Introduction
The lungs serve two primary functions: (1) to facilitate oxygen uptake and delivery to the vital organs and peripheral tissues and (2) to eliminate carbon dioxide and help maintain pH balance. When these primary functions fail, acute respiratory failure occurs. Management of acute respiratory failure depends on the etiology underlying the presentation; however, there is significant overlap in the stabilization and early treatment of patients with respiratory issues.
Early stabilization of the patient with acute respiratory failure
The first response to a critically ill patient should include immediate assessment and stabilization. This is accomplished by systematically identifying problems with circulation and breathing and simultaneously providing appropriate support. In 2010, the American Heart Association published new guidelines recommending an adjustment to the well-known mnemonic Airway, Breathing, Circulation that prioritized circulatory support prior to respiratory support.1 However, for the purposes of this review, we will focus primarily on the parallel assessment and management of airway and breathing in patients with acute respiratory failure.
Airway and Breathing
Assessment of breathing pattern, frequency of respirations, and adequacy of oxygenation and ventilation should be performed simultaneously with the airway examination. The airway examination includes an evaluation of the patient’s mental status as well as a focused exam of the oropharynx, chest wall, lungs, and heart. Obstruction of the airway should be addressed as soon as possible.
Oxygenation is often first assessed by pulse oximetry. Ventilation is typically evaluated through the measurement of the partial pressure of carbon dioxide (pCO2) in arterial or venous blood. Non-invasive evaluation of pCO2 can be achieved through the measurement of end-tidal CO2 via capnometry or capnography. Capnometry delivers a numerical value alone. However, capnography also provides a waveform for the end-tidal CO2 that is more reliable and allows for real-time evaluation of cardiac output and pulmonary blood flow.2,3 Capnography is most frequently employed to confirm placement of an endotracheal tube into the trachea, to monitor adequacy of chest compressions during CPR, and to evaluate for return of spontaneous circulation after cardiac arrest.3,4 Early strategies to address issues with oxygenation or ventilation include chin tilt, jaw thrust, removal of foreign body, bag-valve mask ventilation, insertion of a nasal or oral airway, endotracheal intubation, or tracheotomy.
Identifying the type of acute respiratory failure
Early diagnostic maneuvers can help to distinguish between acute hypoxemic and hypercapnic respiratory failure. To start, we obtain oxygen saturation by pulse oximetry (SpO2), oxygen saturation from arterial blood (SaO2), partial pressure of oxygen in arterial blood (PaO2), partial pressure of carbon dioxide in arterial blood (PaCO2), and hemoglobin level from a complete blood count.
While arterial blood gas values remain the gold standard for evaluating the cause of acute respiratory failure, peripheral venous blood gas sampling has gained popularity for its ease of acquisition.5,6 Several observational studies have demonstrated correlation of pH and pCO2 between arterial and peripheral venous blood that justifies the use of the venous blood as a surrogate for arterial samples.7,8 However, a meta-analysis published in 2014 identified wide variations between arterial and peripheral venous pCO2 and pO2 measurements and also noted clinically significant differences in diagnoses when venous blood gases were used to predict arterial values.9 That said, pH was noted to correlate with reasonable accuracy in this study and has also demonstrated consistency when measured from peripheral venous blood gases in hypotensive patients.7,9 Taken together, these data suggest that there is a role for venous blood sampling in the evaluation of an acutely ill patient, but we caution against the sole use of venous blood in lieu of arterial blood in the diagnosis of acute respiratory failure, particularly for acute hypoxemic respiratory failure.
Acute hypoxemic respiratory failure
Acute hypoxemic respiratory failure is defined by the inability of the respiratory system to maintain an adequate blood oxygen level to preserve normal organ function. Clinically, oxygen saturation and PaO2 are used as surrogate measures to assess adequate blood oxygen content. Hypoxemic respiratory failure is thus defined by a PaO2 < 60 mmHg or SaO2 < 88%. There are five distinct but interconnected mechanisms that result in hypoxemia, which we detail below. Clinical characteristics and disease-specific examples are included in Table 1.
Table 1:
Mechanisms of hypoxemia
| Mechanism of Hypoxemia | Clinical Characteristics | Examples |
|---|---|---|
| V/Q mismatch |
|
|
| Shunt |
|
|
| Diffusion limitation |
|
|
| Hypoventilation |
|
|
| Low inspired FiO2 |
|
|
| Oxygen delivery-consumption imbalance |
|
|
Causes of hypoxemia
The causes of hypoxemia are understood through the pathophysiologic mechanisms that underlie them: V/Q mismatch, shunt, diffusion limitation, hypoventilation, low inspired oxygen tension, oxygen delivery-consumption mismatch (Table 1).
V/Q mismatch
V/Q mismatch is the most common cause of hypoxemia in critically ill patients and is defined by a change to the typical ratio of ventilation and perfusion within in the lung. Hypoxemia due to V/Q mismatch can be corrected with the addition of supplemental oxygen.10
Shunt
The most extreme example of reduced V/Q ratio is anatomic shunt, which occurs when there is complete impairment in ventilation despite adequate blood flow. Because the capillary blood is not exposed to ventilated lung units, hypoxemia due to shunt does not demonstrate the same improvement with supplemental oxygen as V/Q mismatch.11 As the proportion of shunted blood, or shunt fraction, rises, progressive hypoxemia will become less responsive to supplemental oxygen. When the shunt fraction rises above 50%, PaO2 is essentially independent of the fraction of inspired oxygen (FiO2). At that point, supplemental oxygen therapy is typically ineffective.12
Diffusion limitation
Diffusion limitation occurs when oxygen is unable to move across the alveolus into the capillary blood despite an unchanged V/Q ratio. Therefore, conditions that increase either membrane thickness (e.g., interstitial lung disease or pulmonary hypertension) or rate of capillary blood flow (e.g., increased cardiac output that can occur during exertion) can worsen diffusion and lead to hypoxemia.11 Supplemental oxygen can correct hypoxemia generated by diffusion limitation.13
Hypoventilation
Hypoventilation can result in hypoxemia because of reduction in the partial pressure of oxygen in the alveolus (PAO2) without concomitant changes in the ability of the capillary blood to extract oxygen.11 Supplemental oxygen will improve hypoxemia from hypoventilation but does not improve hypercapnia.
Low inspired oxygen tension (PiO2)
With reductions in PiO2 at high altitude (including on aircrafts), hypoxemia is caused by the resulting low PAO2 and is improved with the addition of supplemental oxygen.11
Oxygen delivery-consumption mismatch
When oxygen demand by the peripheral tissues is exceeded by supply—as in states of hypermetabolism, low cardiac output, low oxygen carrying capacity—hypoxemia can result. In these cases, provision of supplemental oxygen and treatment targeted toward correcting the underlying supply-demand mismatch will improve hypoxemia.
Evaluation of acute hypoxemic respiratory failure
We begin the evaluation for acute hypoxemic respiratory failure by checking a peripheral oxygen saturation (SpO2) to determine if hypoxemia is present. If the SpO2 is abnormal or the SpO2 is normal, but the patient demonstrates clinical evidence of respiratory failure (e.g., dyspnea, increased work of breathing), we perform an arterial blood gas for evaluation of arterial oxygen saturation (SaO2). If the SaO2 is low, we calculate the A-a gradient.
The A-a gradient is the difference between the PAO2 and the PaO2. The PAO2 is calculated using the alveolar gas equation (Figure 1), and the PaO2 is measured from arterial blood. This equation allows for identification of abnormalities in oxygen absorption in the lung. Because the lung demonstrates variable ventilation-perfusion ratios in the normal state, a small difference in PAO2 and PaO2 is expected. A calculation of the normal A-a gradient for a person breathing room air at sea level is demonstrated in Figure 2. The normal A-a gradient also increases with age. The age-adjustment equation for the normal A-a gradient is shown in Figure 3.
Figure 1:

The alveolar gas equation
Figure 2:
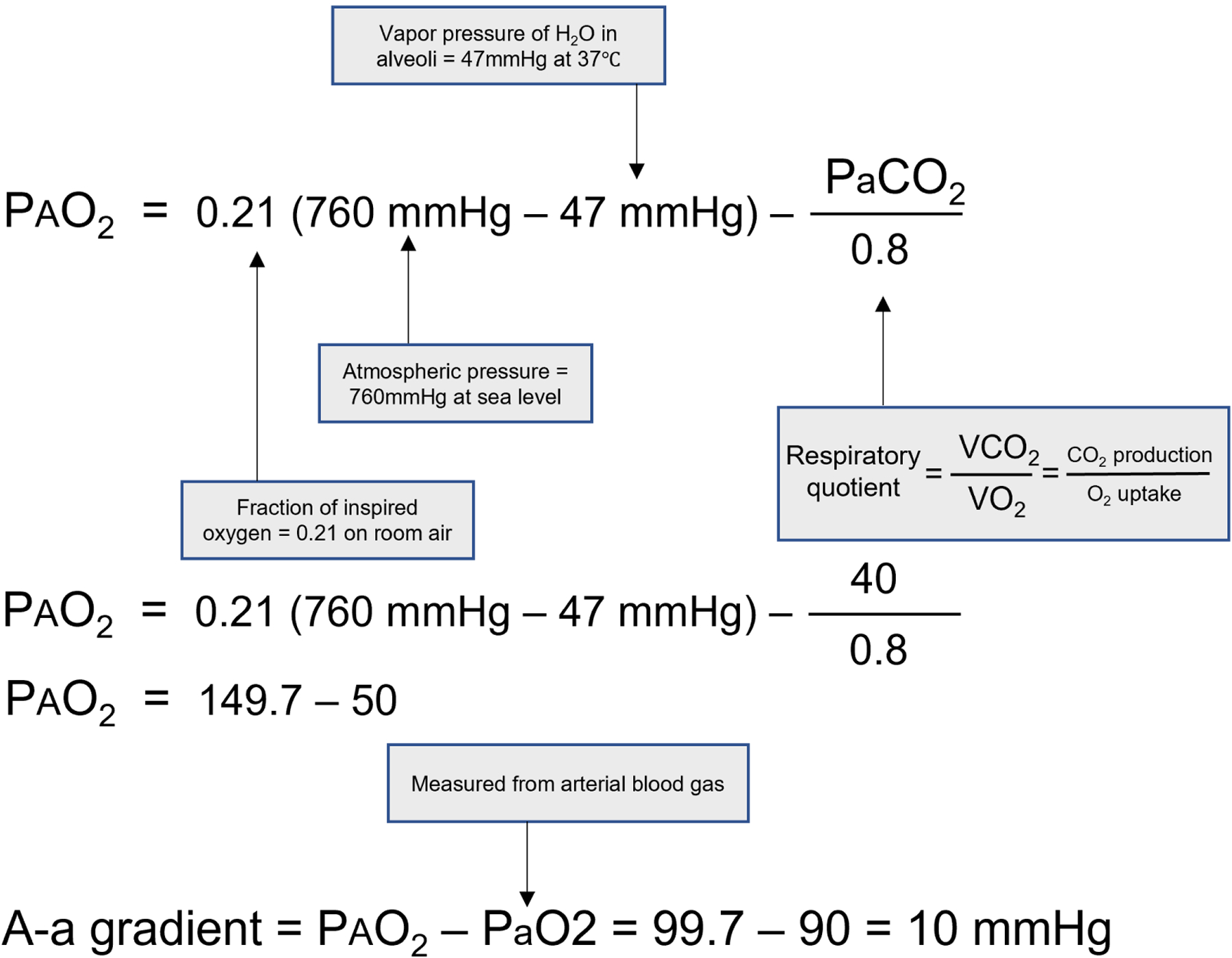
Calculation of the normal A-a gradient
Figure 3:

Age-adjusted A-a gradient equation
If the A-a gradient is wider than expected, the etiology of hypoxemia can be narrowed to V/Q mismatch, shunt, or diffusion limitation. To delineate further, we begin with a chest x-ray to evaluate for pneumothorax, alveolar filling, or interstitial process. In select populations, cross-sectional imaging of the chest may be warranted in follow up to the chest x-ray or as the initial imaging method. Computed tomography (CT) can assist with the diagnosis of infectious etiologies, particularly fungal infections, interstitial disease or inflammatory disorders impacting the lung, as well as for pulmonary embolism via angiography.14 Specifically, we proceed with CT of the chest, instead of chest x-ray, in immunocompromised patients or when there is high suspicion of the previously mentioned conditions. If there is clinical concern for shunt, as demonstrated by limited response to supplemental oxygen, CT angiography of the chest or cardiac ultrasound with shunt study can be pursued. Contribution from an oxygen supply-demand mismatch can also be measured from a central venous oxygen saturation. If the central venous oxygen saturation is low, oxygen consumption by peripheral tissues exceeds delivery, which is indicative of hypermetabolic states, impaired cardiac output, or anemia.
Management of acute hypoxemic respiratory failure
While the management of hypoxemia is focused primarily on treatment of the underlying disorder, all hypoxemic patients should be provided with supplemental oxygen therapy to maintain adequate oxygen delivery to tissues. The optimal oxygen target is still under investigation. Many sources argue for a conservative oxygen approach, targeting PaO2 between 55 mmHg and 80 mmHg.15
Conservative approaches have theoretical basis, as hyperoxemia may result in tissue injury involving multiple systems including the lungs and central nervous system.16 Some clinical trials have also supported this. A meta-analysis published in 2018 examining data from 25 randomized controlled trials (RCTs) identified an increased risk of mortality with liberal oxygen strategies (SpO2 > 96%) compared with conservative strategies.17 On the other hand, other data have demonstrated a signal for increased mesenteric ischemia and 90-day mortality with conservative oxygen strategies (SpO2 88–92%) in patients with severe ARDS, suggesting a possible benefit to targeting an intermediate oxygen saturation (SpO2 92–96%).18 More recent data on this topic have been conflicting, as a large RCT examining liberal, intermediate, and conservative oxygen strategies found no significant difference in ventilator-free days between groups and no increased signal for harm in any group, supporting the conclusion that an SpO2 between 90 to 98% may be safe.19
Monitoring for hypoxemia is complicated further by recent studies that have identified limitations in non-invasive oxygen monitoring. Pulse oximetry has been criticized for both inaccuracy as well as systematic bias based on skin tone.20–23 A large 2020 multicenter study identified occult hypoxemia (defined by having a SpO2 92–96% on pulse oximetry but a SaO2 < 88% in the arterial blood) in 11.4% of Black patients compared with 3.6% of White patients.23
Given this concern, early arterial blood sampling for patients in whom respiratory failure is suspected based on clinical exam, but not by SpO2 measurement, should be performed to evaluate the arterial oxygen saturation.21,23 Taking into account the emerging data suggesting that higher oxygen targets may not result in harm for patients receiving mechanical ventilation, one might consider using higher SpO2 thresholds in patients at risk for pulse oximeter inaccuracy (i.e., individuals with darker skin tone).17,19,23
Based on these data, we generally believe that a SpO2 within a range of 90 to 98% is likely to be safe, and we consider employing a more liberal strategy (e.g., 92 to 98%) in patients with darker skin tones in whom SaO2 or PaO2 have not or cannot be measured.
Oxygen delivery modalities
Treatment of hypoxemic respiratory failure can be achieved via multiple different modalities. The most accessible of these devices is conventional oxygen therapy, which can be delivered via standard nasal cannula or facemask at flow rates up to six liters of oxygen per minute. For each liter of oxygen per minute delivered by standard nasal cannula, the expected FiO2 that is delivered increases by approximately 4% above the FiO2 of room air (i.e., 21%).24 Table 2 provides further breakdown of oxygen delivery devices and estimated FiO2.
Table 2:
Oxygen delivery devices
| Oxygen delivery device | Deliverable flow rates (L·min−1) | Estimated FiO2 (%) |
|---|---|---|
| Nasal cannula | 1–2 | 24–28 |
| 3–4 | 20–35 | |
| 5–6 | 38–44 | |
| Simple face mask | 5–10 | 35–55 |
| Venturi mask* | 5–15 | 24–50 |
| Non-rebreather mask* | 10–15 | 80–95 |
| High-flow nasal cannula* | Up to 60 | 30–100 |
High-flow oxygen delivery modalities
Critically ill patients sometimes increase their minute ventilation above the oxygen delivery rates achieved by standard nasal cannulas or simple facemasks (i.e., > 6 LPM). This results in a dilution of the administered oxygen supply with additional room air. To overcome this problem in hypoxemic patients, high flow nasal cannulas (HFNC) were developed with flow rates of supplemental oxygen up to 60 liters per minute. Non-rebreather masks with oxygen reservoirs can also serve to maximally concentrate FiO2 to prevent dilution by room air.
The COVID-19 pandemic magnified the intensity of research devoted to respiratory support devices such as HFNC and non-invasive ventilation (NIV) in hypoxemic patients. However, this research was met with mixed results.25–27 A trial of patients with COVID-19 that compared HFNC to NIV via helmet interface found that NIV may reduce the risk of mechanical ventilation without impacting mortality.26 Though interpretation can be challenging, some have raised the possibility that patients who received NIV and eventually required mechanical ventilation may have experienced self-inflicted lung injury from large tidal volumes that resulted from the use of NIV.28 Despite this, a subsequent trial comparing NIV through continuous positive airway pressure (CPAP) or HFNC with conventional oxygen therapy in patients with COVID-19 found a mortality reduction with CPAP compared with conventional oxygen (36% vs 44%, respectively) but no difference in mortality between HFNC and conventional oxygen.25
Despite this, the American College of Physicians published a clinical practice guideline in 2021 that recommended HFNC over NIV for all individuals with acute hypoxemic respiratory failure.30 This recommendation was based largely on a 2015 RCT that demonstrated mortality benefit with the use of HFNC over NIV.31 The study included roughly 200 patients with acute hypoxemic respiratory failure due to multiple etiologies and documented an absolute risk reduction of mortality of nearly 16% (mortality rates of 12.4% vs. 28.2%, respectively), an increase in ventilator-free days, and a reduced risk of hospital-acquired pneumonia in patients receiving HFNC compared with NIV.30,31 Evidence also suggests improvement in patient dyspnea with HFNC compared with conventional oxygen therapy, suggesting that some increase in work of breathing may be appropriately addressed by HFNC alone.30 Further cited benefits of HFNC include improved patient comfort and less interface displacement, thus fewer interruptions to ventilatory support, when compared with NIV.32
Accordingly, we employ conventional oxygen therapy to patients with hypoxemia but normal work of breathing. In patients with persistent hypoxemia that requires > 6 LPM of supplemental oxygen (or approximately 40% FiO2), we use HFNC. For patients with acute hypoxemic respiratory failure, dyspnea, or elevated work of breathing, we use HFNC. If there is persistently elevated work of breathing despite improvement in hypoxemia in patients with COVID-19, we consider a trial of NIV prior to intubation and mechanical ventilation.25,26
In certain cases, we consider NIV as the initial treatment. In patients presenting with acute cardiogenic pulmonary edema, use of NIV (including CPAP) has been shown to improve respiratory distress (defined by symptoms of dyspnea and derangements in physiologic parameters like heart and respiratory rate) when compared to standard oxygen therapy.33 Further studies have identified reduced in-hospital mortality with use of NIV in this population.34 NIV for patients with obstructive lung disease has also been well-studied. Robust data from research spanning more than three decades has substantiated the use of NIV for respiratory failure resulting from acute exacerbations of chronic obstructive pulmonary disease (COPD).35 As a result, the European Respiratory Society/American Thoracic Society guideline for management of COPD exacerbations issued a strong recommendation for the use of NIV in 2017.36 We initiate NIV as first-line treatment for hypoxemia in patients with acute cardiogenic pulmonary edema or acute exacerbations of COPD while disease-specific interventions are begun. In patients who demonstrate inadequate response to the above therapies or exhibit signs of hemodynamic instability, endotracheal intubation and mechanical ventilation are necessary.
Indications for intubation
Despite its importance to critical care, the threshold at which to intubate a patient for acute hypoxic respiratory failure remains uncertain.37–39 Some support an early intubation strategy to limit exposure to injuriously large tidal volumes that may result from severe dyspnea and ventilatory support mechanisms like HFNC and NIV.40 Recent data, including those that emerged from the COVID-19 pandemic, have afforded little clarity. Several observational studies have correlated pre-intubation HFNC use with elevated mortality possibly due to delay of intubation and mechanical ventilation.41,42 However, a 2021 meta-analysis of 12 studies evaluating outcomes associated with the timing of intubation (early (i.e., within 24 hours) versus late intubation) in patients with acute hypoxic respiratory failure due to COVID-19 did not reveal significant differences in all-cause mortality between groups.29 Conversely, a more recent observational study examining a historical cohort of patients with COVID-19 identified an increase in mortality associated with a pre-intubation HFNC trial lasting more than 48 hours.43 Further data are warranted to determine the safety of proceeding with HFNC past 48 hours for patients with acute hypoxic respiratory failure.
Given the equipoise, we tend not to employ a standardized time limit on HFNC or NIV trial prior to intubation for all-comers with acute hypoxic respiratory failure. Rather, we implement a case-by-case trial with close monitoring to determine whether and when to move to invasive mechanical ventilation. In general, when HFNC or NIV fail to adequately manage a patient’s respiratory failure or markers of tissue hypoperfusion become evident, we proceed with endotracheal intubation and invasive mechanical ventilation. Of course, in every case the decision to initiate mechanical ventilation should be weighed carefully with a patient’s preferences, the risks of endotracheal intubation, and the risks of mechanical ventilation.38
Patients with acute hypoxemic respiratory failure are challenging in the peri-intubation period. Acute hypoxemic respiratory failure has been described as one of several conditions that present a “physiologically difficult airway.”44–46 Patients who are hypoxemic, regardless of the mechanism, are at high risk of developing worsening oxygenation during rapid sequence induction (RSI) due to the cessation of respiratory function with neuromuscular blockade. This process makes intubation more likely to be associated with downstream complications, such as arrhythmia, hemodynamic instability, or cardiac arrest.45,47,48
One of the most important interventions for patients with hypoxemia is adequate preoxygenation, which the Society for Airway Management recommends should last for at least three minutes prior to intubation and be delivered either through HFNC or NIV. Adequate preoxygenation can provide more time between initiation of RSI and first desaturation by creating a large alveolar oxygen reserve, which has been shown to increase the chance of first-pass success for intubation.45,49
Apneic oxygenation (i.e., continued delivery of oxygen during the period of apnea that follows neuromuscular blockade but precedes intubation during RSI) has received attention as a possible method by which to prevent desaturation in the peri-intubation period, though data have been mixed. In observational studies, passive apneic oxygenation is associated with fewer desaturations and more first-pass success.50–52 However, a 2016 RCT evaluating the role of passive apneic oxygenation found no significant difference in oxygen saturations between patients receiving 15 L passive apneic oxygenation by high flow nasal cannula compared to those who did not.53 More robust evidence supports the use of bag-valve mask ventilation for prevention of hypoxemia during the apneic period without evidence of increased risk for aspiration.54 Given possible benefit and limited evidence of harm, we employ apneic oxygenation via bag-valve mask ventilation when available in patients with acute hypoxic respiratory failure undergoing RSI.49–52 If available, video laryngoscopy should be considered as the primary method for endotracheal intubation over direct laryngoscopy, as data suggest that its use substantially increases first-pass success in critically ill patients.55 In patients with refractory hypoxemia, inhaled pulmonary vasodilators or awake fiberoptic intubations could be considered to reduce harms associated with RSI.44,45
In patients exhibiting signs of circulatory collapse and respiratory failure, resuscitation and RSI should be considered simultaneously, though resuscitation should not delay intubation if needed.56 In a trial of roughly 1,000 patients in intensive care units undergoing endotracheal intubation, fluid bolus of 500 mL of crystalloid during RSI had no significant impact on reducing hypotension (defined by drop in systolic blood pressure < 65 mmHg or increased dose of vasopressors).56 For those patients who demonstrate hemodynamic instability and respiratory failure, vasopressor therapy should be employed to maintain blood pressure targets during intubation. Fluid resuscitation should be aimed at treating hypovolemia if present.56,57
Acute hypercapnic respiratory failure
Hypercapnia occurs when the lungs are unable to eliminate carbon dioxide (CO2) to keep levels at or below 45 mmHg. Once hypercapnia has been identified, it is important to understand whether the source is a primary respiratory or neurologic dysfunction or whether the elevated PaCO2 is compensatory, in response to a primary metabolic alkalosis.
An arterial blood gas and blood chemistry panel, along with clinical history and physical exam, are essential in identifying the presence of acute hypercapnic respiratory failure. Acute hypercapnic respiratory failure is diagnosed when the PaCO2 is ≥ 45 mmHg and the blood pH is acidemic, defined by a pH < 7.35. Chronic hypercapnic respiratory failure is diagnosed when the PaCO2 is ≥ 45 mmHg, the increase in the PaCO2 is not compensatory for a primary metabolic alkalosis, and the blood pH is normal or near normal. Table 3 provides expected changes in pH and PaCO2 in acute and chronic hypercapnic respiratory failure.
Table 3.
Expected changes in pH and PaCO2 in acute and chronic hypercapnic respiratory failure
| Increase in PaCO2 | Increase in HCO3 | Decrease in pH | |
|---|---|---|---|
| Acute hypercapnia | 10 mmHg | 1 mEq/L | 0.08 |
| Chronic hypercapnia | 10 mmHg | 3.5 mEq/L | 0.03 |
Causes of hypercapnia
Hypercapnic respiratory failure results from alveolar hypoventilation, increased fraction of dead space, increased production of carbon dioxide, or a combination thereof. CO2 production can also sometimes overwhelm elimination, usually in patients with compromised baseline pulmonary reserve, which can result in elevations in PaCO2 in the absence of a new pulmonary problem.58 The mechanisms of hypercapnia can be understood through the mnemonic “won’t breathe, can’t breathe, can’t breathe enough.”
“Won’t breathe” causes of hypercapnia include central disorders of hypoventilation which result in reduced respiratory drive and subsequent alveolar hypoventilation. Common causes of central hypoventilation include stroke, use of sedative medications, central and obstructive sleep apnea, obesity hypoventilation, and hypothyroidism.59 These disorders are characterized by a normal A-a gradient, indicating a reduction in alveolar ventilation.
“Can’t breathe” disorders result from limited ventilatory capacity of the lungs, including advanced obstructive lung disease, diseases of the chest wall, or neuromuscular disease that prevent the adequate functioning of respiratory muscles without impairing central respiratory drive. Clinical history and exam are essential in distinguishing these disorders and could include review of prior pulmonary function testing or imaging.
Finally, “can’t breathe enough” disorders result from an overproduction of CO2 that overwhelms lung function or new elevations in the proportion of dead space ventilation that cannot be overcome by compensatory increases in minute ventilation.59 A more complete list of the disorders causing hypercapnia, stratified by category, is included in Table 4.
Table 4:
Mechanisms of hypercapnia
| Mechanism of hypercapnia | Category | Pathophysiology | Examples |
|---|---|---|---|
| Hypoventilation | “Won’t breathe” | Decreased central respiratory drive |
|
| Hypoventilation | “Can’t breathe” | Impaired function of respiratory muscles due to altered neuromuscular function |
|
| Hypoventilation | “Can’t breathe” | Chest wall disorders and pleural disease |
|
| Increased proportion of dead space | “Can’t breathe” | Airway obstruction resulting in elevated V/Q ratios |
|
| Increased proportion of dead space | “Can’t breathe” | Pulmonary vascular disease resulting in elevated V/Q ratios |
|
| Increased CO2 production | “Can’t breathe enough” | CO2 production overwhelms pulmonary CO2 elimination |
|
Evaluation of acute hypercapnic respiratory failure
To evaluate for the presence of acute hypercapnic respiratory failure, we obtain pulse oximetry, an arterial blood gas measurement, and a full blood chemistry panel. Evaluation of the arterial blood gas should be performed to identify acute, chronic, or acute on chronic hypercapnic respiratory failure. Pulse oximetry measurement or SaO2 measurement can identify patients with mixed hypoxemic and hypercapnic respiratory failure. Calculation of the A-a gradient should be performed regardless of the presence of hypoxemia as a normal A-a gradient in a patient with hypercapnic respiratory failure signifies alveolar hypoventilation or increased CO2 production.
Patients with hypercapnia may report symptoms of shortness of breath, headache, somnolence, or they may demonstrate increased work of breathing, agitation, or anxiety.60 As PaCO2 rises and pH decreases, multiple organ systems may be impacted. Cardiac and diaphragmatic contractility are reduced, resulting in increased risk for arrhythmia, circulatory collapse, and further perpetuating CO2 retention.58,61 Acute hypercapnia can result in depressed mentation and decreased respiratory drive, typically occurring when PaCO2 rises above 75 mmHg in normally-eucapnic individuals.60 Cerebral blood flow is increased as a result of elevated PaCO2, which can result in elevated intracranial pressures, seizures, and coma in severe cases.60
The initial clinical evaluation is aimed at identifying the mechanism of hypercapnia. In all patients with hypercapnic respiratory failure, in addition to early stabilization and assessment of airway, breathing, circulation, we perform a neurologic exam that includes evaluation for focal neurologic deficit (cranial nerve exam, speech evaluation, muscle strength testing) as well as an assessment of global mental status. From there, further examination and evaluation can be guided by the patient’s breathing pattern.
If there are signs of reduced respiratory drive (i.e. low respiratory rate) in the setting of hypercapnia, we consider central, “won’t breathe” etiologies. If accompanying neurologic findings are consistent with acute stroke (e.g., facial droop, dysarthria, muscle weakness, sensation changes), we pursue immediate imaging and expert consultation. In addition, we perform a simultaneous medication review, focused history, or drug screen to identify medications or substances that may blunt the respiratory drive and result in global mental status changes including narcotics, benzodiazepines, barbiturates, or alcohol. If history or lab testing indicate exposure to respiratory depressants, appropriate reversal agents should be administered.
If there are signs of increased respiratory effort (i.e. tachypnea) in the setting of hypercapnia, we consider “can’t breathe” or “can’t breathe enough” etiologies. We quickly review the patient’s medical history for neuromuscular disorders, chest wall disease, pleural disease, or obstructive lung disease which may result in impairment of respiratory efficiency and increased dead space ventilation. We also perform a focused physical exam with attention to breath sounds (e.g., degree of air movement and character of breath sounds) and chest wall findings to help differentiate between obstructive lung disease and neuromuscular causes of hypercapnia. Because low cardiac output can increase dead space fraction and hypercapnia by way of reduced pulmonary perfusion and increased thoracic gas volume, careful assessment for and treatment of concomitant shock is paramount.62
Management of acute hypercapnic respiratory failure
The primary goal of management for patients with acute hypercapnic respiratory failure is to provide respiratory support to increase work of breathing. For patients who are candidates, NIV is typically the first-line therapy. We select patients for NIV based on several criteria. First, patients receiving NIV must be spontaneously breathing. Second, patients must demonstrate the ability to protect their airway and remove the NIV mask in the event of emesis as aspiration of gastric secretions presents a major risk to those receiving NIV. There are situations that arise in which patients who do not meet these criteria may be trialed on NIV, but these individuals should be monitored closely, ideally in a critical care setting.
Emerging evidence has suggested that HFNC can be safely used in patients with hypercapnic respiratory failure who cannot tolerate or are not candidates for NIV. One meta-analysis published in 2020 concluded that HFNC was non-inferior to NIV in preventing intubation in patients with mild to moderate hypercapnia and cited no significant differences in blood gas analysis or respiratory rate between support mechanisms in the included studies.63 Further attention is being directed toward the use of HFNC in the treatment of COPD and chronic hypercapnia with promising early results.64 Given this, we consider use of HFNC for patients with acute hypercapnic respiratory failure who are not candidates for NIV.
However, targeting normal or higher oxygen saturation ranges has the potential to result in an alteration of physiologic hypoxic vasoconstriction in individuals with COPD which could conversely worsen dead space ventilation and hypercapnia. Oxygen saturations of 93% or greater have been correlated with elevated mortality in hospitalized patients with acute exacerbations of COPD.65 In addition, treatment of hypoventilation alone can improve oxygenation in patients with hypercapnic respiratory failure.66 Thus, we target an SpO2 range of 88–92% in this population and careful monitor HFNC or NIV trials.
SpO2, SaO2, PaCO2, and clinical status should be serially evaluated during any trial of NIV or HFNC. End tidal CO2 monitoring can be used in these circumstances but caution should be taken when interpreting the results. End tidal CO2 measurement is impacted not just by minute ventilation but also by other physiologic parameters including cardiac output and ventilation-perfusion matching. Thus, it can at times be an unreliable surrogate for PaCO2 in patients with alterations to normal respiratory and cardiac function.67 Monitoring the difference between the end tidal CO2 and the PaCO2, or CO2 gradient, to assess for adequacy of ventilatory support during NIV trial has been proposed but has not been sufficiently studied.69 Given this, we do not routinely use end tidal CO2 to estimate PaCO2 in the setting of hypercapnic respiratory failure. While transcutaneous CO2 monitoring appears to have better accuracy in estimating PaCO2 when compared with end tidal CO2 and could be considered for monitoring response to ventilatory support over time, we favor serial blood gas collection for pH and PaCO2 assessment over noninvasive monitoring at this time.70
If during a trial of NIV or HFNC, the pH does not normalize, the PaCO2 does not improve, or the patient’s clinical status worsens despite adequate ventilatory support (which includes ensuring mask fit, minute ventilation, and adjusting NIV settings), then intubation and invasive mechanical ventilation are indicated.71 The appropriate length of a NIV trial prior to intubation for patients with hypercapnic respiratory failure is unknown. It is estimated that benefit from initiation of NIV in the setting of acute exacerbation of COPD should be seen within 1–4 hours.72 Further, a relatively small retrospective cohort study published in 2021 documented increased 30-day mortality and increased ventilator dependence for patients who failed a trial of NIV or HFNC after more than six hours compared with those who failed in fewer than six hours.73 Given these data, we generally restrict trials of NIV support for hypercapnic respiratory failure to four hours and proceed with intubation and mechanical ventilation if no benefit is observed within this timeframe.
Intubation for hypercapnic respiratory failure
Severe pH derangements from hypercapnic respiratory failure tend to correct quickly with restoration of alveolar ventilation.44 During RSI for endotracheal intubation, attention should be paid to the impact of apnea on worsening acidemia, which could result in complications.44,46 In these cases, one might consider avoiding neuromuscular blockade entirely by utilizing alternate sedative agents or pursuing awake fiberoptic intubation.46,74,75
In most cases, we continue ventilation with NIV with a respiratory rate or bag-valve mask during the apneic period of RSI.54 Advanced surgical airways should be considered in select patients with hypercapnia due to upper airway obstruction for whom endotracheal intubation is not feasible. This is optimally performed with a multidisciplinary approach that involves critical care, anesthesia, and surgical teams.
Management of mixed hypoxemic and hypercapnic respiratory failure
Given the overlap between mechanisms causing hypoxemia and hypercapnia, some patients present with both derangements. While treatment should always be aimed towards addressing the primary or most severe dysfunction, it may be difficult to differentiate the type of respiratory failure in the initial stabilization period. Some have proposed that NIV, when employed in the wrong patient populations, could cause harm by generating self-inflicted lung injury.30,40 While there may be some conceptual basis for this, there is limited evidence to suggest a clinically meaningful signal for harm. Furthermore, data borne out of COVID-19 argue that use of NIV may result in fewer intubations without increasing mortality.25,26 In patients with increased work of breathing and concern for hypercapnic respiratory failure, we initiate NIV. If concomitant hypoxemia is identified, we initiate supplemental oxygen to maintain PaO2 > 60 mmHg with SaO2 88–92%, given the potential for hyperoxemia to worsen hypercapnia in select populations.
Conclusions
Figure 4 describes our algorithm for diagnosis and early management of acute respiratory failure. It is imperative that the diagnosis and early management of acute respiratory failure happen in parallel. Early stabilization can be achieved by implementing an organized approach to assessment and management. Further stratification of disease is based on clinical history, physical exam, and rapid laboratory assessment. Calculation of the A-a gradient can help clarify the underlying disease pathology for both hypoxemia and hypercapnia. Treatment of acute respiratory failure varies based upon the underlying the presentation. However, oxygen and ventilatory support mechanisms, such as HFNC, NIV, or invasive mechanical ventilation, should aim to maintain a PaO2 > 60 mmHg, SaO2 > 88%, normal pH, and reduced dyspnea.
Figure 4:

Diagnosis and early management of respiratory failure
Key Points:
Acute hypoxemic respiratory failure is defined by PaO2 < 60 mmHg or SaO2 < 88% and may results from V/Q mismatch, shunt, hypoventilation, diffusion limitation, or low inspired oxygen tension.
Acute hypercapnic respiratory failure is defined by PaCO2 ≥ 45 mmHg and pH < 7.35 and may result from alveolar hypoventilation, increased fraction of dead space, or increased production of carbon dioxide.
Early diagnostic maneuvers, such as measurement of SpO2 and arterial blood gas, can differentiate the type of respiratory failure and guide next steps in evaluation and management.
Treatment should be directed at the primary derangement and targeted toward maintaining adequate tissue oxygenation and a normal pH with supportive modalities such as high-flow nasal cannula, non-invasive ventilation, or invasive mechanical ventilation.
Synopsis.
Acute hypoxemic respiratory failure is defined by PaO2 < 60 mmHg or SaO2 < 88% and may results from V/Q mismatch, shunt, hypoventilation, diffusion limitation, or low inspired oxygen tension. Acute hypercapnic respiratory failure is defined by PaCO2 ≥ 45 mmHg and pH < 7.35 and may result from alveolar hypoventilation, increased fraction of dead space, or increased production of carbon dioxide. Early diagnostic maneuvers, such as measurement of SpO2 and arterial blood gas, can differentiate the type of respiratory failure and guide next steps in evaluation and management. Treatment should be directed at the primary derangement and targeted toward maintaining adequate tissue oxygenation and a normal pH with supportive modalities such as high-flow nasal cannula, non-invasive ventilation, or invasive mechanical ventilation.
Clinics Care Points:
The initial approach to the patient with acute respiratory failure depends on the extent to which hypoxemia or hypercapnia are present and should consist of parallel assessment of and support for airway, breathing, and oxygen delivery to the tissues.
Treatment should be directed towards reversing the primary respiratory derangement.
Supportive respiratory technology, such as high-flow nasal cannula, non-invasive ventilation, or invasive mechanical ventilation, are critical to ensuring adequate oxygenation and ventilation for the critically ill patient.
Funding:
Funding provided through NHLBI K23 HL140165 and NHLBI R01 HL157361
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: This manuscript does not necessarily represent the view of the U.S. Government or the Department of Veterans Affairs.
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. Nov 2 2010;122(18 Suppl 3):S640–56. doi:10.1161/circulationaha.110.970889 [DOI] [PubMed] [Google Scholar]
- 2.Deakin CD, Morrison LJ, Morley PT, et al. Part 8: Advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation. Oct 2010;81 Suppl 1:e93–e174. doi:10.1016/j.resuscitation.2010.08.027 [DOI] [PubMed] [Google Scholar]
- 3.Brian KW, David NC, Ruben DR. Capnography/Capnometry During Mechanical Ventilation: 2011. Respiratory Care. 2011;56(4):503. doi:10.4187/respcare.01175 [DOI] [PubMed] [Google Scholar]
- 4.Lecompte-Osorio P, Pearson SD, Pieroni CH, et al. Bedside estimates of dead space using end-tidal CO(2) are independently associated with mortality in ARDS. Crit Care. Sep 15 2021;25(1):333. doi:10.1186/s13054-021-03751-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis MD, Walsh BK, Sittig SE, Restrepo RD. AARC clinical practice guideline: blood gas analysis and hemoximetry: 2013. Respir Care. Oct 2013;58(10):1694–703. doi:10.4187/respcare.02786 [DOI] [PubMed] [Google Scholar]
- 6.Rowling SC, Fløjstrup M, Henriksen DP, et al. Arterial blood gas analysis: as safe as we think? A multicentre historical cohort study. ERJ Open Res. Jan 2022;8(1)doi:10.1183/23120541.00535-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad H, Vempalli N, Agrawal N, et al. Correlation and agreement between arterial and venous blood gas analysis in patients with hypotension-an emergency department-based cross-sectional study. Int J Emerg Med. Mar 10 2023;16(1):18. doi:10.1186/s12245-023-00486-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold TDW, Miller M, van Wessem KP, Evans JA, Balogh ZJ. Base Deficit From the First Peripheral Venous Sample: A Surrogate for Arterial Base Deficit in the Trauma Bay. Journal of Trauma and Acute Care Surgery. 2011;71(4) [DOI] [PubMed] [Google Scholar]
- 9.Byrne AL, Bennett M, Chatterji R, Symons R, Pace NL, Thomas PS. Peripheral venous and arterial blood gas analysis in adults: are they comparable? A systematic review and meta-analysis. Respirology. Feb 2014;19(2):168–175. doi:10.1111/resp.12225 [DOI] [PubMed] [Google Scholar]
- 10.Sarkar M, Niranjan N, Banyal PK. Mechanisms of hypoxemia. Lung India. Jan-Feb 2017;34(1):47–60. doi:10.4103/0970-2113.197116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johan P, Robb WG. Gas exchange and ventilation–perfusion relationships in the lung. European Respiratory Journal. 2014;44(4):1023. doi:10.1183/09031936.00037014 [DOI] [PubMed] [Google Scholar]
- 12.Marino PL. Hypoxemia and Hypercapnia. Marino’s: The ICU Book, Fourth Edition. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2014:chap 20. [Google Scholar]
- 13.RN P. Chapter 4, Oxygen Transport. Regulation of Tissue Oxygenation. Morgan & Claypool Life Sciences; 2011. [PubMed] [Google Scholar]
- 14.Washington L, Khan A, Mohammed TL, et al. ACR Appropriateness Criteria on acute respiratory illness. J Am Coll Radiol. Oct 2009;6(10):675–80. doi:10.1016/j.jacr.2009.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. May 4 2000;342(18):1301–8. doi:10.1056/nejm200005043421801 [DOI] [PubMed] [Google Scholar]
- 16.Hafner S, Beloncle F, Koch A, Radermacher P, Asfar P. Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update. Ann Intensive Care. Dec 2015;5(1):42. doi:10.1186/s13613-015-0084-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. Apr 28 2018;391(10131):1693–1705. doi:10.1016/s0140-6736(18)30479-3 [DOI] [PubMed] [Google Scholar]
- 18.Barrot L, Asfar P, Mauny F, et al. Liberal or Conservative Oxygen Therapy for Acute Respiratory Distress Syndrome. N Engl J Med. Mar 12 2020;382(11):999–1008. doi:10.1056/NEJMoa1916431 [DOI] [PubMed] [Google Scholar]
- 19.Semler MW, Casey JD, Lloyd BD, et al. Oxygen-Saturation Targets for Critically Ill Adults Receiving Mechanical Ventilation. New England Journal of Medicine. 2022;387(19):1759–1769. doi:10.1056/NEJMoa2208415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Meter A, Williams U, Zavala A, et al. Beat to Beat: A Measured Look at the History of Pulse Oximetry. Journal of Anesthesia History. 2017/01/01/ 2017;3(1):24–26. doi: 10.1016/j.janh.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 21.Bickler PE, Feiner JR, Severinghaus JW. Effects of skin pigmentation on pulse oximeter accuracy at low saturation. Anesthesiology. Apr 2005;102(4):715–9. doi:10.1097/00000542-200504000-00004 [DOI] [PubMed] [Google Scholar]
- 22.Gupta S, Medikayala S, Singh B, Bhatt H, Singh S. Leukocytosis and Spurious Hypoxemia. Cureus. Jun 2021;13(6):e15942. doi:10.7759/cureus.15942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial Bias in Pulse Oximetry Measurement. New England Journal of Medicine. 2020/12/17 2020;383(25):2477–2478. doi:10.1056/NEJMc2029240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardavella G, Karampinis I, Frille A, Sreter K, Rousalova I. Oxygen devices and delivery systems. Breathe (Sheff). Sep 2019;15(3):e108–e116. doi:10.1183/20734735.0204-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkins GD, Ji C, Connolly BA, et al. Effect of Noninvasive Respiratory Strategies on Intubation or Mortality Among Patients With Acute Hypoxemic Respiratory Failure and COVID-19: The RECOVERY-RS Randomized Clinical Trial. JAMA. 2022;327(6):546–558. doi:10.1001/jama.2022.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grieco DL, Menga LS, Cesarano M, et al. Effect of Helmet Noninvasive Ventilation vs High-Flow Nasal Oxygen on Days Free of Respiratory Support in Patients With COVID-19 and Moderate to Severe Hypoxemic Respiratory Failure: The HENIVOT Randomized Clinical Trial. Jama. May 4 2021;325(17):1731–1743. doi:10.1001/jama.2021.4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ospina-Tascón GA, Calderón-Tapia LE, García AF, et al. Effect of High-Flow Oxygen Therapy vs Conventional Oxygen Therapy on Invasive Mechanical Ventilation and Clinical Recovery in Patients With Severe COVID-19: A Randomized Clinical Trial. Jama. Dec 7 2021;326(21):2161–2171. doi:10.1001/jama.2021.20714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Battaglini D, Robba C, Ball L, et al. Noninvasive respiratory support and patient self-inflicted lung injury in COVID-19: a narrative review. Br J Anaesth. Sep 2021;127(3):353–364. doi:10.1016/j.bja.2021.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papoutsi E, Giannakoulis VG, Xourgia E, Routsi C, Kotanidou A, Siempos II. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies. Critical Care. 2021/03/25 2021;25(1):121. doi:10.1186/s13054-021-03540-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qaseem A, Etxeandia-Ikobaltzeta I, Fitterman N, Williams JW, Kansagara D. Appropriate Use of High-Flow Nasal Oxygen in Hospitalized Patients for Initial or Postextubation Management of Acute Respiratory Failure: A Clinical Guideline From the American College of Physicians. Annals of Internal Medicine. 2021/07/20 2021;174(7):977–984. doi:10.7326/M20-7533 [DOI] [PubMed] [Google Scholar]
- 31.Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. Jun 4 2015;372(23):2185–96. doi:10.1056/NEJMoa1503326 [DOI] [PubMed] [Google Scholar]
- 32.Papazian L, Corley A, Hess D, et al. Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. Intensive Care Med. Sep 2016;42(9):1336–49. doi:10.1007/s00134-016-4277-8 [DOI] [PubMed] [Google Scholar]
- 33.Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. Jul 10 2008;359(2):142–51. doi:10.1056/NEJMoa0707992 [DOI] [PubMed] [Google Scholar]
- 34.Mariani J, Macchia A, Belziti C, et al. Noninvasive ventilation in acute cardiogenic pulmonary edema: a meta-analysis of randomized controlled trials. J Card Fail. Oct 2011;17(10):850–9. doi:10.1016/j.cardfail.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 35.Shah NM, D’Cruz RF, Murphy PB. Update: non-invasive ventilation in chronic obstructive pulmonary disease. J Thorac Dis. Jan 2018;10(Suppl 1):S71–s79. doi:10.21037/jtd.2017.10.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wedzicha JAEC-C, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. Mar 2017;49(3)doi:10.1183/13993003.00791-2016 [DOI] [PubMed] [Google Scholar]
- 37.Pisano A, Yavorovskiy A, Verniero L, Landoni G. Indications for Tracheal Intubation in Patients With Coronavirus Disease 2019 (COVID-19). J Cardiothorac Vasc Anesth. May 2021;35(5):1276–1280. doi:10.1053/j.jvca.2020.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabrini L, Ghislanzoni L, Severgnini P, et al. Early versus late tracheal intubation in COVID-19 patients: a “pros/cons” debate also considering heart-lung interactions. Minerva Cardiol Angiol. Oct 2021;69(5):596–605. doi:10.23736/s2724-5683.20.05356-6 [DOI] [PubMed] [Google Scholar]
- 39.Tobin MJ, Laghi F, Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care. Jun 9 2020;10(1):78. doi:10.1186/s13613-020-00692-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grieco DL, Menga LS, Eleuteri D, Antonelli M. Patient self-inflicted lung injury: implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol. Sep 2019;85(9):1014–1023. doi:10.23736/s0375-9393.19.13418-9 [DOI] [PubMed] [Google Scholar]
- 41.Kang BJ, Koh Y, Lim CM, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. Apr 2015;41(4):623–32. doi:10.1007/s00134-015-3693-5 [DOI] [PubMed] [Google Scholar]
- 42.Miller DC, Pu J, Kukafka D, Bime C. Failure of High Flow Nasal Cannula and Subsequent Intubation Is Associated With Increased Mortality as Compared to Failure of Non-Invasive Ventilation and Mechanical Ventilation Alone: A Real-World Retrospective Analysis. J Intensive Care Med. Jan 2022;37(1):41–45. doi:10.1177/0885066620968041 [DOI] [PubMed] [Google Scholar]
- 43.López-Ramírez VY, Sanabria-Rodríguez OO, Bottia-Córdoba S, Muñoz-Velandia OM. Delayed mechanical ventilation with prolonged high-flow nasal cannula exposure time as a risk factor for mortality in acute respiratory distress syndrome due to SARS-CoV-2. Intern Emerg Med. Mar 2023;18(2):429–437. doi:10.1007/s11739-022-03186-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mosier JM. Physiologically difficult airway in critically ill patients: winning the race between haemoglobin desaturation and tracheal intubation. Br J Anaesth. Jul 2020;125(1):e1–e4. doi:10.1016/j.bja.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 45.Kornas RL, Owyang CG, Sakles JC, Foley LJ, Mosier JM. Evaluation and Management of the Physiologically Difficult Airway: Consensus Recommendations From Society for Airway Management. Anesth Analg. Feb 1 2021;132(2):395–405. doi:10.1213/ane.0000000000005233 [DOI] [PubMed] [Google Scholar]
- 46.Mosier JM, Joshi R, Hypes C, Pacheco G, Valenzuela T, Sakles JC. The Physiologically Difficult Airway. West J Emerg Med. Dec 2015;16(7):1109–17. doi:10.5811/westjem.2015.8.27467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mort TC. The incidence and risk factors for cardiac arrest during emergency tracheal intubation: a justification for incorporating the ASA Guidelines in the remote location. J Clin Anesth. Nov 2004;16(7):508–16. doi:10.1016/j.jclinane.2004.01.007 [DOI] [PubMed] [Google Scholar]
- 48.Russotto V, Myatra SN, Laffey JG, et al. Intubation Practices and Adverse Peri-intubation Events in Critically Ill Patients From 29 Countries. Jama. Mar 23 2021;325(12):1164–1172. doi:10.1001/jama.2021.1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis DP, Lemieux J, Serra J, Koenig W, Aguilar SA. Preoxygenation reduces desaturation events and improves intubation success. Air Med J. Mar-Apr 2015;34(2):82–5. doi:10.1016/j.amj.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 50.Wimalasena Y, Burns B, Reid C, Ware S, Habig K. Apneic oxygenation was associated with decreased desaturation rates during rapid sequence intubation by an Australian helicopter emergency medicine service. Ann Emerg Med. Apr 2015;65(4):371–6. doi:10.1016/j.annemergmed.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 51.Sakles JC, Mosier JM, Patanwala AE, Arcaris B, Dicken JM. First Pass Success Without Hypoxemia Is Increased With the Use of Apneic Oxygenation During Rapid Sequence Intubation in the Emergency Department. Acad Emerg Med. Jun 2016;23(6):703–10. doi:10.1111/acem.12931 [DOI] [PubMed] [Google Scholar]
- 52.Sakles JC. Maintenance of Oxygenation During Rapid Sequence Intubation in the Emergency Department. https://doi.org/10.1111/acem.13271. Academic Emergency Medicine. 2017/11/01 2017;24(11):1395–1404. doi: 10.1111/acem.13271 [DOI] [PubMed] [Google Scholar]
- 53.Semler MW, Janz DR, Lentz RJ, et al. Randomized Trial of Apneic Oxygenation during Endotracheal Intubation of the Critically Ill. Am J Respir Crit Care Med. Feb 1 2016;193(3):273–80. doi:10.1164/rccm.201507-1294OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casey JD, Janz DR, Russell DW, et al. Bag-Mask Ventilation during Tracheal Intubation of Critically Ill Adults. New England Journal of Medicine. 2019/02/28 2019;380(9):811–821. doi:10.1056/NEJMoa1812405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prekker ME, Driver BE, Trent SA, et al. Video versus Direct Laryngoscopy for Tracheal Intubation of Critically Ill Adults. N Engl J Med. Aug 3 2023;389(5):418–429. doi:10.1056/NEJMoa2301601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russell DW, Casey JD, Gibbs KW, et al. Effect of Fluid Bolus Administration on Cardiovascular Collapse Among Critically Ill Patients Undergoing Tracheal Intubation: A Randomized Clinical Trial. Jama. Jul 19 2022;328(3):270–279. doi:10.1001/jama.2022.9792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janz DR, Casey JD, Semler MW, et al. Effect of a fluid bolus on cardiovascular collapse among critically ill adults undergoing tracheal intubation (PrePARE): a randomised controlled trial. Lancet Respir Med. Dec 2019;7(12):1039–1047. doi:10.1016/s2213-2600(19)30246-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Juan G, Calverley P, Talamo C, Schnader J, Roussos C. Effect of carbon dioxide on diaphragmatic function in human beings. N Engl J Med. Apr 5 1984;310(14):874–9. doi:10.1056/nejm198404053101402 [DOI] [PubMed] [Google Scholar]
- 59.Feller-Kopman DJ, Schwartzstein, Richard M. The evaluation, diagnosis, and treatment of the adult patient with acute hypercapnic respiratory failure. In: Stoller JK, Finlay, Geraldine, ed. UpToDate. Wolters Kluwer; 2022. Accessed June 6, 2023. https://www.uptodate.com/contents/the-evaluation-diagnosis-and-treatment-of-the-adult-patient-with-acute-hypercapnic-respiratory-failure [Google Scholar]
- 60.Drechsler M, Morris J. Carbon Dioxide Narcosis. StatPearls. StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; 2023. [PubMed] [Google Scholar]
- 61.Price HL. Effects of carbon dioxide on the cardiovascular system. Anesthesiology. Nov-Dec 1960;21:652–63. doi:10.1097/00000542-196011000-00009 [DOI] [PubMed] [Google Scholar]
- 62.Bayat S, Albu G, Layachi S, et al. Acute hemorrhagic shock decreases airway resistance in anesthetized rat. J Appl Physiol (1985). Aug 2011;111(2):458–64. doi:10.1152/japplphysiol.00024.2011 [DOI] [PubMed] [Google Scholar]
- 63.Huang Y, Lei W, Zhang W, Huang JA. High-Flow Nasal Cannula in Hypercapnic Respiratory Failure: A Systematic Review and Meta-Analysis. Can Respir J. 2020;2020:7406457. doi:10.1155/2020/7406457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pitre T, Abbasi S, Su J, Mah J, Zeraatkar D. Home high flow nasal cannula for chronic hypercapnic respiratory failure in COPD: A systematic review and meta-analysis. Respir Med. Nov-Dec 2023;219:107420. doi:10.1016/j.rmed.2023.107420 [DOI] [PubMed] [Google Scholar]
- 65.Echevarria C, Steer J, Wason J, Bourke S. Oxygen therapy and inpatient mortality in COPD exacerbation. Emerg Med J. Mar 2021;38(3):170–177. doi:10.1136/emermed-2019-209257 [DOI] [PubMed] [Google Scholar]
- 66.Hanson CW 3rd, Marshall BE, Frasch HF, Marshall C. Causes of hypercarbia with oxygen therapy in patients with chronic obstructive pulmonary disease. Crit Care Med. Jan 1996;24(1):23–8. doi:10.1097/00003246-199601000-00007 [DOI] [PubMed] [Google Scholar]
- 67.Jabre P, Jacob L, Auger H, et al. Capnography monitoring in nonintubated patients with respiratory distress. Am J Emerg Med. Nov 2009;27(9):1056–9. doi:10.1016/j.ajem.2008.08.017 [DOI] [PubMed] [Google Scholar]
- 68.Campion EM, Robinson CK, Brant N, et al. End-tidal carbon dioxide underestimates plasma carbon dioxide during emergent trauma laparotomy leading to hypoventilation and misguided resuscitation: A Western Trauma Association Multicenter Study. J Trauma Acute Care Surg. Nov 2019;87(5):1119–1124. doi:10.1097/ta.0000000000002469 [DOI] [PubMed] [Google Scholar]
- 69.Defilippis V, D’Antini D, Cinnella G, Dambrosio M, Schiraldi F, Procacci V. End-tidal arterial CO2 partial pressure gradient in patients with severe hypercapnia undergoing noninvasive ventilation. Open Access Emerg Med. 2013;5:1–7. doi:10.2147/oaem.s43070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lermuzeaux M, Meric H, Sauneuf B, et al. Superiority of transcutaneous CO2 over end-tidal CO2 measurement for monitoring respiratory failure in nonintubated patients: A pilot study. J Crit Care. Feb 2016;31(1):150–6. doi:10.1016/j.jcrc.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 71.Davidson AC, Banham S, Elliott M, et al. BTS/ICS guideline for the ventilatory management of acute hypercapnic respiratory failure in adults. Thorax. Apr 2016;71 Suppl 2:ii1–35. doi:10.1136/thoraxjnl-2015-208209 [DOI] [PubMed] [Google Scholar]
- 72.Plant PK, Owen JL, Elliott MW. Non-invasive ventilation in acute exacerbations of chronic obstructive pulmonary disease: long term survival and predictors of in-hospital outcome. Thorax. Sep 2001;56(9):708–12. doi:10.1136/thorax.56.9.708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nishikimi M, Nishida K, Shindo Y, et al. Failure of non-invasive respiratory support after 6 hours from initiation is associated with ICU mortality. PLoS One. 2021;16(4):e0251030. doi:10.1371/journal.pone.0251030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merelman AH, Perlmutter MC, Strayer RJ. Alternatives to Rapid Sequence Intubation: Contemporary Airway Management with Ketamine. West J Emerg Med. May 2019;20(3):466–471. doi:10.5811/westjem.2019.4.42753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lentz S, Grossman A, Koyfman A, Long B. High-Risk Airway Management in the Emergency Department. Part I: Diseases and Approaches. J Emerg Med. Jul 2020;59(1):84–95. doi:10.1016/j.jemermed.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]


