Abstract
Alterations in DNA methylation play an important pathophysiological role in the development and progression of colorectal cancer. We comprehensively profiled DNA methylation alterations in 165 Korean patients with colorectal cancer (CRC), and conducted an in-depth investigation of cancer-specific methylation patterns. Our analysis of the tumor samples revealed a significant presence of hypomethylated probes, primarily within the gene body regions; few hypermethylated sites were observed, which were mostly enriched in promoter-like and CpG island regions. The CpG Island Methylator Phenotype-High (CIMP-H) exhibited notable enrichment of microsatellite instability-high (MSI-H). Additionally, our findings indicated a significant correlation between methylation of the MLH1 gene and MSI-H status. Furthermore, we found that the CIMP-H had a higher tendency to affect the right-side of the colon tissues and was slightly more prevalent among older patients. Through our methylome profile analysis, we successfully verified the methylation patterns and clinical characteristics of Korean patients with CRC. This valuable dataset lays a strong foundation for exploring novel molecular insights and potential therapeutic targets for the treatment of CRC.
Keywords: Colorectal cancer, CpG island methylator phenotype (CIMP), DNA methylation, MutL homolog 1 (MLH1)
INTRODUCTION
Colorectal cancer (CRC) is the third most diagnosed cancer and the second leading cause of cancer-related deaths worldwide (1). Epigenetic alterations play a crucial role in the pathogenesis of CRC (2), and increasing evidence suggests that epigenetic changes induced by bacteria and host diet play an important role in the carcinogenesis and prognosis of CRC (3, 4). One oncogenic mechanism is hypermethylation of promoter regions that cause epigenetic silencing of tumor suppressor genes; however, genome-wide hypomethylation is often observed in patients with CRC (5). Nevertheless, little is known about the role of global hypomethylation at CpG sites outside gene promoters in cancer development (6).
Epigenetic alterations are often accompanied by altered gene expressions. The occurrence of CRC is predominantly attributed to chromosomal instability (CIN), which is closely related to global hypomethylation (7). About 10-15% of CRCs are caused by a defect in the DNA mismatch repair (MMR) system, which can arise from genetic mutations in MMR genes or silencing of MLH1 gene due to hypermethylation of their promoter regions (8). Microsatellite instability (MSI) is a genetic condition characterized by increased susceptibility to mutations due to impaired DNA-MMR mechanisms. CRCs exhibiting high MSI (MSI-H) are associated with a distinct clinical and pathological profile (9). CpG island methylator phenotype (CIMP) is a subset of CRCs distinguished by the inactivation of tumor suppressor genes, caused by hypermethylation of promoter CpG island sites; CIMP is known to have substantial effects on the development, progression, and prognosis of CRC (10-13). Presence of CIMP in CRC has been associated with distinct clinical and molecular features, and it is typically seen in association with MSI and BRAF mutations (14). Hence, DNA methylation has emerged as a potential predictive biomarker for therapeutic interventions, showing significant correlations with treatment outcomes in some studies (15, 16). However, majority of the available evidence was derived from small-sized studies.
In this study, we analyzed a comprehensive collection of methylation datasets containing clinical and molecular information. We aimed to obtain an integrated methylome profile that can be used for future research. These data are expected to have a variety of applications, ranging from deciphering the molecular characteristics of cancer subtypes to exploring novel biomarkers, identifying prognostic signatures, and analyzing treatment-related variations.
RESULTS
Clinical characteristics of patients with CRC
We analyzed 165 tumors from Korean patients with CRC who underwent surgical resection in South Korea, and matched them with normal samples. The patient characteristics are presented in Table 1 and Supplementary Fig. 1. The median age of the patients was 63 years (range: 23-90 years), where more than half of the patients (N = 96, 58.2%) were aged > 60 years. Ninety-eight (59.4%) patients were male, and approximately two-thirds of the patients had tumors on the left side (N = 64, 38.8%) at stage III (N = 107, 65%). Most patients had moderately differentiated adenocarcinoma (N = 107, 64.8%), and 15 patients (9.1%) had mucinous adenocarcinoma. Most patients had tumors with microsatellite stability (MSS) (N = 132, 80%); 27 (16.4%) patients had tumors with high MSI (MSI-H).
Table 1.
Clinical characteristics of 165 CRC patients
| Clinicopathological factors | Number of subject (%) |
|---|---|
| Sex | |
| Female | 67 (40.6) |
| Male | 98 (59.4) |
| Age (median: 63) | |
| ≤ 60 | 69 (41.8) |
| > 60 | 96 (58.2) |
| Location* | |
| Right-sided | 58 (35.2) |
| Left-sided | 64 (38.8) |
| Rectum | 41 (24.9) |
| Synchronous | 2 (1.2) |
| MSI | |
| MSI-H | 27 (16.4) |
| MSI-L | 2 (1.2) |
| MSS | 132 (80) |
| NA | 4 (2.5) |
| Tumor stage (AJCC 8th edition) | |
| I | 10 (6.1) |
| II | 39 (23.6) |
| III | 107 (64.8) |
| IV | 9 (5.5) |
| T stage | |
| II | 15 (9.1) |
| III | 107 (64.8) |
| IV | 43 (26.1) |
| N stage | |
| N0 | 49 (29.7) |
| N1 | 82 (49.7) |
| N2 | 34 (20.6) |
| M stage | |
| M0 | 156 (94.5) |
| M1 | 9 (5.5) |
| Differentiation** | |
| WD | 35 (21.2) |
| MD | 107 (64.8) |
| PD | 8 (4.8) |
| Mucinous | 15 (9.1) |
| Pathology | |
| Adenocarcinoma | 150 (90.9) |
| Mucinous carcinoma | 15 (9.1) |
*Descending, rectosigmoid, sigmoid, splenic flexure tumors are represented as left-sided; Appendix, ascending, cecum, hepatic flexure, ileocecal valve, and transverse tumors or multiple presence of these tumors are represented as right-sided. Synchronous represents the presence of multiple tumors (left- and right-sided). **WD, MD and PD represent Well-Differentiated, Moderately-differentiated, and Poorly-Differentiated, respectively. NA denotes the absence of information.
Preprocessing of DNA methylation
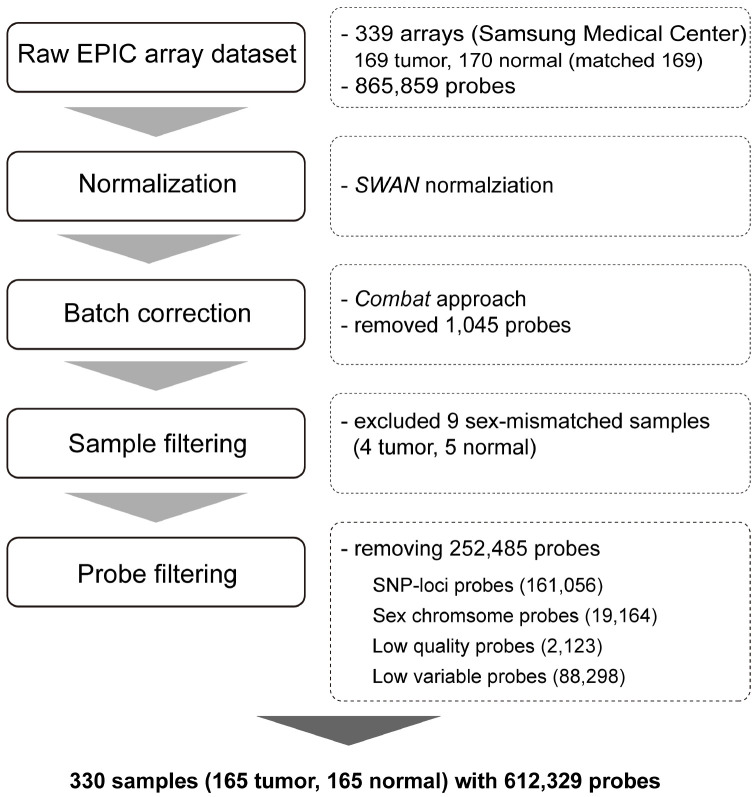
From the methylation EPIC array used, raw intensities of 865,859 probes were obtained for all 165 tumors and matched normal samples. To evaluate the quality of the probes, we examined their overall beta value distribution, control strip plots, bisulfite conversion efficiency, extension quality, and specificity (Supplementary Fig. 2). After array normalization between type I and type II probes, we corrected the data for known batch effects and filtered 1,045 probes using the ComBat method (17, 18). We also removed additional probes, such as those for sex chromosome methylation data (19,164 probes), those for known SNP sites (161,056 probes), those which we failed to identify (2,123 probes) based on P-values of each detection ratio, and those with beta value differences exceeding 0.1 (88,298 probes). The filtering process yielded 612,329 high-quality probes for further analyses (Fig. 1).
Fig. 1.
Schematic representation of preprocessing of EPIC array methylation profile. This study involved the processing of 339 raw EPIC array-based methylation profiles, comprising 169 tumor samples and 170 normal samples. Preprocessing was conducted in accordance with the minfi pipeline. Normalization was facilitated using the subset-quantile within array normalization (SWAN) method, and batch correction was accomplished using the ComBat method. Nine samples that did not match between methylation-based sex prediction and clinical information were excluded. After filtering out the probes with poor performance, SNPs, sex chromosomes, and low-variable sites, the final methylation profiles were composed of 165 tumor and 165 normal samples (164 of which were matched) with the methylation quantification of 612,329 probes.
Methylation patterns in tumors
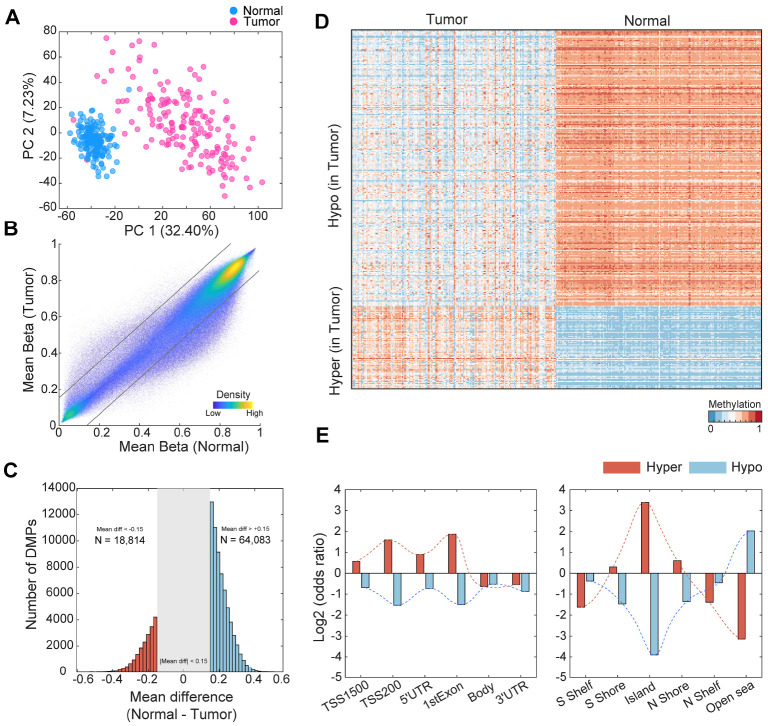
We conducted principal component analysis (PCA) to assess whether well-curated samples and high-quality probe information could effectively distinguish the DNA methylation characteristics between normal and tumor cells. PC1 accounted for 32.4% of the variation in the samples, providing sufficient evidence of intrinsic differences between normal and tumor tissues (Fig. 2A). Next, we performed a comparative analysis of methylation sites in normal and tumor tissues and discovered 82,897 differentially methylated positions (DMP) (19, 20). Hypermethylation was defined when the tumor methylation value was 0.15 higher than that of normal tissue (q-value < 0.000001, 18,814 sites). Conversely, hypomethylation was defined when the tumor methylation value was 0.15 lower than that of normal cells (q-value < 0.000001, 64,083 sites) (Fig. 2B, C). The average methylation pattern was generally higher in normal samples (tumor: 0.5586, normal: 0.5884), whereas hypomethylation was more prevalent in tumor samples (Fig. 2C, D). The overall occurrence of these hypomethylation patterns in tumors was consistent with the association between genomic instability and tumor initiation (Fig. 2C) (21). To analyze the methylation patterns in specific genomic regions, we conducted an enrichment analysis for hyper- and hypomethylation in each region. While gene body regions and 3’ untranslated regions (UTRs) were not hypermethylated, promoter-like regions such as TSS1500, TSS200, 5’ UTR, and first exons harbored a significant number of hypermethylated sites in tumors (Fig. 2E). Furthermore, CpG islands and nearby regions were also highly enriched in hypermethylated sites. In contrast, only open sea regions showed hypomethylation enrichment in tumors (Fig. 2E).
Fig. 2.
Differences in DNA methylation between colon tumor and normal tissues. (A) Principal component plot including data from 612,329 preprocessed cg probes. (B) Scatter plot of mean beta values between normal and tumor samples. Each gray line represents the criteria of differentially methylated probes (|mean difference| < 0.15). (C) Distribution of the number of DMPs between tumor and normal samples. (D) Heatmap of beta values for differentially methylated probes. Used probes were selected based on top 5% of total hyper- and hypomethylated probes according to mean differences between normal and tumor samples. (E) Odds ratios of the number of defined DMPs based on genomic regions. Left: gene body structure, Right: around CpG island.
CIMP subgroup for 165 colon tumor samples
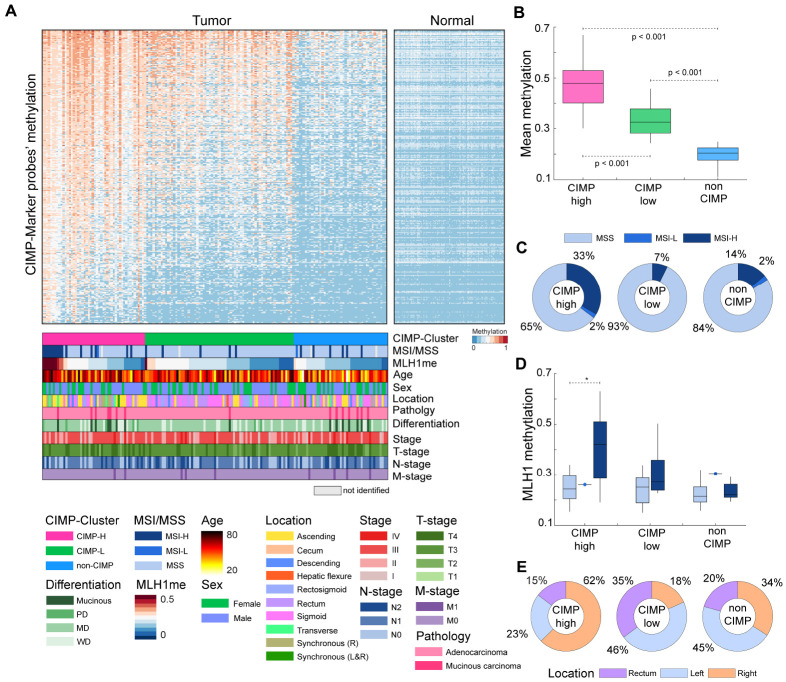
Considering that CpG islands and promoter regions contain multiple hypermethylation sites (Fig. 2E), we conducted a more extensive investigation of CIMP, which is characterized by simultaneous hypermethylation of multiple CpG islands located in the promoter regions of specific genes. Additionally, CIMP is associated with distinct clinicopathological features, including MSI, age, and tumor location (13). For CIMP analysis, we used a set of 4,327 probes of 258 CIMP marker genes (22). Among them, we selected 2,352 highly variable probes from 165 samples (standard deviation > 0.15). Using the K-means algorithm with 100 iterations of these probes, tumor samples were clustered into CIMP-high (CIMP-H; N = 49, 29.7%), CIMP-low (CIMP-L; N = 71, 43%), or non-CIMP (N = 45, 27.3%) groups based on average methylation levels (Fig. 3A); mean of methylation levels in the three groups showed significant differences (t-test, P < 0.001) (Fig. 3B). We then stratified the tumor samples based on CIMP and clinical features (Fig. 3A). Among the CIMP-H clustered patients, 35% exhibited MSI status; in contrast, MSI was detected only in 7% of CIMP-L and 16% of non-CIMP patients (Fig. 3C). This result showed a significant enrichment of patients with MSI-H or MSI-L within the CIMP-H group (Fisher’s exact test, P < 0.001). Subsequently, we investigated the methylation status of MLH1, a gene responsible for DNA mismatch repair. Defects in MLH1 are known to be associated with MSI. Overall, MLH1 methylation levels were higher in CIMP-H (mean methylation: 0.30) than in CIMP-L (mean methylation: 0.25) or non-CIMP (mean methylation: 0.23) groups. Additionally, high correlation was observed between MLH1 methylation and MSI-H in the CIMP-H (Fig. 3D). Among patients with CIMP-H and MSI-H, the mean MLH1 methylation level was 0.41, which was significantly higher than that in patients with CIMP-H and MSS (t-test, P < 0.001). Furthermore, the CIMP status was correlated with patient age and the anatomical location of tumors in the large intestine. In the CIMP-H group, 30 tumors (62%) were collected from the right-sided colon, 11 (23%) from the rectum, and 7 (15%) from left-sided colon (Fig. 3E), without considering synchronous tumors (i.e., presence in both left and right sides). The CIMP-L and non-CIMP contained 13 (18%) and 15 (34%) right-sided colon tumors, respectively. These results revealed a higher enrichment of right-sided colon tumor samples in the CIMP-H (Fisher’s exact test, P < 0.01). When comparing age distribution according to CIMP, the mean age of CIMP-H patients was 65.8 years, which is slightly higher than that of CIMP-L (mean age: 63.7 years), and significantly higher than that of non-CIMP (mean age: 55.8 years) groups (t-test, P < 0.05). Next, we compared the patient’s survival outcome on the basis of CIMP. Patients who were classified as CIMP-H showed a worse prognosis than those classified as non-CIMP (Supplementary Fig. 3A). No significant prognostic differences were observed between CIMP-H and CIMP-L. To further investigate this observation, we identified potential prognostic methylation markers by conducting univariate Cox regression analysis using CIMP-H marker probes. We discovered 80 significant probes capable of differentiating patient outcomes (Supplementary Fig. 3B and Supplementary Table 1). For example, patients who were hypermethylated at cg19074340, located in the WT1 promoter region, had a worse prognosis than those who had hypomethylation at this site. We also compared overall methylation of gene promoters between MSI and MSS within CIMP-H samples. Our observations revealed several genes, including WNT1, SFRP2, NFAR5, and AXIN2, were hypermethylated in MSI-H and were associated with the WNT signaling pathway (Supplementary Fig. 4A). For instance, the promoter of SFRP2 gene was significantly hypermethylated in MSS within CIMP-H (Supplementary Fig. 4B), and the promoter of WNT1 gene was hypermethylated in MSI-H within CIMP-H (Supplementary Fig. 4C).
Fig. 3.
CpG island methylator phenotype (CIMP) subgroups for 165 colon tumors samples. (A) Clustering of colon tumor samples based on CIMP marker probes. Synchronous represents the presence of multiple tumors (L&R: left- and right-sided, R: right-sided). WD, MD, and PD represent Well-Differentiated, Moderately-Differentiated, and Poorly-Differentiated, respectively. (B) Boxplot for patients’ mean CIMP marker probe methylation levels according to CIMP and MSI status. (C) Proportion of MSI and MSS status probes according to CIMP status. (D) Boxplot for the distribution of MLH1 gene methylation according to CIMP and MSI status. Notation “*” represents the significance of comparisons (t-test, P < 0.05). (E) Proportion of tissue location status of patients according to CIMP status. Descending, rectosigmoid, sigmoid, splenic flexure tumors are represented as left-sided; appendix, ascending, cecum, hepatic flexure, ileocecal valve, and transverse tumors are represented as right-sided.
DISCUSSION
In this study, we aimed to obtain integrated methylome profiles for different clinical and pathological subtypes of colorectal cancer. Integrating methylome profiles with patient data will create a valuable resource that will enable interpretation of the molecular mechanisms of methylation changes associated with cancer development (23). Our dataset showed that tumor samples contained a larger number of hypomethylated sites than hypermethylated sites. In addition, promoter-like regions and CpG island regions contained a higher proportion of hypermethylated sites, whereas hypomethylated probes were predominantly located in open sea regions. We clustered Korean patients with CRC into three distinct CIMP classes, namely CIMP-H, CIMP-L, and non-CIMP. The CIMP-H group more frequently developed right-sided colorectal tumors with elevated MLH1 gene methylation and enrichment of MSI patients, consistent with previous findings (24, 25). The CIMP-H group was significantly enriched in MSI, with elevated MLH1 methylation, and a significant relationship between MLH1 methylation and MSI-H was observed. CIMP was also correlated with the anatomical location of the tumors and patient age, with a higher proportion of right-sided colon tumors and slightly older patients in the CIMP-H group.
DNA methylation biomarkers have been associated with advanced disease stages or poor outcomes in patients with CRC, but there is insufficient evidence for their use in routine clinical practice for CRC, given the difficulties in their validation (15, 16, 26). Although a single-medical-center design and the relatively small study population may limit its applicability, our study showed characteristics of age, sex, and anatomical locations similar to those in other studies based on Korea National Cancer Incidence Database (27, 28), which, to some extent, could be representative of the general frequency of Korean patients with CRC. In addition, given the limited reports on the methylome of Korean patients with CRC, our dataset is capable of representing clinical features of CRC in Korea. We also investigated the correlation between prognosis of patients and CIMP; patients with CIMP-H displayed a worse prognosis than those classified as non-CIMP. Using univariate Cox regression models, we identified 80 significant methylation markers, such as cg19074340 in the WT1 gene promoter region, that were strongly associated with patient prognosis. Furthermore, we found that SFRP2 and WNT1 genes with hypermethylated promoters in MSI or MSS subtypes within CIMP-H samples were extensively associated with WNT signaling pathway. This detailed exploration of gene methylation in the context of CIMP enhances our understanding of the complex molecular interactions that drive colorectal cancer prognosis. Further research is warranted to refine these markers and explore their potential therapeutic implications.
Overall, we highlighted the clinical characteristics of Korean patients with CRC and provided insights into the methylation patterns in colon tumors compared with those in normal tissues. These results constitute a representative dataset for validating the relationship between clinical and epigenetic features. In parallel with our efforts to construct a more comprehensive methylome profile, further studies based on this profiling could contribute to the understanding of CRC development and may have implications for molecular characterization, biomarker discovery, and treatment analysis.
MATERIALS AND METHODS
Ethics approval statement
This study was approved by Samsung Medical Center Institutional Review Board (IRB 2017-01-131) and Yonsei University Institutional review board (approval number: 7001988-201910-BR-727-02).
Study population
We collected data from 170 patients who underwent pathological confirmation of colorectal cancer; this involved a detailed procedure. The methylation dataset comprised 339 arrays distributed by Samsung Medical Center. From this total, 169 tumor and 170 normal (matched 169) samples were identified. All normal samples were obtained from the margins of the resected specimens farthest from the tumor site. After excluding four tumor and five normal samples that were mismatched with clinical sex information based on methylome-based criteria, 165 tumor and 165 normal (164 matched) samples were analyzed using the EPIC array and subjected to preprocessing and quality control for downstream analysis.
DNA extraction and EPIC array-based methylation assay
Genomic DNA was isolated from tumor and adjacent normal tissues using a PureLinkTM Genomic DNA Mini Kit (Invitrogen, Waltham, MA, USA). The quality of the genomic DNA was checked using NanoDropⓇ (ND-2000, Waltham, MA, USA) and gel electrophoresis (1% agarose gel, 100 V, 30 min). Intact genomic DNA was diluted to 50 ng/μl based on Quant-iT Picogreen (Invitrogen, Waltham, MA, USA) quantitation and subjected to bisulfite conversion using EZ DNA Methylation Kit (ZymoResearch, USA). Subsequently, the converted genomic DNA was amplified up to 1,000-fold through whole-genome amplification and then hybridized to Infinium MethylationEPIC BeadChip (V1; WG-317-1003, Illumina, San Diego, CA, USA) following the standard recommended Illumina protocol. After completing the single-base extension in the sd, the BeadChip was imaged using the iScanTM system (SY-101-1001, Illumina, San Diego, CA, USA) to yield raw data in the IDAT format.
Normalization, batch correction, and probe filtering
The EPIC array dataset was processed using the established minfi pipeline for Illumina methylation arrays (19). Raw signal intensities from 865,859 probes were extracted, followed by the application of subset-quantile within array normalization (SWAN) to correct for technical differences between type I and type II probes (18). Batch effects were addressed using surrogate variable analysis (SVA) and the ComBat method (17). To handle batch correction, we used sentrix ID information because the dataset consisted of one set before 2042203330001 and another set after 2042203330001. In the batch correction process, we manually removed 1,045 probes based on the Illumina EPIC array manual version 1.05B. Subsequently, poorly performing probes, sex chromosomes, and known SNP sites were excluded from downstream analysis. Additionally, if the beta range was < 0.1, the probes were removed. Ultimately, 612,329 probe methylation beta values remained for 165 tumor and 165 normal samples.
Funding Statement
ACKNOWLEDGEMENTS This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science & ICT (grant number: NRF-2017M3A9A7050614, NRF-2017M3A9A7050803, and NRF-2017M3A9A7050610). It was additionally supported by a grant from the National Research Foundation of Korea (NRF-2020M3A9I6A01036057).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
DATA AVAILABILITY
The raw IDAT files and processed methylation profiles are available at Korea BioData Station (KBDS, https://kbds.re.kr/) under the accession ID KAP240420 (29).
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Goel A, Boland CR. Epigenetics of colorectal cancer. Gastroenterology. 2012;143:1442–1460. doi: 10.1053/j.gastro.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun D, Chen Y, Fang JY. Influence of the microbiota on epigenetics in colorectal cancer. Natl Sci Rev. 2019;6:1138–1148. doi: 10.1093/nsr/nwy160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esteban-Gil A, Pérez-Sanz F, García-Solano J, et al. ColPortal, an integrative multiomic platform for analysing epigenetic interactions in colorectal cancer. Sci Data. 2019;6:255. doi: 10.1038/s41597-019-0198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng JM, Yu J. Promoter hypermethylation of tumour suppressor genes as potential biomarkers in colorectal cancer. Int J Mol Sci. 2015;16:2472–2496. doi: 10.3390/ijms16022472.ae342d5f6d1749fba2263231ff8436d0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fatemi N, Tierling S, Es HA, et al. DNA methylation biomarkers in colorectal cancer: clinical applications for precision medicine. Int J Cancer. 2022;151:2068–2081. doi: 10.1002/ijc.34186. [DOI] [PubMed] [Google Scholar]
- 7.Jung G, Hernandez-Illan E, Moreira L, Balaguer F, Goel A. Epigenetics of colorectal cancer: biomarker and therapeutic potential. Nat Rev Gastroenterol Hepatol. 2020;17:111–130. doi: 10.1038/s41575-019-0230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham JM, Christensen ER, Tester DJ, et al. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res. 1998;58:3455–3460. [PubMed] [Google Scholar]
- 9.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 10.Issa JP. CpG island methylator phenotype in cancer. Nat Rev Cancer. 2004;4:988–993. doi: 10.1038/nrc1507. [DOI] [PubMed] [Google Scholar]
- 11.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 12.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mojarad EN, Kuppen PJ, Aghdaei HA, Zali MR. The CpG island methylator phenotype (CIMP) in colorectal cancer. Gastroenterol Hepatol Bed Bench. 2013;6:120–128. doi: 10.32388/ezc1s6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 15.Ahn JB, Chung WB, Maeda O, et al. DNA methylation predicts recurrence from resected stage III proximal colon cancer. Cancer. 2011;117:1847–1854. doi: 10.1002/cncr.25737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhee YY, Kim MJ, Bae JM, et al. Clinical outcomes of patients with microsatellite-unstable colorectal carcinomas depend on L1 methylation level. Ann Surg Oncol. 2012;19:3441–3448. doi: 10.1245/s10434-012-2410-7. [DOI] [PubMed] [Google Scholar]
- 17.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–883. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maksimovic J, Gordon L, Oshlack A. SWAN: subset-quantile within array normalization for Illumina Infinium HumanMethylation450 BeadChips. Genome Biol. 2012;13:R44. doi: 10.1186/gb-2012-13-6-r44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aryee MJ, Jaffe AE, Corrada-Bravo H, et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30:1363–1369. doi: 10.1093/bioinformatics/btu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheaffer KL, Elliott EN, Kaestner KH. DNA hypomethylation contributes to genomic instability and intestinal cancer initiation. Cancer Prev Res (Phila) 2016;9:534–546. doi: 10.1158/1940-6207.CAPR-15-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McInnes T, Zou D, Rao DS, et al. Genome-wide methylation analysis identifies a core set of hypermethylated genes in CIMP-H colorectal cancer. BMC Cancer. 2017;17:228. doi: 10.1186/s12885-017-3226-4.b983699b554c4626aa4eff65786a812f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller D, Gyorffy B. DNA methylation-based diagnostic, prognostic, and predictive biomarkers in colorectal cancer. Biochim Biophys Acta Rev Cancer. 2022;1877:188722. doi: 10.1016/j.bbcan.2022.188722. [DOI] [PubMed] [Google Scholar]
- 24.Worthley DL, Leggett BA. Colorectal cancer: molecular features and clinical opportunities. Clin Biochem Rev. 2010;31:31–38. [PMC free article] [PubMed] [Google Scholar]
- 25.Jia M, Gao X, Zhang Y, Hoffmeister M, Brenner H. Different definitions of CpG island methylator phenotype and outcomes of colorectal cancer: a systematic review. Clin Epigenetics. 2016;8:25. doi: 10.1186/s13148-016-0191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inamura K, Yamauchi M, Nishihara R, et al. Tumor LINE-1 methylation level and microsatellite instability in relation to colorectal cancer prognosis. J Natl Cancer Inst. 2014;106:dju195. doi: 10.1093/jnci/dju195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hur H, Oh CM, Won YJ, Oh JH, Kim NK. Characteristics and survival of Korean patients with colorectal cancer based on data from the Korea Central Cancer Registry Data. Ann Coloproctol. 2018;34:212–221. doi: 10.3393/ac.2018.08.02.1.62111a9ff66c4712acf3ba4e9769723c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baek SK, Lee JS, Hwang IG, et al. Clinical characteristics and survival of colorectal cancer patients in Korea stratified by age. Korean J Int Med. 2021;36:985–991. doi: 10.3904/kjim.2019.066.a019ece058fd4542bbc8e70d4c4284dc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee B, Hwang S, Kim PG, et al. Introduction of the Korea BioData Station (K-BDS) for sharing biological data. Genomics Inf. 2023;21:e12. doi: 10.5808/gi.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.