Abstract
Neurofibromatosis type 1 (NF1) is an autosomally dominant tumor suppressor syndrome and multisystem disease. Central giant-cell granulomas (CGCGs) can be seen in patients with NF1. A 21-year-old female was diagnosed with two CGCGs, one in the mandible and then one in the maxilla, in a 7-year period. Increased incidence of CGCGs in NF1 patients was thought to be caused by an underlying susceptibility to developing CGCG-like lesions in qualitatively abnormal bone, such as fibrous dysplasia. However, germline and somatic truncating second-hit mutations in the NF1 gene have been detected in NF1 patients with CGCGs, validating that they are NF1-associated lesions. Oral manifestations in patients with NF1 are very common. Knowledge of these manifestations and the genetic link between NF1 and CGCGs will enhance early detection and enable optimal patient care.
Keywords: Neurofibromatosis type 1, Central giant-cell granuloma, Cone-beam computed tomography
I. Introduction
Neurofibromatosis type 1 (NF1) is an autosomally dominant tumor suppressor syndrome, an inherited and multisystem disease that affects about 1 in 3,000 children at birth, regardless of ethnicity, sex, or race1. It is also known as Von Recklinghausen’s disease and is one of the most common hereditary neurocutaneous disorders in humans, causing a predisposition to benign and malignant neoplasms2. This disease is caused by a spectrum of mutations affecting the NF1 gene (located at 17q11.2) that encodes for a tumor-suppressor protein called neurofibromin1,3. About 50% of NF1 cases occur in patients with unaffected parents, and they are believed to represent spontaneous mutations4. The heterogeneity of NF1 mutations and relatively high rate of new mutations makes molecular testing difficult. However, in most cases, a diagnosis of NF1 can be based on clinical diagnostic criteria that were originally established by the 1987 National Institutes of Health Neurofibromatosis Consensus Development Conference.
Clinically, NF1 is characterized by café-au-lait spots, freckling, skin neurofibromas, plexiform neurofibromas, bony lesions (present in 50% to 70% of patients) that can affect the entire skeleton, Lisch nodules, optic gliomas, and tumors of the central nervous system2,5. There is great phenotypic variability, with 50% of patients presenting with only minor manifestations5.
Neurofibromas are benign soft-tissue tumors, and they are the most common tumor of NF1, seen in about 60% of patients. Plexiform neurofibromas are internal neurofibromas that have an increased rate of malignant transformation. They are seen in 2%-16% of patients and are the leading cause of death in NF1 patients. Other types of non-neurologic cancers are also seen in NF1 patients with greater frequency than in the general population3,4.
Oral manifestations of NF1, including enlarged fungiform papillae, macroglossia, enlarged mandibular foramen/canal and mental foramen, osseous dysplasia, branched mandibular canal, widening of the coronoid notch, deformity of the mandibular condylar head, lengthening of the condylar neck, irregularity of the inferior mandibular cortex, intrabony cyst-like lesions, and thinning and lateral bowing of the ramus, can be found in 72% of patients4,6. Recently, central giant-cell granulomas (CGCGs), especially in the mandible, have been described as a common entity in patients with NF1. Both germline and somatic truncating second-hit mutations in the NF1 gene have been detected in CGCGs from NF1 patients1-3, validating that these are NF1-associated lesions7.
CGCGs are benign central lesions of bone with a variably aggressive nature. They represent a non-neoplastic, reactive process that mostly involves the jaws, particularly the mandible. They are characterized by aggregates of multinucleated giant cells on a background of cellular vascular fibrous connective tissue and spindle-shaped mononuclear stromal cells, often with extravasation of red blood cells. CGCGs account for about 7% of all benign tumors of the jaw, usually appear in the second decade of life, and the incidence in females compared with males is 2:1. Currently no reliable criteria are available for correlating clinical aggressiveness with histological presentation, so aggressiveness is mostly determined by radiographic presentation and clinical symptoms. Recurrences are common, with an estimated 5-year disease-free success rate of only 75% following conventional surgical therapy. However, the recurrence rate depends on the aggressiveness of the lesion and the treatment performed4,8-12.
Most CGCGs present as a painless, slow-growing swelling of the jaw, most commonly on the mandible and anterior to the first molars. Pain and sensory disturbance are rare, but intraoral swelling, sometimes with a bluish-brown aspect, can be present. Radiological findings are diverse, ranging from small radiolucent unilocular lesions to large multilocular lesions with granular and wispy septa. They have well-defined borders with varying degrees of cortical expansion and cortical perforation. They usually displace teeth or tooth germs and resorb roots (which is a radiographic sign of aggressiveness). Radiographically, the top differential diagnoses for this entity are ameloblastoma, odontogenic myxoma, and aneurysmal bone cyst13,14.
Here, we present the case of a patient with NF1 who had a CGCG in the mandible, with 7 years of follow up since the onset of symptoms.
II. Case Report
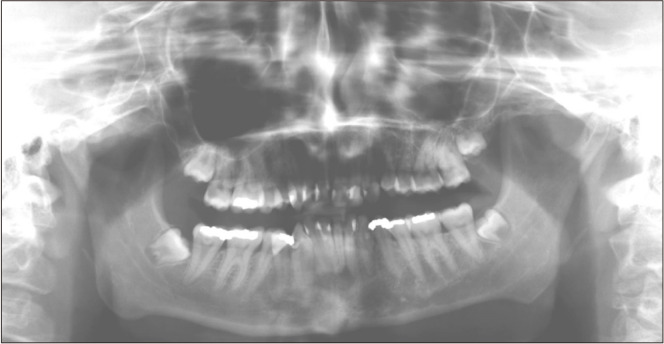
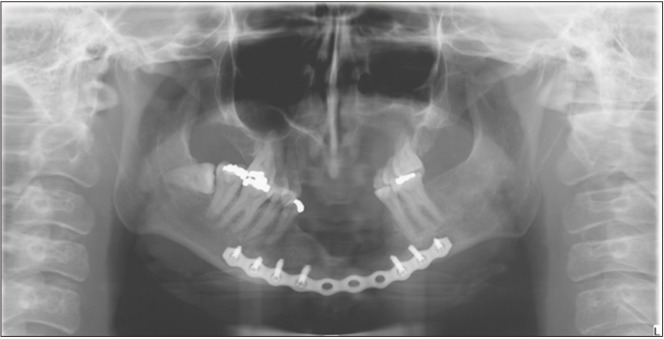
In January 2016, a 21-year-old female with a relevant medical/dental history of attention-deficit/hyperactivity disorder, depression, obsessive compulsive disorder, autism, developmental delay, hypothyroidism, congenital heart defect, NF1, and multiple carious teeth, presented to the University of Florida (UF) College of Dentistry with a chief complaint of facial swelling and pain lasting more than 24 hours. During the extraoral evaluation, the patient experienced tenderness to palpation on the mandibular region, swelling of the angle and submandibular area, and slight pain during wide opening. Intraorally, there was no evidence of swelling. A panoramic radiograph was analyzed by her general dentist, who did not find the cause of the swelling.(Fig. 1) The partially impacted tooth 3.8 was considered the source of pain and extracted. Pain and anti-inflammatory medication were prescribed, and the pain eventually subsided.
Fig. 1.
First panoramic radiograph (2016).
On the following appointment in February 2017, the patient presented to the UF College of Dentistry with multiple new carious lesions and poor dental hygiene. Due to financial concerns, restoration and extraction of teeth 1.8, 1.7, 1.3-2.2, 2.5-3.7, and 3.3-4.3 were performed. Later that year, maxillary and mandibular partial dentures were fabricated for the patient. Over the span of more than a year, the patient complained of an ill-fitting and painful lower denture. Subsequently, implants in the mandibular edentulous region were planned to support a fixed prosthesis. Cone-beam computed tomography (CBCT) was conducted for implant planning.
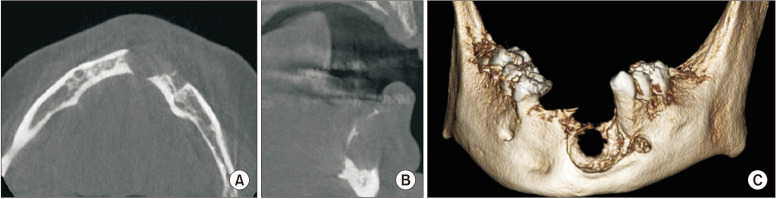
A CBCT analysis (Fig. 2, 3) was performed in March 2019, approximately 3 years after the initial visit and the initial panoramic radiograph. Upon review by an oral and maxillofacial radiologist, the CBCT revealed a well-defined, multiloculated, radiolucent entity in the left parasymphyseal region. This entity extended from tooth 3.5 to the mandibular symphysis and from the alveolar crest to the inferior third of the mandible. The alveolar crest and buccal cortex were thinned and mainly resorbed. The root of 3.5 was distally displaced without evidence of root resorption. The surrounding bone was sclerotic. A thin septum dividing the lesion from the left mental foramen was noted. The left inferior alveolar canal was slightly enlarged compared with the contralateral side. Given the radiographic appearance and history of NF1, a CGCG and neurofibroma were considered as differential diagnoses. Widening of the periodontal ligament and root resorption was also noted on tooth 2.3.(Fig. 4) A histopathological evaluation was recommended for the mandibular lesion and the lesion associated with tooth 2.3.
Fig. 2.
Cone-beam computed tomography panoramic reconstruction of the mandibular lesion.
Fig. 3.
Cone-beam computed tomography axial (A), cross-sectional (B), and volume rendering (C) views of the mandibular lesion.
Fig. 4.
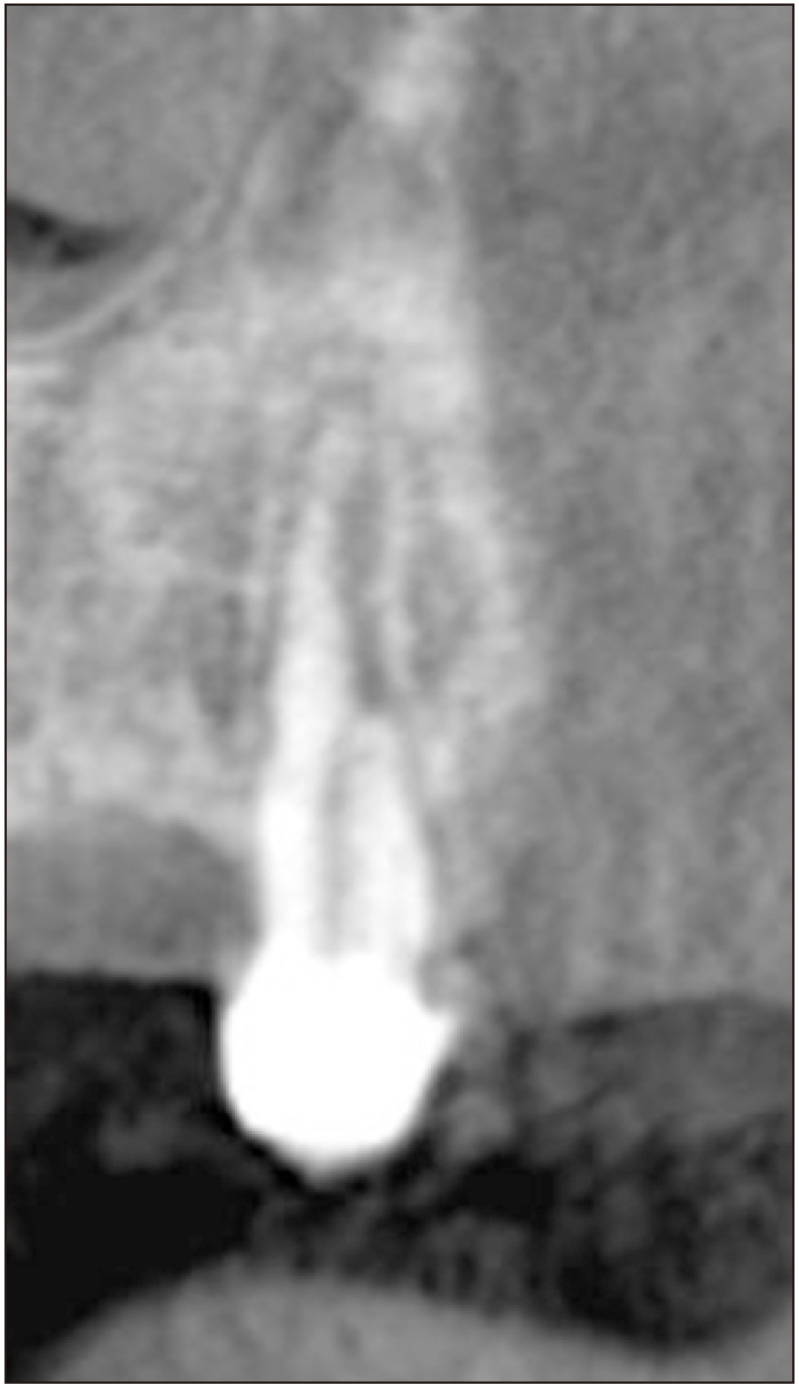
Coronal cone-beam computed tomography slice of tooth 2.3.
The biopsy result confirmed the mandibular entity to be a CGCG, whereas the lesion associated with tooth 2.3 was a periapical granuloma. Surgical removal of the CGCG and extraction of tooth 2.3 were recommended. However, due to endocrinology concerns about the anesthesia, surgery was postponed, and intralesional steroid injections were recommended to shrink the CGCG until the patient could obtain medical clearance for surgery. However, the patient refused the intralesional injections.
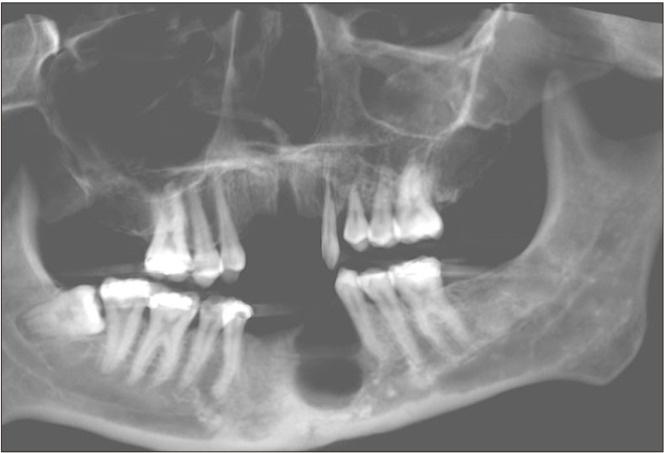
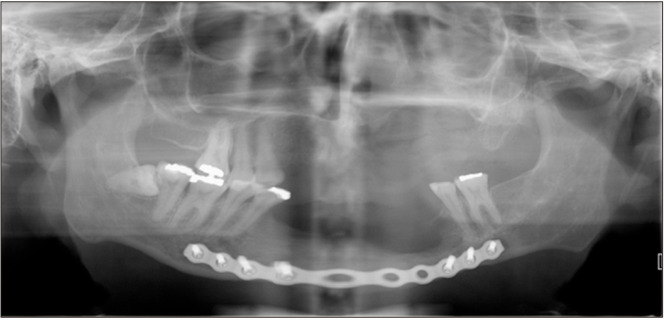
In May 2020, the patient obtained clearance for surgery. A panoramic radiograph was taken (Fig. 5), and both the lesion associated with tooth 2.3 and the mandibular lesion had increased considerably in size. The surgical intervention was performed in June 2020 and included enucleation and curettage of the CGCG, extraction of teeth 2.3 and 3.5, and placement of a mandibular reconstruction plate and a bone graft in the left parasymphyseal region of the mandible.
Fig. 5.
Panoramic radiograph from 2020.
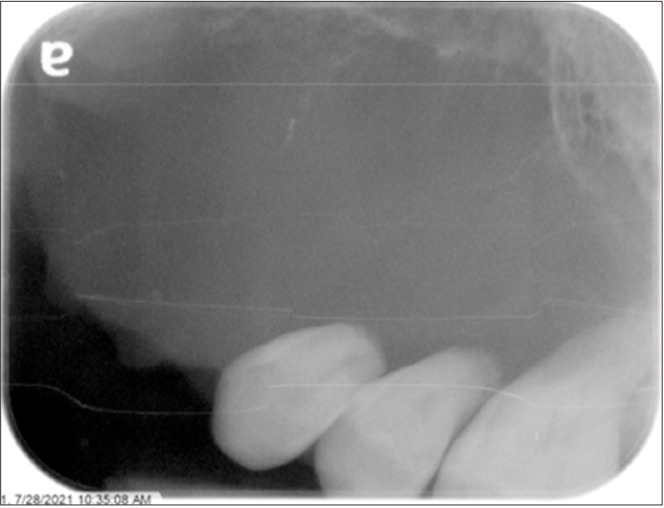
In July 2021, the patient came back complaining of spontaneous pain in the upper left quadrant and mild left facial swelling. Periapical and panoramic radiographs were performed (Fig. 6, 7), and a well-defined, expansile, soft tissue-density lesion was noted in the left maxilla. It extended from the site of the missing 2.3 to the mesial of tooth 2.6 and from the alveolar crest to the floor of the left nasal cavity and maxillary sinus, which appeared to be superiorly displaced. Significant root resorption was noted on teeth 2.4 to 2.6. Multi-detector computed tomography (MDCT) with contrast was requested to better evaluate the lesion.
Fig. 6.
Periapical radiograph of the right maxilla performed in July 2021.
Fig. 7.
Panoramic radiograph performed in July 2021.
The MDCT revealed a 30 mm×25 mm mass involving the left side of the maxilla and hard palate supplied by branches of the distal lingual artery and the facial artery. The radiographic appearance was most consistent with a benign or malignant tumor, until proven otherwise.
A biopsy of the site revealed a large proliferation of multinucleated giant cells distributed in a highly cellular and vascular fibrous connective tissue stroma. Numerous multinucleated giant cells exhibited multiple hyperchromatic, round to ovoid nuclei with abundant eosinophilic cytoplasm, surrounded by trabecular viable bone that showed resorption and infiltration by the giant cells. The diagnosis of CGCG was confirmed.
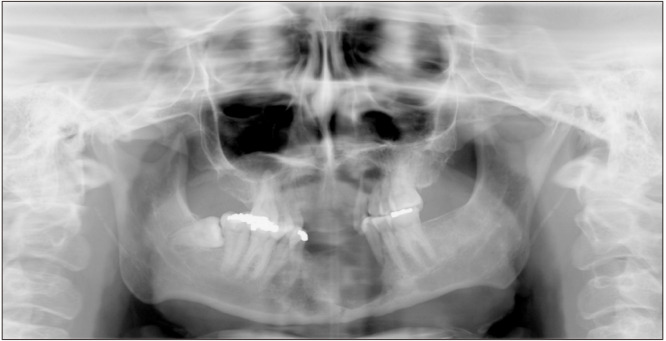
Enucleation and curettage of the left maxillary CGCG and the extraction of teeth 2.4 to 2.6 were performed in February 2022. A panoramic radiograph was obtained immediately after surgery.(Fig. 8) The postoperative biopsy of the specimen confirmed the preoperative diagnosis of CGCG.
Fig. 8.
Postoperative panoramic radiograph.
III. Discussion
NF1 is an inherited disorder caused by a spectrum of mutations affecting the NF1 gene. Increased incidence of CGCGs in the mandible or maxilla of NF1 patients is documented4,5. However, in the past it was thought to be a coincidental association or to reflect an underlying susceptibility of NF1 patients to developing CGCG-like lesions in qualitatively abnormal bone, such as fibrous dysplasia4. However, a genetic link has been demonstrated: a second mutation on the NF1 gene of neoplastic spindle-shaped cells in CGCGs has demonstrated the genetic association between the two conditions1.
In our case, an oral and maxillofacial radiologist detected the mandibular CGCG on a CBCT requested for implant planning purposes. The mandibular lesion with subtle radiographic changes was not documented by the dentist when the first panoramic radiograph was taken three years prior. In the Fig. 1, we can see a tortuous anterior third of the left inferior alveolar canal with a radiolucency apical to tooth 3.3. An additional radiolucent region can be seen on the apical portion of the mandibular incisors. However, the latter could also represent an artifact due to improper patient positioning. The presence of a palatoglossal airspace precluded the evaluation of tooth 2.3.
At this time, the lesion associated with tooth 2.3 was diagnosed as a granuloma, and the tooth was extracted. Given the posterior development of a CGCG in this area, the question of an erroneous initial diagnosis arises.
NF1 is a common genetic disease with extremely variable expressivity. Oral manifestations are very common, so dentists should be aware of the characteristics of this disease. A knowledge of these manifestations will enhance early detection and enable optimal patient care.
CGCGs can be classified as aggressive or non-aggressive based on modified criteria proposed by Chuong and colleagues and Kaban and colleagues and explained by Chrcanovic et al.9. Lesions that present at least 3 of the features listed in Table 1 are classified as aggressive CGCGs. In our case, the mandibular CGCG met 3 of the criteria (tooth displacement, cortical bone thinning, and cortical perforation) and was considered aggressive. The maxillary CGCG met all criteria except size larger than 5 cm and was also considered aggressive. The distinction between aggressive and non-aggressive behaviors is critical for establishing the correct treatment plan.
Table 1.
Central giant-cell granulomas (CGCG) aggressiveness criteria9
| CGCG aggressiveness features (must have 3 of the following to be considered aggressive) |
| 1. Size >5 cm |
| 2. Rapid growth |
| 3. Root resorption |
| 4. Tooth displacement |
| 5. Cortical bone thinning |
| 6. Perforation of the cortical bone |
At present, surgical curettage is the therapy most frequently applied for CGCGs, with recurrence rates of about 9% and 37.2% for non-aggressive and aggressive CGCGs, respectively. Higher recurrence rates have been seen in young patients, especially young males. However, no difference in recurrence was noted in the maxilla versus the mandible. The combination of curettage and enucleation, which was the surgical approach performed in our patient, has an 11.8% and 22.2% rate of recurrence for non-aggressive and aggressive lesions, respectively. The high recurrence rates are the major drawback of curettage and enucleation10. A more radical surgical intervention, such as segmental resection, has lower recurrence and higher success rates. However, it is associated with morbidities such as increasing the risk of damage to vital structures, facial disfigurement, and functional impairment due to bone destruction9.
Intralesional injections of corticosteroids have been considered a conservative and effective alternative approach, aiming to minimize the unintended consequences of invasive surgical procedures, and they have been shown to work efficiently in non-aggressive lesions11. Although injections are less effective in aggressive entities, a 2018 study by Chrcanovic et al.9 reported that out of 33 aggressive CGCGs, 18 (54.5%) showed complete regression, 6 (18.2%) showed partial regression, and 9 (27.3%) were non-responsive to intralesional injections of corticosteroids. It has been established in the literature that mandibular CGCGs in young patients respond better to intralesional corticoid injections than lesions in older patients or in the maxilla15. In 2016, Dolanmaz et al.10 published a review of the literature regarding intralesional injections of corticosteroids, and they concluded that this treatment was safe and effective for CGCGs, especially non-aggressive ones.
The scientific literature indicates that aggressive CGCGs recur more often than non-aggressive lesions. Even though CGCGs sometimes show poor response to corticoid injections or surgical curettage, a combination of both treatment strategies should be considered in aggressive cases to reduce the morbidities associated with radical surgery. The best protocol for managing aggressive and non-aggressive CGCG lesions remains to be determined9,13,16. In our patient, the surgical intervention was postponed due to anesthetic concerns, and intralesional injections of corticosteroid were recommended to shrink the lesion before surgery. However, the patient refused this alternative and preferred to wait for clearance for the surgical intervention.
Last, it is interesting to mention that the gene responsible for NF1 encodes neurofibromin, which is a tumor suppressor protein, the loss of which leads to an increased risk of developing tumors. Thus, in 2016, Friedrich et al.1 proposed that CGCGs have a tumoral origin instead of being reactive lesions when they are associated with syndromes such as NF1.
In conclusion, CGCGs are rare, benign, intraosseous lesions with aggressive potential that can be locally destructive. Aggressive CGCGs recur more often than non-aggressive ones. Although they sometimes show a poor response to corticoid injections or surgical curettage, a combination of those treatment strategies should be considered in aggressive cases to reduce the morbidities associated with radical surgery.
CGCGs commonly occur in patients with NF1, and all dental radiographs from NF1 patients should be systematically analyzed for any neural or giant-cell lesions. Dentists, especially oral and maxillofacial radiologists, need to be aware of this genetic predisposition so they can offer prompt, accurate diagnoses. Early detection and systematic radiographic follow-ups can enable the use of the least invasive treatment options.
Footnotes
Authors’ Contributions
M.B.G. wrote the manuscript, collected data, participated in study design and drafted the manuscript. A.G. and C.D.M. participated in study design, coordination and manuscript drafting. R.J. and J.H. participated in study design and coordination. V.D. participated in data collection. All authors read and approved the final manuscript.
Funding
No funding to declare.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Friedrich RE, Grob TJ, Hollants S, Zustin J, Spaepen M, Mautner VF, et al. Recurrent multilocular mandibular giant cell granuloma in neurofibromatosis type 1: evidence for second hit mutation of NF1 gene in the jaw lesion and treatment with curettage and bone substitute materials. J Craniomaxillofac Surg. 2016;44:1054–60. doi: 10.1016/j.jcms.2016.05.010. https://doi.org/10.1016/j.jcms.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Chrcanovic BR, Gomez RS, Freire-Maia B. Neurofibromatosis type 1 associated with bilateral central giant cell granuloma of the mandible. J Craniomaxillofac Surg. 2011;39:538–43. doi: 10.1016/j.jcms.2010.10.014. https://doi.org/10.1016/j.jcms.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Friedrich RE, Mautner VF, Scheuer HA. Loss of heterozygosity in tumor cells of a recurrent mandibular giant cell granuloma in neurofibromatosis type 1. Anticancer Res. 2007;27:2079–83. [PubMed] [Google Scholar]
- 4.Edwards PC, Fantasia JE, Saini T, Rosenberg TJ, Sachs SA, Ruggiero S. Clinically aggressive central giant cell granulomas in two patients with neurofibromatosis 1. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:765–72. doi: 10.1016/j.tripleo.2005.10.038. https://doi.org/10.1016/j.tripleo.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 5.Ruggieri M, Pavone V, Polizzi A, Albanese S, Magro G, Merino M, et al. Unusual form of recurrent giant cell granuloma of the mandible and lower extremities in a patient with neurofibromatosis type 1. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:67–72. doi: 10.1016/S1079-2104(99)70297-0. https://doi.org/10.1016/s1079-2104(99)70297-0. [DOI] [PubMed] [Google Scholar]
- 6.D'Ambrosio JA, Langlais RP, Young RS. Jaw and skull changes in neurofibromatosis. Oral Surg Oral Med Oral Pathol. 1988;66:391–6. doi: 10.1016/0030-4220(88)90252-6. https://doi.org/10.1016/0030-4220(88)90252-6. [DOI] [PubMed] [Google Scholar]
- 7.Stewart DR, Brems H, Gomes AG, Ruppert SL, Callens T, Williams J, et al. Jaffe-Campanacci syndrome, revisited: detailed clinical and molecular analyses determine whether patients have neurofibromatosis type 1, coincidental manifestations, or a distinct disorder. Genet Med. 2014;16:448–59. doi: 10.1038/gim.2013.163. https://doi.org/10.1038/gim.2013.163. [DOI] [PubMed] [Google Scholar]
- 8.Krammer U, Wimmer K, Wiesbauer P, Rasse M, Lang S, Müllner-Eidenböck A, et al. Neurofibromatosis 1: a novel NF1 mutation in an 11-year-old girl with a giant cell granuloma. J Child Neurol. 2003;18:371–3. doi: 10.1177/08830738030180051901. https://doi.org/10.1177/08830738030180051901. [DOI] [PubMed] [Google Scholar]
- 9.Chrcanovic BR, Gomes CC, Gomez RS. Central giant cell lesion of the jaws: an updated analysis of 2270 cases reported in the literature. J Oral Pathol Med. 2018;47:731–9. doi: 10.1111/jop.12730. https://doi.org/10.1111/jop.12730. [DOI] [PubMed] [Google Scholar]
- 10.Dolanmaz D, Esen A, Mihmanlı A, Işık K. Management of central giant cell granuloma of the jaws with intralesional steroid injection and review of the literature. Oral Maxillofac Surg. 2016;20:203–9. doi: 10.1007/s10006-015-0530-5. https://doi.org/10.1007/s10006-015-0530-5. [DOI] [PubMed] [Google Scholar]
- 11.Batista Severo ML, de Sousa Lopes MLD, da Costa Miguel MC, Germano AR, Nogueira RLM, Turatti E, et al. Immunoexpression of calcitonin and glucocorticoid receptors in central giant cell lesions of the jaws. J Oral Pathol Med. 2018;47:907–13. doi: 10.1111/jop.12766. https://doi.org/10.1111/jop.12766. [DOI] [PubMed] [Google Scholar]
- 12.Moura LB, Tarquinio SBC, Gomes APN, Schinestsck AR, Torriani MA. Modified approach to central giant cell lesion. J Clin Pediatr Dent. 2018;42:292–4. doi: 10.17796/1053-4628-42.4.9. https://doi.org/10.17796/1053-4628-42.4.9. [DOI] [PubMed] [Google Scholar]
- 13.de Lange J, van den Akker HP, van den Berg H. Central giant cell granuloma of the jaw: a review of the literature with emphasis on therapy options. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:603–15. doi: 10.1016/j.tripleo.2007.04.003. https://doi.org/10.1016/j.tripleo.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Koenig LJ, Tamimi D, Petrikowski CG, Perschbacher SE, Ruprecht A, Benson BW, et al. Diagnostic imaging: oral and maxillofacial. 2nd ed. Elsevier; 2017. [Google Scholar]
- 15.Yanik S, Aras MH. Management of central giant cell granuloma of mandible using intralesional corticosteroids: case report and review of literature. J Oral Maxillofac Surg. 2013;71:721–2. doi: 10.1016/j.joms.2012.10.013. https://doi.org/10.1016/j.joms.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 16.de Mendonça RP, Mitre GP, Real FH, da Silva Kataoka MS, de Melo Alves Júnior S, Vianna P, et al. Central giant cell granuloma treated with intralesional corticosteroid injections and bisphosphonates: a long-term follow-up case study. Head Neck Pathol. 2020;14:497–502. doi: 10.1007/s12105-019-01053-x. https://doi.org/10.1007/s12105-019-01053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]