Abstract
Papillary thyroid cancer (PTC) is the most common endocrine malignancy. The incidence of PTC has increased annually worldwide. Thus, PTC diagnosis and treatment attract more attention. Noncoding RNAs (lncRNAs) play crucial roles in PTC progression and act as prognostic biomarkers. Moreover, microRNAs (miRNAs) and epithelial-mesenchymal transition (EMT)-associated proteins have potential biomarkers for diagnosing and treating PTC. However, the correlation of lncRNAs with miRNAs and EMT-associated proteins needs further clarification. The present review highlights the recent advances of lncRNAs in PTC. We significantly summarized the two molecular regulatory mechanisms in PTC progress, including lncRNAs-miRNAs-protein signaling axes and lncRNAs-EMT pathways. This review will help our understanding of the association between lncRNAs and PTC and may assist us in evaluating the prognosis for PTC patients. Taken together, targeting the lncRNAs regulatory network has promising applications in diagnosing and treating PTC.
Keywords: Papillary thyroid cancer, lncRNAs, miRNAs, EMT, Prognosis, Diagnosis
Highlights
-
•
Non-coding RNAs (lncRNAs) serve as crucial players in PTC progression and prognostic markers.
-
•
MicroRNAs (miRNAs) and epithelial-mesenchymal transition (EMT)-associated proteins hold potential as PTC diagnostic and treatment markers.
-
•
The review emphasizes recent advancements in understanding the role of lncRNAs in PTC.
1. Introduction
Over the past few decades, the incidence of thyroid cancer has been steadily increasing worldwide [[1], [2], [3]]. Among thyroid cancer cases, papillary thyroid cancer (PTC) is the most common histological subtype, accounting for 89.1% of all cases [4]. As the most common endocrine malignancy [5], thyroid cancer diagnosis and treatment attract more attention. In addition to conventional imaging and treatment methods such as surgery and radiation therapy, molecular and biomarker analysis has become an essential supplement to traditional pathological assessments of thyroid cancer in recent years [6]. These tools represent valuable resources for improving thyroid cancer diagnosis and clinical management [7]. As indicated by research, with advancements in early tumor diagnosis and novel treatment approaches such as proteogenomics, DNA methylation, lncRNAs, and microRNAs (miRNAs), the survival rate of cancer patients is improving [8]. Several key points include: specific biomarkers participate in thyroid cancer cell processes like proliferation, migration, and apoptosis [9]; some relate to the tumor microenvironment, affecting prognosis [10]; other biomarkers help tumor cells evade the immune system [11,12], highlighting immunotherapy's potential as an effective treatment option.
As mentioned earlier, the majority of thyroid cancers are PTC. As a well-differentiated subtype of malignant tumors, PTC is generally considered to have a good prognosis [13]. However, studies have indicated that PTC can progress toward a poorly differentiated state, significantly decreasing life expectancy [14,15]. Furthermore, the diagnosis of PTC currently relies mainly on histopathological biopsy and fine-needle aspiration biopsy. These diagnostic methods are time-consuming in clinical practice, and the sampled tissues can significantly influence the test results. Therefore, it is crucial to identify tumor biomarkers that can be used for early and precise diagnosis. Here are some common PTC biomarkers:1.long noncoding RNAs (lncRNAs): lncRNAs also display altered expression levels in PTC, such as NEAT1 [16], H19 [17], HAGLROS [18], MFSD4A-AS1 [19] and MALAT1 [20]. These lncRNAs can be utilized as biomarkers to aid diagnosis and prognostic evaluation. 2. Altered expression levels of proteins: In PTC, some proteins exhibit altered expression levels, such as cell adhesion molecules (CAMs) in the epithelial-mesenchymal transition (EMT) pathway associated with N-cadherin, E-cadherin, and P-cadherin [21]. These proteins can serve as biomarkers to facilitate diagnosis and prognostic evaluation of tumors. 3.microRNAs (miRNAs): TO et al. have proposed that miRNAs can be used as a novel biomarker for tumors, and employing miRNAs in conjunction with gene treatments can effectively prevent the growth of tumors [22]. Similarly, several miRNAs in PTC demonstrate changed expression levels, such as miR-146b, miR-221, and miR-222 [23]. These miRNAs also have potential use as biomarkers for diagnosing, prognostic assessment, and treating PTC.
Numerous evidence indicate that the three types of biomarkers mentioned above are interconnected. This review mainly investigates the correlation of lncRNAs with miRNAs and EMT-associated proteins in PTC progress (Fig. 1). In addition, we suggest some potential approaches for diagnosing and treating PTC.
Fig. 1.
LncRNAs' correlation with miRNAs and EMT-associated proteins in proliferation and metastasis of papillary thyroid carcinoma (PTC).
2. LncRNAs-miRNAs-protein signaling axes
LncRNAs are a small subclass of transcriptional molecules, ranging in length from 200 nucleotides to 100 kilobases [24]. LncRNAs play a crucial role in tumorigenesis, angiogenesis, proliferation, migration, apoptosis, and differentiation [25]. Recently, more and more evidence has shown that lncRNAs can act as competitive endogenous RNAs (ceRNAs) to regulate the biological functions of miRNAs [[26], [27], [28]]. Herein, we will provide a detailed description of the regulatory mechanisms involved in PTC.
CeRNAs are RNA molecules that regulate microRNA (miRNA) activity through "sponging." This process is based on the hypothesis that different RNA molecules, such as lncRNAs, circular RNAs (circRNAs), and messenger RNAs (mRNAs), compete for binding to miRNAs response elements (MREs) on miRNAs [29,30].
MiRNAs play a crucial role in post-transcriptional gene regulation by binding to complementary sequences on target mRNAs [31]. This leads to mRNA degradation or translation inhibition, ultimately decreasing protein production. ceRNAs can sequester miRNAs by sharing MREs, preventing them from binding to their target mRNAs [32]. In this way, ceRNAs indirectly regulate gene expression by modulating the availability of miRNAs. Based on this mechanism, we summarize tumor-promoting lncRNAs in PTC (Table 1). Moreover, modern biology's molecular and genetic tools have greatly improved the understanding of the molecules and signaling axes that regulate PTC growth, proliferation, migration, and metastasis.
Table 1.
Summary of tumor-promoting lncRNAs in papillary thyroid cancer(PTC).
| lncRNAs | Variant | Expression | Functions | References |
|---|---|---|---|---|
| PWRN2 | PTC | High | Sponging miR-325 to upregulate DDX5. | [33] |
| FAM230B | PTC | High | Sponging miR-378a-3p to upregulate WNT5A. | [35] |
| n384546 | PTC | High | Sponging miR-145-5p to upregulate AKT3. | [39] |
| NORAD | PTC | High | Sponging miR-451 to upregulate IL-6R. | [40] |
| XIST | PTC | High | Sponging miR-101-3p to upregulate CLDN1. | [58] |
| NEAT1_2 | PTC | High | Sponging miR-106b-5p to upregulate ATAD2. | [62] |
| MIAT | PTC | High | Sponging miR-150-5p to upregulate EZH2. | [71] |
2.1. PWRN2-miR-325-DDX5 axis
The PWRN2-miR-325-DDX5 axis is crucial in regulating the development of PTC. Specifically, lncRNAs PWRN2 promotes proliferation and migration in PTC by upregulating DEAD-box helicase 5 (DDX5) through sponging miR-325. Overexpression of PWRN2 enhances proliferative and migratory potentials in PTC cells, but this effect can be partially reversed by overexpressing miR-325. The role of the PWRN2/miR-325/DDX5 axis in aggravating the development of PTC was confirmed through luciferase assays and rescue experiments [33]. Therefore, a potential therapeutic approach for PTC is to develop drugs or targeted therapies against critical molecules in the PWRN2/miR-325/DDX5 axis, such as miR-325 and DDX5, and finally inhibit PTC cell proliferation and migration. Furthermore, after carefully searching the relevant literature, it has not been found that PWRN2 is involved in the progression of other malignant tumors. Therefore, it may now be considered as a specific biomarker for PTC.
2.2. FAM230B-miR-378a-3p-WNT5A axis
FAM230B functions as a miRNA sponge to competitively protect WNT5A mRNA from degradation by miR-378a-3p. FAM230B, as a long noncoding RNA, can competitively bind to the 3′UTR region of WNT5A mRNA with miR-378a-3p to promote the metastasis of PTC. Elevating miR-378a-3p results in the degradation of WNT5A mRNA, which reduces the expression of the WNT5A protein involved in cell migration and invasion [34,35]. Hence, miR-378a-3p, WNT5A, and FAM230B exhibit potential as targets for hindering PTC metastasis in the future. Despite studies suggesting a potential association between FAM230B and the progression of gastric cancer [36] and colorectal cancer [37], it is more important to note that the FAM230B-miR-378a-3p-WNT5A regulatory axis appears to be specifically involved in PTC based on current research. Therefore, precision treatment approaches targeting this regulatory axis still hold great promise for PTC.
2.3. n384546-miR-145-5p-AKT3 axis
A novel lncRNA n384546 promotes PTC development by acting as a competing endogenous RNA of miR-145-5p to regulate v-akt murine thymoma viral oncogene homolog 3 (AKT3), which participates in the induction of proliferation, invasion, and metastasis of malignant tumors, and inhibition of apoptosis [38]. Specifically, lncRNA n384546 reduces the inhibitory effect of miR-145-5p on AKT3 by sequestering miR-145-5p, increasing AKT3 expression. This process can promote PTC cell proliferation and metastasis [39]. It is worth mentioning that current research has not indicated the involvement of lncRNA n384546 in the progression of other malignant tumors. Therefore, it can potentially serve as a specific tumor biomarker for PTC.
2.4. NORAD-miR-451-IL-6R axis
In PTC, the NORAD-miR-451-IL-6R axis significantly impacts tumor development and progression [40]. NORAD is a lncRNA highly expressed in PTC cells and has a pro-oncogenic effect. Inhibition of NORAD can suppress tumor cell proliferation and metastasis. However, MiR-451, a microRNA, has low expression levels in PTC [41]. NORAD can inhibit the function of miR-451 through competitive binding with endogenous RNA molecules, known as the "ceRNA mechanism", which promotes the development of PTC since miR-451 inhibits tumor cell proliferation and migration [42]. IL-6R is a target gene of miR-451. When NORAD inhibits miR-451, IL-6R expression increases [43,44]. Increasing IL-6R may accelerate tumor development and increase tumors' invasion and metastasis [45]. After reviewing the relevant literature, we found that NORAD is also involved in the progression of other cancers, including breast cancer [46,47], gastric cancer [48,49], bladder cancer [50], osteosarcoma [51], hepatocellular carcinoma [52], pancreatic cancer [53] and colorectal cancer [54]. However, NORAD affects different downstream target molecules in each malignant tumor type. The NORAD-miR-451-IL-6R axis explicitly influences the progression of PTC. Therefore, we can consider targeting this regulatory axis for the precision treatment of PTC.
2.5. XIST-miR-101-3p-CLDN1 axis
lncRNA XIST is upregulated in PTC tissues and cell lines. Studies have shown that XIST can regulate the migration and invasion of PTC cells by acting as a sponge (competitive endogenous RNA) for miR-101-3p. CLDN1 (Claudin-1) is a tight junction protein, and its aberrant expression is associated with the proliferation, migration, and invasion of various cancers [[55], [56], [57]]. Research has revealed that CLDN1 is a target gene of miR-101-3p. XIST can upregulate the expression of CLDN1 by sponging miR-101-3p, thereby promoting the migration and invasion of PTC cells [58]. In summary, XIST plays a regulatory role in developing PTC through the miR-101-3p/CLDN1 axis. XIST is also proved to be involved in the progression of other malignant tumors through complex pathways [[59], [60], [61]].
2.6. NEAT1_2- miR-106b-5p- ATAD2 axis
NEAT1_2 is overexpressed in PTC tissues. In the NEAT1_2-miR-106b-5p-ATAD2 axis, NEAT1_2 upregulates the expression of ATAD2 by acting as a ceRNA to sponge miR-106b-5p. This regulatory effect promotes PTC cells' growth, invasion, and metastasis and inhibits cell apoptosis [62]. This finding reveals the important role of NEAT1_2 in the pathogenesis of PTC and provides new ideas for future therapeutic strategies targeting PTC. Meanwhile, there are studies suggesting that NEAT1_2 is associated with the sensitivity of hepatocellular carcinoma to radiation therapy [63] and also plays a role in the regulation of gastric cancer progression [64]. However, unlike its specific regulatory axis in PTC, where the mechanism is well-defined, the exact regulatory axis of NEAT1_2 in liver and gastric cancer has not yet been identified.
2.7. MIAT-miR-150-5p-EZH2 axis
The MIAT-miR-150-5p-EZH2 axis plays a significant role in the progression of PTC through molecular interactions and regulatory mechanisms. In this case, MIAT sponges miR-150-5p, which leads to the upregulation of EZH2, a critical factor in promoting tumor malignancy, invasion, and poor prognosis [[65], [66], [67], [68]]. EZH2, a core component of the polycomb repressive complex 2 (PRC2), catalyzes the tri-methylation of histone 3 at lysine 27, thereby contributing to the epigenetic regulation of gene expression [69,70]. Elevated EZH2 expression is associated with various cancers, including PTC. The interaction between MIAT and mir-150-5p modulates EZH2 expression, ultimately promoting PTC tumors' development, invasion, and metastasis [71]. As a well-studied lncRNA, MIAT is currently recognized to participate in the regulation of digestive cancers, including gastric cancer [72], hepatocellular carcinoma [73], colorectal cancer [74], as well as genitourinary cancers such as ovarian cancer [75,76], breast cancer [77], cervical cancer [78], and prostate cancer [79]. The specific regulatory mechanisms of MIAT vary among different types of cancers, indicating its involvement in complex networks during cancer regulation. Thus, we suggest that targeting MIAT for future therapies or utilizing it as a cancer biomarker would yield significant benefits for various types of cancer, specifically for PTC.
3. LncRNAs impact PTC progression via EMT pathways
The impact of E-cadherin, N-cadherin, and vimentin on cancer progression is mainly related to the process of EMT [80,81]. This biological process involves cells losing their epithelial characteristics and acquiring mesenchymal properties. It is well-known that increasing the ability of cell migration and invasion can promote tumor metastasis. E-cadherin is an adhesion protein in epithelial cells that maintains tight connections between cells [82]. During EMT, E-cadherin's expression decreases, weakening intercellular connections, which makes it easier for cancer cells to detach from the primary tumor site and enter surrounding tissues or blood vessels, ultimately forming distant metastases [83,84]. N-cadherin is an adhesion protein in mesenchymal cells. When cancer cells undergo EMT, the expression of N-cadherin increases, which gives cancer cells more robust migration and invasion abilities, thereby promoting cancer progression [[85], [86], [87]]. Vimentin is a specific cytoskeletal protein found in mesenchymal cells. During EMT, vimentin expression increases and helps cancer cells gain higher migration ability and resistance against apoptosis Triviño López and González [88], making it easier for tumors to invade surrounding tissues and blood vessels, forming secondary tumors [89,90].
Emerging evidence has revealed a potential contribution of lncRNAs to tumor development. As shown in Table 2, we summarized the relationship between lncRNAs and EMT in PTC. Moreover, numerous studies have suggested that lncRNAs are involved in the process of EMT in PTC through a variety of pathways.
Table 2.
Summary of lncRNAs associated with EMT in PTC.
| lncRNAs | Variant | Expression | Functions | References |
|---|---|---|---|---|
| H19 | PTC | High | Facilitating the process of EMT in cells. | [91] |
| CASC2 | PTC | Low | Inhibiting the process of EMT in cells. | [95] |
| ABCA1 | PTC | High | Facilitating the process of EMT in cells. | [97] |
| LOC389641 | PTC | High | Facilitating the process of EMT in cells. | [111] |
| DOCK9-AS2 | PTC | High | Facilitating the process of EMT in cells. | [114] |
| MALAT1 | PTC | High | Facilitating the process of EMT in cells. | [115] |
3.1. LncRNA H19-EMT
LncRNA H19 enhances the migration and invasion of PTC cells by regulating the mRNA and protein expression of E-Cadherin and vimentin, classic EMT markers. Inhibiting lncRNA H19 resulted in upregulating E-cadherin mRNA and protein levels while downregulating vimentin mRNA and protein levels [91]. These findings suggest that lncRNA H19 plays a role in PTC development through the EMT pathway. H19 is also involved in the regulation of various types of cancer through pathways other than EMT, such as colorectal cancer and lung cancer [[92], [93], [94]]. This further illustrates that H19 can serve as an effective target for precision treatment of cancer.
3.2. LncRNA CASC2-EMT
Overexpression of lncRNA CASC2 significantly decreases mRNA and protein expression of mesenchymal cell markers zinc finger E-box binding homeobox 1(ZEB1) and N-cadherin while increasing mRNA and protein expression of epithelial cell marker E-cadherin [95]. These findings indicate that overexpressing lncRNA CASC2 impedes PTC cell invasion by impacting the EMT pathway. CASC2 plays a crucial regulatory role in human cancer and has been validated in other types of cancer as well [96].
3.3. LncRNA ABCA1-EMT
lncRNA ABCA1 regulates the extracellular signal-regulated kinase (ERK) signaling pathway [97], which is critical in various biological processes such as cell proliferation, differentiation, migration, and survival [98]. The activation of the ERK signaling pathway leads to phosphorylation and activation of Fra-1 (Fos-related antigen 1), an immediate early gene belonging to the AP-1 transcription factor family [99,100]. Fra-1 plays a crucial role in many types of cancer, including tumor occurrence, invasion, and metastasis [[101], [102], [103]]. After activation, Fra-1 can further regulate the transcription of ZEB1. ZEB1 is a key EMT transcription factor that promotes the EMT process by inhibiting the expression of epithelial cell-related genes such as E-cadherin while activating the expression of mesenchymal cell-related genes such as N-cadherin and Vimentin [[104], [105], [106], [107], [108], [109]], characterized by loss of epithelial cell characteristics, acquisition of mesenchymal cell characteristics, and the enhanced ability for cellular migration and invasion which promotes tumor metastasis. ABCA1 has been shown to play a role in other types of cancer as well, but not all through the EMT pathway [110].
3.4. LncRNA LOC389641-EMT
The downregulation of lncRNA LOC389641 suppressed PTC cell proliferation, migration, and invasion abilities [111]. Moreover, Si-LOC389641(LOC389641 gene silencing) transfected cell lines showed higher E-cadherin and lower N-cadherin and vimentin expression. These results suggest that lncRNA LOC389641 may promote EMT in PTC, associated with tumor progression. In addition to PTC, research also suggests that LOC389641 can influence the progression of pancreatic cancer and lung adenocarcinoma through other pathways [112,113].
3.5. LncRNA DOCK9-AS2-EMT
lncRNA DOCK9-AS2 plays a crucial role in influencing PTC progression through EMT by a series of interconnected mechanisms. Firstly, it is upregulated in PTC tissues and cell lines and found in exosomes of PTC patients and cell lines. Furthermore, lncRNA DOCK9-AS2 upregulation promotes PTC cell proliferation, motility, EMT, and stemness, suggesting that targeting lncRNA DOCK9-AS2 could potentially hinder PTC progression. Secondly, exosomes from PTC-CSCs increase lncRNA DOCK9-AS2 levels, facilitating PTC progression both in vitro and in vivo, which implies that lncRNA DOCK9-AS2 may affect exosome-mediated communication between non-Cancer Stem Cells (CSCs) and CSCs in PTC, ultimately driving PTC progression. Thirdly, lncRNA DOCK9-AS2 positively regulates the Wnt/β-catenin pathway by increasing β-catenin levels without affecting GSK-3β and p-GSK-3β. Located in both the nucleus and cytoplasm of PTC cells, DOCK9-AS2 interacts with the transcription factor SP1 in the nucleus, targeting CTNNB1, and acts as a ceRNA by sequestering miR-1972 in the cytoplasm, thereby regulating CTNNB1 [114]. As a result, lncRNA DOCK9-AS2 activates the Wnt/β-catenin pathway through SP1 and miR-1972-regulated CTNNB1, leading to accelerated PTC progression. As an emerging lncRNA, DOCK9-AS2 has only been found to promote the progression of PTC so far. However, whether it plays a role in other types of cancer remains to be further studied. Whether it can serve as a specific biomarker for PTC is interesting.
3.6. LncRNA MALAT1-EMT
lncRNA MALAT1, a well-known lncRNA, has been shown to impact the progression of PTC through the EMT pathway in several ways. In normal thyroid tissue and thyroid neoplasms, MALAT1 expression increases during the progression from normal tissue to benign neoplasms and then to well-differentiated thyroid cancers, such as PTCs and follicular carcinoma (FCs). Notably, lncRNA MALAT1 expression is upregulated in PTC cells during TGF-β-induced EMT. Previous studies have demonstrated that EMT in thyroid cell lines is associated with increased cell proliferation, cell migration, tumor growth in immunodeficient mice, and the number of cancer stem cells [115]. As a result, lncRNA MALAT1 could play a vital role in cancer stem cell proliferation in thyroid tumors, thereby influencing the progression of PTC through the EMT pathway. MALAT1 can regulate multiple steps in tumor development, and its diagnostic and prognostic significance has been demonstrated in various types of cancer [116].
4. Therapeutic approaches
LncRNAs-miRNAs-protein signaling axes are shown in Fig. 2. These findings shed light on the complex molecular mechanisms underlying PTC tumorigenesis and provide valuable insights for developing novel diagnostic and therapeutic strategies for PTC. Based on the above, potential treatment options could be considered: utilize gene editing techniques (such as CRISPR-Cas) to modify ceRNAs and regulate their binding ability with miRNAs [117]. This approach enables the development of gene therapy methods that accurately control the interactions between ceRNAs and miRNA (Fig. 3). Gene therapy also involves introducing a vector encoding for a particular miRNA into cells to regulate its expression [118]. This method can increase the expression of specific miRNAs, affecting tumor biology processes (Fig. 3). Specific proteins derived from miRNA transcription can promote tumor progression, while others have the opposite effect [[119], [120], [121]]. Therefore, we can use miRNA inhibitors to simulate the "ceRNA" mechanism and increase the number of tumor-suppressing proteins, thereby controlling tumor progression. This approach shows promise as a potential therapeutic strategy for cancer. MiRNA inhibitors bind to miRNAs and prevent them from binding to target mRNAs, inhibiting their biological function [122], which can hinder the growth and spread of cancer cells. (Fig. 4). Furthermore, miRNA mimics are artificially synthesized molecules that mimic natural miRNAs' function [122]. Introducing them into cells can increase the activity of specific miRNAs, finally regulating gene expression related to tumors.
Fig. 2.
Seven types of lncRNAs-miRNAs-proteins and their effects on PTC. LncRNAs competitively bind miRNAs with mRNA, resulting in the mRNAs of tumor-associated proteins not being degraded, thereby promoting the proliferation, migration, and metastasis of PTC.
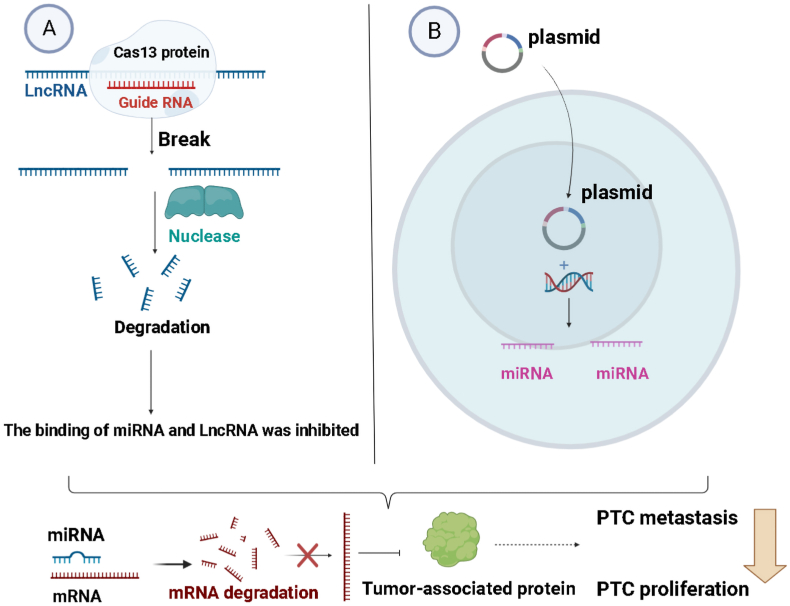
Fig. 3.
LncRNA and miRNA can decrease mRNA stability or suppress its translation, further influencing the proliferation and metastasis of PTC. A. Upon recognition and binding to the target RNA through its guide RNA (gRNA), Cas13 induces site-specific cleavage of the target RNA molecule. Subsequently, the cleaved RNA fragments are typically degraded by cellular nucleases. B. Upon entry into the target cells, the viral vector introduces the miRNA sequence into the cell's genome. This miRNA sequence is recognized by the cellular transcription machinery and transcribed into miRNA.
Fig. 4.
miRNA regulates PTC proliferation, migration, and metastasis. miRNA inhibitors bind to particular miRNA molecules, preventing their complementary interaction with target mRNAs and restoring tumor suppressor protein. This restoration may inhibit PTC proliferation, migration, and metastasis, ultimately contributing to an anti-tumor effect.
We have also summarized the role of lncRNAs-EMT pathways in PTC progression. However, further research is required to fully understand the association between lncRNAs and EMT in PTC, which may have diagnostic and prognostic implications. Previous research indicates that lncRNAs can induce EMT through the PI3K-AKT and Wnt/β-catenin pathways, thereby promoting tumor invasion, metastasis, and prognosis. Based on the above, some potential therapeutic strategies could be considered: 1. Inhibitors targeting EMT-induced pathways: developed drugs target the vital EMT signaling pathways, such as transforming growth factor beta (TGF-β) signaling pathway [123,124], Wnt/β-catenin signaling pathway (Fig. 5), PI3K/Akt signaling pathway (Fig. 6), to prevent tumor cell invasion and metastasis [125]. 2. Targeted therapy: antibody or small molecule drugs target the specific cell surface markers on tumor cells like N-cadherin and vimentin [126,127].
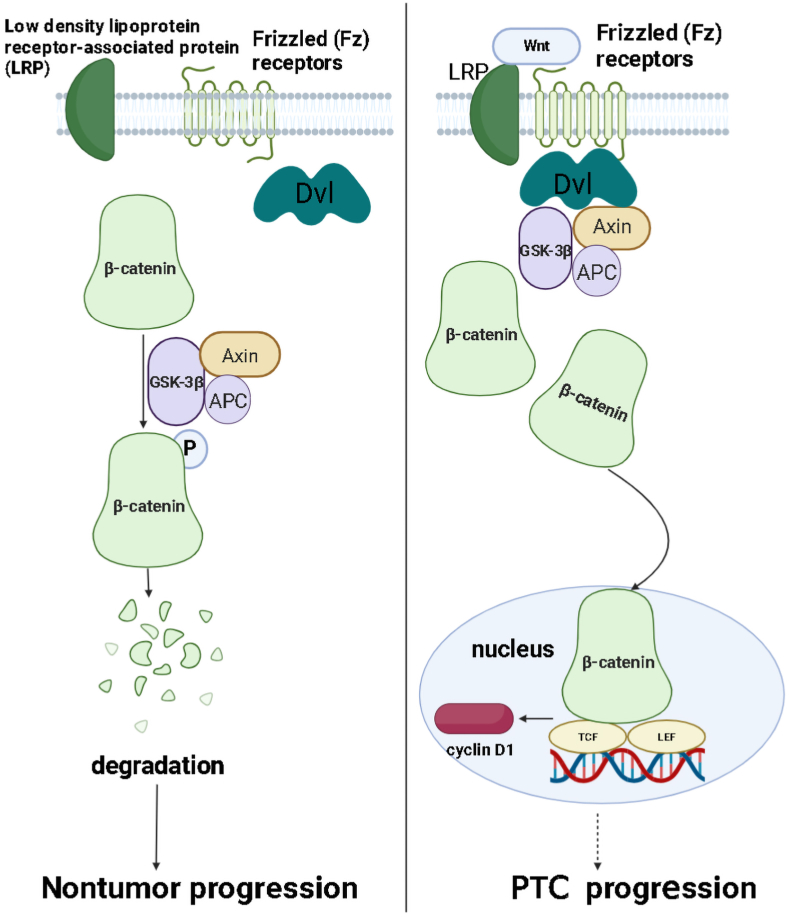
Fig. 5.
The Wnt/β-catenin signaling pathway potentially contributes to PTC progress. β-catenin is phosphorylated and degraded when the Wnt signal is absent. However, upon activation of the Wnt signal by binding to Frizzled family proteins and its ligand Wnt, the protein tyrosine kinase Dishevelled (Dvl) gets activated, protecting phosphorylated β-catenin from degradation. The β-catenin then enters the nucleus through nuclear pore complexes where it binds with TCF/LEF transcription factors to promote transcription of target genes such as c-Myc and cyclin D1 involved in cellular proliferation.
Fig. 6.
The PI3K/Akt signaling pathway induces PTC proliferation. The associated protein initially binds to its cell surface receptor(receptor tyrosine kinases, RTK). RTK activates protein kinases like PI3K, a crucial signaling molecule in cellular growth, proliferation, and migration. Once activated by PI3K, Akt produces phosphatidylinositol-3,4,5-triphosphate (PIP3), a serine/threonine kinase found in cells. Akt is essential in regulating cellular processes, including cell growth, apoptosis, and proliferation through phosphorylation of downstream target proteins(p53/BAD/mTOR/NF-κB), finally promoting PTC proliferation via inhibiting apoptosis and increasing growth.
5. Conclusions and perspectives
PTC, the most common type of thyroid cancer, accounts for about 80% of all thyroid cancers [128]. Although PTC generally has a good prognosis, 10% of patients suffer from local recurrence and distant metastasis. Thus, it is essential to clarify the mechanisms of PTC and identify the latent biomarkers for improving the prognosis of PTC.
Numerous pieces of evidence have identified some biomarkers that could assist in diagnosing PTC. Zhang et al. suggested that the down-regulation of lncRNA DANCR could be a diagnostic biomarker for PTC [129]. Lan et al. proposed that lncRNA NONHSAT037832 has high diagnostic value in distinguishing PTC from non-cancerous diseases and identifying PTC with lymph node metastasis and larger tumors (≥3 cm) [130]. Generally, combining several biomarkers can increase the accuracy of diagnosis. For example, Kim et al. found that the combination of LRRC52-AS1, LINC02082, and UNC5B-AS1 lncRNAs accurately diagnose PTC [131]. To improve the diagnostic accuracy of PTC more conveniently and increase its clinical utility, Wu et al. identified seven downregulated circulating lncRNAs (TCONS_00516390, TCONS_00336559, TCONS_00311568, TCONS_00321917, TCONS_00336522, TCONS_00282483, and TCONS_00494326) with the ability to predict the occurrence of PTC [132]. In addition to lncRNAs, miRNAs are also considered necessary in the diagnosis and prognosis of PTC [133].
Taken together, we not only describe lncRNAs as novel candidate biomarkers for the clinical diagnosis of PTC and potential indicators for prognosis. Moreover, miRNAs have potential use as biomarkers for diagnosis, prognosis, and treatment of PTC. EMT-associated proteins can also be biomarkers to facilitate PTC diagnosis and prognostic evaluation. Specifically, we investigate lncRNA's interaction with miRNAs and EMT-associated proteins. More importantly, we can comprehensively analyze pivotal mechanisms in PTC, including lncRNAs-miRNAs-protein signaling axes and lncRNAs-EMT pathways. These signaling pathways can help understand thyroid cancer's development mechanisms better while providing new strategies for diagnosis or treatment.
In recent years, personalized treatment for patients diagnosed with solid tumors has resulted in several advancements [134]. Personalized cancer medicine can have fewer side effects than other types of therapy because it is designed to be more specific [135]. Personalized treatment may affect healthy cells less and cells involved in cancer more and improve the patient's prognosis [136,137]. As mentioned in the previous section on treatment modalities, we summarize the mechanisms of lncRNA involvement in the progression of PTC. Based on these findings, we have provided some feasible or worthy of further research strategies for clinical diagnosis and treatment. These include regulating the expression of lncRNAs targeting miRNAs in vivo and developing drugs that target EMT-related pathways. Delightfully, our findings can provide a reference for PTC patients' clinical treatment and serve as a valuable resource for clinical researchers in this field. Furthermore, developing personalized treatment plans based on PTC patient-specific information such as gene expression profiles, genetic mutations, and pathological subtypes is beneficial. Correspondingly, combining the therapeutic methods could achieve a more effective treatment outcome.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This work was supported by the Natural Science Basic Research Program of Shaanxi (2022JZ-60) and the Exploration Research Foundation of the Second Affiliated Hospital of Xi'an Jiaotong University (2020YJ(ZYTS) 607 and 608).
Data availability
Data will be made available on request.
CRediT authorship contribution statement
Yuanhao Su: Writing – original draft. Lin Mei: Validation. Tiantian Jiang: Validation. Zhidong Wang: Writing – review & editing. Yuanyuan Ji: Writing – review & editing, Visualization, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Contributor Information
Zhidong Wang, Email: xawzd@163.com.
Yuanyuan Ji, Email: jiyuanyuan1234@163.com.
References
- 1.Cho B.Y., Choi H.S., Park Y.J., Lim J.A., Ahn H.Y., Lee E.K., Kim K.W., Yi K.H., Chung J.K., Youn Y.K., et al. Changes in the clinicopathological characteristics and outcomes of thyroid cancer in Korea over the past four decades. Thyroid. 2013;23(7):797–804. doi: 10.1089/thy.2012.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung C.K., Little M.P., Lubin J.H., Brenner A.V., Wells S.A., Jr., Sigurdson A.J., Nikiforov Y.E. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J. Clin. Endocrinol. Metab. 2014;99(2):E276–E285. doi: 10.1210/jc.2013-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaccarella S., Franceschi S., Bray F., Wild C.P., Plummer M., Dal Maso L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N. Engl. J. Med. 2016;375(7):614–617. doi: 10.1056/NEJMp1604412. [DOI] [PubMed] [Google Scholar]
- 4.Megwalu U.C., Moon P.K. Thyroid cancer incidence and mortality trends in the United States: 2000-2018. Thyroid. 2022;32(5):560–570. doi: 10.1089/thy.2021.0662. [DOI] [PubMed] [Google Scholar]
- 5.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 6.Abdullah M.I., Junit S.M., Ng K.L., Jayapalan J.J., Karikalan B., Hashim O.H. Papillary thyroid cancer: genetic alterations and molecular biomarker investigations. Int. J. Med. Sci. 2019;16(3):450–460. doi: 10.7150/ijms.29935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oskouie A.A., Ahmadi M.S., Taherkhani A. Identification of prognostic biomarkers in papillary thyroid cancer and developing non-invasive diagnostic models through integrated bioinformatics analysis. MicroRNA. 2022;11(1):73–87. doi: 10.2174/2211536611666220124115445. [DOI] [PubMed] [Google Scholar]
- 8.Cheng H., Yang Q., Wang R., Luo R., Zhu S., Li M., Li W., Chen C., Zou Y., Huang Z., et al. Emerging advances of detection strategies for tumor-derived exosomes. Int. J. Mol. Sci. 2022;23(2) doi: 10.3390/ijms23020868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J., Zheng X., Liu H. Hsa_circ_0102272 serves as a prognostic biomarker and regulates proliferation, migration and apoptosis in thyroid cancer. J. Gene Med. 2020;22(9) doi: 10.1002/jgm.3209. [DOI] [PubMed] [Google Scholar]
- 10.Mao M., Huang R.Z., Zheng J., Liang H.Q., Huang W.H., Liu J., Li J.H. OGDHL closely associates with tumor microenvironment and can serve as a prognostic biomarker for papillary thyroid cancer. Cancer Med. 2021;10(2):728–736. doi: 10.1002/cam4.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 12.Qin X.J., Lin X., Xue G., Fan H.L., Wang H.Y., Wu J.F., Pei D. CXCL10 is a potential biomarker and associated with immune infiltration in human papillary thyroid cancer. Biosci. Rep. 2021;41(1) doi: 10.1042/BSR20203459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark O.H. Thyroid cancer and lymph node metastases. J. Surg. Oncol. 2011;103(6):615–618. doi: 10.1002/jso.21804. [DOI] [PubMed] [Google Scholar]
- 14.Luo H., Xia X., Kim G.D., Liu Y., Xue Z., Zhang L., Shu Y., Yang T., Chen Y., Zhang S., et al. Characterizing dedifferentiation of thyroid cancer by integrated analysis. Sci. Adv. 2021;7(31) doi: 10.1126/sciadv.abf3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing M., Haugen B.R., Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381(9871):1058–1069. doi: 10.1016/S0140-6736(13)60109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun W., Qin Y., Wang Z., Dong W., He L., Zhang T., Zhang H. The neat1_2/miR-491 Axis modulates papillary thyroid cancer invasion and metastasis through TGM2/NFκb/FN1 signaling. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.610547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan X., Sun W., Dong W., Wang Z., Zhang T., He L., Zhang H. Downregulation of long noncoding RNA H19 contributes to the proliferation and migration of papillary thyroid carcinoma. Gene. 2018;646:98–105. doi: 10.1016/j.gene.2017.12.051. [DOI] [PubMed] [Google Scholar]
- 18.Zeng Z., Tang S., Chen L., Hou H., Liu Y., Li J. LncRNA HAGLROS contribute to papillary thyroid cancer progression by modulating miR-206/HMGA2 expression. Aging (Albany NY) 2023;15(24):14930–14944. doi: 10.18632/aging.205321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X., Zhang C., Wang X., Cui C., Cui H., Zhu B., Chen A., Zhang L., Xin J., Fu Q., et al. Long non-coding RNA MFSD4A-AS1 promotes lymphangiogenesis and lymphatic metastasis of papillary thyroid cancer. Endocr. Relat. Cancer. 2023;30(3) doi: 10.1530/ERC-22-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng Y., Fang X., Yao H., Zhang Y., Shi J. MiR-146b-5p regulates the expression of long noncoding RNA MALAT1 and its effect on the invasion and proliferation of papillary thyroid cancer. Cancer Biother. Radiopharm. 2021;36(5):433–440. doi: 10.1089/cbr.2019.3322. [DOI] [PubMed] [Google Scholar]
- 21.Da C., Wu K., Yue C., Bai P., Wang R., Wang G., Zhao M., Lv Y., Hou P. N-cadherin promotes thyroid tumorigenesis through modulating major signaling pathways. Oncotarget. 2017;8(5):8131–8142. doi: 10.18632/oncotarget.14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Şahin T., Yılmaz B., Yeşilyurt N., Cicia D., Szymanowska A., Amero P., Ağagündüz D., Capasso R. Recent insights into the nutritional immunomodulation of cancer-related microRNAs. Phytother Res. 2023;37(10):4375–4397. doi: 10.1002/ptr.7937. [DOI] [PubMed] [Google Scholar]
- 23.Cai S., Ma J., Wang Y., Cai Y., Xie L., Chen X., Yang Y., Peng Q. Biomarker value of miR-221 and miR-222 as potential substrates in the differential diagnosis of papillary thyroid cancer based on data synthesis and bioinformatics approach. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.794490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anastasiadou E., Jacob L.S., Slack F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer. 2018;18(1):5–18. doi: 10.1038/nrc.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Gesualdo F., Capaccioli S., Lulli M. A pathophysiological view of the long non-coding RNA world. Oncotarget. 2014;5(22):10976–10996. doi: 10.18632/oncotarget.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu L., Ding J., Chen C., Wu Z.J., Liu B., Gao Y., Chen W., Liu F., Sun W., Li X.F., et al. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29(5):653–668. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Wang J., Liu X., Wu H., Ni P., Gu Z., Qiao Y., Chen N., Sun F., Fan Q. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res. 2010;38(16):5366–5383. doi: 10.1093/nar/gkq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan B., Yao J., Liu J.Y., Li X.M., Wang X.Q., Li Y.J., Tao Z.F., Song Y.C., Chen Q., Jiang Q. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ. Res. 2015;116(7):1143–1156. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 29.Karreth F.A., Pandolfi P.P. ceRNA cross-talk in cancer: when ce-bling rivalries go awry. Cancer Discov. 2013;3(10):1113–1121. doi: 10.1158/2159-8290.CD-13-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146(3):353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 32.Tay Y., Rinn J., Pandolfi P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505(7483):344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xin C.H., Li Z. LncRNA PWRN2 stimulates the proliferation and migration in papillary thyroid carcinoma through the miR-325/DDX5 axis. Eur. Rev. Med. Pharmacol. Sci. 2020;24(19):10022–10027. doi: 10.26355/eurrev_202010_23216. [DOI] [PubMed] [Google Scholar]
- 34.Prasad C.P., Chaurasiya S.K., Guilmain W., Andersson T. WNT5A signaling impairs breast cancer cell migration and invasion via mechanisms independent of the epithelial-mesenchymal transition. J. Exp. Clin. Cancer Res. 2016;35(1):144. doi: 10.1186/s13046-016-0421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Q., Feng J., Yin S., Ma S., Wang J., Yi H. LncRNA FAM230B promotes the metastasis of papillary thyroid cancer by sponging the miR-378a-3p/WNT5A axis. Biochem. Biophys. Res. Commun. 2021;546:83–89. doi: 10.1016/j.bbrc.2021.01.109. [DOI] [PubMed] [Google Scholar]
- 36.Cui Y., Pu R., Ye J., Huang H., Liao D., Yang Y., Chen W., Yao Y., He Y. LncRNA FAM230B promotes gastric cancer growth and metastasis by regulating the miR-27a-5p/top2a Axis. Dig. Dis. Sci. 2021;66(8):2637–2650. doi: 10.1007/s10620-020-06581-z. [DOI] [PubMed] [Google Scholar]
- 37.Li N., Zhou C., Yang F. lncRNA FAM230B is highly expressed in colorectal cancer and suppresses the maturation of miR-1182 to increase cell proliferation. Open Med. 2022;17(1):1559–1567. doi: 10.1515/med-2022-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehman H.L., Van Laere S.J., van Golen C.M., Vermeulen P.B., Dirix L.Y., van Golen K.L. Regulation of inflammatory breast cancer cell invasion through Akt1/PKBα phosphorylation of RhoC GTPase. Mol. Cancer Res. 2012;10(10):1306–1318. doi: 10.1158/1541-7786.MCR-12-0173. [DOI] [PubMed] [Google Scholar]
- 39.Feng J., Zhou Q., Yi H., Ma S., Li D., Xu Y., Wang J., Yin S. A novel lncRNA n384546 promotes thyroid papillary cancer progression and metastasis by acting as a competing endogenous RNA of miR-145-5p to regulate AKT3. Cell Death Dis. 2019;10(6):433. doi: 10.1038/s41419-019-1637-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi L., Cong Y.Z., Wang Z.J. LncRNA NORAD promotes thyroid carcinoma progression by targeting miR-451. Eur. Rev. Med. Pharmacol. Sci. 2021;25(20):6187–6195. doi: 10.26355/eurrev_202110_26989. [DOI] [PubMed] [Google Scholar]
- 41.Duan Y., Zhang Y., Peng W., Jiang P., Deng Z., Wu C. MiR-7-5pand miR-451 as diagnostic biomarkers for papillary thyroid carcinoma in formalin-fixed paraffin-embedded tissues. Pharmazie. 2020;75(6):266–270. doi: 10.1691/ph.2020.0335. [DOI] [PubMed] [Google Scholar]
- 42.Wang R., Wang Z.X., Yang J.S., Pan X., De W., Chen L.B. MicroRNA-451 functions as a tumor suppressor in human non-small cell lung cancer by targeting ras-related protein 14 (RAB14) Oncogene. 2011;30(23):2644–2658. doi: 10.1038/onc.2010.642. [DOI] [PubMed] [Google Scholar]
- 43.Liu S.Y., Deng S.Y., He Y.B., Ni G.X. miR-451 inhibits cell growth, migration and angiogenesis in human osteosarcoma via down-regulating IL 6R. Biochem. Biophys. Res. Commun. 2017;482(4):987–993. doi: 10.1016/j.bbrc.2016.11.145. [DOI] [PubMed] [Google Scholar]
- 44.Liu X., Zhang A., Xiang J., Lv Y., Zhang X. miR-451 acts as a suppressor of angiogenesis in hepatocellular carcinoma by targeting the IL-6R-STAT3 pathway. Oncol. Rep. 2016;36(3):1385–1392. doi: 10.3892/or.2016.4971. [DOI] [PubMed] [Google Scholar]
- 45.Rokavec M., Öner M.G., Li H., Jackstadt R., Jiang L., Lodygin D., Kaller M., Horst D., Ziegler P.K., Schwitalla S., et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J. Clin. Invest. 2014;124(4):1853–1867. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou K., Ou Q., Wang G., Zhang W., Hao Y., Li W. High long non-coding RNA NORAD expression predicts poor prognosis and promotes breast cancer progression by regulating TGF-β pathway. Cancer Cell Int. 2019;19:63. doi: 10.1186/s12935-019-0781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan B.S., Yang M.C., Singh S., Chou Y.C., Chen H.Y., Wang M.Y., Wang Y.C., Chen R.H. LncRNA NORAD is repressed by the YAP pathway and suppresses lung and breast cancer metastasis by sequestering S100P. Oncogene. 2019;38(28):5612–5626. doi: 10.1038/s41388-019-0812-8. [DOI] [PubMed] [Google Scholar]
- 48.Yu S.Y., Peng H., Zhu Q., Wu Y.X., Wu F., Han C.R., Yan B., Li Q., Xiang H.G. Silencing the long noncoding RNA NORAD inhibits gastric cancer cell proliferation and invasion by the RhoA/ROCK1 pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23(9):3760–3770. doi: 10.26355/eurrev_201905_17802. [DOI] [PubMed] [Google Scholar]
- 49.Tao W., Li Y., Zhu M., Li C., Li P. LncRNA NORAD promotes proliferation and inhibits apoptosis of gastric cancer by regulating miR-214/Akt/mTOR Axis. OncoTargets Ther. 2019;12:8841–8851. doi: 10.2147/OTT.S216862. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Li Q., Li C., Chen J., Liu P., Cui Y., Zhou X., Li H., Zu X. High expression of long noncoding RNA NORAD indicates a poor prognosis and promotes clinical progression and metastasis in bladder cancer. Urol. Oncol. 2018;36(6):310.e315–310.e322. doi: 10.1016/j.urolonc.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 51.Wang X., Zou J., Chen H., Zhang P., Lu Z., You Z., Sun J. Long noncoding RNA NORAD regulates cancer cell proliferation and migration in human osteosarcoma by endogenously competing with miR-199a-3p. IUBMB Life. 2019;71(10):1482–1491. doi: 10.1002/iub.2064. [DOI] [PubMed] [Google Scholar]
- 52.Tian Q., Yan X., Yang L., Liu Z., Yuan Z., Shen Z., Zhang Y. lncRNA NORAD promotes hepatocellular carcinoma progression via regulating miR-144-3p/SEPT2. Am J Transl Res. 2020;12(5):2257–2266. [PMC free article] [PubMed] [Google Scholar]
- 53.Li H., Wang X., Wen C., Huo Z., Wang W., Zhan Q., Cheng D., Chen H., Deng X., Peng C., et al. Long noncoding RNA NORAD, a novel competing endogenous RNA, enhances the hypoxia-induced epithelial-mesenchymal transition to promote metastasis in pancreatic cancer. Mol. Cancer. 2017;16(1):169. doi: 10.1186/s12943-017-0738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J., Li X.Y., Hu P., Ding Y.S. lncRNA NORAD contributes to colorectal cancer progression by inhibition of miR-202-5p. Oncol. Res. 2018;26(9):1411–1418. doi: 10.3727/096504018X15190844870055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwon M.J. Emerging roles of claudins in human cancer. Int. J. Mol. Sci. 2013;14(9):18148–18180. doi: 10.3390/ijms140918148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh A.B., Sharma A., Dhawan P. Claudin family of proteins and cancer: an overview. J Oncol. 2010 doi: 10.1155/2010/541957. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsukita S., Tanaka H., Tamura A. The claudins: from tight junctions to biological systems. Trends Biochem. Sci. 2019;44(2):141–152. doi: 10.1016/j.tibs.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Du Y.L., Liang Y., Cao Y., Liu L., Li J., Shi G.Q. LncRNA XIST promotes migration and invasion of papillary thyroid cancer cell by modulating MiR-101-3p/CLDN1 Axis. Biochem. Genet. 2021;59(2):437–452. doi: 10.1007/s10528-020-09985-8. [DOI] [PubMed] [Google Scholar]
- 59.Liu H., Wang D., Kan S., Hao M., Chang L., Lu P., Liu Y., Jin Y., Liu W. The role of lncRNAs and XIST in oral cancer. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.826650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eldesouki S., Samara K.A., Qadri R., Obaideen A.A., Otour A.H., Habbal O., Bm Ahmed S. XIST in brain cancer. Clin. Chim. Acta. 2022;531:283–290. doi: 10.1016/j.cca.2022.04.993. [DOI] [PubMed] [Google Scholar]
- 61.Ma Y., Zhu Y., Shang L., Qiu Y., Shen N., Wang J., Adam T., Wei W., Song Q., Li J., et al. LncRNA XIST regulates breast cancer stem cells by activating proinflammatory IL-6/STAT3 signaling. Oncogene. 2023;42(18):1419–1437. doi: 10.1038/s41388-023-02652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun W., Lan X., Zhang H., Wang Z., Dong W., He L., Zhang T., Zhang P., Liu J., Qin Y. NEAT1_2 functions as a competing endogenous RNA to regulate ATAD2 expression by sponging microRNA-106b-5p in papillary thyroid cancer. Cell Death Dis. 2018;9(3):380. doi: 10.1038/s41419-018-0418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen X., Zhang N. Downregulation of lncRNA NEAT1_2 radiosensitizes hepatocellular carcinoma cells through regulation of miR-101-3p/WEE1 axis. Cell Biol. Int. 2019;43(1):44–55. doi: 10.1002/cbin.11077. [DOI] [PubMed] [Google Scholar]
- 64.Fu R., Wang X., Hu Y., Du H., Dong B., Ao S., Zhang L., Sun Z., Zhang L., Lv G., et al. Solamargine inhibits gastric cancer progression by regulating the expression of lncNEAT1_2 via the MAPK signaling pathway. Int. J. Oncol. 2019;54(5):1545–1554. doi: 10.3892/ijo.2019.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bachmann I.M., Halvorsen O.J., Collett K., Stefansson I.M., Straume O., Haukaas S.A., Salvesen H.B., Otte A.P., Akslen L.A. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J. Clin. Oncol. 2006;24(2):268–273. doi: 10.1200/JCO.2005.01.5180. [DOI] [PubMed] [Google Scholar]
- 66.Bracken A.P., Pasini D., Capra M., Prosperini E., Colli E., Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22(20):5323–5335. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sauvageau M., Sauvageau G. Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell. 2010;7(3):299–313. doi: 10.1016/j.stem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Varambally S., Dhanasekaran S.M., Zhou M., Barrette T.R., Kumar-Sinha C., Sanda M.G., Ghosh D., Pienta K.J., Sewalt R.G., Otte A.P., et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419(6907):624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 69.Zeng J., Zhang J., Sun Y., Wang J., Ren C., Banerjee S., Ouyang L., Wang Y. Targeting EZH2 for cancer therapy: from current progress to novel strategies. Eur. J. Med. Chem. 2022;238 doi: 10.1016/j.ejmech.2022.114419. [DOI] [PubMed] [Google Scholar]
- 70.Han Li C., Chen Y. Targeting EZH2 for cancer therapy: progress and perspective. Curr. Protein Pept. Sci. 2015;16(6):559–570. doi: 10.2174/1389203716666150409100233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo K., Qian K., Shi Y., Sun T., Wang Z. LncRNA-MIAT promotes thyroid cancer progression and function as ceRNA to target EZH2 by sponging miR-150-5p. Cell Death Dis. 2021;12(12):1097. doi: 10.1038/s41419-021-04386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sha M., Lin M., Wang J., Ye J., Xu J., Xu N., Huang J. Long non-coding RNA MIAT promotes gastric cancer growth and metastasis through regulation of miR-141/DDX5 pathway. J. Exp. Clin. Cancer Res. 2018;37(1):58. doi: 10.1186/s13046-018-0725-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiang Y., Huang Y., Sun H., Pan Y., Wu M., Zhang J. Deregulation of miR-520d-3p promotes hepatocellular carcinoma development via lncRNA MIAT regulation and EPHA2 signaling activation. Biomed. Pharmacother. 2019;109:1630–1639. doi: 10.1016/j.biopha.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 74.Liu Z., Wang H., Cai H., Hong Y., Li Y., Su D., Fan Z. Long non-coding RNA MIAT promotes growth and metastasis of colorectal cancer cells through regulation of miR-132/Derlin-1 pathway. Cancer Cell Int. 2018;18:59. doi: 10.1186/s12935-017-0477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitra R., Chen X., Greenawalt E.J., Maulik U., Jiang W., Zhao Z., Eischen C.M. Decoding critical long non-coding RNA in ovarian cancer epithelial-to-mesenchymal transition. Nat. Commun. 2017;8(1):1604. doi: 10.1038/s41467-017-01781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shao S., Tian J., Zhang H., Wang S. LncRNA myocardial infarction-associated transcript promotes cell proliferation and inhibits cell apoptosis by targeting miR-330-5p in epithelial ovarian cancer cells. Arch. Med. Sci. 2018;14(6):1263–1270. doi: 10.5114/aoms.2018.75535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alipoor F.J., Asadi M.H., Torkzadeh-Mahani M. MIAT lncRNA is overexpressed in breast cancer and its inhibition triggers senescence and G1 arrest in MCF7 cell line. J. Cell. Biochem. 2018;119(8):6470–6481. doi: 10.1002/jcb.26678. [DOI] [PubMed] [Google Scholar]
- 78.Zhang L., Ge S., Cao B. Long non-coding RNA MIAT promotes cervical cancer proliferation and migration. J. Biochem. 2020;168(2):183–190. doi: 10.1093/jb/mvaa037. [DOI] [PubMed] [Google Scholar]
- 79.Crea F., Venalainen E., Ci X., Cheng H., Pikor L., Parolia A., Xue H., Nur Saidy N.R., Lin D., Lam W., et al. The role of epigenetics and long noncoding RNA MIAT in neuroendocrine prostate cancer. Epigenomics. 2016;8(5):721–731. doi: 10.2217/epi.16.6. [DOI] [PubMed] [Google Scholar]
- 80.De Craene B., Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer. 2013;13(2):97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 81.Peinado H., Olmeda D., Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer. 2007;7(6):415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 82.van Roy F., Berx G. The cell-cell adhesion molecule E-cadherin. Cell. Mol. Life Sci. 2008;65(23):3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lambert A.W., Pattabiraman D.R., Weinberg R.A. Emerging biological principles of metastasis. Cell. 2017;168(4):670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nieto M.A., Huang R.Y., Jackson R.A., Thiery J.P. Emt. Cell. 2016;166(1):21–45. doi: 10.1016/j.cell.2016.06.028. 2016. [DOI] [PubMed] [Google Scholar]
- 85.Hazan R.B., Phillips G.R., Qiao R.F., Norton L., Aaronson S.A. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J. Cell Biol. 2000;148(4):779–790. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Islam S., Carey T.E., Wolf G.T., Wheelock M.J., Johnson K.R. Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J. Cell Biol. 1996;135(6 Pt 1):1643–1654. doi: 10.1083/jcb.135.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nieman M.T., Prudoff R.S., Johnson K.R., Wheelock M.J. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J. Cell Biol. 1999;147(3):631–644. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Triviño López A., González I.C. An immunohistochemical study of epithelial membrane antigen, cytokeratin, and vimentin in papillary thyroid carcinoma: recognition of lethal and favorable prognostic types. Cancer. 1993;72(7):2286–2287. doi: 10.1002/1097-0142(19931001)72:7<2286::aid-cncr2820720738>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 89.Wei J., Xu G., Wu M., Zhang Y., Li Q., Liu P., Zhu T., Song A., Zhao L., Han Z., et al. Overexpression of vimentin contributes to prostate cancer invasion and metastasis via src regulation. Anticancer Res. 2008;28(1a):327–334. [PubMed] [Google Scholar]
- 90.Jin H., Morohashi S., Sato F., Kudo Y., Akasaka H., Tsutsumi S., Ogasawara H., Miyamoto K., Wajima N., Kawasaki H., et al. Vimentin expression of esophageal squamous cell carcinoma and its aggressive potential for lymph node metastasis. Biomed Res. 2010;31(2):105–112. doi: 10.2220/biomedres.31.105. [DOI] [PubMed] [Google Scholar]
- 91.W.Q. Liang, D. Zeng, C.F. Chen, S.M. Sun, X.F. Lu, C.Y. Peng, H.Y. Lin, Long Noncoding RNA H19 Is a Critical Oncogenic Driver and Contributes to Epithelial-Mesenchymal Transition in Papillary Thyroid Carcinoma, (1179-1322 (Print)). [DOI] [PMC free article] [PubMed]
- 92.Chen S., Shen X. Long noncoding RNAs: functions and mechanisms in colon cancer. Mol. Cancer. 2020;19(1):167. doi: 10.1186/s12943-020-01287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang R., Pan T., Xiang Y., Zhang M., Xie H., Liang Z., Chen B., Xu C., Wang J., Huang X., et al. Curcumenol triggered ferroptosis in lung cancer cells via lncRNA H19/miR-19b-3p/FTH1 axis. Bioact. Mater. 2022;13:23–36. doi: 10.1016/j.bioactmat.2021.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hashemi M., Moosavi M.S., Abed H.M., Dehghani M., Aalipour M., Heydari E.A., Behroozaghdam M., Entezari M., Salimimoghadam S., Gunduz E.S., et al. Long non-coding RNA (lncRNA) H19 in human cancer: from proliferation and metastasis to therapy. Pharmacol. Res. 2022;184 doi: 10.1016/j.phrs.2022.106418. [DOI] [PubMed] [Google Scholar]
- 95.Zhou T., Zhong M., Zhang S., Wang Z., Xie R., Xiong C., Lv Y., Chen W., Yu J. LncRNA CASC2 expression is down- regulated in papillary thyroid cancer and promotes cell invasion by affecting EMT pathway. Cancer Biomark. 2018;23(2):185–191. doi: 10.3233/CBM-181198. [DOI] [PubMed] [Google Scholar]
- 96.Palmieri G., Paliogiannis P., Sini M.C., Manca A., Palomba G., Doneddu V., Tanda F., Pascale M.R., Cossu A. Long non-coding RNA CASC2 in human cancer. Crit. Rev. Oncol. Hematol. 2017;111:31–38. doi: 10.1016/j.critrevonc.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 97.Park J.H., Myung J.K., Lee S.J., Kim H., Kim S., Lee S.B., Jang H., Jang W.I., Park S., Yang H., et al. ABCA1-Mediated EMT promotes papillary thyroid cancer malignancy through the ERK/Fra-1/ZEB1 pathway. Cells. 2023;12(2) doi: 10.3390/cells12020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun Y., Liu W.Z., Liu T., Feng X., Yang N., Zhou H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015;35(6):600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 99.Hess J., Angel P., Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J. Cell Sci. 2004;117(Pt 25):5965–5973. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- 100.Bejjani F., Evanno E., Zibara K., Piechaczyk M., Jariel-Encontre I. The AP-1 transcriptional complex: local switch or remote command? Biochim. Biophys. Acta Rev. Canc. 2019;1872(1):11–23. doi: 10.1016/j.bbcan.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 101.Iskit S., Schlicker A., Wessels L., Peeper D.S. Fra-1 is a key driver of colon cancer metastasis and a Fra-1 classifier predicts disease-free survival. Oncotarget. 2015;6(41):43146–43161. doi: 10.18632/oncotarget.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Desmet C.J., Gallenne T., Prieur A., Reyal F., Visser N.L., Wittner B.S., Smit M.A., Geiger T.R., Laoukili J., Iskit S., et al. Identification of a pharmacologically tractable Fra-1/ADORA2B axis promoting breast cancer metastasis. Proc Natl Acad Sci U S A. 2013;110(13):5139–5144. doi: 10.1073/pnas.1222085110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheng F., Su L., Yao C., Liu L., Shen J., Liu C., Chen X., Luo Y., Jiang L., Shan J., et al. SIRT1 promotes epithelial-mesenchymal transition and metastasis in colorectal cancer by regulating Fra-1 expression. Cancer Lett. 2016;375(2):274–283. doi: 10.1016/j.canlet.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 104.Okugawa Y., Toiyama Y., Tanaka K., Matsusita K., Fujikawa H., Saigusa S., Ohi M., Inoue Y., Mohri Y., Uchida K., et al. Clinical significance of Zinc finger E-box Binding homeobox 1 (ZEB1) in human gastric cancer. J. Surg. Oncol. 2012;106(3):280–285. doi: 10.1002/jso.22142. [DOI] [PubMed] [Google Scholar]
- 105.Zhang J., Lu C., Zhang J., Kang J., Cao C., Li M. Involvement of ZEB1 and E-cadherin in the invasion of lung squamous cell carcinoma. Mol. Biol. Rep. 2013;40(2):949–956. doi: 10.1007/s11033-012-2136-4. [DOI] [PubMed] [Google Scholar]
- 106.Shen A., Zhang Y., Yang H., Xu R., Huang G. Overexpression of ZEB1 relates to metastasis and invasion in osteosarcoma. J. Surg. Oncol. 2012;105(8):830–834. doi: 10.1002/jso.23012. [DOI] [PubMed] [Google Scholar]
- 107.Spaderna S., Schmalhofer O., Wahlbuhl M., Dimmler A., Bauer K., Sultan A., Hlubek F., Jung A., Strand D., Eger A., et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008;68(2):537–544. doi: 10.1158/0008-5472.CAN-07-5682. [DOI] [PubMed] [Google Scholar]
- 108.Zhang P., Sun Y., Ma L. ZEB1: at the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle. 2015;14(4):481–487. doi: 10.1080/15384101.2015.1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Y., Zhang N., Wang Y., Xu M., Liu N., Pang X., Cao J., Ma N., Pang H., Liu L., et al. Zinc finger E-box binding homeobox 1 promotes invasion and bone metastasis of small cell lung cancer in vitro and in vivo. Cancer Sci. 2012;103(8):1420–1428. doi: 10.1111/j.1349-7006.2012.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu K., Zou L., Lei X., Yang X. Roles of ABCA1 in cancer. Oncol. Lett. 2022;24(4):349. doi: 10.3892/ol.2022.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen C.Z., Wen J.L., Lin B.Y., Zheng C., Quan R.D., Zhang X.H., Qu J.M. LOC389641 promotes papillary thyroid cancer progression by regulating the EMT pathway. Biomark Med. 2020;14(11):969–980. doi: 10.2217/bmm-2020-0080. [DOI] [PubMed] [Google Scholar]
- 112.Ji T., Ma K., Wu H., Cao T. A substance P (SP)/Neurokinin-1 receptor Axis promotes perineural invasion of pancreatic cancer and is affected by lncRNA LOC389641. J Immunol Res. 2022 doi: 10.1155/2022/5582811. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiao L., Li Y., Zeng X., Zhou Z., Hu S., Zhang S., Zhou Y., Zhang Z., Zhao H., Zhao H., et al. Silencing of LOC389641 impairs cell proliferation and induces autophagy via EGFR/MET signaling in lung adenocarcinoma. Aging (Albany NY) 2020;13(2):2539–2552. doi: 10.18632/aging.202286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dai W., Jin X., Han L., Huang H., Ji Z., Xu X., Tang M., Jiang B., Chen W. Exosomal lncRNA DOCK9-AS2 derived from cancer stem cell-like cells activated Wnt/β-catenin pathway to aggravate stemness, proliferation, migration, and invasion in papillary thyroid carcinoma. Cell Death Dis. 2020;11(9):743. doi: 10.1038/s41419-020-02827-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang R., Hardin H., Huang W., Chen J., Asioli S., Righi A., Maletta F., Sapino A., Lloyd R.V. MALAT1 long non-coding RNA expression in thyroid tissues: analysis by in situ hybridization and real-time PCR. Endocr. Pathol. 2017;28(1):7–12. doi: 10.1007/s12022-016-9453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goyal B., Yadav S.R.M., Awasthee N., Gupta S., Kunnumakkara A.B., Gupta S.C. Diagnostic, prognostic, and therapeutic significance of long non-coding RNA MALAT1 in cancer. Biochim. Biophys. Acta Rev. Canc. 2021;1875(2) doi: 10.1016/j.bbcan.2021.188502. [DOI] [PubMed] [Google Scholar]
- 117.Matboli M., Kamel M.M., Essawy N., Bekhit M.M., Abdulrahman B., Mohamed G.F., Eissa S. Identification of novel insulin resistance related ceRNA network in T2DM and its potential editing by CRISPR/Cas9. Int. J. Mol. Sci. 2021;22(15) doi: 10.3390/ijms22158129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bak R.O., Hollensen A.K., Mikkelsen J.G. Managing microRNAs with vector-encoded decoy-type inhibitors. Mol. Ther. 2013;21(8):1478–1485. doi: 10.1038/mt.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Patil M.R., Bihari A. A comprehensive study of p53 protein. J. Cell. Biochem. 2022;123(12):1891–1937. doi: 10.1002/jcb.30331. [DOI] [PubMed] [Google Scholar]
- 120.Zawacka-Pankau J., Kostecka A., Sznarkowska A., Hedström E., Kawiak A. p73 tumor suppressor protein: a close relative of p53 not only in structure but also in anti-cancer approach? Cell Cycle. 2010;9(4):720–728. doi: 10.4161/cc.9.4.10668. [DOI] [PubMed] [Google Scholar]
- 121.Wu E.H., Wu K.K., Wong Y.H. Tuberin: a stimulus-regulated tumor suppressor protein controlled by a diverse array of receptor tyrosine kinases and G protein-coupled receptors. Neurosignals. 2006;15(5):217–227. doi: 10.1159/000101333. [DOI] [PubMed] [Google Scholar]
- 122.Thomas M., Lange-Grünweller K., Dayyoub E., Bakowsky U., Weirauch U., Aigner A., Hartmann R.K., Grünweller A. PEI-complexed LNA antiseeds as miRNA inhibitors. RNA Biol. 2012;9(8):1088–1098. doi: 10.4161/rna.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hao Y., Baker D., Ten Dijke P. TGF-β-Mediated epithelial-mesenchymal transition and cancer metastasis. Int. J. Mol. Sci. 2019;20(11) doi: 10.3390/ijms20112767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peinado H., Quintanilla M., Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J. Biol. Chem. 2003;278(23):21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- 125.Babaei G., Aziz S.G., Jaghi N.Z.Z. EMT, cancer stem cells and autophagy; the three main axes of metastasis. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110909. [DOI] [PubMed] [Google Scholar]
- 126.Shash L.S., Ibrahim R.A., Elgohary S.A. E-Cadherin and N-cadherin immunohistochemical expression in proliferating urothelial lesions: potential novel cancer predictive EMT profiles. Appl. Immunohistochem. Mol. Morphol. 2021;29(9):657–666. doi: 10.1097/PAI.0000000000000940. [DOI] [PubMed] [Google Scholar]
- 127.Na T.Y., Schecterson L., Mendonsa A.M., Gumbiner B.M. The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc Natl Acad Sci U S A. 2020;117(11):5931–5937. doi: 10.1073/pnas.1918167117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carling T., Udelsman R. Thyroid cancer. Annu. Rev. Med. 2014;65:125–137. doi: 10.1146/annurev-med-061512-105739. [DOI] [PubMed] [Google Scholar]
- 129.Zhang K., Lv J., Peng X., Liu J., Li C., Li J., Yin N., Li H., Li Z. Down-regulation of DANCR acts as a potential biomarker for papillary thyroid cancer diagnosis. Biosci. Rep. 2019;39(4) doi: 10.1042/BSR20181616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lan X., Sun W., Zhang P., He L., Dong W., Wang Z., Liu S., Zhang H. Downregulation of long noncoding RNA NONHSAT037832 in papillary thyroid carcinoma and its clinical significance. Tumour Biol. 2016;37(5):6117–6123. doi: 10.1007/s13277-015-4461-4. [DOI] [PubMed] [Google Scholar]
- 131.Kim D., Yu J., Kim J., Hwang Y.A., Kim J.K., Ku C.R., Yoon J.H., Kwak J.Y., Nam K.H., Lee E.J. Use of long non-coding RNAs for the molecular diagnosis of papillary thyroid cancer. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.924409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu S.C., Chi S.Y., Rau C.S., Kuo P.J., Huang L.H., Wu Y.C., Wu C.J., Lin H.P., Hsieh C.H. Identification of circulating biomarkers for differentiating patients with papillary thyroid cancers from benign thyroid tumors. J. Endocrinol. Invest. 2021;44(11):2375–2386. doi: 10.1007/s40618-021-01543-2. [DOI] [PubMed] [Google Scholar]
- 133.Rogucki M., Buczyńska A., Krętowski A.J., Popławska-Kita A. The importance of miRNA in the diagnosis and prognosis of papillary thyroid cancer. J. Clin. Med. 2021;10(20) doi: 10.3390/jcm10204738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gambardella V., Tarazona N., Cejalvo J.M., Lombardi P., Huerta M., Roselló S., Fleitas T., Roda D., Cervantes A. Personalized medicine: recent progress in cancer therapy. Cancers. 2020;12(4) doi: 10.3390/cancers12041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.van de Haar J., Hoes L., Voest E. Advancing molecular tumour boards: highly needed to maximise the impact of precision medicine. ESMO Open. 2019;4(2) doi: 10.1136/esmoopen-2019-000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stricker T., Catenacci D.V., Seiwert T.Y. Molecular profiling of cancer--the future of personalized cancer medicine: a primer on cancer biology and the tools necessary to bring molecular testing to the clinic. Semin. Oncol. 2011;38(2):173–185. doi: 10.1053/j.seminoncol.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 137.Verma M. Personalized medicine and cancer. J. Personalized Med. 2012;2(1):1–14. doi: 10.3390/jpm2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Data will be made available on request.