°C to 23°C, and later transported in a specially designed egg truck with temperature set to 20°C. Immediately upon arrival, fertile eggs were automatically sorted (Yamasa CHSL 54,000 sorting machine, Rinpolis, São Paulo, Brazil) to eliminate eggs unsuitable for hatching (e.g., cracked, dirty, undersized, or oversized eggs) and accommodated in incubation trays and trolleys (storage room at 18°C, RH 60–80%). On the next day, 15,976 eggs had their SG estimated by the flotation method using salt solutions with concentrations of 1.065, 1.070, 1.075, 1.080, and 1.085 with water at a constant temperature of 18°C (Voisey and Hunt, 1974). For every 300 eggs the salt solutions were rechecked for accuracy with a hydrometer. Then, eggs were separated in different incubation trays according to their respective SG category and assessed for eggshell color using an electronic colorimeter (Nix Pro Color Sensor, Nix Sensor Ltd., Ontario, Canada). Colorimeter readings were standardized using Zinpro's Eggshell Color Guide (Zinpro Corp., Eden Praire, MN) where eggs were classified as follows: 1) dark color when Lightness (L) in the NIX CIELab System ranged from 64.81 to 46.63 (color guide categories 6–9); 2) intermediate color when L ranged from 77.83 to 64.86 (color guide categories 3–5) and 3) light color when L ranged from 77.88 to 88.29 (color guide categories 1–2). On the fifth day of storage, eggs classified as light and dark within each SG were individually marked, weighed, and examined for their translucency using Zinpro BlueBox (Zinpro Corp., Eden Praire, MN). Such tool allowed us to grade eggs into 3 translucency scores: 1) slight (eggshells showing few and small translucent spots); 2) medium (more translucency spots widely distributed throughout the eggshell); and 3) severe (presence of several spots and even larger windows of translucency all over the eggshell).
Upon completion of these evaluations, eggs were preheated at 23.8°C for 4 h and then at 25.0°C for another 4 h, and incubation temperature and relative humidity were programmed to gradually decrease from 37.94°C to 37.66°C and from 56.24 to 46.7%, respectively. These settings were established from previous tests in this single-stage incubator to guarantee that eggshell temperature was maintained around 37.7°C throughout incubation. Egg turning was performed hourly until the time of in ovo vaccination and egg transfer (456 h). All eggs were individually weighed again approximately 2 h before transfer to monitor egg weight loss. The hatcher was configured to keep temperature at 36.9°C and relative humidity of 55% for the first 24 h, and 36.7°C and relative humidity of 61% for the remaining 24 h. At 508 h, chicks were removed from the hatchers and sent to the processing area.
Hatching Events, Hatchability and Embryonic Mortalities
Hatchlings from each tray (properly tagged with their respective SG and eggshell color category) were transferred to previously marked boxes for weighing and quality analysis, whereas unhatched eggs were opened and macroscopically categorized into the following groups: unfertile; embryonic mortality at 0 to 4 d; embryonic mortality at 5 to 10 d; embryonic mortality at 11 to 18 d; embryonic mortality at 19 to 21 d; pipped eggs, and contaminated eggs. As aforementioned, whenever the number of eggs with the same SG and color did not complete a hatcher tray, screen dividers were placed to allow the separation of chicks and unhatched belonging to different categories (e.g., 1.065 light/1.065 dark). Individual marking of the eggs (numbers written on the shell) also allowed tracking of the translucency scores to which each unhatched egg belonged.
Statistical Analysis
Statistical analysis was performed using the Statistical Analysis System 9.3 software (SAS institute, Cary, NC). A chi-square test of independence was performed to examine the relation between eggshell translucency and SG, and between eggshell translucency and color. Variables were initially tested for variance homogeneity and data normality (Guided Data Analysis), and whenever these assumptions were not validated the data were transformed. If transformations were unsuccessful, nonparametric tests were used for conducting statistical analysis. The experimental unit was sets of 30 eggs. The interaction effect between eggshell translucency, specific gravity, and color was tested by general linear model procedures. For parametric variables, differences between groups were analyzed using ANOVA followed by Duncan tests, whereas nonparametric variables were analyzed using Kruskal-Wallis followed by Wilcoxon test. A probability value of P < 0.05 was considered statistically significant. Results are reported as untransformed means ± SEM.
RESULTS
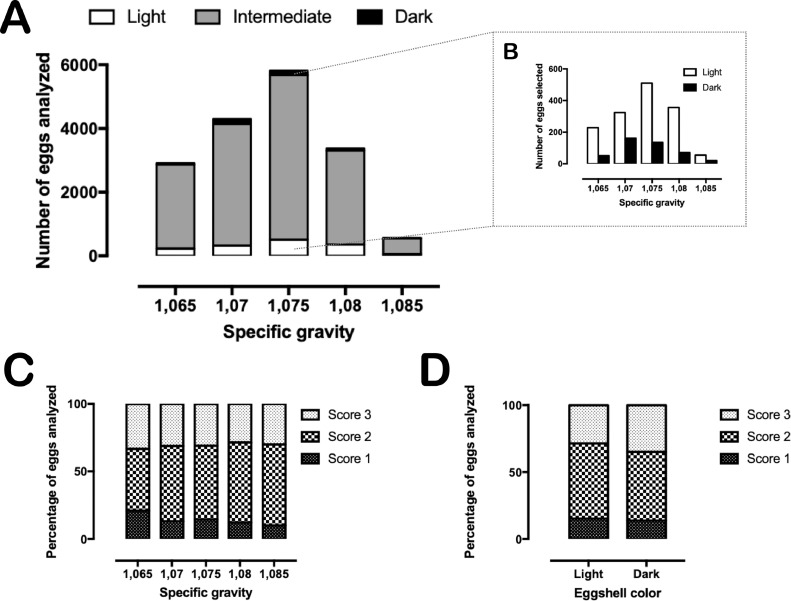
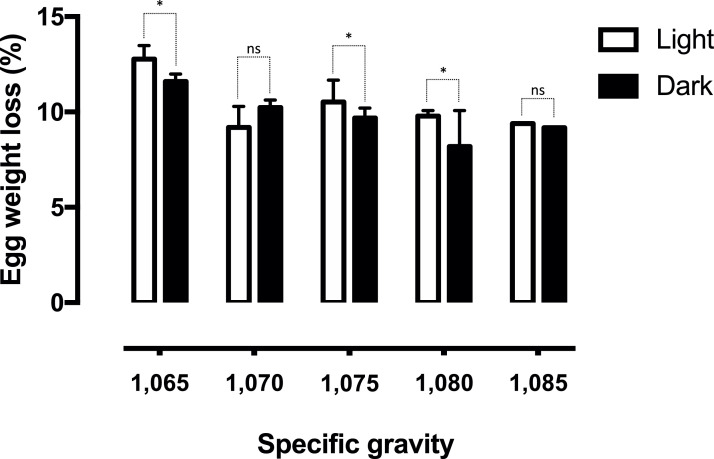
Of the 15,976 eggs analyzed for SG and shell color, we managed to select 1,545 eggs as light and dark within each SG category using colorimeter readings (7.6% and 2.1% of total eggs, respectively – Figures 1A and 1B). After a second round of evaluation (considering egg shape, cleanliness, cracked shells, etc.), 1,204 good-quality hatching eggs were incubated and transferred, of which 1,138 were considered fertile during hatch debris breakouts. Overall, translucency scores 1, 2, and 3 accounted for 14.5, 54.6, and 30.9% of the total eggs analyzed, respectively. The distribution of translucency scores within each specific gravity and eggshell color is detailed in Figures 1C and 1D. A chi-square test of independence showed that there was no correlation between eggshell translucency and specific gravity, χ2 [8, N = 1138] = 13.67, P = 0.090, nor between translucency and color, χ2 [2, N = 1,138] = 4.93, P = 0.084. Additionally, no interactions between eggshell translucency and SG or between translucency and color were found for the analyzed variables (Table 1). The only interaction observed was between SG and color regarding egg weight loss, where light-shelled eggs in most SG categories lost more weight during incubation (Figure 2).
Figure 1.
– Overall distribution of the analyzed eggs as per eggshell translucency, specific gravity and color (a total of 15,976 eggs from Ross 308AP breeders with 51 wk of age). (A) Number of eggs according to their specific gravity category and eggshell color; (B) Number of light and dark eggs within each specific gravity category selected in our study to be monitored for eggshell translucency and incubation parameters; (C) Distribution of eggshell translucency scores within each specific gravity category of selected light and dark eggs (no association between eggshell translucency and specific gravity was observed, χ2 (8, N = 1138) = 13.67, P = 0.090); (D) Distribution of eggshell translucency scores within each color category (no association between eggshell translucency and color was observed, χ2 (2, N = 1138) = 4.93, P = 0.084).
Table 1.
Probability values for the effects of eggshell translucency (scores 1, 2, and 3), specific gravity (≥1.065, 1.070, 1.075, 1.080, and ≤1.085) and color (light and dark) as well as their interactions on egg weight loss, hatchability, embryonic mortalities, contamination and pipped eggs.
| Variables | Translucency | Specific gravity | Color | Interaction effect |
||
|---|---|---|---|---|---|---|
| Translucency × specific gravity | Translucency × color | Specific gravity × color | ||||
| Egg weight loss | 0.0273 | < 0.0001 | 0.3243 | 0.1197 | 0.8061 | 0.0279 |
| Hatchability (%) | 0.0225 | 0.0343 | 0.9239 | 0.3401 | 0.5572 | 0.0520 |
| Embryonic mortality % (0–4 d) | 0.0606 | 0.2869 | 0.4561 | 0.6224 | 0.6135 | 0.2815 |
| Embryonic mortality % (5–10 d) | 0.3606 | 0.4459 | 0.9176 | 0.8845 | 0.5821 | 0.5800 |
| Embryonic mortality % (11–18 d) | 0.0122 | 0.8016 | 0.0479 | 0.5545 | 0.2141 | 0.6036 |
| 0.5658 | 0.1238 | 0.0479 | 0.0600 | 0.4000 | 0.9447 | |
| Pipped eggs | 0.0886 | 0.4514 | 0.7704 | 0.8864 | 0.7386 | 0.9410 |
| Contaminated eggs | 0.1048 | 0.0258 | 0.1768 | 0.0700 | 0.2031 | 0.2942 |
Figure 2.
– Weight loss during incubation of light and dark eggs within different specific gravity categories. Asterisks and NS indicate significant (P < 0.05) and nonsignificant (P > 0.05) differences of eggshell color within each specific gravity category, respectively.
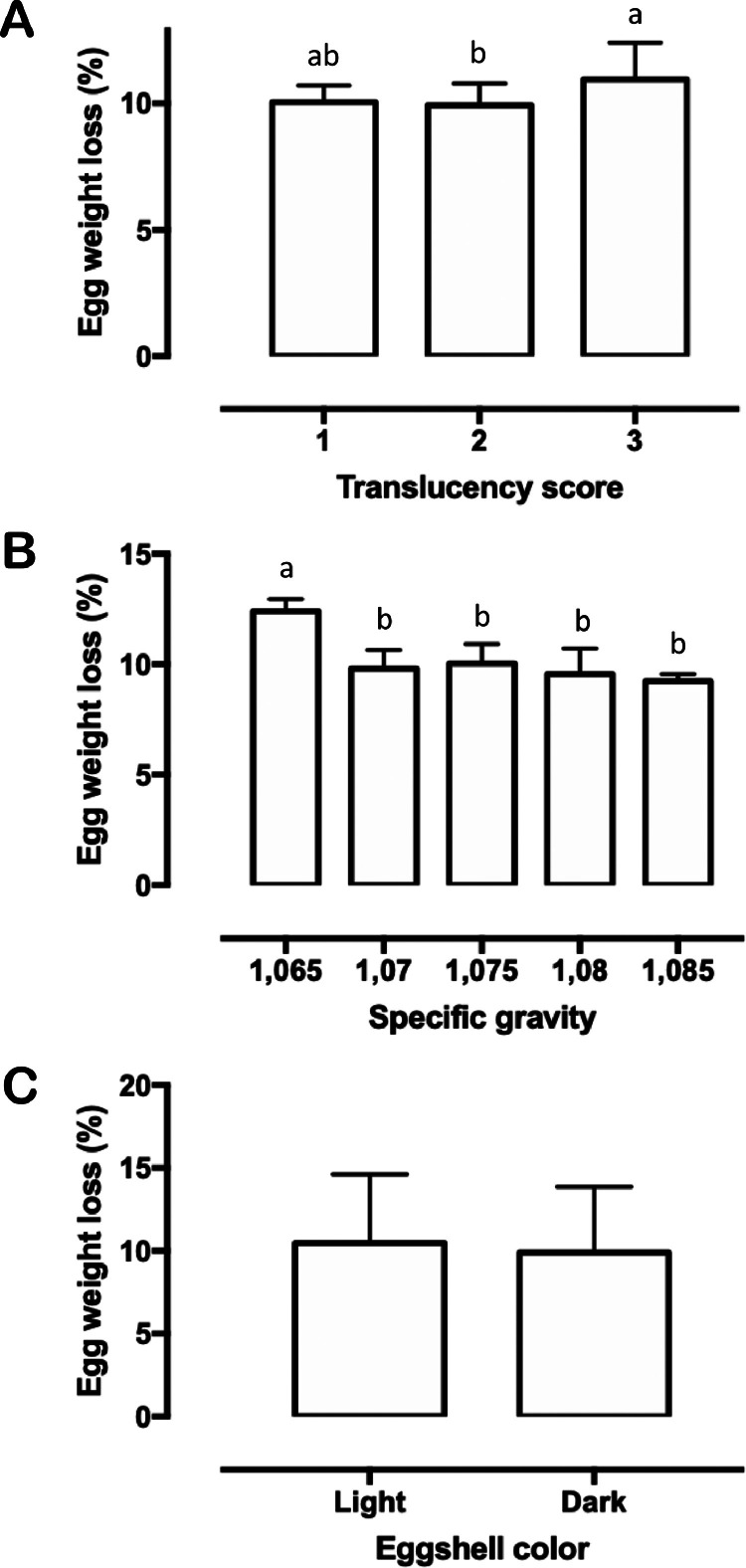
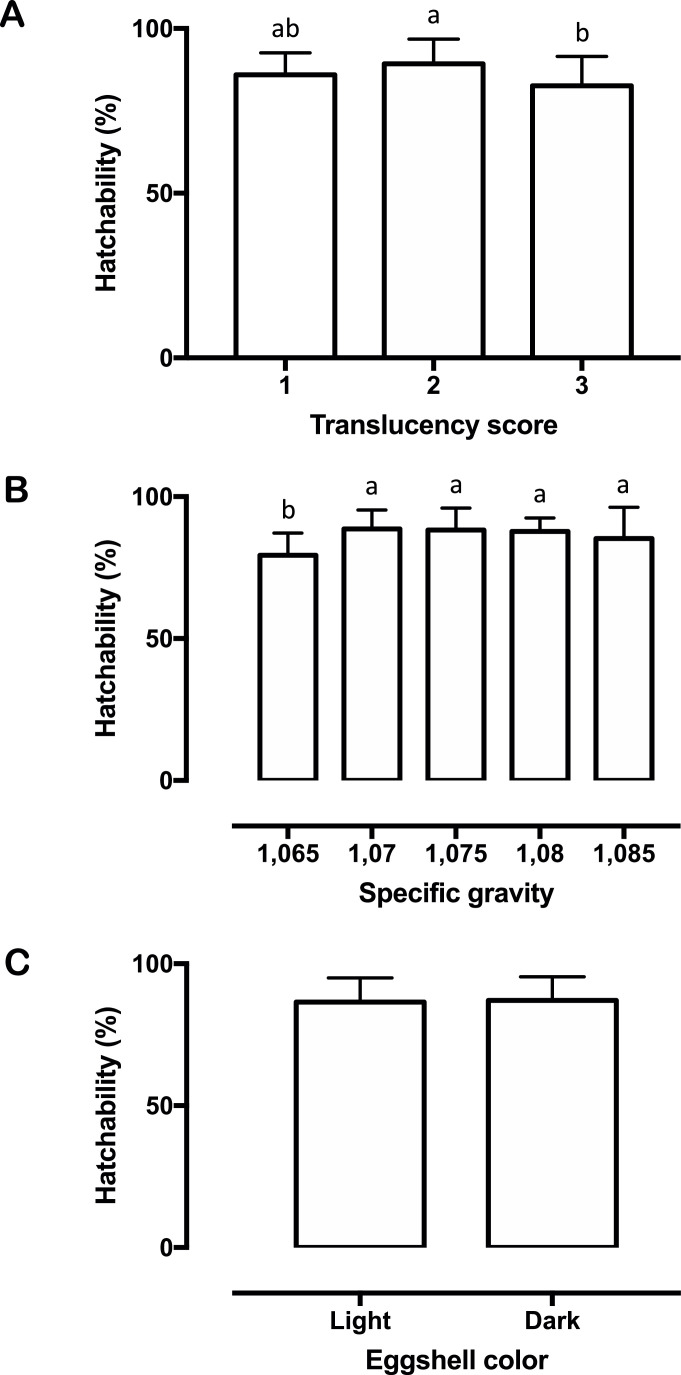
Eggshell translucency influenced egg weight loss, hatchability, and embryonic mortality on 11 to 18 d of incubation, with score 3 differing from 2 in the first 2 parameters and from 1 in the last parameter (Figure 3, Figure 4, Table 2). Egg weight loss and hatchability were equally affected by SG with the lowest category diverging from the others. The percentage of contaminated eggs in SG < 1.065 was higher than in categories 1.070, 1.075, and 1.080 (Figures 3 and 4, Table 3). Light-shelled eggs showed higher embryonic mortalities on 11 to 18 and 19 to 21 d of incubation than eggs with dark shells (Table 4).
Figure 3.
– Means (± SEM) of egg weight loss during incubation according to (A) shell translucency scores; (B) specific gravity; and (C) shell color. Different letters indicate significant differences between groups (P < 0.05).
Figure 4.
– Means (± SEM) of hatchability according to (A) shell translucency scores; (B) specific gravity; and (C) shell color. Different letters indicate significant differences between groups (P < 0.05).
Table 2.
Mean ± SEM of hatching parameters according to eggshell translucency.
| Translucency score1 |
|||
|---|---|---|---|
| Variables | 1 | 2 | 3 |
| Hatchability (%) | 85.93 ± 2.74 ab | 89.32 ± 1.62a | 81.48 ± 2.24b |
| Egg weight loss (%) | 10.05 ± 0.27ab | 9.92 ± 0.18b | 10.95 ± 0.41a |
| Embryonic mortality (%) (0-4 d) | 4.04 ± 1.64 | 3.44 ± 0.81 | 7.66 ± 1.68 |
| Embryonic mortality (%) (5-10 d) | 1.19 ± 0.75 | 1.76 ± 0.49 | 0.59 ± 0.40 |
| Embryonic mortality (%) (11-18 d) | 0.00 ± 0.00b | 2.06 ± 0.47ab | 3.99 ± 0.90a |
| Embryonic mortality (%) (19-21 d) | 2.12 ± 1.36 | 0.83 ± 0.33 | 1.54 ± 0.70 |
| Pipped eggs (%) | 1.77 ± 0.79 | 0.49 ± 0.27 | 0.28 ±0.28 |
| Contaminated eggs (%) | 4.35 ± 2.50 | 1.90 ± 0.58 | 4.41 ± 1.01 |
Score 1 being the least mottled (n = 165 eggs), score 2 the moderately mottled (n = 622 eggs), and score 3 the heavily mottled (n = 351 eggs).
Columns with different superscripts differ significantly (P < 0.05).
Table 3.
Mean ± SEM of hatching parameters according to specific gravity.
| Specific gravity1 |
|||||
|---|---|---|---|---|---|
| Variables | ≥1.065 | 1.070 | 1.075 | 1.080 | ≤1.085 |
| Hatchability (%) | 79.27 ± 2.42b | 88.61 ± 2.01a | 88.23 ± 2.44a | 87.72 ± 1.69a | 85.27 ± 6.33a |
| Egg weight loss (%) | 12.40 ± 0.20a | 9.80 ± 0.25b | 10.03 ± 0.27b | 9.55 ± 0.41b | 9.23 ± 0.17b |
| Embryonic mortality (%) (0–4 d) | 5.12 ± 0.65 | 3.16 ± 0.85 | 5.92 ± 1.48 | 5.56 ± 1.73 | 2.26 ± 1.13 |
| Embryonic mortality (%) (5–10 d) | 2.68 ± 1.10 | 1.24 ± 0.69 | 0.68 ± 0.45 | 1.44 ± 0.71 | 3.03 ± 3.03 |
| Embryonic mortality (%) (11–18 d) | 3.01 ± 1.00 | 1.94 ± 0.73 | 2.03 ± 0.74 | 1.69 ± 0.89 | 2.27 ±1.32 |
| Embryonic mortality (%) (19–21 d) | 2.16 ± 0.70 | 0.95 ± 0.48 | 0.34 ± 0.34 | 2.29 ± 0.69 | 3.03 ± 3.03 |
| Pipped eggs (%) | 1.02 ± 0.66 | 1.28 ± 0.99 | 1.06 ± 0.54 | 0.45 ± 0.80 | 1.11 ± 1.11 |
| Contaminated eggs (%) | 6.74 ± 1.24a | 2.82 ± 1.01b | 1.71 ± 1.05b | 1.27 ± 0.90b | 3.03 ±3.03ab |
Number of eggs per SG groups: ≥1.065 (n = 214); 1.070 (n = 324); 1.075 (n = 297); 1.080 (n = 232); and ≤1.085 (n = 71).
Columns with different superscripts differ significantly (P < 0.05).
Table 4.
Mean ± SEM of hatching parameters according to eggshell color.
| Eggshell color |
||
|---|---|---|
| Variables | Light | Dark |
| Hatchability (%) | 87.35 ± 1.52 | 87.59 ± 1.87 |
| Egg weight loss (%) | 10.45 ± 0.27 | 10.02 ± 0.29 |
| Embryonic mortality (%) (0–4 d) | 3.77 ± 0.52 | 5.25 ± 1.23 |
| Embryonic mortality (%) (5–10 d) | 1.37 ± 0.43 | 1.55 ± 0.64 |
| Embryonic mortality (%) (11–18 d) | 2.74 ± 0.62a | 1.09 ± 0.51b |
| Embryonic mortality (%) (19–21 d) | 1.20 ± 0.35 a | 0.66 ± 0.36b |
| Pipped eggs (%) | 0.44 ± 0.24 | 1.14 ± 0.43 |
| Contaminated eggs (%) | 3.10 ± 0.76 | 2.69 ± 0.74 |
1Number of eggs per eggshell color groups: light (n = 701) and dark (n = 437).
Columns with different superscripts differ significantly (P < 0.05).
DISCUSSION
In recent years, much research has been conducted on eggshell translucency, especially with respect to the characteristics, incidence, influencing factors, and impact of this abnormality on table eggs (Leach and Gross, 1983; Chousalkar et al., 2010; Wang et al., 2017; Wang et al., 2019; Wong et al., 2020; Zhang et al., 2021; Ren et al., 2023). However, despite the belief that eggshell translucency can affect incubation and hatching parameters, concrete data are scarce (Galindo et al., 2022; van den Brand et al., 2023). To fill this gap, we examined whether translucency is in any way linked to SG or eggshell color and what role it plays in broiler egg weight loss, hatchability, and embryonic mortalities. Herein, no relationship was found between translucency and the other 2 eggshell features, and the only confirmed interaction was between SG and color for egg weight loss. The lack of association or interaction between eggshell translucency and SG is plausible considering that the former refers to nonuniform distribution of moisture throughout the eggshell and does not seem to be connected to eggshell thickness as SG (Ahmad et al., 1976; Foster and Weatherup, 1979; Peebles and Brake, 1987; Narushin, 1997; Cheng and Ning, 2023). In fact, the literature contains conflicting data around eggshell thickness in translucent eggs as there are studies that claim they are thicker, thinner, or have no differences when compared to nontranslucent eggs (Holst et al., 1932; Almquist and Burmester, 1934; Talbot and Tyler, 1974; Wang et al., 2017; van den Brand et al., 2023). In contrast, SG is perhaps the easiest and most widely nondestructive method used to estimate shell thickness given its significant correlation with this parameter (Frank et al., 1964; Voisey and Hunt, 1974; Ahmad et al., 1976; Foster and Weatherup, 1979; Peebles and Brake, 1987; Narushin, 1997). Thus, while observing that both parameters showed effects on variables such as egg weight loss and hatchability separately, it seems likely that they are not interconnected because one derives from ultrastructural disorganization of the mammillary and palisade layers, and the other relates to eggshell thickness itself. The same line of thinking applies to the absence of a relationship between translucency and eggshell color since increasing evidence points to the eggshell membrane as a critical factor affecting translucency (due to its influence on the density and organization of the nucleation sites and mammillary knobs), whereas eggshell pigmentation depends on the amount and type of pigments deposited throughout the eggshell and does not appear to be linked to the eggshell membrane formation (Leach and Gross, 1983; Chousalkar et al., 2010; Sparks, 2011; Wang et al., 2017; Ren et al., 2023). Unfortunately, the only information available on the subject reports that eggshell color and translucency are somehow related, with darker and less mottled eggs exhibiting better hatchability and chick weight, although the possible reasons for this effect have not been discussed (Galindo et al., 2022).
As aforementioned, the current analysis also revealed that SG and eggshell color interacted with light-shelled eggs in most SG categories losing more weight over the course of incubation than dark ones. Yet this circumstance did not interfere with hatchability. The assumption that light-shelled eggs do not hatch as well as those with dark shells is widespread among broiler breeders, although there is no consensus on the subject among researchers. Significant but low correlations between these 2 characteristics have been described, leading several authors to conclude that color alone cannot be applied reliably as an eggshell quality indicator (Godfrey and Jaap, 1949; Grover et al., 1980; Joseph et al., 1999; Ingram et al., 2008; Aygun, 2014). According to these studies, dark-shelled eggs usually exhibit higher SG than light-shelled ones. Moreover, Yang et al. (2009) noticed that color was positively correlated with shell strength, weight and thickness, result that for the authors could be related to a longer length of stay of the egg in the shell gland. However, when analyzing the influence of color on eggshell quality and composition in different layer breeds, Drabik et al. (2021) observed no effect of this feature on SG. Interestingly, none of the previous research with broiler breeders examined the interaction effect of eggshell color and SG on incubation parameters (Godfrey and Jaap, 1949; Joseph et al., 1999; Ingram et al., 2008). Hence, we believe that the differences in weight loss between light and dark eggs within SG categories may be explained by small variations in shell thickness, but because these losses are within an acceptable range, they did not show an additive effect on hatchability.
According to a recent publication (Javůrková and Mikšík, 2023) the eggshell pigment (protoporphyrin IX) provides primary antimicrobial protection for eggs, acting as an antioxidant, beyond to be positively correlated with 17α-hydroxy-pregnenolone in the egg albumen. This could potentially explain the greater late mortality of embryos originated from light-shelled eggs. Within the same lineage, chicks hatched from light-shelled eggs are less vigorous than the ones from brown-shelled eggs, they reach the slaughter age weighting less than the ones from brown-shelled eggs and their carcasses present more fat and less lean meat, showing that they were less efficient in depositing protein (Ebbing et al., 2019).
In the current study, translucency alone had a significant impact on egg weight loss, hatchability, and embryonic mortality at 11 to 18 d. Greater egg weight loss was also detected in high translucent eggs from broiler breeders fed with inorganic trace minerals, but information regarding their hatchability was not disclosed (van den Brand et al., 2023). Ultrastructural disorganization of the shell layers in translucent eggs apparently increases in size but decreases in number the mammillary knobs, and forms long continuous grooves between the cones (Leach and Gross, 1983; Chousalkar et al., 2010). These irregularities may trigger changes in eggshell conductance which in turn may lead to increased water loss from inside the egg to the external environment. For example, Ray et al. (2015) reported that high translucent eggs displayed more externally branched pores than those with low translucency, a trait that likely influences weight loss during incubation. Although eggs classified with less mottled shells lost more weight than those with moderately mottled shells, this loss was not different as observed between eggs with moderately and heavily mottled shells. One explanation for this outcome may be the low representativeness in our sample of score 1 eggs (165 eggs) when compared to those shown by scores 2 and 3 (with 622 and 351 eggs, respectively). This sampling issue may also account for the lack of significance between scores 1 and 3 with regard to hatchability, despite the former having a better average result (similar to score 2 eggs that were significantly higher). On average, eggs of scores 1 and 2 had around 4.45% and 7.84% more hatchability than those of score 3, rates close to that provided by Galindo et al. (2022) (6.91%) but lower than that described by Hebbink (2018) (>10%). Considering our findings, this lower hatchability observed in heavily mottled eggs may have been caused by a higher embryonic mortality (especially between 11 and 18 d of incubation), but the reasons for this remain unknown.
Traditionally, SG has been one of the most widely used nondestructive measures of shell quality for both table and hatching eggs because of its high and positive correlation with eggshell weight, thickness and strength (Godfrey and Jaap, 1949; McDaniel et al., 1979; Hamilton, 1982; Peebles and Brake, 1987; Bennett, 1992; Roque and Soares, 1994). Most of these surveys have also shown a positive relationship between SG and hatchability, although some authors argue it may not be a consistent index of hatchability in different ages of birds. Overall, eggs with lower SG exhibit the highest weight losses during incubation and the lowest hatchability (Godfrey and Jaap, 1949; McDaniel et al., 1979; McDaniel et al., 1981; Hamilton, 1982; Bennett, 1992; Roque and Soares, 1994). In line with this information, our results confirmed that eggs with SG below 1.070 differ from the others in terms of weight loss and hatchability. In addition, we also perceived an increase in the percentage of contaminated eggs, which we believe is linked to the higher susceptibility of thin-shelled eggs to crack during handling. A further reason for this increased contamination relies on earlier findings that whole eggs with lower specific gravity are less resistant to bacterial penetration (Orel, 1959; Sauter and Petersen, 1969; Sauter and Petersen, 1974).
Another point that has been the source of great controversy refers to the effect of shell color on hatching eggs. This is because, to date, most studies involving eggshell color have only estimated its correlation with other shell quality indicators (which in general was low), and those few that have investigated hatchability obtained mixed results (Funk and Forward, 1949; Godfrey, 1949; Godfrey and Jaap, 1949; Grover et al., 1980; Joseph et al., 1999; Ingram et al., 2008; Yang et al., 2009; Aygun, 2014; Baylan et al., 2017; Drabik et al., 2021). Here, light-shelled eggs only differed from dark-shelled eggs with regard to late embryonic mortality. A similar outcome to that was obtained by Baylan et al. (2017), who also found no differences in hatchability of fertile eggs with light, medium, and dark shells. Conversely, Shafey et al. (2005) noted that lightly pigmented fertile brown eggs hatched better than medium pigmented ones when incubated without light, whereas heavily pigmented brown eggs had intermediate hatchability. Furthermore, these 3 groups of brown eggs did not show any difference in weight loss throughout incubation. Previous authors have claimed that dark- or medium-shelled eggs hatched more compared to light-shelled eggs (Funk and Forward, 1949; Godfrey and Jaap, 1949; Galindo et al., 2022). However, in one of these studies an abnormality was noted in 1 strain of New Hampshires breeders (Oklahoma) a correlation was found between shell color and hatchability while in another (Ohio) it was not. Therefore, we believe that part of these discrepancies around the effects of shell color on hatching parameters may be the result of other factors such as breeds or methods for determining shell color. For example, our study and Baylan et al. (2017) analyzed eggs from Ross breeders and used electronic colorimeters, whereas Funk and Forward (1949) and Godfrey and Jaap (1949) monitored eggs from New Hampshires breeders and performed visual grading. Unlike the above mentioned, Shafey et al. (2005) examined eggs from Hybro breeders and used visual grading. Thus, further research addressing eggshell color under the similar conditions (i.e., chicken breed, objective methods for color determination, large samples, etc.) are needed to advance our understanding of its influence on incubation variables.
In summary, our data indicate that despite its individual effect, translucency had no relationship or interaction with SG or shell color over incubation parameters. Meanwhile, we ascertained an interaction between SG and eggshell color in which light-shelled eggs tended to lose more weight throughout incubation. High translucency eggs in general lost more weight and hatched less, but these differences were only significant when compared to medium translucency eggs. Eggs with SG lower than 1.070 displayed the greatest weight loss, lowest hatchability and highest contamination. Eggshell color alone only impacted late embryonic mortality but without major consequences on hatchability.
ACKNOWLEDGMENTS
The authors would like to thank Zinpro Animal Nutrition Inc. for financially supporting this study and for lending their electronic colorimeter and BlueBox. We are very grateful to Ad'Oro S.A. for allowing us to conduct all our analyses in their hatchery. Special gratitude goes to Drs Marcílio Nichi and Marcos José Batista dos Santos for their assistance during the statistical analysis. We also thank Drs. Alba Fireman and Ivânio José Martins Bueno for the discussions of the data and critical reading of the manuscript.
Ethics Statement: Does not apply.
Data Availability Statement: The data that support the findings of this study are available from the corresponding author, [RJGP], upon reasonable request.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Ahmad M.M., Froning G.W., Mather F.B., Bashford L.L. Relationships of egg specific gravity and shell thickness to quasi-static compression tests. Poult. Sci. 1976;55:1282–1289. [Google Scholar]
- Almquist H.J., Burmester B.R. Characteristics of an abnormal type of egg shell. Poult. Sci. 1934;13:116–122. [Google Scholar]
- Aygun A. The relationship between eggshell color and egg quality traits in table eggs. Indian J. Anim. Res. 2014;48:290. [Google Scholar]
- Bain M.M. Eggshell strength: A relationship between the mechanism of failure and the ultrastructural organisation of the mammillary layer. Br. Poult. Sci. 1992;33:303–319. [Google Scholar]
- Bain M.M., MacLeod N., Thomson R., Hancock J.W. Microcracks in eggs. Poult. Sci. 2006;85:2001–2008. doi: 10.1093/ps/85.11.2001. [DOI] [PubMed] [Google Scholar]
- Baylan M., Celik L.B., Akpinar G.C., Alasahan S., Kucukgul A., Dogan S.C. Influence of eggshell colour on egg yolk antibody level, incubation results, and growth in broiler breeders. Rev. Bras. Zootec. 2017;46:105–112. [Google Scholar]
- Bennett C.D. The influence of shell thickness on hatchability in commercial broiler breeder flocks. J. Appl. Poult. Res. 1992;1:61–65. [Google Scholar]
- Cheng X., Ning Z. Research progress on bird eggshell quality defects: a review. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chousalkar K.K., Flynn P., Sutherland M., Roberts J.R., Cheetham B.F. Recovery of Salmonella and Escherichia coli from commercial egg shells and effect of translucency on bacterial penetration in eggs. Int. J. Food Microbiol. 2010;142:207–213. doi: 10.1016/j.ijfoodmicro.2010.06.029. [DOI] [PubMed] [Google Scholar]
- De Ketelaere B., Bamelis F., Kemps B., Decuypere E., De Baerdemaeker J. Non-destructive measurements of the egg quality. Worlds. Poult. Sci. J. 2004;60:289–302. [Google Scholar]
- Drabik K., Karwowska M., Wengerska K., Próchniak T., Adamczuk A., Batkowska J. The variability of quality traits of table eggs and eggshell mineral composition depending on hens’ breed and eggshell color. Animals. 2021;11:1204. doi: 10.3390/ani11051204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbing M.A., Vieira S.L., Stefanello C., Berwanger E., Mayer A., Maria D.D., Fireman A.K. An investigation on iron sources fed to broiler breeder hens and the corresponding color of laid eggshells on the performance of the resulting progeny. J. Appl. Poult. Res. 2019;28:184–193. [Google Scholar]
- Foster W.H., Weatherup S.T.C. The use of specific gravity of the egg to estimate shell thickness. Br. Poult. Sci. 1979;20:439–443. [Google Scholar]
- Frank F.R., Swanson M.H., Burger R.E. The relationships between selected physical characteristics and the resistance to shell failure of gallus domesticus eggs. Poult. Sci. 1964;43:1228–1235. doi: 10.3382/ps.0440063. [DOI] [PubMed] [Google Scholar]
- Funk E.M., Forward J.F. The relationship of egg shell color to hatchability in New Hampshires. Poult. Sci. 1949;28:577–580. [Google Scholar]
- Galindo O., Never D., Krehling J., Burin R., Carvalho P., Almeida L., Urrutia A., Munoz L., Lobo C., Bailey M., Chaves-Cordoba B., Rebollo M., Macklin K. Effect of translucency and eggshell color on broiler egg hatchability and chick weight. Page 46 in Poultry Science Association 111th Annual Meeting Abstracts; San Antonio; Poultry Science Association; 2022. [Google Scholar]
- Godfrey G.F. Shell color as a measure of egg shell quality. Poult. Sci. 1949;28:150–151. [Google Scholar]
- Godfrey G.F., Jaap R.G. The relationship of specific gravity, 14-day incubation weight-loss and egg shell color to hatchability and egg shell quality. Poult. Sci. 1949;28:874–889. [Google Scholar]
- Grover R.M., Anderson D.L., Damon R.A. The correlation between egg shell color and specific gravity as a measure of shell strength. Poult. Sci. 1980;59:1335–1336. [Google Scholar]
- Hamilton R.M.G. Methods and factors that affect the measurement of egg shell quality. Poult. Sci. 1982;61:2022–2039. [Google Scholar]
- Hebbink, L. 2018. Eggshell mottling, does it affect incubation results? PasReform Webpage/Knowledge. Acessed June. 2023. https://www.pasreform.com/en/knowledge/126/eggshell-mottling-does-it-affect-incubation-results.
- Holst W.F., Almquist H.J., Lorenz F.W. A study of shell texture of the hen's egg. Poult. Sci. 1932;11:144–149. [Google Scholar]
- Ingram D.R., Hatten L.F., III, Homan K.D. A study on the relationship between eggshell color and eggshell quality in commercial broiler breeders. Int. J. Poult. Sci. 2008;7:700–703. [Google Scholar]
- Ismail I., Rizk Y., Awadien N., Tawfeek F., El-Wardany I. Effects Of short-term thermal manipulation during late embryogenesis on hatching traits and post hatched subsequent performance of Mamoura strain chicks. J. Anim. Poult. Prod. 2016;7:145–151. [Google Scholar]
- Javůrková V.G., Mikšík I. New insights into the relationships between egg maternal components: the interplays between albumen steroid hormones, proteins and eggshell protoporphyrin. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2023;279 doi: 10.1016/j.cbpa.2023.111401. [DOI] [PubMed] [Google Scholar]
- Joseph N.S., Robinson N.A., Renema R.A., Robinson F.E. Shell quality and color variation in broiler breeder eggs. J. Appl. Poult. Res. 1999;8:70–74. [Google Scholar]
- Ketta M., Tůmová E. Eggshell structure, measurements, and quality-affecting factors in laying hens: a review. Czech J. Anim. Sci. 2016;61:299–309. doi: 10.17221/46/2015-CJAS. [DOI] [Google Scholar]
- Kibala L., Rozempolska-Rucinska I., Kasperek K., Zieba G., Lukaszewicz M. Eggshell qualities as indicative of eggshell strength for layer selection. Rev. Bras. Ciência Avícola. 2018;20:99–102. [Google Scholar]
- King'ori A. Review of the factors that influence egg fertility and hatchability in poultry. Int. J. Poult. Sci. 2011;10:484–492. [Google Scholar]
- Kroetz Neto F., Gonzales E., Novaes G., Pereira R. Beneficial impact of hypercapnic conditions during early incubation on broiler hatchability. Embryo mortality and postnatal performance. Brazilian J. Poult. Sci. 2023;25:1–12. [Google Scholar]
- Leach R.M., Gross J.R. The effect of manganese deficiency upon the ultrastructure of the eggshell. Poult. Sci. 1983;62:499–504. doi: 10.3382/ps.0620499. [DOI] [PubMed] [Google Scholar]
- McDaniel G.R., Brake J., Eckman M.K. Factors affecting broiler breeder performance. Poult. Sci. 1981;60:1792–1797. [Google Scholar]
- McDaniel G.R., Roland D.A., Coleman M.A. The effect of egg shell quality on hatchability and embryonic mortality. Poult. Sci. 1979;58:10–13. [Google Scholar]
- Narushin V.G. Non-destructive measurements of egg parameters and quality characteristics. Worlds. Poult. Sci. J. 1997;53:141–153. [Google Scholar]
- Narushin V.G., Romanov M.N. Egg physical characteristics and hatchability. Worlds. Poult. Sci. J. 2002;58:297–303. [Google Scholar]
- Orel V. The pseudomonas spoilage of eggs laid by individual hens. Poult. Sci. 1959;38:8–12. [Google Scholar]
- Peebles E.D., Brake J. Eggshell quality and hatchability in broiler breeder eggs. Poult. Sci. 1987;66:596–604. [Google Scholar]
- Ray A., Roberts J.R., Flavel R., Chousalkar K.K. Eggshell penetration by Salmonella Typhimurium in table eggs: examination of underlying eggshell structures by micro-computed tomography and scanning electron microscopy. Food Res. Int. 2015;78:34–40. doi: 10.1016/j.foodres.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Ren H.-L., Zhao X.-Y., Di K.-Q., Li L.-H., Hao E.-Y., Chen H., Zhou R.-Y., Nie C.-S., Wang D.-H. Eggshell translucency in late-phase laying hens and its effect on egg quality and physiological indicators. Front. Vet. Sci. 2023;10:1–10. doi: 10.3389/fvets.2023.1133752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque L., Soares M.C. Effects of eggshell quality and broiler breeder age on hatchability. Poult. Sci. 1994;73:1838–1845. doi: 10.3382/ps.0731838. [DOI] [PubMed] [Google Scholar]
- Sauter E.A., Petersen C.F. The effect of egg shell quality on penetration by Pseudomonas fluorescens. Poult. Sci. 1969;48:1525–1528. doi: 10.3382/ps.0481525. [DOI] [PubMed] [Google Scholar]
- Sauter E.A., Petersen C.F. The effect of egg shell quality on penetration by various Salmonellae. Poult. Sci. 1974;53:2159–2162. doi: 10.3382/ps.0532159. [DOI] [PubMed] [Google Scholar]
- Shafey T.M., AL-Batshan H.A., Ghannam M.M., Al-Ayed M.S. Effect of intensity of eggshell pigment and illuminated incubation on hatchability of brown eggs. Br. Poult. Sci. 2005;46:190–198. doi: 10.1080/00071660500065789. [DOI] [PubMed] [Google Scholar]
- Sparks N.H.C. Eggshell Pigments–from formation to deposition. Avian Biol. Res. 2011;4:162–167. [Google Scholar]
- Talbot C.J., Tyler C. A study of the fundamental cause of natural translucent areas in egg shells. Br. Poult. Sci. 1974;15:197–204. [Google Scholar]
- Tallentire C.W., Leinonen I., Kyriazakis I. Artificial selection for improved energy efficiency is reaching its limits in broiler chickens. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-19231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler C., Geake F.H. The effect of water on egg shell strength including a study of the translucent areas of the shell. Br. Poult. Sci. 1964;5:277–284. [Google Scholar]
- van den Brand H., Hubers T., van den Anker I., Torres C.A., Frehen E., Ooms M., Arts J., Laurenssen B.F.A., Heetkamp M.J.W., Kemp B., Molenaar R. Effects of trace minerals source in the broiler breeder diet and eggshell translucency on embryonic development of the offspring. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisey P.W., Hunt J.R. Measurement of eggshell strength. J. Texture Stud. 1974;5:135–182. [Google Scholar]
- Wang D.-H., Chen H., Zhou R.-Y., Huang C.-X., Gao H.-X., Fan B.-L., Liu G.-J., Ning Z.-H. Study of measurement methods on phenotype of translucent eggs. Poult. Sci. 2019;98:6677–6683. doi: 10.3382/ps/pez539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.-H., Li Y.-J., Liu L., Liu J.-S., Bao M., Yang N., Zhuo-Cheng H., Ning Z.-H. Traits of eggshells and shell membranes of translucent eggs. Poult. Sci. 2017;96:351–358. doi: 10.3382/ps/pew328. [DOI] [PubMed] [Google Scholar]
- Wolc A., White I.M., Olori V.E., Hill W.G. Inheritance of fertility in broiler chickens. Genet. Sel. Evol. 2009;41:1–9. doi: 10.1186/1297-9686-41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolc A., White I.M.S., Hill W.G., Olori V.E. Inheritance of hatchability in broiler chickens and its relationship to egg quality traits. Poult. Sci. 2010;89:2334–2340. doi: 10.3382/ps.2009-00614. [DOI] [PubMed] [Google Scholar]
- Wong H.C., Ng E.Y., Cheng L.-H., Gun S., Yen K.S. Classification of inhomogeneous eggshell-mottling patterns using a pretrained convolutional neural network. J. Electron. Imaging. 2020;29:1. [Google Scholar]
- Yang H.M., Wang Z.Y., Lu J. Study on the relationship between eggshell colors and egg quality as well as shell ultrastructure in Yangzhou chicken. African J. Biotechnol. 2009;8:2898–2902. [Google Scholar]
- Zhang H.-D., Zhao X.-F., Ren Z.-Z., Tong M.-Q., Chen J.-N., Li S.-Y., Chen H., Wang D.-H. Comparison between different breeds of laying hens in terms of eggshell translucency and its distribution in various ends of the eggshell. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101510. [DOI] [PMC free article] [PubMed] [Google Scholar]